Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1317
Revised: February 22, 2024
Accepted: April 1, 2024
Published online: June 15, 2024
Processing time: 168 Days and 11.6 Hours
Diabetic retinopathy (DR) is the primary cause of visual problems in patients with diabetes. The Heyingwuzi formulation (HYWZF) is effective against DR.
To determine the HYWZF prevention mechanisms, especially those underlying mitophagy.
Human retinal capillary endothelial cells (HRCECs) were treated with high glucose (hg), HYWZF serum, PX-478, or Mdivi-1 in vitro. Then, cell counting kit-8, transwell, and tube formation assays were used to evaluate HRCEC proliferation, invasion, and tube formation, respectively. Transmission electron microscopy was used to assess mitochondrial morphology, and Western blotting was used to determine the protein levels. Flow cytometry was used to assess cell apoptosis, reactive oxygen species (ROS) production, and mitochondrial membrane potential. Moreover, C57BL/6 mice were established in vivo using streptozotocin and treated with HYWZF for four weeks. Blood glucose levels and body weight were monitored continuously. Changes in retinal characteristics were evaluated using hematoxylin and eosin, tar violet, and periodic acid-Schiff staining. Protein levels in retinal tissues were determined via Western blotting, immunohistochemistry, and immunostaining.
HYWZF inhibited excessive ROS production, apoptosis, tube formation, and invasion in hg-induced HRCECs via mitochondrial autophagy in vitro. It increased the mRNA expression levels of BCL2-interacting protein 3 (BNIP3), FUN14 domain-containing 1, BNIP3-like (BNIP3L, also known as NIX), PARKIN, PTEN-induced kinase 1, and hypoxia-inducible factor (HIF)-1α. Moreover, it downregulated the protein levels of vascular endothelial cell growth factor and increased the light chain 3-II/I ratio. However, PX-478 and Mdivi-1 reversed these effects. Additionally, PX-478 and Mdivi-1 rescued the effects of HYWZF by decreasing oxidative stress and apoptosis and increasing mitophagy. HYWZF intervention improved the symptoms of diabetes, tissue damage, number of acellular capillaries, and oxidative stress in vivo. Furthermore, in vivo experiments confirmed the results of in vitro experiments.
HYWZF alleviated DR and associated damage by promoting mitophagy via the HIF-1α/BNIP3/NIX axis.
Core Tip: Our study demonstrates the protective effects and action mechanisms of the Heyingwuzi formulation against diabetic retinopathy (DR) in vivo and in vitro. Here, we developed a new treatment formulation for DR based on traditional Chinese medicine and revealed its action mechanism involving the promotion of mitophagy via regulation of the hypoxia-inducible factor-1α/BCL2-interacting protein 3 (BNIP3)/BNIP3-like (BNIP3L, also known as NIX) axis.
- Citation: Wu JJ, Zhang SY, Mu L, Dong ZG, Zhang YJ. Heyingwuzi formulation alleviates diabetic retinopathy by promoting mitophagy via the HIF-1α/BNIP3/NIX axis. World J Diabetes 2024; 15(6): 1317-1339
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1317.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1317
Diabetes mellitus (DM) is a common metabolic disease characterized by chronic hyperglycemia, possibly leading to multi-organ damage, with no specific treatment[1]. The World Health Organization reports 422 million adults (8.5%) with DM worldwide, and this number is expected to increase to 592 million by 2035[2,3]. DM may induce various complications, such as cardiovascular, kidney, and eye diseases, posing a huge economic burden on affected individuals and healthcare systems. Diabetic retinopathy (DR) has attracted attention as the main cause of visual impairment and blindness in patients with diabetes[4]. The number of affected individuals is increasing annually with the increase in the incidence of diabetes and lifespan of patients[5]. Currently, pan-retinal photocoagulation (PRP) and anti-vascular endothelial cell growth factor (VEGF) therapy are the main treatments for DR. Although both methods are moderately effective, PRP may cause the loss of peripheral vision and macular edema, whereas anti-VEGF agents have a short duration of action[6,7]. Therefore, the development of novel and effective strategies is necessary for DR treatment.
Recently, traditional Chinese medicine is widely used for the comprehensive treatment of DM by improving the permeability of the blood-retinal barrier, retinal edema, and blood supply. These traditional medicines are widely used for clinical treatment and research. Heyingwuzi formulation (HYWZF) consists of Rehmannia glutinosa (R. glutinosa, 10 g), Angelica sinensis (10 g), figwort root (10 g), honeysuckle (10 g), dandelion (30 g), danshen (15 g), barbary wolfberry fruit (10 g), sealwort (10 g), pale butterfly bush flower (10 g), glossy privet fruit (10 g), chuanxiong (10 g), Cuscuta (15 g), Chushizi (10 g), white chrysanthemum (10 g), and Plantago (15 g). R. glutinosa is a common bulk medicinal plant widely used in China owing to its active ingredients. R. glutinosa improves diabetic damage via the advanced glycation end products/receptor for AGE/hypoxia-inducible factor (HIF)-1α and nuclear factor-κB/NLR family pyrin domain-containing 3 signaling pathways[8,9]. Plantago extract reduces the blood glucose levels in streptozotocin (STZ)-induced diabetic mice[10]. Salvianolic acid A is an active phenolic acid derived from danshen that alleviates diabetic complications[11]. Dandelion is used in herbal medicine for its choleretic, antirheumatic, and diuretic properties[12]. Dandelion water extract supplements improve the lipid metabolism and prevent complications in diabetic rats[13]. Therefore, we hypothesized that HYWZF also alleviates DR-induced damage.
Mitophagy is a type of self-regulation in which damaged mitochondria are broken down and reused to maintain the balance of material and energy metabolism[14]. Many studies have reported an association between mitophagy and DM. Zhang et al[15] reported that the upregulation of mitophagy and antioxidant enzyme activity reduces the oxidative stress in the liver, thereby exerting protective effects against diabetes. Tang et al[16] revealed that melatonin plays a protective role in diabetic nephropathy via the AMP-activated protein kinase/PTEN-induced kinase 1 mitophagy pathway. A recent study reported that HIF-1α upregulates mitochondrial autophagy in the spinal cord of mice with diabetic neuropathic pain by regulating the Parkin signaling pathway[17]. HIF-1α triggers mitophagy in nucleus pulposus cells via BCL2-interacting protein 3 (BNIP3). However, whether HYWZF mediates mitophagy via HIF-1α to reduce DR injury remains unknown.
Mitophagy occurs via two main pathways: Receptor- and non-receptor-mediated pathways. The receptor-dependent pathway involves mitochondrial outer membrane proteins, such as BNIP3, BNIP3-like (BNIP3L, also known as NIX), and FUN14 domain-containing 1 (FUNDC1)[18]. As DM is a starvation-stimulated disease, we aimed to characterize the receptor-mediated mitophagy pathway and explore whether HIF-1α regulates mitophagy in this study. Consistent with previous reports, we found that HYWZF promoted mitophagy via HIF-1α. Therefore, this study provides a strong foundation for the development of effective DR treatment methods.
Male C57BL/6 mice (8-wk-old, 22-30 g) purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., LTD were housed in a temperature-controlled room (22 °C ± 2 °C) under a 12 h/h light/dark cycle with free access to food and water. The model group was intraperitoneally injected with STZ (50 mg/kg/d) for five days to induce diabetes[19], whereas the control group (con) was injected with an equal volume of sodium citrate buffer (10 mg/mL). The model group mice were randomly divided into three different groups (n = 6 each): Diabetic model (mod), HYWZF-low-dose (HYWZF-L), and HYWZF-high-dose (HYWZF-H) groups. Mice in the HYWZF-L and HYWZF-H groups were orally administered low (12 g/kg/d) and high (24 g/kg/d) doses of HYWZF, respectively, for four weeks. Mice in the control and model groups were administered equal volumes of sterilized water.
Mice in HYWZF-treated groups were administered 60.125 g/kg of HYWZF twice for three days. Mice in the control group received equal volumes of normal saline. Two hours after the final administration, mice were anesthetized using isoflurane. The collected blood was allowed to stand at room temperature and centrifuged to obtain the supernatant after inactivation at 56 °C for 30 min.
Human retinal capillary endothelial cells (HRCECs; BFN60804011) were purchased from Qingqi Biotechnology Development Co., Ltd. Normal (5.5 mM) or high glucose medium (33 mM) was used to culture the cells at 37 °C and 5% CO2. After the cells adhered to the wall, serum starvation was carried out for 24 h. After replacing with a fresh medium, the cells were cultured for 48 h. Cells cultured in the high glucose medium were randomly divided into five groups: high glucose (hg), hg + negative serum (NEG), hg + 10% HYWZF serum (hg + ps), hg + 10% HYWZF serum + HIF-1α inhibitor (hg + ps + PX-478), hg + 10% HYWZF serum + mitochondrial inhibitor (hg + ps + Mdivi-1) groups. After high glucose treatment, the hg + ns group was treated with the NEG, whereas the hg + ps + HIF-1α inhibitor and hg + ps + mitochondrial inhibitor groups were treated with PX-478 (10 μM, S7612) and Mdivi-1 (25 μM, S7162), respectively, for 2 h. Then, hg + ps, hg + ps + HIF-1α inhibitor, and hg + ps + mitochondrial inhibitor groups were treated with 10% HYWZF serum for 48 h.
Cell viability was determined using the cell counting kit (CCK)-8 kit (BS350A; Biosharp, China). Briefly, 100 µL of HRCECs (2 × 103 cells/well) were seeded on a 96-well plate. After the cells were completely attached to the cell wall, 10 µL CCK-8 solution was added to each well and incubated for 2 h. Finally, a microplate reader (Spectra max PLUS 384; Molecular Devices) was used to measure the absorbance at 450 nm.
Cell invasion was assessed using the transwell assay with the transwell chambers (8-μm pore; Corning, United States). HRCECs (1 × 105 cells/mL) suspended in a serum-free culture medium with or without HYWZF were added to the upper chamber. The lower chamber was filled with 600 μL of complete medium. After 48 h, the non-invading cells on the upper chamber were wiped off and the cells on the lower chamber were fixed with methanol and stained with 0.1% crystal violet. After air-drying, digital images were acquired, and the number of invasive cells was counted under a microscope (Olympus BX61, Olympus, Japan).
Matrigel (200 µL) was added to each well of a 96-well plate and placed in an incubator for 30 min. HRCECs (2 × 105 cells/well) were seeded in the matrigel-coated plates and incubated at 37 °C for 16 h, after which the number of tubes was counted in two random fields from each well under a microscope (Olympus BX61, Olympus, Japan).
HRCEC apoptosis was detected using the Annexin V-APC/propidium iodide (PI) apoptosis detection kit (KGA1030; KeyGEN Bio-Tech, China). Cells were seeded in a 6-well plate at a density of 3 × 105 cells/well and treated with HYWZF for 48 h. Then, the cells were digested, washed with phosphate-buffered saline (PBS; AM9624; Thermo Fisher Scientific, United States), and stained with annexin V (5 μL) and PI (5 μL) for 15 min in the dark. Cell apoptosis was assessed using a flow cytometer (CytoFlex; Beckman Coulter).
