Published online Jul 15, 2024. doi: 10.4239/wjd.v15.i7.1398
Revised: March 18, 2024
Accepted: April 22, 2024
Published online: July 15, 2024
Processing time: 148 Days and 15.3 Hours
Diabetic kidney disease (DKD) is one of the complications of diabetes, affecting millions of people worldwide. The relentless progression of this condition can lead to kidney failure, requiring life-altering interventions such as dialysis or transplants. Accumulating evidence suggests that immunologic and inflammatory elements play an important role in initiating and perpetuating the damage in
Core Tip: Targeting Toll-like receptors (TLRs) via microRNAs is a promising therapeutic approach for reducing inflammation and slowing the progression of diabetic kidney disease (DKD). Understanding how TLR4 expression and signaling are linked to microRNAs regulation, may pave the path for future targeted clinical interventions in DKD.
- Citation: Donate-Correa J, González-Luis A, Díaz-Vera J, Hernandez-Fernaud JR. MicroRNA-630: A promising avenue for alleviating inflammation in diabetic kidney disease. World J Diabetes 2024; 15(7): 1398-1403
- URL: https://www.wjgnet.com/1948-9358/full/v15/i7/1398.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i7.1398
Diabetic kidney disease (DKD) is a common complication of diabetes and the leading cause of kidney failure worldwide. The enormous incidence of DKD has motivated the study of this complication in the most recent phase-3 randomized clinical trials testing kidney protection. As a result of these trials, two new renal protective drugs have been introduced into clinical practice for the treatment of chronic kidney disease (CKD) in subjects with and without diabetes[1]: Sodium-glucose cotransporter-2 inhibitors and the non-steroidal mineralocorticoid receptor antagonist finerenone[1,2]. However, novel therapies are needed as the residual risk of progression to end-stage renal disease remains in this population.
Inflammation is prevalent in diabetes,contributing to the onset and progression of DKD[3,4]. In this disease, a myriad of factors, including hyperglycemia, advanced glycation end products, lipid accumulation, lipotoxicity and increased urinary albumin excretion, act on renal cells, activating cellular and molecular mechanisms that lead to sustained kidney inflammation. In addition, immune cells are recruited into the renal tissue and contributing to kidney damage[5]. To date, promising clinical trials designed to evaluate effective renoprotection by anti-inflammatory drugs in patients with diabetes have failed or were prematurely stopped due to safety concerns[6-9].
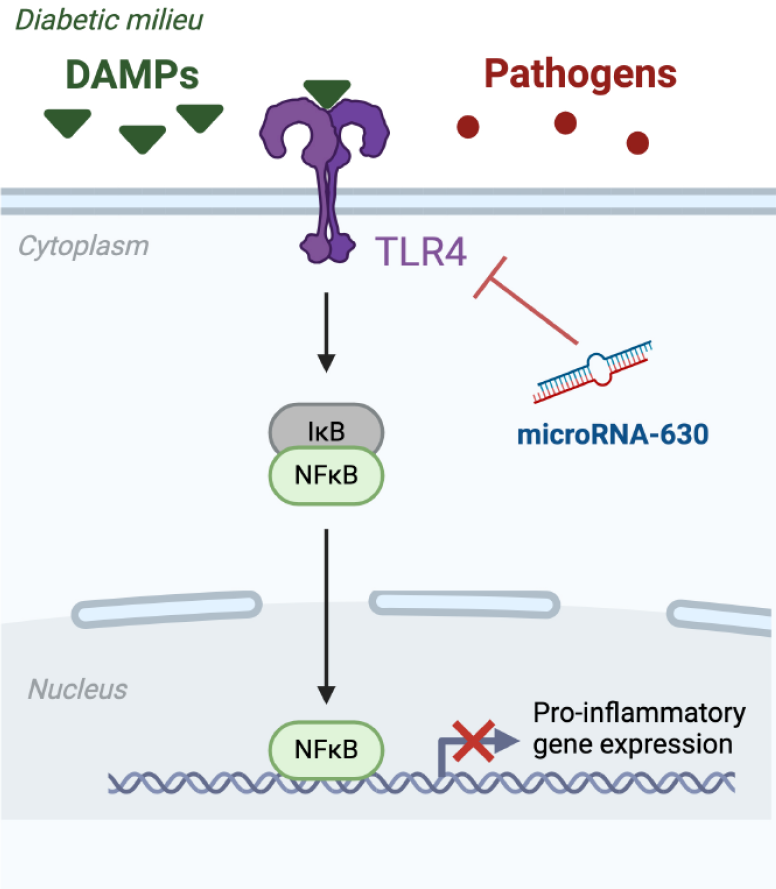
There is striking compelling evidence about the participation of the nuclear factor-kappa B (NF-κB) pathway in the progression of DKD by triggering kidney inflammation and fibrosis[10]. NF-κB is a family of transcription factors involved in different processes, including immune response modulation, cell differentiation and development, inflammation, and tumorigenesis. NF-κB transcription factors are present in cells in an inactive state, forming a complex with inhibitors of NF-κB[10]. Activated NF-κB leads to an increase in the transcription of pro-inflammatory cytokines and chemokines, such as interleukins interleukin-6 (IL-6), IL-1β, and monocyte chemotactic protein-1, thereby initiating local inflammation and leukocyte accumulation[11,12] (Figure 1). The canonical NF-κB pathway responds to diverse stimuli including pathogens which are sensed through multiple classes of pattern-recognition receptors (PRRs) in innate immune cells such as macrophages. Among the cell surface PRRs, Toll-Like receptors (TLRs) have been the most extensively studied. TLRs are a conserved family of pattern recognition receptors that activate downstream inflammatory signaling pathways in response to exogenous microbial pathogens[12], playing a crucial role in the innate immune system. The interplay between TLR4 and NF-κB is a finely tuned mechanism that helps the immune system respond to microbial threats while maintaining balance to prevent excessive inflammation. However, TLRs can activate NF-κB transcription factors in response to nonmicrobial endogenous ligands, namely damage-associated molecular patterns, in addition to microbial pathogens[10]. This activation has been implicated in noninfectious inflammatory conditions such as diabetes[13,14] (Figure 1). TLRs are expressed in various extrinsic and intrinsic cell types in the kidneys including lymphocytes, macrophages, and endothelial and tubular epithelial cells[15]. The activation of renal TLRs, mainly TLR2 and TLR4, has been involved in the development of renal inflammation, leukocyte infiltration, and progressive fibrosis in various acute and CKD diseases, including DKD[16,17]. These observations have generated a new field of study focused on the development of new strategies that target TLR signaling with potential therapeutic utility in DKD.
Among TLRs, TLR2 and TLR4 have been particularly associated with the pathogenesis of renal ischemia-reperfusion injury, acute kidney injury, acute allograft rejection, and DKD[16,18,19]. Compelling evidence suggests that TLR2 and TLR4 are involved in the development of DKD through two pathways. On one hand, the upregulation of several pro-inflammatory endogenous ligands for TLR2 and TLR4 under diabetic conditions is a result of high glucose, hypoxia and hyperlipidemia[20,21]. On the other hand, the diabetic milieu increases TLR2 and TLR4 expression in monocytes, human kidney proximal tubular cells and endothelial cells[9,20,21]. The increased expression of TLRs in DKD amplifies the activation of the innate immune system in response to endogenous diabetes-related ligands, resulting in the development of sustained inflammation and fibrosis in the kidney, which are the hallmarks of DKD[22].
In both animal models and human studies, increased expression of TLR4 has been observed in diabetic kidneys[15]. Recent clinical studies suggest that TLR4, investigated by Wu et al[23], is the major TLR implicated in DKD[23]. Accordingly, kidney biopsies of DKD patients show increased expression of TLR4, but not TLR2, in the tubules[22]. Additionally, in DKD patients, TLR4 levels in renal glomeruli and tubules were found to have a positive correlation with HbA1c levels, albuminuria, interstitial macrophage infiltration, fibrosis, and tubular atrophy score, and a negative correlation with the glomerular filtration rate[20-22].
Experimental studies also suggest that TLR4 is involved in the pathogenesis of DKD. Renal TLR4 expression is increased in type I diabetes mouse models[24]. Nevertheless, TLR4 knock-out in these animals is associated with the amelioration of renal pathology and the reduction of diabetes-related TLR ligands and downstream markers of their activation[16,25,26]. Similarly, inhibiting TLR4 with drugs confers renoprotection to db/db and endothelial nitric oxide synthase knockout diabetes mouse models through different pathways, including metabolic and anti-glomerulosclerosis mechanisms, as well as the inhibition of NF-κB activation[26-29].
The understanding of the intricate relationship between DKD and TLR4 has provided potential therapeutic targets. Modulating TLR4 signaling offers a promising approach to address the underlying inflammation and delay the progression of DKD.
The study by Wu et al[23] in rats explores the therapeutic potential of microRNA-630 in mitigating inflammatory responses associated with DKD. MicroRNAs are short, non-coding RNA molecules, approximately 22 nucleotides in length. They play a pivotal role in regulating gene expression by targeting microRNAs for cleavage or translational repression after binding to the 3’-untranslated region[30]. The human genome contains more than 1000 microRNAs, and it is estimated that approximately 60% of human protein-coding genes may be regulated by microRNAs, suggesting their impact on the regulation of protein synthesis and thus on protein activity. Variations in microRNA expression have been linked to the development of various human diseases and there is increasing evidence pointing to their role in the development of DKD[31-33]. Accordingly, microRNA-302a-3p levels are inversely related to albuminuria in patients with DKD. In vitro experiments suggest its potential role in modulating the renal epithelial-mesenchymal transition process[32]. Conversely, microRNA-184 has been reported to contribute to albuminuria driving renal fibrosis in an experimental rat model of DKD[33]. In addition, microRNA-27a can promote podocyte injury by activating β-catenin in diabetic rats[34].
Wu et al[23], presented evidence of a protective effect of the microRNA-630 on DKD by targeting TLR4 and inhibiting the inflammatory reaction. The authors observed reduced levels of microRNA-630 in the renal tissue of rats with streptozotocin-induced DKD. Furthermore, DKD rats treated with microRNA-630 agomir experienced a significant reduction in albuminuria, glycemia, kidney expression levels of TLR4, and the proinflammatory markers tumor necrosis factor α (TNF-α), IL-1β, and IL6 compared to non-treated rats. In addition, microRNA-630 agomir-injected DKD rats exhibited fewer kidney lesions and reduced infiltration of inflammatory cells. Wu et al[23] also determined the variations in microRNA-630 and TLR4 gene expression levels in the kidney epithelial-like cell line NRK-52E, cultured under high-glucose conditions. Their findings revealed a significant decrease in micorRNA-630 levels and an increase in TLR4 and inflammatory TNF-α, IL-1β, and IL-6 expression. Similarly, high glucose conditions induced a significant decrease in the protein levels of TLR4, α-smooth muscle actin, and collagen IV in NRK-52E cells. Notably, the rise in TLR4, pro-inflammatory cytokines, E-cadherin, α-smooth muscle actin, and collagen IV, which is dependent on high glucose, was abolished when the cells were treated with a mimic of microRNA-630, indicating its relevant regulatory role.
In fact, similar results have recently been observed with microRNA-874 in DKD rats[31]. Consistent with the results obtained by Wu et al[23] with microRNA-630, overexpression of microRNA-874 was able to alleviate kidney injury. Moreover, microRNA-874 reversed the antiproliferative and apoptotic effects in a glucose-induced mouse podocyte model and dramatically attenuated the inflammatory response. Similarly, microRNA-874 was found to be able to reduce TLR4 levels in podocytes.
The evidence presented here suggests that targeting TLR4 and its downstream signaling pathways through microRNAs is a potential avenue for therapeutic intervention in DKD. Inhibitors or modulators of TLR4 signaling are being explored in preclinical and clinical studies as potential treatments to decrease inflammation and delay the progression of kidney disease in diabetic patients.
The study by Wu et al[23] has important implications for the development of novel therapeutic strategies for DKD. MicroRNA-630-based interventions could provide a targeted and precise approach to ameliorate inflammation in diabetic kidneys if the results can be translated to humans. This could potentially slow or even halt the progression of DKD and improve the quality of life for people living with this debilitating complication of diabetes.
As with any groundbreaking research, several challenges and considerations must be addressed. The transition from animal models to human trials is a crucial step that requires careful consideration. Ethical and safety considerations in the development of microRNA-based therapies for DKD include thorough evaluation of long-term safety and efficacy, monitoring for off-target effects, adherence to ethical principles in clinical research, ensuring equitable access and affordability, and compliance with regulatory standards for approval and commercialization. Responsible management of these issues will be critical to the advancement of future microRNA-based therapies for DKD.
The studies on microRNA-630 and its role in alleviating inflammatory responses in rats with DKD offer hope in the field of diabetes-related complications. The potential therapeutic implications of this research are significant and open the door to broader clinical applications, although much work remains to be done. Targeted interventions such as microRNA-630 hold significant promise not only for DKD but also for the treatment of other inflammatory conditions associated with diabetes. In addition, understanding the molecular mechanisms underlying the effects of microRNA-630 may lead to the development of novel therapeutic strategies targeting similar pathways.
Looking ahead, future research in this area could take several directions. First, clinical trials are needed to validate the efficacy and safety of microRNA-630-based interventions in patients with DKD. Longitudinal studies following patients over time would provide valuable insights into the durability of treatment effects and potential long-term benefits. In addition, investigating the interplay between microRNA-630 and other molecular pathways involved in DKD may reveal synergistic therapeutic approaches or identify biomarkers for patient stratification. Moreovoer, off-target effects of microRNA-630 derived from the disruption of normal immune function cloud potentially include an increased susceptibility and recurrence of infections and even reactivation of latent ones.
Furthermore, exploring the potential of microRNA-630 as a diagnostic or prognostic marker for DKD may improve early detection and risk stratification, allowing timely intervention to prevent or slow disease progression. Finally, the evident complexity of DKD demands essential interdisciplinary collaborations integrating expertise from genetics, molecular biology and clinical medicine to advance our understanding of the disease and translate research findings into effective clinical interventions.
The study by Wu et al[23] on the role of microRNA-630 in diabetes is an important milestone but also provides a catalyst for further exploration and innovation in diabetes research. As we further dissect the molecular mechanisms of DKD and continue to develop targeted interventions, we can work toward a future where the burden of diabetes-related renal complications is substantially reduced, improving the lives of patients worldwide.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country of origin: Spain
Peer-review report’s classification
Scientific Quality: Grade B, Grade C, Grade C
Novelty: Grade B, Grade B, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade B
P-Reviewer: Ankrah AO, Netherlands; Wu L, China S-Editor: Li L L-Editor: A P-Editor: Zheng XM
| 1. | Fernandez-Fernandez B, Sarafidis P, Kanbay M, Navarro-González JF, Soler MJ, Górriz JL, Ortiz A. SGLT2 inhibitors for non-diabetic kidney disease: drugs to treat CKD that also improve glycaemia. Clin Kidney J. 2020;13:728-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Ortiz A, Ferro CJ, Balafa O, Burnier M, Ekart R, Halimi JM, Kreutz R, Mark PB, Persu A, Rossignol P, Ruilope LM, Schmieder RE, Valdivielso JM, Del Vecchio L, Zoccali C, Mallamaci F, Sarafidis P; European Renal and Cardiovascular Medicine (EURECA-m) Working Group of the European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney Working Group of the European Society of Hypertension (ESH). Mineralocorticoid receptor antagonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. Nephrol Dial Transplant. 2023;38:10-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 3. | Donate-Correa J, Ferri CM, Sánchez-Quintana F, Pérez-Castro A, González-Luis A, Martín-Núñez E, Mora-Fernández C, Navarro-González JF. Inflammatory Cytokines in Diabetic Kidney Disease: Pathophysiologic and Therapeutic Implications. Front Med (Lausanne). 2020;7:628289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Rayego-Mateos S, Rodrigues-Diez RR, Fernandez-Fernandez B, Mora-Fernández C, Marchant V, Donate-Correa J, Navarro-González JF, Ortiz A, Ruiz-Ortega M. Targeting inflammation to treat diabetic kidney disease: the road to 2030. Kidney Int. 2023;103:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 5. | Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L, Guerrero-Hue M, García-Caballero C, Vázquez-Carballo C, Mas S, Sanz AB, Herencia C, Mezzano S, Gómez-Guerrero C, Moreno JA, Egido J. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 6. | de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM; BEACON Trial Investigators. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 801] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 7. | Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1506] [Cited by in RCA: 1492] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 8. | Packham DK, Wolfe R, Reutens AT, Berl T, Heerspink HL, Rohde R, Ivory S, Lewis J, Raz I, Wiegmann TB, Chan JC, de Zeeuw D, Lewis EJ, Atkins RC; Collaborative Study Group. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, Pohl MA, Rohde RD, Raz I, Yerushalmy Y, Yagil Y, Herskovits T, Atkins RC, Reutens AT, Packham DK, Lewis JB; Collaborative Study Group. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 387] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Wei M, Li Z, Xiao L, Yang Z. Effects of ROS-relative NF-κB signaling on high glucose-induced TLR4 and MCP-1 expression in podocyte injury. Mol Immunol. 2015;68:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Yang S, Zhang J, Wang S, Zhao X, Shi J. SOCS2 overexpression alleviates diabetic nephropathy in rats by inhibiting the TLR4/NF-κB pathway. Oncotarget. 2017;8:91185-91198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5843] [Cited by in RCA: 6712] [Article Influence: 447.5] [Reference Citation Analysis (0)] |
| 14. | Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 15. | Lin M, Tang SC. Toll-like receptors: sensing and reacting to diabetic injury in the kidney. Nephrol Dial Transplant. 2014;29:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23:86-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 17. | Li F, Yang N, Zhang L, Tan H, Huang B, Liang Y, Chen M, Yu X. Increased expression of toll-like receptor 2 in rat diabetic nephropathy. Am J Nephrol. 2010;32:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Chen J, John R, Richardson JA, Shelton JM, Zhou XJ, Wang Y, Wu QQ, Hartono JR, Winterberg PD, Lu CY. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 2011;79:288-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol. 2004;172:2629-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Mudaliar H, Pollock C, Komala MG, Chadban S, Wu H, Panchapakesan U. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Renal Physiol. 2013;305:F143-F154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090-3098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 359] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 22. | Mudaliar H, Pollock C, Ma J, Wu H, Chadban S, Panchapakesan U. The role of TLR2 and 4-mediated inflammatory pathways in endothelial cells exposed to high glucose. PLoS One. 2014;9:e108844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Wu QS, Zheng DN, Ji C, Qian H, Jin J, He Q. MicroRNA-630 alleviates inflammatory reactions in rats with diabetic kidney disease by targeting toll-like receptor 4. World J Diabetes. 2024;15:488-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Cardenas-Gonzalez M, Srivastava A, Pavkovic M, Bijol V, Rennke HG, Stillman IE, Zhang X, Parikh S, Rovin BH, Afkarian M, de Boer IH, Himmelfarb J, Waikar SS, Vaidya VS. Identification, Confirmation, and Replication of Novel Urinary MicroRNA Biomarkers in Lupus Nephritis and Diabetic Nephropathy. Clin Chem. 2017;63:1515-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Jialal I, Major AM, Devaraj S. Global Toll-like receptor 4 knockout results in decreased renal inflammation, fibrosis and podocytopathy. J Diabetes Complications. 2014;28:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Ma J, Chadban SJ, Zhao CY, Chen X, Kwan T, Panchapakesan U, Pollock CA, Wu H. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS One. 2014;9:e97985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Zhu X, Yuan S, Wen S, Liu X, Wang C, Qu Z, Li J, Liu H, Sun L, Liu F. TLR4/NF-κB Signaling Induces GSDMD-Related Pyroptosis in Tubular Cells in Diabetic Kidney Disease. Front Endocrinol (Lausanne). 2019;10:603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 28. | Cha JJ, Hyun YY, Lee MH, Kim JE, Nam DH, Song HK, Kang YS, Lee JE, Kim HW, Han JY, Cha DR. Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology. 2013;154:2144-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Lin M, Yiu WH, Li RX, Wu HJ, Wong DW, Chan LY, Leung JC, Lai KN, Tang SC. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 2013;83:887-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 27800] [Article Influence: 1323.8] [Reference Citation Analysis (0)] |
| 31. | Yao T, Zha D, Gao P, Shui H, Wu X. MiR-874 alleviates renal injury and inflammatory response in diabetic nephropathy through targeting toll-like receptor-4. J Cell Physiol. 2018;234:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Tang WB, Zheng L, Yan R, Yang J, Ning J, Peng L, Zhou Q, Chen L. miR302a-3p May Modulate Renal Epithelial-Mesenchymal Transition in Diabetic Kidney Disease by Targeting ZEB1. Nephron. 2018;138:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Zanchi C, Macconi D, Trionfini P, Tomasoni S, Rottoli D, Locatelli M, Rudnicki M, Vandesompele J, Mestdagh P, Remuzzi G, Benigni A, Zoja C. MicroRNA-184 is a downstream effector of albuminuria driving renal fibrosis in rats with diabetic nephropathy. Diabetologia. 2017;60:1114-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Zhou Z, Wan J, Hou X, Geng J, Li X, Bai X. MicroRNA-27a promotes podocyte injury via PPARγ-mediated β-catenin activation in diabetic nephropathy. Cell Death Dis. 2017;8:e2658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |