INTRODUCTION
Tuberculosis (TB) remains as a significant healthcare challenge in several developing countries even after the global effort to curtail the devastation of the disease under the leadership of international agencies like the United Nations and World Health Organisation (WHO) in the past few years. While undernutrition remains a major risk factor for TB-related morbidity and mortality in resource-poor settings[1], other comorbidities such as advanced kidney disease, human immunodeficiency virus infection and diabetes mellitus (DM) significantly increase the risk of onset and progression of TB[2]. Therefore, healthcare providers should be mindful of these risk factors while diagnosing and managing any patient with TB.
According to the Global Health Observatory of WHO, the median diabetes prevalence in 30 countries with high TB burden in 2021 was 8%, which is expected to increase by 99% by the year 2045[3]. Further, it suggests that DM is associated with a two- to three-fold excess risk of TB, a two-fold excess risk of treatment-related TB mortality, a four-fold higher risk of TB relapse following treatment and a two-fold excess risk of multi-drug resistant TB (MDR-TB). The pooled prevalence of diabetes in TB patients was estimated to be 21% in a meta-analysis of 74 studies from Southeast Asia, higher than in many other regions of the world[4]. The estimated global prevalence of DM in individuals with TB was > 15%, while the total diabetes prevalence was only 9.3% in the year 2019[5,6]. Therefore, an estimated 1.5 million patients worldwide need coordinated care to manage both DM and TB optimally[3].
TB and DM are most common in low- and middle-income nations and these countries report higher rates of TB and DM combination. Six of the ten nations with the largest number of DM patients globally are categorised by the WHO as having a "high burden" of TB, which means they are responsible for 80% of all TB cases globally[3]. Worldwide research on the epidemiology of TB-related diabetes is expanding, and Some areas, such as South India (54%), the Pacific Islands (40%) and northern Mexico (36%), show notably high prevalence rates of diabetes in patients with TB[5]. Understanding the pathobiology, clinical characteristics, appropriate management strategies, and follow-up care is crucial to ensure optimal disease-related clinical outcomes in patients with both conditions.
PATHOBIOLOGY OF TB IN PATIENTS WITH DM
The relationship between TB and DM is bidirectional with each adversely impacting the other in a vicious cycle and compounding the clinical presentation and/or complications associated with them.
Impact of diabetes on TB
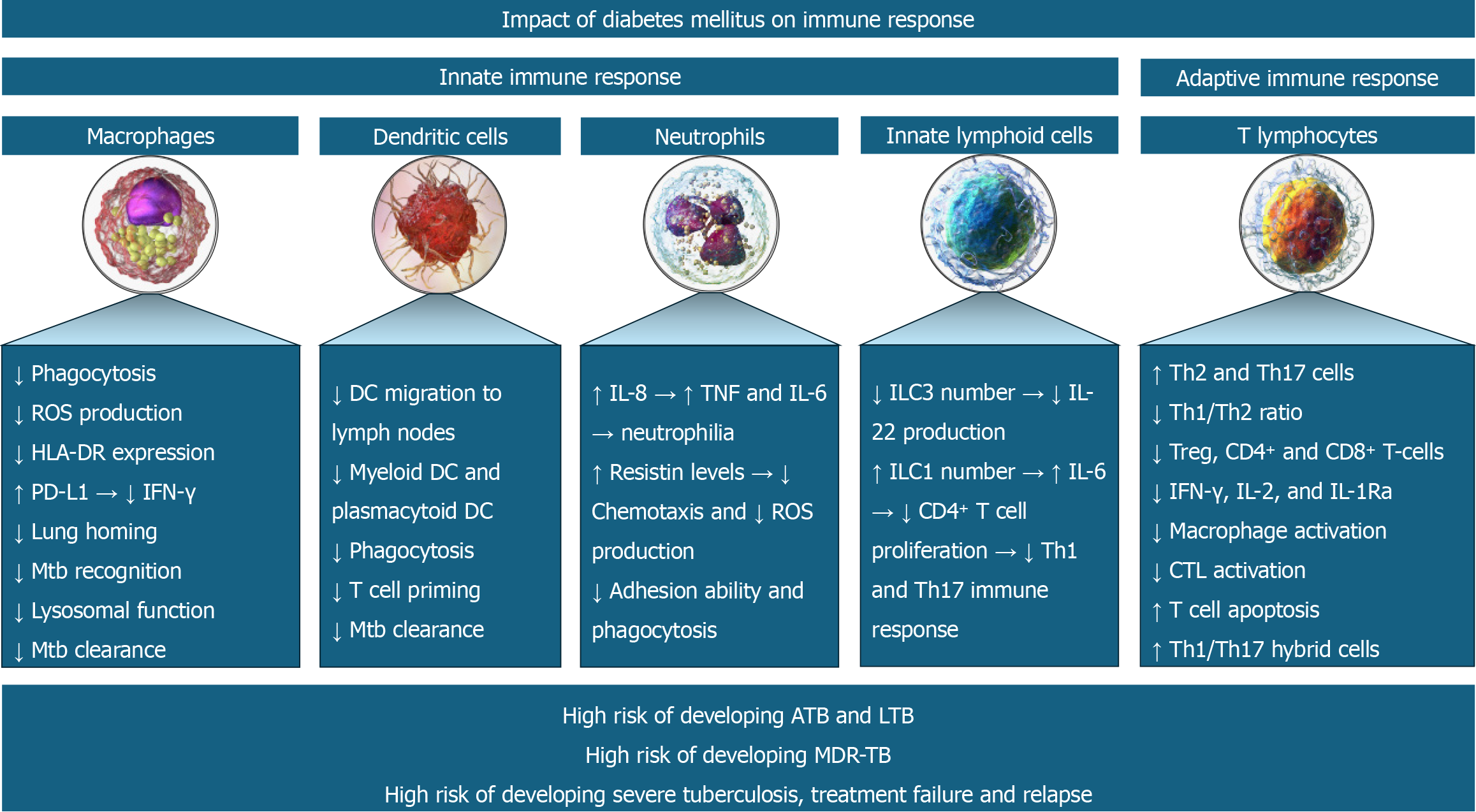
DM is an important risk factor for latent and active TB. Immunological alterations in patients with DM increase the susceptibility to developing active TB. Alterations in both innate and adaptive immune responses mediate the risk of TB in patients with DM[7]. The innate immune response is mediated by macrophages, dendritic cells (DCs), innate lymphoid cells (ILCs), and neutrophils. In contrast, the adaptive immune response is mediated by cytotoxic T lymphocytes (CTLs), T-helper type 1 (Th1), T-helper type 17 (Th17) and regulatory T cells (Tregs)[7]. The immune dysfunction occurs at various levels including T cell abnormalities with altered secretion of cytokines and chemokines, aberrations in innate immunity with impaired functions of neutrophils, macrophages and antigen-presenting cells, and bacterial recognition, abnormalities in B cell function with defective antibody production and alteration in the complement system[8].
Martinez et al[9] reported decreased expression of CD14 and MARCO (macrophage receptor with a collagenous structure – acts as a scavenger) in the alveolar macrophages which are involved in the recognition of mycobacterial cell wall components in hyperglycaemic mice, masking Mycobacterium tuberculosis (Mtb) recognition. DM decreases macrophage function by reducing HLA-DR expression, increasing PD-L1 expression [decreasing Th1 immune response and interferon-gamma (IFN-γ) production], increasing chemokine receptor 2 expression (decreasing monocyte homing to the lungs), and inducing lysosomal dysfunction (decreasing Mtb clearance)[7]. DM is also associated with reduced myeloid and plasmacytoid DC (mDC and pDC) numbers, reduced migration of DCs to lymph nodes for maturation, impaired ability to prime T cells, and thereby impaired Mtb clearance[10].
The chronic low-grade inflammation of DM is associated with increased interleukin (IL)-8, tumor necrosis factor (TNF) and IL-6 production and thereby neutrophil recruitment (neutrophilia)[11]. Raised resistin levels of DM impair the chemotactic function and reactive oxygen species (ROS) generation by neutrophils[12]. DM is associated with decreased ILC3 and increased ILC1 (also known as natural killer cells)[7]. The decreased IL-22 production by the lowered ILC3 frequency decreased the survival of Mtb-infected mice with DM[13]. Similarly, the increased IL-6 production by the raised ILC1 frequency decreased the CD4+ T cell proliferation, Th1 and Th17 immune response and thereby the survival of Mtb-infected mice with diabetes[14]. Though the Th1 number remains unchanged in DM, the Th1/Th2 ratio is decreased due to a marked increase in Th2 and Th17 cells[15]. This is associated with a reduction in Tregs and CD4+ & CD8+ T-cells[16]. Diabetes is associated with a reduction in IL-12 (promoter of Th1 response and IFN-γ production) and an increase in IL-10 (inhibitor of Th1 response), thereby reducing the IFN-γ, IL-2, and IL-1Ra production[17]. The resultant reduced IL-2 reduces the CTL activation and increases the T cell apoptosis[18]. Finally, the reduced IL-1Ra production increases the formation of pathogenic Th1/Th17 hybrid cells that are associated with worse active TB[19].
DM-induced abnormalities in the innate immune system permit easy invasion of respiratory epithelium by the Mtb and prolonged persistence. Abnormalities in the adaptive immune response in the form of dysregulation of the T cell profile and impaired B cell function promote chronic inflammation and granuloma formation[20]. Defective immune response facilitates both the development of primary TB and the reactivation of latent TB. This impaired immune response not only increases the risk of acquiring infection but also increases the severity of infection. The resulting higher bacterial load, poor inflammatory response, reduced bactericidal activity and dissemination may predispose to severe presentations, including the development of military TB[21]. Figure 1 depicts immune system abnormalities predisposing to TB infection and severe presentations of the disease in patients with DM.
Figure 1 Various immune system abnormalities predispose to tuberculosis infection and its severe course in patients with diabetes mellitus.
ATB: Active tuberculosis; LTB: Latent tuberculosis; MDR-TB: Multi-drug resistant tuberculosis.
Impact of TB on glycemia
TB can cause or worsen existing hyperglycaemia. In a meta-analysis by Menon et al[22], newly developed hyperglycaemia was seen in 27.3% of TB patients at baseline. The hyperglycaemia persisted in 50% of subjects at 3-6 months of follow-up. Krishnappa et al[23] demonstrated the adverse impact of TB on glycemia - with 7% of TB patients developing a new diagnosis of DM and 4.5% developing impaired glucose tolerance (IGT). They also reported improvement in blood sugar levels at the end of successful treatment of TB, with 65% of IGT patients reverting to euglycemia following successful treatment of TB.
This hyperglycaemic effect is proposed as secondary to stress, prolonged inflammation, alterations in glucose and lipid metabolism, and the development of insulin resistance among patients with TB. The antitubercular drugs could also influence the pharmacokinetics and pharmacodynamics of the glucose-lowering therapies and worsen the complications of diabetes. Patients with TB and DM carry a higher risk of developing cardiovascular complications such as myocardial infarction and stroke as well as DVT during the early months of TB treatment[24].
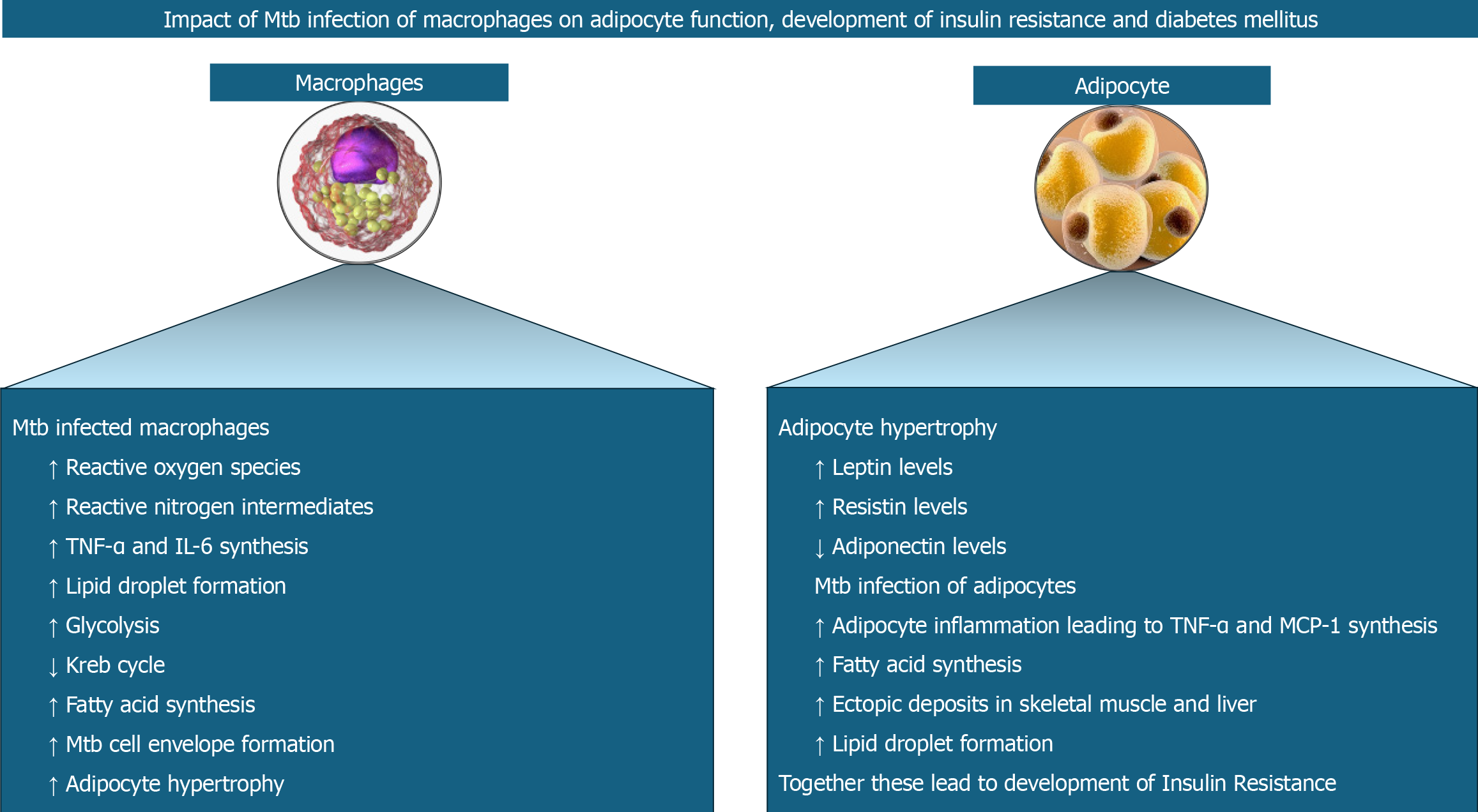
Toll-like receptors (TLRs) enable macrophages to recognize various Mtb proteins[25]. Interaction of proline-proline-glutamic acid (PPE17) or protein Rv1168c of Mtb with TLR2[26] and Mtbhsp60 (heat shock protein 60 of Mtb) with TLR4[27], respectively results in nuclear factor kappa B (NF-kB) signalling, production of ROS and reactive nitrogen intermediates, and expression of pro-inflammatory cytokines including TNF-α and IL-6. Another Mtb protein known as Rv0183, a monoglyceride lipase (mtbMGL) from Mtb, induces myeloid differentiation factor 88 (MyD88)-NF-kB signalling and expression of pro-inflammatory IL-6 and TNF-α[28]. Yet another Mtb protein known as MTP53 (Rv2878c) directly interacts with, and activates transforming growth factor-β-activated kinase 1 or TAK1 independent of TLR2 or MyD88 to enhance pro-inflammatory cytokine expression[29]. Mtb-infected macrophages also secrete matrix metalloproteinases (MMPs), especially MMP-2 and MMP-9, which degrade the extracellular matrix components in the adipose tissue allowing adipocytes to hypertrophy[30].
The adipocyte hypertrophy decreases adiponectin but increases the leptin and resistin expression[21]. Resistin increases lipolysis by increasing the expression of hormone-sensitive lipase[31]. Acting via the mTORC2/Akt pathway in adipocytes, Mtb increases TNF-α and MCP-1[32], with the latter promoting inflammation by attracting monocytes and T lymphocytes[33]. The TNF-α activates protein kinase A, which in turn phosphorylates hormone-sensitive lipase, thereby causing lipolysis in the adipocytes[34]. Ectopic deposition of resultant free fatty acids results in the development of hepatic and skeletal muscle insulin resistance[34].
Mtb protein-TLR interaction on macrophages results in the stabilisation of hypoxia-mediated factor-1α (HIF-1α) in addition to NF-kB signalling and expression of pro-inflammatory cytokines[35]. HIF-1α induces the formation of lipid droplets (a risk factor for DM) and upregulates the glycolytic genes and genes for pro-inflammatory mediators like IL-1β[35]. Lipid droplets are also formed by the TNF-α produced during Mtb infection interacting with TNF receptor-associated factors[36] and various lipases (Rv3091, Rv0183, Rv1592c, Rv2037c, and Rv1683) produced by Mtb present inside the phagosomes[37]. These lipases metabolise host lipids to form fatty acids which are used as the source of energy, as building blocks for cell envelope and as regulators of host immune response. Fatty acids are also produced with the help of the Mtb protein known as ESAT-6 which increases the GLUT-1 (glucose transporter) mediated glucose uptake, upregulates the glycolytic genes, and downregulates the Kreb cycle genes in macrophages[38]. The resultant decreased mitochondrial respiration causes inflammation and thereby worsens insulin resistance[21]. Pyruvate from upregulated glycolysis is converted to lactate and secreted out of the cell. Excess pyruvate is transported into the mitochondria and enters the Kreb cycle resulting in the accumulation of citrate which, on return to the cytoplasm, is converted to acetyl-CoA to be fed into fatty acid biosynthesis[21]. Figure 2 shows the pathobiological aspects of macrophage and adipocyte interactions and the related cellular events perpetuating TB and DM.
Figure 2 Interactions between macrophages and adipocytes with the release of various cytokines and chemokines perpetuate tuberculosis and diabetes mellitus in disease-prone individuals.
Mtb: Mycobacterium tuberculosis; TNF-α: Tumor necrosis factor; IL: Interleukin.
CLINICAL CHARACTERISTICS
Mtb infection in patients with diabetes usually runs a severe clinical course with rapid progression and more severe pulmonary and extrapulmonary manifestations as compared to those without diabetes. Pulmonary TB can often be bilateral and extensive with cavitation and infiltration in those with DM[39]. They also have a higher mycobacterial load. Resistance to anti-tubercular drugs with genotypical drug resistance and late culture conversion are additional concerns in these patients[40]. They may have higher rates of treatment failure, relapse, and mortality compared to TB patients without diabetes. Studies have also reported a 2-fold higher mortality in TB patients with diabetes[41]. Patients with type 2 DM (T2DM) were found to have a 2-fold excess risk of developing MDR-TB[42,43]. Diabetes can also complicate the diagnosis of TB as it can mask some of the usual symptoms of TB or lead to atypical presentations, delaying diagnosis and treatment initiation. The level of HbA1c control has an influence on efficacy of anti-tuberculous therapy as measure by sputum conversion rate and TB focus absorption[44]. Finally, a rising HbA1c trend during and after anti-tuberculous therapy may be a predictor of adverse treatment response in those presenting with HbA1c ≥ 48 mmol/mol at the time of diagnosis of TB[45]. Thus, DM has an impact on the clinical presentation, severity, response to therapy and prognosis of TB.
MANAGEMENT ASPECTS
A high index of clinical suspicion and prompt screening for TB in those with suggestive symptomatology is essential in patients with DM. The co-existence of TB and DM may interfere with the therapeutic intervention of both TB and DM and influence the response to therapies. Although the treatment approach to both Diabetes and TB would not differ significantly, it is important to be aware of drug interactions and to make appropriate modifications to the treatment regimens. It is important to achieve strict glycaemic control for the success of TB treatment. Frequent glucose monitoring and titration of drug doses would be vital to achieve strict control[46]. Steroids given for meningeal and pericardial TB increases the risk of causing hyperglycaemia and this needs to be monitored appropriately[47].
Given the interconnection between TB and diabetes, there is increasing recognition of the need for integrated care for individuals with both TB and diabetes. Measures could be taken for coordinated efforts between TB and diabetes programs to ensure optimal management of both diseases.
FOLLOW-UP CARE
A coordinated approach for long-term care for DM coupled with immediate short-term care for TB management is vital in a successful therapeutic algorithm for patients with both diseases. Ensuring optimal glycaemic control assists in a better response to TB therapies. Compliance with both therapies must be assessed regularly with periodic follow-ups and appropriate testing.
TB and diabetes form a dangerous combination, each exacerbating the other's impact and complicating management. Hence, addressing both diseases together is crucial for better health outcomes. With an increasing diabetes prevalence in pandemic proportions across the globe, there is an urgent need for healthcare providers and professional bodies to be highly vigilant about the dangerous duo of DM and TB. Better preparedness at various levels of health care, a multidisciplinary approach, and coordinated efforts of Diabetes and TB management programs would help to address this dual threat.
EMERGING EVIDENCE ON TB RISK IN PATIENTS WITH T2DM
Ongoing research is expected to shed more light on our knowledge base on the pathobiology, risk factors, prognostication, and management of patients with DM and TB. In their recent study published in the World Journal of Diabetes, Shi et al[48], report factors associated with risk for TB in patients with T2DM from a large cohort of patients from China. This study observed that T2DM subjects with poor glycaemic control, hypoproteinaemia, lymphopenia, history of TB contact, recurrent infections, smoking, and alcohol consumption were associated with an increased risk of developing pulmonary TB. In contrast, being in a married relationship, the presence of hypertension, oral hypoglycaemic agent plus insulin-treated T2DM, overweight, obesity and regular exercise were associated with a reduced risk of developing pulmonary TB in their univariate analysis. While lymphopenia [odds ratio (OR): 17.75], smoking (OR: 12.25), history of TB contact (OR: 6.56) and poor glycaemic control (OR: 3.37) were associated with an excess risk of developing pulmonary TB in patients with T2DM, being overweight (OR: 0.23) and obese (OR: 0.11) posed a lower risk in their multivariate analysis.
The study by Shi et al[48], used a 1:2 ratio case-control design to make data more valuable, wherein the cases and controls were matched for sex, age and T2DM duration. The study used conditional logistic regression analysis. These increased the accuracy in the estimation of the model. However, the shortcoming was the high risk of selection bias, as the samples were collected from hospitals. Although the risk factors for developing TB in patients with DM, and the epidemiological and pathobiological characteristics may be different in other regions of the world, the study by Shi et al[48] provides us with important risk factors for TB in T2DM cases that healthcare providers should be vigilant about at least in the Asian countries so that we can prevent or treat coexistent pulmonary TB in patients with T2DM.
CONCLUSION
The rising prevalence of DM worldwide is acknowledged as a resurgent risk factor and an obstacle to the control of TB. As each of these diseases perpetuates the risk and complications associated with one another, medical professionals should remain highly vigilant with the most up-to-date knowledge about TB and DM, and their co-existence in the same individual to optimise management strategies. More research on epidemiology, pathobiology, clinical characteristics, and diagnostic and therapeutic algorithms should enable improved disease care of this dangerous coexistence. The research data published by Shi et al[48], in the Journal is such an attempt to empower the global scientific fraternity.
ACKNOWLEDGEMENTS
We thank Dr. Marina George Kudiyirickal for providing the audio for the Core Tip of this paper.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country of origin: United Kingdom
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Batta A, India S-Editor: Lin C L-Editor: A P-Editor: Zheng XM