Total RNA was obtained from cells using the Total RNA Kit (19221ES50; YEASEN, China) and reverse-transcribed into cDNA using the PrimeScript RT reagent kit (RR047A; Baori Doctor Biotechnology, China). Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using Tli RNase H Plus (RR820A; Baori Doctor Biotechnology). The reaction conditions for qRT-PCR were as follows: Pre-denaturation heating at 95 °C for 30 s, 40 cycles of denaturation at 95 °C for 5 s, annealing at 56 °C, and extension at 72 °C for 30 s. Relative expression was determined using the 2-ΔΔCt method[20]. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal reference. Each sample was tested thrice. All primer sequences are listed in Table 1.
| Gene | Forward primers | Reverse primers |
| HIF-1α | TGCTCATCAGTTGCCACTTCCAC | CACCCTGTTGCTGTAGCCAAA |
| BNIP3 | AATAATGGGAACGGGGGCAG | CCCCCTTTCTTCATGACGCT |
| NIX | CACACCAGCAGGGACCATAG | TGTGCTCAGTCGCTTTCCAA |
| FUNDC1 | CCCCCTCCCCAAGACTATGA | TGCAAGTCCGAGCAAAAAGC |
| PINK1 | AGTCCATTGGTAAGGGCTGC | AAATCTGCGATCACCAGCCA |
| Parkin | AATCAGAGGGCTCTGGAGGT | TCATCCGAATGGACCGGATT |
| GAPDH | TGACTTCAACAGCGACACCCA | CACCCTGTTGCTGTAGCCAAA |
HRCECs were lysed using the radioimmunoprecipitation assay buffer (P0013; Biyuntian, China). The supernatant was collected, and protein concentration was quantitatively analyzed using the BCA kit (P0009; Biyuntian). Protein samples were treated with the sodium dodecyl sulfate (SDS) loading buffer for 5 min, subjected to SDS-polyacrylamide gel electrophoresis, and transferred onto polyvinylidene fluoride membranes (ISEQ00010; Sigma-Aldrich, United Kingdom). The membranes were blocked with TBST containing 5% skim milk for 1 h and incubated overnight at 4 °C with anti-P62 (A19700; 1:1000; Abclonal, China), anti-light chain 3 (LC3)-B (A19665; 1:1000; Abclonal), anti-β-actin (AC026; 1:50000; Abclonal), anti-BNIP3L (DF8163; 1:3000; Affinity, United States), anti-COX IV (A6564; 1:5000; Abclonal), anti-Beclin1 (A7353; 1:2000; Abclonal), anti-HIF-1α (BS-20399R; 1:2000; Bioss, China), anti-Bcl2 (A19693; 1:2000; Abclonal), anti-caspase-3 (A2156; 1:1000; Abclonal), anti-claudin-5 (AF5216; 1:2000; Affinity), anti-occludin (A2601; 1:1000; Abclonal), anti-VEGF (BS-34032R; 1:1000; Bioss), anti-matrix metalloproteinase (MMP)-9 (A11147; 1:1000; Abclonal), and anti-BNIP3 (BS-4239R; 1:2000; Bioss) antibodies. Then, the membranes were incubated again with goat anti-rabbit IgG (ab150077; 1:1000; Abcam, United Kingdom) secondary antibodies for 1 h at room temperature and captured using the iBright CL750 instrument (A44116; Thermo Fisher Scientific) with the ECL Plus HRP substrate kit (k22030; Abbkine, United States).
High-performance LC (HPLC; Ultimate 3000; Thermo Fisher Scientific) and mass spectrometer (TripleTOF5600+; AB SCIEX, CA, United States) were used to identify the components in the HYWZF serum. A Sepax GP-C18 Column (1.8 µm, 120 Å, 2.1 mm × 150.0 mm) was used to load the serum after activation by methanol and water. Moreover, 0.1% formic acid was used as mobile phase A and 100% ACN as mobile phase B. Each component was analyzed for 21 min at 40 °C at 0.3 mL/min. The similarities among the HPLC fingerprints of six batches of serum samples were evaluated using the Computer-Aided Similarity Evaluation System for Chromatographic Fingerprints of Traditional Chinese Medicine. The fingerprint similarities at 238 nm and 440 nm were greater than or equal to 0.982 and 0.862, respectively. Electrospray ionization positive and negative ion modes were used for MS analysis. Secondary mass spectra were obtained using information-dependent acquisition. MS-DIAL 4.70 software and various databases, including the MassBank, RIKEN tandem mass spectral, and Global Natural Products Social Molecular Networking databases, were used to analyze the HYWZF components.
Cells were uniformly resuspended in the JC-1 working solution (500 µL) and incubated at 37 °C and 5% CO2 for 20 min. After centrifugation at 350 × g for 5 min, the supernatant was discarded, and cells were washed twice with 1 × incubation buffer (1 mL). Then, cells were resuspended in 500 µL of 1 × incubation buffer and analyzed using a flow cytometer.
HRCECs (5 × 105 cells/well) were seeded in 6-well plates and cultured overnight. After PBS washing, cells were collected and incubated with 5 µM 2’,7’-dichlorodihydrofluorescein diacetate for 30 min in an incubator. The fluorescence intensity of the cells was analyzed using a flow cytometer.
HRCECs were fixed with 2.5% glutaraldehyde at 4 °C overnight and washed thrice with PBS. The cells were then fixed with 2% osmium tetroxide at room temperature for 2 h, dehydrated with ethanol (50%, 70%, 80%, 90%, and 100%), and embedded in Epon812 resin (1260804; SPI). The sections were stained with uranyl acetate/lead citrate and examined under a transmission electron microscope (JEM-1400FLASH; Japan).
Superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and ATP activities were determined using the total SOD activity (A001-3-1; Nanjing Jiancheng Bioengineering Institute, China), MDA activity (A003-1-2), GSH-Px activity (A005-1-2), and ATP activity (A016-2-1) detection kits. EB was performed as described by Sun et al[21].
Retinal tissues were fixed with 4% paraformaldehyde for 48 h at 4 °C and embedded in paraffin. Paraffin sections (5 μm) were then deparaffinized, dehydrated with alcohol, and stained with Hematoxylin and eosin (HE) and 0.1% tar violet staining solution (G1700; Beijing Solaibao Technology Co., Ltd., China). After washing with distilled water, the sections were dehydrated with alcohol, cleared with xylene, and mounted using distyrene plasticizer xylene.
Tissue sections were incubated with the periodate solution (G1280; Wuhan Saiweier Biotechnology Co., Ltd., China) for 8 min, dipped in Schiff’s reagent for 15 min, and stained with hematoxylin for 1 min. After staining, the slides were dehydrated using a gradient of alcohol and cleared with xylene for 2 min after each reagent.
After antigen retrieval and peroxidase blocking, tissue slices were incubated with anti-VEGF (05-443; 1:100; Sigma-Aldrich, United States) and zonula occludens (ZO)-1 (21773-1-AP; 1:100; Proteintech, China) antibodies for 24 h at 4 °C, followed by incubation with secondary goat anti-rabbit antibodies (GB23303; 1:100; Servicebio, China) at 37 °C for 30 min. Finally, DAB chromogenic solution (ZLI-9018; 1:20; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., China) was added with hematoxylin as a mild counterstain, and the sections were dehydrated in a graded series of ethanol and sealed.
Tissue slices were blocked with 3% bovine serum albumin (GC305010; Wuhan Sevier Biotechnology Co., Ltd. China) at 25 °C for 30 min and incubated with the anti-lysosome membrane-associated protein (LAMP)-2 (66301-1-IG; 1:100; Proteintech, United States), LC3B (14600-1-AP; 1:100; Proteintech), and Tom20 (11802-1-AP; 1:100; Proteintech) antibodies. The sections were then incubated with goat anti-rabbit IgG (GB22303; 1:100; Servicebio) or goat anti-mouse IgG (GB21301; 1:100; Servicebio) antibodies.
All statistical analyses were conducted using SPSS23.0 (IBM). Statistical significance was set at P < 0.05. Results are expressed as the mean ± SD. One-way analysis of variance was used to compare multiple groups.
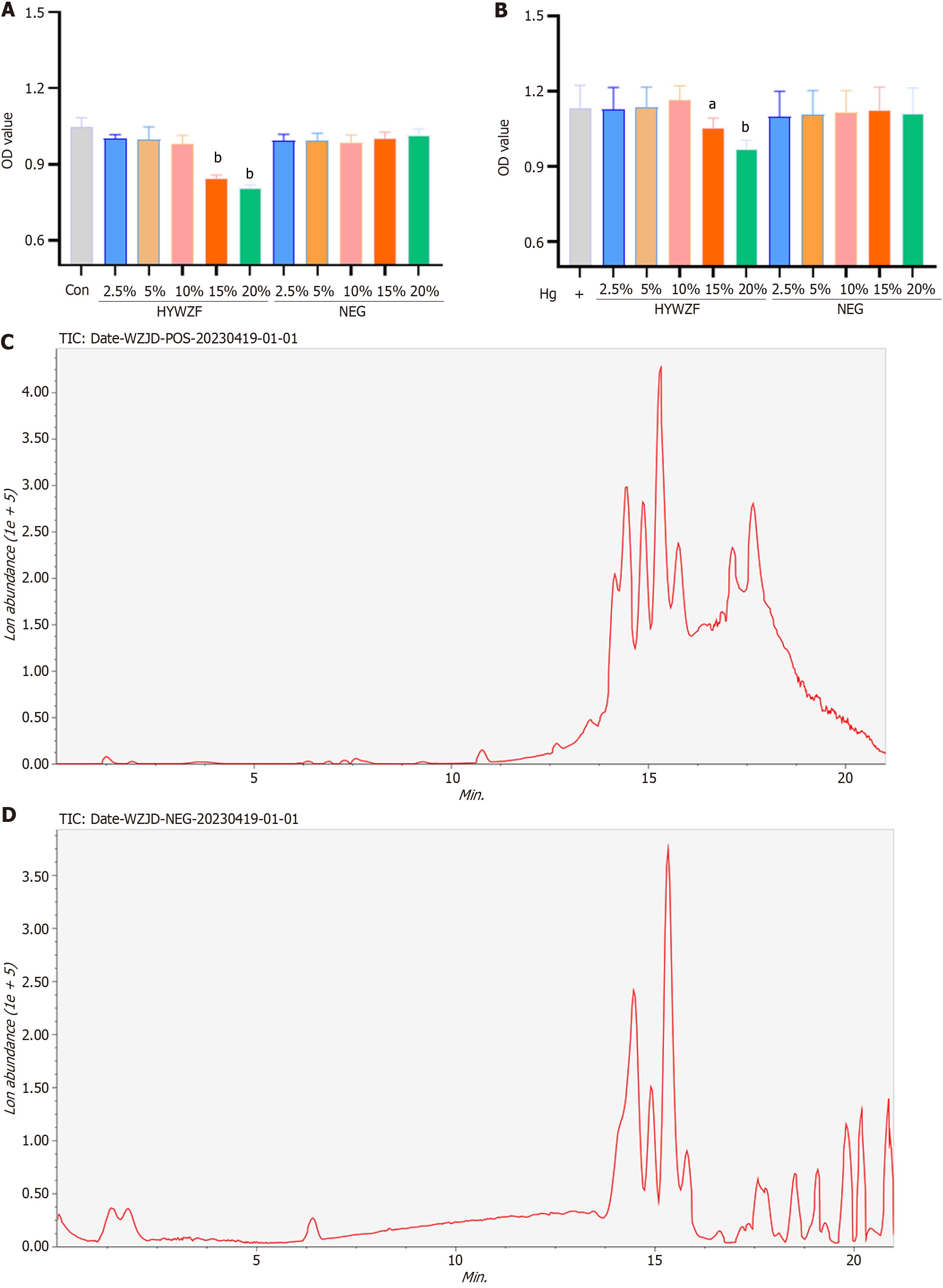
CCK-8 assay was used to determine the effects of HYWZF serum on HRCECs in a medium-to high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (HyClone, China). As shown in Figure 1A and B, 15% HYWZF serum inhibited the cell viability in normal and high-glucose DMEM, whereas 10% HYWZF serum did not affect the cell viability. Chromatographic analysis (Figure 1C and D) revealed 114 bioactive constituents in HYWZF (Table 2; Supplementary Table 1).
| No. | Title | RT (min) | Area | Ontology |
| 1 | Phosphatidylethanolamine lyso 22 | 13.94715 | 1899972.00 | 2-acyl-sn-glycero-3-phosphoethanolamines |
| 2 | Phosphatidylethanolamine lyso 18 | 15.32940 | 226900.40 | 2-acyl-sn-glycero-3-phosphoethanolamines |
| 3 | Citric acid | 1.820983 | 184061.70 | Tricarboxylic acids and derivatives |
| 4 | sn-Glycero-3-phosphocholine | 14.45802 | 144480.10 | Glycerophosphocholines |
| 5 | (2R,3R,6R,8R,9S,12S,13R,14R,15R,16R)-6,8,14,15-tetrahydroxy-2,6,13,16-tetramethyl-3-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-10-oxatetracyclo[7.6.1.0,.0,]hexadec-4-en-11-one | 14.30263 | 143726.20 | Eudesmanolides, secoeudesmanolides, and derivatives |
| 6 | Phosphatidylethanolamine lyso 20 | 13.50750 | 80798.77 | 2-acyl-sn-glycero-3-phosphoethanolamines |
| 7 | 2-[(4S,5S,5aS,9aS)-4-methoxy-6,6,9a-trimethyl-5-[(2E,4E,6E)-octa-2,4,6-trienoyl]oxy-1-oxo-4,5,5a,7,8,9-hexahydro-3H-benzo[e]isoindol-2-yl]pentanedioic acid | 15.12730 | 50046.63 | Glutamic acid and derivatives |
| 8 | Phosphatidylethanolamine lyso alkenyl 16 | 15.92828 | 20239.35 | Glycerophosphoethanolamines |
| 9 | USNIC ACID | 7.950984 | 17973.83 | Acetophenones |
| 10 | FORMONONETIN | 11.89108 | 16466.15 | 4’-O-methylisoflavones |
| 11 | PFCA-unsaturated | 1.420167 | 15911.72 | PFSA |
| 12 | 3-hydroxy-1a,5-bis(hydroxymethyl)-5,6b-dimethyl-1,3,3a,4,6,6a-hexahydrocyclopropa[e]inden-2-one | 11.26097 | 15753.38 | Cyclic alcohols and derivatives |
| 13 | 4-[5-[[4-[5-[acetyl(hydroxy)amino]pentylamino]-4-oxobutanoyl]-hydroxyamino]pentylamino]-4-oxobutanoic acid | 14.49665 | 426921.60 | N-acyl amines |
| 14 | LPE 16:0 | 14.81610 | 94029.74 | Lipids |
| 15 | Cytarabine | 1.46050 | 28727.73 | Pyrimidine nucleosides |
| 16 | Indole-3-acetyl-L-alanine | 7.712983 | 17271.44 | Amino acids |
| 17 | Taurocholic acid | 13.89302 | 14700.46 | Trihydroxy bile acids, alcohols, and derivatives |
| 18 | 10-Hydroxydecanoic acid | 11.39715 | 11104.43 | Medium-chain hydroxy acids and derivatives |
| 19 | Phosphatidylethanolamine lyso 16 | 15.29023 | 178188.7 | 2-acyl-sn-glycero-3-phosphoethanolamines |
| 20 | Actrarit | 6.760533 | 23273.13 | Benzene and substituted derivatives |
| 21 | Pyroglutamic acid | 1.820983 | 19421.45 | Alpha amino acids and derivatives |
| 22 | Atenolol acid | 11.30097 | 12153.77 | Phenol ethers |
| 23 | D-PANTOTHENIC ACID | 1.94130 | 22119.00 | Secondary alcohols |
| 24 | Acacetin | 18.55157 | 15547.79 | 4’-O-methylated flavonoids |
| 25 | (1R,6R,10S,11R,13S,15R)-1,6-dihydroxy-8-(hydroxymethyl)-4,12,12,15-tetramethyl-5-oxotetracyclo[8.5.0.0,.0,]pentadeca-3,8-dien-13-yl hexadecanoate | 14.02730 | 61856.96 | Phorbol esters |
| 26 | arctigenin | 11.81143 | 24300.10 | Dibenzylbutyrolactone lignans |
| 27 | 4-Hydroxy-4-methyl-2-pentanone | 20.19918 | 11647.11 | Beta-hydroxy ketones |
| 28 | 12-hydroxyeicosapentaenoic acid | 14.65860 | 84224.38 | Hydroxyeicosapentaenoic acids |
| 29 | (+/-)-Synephrine | 20.86260 | 72999.33 | 1-hydroxy-2-unsubstituted benzenoids |
| 30 | Phosphatidylcholine lyso 16 | 15.93340 | 59974.72 | 2-acyl-sn-glycero-3-phosphocholines |
| 31 | 4-O-Methylphloracetophenone | 1.17735 | 19537.06 | Alkyl-phenylketones |
| 32 | 5-Methoxy-3-indoleacetic acid | 8.153967 | 13901.50 | Indole-3-acetic acid derivatives |
| 33 | (1R,9S,10S)-3,4-dihydroxy-11,11-dimethyl-5-(propan-2-yl)-16-oxatetracyclo[7.5.2.0,.0,]hexadeca-2(7),3,5-triene-8,15-dione | 15.36857 | 66856.04 | Diterpene lactones |
| 34 | 3-Isobutylglutaric acid | 8.214517 | 16494.50 | Methyl-branched fatty acids |
| 35 | LPC 18:1 | 15.80038 | 1556111.00 | Lipids |
| 36 | Phosphatidylcholine lyso 18 | 16.44933 | 19155.42 | 2-acyl-sn-glycero-3-phosphocholines |
| 37 | Reserpine | 15.13208 | 27432.26 | Yohimbine alkaloids |
| 38 | ISOPALMITIC ACID | 10.76080 | 133306.00 | Long-chain fatty acids |
| 39 | Celastrol | 16.38930 | 16379.43 | Triterpenoids |
| 40 | (4E,8E)-10-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-2-benzofuran-5-yl)-4,8-dimethyldeca-4,8-dienoic acid | 15.60355 | 67788.85 | Terpene lactones |
| 41 | icos-19-ene-1,2,4-triol | 11.94487 | 16965.70 | Long-chain fatty alcohols |
| 42 | FT-thioether | 7.47550 | 402198.90 | PFSA |
| 43 | 5-Methoxypsoralen | 1.379833 | 717938.30 | 5-methoxypsoralens |
| 44 | Chaulmoogric Acid | 13.66750 | 19960.99 | Long-chain fatty acids |
| 45 | Cinnamoylglycine | 7.752817 | 16160.29 | N-acyl-alpha amino acids |
| 46 | Griseofulvin | 7.03820 | 19173.55 | Benzofurans |
| 47 | Ketoisovaleric acid | 1.841267 | 9078.108 | Short-chain keto acids and derivatives |
| 48 | Glycocholic Acid | 15.75758 | 439946.10 | Glycinated bile acids and derivatives |
| 49 | Phosphatidylserine 18 | 15.56438 | 30450.24 | Phosphatidylserines |
| 50 | [(6Z,10Z)-6-(acetyloxymethyl)-10-(hydroperoxymethyl)-3-methylidene-2-oxo-3a,4,5,8,9,11a-hexahydrocyclodeca[b]furan-4-yl] 2-methylbutanoate | 9.359233 | 23463.89 | Germacranolides and derivatives |
| 51 | (2E,4E)-1-[(2R,6S,14S,22S,25R)-25-(3,3-dimethyloxiran-2-yl)-15-methyl-1,3,13,15-tetraazaheptacyclo[18.4.1.0,.0,.0,.0,.0,]pentacosa-7,9,11,16(21),17,19-hexaen-3-yl]hexa-2,4-dien-1-one | 14.41933 | 93651.37 | Alpha carbolines |
| 52 | 2-[5-[2-[2-[5-(2-hydroxypropyl)oxolan-2-yl]propanoyloxy]propyl]oxolan-2-yl]propanoic acid | 12.90287 | 30013.75 | Dicarboxylic acids and derivatives |
| 53 | Bufotalin | 15.83517 | 45015.64 | Bufanolides and derivatives |
| 54 | Furostane base -2H + O-Hex, O-Hex-dHex-dHex-dHex | 9.319567 | 21781.36 | Steroidal saponins |
| 55 | C18(Plasm)-18:1 PC | 11.90607 | 40133.82 | 1-(1Z-alkenyl),2-acyl-glycerophosphocholines |
| 56 | DErySphingosine | 10.83890 | 38805.35 | 1,2-aminoalcohols |
| 57 | C12-AE1S (TENTATIVE) | 12.62238 | 29299.55 | Sulfuric acid monoesters |
| 58 | LPC 18:3 | 13.62767 | 173150.50 | Lipids |
| 59 | Nifekalant | 6.64155 | 22225.56 | Phenylpropylamines |
| 60 | beta-N-Methylaminoalanine | 13.10987 | 30146.63 | Alpha amino acids |
| 61 | Serine-Cholic Acid | 15.32113 | 551130.30 | Glycinated bile acids and derivatives |
| 62 | methyl 4-((10R,13R)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl) pentanoate | 15.44695 | 19387.68 | Monohydroxy bile acids, alcohols, and derivatives |
| 63 | Diatoxanthin | 7.272067 | 14265.04 | Triterpenoids |
| 64 | Argatroban | 15.12730 | 47349.21 | Dipeptides |
| 65 | 9-Methoxy-2,2-dimethyl-2,6-dihydro-pyrano[3,2-c]quinolin-5-one | 1.35255 | 17009.10 | Pyranoquinolines |
| 66 | Phosphatidylcholine 16 | 7.83215 | 48466.42 | Phosphatidylcholines |
| 67 | Glutamine | 1.33950 | 40752.10 | Alpha amino acids |
| 68 | (E)-5-hydroxy-N-[3-[5-[3-[[(E)-5-hydroxy-3-methylpent-2-enoyl]amino]propyl]-3,6-dioxopiperazin-2-yl]propyl]-3-methylpent-2-enamide | 11.65172 | 14764.10 | Alpha amino acids and derivatives |
| 69 | LPC 16:0 | 14.89577 | 1942964.00 | Lipids |
| 70 | Shanzhiside methyl ester | 14.03128 | 26912.56 | Iridoid O-glycosides |
| 71 | 9-Trans-Palmitelaidic acid | 18.88537 | 58417.61 | Long-chain fatty acids |
| 72 | Sebacic acid | 8.96275 | 28959.26 | Medium-chain fatty acids |
| 73 | Vindoline | 13.89302 | 60721.01 | Plumeran-type alkaloids |
| 74 | 9Z,12Z-Linoleic acid (NMR) | 19.25802 | 313966.20 | Lineolic acids and derivatives |
| 75 | 3-Indoxyl sulfate | 6.68105 | 322077.40 | Arylsulfates |
| 76 | a-Linolenic acid (NMR) | 18.11325 | 80101.46 | Lineolic acids and derivatives |
| 77 | Arachidonic acid | 18.92453 | 253179.40 | Long-chain fatty acids |
| 78 | Taurine | 8.30815 | 5742.452 | Organosulfonic acids |
| 79 | LPC 18:2 | 14.18563 | 1163369.00 | Lipids |
| 80 | Oleic acid | 20.54262 | 181184.10 | Long-chain fatty acids |
| 81 | Conessine | 12.99227 | 21960.55 | Conanine-type alkaloids |
| 82 | PROTOVERATRINE A | 1.379833 | 21784.06 | Cerveratrum-type alkaloids |
| 83 | 4-Nitrophenol | 20.19397 | 10567.01 | Nitrophenols |
| 84 | methyl orsellinate | 1.313333 | 25321.51 | P Hydroxybenzoic acid alkyl esters |
| 85 | Atalaphylline | 13.38752 | 47479.44 | Acridones |
| 86 | PFSA-pentafluorosulfide | 1.17735 | 85352.33 | PFSA |
| 87 | piperazine-2,5-dione | 1.841267 | 12361.39 | Alpha amino acids and derivatives |
| 88 | N-(4-((6aR,8aS)-4-hydroxy-6a,8a,9-trimethyl-3,4,5,6,6a,6b,7,8,8a,8b,11a,12,12a,12b-tetradecahydro-1H-naphtho[2’,1’:4,5]indeno[2,1-b]furan-10-yl)-2-methylbutyl)acetamide | 15.32113 | 1344867.00 | Furostanes and derivatives |
| 89 | 6-(furan-3-yl)-6,8,12,16,21-pentahydroxy-7,15-dimethyl-9-oxo-3,17,19-trioxaheptacyclo[9.9.3.0,.0,.0,.0,.0,]tricosan-14-yl 2-methylbutanoate | 13.50750 | 38900.18 | Limonoids |
| 90 | Gedunol | 15.79632 | 305832.30 | Limonoids |
| 91 | 2,6-di-tert-butyl-4-methylphenol | 1.70115 | 164993.80 | Phenylpropanes |
| 92 | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine | 16.57080 | 1376141.00 | Phosphatidylcholines |
| 93 | Trifluoroacetic acid | 16.21035 | 175237.70 | PFSA |
| 94 | Germinaline | 1.379833 | 26898.38 | Alkaloids |
| 95 | Phytosphingosine (not validated, isomer of 1696) | 10.79983 | 58379.87 | Lipids |
| 96 | Choline | 15.32113 | 1686757.00 | Cholines |
| 97 | Decanoic acid | 19.0867 | 11618.62 | Medium-chain fatty acids |
| 98 | 7-ethenyl-1,4a,7-trimethyl-3,4,6,8,8a,9,10,10a-octahydro-2H-phenanthrene-1-carboxylic acid | 17.87777 | 111714.60 | Diterpenoids |
| 99 | Uric acid | 1.820983 | 69064.27 | Xanthines |
| 100 | vanillic acid | 1.313333 | 53861.45 | M-methoxybenzoic acids and derivatives |
| 101 | Tuberostemonine | 12.69258 | 91277.17 | Stichoneurine-type alkaloids |
| 102 | (1R,2R,5R,5’S,6S,8aS)-6-(acetyloxy)-2,5,8a-trimethyl-5’’-oxo-octahydro-2H-dispiro[naphthalene-1,2’:5’,3’-bis(oxolane)]-5-ylmethyl acetate | 15.32113 | 668259.40 | Diterpene lactones |
| 103 | (R)-4-aminoisoxazolidin-3-one | 1.934117 | 30480.44 | Alpha amino acids and derivatives |
| 104 | (1S,3R,6S,6aR,6bR,8S,9S,11R,11aR,12R,12aR,14R)-1-ethyl-6,8,11-trihydroxy-3-methyl-10-methylenetetradecahydro-3,6a,12-(epiethane[1,1,2]triyl)-9,11a-methanoazuleno[2,1-b]azocine 1-oxide | 12.69258 | 247497.70 | Kaurane diterpenoids |
| 105 | Secoisolariciresinol | 12.26407 | 15677.30 | Dibenzylbutanediol lignans |
| 106 | Cyclamate | 6.110917 | 33820.46 | Cyclamates |
| 107 | Docosahexanoic acid | 18.55157 | 591446.80 | Very long-chain fatty acids |
| 108 | anthothecol | 14.03128 | 23028.57 | Limonoids |
| 109 | (1R,4aR,5S)-5-(3-hydroxy-3-methylpent-4-enyl)-1,4a-dimethyl-6-methylidene-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylic acid | 15.60355 | 517448.60 | Diterpenoids |
| 110 | (6aR,8aS)-11-(3-acetamido-2-methylpropyl)-6a,8a,9-trimethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,8b,9,10,12,12a,12b-hexadecahydropentaleno[2,1-a]phenanthren-4-yl acetate | 14.89468 | 53006.64 | Steroid esters |
| 111 | 4-((9S)-3,5,14-trihydroxy-10-((E)-((1-hydroxybutan-2-yl)imino)methyl)-13-methylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one | 13.89302 | 42323.20 | Cardenolides and derivatives |
| 112 | N-ACETYL-L-LEUCINE | 7.51500 | 24397.29 | Leucine and derivatives |
| 113 | 2-Mercaptobenzothiazole | 17.58292 | 35381.59 | Benzothiazoles |
| 114 | Pristimerin | 17.16623 | 48255.61 | Triterpenoids |
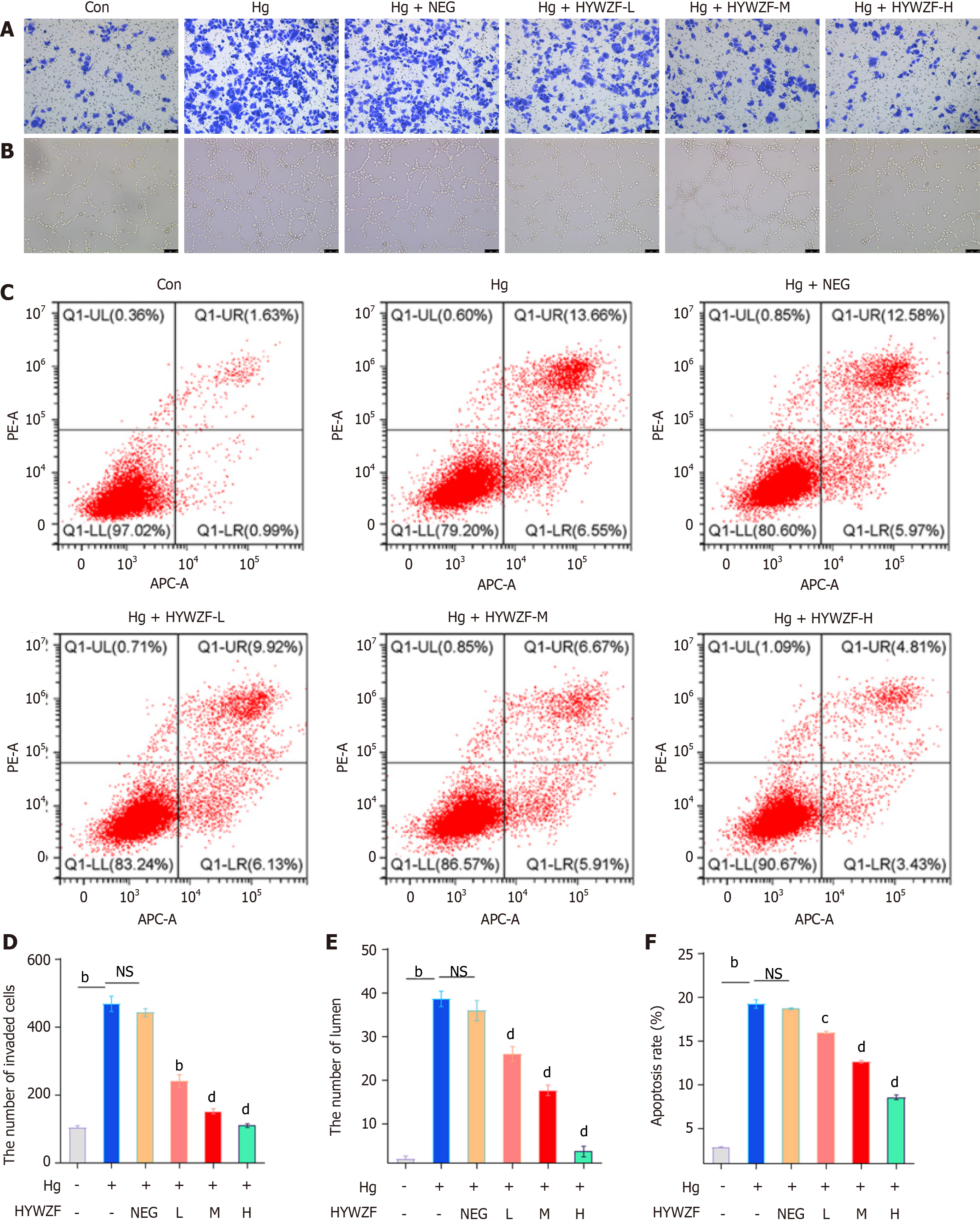
Here, we found that hg significantly increased the invasion of cells (Figure 2A and D). Compared to the con group, HYWZF-treated groups exhibited reduced tube formation (Figure 2B and E). Flow cytometry was used to determine whether blocking apoptosis aids in the prevention of DR. As shown in Figure 2C and F, high dose of HYWZF serum reduced the apoptosis rate to 9.03% compared to that in the control group. Notably, HYWZF serum inhibited the invasion, tube formation, and apoptosis in HRCECs in a dose-dependent manner.
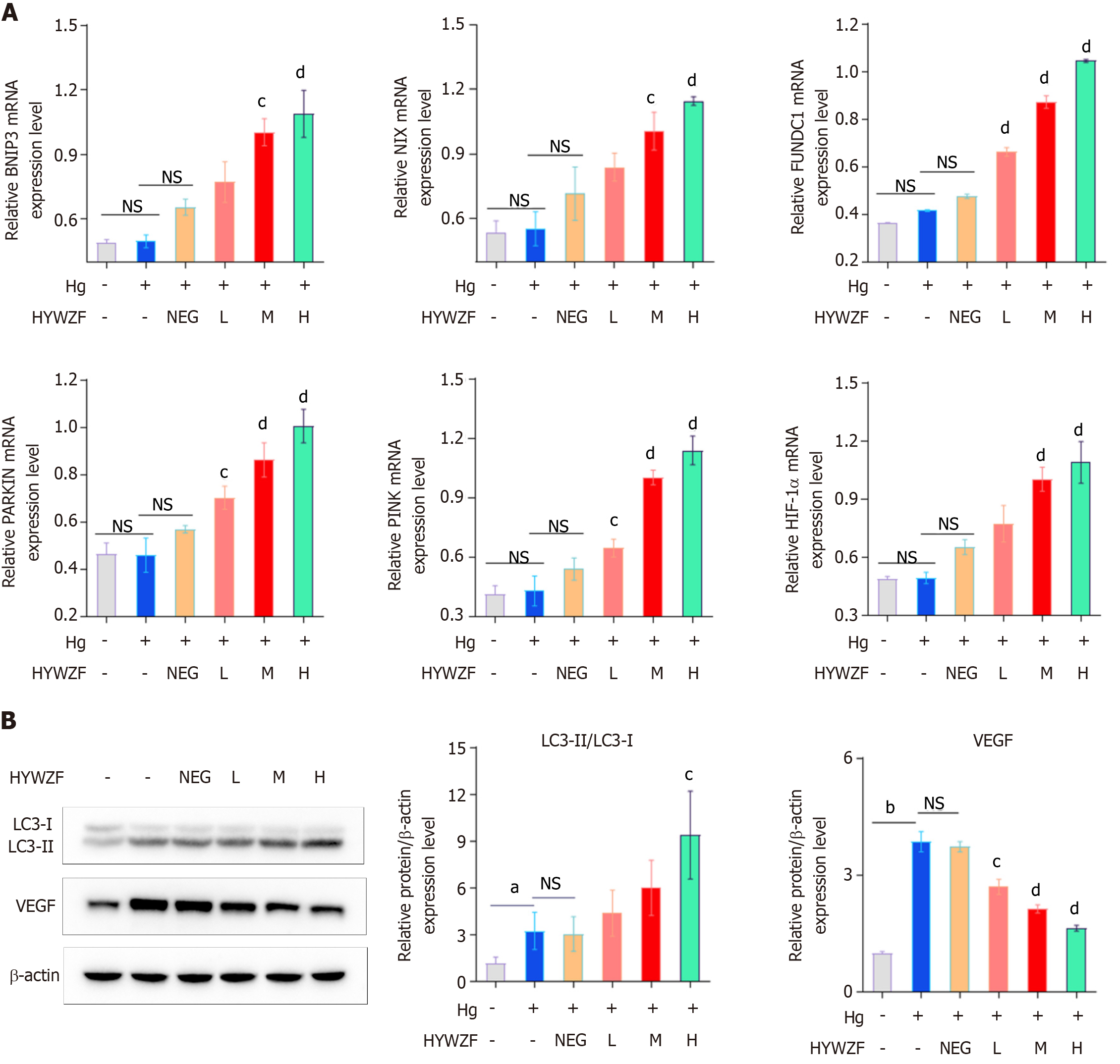
Mitophagy protects B cells from diabetic damage[22]. Therefore, this study aimed to explore the role of mitophagy in DR. qPCR was performed to assess the expression levels of mitophagy-related biomarkers. Figure 3A shows that HYWZF serum increased the mRNA expression levels of BNIP3, FUNDC1, and NIX, and high doses of HYWZF serum exhibited the most potent effects. We also verified the effects of mitophagy in cells via Western blotting. Compared with the hg group, HYWZF increased the ratio of LC3-II/LC3-I and downregulated the VEGF protein levels (Figure 3B). Therefore, 10% HYWZF serum was used for subsequent experiments.
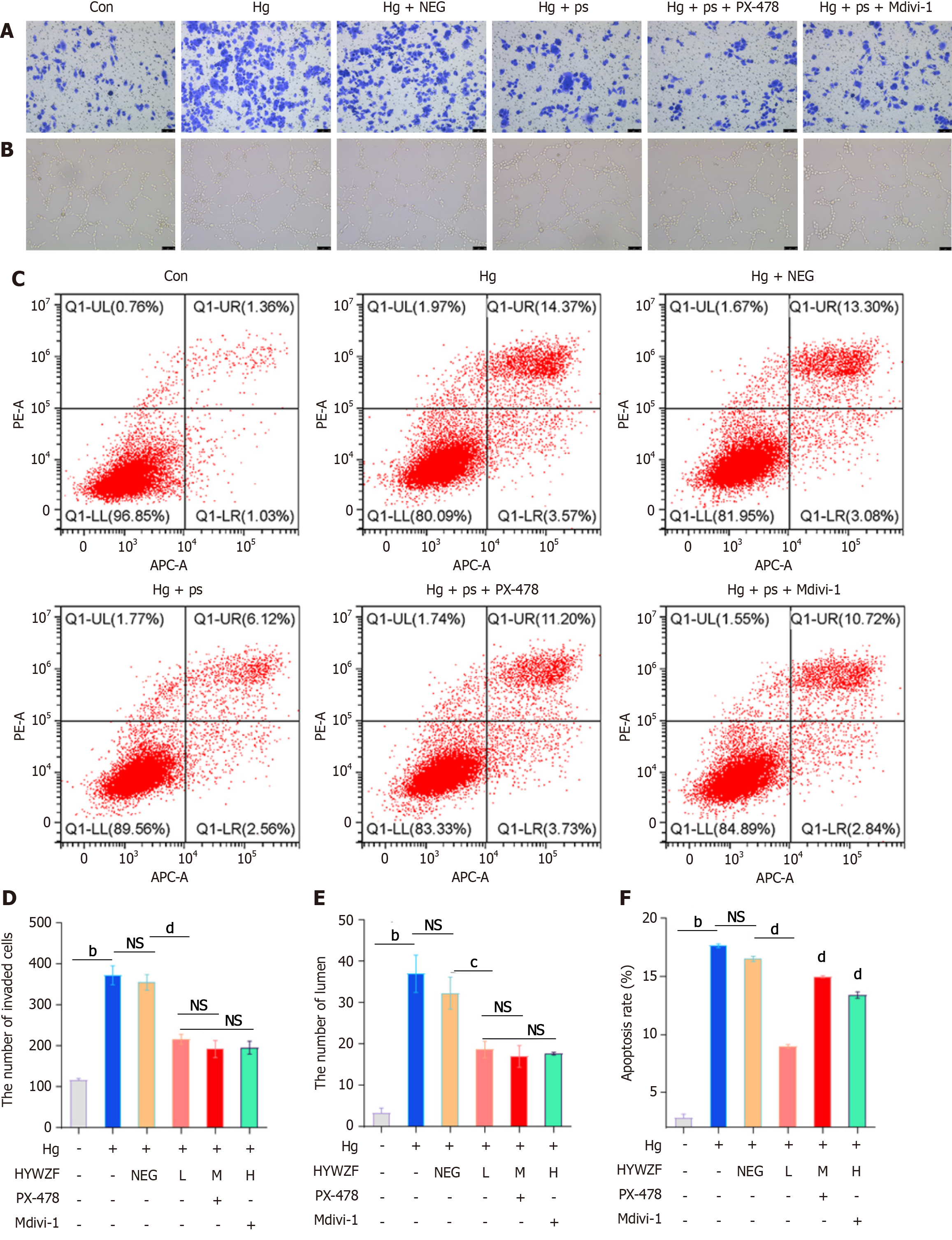
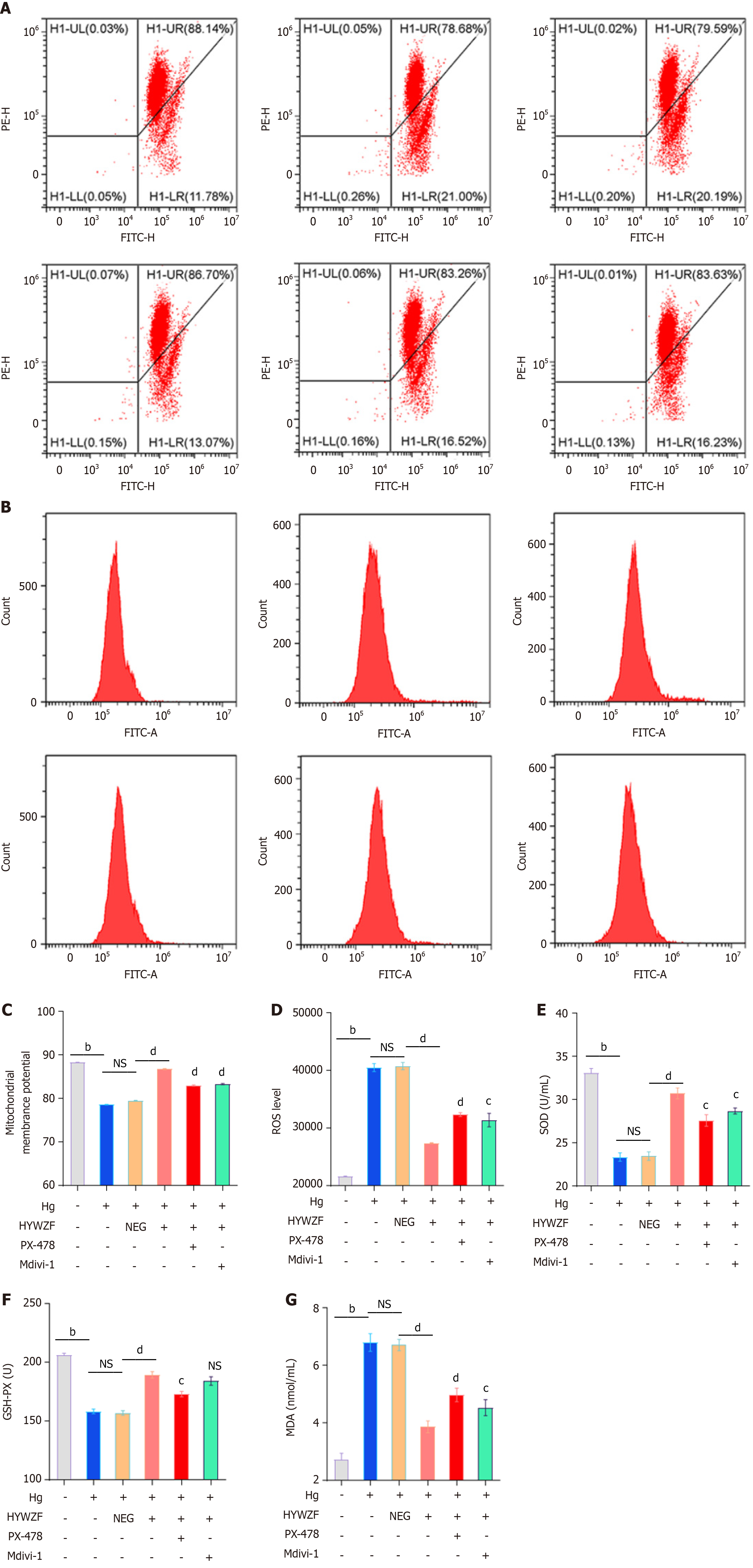
To better understand mitochondrial autophagy and determine whether HIF-1α affects the morphology of HRCECs, inhibitors were used in experiments. In contrast to the hg group, 10% HYWZF serum (hg + ps)-treated group exhibited significantly decreased cell invasion (Figure 4A and D). As shown in Figure 4B and E, the tube-forming capacity was significantly decreased in HYWZF-treated groups compared to that in the hg group. Moreover, HYWZF reduced apoptosis compared to that observed in the hg group (Figure 4C and F). HYWZF increased the mitochondrial membrane potential (Figure 5A and C) and reduced the intracellular reactive oxygen species (ROS) content (Figure 5B and D). Additionally, HYWZF significantly enhanced the activities of SOD and GSH-PX but decreased that of MDA (Figure 5E-G).
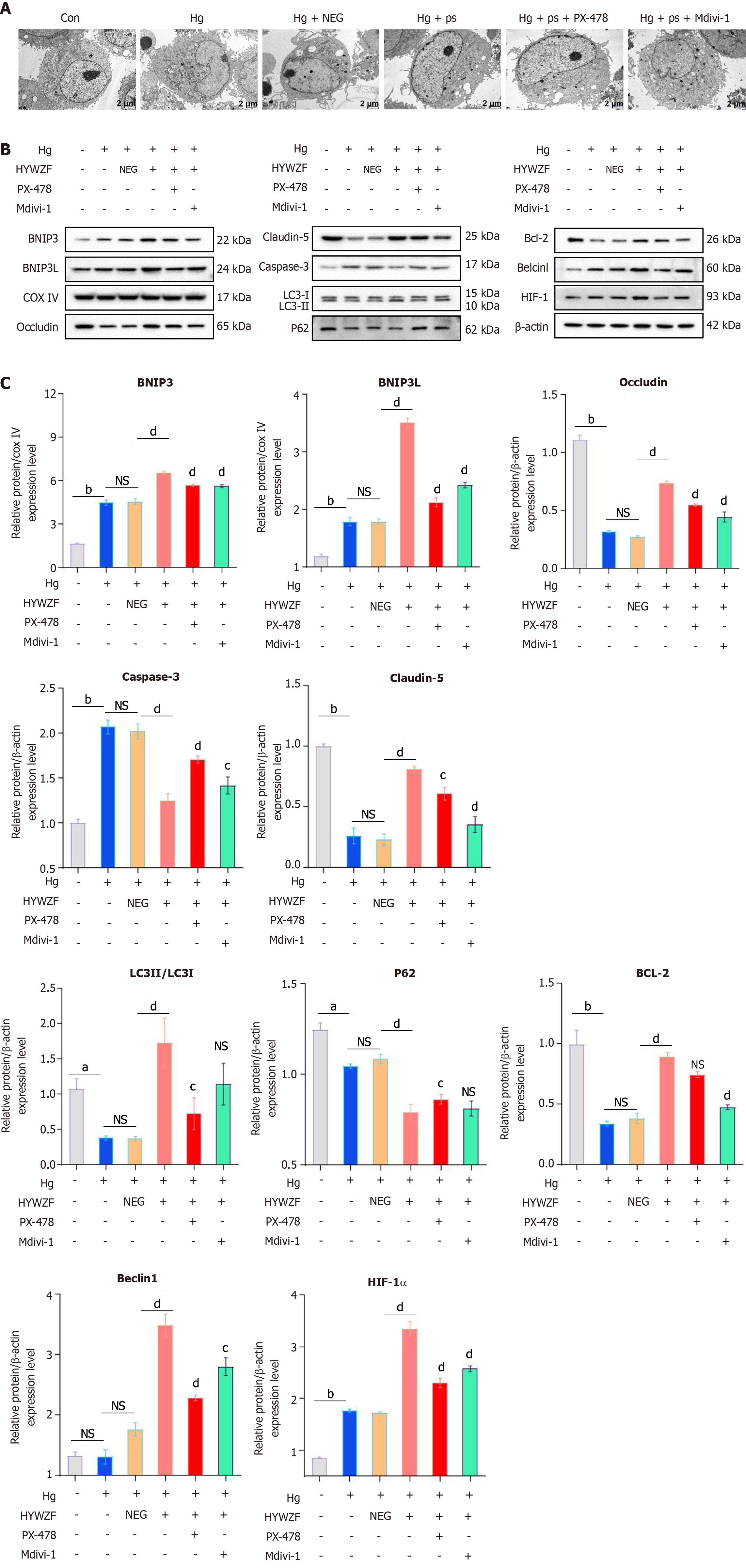
HYWZF rescued mitochondrial swelling and vacuolation (Figure 6A). Western blotting revealed that HYWZF increased the levels of the mitophagy proteins, BNIP3 and BNIP3L, indicating that HYWZF promoted mitophagy. HYWZF also upregulated the levels of claudin-5 and occludin, indicating that HYWZF affected the intercellular interactions (Figure 6B and C). Additionally, HYWZF upregulated the HIF-1α, Beclin1, BNIP3, BNIP3L, Bcl2, and Belin1 levels and LC3I/LC3II ratio and downregulated the caspase-3 and p62 levels in hg-induced cells. However, PX-478 and Mdivi-1 reversed these effects, suggesting that HYWZF targets the HIF-1α/BNIP3/NIX axis to alleviate hg-induced mitophagy in HRCECs. These results demonstrate the protective effects of HYWZF against DR.
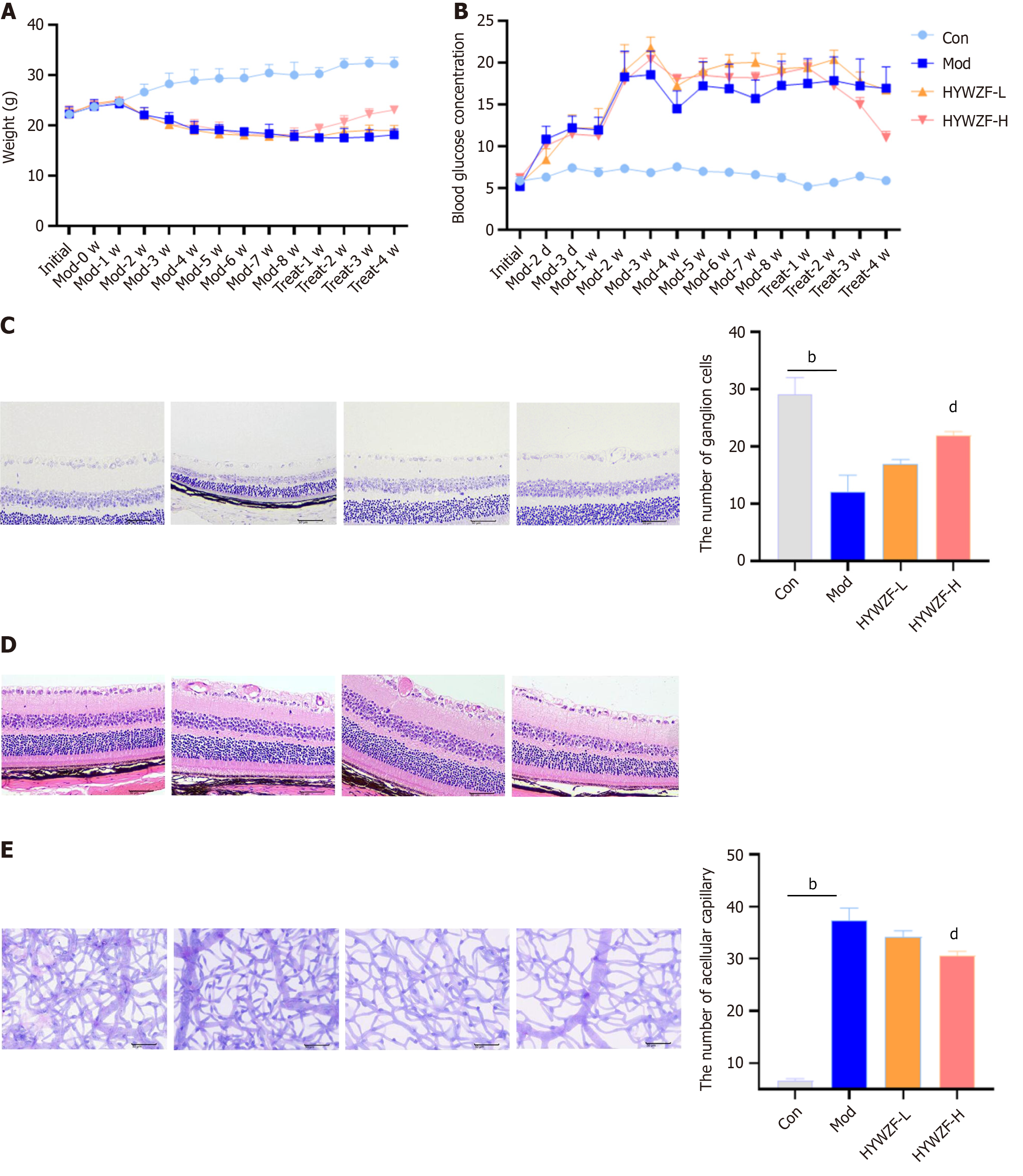
To further investigate the effects of HYWZF, C57BL/6 mice were used as the experimental animal model. Mice with blood glucose levels above 16.7 mmol/L were classified as the diabetic group. After 12 wk of hyperglycemia induction, mice with fundus hemorrhage and exudation were classified as the DR group. Both low (12 g/kg/d)- and high (48 g/kg/d)- dose HYWZF treatment improved the overall state of DR mice compared to that of the model group.
Body weight gradually increased after HYWZF treatment (Figure 7A), whereas that of the model group decreased. Blood glucose levels also decreased after HYWZF treatment (Figure 7B). Tar violet staining revealed that the numbers of ganglion cells were higher in the HYWZF-L and HYWZF-H groups than in the model group (Figure 7C). Retinal histopathological damage was ameliorated by HYWZF treatment (Figure 7D). Moreover, number of apoptotic cells in the model group was higher than that in the control group (Figure 7E). These data suggest that HYWZF alleviates the pathological damage in the retinal tissues of diabetic mice.
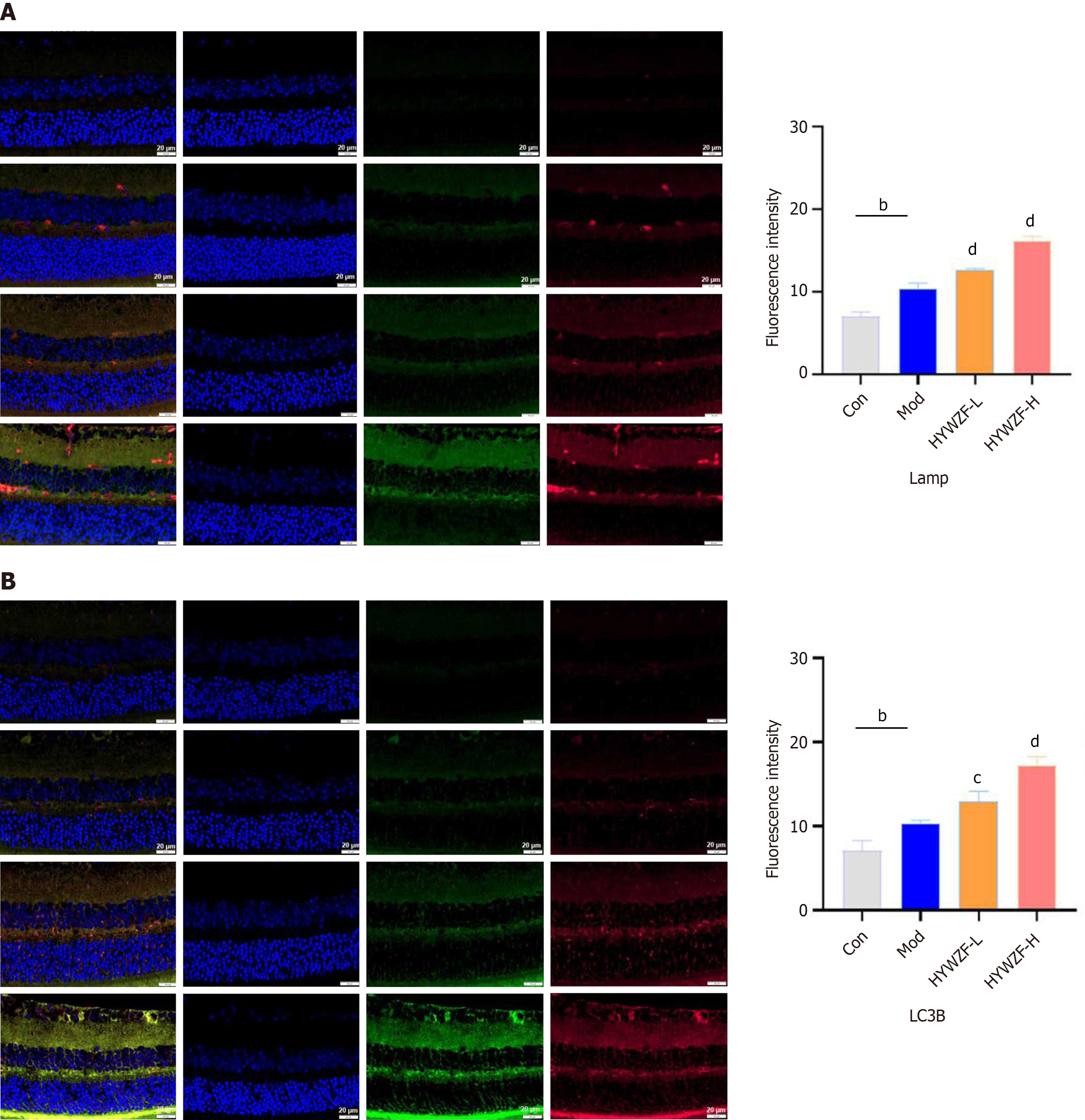
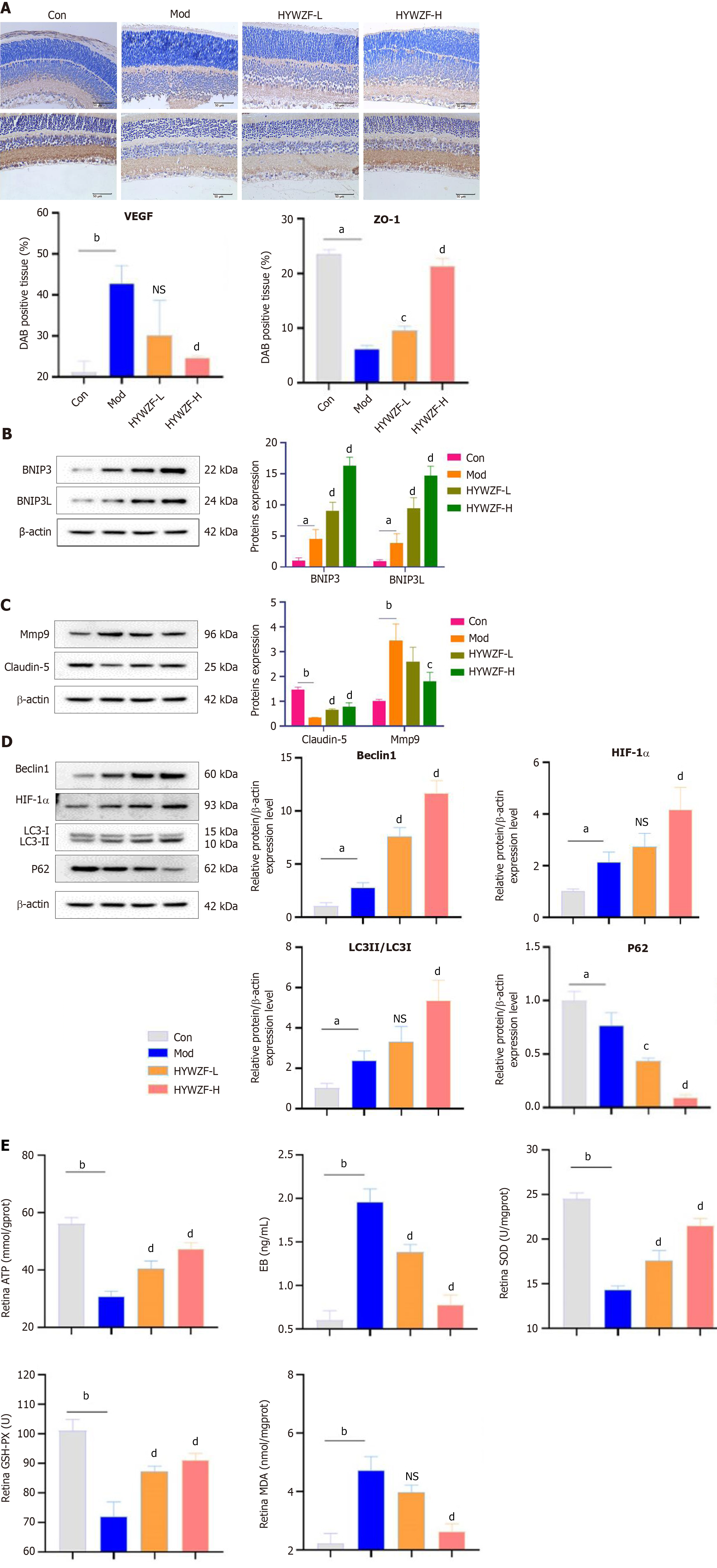
Immunofluorescence analysis revealed increased colocalization of Tom20 with LAMP-2 and LC3B after HYWZF treatment, indicating that HYWZF induced mitochondrial autophagy (Figure 8). Expression levels of VEGF were decreased, whereas those of ZO-1 were increased in the tissues after HYWZF treatment (Figure 9A). Western blotting revealed that the expression levels of tight junction protein (claudin-5), HIF-1α, Beclin1, BNIP3, and BNIP3L were significantly higher in the HYWZF-treated groups than in the model group. However, mmp9 and p62 levels were significantly downregulated by HYWZF (Figure 9B-D). Additionally, the activities of ATP, SOD, and GSH-PX were significantly higher and those of EB and MDA were lower in the retinal samples of the HYWZF-treated groups than in those of the model group (Figure 9E). These results suggest that HYWZF mediates mitophagy via the HIF-1α/BNIP3/NIX axis to reduce oxidative stress, thereby improving the pathological damage in diabetic mice.
DR is the most prominent manifestation of diabetic microangiopathy and a serious complication of diabetes[23]. Retinal endothelial cell dysfunction is the main pathological process responsible for DR[24]. Hyperglycemia promotes abnormal cell division and proliferation by affecting the expression levels of cyclins and tumor-related genes, thereby promoting the pathogenesis of DR[25]. Identifying effective methods to prevent and treat abnormal cell division and proliferation is of major clinical importance. In this study, HYWZF alleviated hyperglycemia in diabetic mice and ameliorated the pathological manifestations of DR, including basement membrane thickening, extracellular matrix expansion, and increased vascular permeability[26]. HYWZF decreased MDA content, increased SOD and GSH-PX activities, and inhibited the apoptosis of HRCECs and retinal tissue. Therefore, we hypothesized that the protective effects of HYWZF are associated with the inhibition of oxidative stress and apoptosis; however, the underlying mechanism warrant further investigations.
In diabetes, hyperglycemia causes oxidative damage by stimulating ROS production[27]. The ROS produced during hyperglycemia can directly affect the expression and distribution of tight junction proteins[28]. ZO-1 is a cytoplasmic attachment protein that can connect the transmembrane proteins claudin-1 and claudin-5 to the cytoskeleton and also participates in the process of polymerization to form tight junctions[29,30]. One study has reported reduced ZO-1 expression in diabetic nephropathy[31]. This result found that HYWZF treatment increased the expression levels of ZO-1, occludin, and claudin-5 proteins, indicating that HYWZF affected tissue integrity and barrier function. HE staining revealed that the retinal tissues in the model group were significantly swollen and infiltrated with inflammatory cells, indicating the presence of a large number of free radicals appeared[32]. Flow cytometry analysis further proved that there were a large number of ROS in the model group. SOD, a biological enzyme found in cells, neutralizes superoxide anion free radicals produced during metabolic processes and protects organisms. The level of MDA in the retina reflects the level of tissue cell oxidative damage[33], while SOD activity reflects the ability of the cell to resist oxidation and repair. They remain in a constant state of balance under physiological conditions. An increase in MDA levels or a decrease in SOD levels indicates significant oxidative damage to retinal tissue cells[34]. Ahmed et al[35] found that Solidago virgaurea increased the level of SOD and downregulated MDA in type 1 diabetes. In this study, an increase in SOD and GSH-PX was found after drug treatment, as well as a low MDA level, indicating that HYWZF could enhance the ability of cells to scavenge oxygen free radicals and reduce the damage caused by peroxidation in tissue cells.
A previous study suggested that mitophagy plays a crucial role in eliminating defective or dysfunctional mitochondria, which are important for the preservation of mitochondrial homeostasis[36-38]. Mitochondria contain less cytochrome c and more pro-apoptotic protein, Bcl-2-associated X (Bax); they tend to swell, break, and have lower membrane potential[39,40]. Bax modulates the mitochondrial membrane potential to increase the release of cytochrome C[41]. Dysfunctional mitochondria are also associated with the death of various retinal cells[42]. Consistent with these findings, the present study showed that mitochondria were swollen and mitochondrial damage occurred in HRCEC in DR. Data also showed that high glucose enhanced ROS production and oxidative stress, whereas HYWZF improved ROS production and oxidative stress by increasing mitochondrial autophagy. LC3 and Beclin-1 are important and reliable markers of autophagy[43]. Our data revealed that the protein levels of Beclin-1, LC3, BNIP3, and BNIP3L (NIX) were upregulated in the retinas of the diabetic mice. After HYWZF treatment, compared to the diabetic group, the level of autophagy was further improved, indicating that HYWZF promoted autophagy in the retina of diabetic mice, accelerated the removal of denatured and damaged proteins, and protected the retinal tissue of mice with diabetes. Furthermore, our study demonstrated that HIF-1α/BNIP3-mediated mitophagy protects against DR by reducing apoptosis.
HIF-1α is an important transcription factor affecting mitophagy, and its expression and activity are closely regulated by the cellular oxygen concentration[44]. It is also involved in various physiological processes, such as angiogenesis, cartilage development, neuroembryogenesis, and cancer development[45]. BNIP3 and NIX are the downstream target genes of HIF-1α that are involved in the regulation of autophagosome-lysosome fusion and act as important mitophagy receptors and adaptors. BNIP3 binds to LC3 to promote mitophagy[46]. The chemical structure of HIF-1α is extremely unstable. In the normoxic environment, hypoxia-inducible factor inhibitor 1 prevents the binding of HIF-1α with transcriptional cofactor p300/CBP and inhibits its transcriptional activity[47]. As the oxygen concentration decreases, the activity of FIH-1 also decreases, leading to the stable expression of HIF-1α[48]. In this study, HIF-1α levels increased due to retinal tissue hypoxia, leading to mitophagy. BNIP3 and NIX levels significantly increased after HYWZF treatment. Therefore, we hypothesized that HIF-1α alleviates hg-induced HRCEC apoptosis via mitophagy by enhancing the expression of BNIP3. Although the protective effects of HIF-1α against diabetes have been demonstrated, those of HYWZF against DR via HIF-1α remain unclear. To investigate the protective effects of HIF-1α, we used PX-478 and Mdivi-1 to regulate HIF-1α expression in HRCECs. Our results suggest that increased HIF-1α expression improves the survival of HRCECs.
This study demonstrated that HYWZF increases the HIF-1α levels; however, the mechanism underlying HIF-1α regulation remains unclear. HIF-1α stability is regulated by various post-translational modifications, including hydroxylation, ubiquitination, SUMOylation, acetylation, methylation, and phosphorylation[49]. The mechanisms by which HYWZF increases autophagy and regulates the stable expression of HIF-1α under hyperglycemic conditions warrant further investigation. Overall, this study revealed that HYWZF protected against DR by mediating mitophagy via the HIF-1α/BNIP3/NIX pathway, suggesting its potential as a therapeutic agent for DR treatment.
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | Sheng C, Guo Y, Hou W, Chen H, Liu H, Wang L. The effect of insulin and kruppel like factor 10 on osteoblasts in the dental implant osseointegration in diabetes mellitus patients. Bioengineered. 2022;13:14259-14269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Lu S, Liao Z, Lu X, Katschinski DM, Mercola M, Chen J, Heller Brown J, Molkentin JD, Bossuyt J, Bers DM. Hyperglycemia Acutely Increases Cytosolic Reactive Oxygen Species via O-linked GlcNAcylation and CaMKII Activation in Mouse Ventricular Myocytes. Circ Res. 2020;126:e80-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 3. | Fu Y, Chen M, Si L. Multimorbidity and catastrophic health expenditure among patients with diabetes in China: a nationwide population-based study. BMJ Glob Health. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Huang H, He J, Johnson D, Wei Y, Liu Y, Wang S, Lutty GA, Duh EJ, Semba RD. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1α-VEGF pathway inhibition. Diabetes. 2015;64:200-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Du P, Wang J, Han Y, Feng J. Blocking the LncRNA MALAT1/miR-224-5p/NLRP3 Axis Inhibits the Hippocampal Inflammatory Response in T2DM With OSA. Front Cell Neurosci. 2020;14:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Zhao F, Gao X, Ge X, Cui J, Liu X. Cyanidin-3-o-glucoside (C3G) inhibits vascular leakage regulated by microglial activation in early diabetic retinopathy and neovascularization in advanced diabetic retinopathy. Bioengineered. 2021;12:9266-9278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Liu QP, Chen YY, Yu YY, An P, Xing YZ, Yang HX, Zhang YJ, Rahman K, Zhang L, Luan X, Zhang H. Bie-Jia-Ruan-Mai-Tang, a Chinese Medicine Formula, Inhibits Retinal Neovascularization in Diabetic Mice Through Inducing the Apoptosis of Retinal Vascular Endothelial Cells. Front Cardiovasc Med. 2022;9:959298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Choi HJ, Jang HJ, Chung TW, Jeong SI, Cha J, Choi JY, Han CW, Jang YS, Joo M, Jeong HS, Ha KT. Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia. 2013;86:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Liu NA, Liu JQ, Liu Y, Zhu Q, Zheng D, Li F, Meng LZ, Qiu M. Rehmannia Glutinosa Polysaccharide Regulates Bone Marrow Microenvironment via HIF-1α/NF-κB Signaling Pathway in Aplastic Anemia Mice. An Acad Bras Cienc. 2023;95:e20220672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Huong DTV, Giang PM, Yen NH, Nguyen ST. Extracts Reduce Blood Glucose in Streptozotocin-Induced Diabetic Mice. J Chem. 2021;2021:6688731. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Wang K, Yang Q, Ma Q, Wang B, Wan Z, Chen M, Wu L. Protective Effects of Salvianolic Acid A against Dextran Sodium Sulfate-Induced Acute Colitis in Rats. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Li Y, Chen Y, Sun-Waterhouse D. The potential of dandelion in the fight against gastrointestinal diseases: A review. J Ethnopharmacol. 2022;293:115272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Cho SY, Park JY, Park EM, Choi MS, Lee MK, Jeon SM, Jang MK, Kim MJ, Park YB. Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clin Chim Acta. 2002;317:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Ma L, Ainsworth HC, Snipes JA, Murea M, Choi YA, Langefeld CD, Parks JS, Bharadwaj MS, Chou JW, Hemal AK, Petrovic S, Craddock AL, Cheng D, Hawkins GA, Miller LD, Hicks PJ, Saleem MA, Divers J, Molina AJA, Freedman BI. APOL1 Kidney-Risk Variants Induce Mitochondrial Fission. Kidney Int Rep. 2020;5:891-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Tan X, Cao Y, An X, Chen J, Yang L. Punicalagin Protects against Diabetic Liver Injury by Upregulating Mitophagy and Antioxidant Enzyme Activities. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Tang H, Yang M, Liu Y, Zhu X, Liu S, Liu H, Sun L, Song P. Melatonin alleviates renal injury by activating mitophagy in diabetic nephropathy. Front Endocrinol (Lausanne). 2022;13:889729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | He J, Qin Z, Chen X, He W, Li D, Zhang L, Le Y, Xiong Q, Zhang B, Wang H. HIF-1α Ameliorates Diabetic Neuropathic Pain via Parkin-Mediated Mitophagy in a Mouse Model. Biomed Res Int. 2022;2022:5274375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Madhu V, Boneski PK, Silagi E, Qiu Y, Kurland I, Guntur AR, Shapiro IM, Risbud MV. Hypoxic Regulation of Mitochondrial Metabolism and Mitophagy in Nucleus Pulposus Cells Is Dependent on HIF-1α-BNIP3 Axis. J Bone Miner Res. 2020;35:1504-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Cannon MV, Silljé HH, Sijbesma JW, Khan MA, Steffensen KR, van Gilst WH, de Boer RA. LXRα improves myocardial glucose tolerance and reduces cardiac hypertrophy in a mouse model of obesity-induced type 2 diabetes. Diabetologia. 2016;59:634-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3:71-85. [PubMed] |
| 21. | Sun Y, Jiang X, Pan R, Zhou X, Qin D, Xiong R, Wang Y, Qiu W, Wu A, Wu J. Escins Isolated from Aesculus chinensis Bge. Promote the Autophagic Degradation of Mutant Huntingtin and Inhibit its Induced Apoptosis in HT22 cells. Front Pharmacol. 2020;11:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Sidarala V, Pearson GL, Parekh VS, Thompson B, Christen L, Gingerich MA, Zhu J, Stromer T, Ren J, Reck EC, Chai B, Corbett JA, Mandrup-Poulsen T, Satin LS, Soleimanpour SA. Mitophagy protects β cells from inflammatory damage in diabetes. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Li M, Wang W, He S. Carvedilol activates nuclear factor E2-related factor 2/ antioxidant response element pathway to inhibit oxidative stress and apoptosis of retinal pigment epithelial cells induced by high glucose. Bioengineered. 2022;13:735-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Yang Q, Li S, Zhou Z, Yang X, Liu Y, Hao K, Fu M. Trimetazidine mitigates high glucose-induced retinal endothelial dysfunction by inhibiting PI3K/Akt/mTOR pathway-mediated autophagy. Bioengineered. 2022;13:7515-7527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Huang W, Hua H, Xiao G, Yang X, Yang Q, Jin L. ZC3HAV1 promotes the proliferation and metastasis via regulating KRAS in pancreatic cancer. Aging (Albany NY). 2021;13:18482-18497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ye L, Guo H, Wang Y, Peng Y, Zhang Y, Li S, Yang M, Wang L. Exosomal circEhmt1 Released from Hypoxia-Pretreated Pericytes Regulates High Glucose-Induced Microvascular Dysfunction via the NFIA/NLRP3 Pathway. Oxid Med Cell Longev. 2021;2021:8833098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Liu ZJ, Zhao W, Lei HY, Xu HL, Lai LY, Xu R, Xu SY. High Glucose Enhances Bupivacaine-Induced Neurotoxicity via MCU-Mediated Oxidative Stress in SH-SY5Y Cells. Oxid Med Cell Longev. 2019;2019:7192798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:3666-3676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Wang H, Segaran RC, Chan LY, Aladresi AAM, Chinnathambi A, Alharbi SA, Sethi G, Tang FR. Gamma Radiation-Induced Disruption of Cellular Junctions in HUVECs Is Mediated through Affecting MAPK/NF-κB Inflammatory Pathways. Oxid Med Cell Longev. 2019;2019:1486232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Zhang J, Sadowska GB, Chen X, Park SY, Kim JE, Bodge CA, Cummings E, Lim YP, Makeyev O, Besio WG, Gaitanis J, Banks WA, Stonestreet BS. Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus. FASEB J. 2015;29:1739-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Rincon-Choles H, Vasylyeva TL, Pergola PE, Bhandari B, Bhandari K, Zhang JH, Wang W, Gorin Y, Barnes JL, Abboud HE. ZO-1 expression and phosphorylation in diabetic nephropathy. Diabetes. 2006;55:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Cao C, Zhao W, Chen X, Shen B, Wang T, Wu C, Rong X. Deciphering the action mechanism of paeoniflorin in suppressing pancreatic cancer: A network pharmacology study and experimental validation. Front Pharmacol. 2022;13:1032282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Wu L, Cen Y, Feng M, Zhou Y, Tang H, Liao X, Wang Y, Wang M, Zhou M. Metformin Activates the Protective Effects of the AMPK Pathway in Acute Lung Injury Caused by Paraquat Poisoning. Oxid Med Cell Longev. 2019;2019:1709718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Silva KA, Dong J, Dong Y, Schor N, Tweardy DJ, Zhang L, Mitch WE. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. J Biol Chem. 2015;290:11177-11187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 35. | Sanad FA, Ahmed SF, El-Tantawy WH. Antidiabetic and hypolipidemic potentials of Solidago virgaurea extract in alloxan-induced diabetes type 1. Arch Physiol Biochem. 2022;128:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Wu X, Li X, Liu Y, Yuan N, Li C, Kang Z, Zhang X, Xia Y, Hao Y, Tan Y. Hydrogen exerts neuroprotective effects on OGD/R damaged neurons in rat hippocampal by protecting mitochondrial function via regulating mitophagy mediated by PINK1/Parkin signaling pathway. Brain Res. 2018;1698:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Fu ZJ, Wang ZY, Xu L, Chen XH, Li XX, Liao WT, Ma HK, Jiang MD, Xu TT, Xu J, Shen Y, Song B, Gao PJ, Han WQ, Zhang W. HIF-1α-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 2020;36:101671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 38. | Amorim R, Simões ICM, Teixeira J, Cagide F, Potes Y, Soares P, Carvalho A, Tavares LC, Benfeito S, Pereira SP, Simões RF, Karkucinska-Wieckowska A, Viegas I, Szymanska S, Dąbrowski M, Janikiewicz J, Cunha-Oliveira T, Dobrzyń A, Jones JG, Borges F, Wieckowski MR, Oliveira PJ. Mitochondria-targeted anti-oxidant AntiOxCIN(4) improved liver steatosis in Western diet-fed mice by preventing lipid accumulation due to upregulation of fatty acid oxidation, quality control mechanism and antioxidant defense systems. Redox Biol. 2022;55:102400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Lin Q, Li S, Jiang N, Jin H, Shao X, Zhu X, Wu J, Zhang M, Zhang Z, Shen J, Zhou W, Gu L, Lu R, Ni Z. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy. 2021;17:2975-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 239] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 40. | El-Rahman GIA, Behairy A, Elseddawy NM, Batiha GE, Hozzein WN, Khodeer DM, Abd-Elhakim YM. Saussurea lappa Ethanolic Extract Attenuates Triamcinolone Acetonide-Induced Pulmonary and Splenic Tissue Damage in Rats via Modulation of Oxidative Stress, Inflammation, and Apoptosis. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Wingard MC, Dalal S, Shook PL, Myers R, Connelly BA, Thewke DP, Singh M, Singh K. Deficiency of ataxia-telangiectasia mutated kinase modulates functional and biochemical parameters of the heart in response to Western-type diet. Am J Physiol Heart Circ Physiol. 2021;320:H2324-H2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Lam CH, Cheung JK, Tse DY, Lam TC. Proteomic Profiling Revealed Mitochondrial Dysfunction in Photoreceptor Cells under Hyperglycemia. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 43. | Lee SJ, Kim YA, Park KK. Anti-Fibrotic Effect of Synthetic Noncoding Decoy ODNs for TFEB in an Animal Model of Chronic Kidney Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Liu XW, Lu MK, Zhong HT, Wang LH, Fu YP. Panax Notoginseng Saponins Attenuate Myocardial Ischemia-Reperfusion Injury Through the HIF-1α/BNIP3 Pathway of Autophagy. J Cardiovasc Pharmacol. 2019;73:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 45. | Yang C, Zhong ZF, Wang SP, Vong CT, Yu B, Wang YT. HIF-1: structure, biology and natural modulators. Chin J Nat Med. 2021;19:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Su L, Zhang J, Gomez H, Kellum JA, Peng Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy. 2023;19:401-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 375] [Article Influence: 187.5] [Reference Citation Analysis (0)] |
| 47. | Terán G, Li H, Catrina SB, Liu R, Brighenti S, Zheng X, Grünler J, Nylén S, Carow B, Rottenberg ME. High Glucose and Carbonyl Stress Impair HIF-1-Regulated Responses and the Control of Mycobacterium tuberculosis in Macrophages. mBio. 2022;13:e0108622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 48. | Bao Y, Wang Z, Liu B, Lu X, Xiong Y, Shi J, Li P, Chen J, Zhang Z, Chen M, Wang L, Wu Z. A feed-forward loop between nuclear translocation of CXCR4 and HIF-1α promotes renal cell carcinoma metastasis. Oncogene. 2019;38:881-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Hafizi R, Imeri F, Wenger RH, Huwiler A. S1P Stimulates Erythropoietin Production in Mouse Renal Interstitial Fibroblasts by S1P(1) and S1P(3) Receptor Activation and HIF-2α Stabilization. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |