Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1280
Revised: February 29, 2024
Accepted: April 26, 2024
Published online: June 15, 2024
Processing time: 143 Days and 8.5 Hours
Peripheral vascular disease (PVD) is a common complication of type 2 diabetes mellitus (T2DM). Patients with T2DM have twice the risk of PVD as nondiabetic patients.
To evaluate left ventricular (LV) systolic function by layer-specific global longitudinal strain (GLS) and peak strain dispersion (PSD) in T2DM patients with and without PVD.
Sixty-five T2DM patients without PVD, 57 T2DM patients with PVD and 63 normal controls were enrolled in the study. Layer-specific GLS [GLS of the epimyocardium (GLSepi), GLS of the middle myocardium (GLSmid) and GLS of the endocardium (GLSendo)] and PSD were calculated. Receiver operating characteristic (ROC) analysis was performed to calculate the sensitivity and specificity of LV systolic dysfunction in T2DM patients with PVD. We calculated Pearson’s correlation coefficients between biochemical data, echocardiographic characteristics, and layer-specific GLS and PSD.
There were significant differences in GLSepi, GLSmid and GLSendo between normal controls, T2DM patients without PVD and T2DM patients with PVD (P < 0.001). Trend tests revealed a ranking of normal controls > T2DM patients without PVD > T2DM patients with PVD in the absolute value of GLS (P < 0.001). PSD differed significantly between the three groups, and the trend ranking was as follows: normal controls < T2DM patients without PVD < T2DM patients with PVD (P < 0.001). ROC analysis revealed that the combination of layer-specific GLS and PSD had high diagnostic efficiency for detecting LV systolic dysfunction in T2DM patients with PVD. Low-density lipoprotein cholesterol was positively correlated with GLSepi, GLSmid and PSD (P < 0.05), while LV ejection fraction was negatively correlated with GLSepi, GLSmid and GLSendo in T2DM patients with PVD (P < 0.01).
PVD may aggravate the deterioration of LV systolic dysfunction in T2DM patients. Layer-specific GLS and PSD can be used to detect LV systolic dysfunction accurately and conveniently in T2DM patients with or without PVD.
Core Tip: Left ventricular (LV) systolic function is impaired in type 2 diabetes mellitus (T2DM) patients, especially those with peripheral vascular disease (PVD). Layer-specific global longitudinal strain (GLS) and PSD can be used to detect LV systolic dysfunction flexibly, accurately and conveniently. The level of low-density lipoprotein cholesterol was positively correlated with global longitudinal strain of the epimyocardium, global longitudinal strain of the middle myocardium and PSD. PVD may aggravate the LV systolic dysfunction in T2DM patients. Layer-specific GLS and PSD can be used to detect LV systolic dysfunction flexibly, accurately and conveniently in T2DM patients with or without PVD.
- Citation: Li GA, Huang J, Fan L. Evaluation of left ventricular systolic function in type 2 diabetes mellitus patients with and without peripheral vascular disease. World J Diabetes 2024; 15(6): 1280-1290
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1280.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1280
Peripheral vascular disease (PVD) of the lower extremities affects more than 200 million people worldwide, and its prevalence is expected to increase in the coming years[1,2]. Compared with coronary heart disease (CHD) and/or carotid artery disease, PVD has not been well studied[3]. PVD is a common complication of type 2 diabetes mellitus (T2DM), as are cardiovascular events, neural degeneration, kidney failure, blindness, and dementia. Patients with T2DM have twice the risk of PVD in as nondiabetic patients[4]. Both the microvasculature and large vessels are affected by classical cross-talk[5].
T2DM may progress to heart failure even in the absence of myocardial ischaemia and hypertension[6,7]. Cardiac magnetic resonance imaging and echocardiography are the most commonly used methods for detecting subclinical left ventricle (LV) systolic and diastolic dysfunction in T2DM patients[8-10]. Layer-specific global longitudinal strain (GLS) and peak strain dispersion (PSD), derived from two-dimensional speckle tracking echocardiography (STE), accurately detect LV systolic dysfunction in patients with most cardiovascular diseases, such as cardiomyopathy[11,12], coronary artery disease (CAD)[13], and hypertension[14].
Even in the subclinical stage of LV ejection fraction (LVEF) preservation, the LV systolic function of T2DM patients may be impaired, and subclinical dysfunction can be evaluated by STE[15-17]. Whether PVD aggravates the impairment of LV systolic function in T2DM patients has not been further investigated. We propose that the LV systolic function of T2DM patients is further impaired by PVD. We added STE to the diagnostic repertoire of this patient population to evaluate LV systolic function impairment early in T2DM patients with PVD and provide a further reference for reducing the incidence of adverse cardiovascular events. The purpose of this study was to evaluate LV systolic function in T2DM patients with PVD by layer-specific GLS and PSD, observe whether LV systolic function in T2DM patients with PVD is further impaired, and calculate the sensitivity and specificity of the two techniques in evaluating LV systolic function in T2DM patients with PVD.
We enrolled 65 T2DM patients without PVD and 57 T2DM patients with PVD. T2DM was diagnosed according to the American Diabetes Association criteria[18]. T2DM patients with PVD were defined by an ankle-brachial index < 0.9. Patients with a history of arrhythmia, CAD, myocardial infarction, cardiomyopathy, valvular disease, thyroid disease, neoplastic disease, or kidney failure were excluded from the study. Sixty-three normal subjects of similar age and sex were enrolled as controls.
The levels of fasting plasma glucose (FPG), glycated haemoglobin (HbA1c), total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), lipoprotein a (LPA), blood urea nitrogen (BUN), and serum creatinine (SCr) were measured in hospital.
Conventional echocardiography was performed with a GE Vivid E9 ultrasound diagnostic system equipped with an M5s 3.5-5 MHz transducer (GE Vingmed Ultrasound, Horten, Norway). M-mode in the parasternal long-axis view of the LV was used to measure the left atrial diameter (LAd), interventricular septum thickness (IVSd), LV posterior wall thickness (LVPWd), and LV diameter (LVDd) in the end-diastole period, and the mitral annular plane systolic excursion (MAPSE) was measured in the apical four-chamber view. Simpson′s biplane method was used to measure LV end-diastolic (LVEDV) and end-systolic volume (LVESV) and to calculate LVEF. Pulsed wave Doppler imaging of the mitral valve was used to measure the peak early and late diastolic mitral annular velocities (E and A, respectively), and the ratio of E/A was then calculated. The peak early (e′) diastolic annular velocities were obtained by averaging the values at the septum and lateral positions using tissue Doppler imaging, and E/e′ was calculated.
Three consecutive cardiac cycles of apical three-, four- and two-chamber views were recorded for off-line analyses. Layer-specific GLS and PSD were measured by EchoPAC software (EchoPAC Version: 203, GE Vingmed Ultrasound, Norway).
All the data analyses were performed with SPSS 26.0 software (SPSS, Chicago, IL, United States). The normality of all variables was assessed by the Shapiro-Wilk test. Variables were compared between the T2DM patients, T2DM patients with PVD and normal controls by one-way analysis of variance or the Kruskal-Wallis rank sum test, as appropriate. We defined layer-specific GLS and PSD values in the normal controls as the normal state and considered the values of T2DM patients with PVD to be abnormal. These values in T2DM patients with PVD were determined through receiver operating characteristic (ROC) curve analysis by MedCalc software. Correlations between biochemical, echocardiographic, and layer-specific GLS and PSD values were tested using Pearson or Spearman correlation tests, as appropriate. The categorical variables are presented as frequencies and percentages. Normally distributed data are presented as mean ± SD, non-normally distributed data as median (interquartile range). A P value < 0.05 was considered significant for all tests.
Twenty random patients among all enrolled subjects were selected for interobserver and interobserver variability analysis of the GLS of the epimyocardial region (GLSepi), GLS of the middle myocardial region (GLSmid), GLS of the endomyocardial region (GLSendo) and PSD.
Significant differences were detected in weight, body mass index, SBP, DBP, HR, HbA1c, HDL-C, FPG and BUN between normal controls, T2DM patients without PVD and T2DM patients with PVD (P < 0.05). No significant differences were found in age, sex, height, BSA, TG, TC, LDL-C, LPA or SCr between the normal controls, T2DM patients without PVD and T2DM patients with PVD (P > 0.05; Table 1).
| Clinical parameters | Normal controls (n = 63) | T2DM without PVD (n = 65) | T2DM with PVD (n = 57) | P value |
| Age, yr | 53.29 ± 7.55 | 54.75 ± 7.72 | 56.73 ± 7.76 | 0.053 |
| Male, n (%) | 31 (49) | 38 (58) | 38 (67) | 0.103 |
| Height, cm | 164.84 ± 7.07 | 165.83 ± 8.61 | 164.20 ± 6.98 | 0.492 |
| Weight, kg | 63.56 ± 10.09 | 68.95 ± 11.76a | 67.32 ± 11.35 | 0.021 |
| BMI, kg/m2 | 23.33 ± 2.91 | 24.97 ± 3.12 | 24.89 ± 3.40a | 0.005 |
| BSA, m2 | 1.67 ± 0.16 | 1.74 ± 0.19 | 1.71 ± 0.17 | 0.054 |
| SBP, mmHg | 77.90 ± 0.57 | 82.17 ± 10.81a | 80.18 ± 10.58 | 0.001 |
| DBP, mmHg | 123.38 ± 11.12 | 132.77 ± 16.29a | 130.70 ± 17.30a | 0.049 |
| HR, bpm | 66.95 ± 8.61 | 75.58 ± 9.91a | 74.80 ± 8.20a | < 0.001 |
| HbA1c, % | 5.48 ± 0.35 | 9.47 ± 2.39a | 9.94 ± 2.39a | < 0.001 |
| TC, mmol/L | 4.53 ± 0.85 | 4.36 ± 1.06 | 4.55 ± 1.03 | 0.550 |
| TG, mmol/L | 1.30 (0.89, 1.80) | 1.52 (0.93, 2.43) | 1.64 (1.01, 2.07) | 0.073 |
| HDL-C, mmol/L | 1.25 ± 0.33 | 1.08 ± 0.29 | 1.08 ± 0.34 | 0.013 |
| LDL-C, mmol/L | 2.65 ± 0.70 | 2.58 ± 0.84 | 2.70 ± 0.83 | 0.729 |
| LPA, g/L | 0.17 (0.09, 0.29) | 0.12 (0.06, 0.32) | 0.16 (0.09, 0.25) | 0.772 |
| FPG, mmol/L | 4.99 (4.57, 5.28) | 10.02 (7.44, 13.64)a | 10.25 (8.29, 14.07)a | < 0.001 |
| BUN, mmol/L | 4.90 (3.60, 6.40) | 5.65 (4.70, 6.58) | 6.00 (4.70, 7.40)a | 0.015 |
| SCr, μmol/L | 65.00 (56.00, 76.00) | 61.00 (51.75, 81.95) | 61.70 (52.20, 73.00) | 0.772 |
| Medication (%) | ||||
| ACEI/ARB | - | 13 (20) | 12 (21) | |
| Calcium channel blocker | - | 10 (15) | 20 (35) | |
| β-blocker | - | 1 (2) | 2 (4) | |
| SGLT-2 inhibitor | - | 14 (22) | 19 (33) | |
| Metformin | - | 36 (55) | 36 (63) | |
| Insulin | - | 41 (63) | 42 (74) |
The LAd and E/e′ in the T2DM patients were significantly higher than those in the normal controls, while the E, A, E/A and e′ were significantly lower. No significant differences were found in IVSd, LVPWd, LVDd, LVEDV, LVESV or LVEF between normal controls, T2DM patients without PVD and T2DM patients with PVD (P > 0.05; Table 2).
| Echocardiographic parameters | Normal controls (n = 63) | T2DM without PVD (n = 65) | T2DM with PVD (n = 57) | P value |
| LAd, mm | 34.42 ± 2.76 | 35.47 ± 3.25 | 36.17 ± 3.82a | 0.015 |
| IVSd, mm | 9.39 ± 0.74 | 9.20 ± 0.97 | 9.27 ± 0.97 | 0.477 |
| LVPWd, mm | 9.05 ± 0.77 | 8.98 ± 0.99 | 9.13 ± 1.06 | 0.685 |
| LVDd, mm | 46.35 ± 2.97 | 45.42 ± 3.60 | 46.60 ± 3.24 | 0.110 |
| LVEDV, mL | 76.76 ± 14.89 | 74.02 ± 16.74 | 78.04 ± 15.98 | 0.358 |
| LVESV, mL | 26.75 ± 6.53 | 26.12 ± 7.13 | 26.63 ± 6.47 | 0.857 |
| LVEF, % | 65.19 ± 3.98 | 64.98 ± 3.39 | 65.81 ± 4.16 | 0.478 |
| MAPSE, mm | 14.44 ± 1.49 | 14.16 ± 1.85 | 13.23 ± 1.87a,b | 0.001 |
| E, m/s | 0.84 ± 0.13 | 0.75 ± 0.15a | 0.79 ± 0.13a | 0.001 |
| A, m/s | 0.70 ± 0.15 | 0.74 ± 0.18 | 0.81 ± 0.17a,b | 0.002 |
| E/A | 1.24 ± 0.28 | 1.08 ± 0.34a | 1.01 ± 0.27a | < 0.001 |
| e′, m/s | 0.11 ± 0.02 | 0.09 ± 0.02a | 0.09 ± 0.02a | < 0.001 |
| E/e′ | 8.09 ± 1.54 | 8.66 ± 1.53 | 9.45 ± 1.92a,b | < 0.001 |
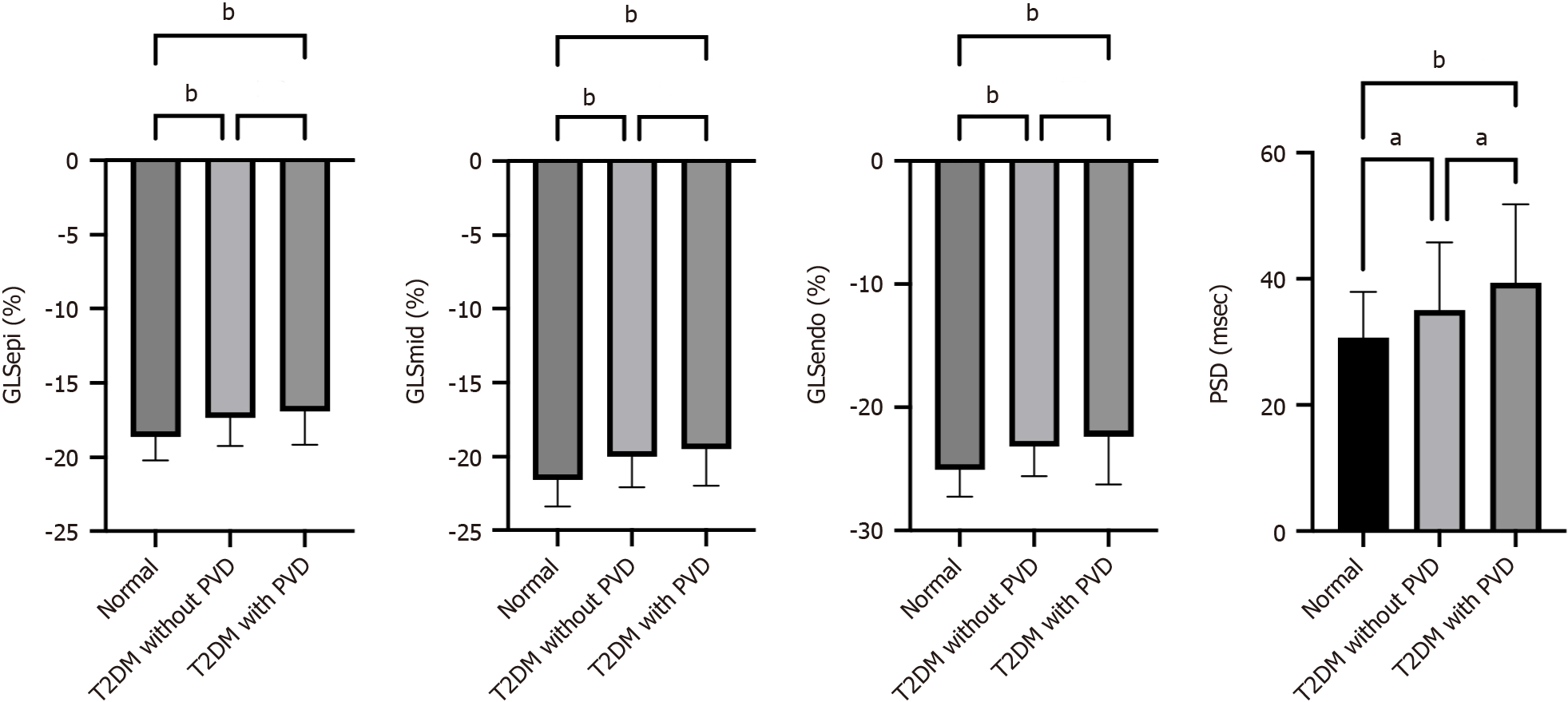
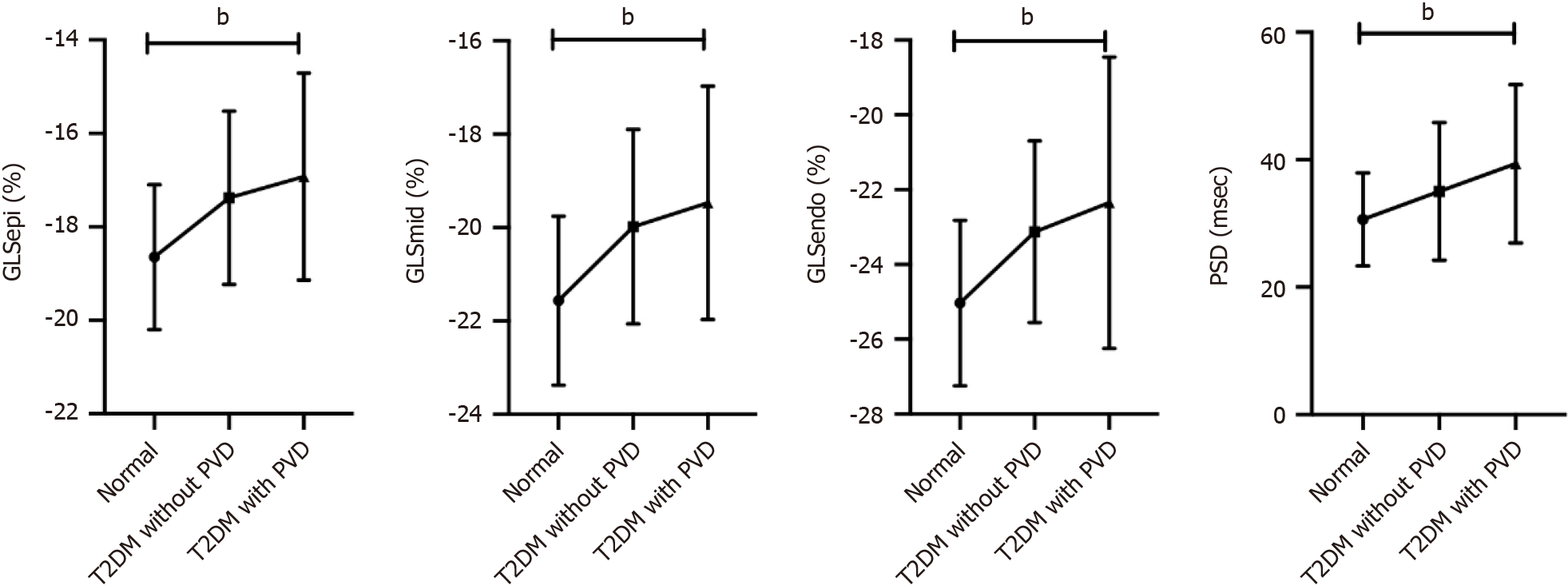
GLSepi, GLSmid and GLSendo differed between normal controls, T2DM patients without PVD and T2DM patients with PVD (P < 0.001), the trend tests showing a ranking of normal controls > T2DM without PVD > T2DM with PVD for the absolute values of all three (P < 0.001). There was a significant difference in PSD between the three groups, and the trend test results were as follows: normal controls < T2DM without PVD < T2DM with PVD (P < 0.001; Figures 1 and 2, and Table 3).
| Normal controls (n = 63) | T2DM without PVD (n = 65) | T2DM with PVD (n = 57) | P value | P trend | |
| GLSepi, % | -18.65 ± 1.55 | -17.37 ± 1.85a | -16.92 ± 2.21a | < 0.001 | < 0.001 |
| GLSmid, % | -21.57 ± 1.81 | -19.98 ± 2.08a | -19.47 ± 2.50a | < 0.001 | < 0.001 |
| GLSendo, % | -25.03 ± 2.21 | -23.12 ± 2.43a | -22.72 ± 2.85a | < 0.001 | < 0.001 |
| PSD, msec | 30.63 ± 7.29 | 35.03 ± 10.81a | 39.37 ± 12.42a,b | < 0.001 | < 0.001 |
Pairwise comparisons of groups revealed a significant difference in PSD between T2DM patients without PVD and T2DM patients with PVD (P < 0.05). There was no significant difference in layer-specific GLS between these two groups (P > 0.05).
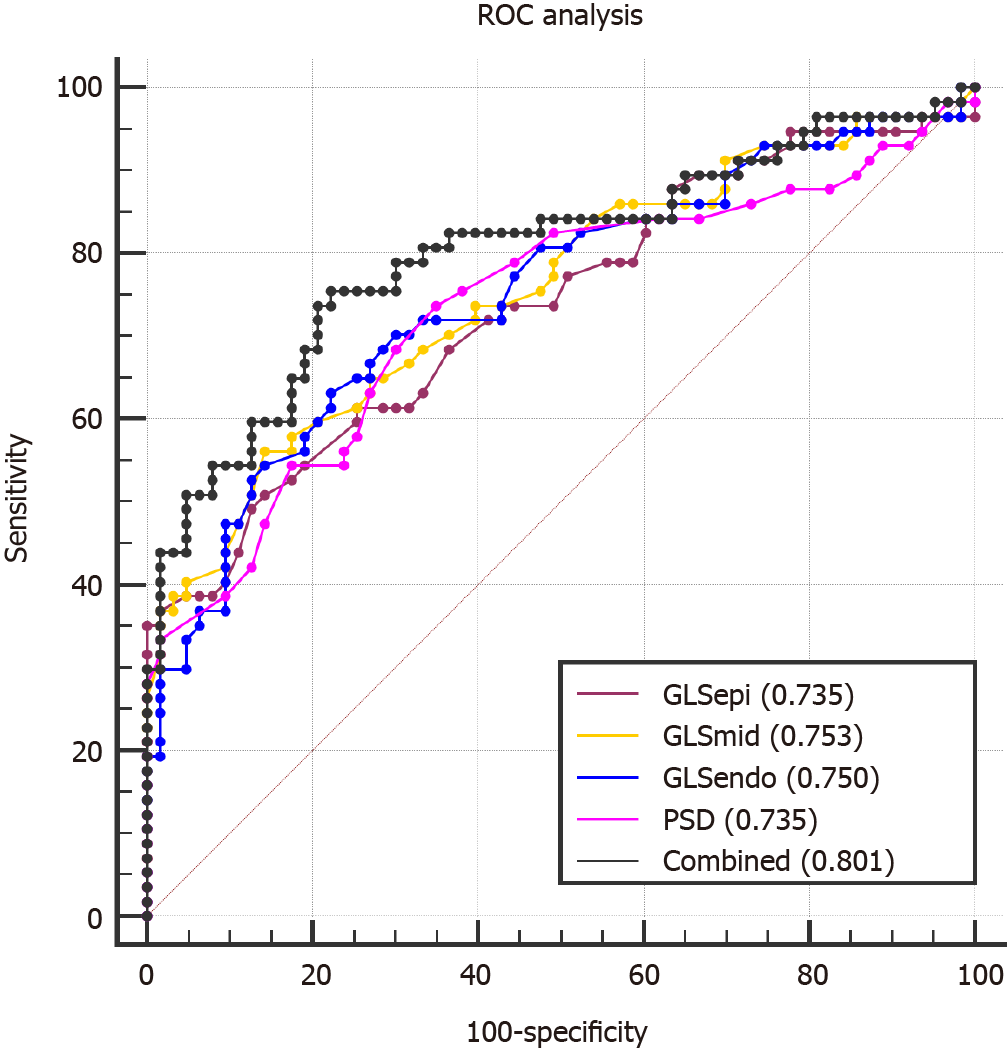
The area under the ROC curve (AUC) of the combination of GLSepi, GLSmid, GLSendo and PSD was 0.801, and the best cut-off value was 0.44, which had a sensitivity of 75.44% and specificity of 77.78%. This AUC was greater than the AUCs of the individual indices alone.
There were no significant differences between the AUCs of layer-specific LV GLS and PSD (P > 0.05; Table 4 and Figure 3).
| ROC | GLSepi | GLSmid | GLSendo | PSD | Combined |
| Sensitivity, % | 50.88 | 56.14 | 66.16 | 73.68 | 75.44 |
| Specificity, % | 85.71 | 85.71 | 77.78 | 65.08 | 77.78 |
| Youden index | 0.3659 | 0.4185 | 0.4094 | 0.3876 | 0.5322 |
| AUC (95%CI) | 0.735 (0.647-0.811) | 0.753 (0.666-0.827) | 0.750 (0.663-0.825) | 0.735 (0.646-0.811) | 0.801 (0.719-0.869) |
| Associated criterion | -17.0 | -19.8 | -23.5 | 32 | 0.44 |
| P value | 0.064 | 0.127 | 0.103 | 0.050 |
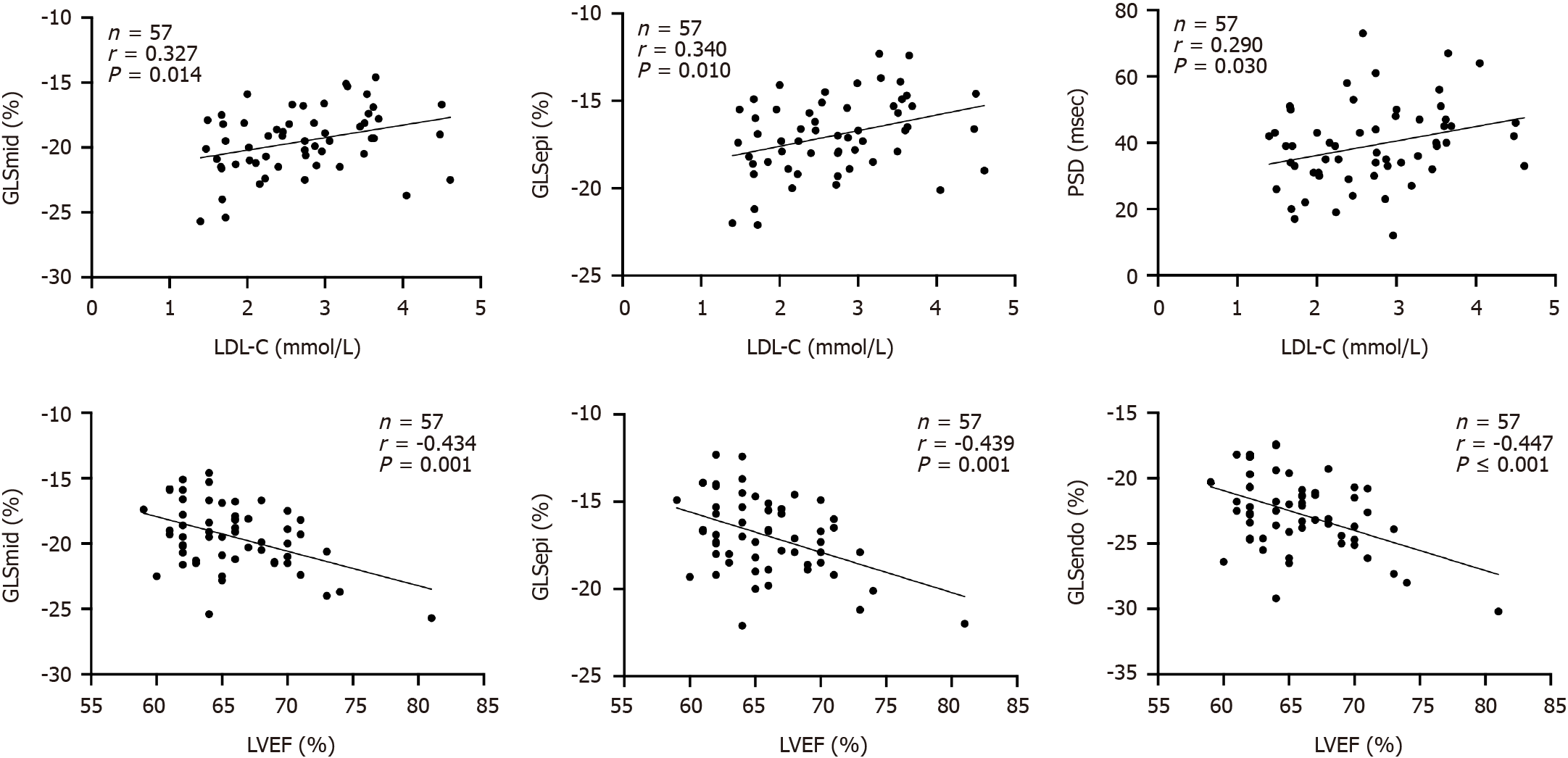
In T2DM patients with PVD, LDL-C was positively correlated with GLSepi, GLSmid and PSD (P < 0.05), while LVEF was negatively correlated with GLSepi, GLSmid and GLSendo (P < 0.01). BUN was positively correlated with GLSepi and PSD (P < 0.05). SCr was positively correlated with GLSepi (P < 0.05). MAPSE was negatively correlated with GLSmid (P < 0.05; Table 5 and Figure 4).
| Varibles | GLSepi | GLSmid | GLSendo | PSD | ||||
| r value | P value | r value | P value | r value | P value | r value | P value | |
| BMI | 0.089 | 0.510 | 0.093 | 0.491 | -0.009 | 0.945 | -0.017 | 0.900 |
| HbA1c | -0.011 | 0.935 | 0.028 | 0.839 | -0.019 | 0.887 | -0.225 | 0.095 |
| TC | 0.190 | 0.158 | 0.179 | 0.182 | 0.040 | 0.767 | 0.293 | 0.027 |
| TG | -0.126 | 0.349 | -0.153 | 0.257 | -0.148 | 0.271 | 0.017 | 0.889 |
| HDL-C | -0.033 | 0.812 | -0.029 | 0.834 | -0.165 | 0.228 | 0.093 | 0.499 |
| LDL-C | 0.340 | 0.010 | 0.327 | 0.014 | 0.204 | 0.131 | 0.290 | 0.030 |
| LPA | 0.159 | 0.254 | 0.134 | 0.339 | 0.184 | 0.187 | -0.103 | 0.465 |
| BUN | 0.293 | 0.033 | 0.248 | 0.074 | 0.149 | 0.287 | 0.292 | 0.034 |
| SCR | 0.301 | 0.027 | 0.264 | 0.053 | 0.166 | 0.231 | 0.205 | 0.137 |
| LVEF | -0.434 | 0.001 | -0.439 | 0.001 | -0.447 | < 0.001 | -0.109 | 0.420 |
| MAPSE | -0.248 | 0.063 | -0.313 | 0.018 | -0.260 | 0.051 | -0.111 | 0.411 |
The intraclass correlation coefficients of layer-specific GLS and PSD were both greater than 0.95 (Table 6).
| Variable | Inter-observer variability | Intra-observer variability | ||
| ICC | 95%CI | ICC | 95%CI | |
| GLSendo | 0.955 | 0.887-0.982 | 0.964 | 0.910-0.986 |
| GLSmid | 0.963 | 0.906-0.985 | 0.970 | 0.924-0.988 |
| GLSepi | 0.960 | 0.900-0.984 | 0.970 | 0.925-0.988 |
| PSD | 0.956 | 0.889-0.983 | 0.979 | 0.946-0.992 |
The findings of this study were as follows: (1) LV systolic function was impaired in both T2DM patients without PVD and T2DM patients with PVD, and LV systolic dysfunction was more serious in T2DM patients with PVD; (2) layer-specific GLS and PSD can be used to detect LV systolic dysfunction flexibly, accurately and conveniently; and (3) LDL-C was positively correlated with GLSepi, GLSmid and PSD.
The most important complications of T2DM are vascular complications, which are the primary cause of death in patients with T2DM. PVD is considered one of the macrovascular complications in T2DM patients, and the complications include CHD, heart failure, and cerebrovascular disease[19].
Layer-specific GLS and PSD can predict LV systolic function and synchronism. Huang et al[20] used layer-specific GLS to evaluate LV systolic function in primary hypertension patients and found that LV systolic function was impaired. Layer-specific GLS has also been used to detect LV systolic function in hypertrophic cardiomyopathy, acute coronary syndrome, Anderson-Fabry disease, and other conditions[12,21,22]. Ji and Zhang[23] used PSD to assess LV systolic synchrony in patients with systemic lupus erythematosus (SLE) and found that LV systolic synchrony was impaired. PSD can be used as a new, reliable index to evaluate LV systolic synchrony. PSD has also been used to evaluate LV systolic function and synchrony in hypertrophic cardiomyopathy, rheumatoid arthritis, and diabetes mellitus[24-26]. Therefore, using layer-specific GLS and PSD to evaluate LV systolic function is considered accurate and reliable.
Our research revealed that T2DM patients with and without PVD had decreased layer-specific GLS and increased PSD but similar LVEDV, LVESV, and LVEF to those of normal controls. Sustained hyperglycaemia can cause myocardial hypertrophy, fibrosis and decreased myocardial compliance, ultimately resulting in LV systolic dysfunction[27,28]. By comparing the three groups, we only found that the PSD in T2DM patients with PVD was greater than that in T2DM patients without a PVD and greater than that in normal controls. We concluded that T2DM patients with PVD are more likely to progress to more severe LV systolic dysfunction. PVD causes inflammatory damage to vessel walls due to endothelial cell dysfunction, resulting in stenosis and occlusion[29]. Hyperglycaemia blocks endothelial NO synthase and increases the amount of reactive oxygen species, causing vascular wall damage[30], and C-reactive protein, a risk factor for PVD, is elevated in patients with DM[3,31].
ROC analysis revealed that both layer-specific GLS and PSD had good predictive value for LV systolic dysfunction in T2DM patients with PVD. The combination of layer-specific GLS and PSD had a high AUC for evaluating LV systolic dysfunction, and the combined index also had the best predictive value for cardiac dysfunction in T2DM patients with PVD.
LDL-C was positively correlated with GLSepi, GLSmid and PSD in T2DM patients with PVD, which indicated that LDL-C should be controlled in T2DM patients when they have been diagnosed with PVD.
First, the sample size of the study was relatively small. A larger sample size could improve the robustness and generalizability of the findings. Second, the study was conducted at a single centre, which may limit the generalizability of the findings to broader populations. Multicentre studies involving diverse demographic and geographic populations could enhance the external validity of the results.
PVD may aggravate the deterioration of LV systolic dysfunction in T2DM patients. Layer-specific GLS and PSD can be used to detect LV systolic dysfunction flexibly, accurately and conveniently in T2DM patients with or without PVD.
We would like to thank the Department of Echocardiography, Cardiology and Endocrinology, The Affiliated Changzhou Second People’s Hospital with Nanjing Medical University.
| 1. | Giannopoulos S, Armstrong EJ. Diabetes mellitus: an important risk factor for peripheral vascular disease. Expert Rev Cardiovasc Ther. 2020;18:131-137. [PubMed] [DOI] [Full Text] |
| 2. | Correction to: 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e790. [PubMed] [DOI] [Full Text] |
| 3. | Yu HI, Sheu WH, Song YM, Liu HC, Lee WJ, Chen YT. C-reactive protein and risk factors for peripheral vascular disease in subjects with Type 2 diabetes mellitus. Diabet Med. 2004;21:336-341. [PubMed] [DOI] [Full Text] |
| 4. | Walters DP, Gatling W, Mullee MA, Hill RD. The prevalence, detection, and epidemiological correlates of peripheral vascular disease: a comparison of diabetic and non-diabetic subjects in an English community. Diabet Med. 1992;9:710-715. [PubMed] [DOI] [Full Text] |
| 5. | Boutouyrie P, Climie RE, Bruno RM. Type 2 Diabetes Mellitus, Interaction Between Left Ventricle and Large Arteries. Am J Hypertens. 2022;35:388-390. [PubMed] [DOI] [Full Text] |
| 6. | Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschöpe C, Hoes AW, Seferović JP, Logue J, McDonagh T, Riley JP, Milinković I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F, McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853-872. [PubMed] [DOI] [Full Text] |
| 7. | Kenny HC, Abel ED. Heart Failure in Type 2 Diabetes Mellitus. Circ Res. 2019;124:121-141. [PubMed] [DOI] [Full Text] |
| 8. | Shen LT, Jiang L, Zhu YW, Shen MT, Huang S, Shi R, Li Y, Yang ZG. Additive effect of aortic regurgitation degree on left ventricular strain in patients with type 2 diabetes mellitus evaluated via cardiac magnetic resonance tissue tracking. Cardiovasc Diabetol. 2022;21:37. [PubMed] [DOI] [Full Text] |
| 9. | Zhang Y, Wang J, Ren Y, Yan WF, Jiang L, Li Y, Yang ZG. The additive effects of kidney dysfunction on left ventricular function and strain in type 2 diabetes mellitus patients verified by cardiac magnetic resonance imaging. Cardiovasc Diabetol. 2021;20:11. [PubMed] [DOI] [Full Text] |
| 10. | Silverii GA, Toncelli L, Casatori L, Bossini R, Nannelli F, Pala L, Mannucci E. Assessment of left ventricular global longitudinal strain in patients with type 2 diabetes: Relationship with microvascular damage and glycemic control. Nutr Metab Cardiovasc Dis. 2022;32:994-1000. [PubMed] [DOI] [Full Text] |
| 11. | Tang S, Guan L, Tayier B, Mu Y. ECHO provides layer-specific insight of both myocardial deformation and microcirculation dysfunction in dilated cardiomyopathy patients: Clinical value of combined application of left ventricular layer-specific strain and myocardial contrast echocardiography. J Clin Ultrasound. 2023;51:753-761. [PubMed] [DOI] [Full Text] |
| 12. | Chen Z, Li C, Li Y, Rao L, Zhang X, Long D. Layer-specific strain echocardiography may reflect regional myocardial impairment in patients with hypertrophic cardiomyopathy. Cardiovasc Ultrasound. 2021;19:15. [PubMed] [DOI] [Full Text] |
| 13. | Hagemann CA, Hoffmann S, Hagemann RA, Fritz-Hansen T, Olsen FJ, Jørgensen PG, Biering-Sørensen T. Usefulness of layer-specific strain in diagnosis of coronary artery disease in patients with stable angina pectoris. Int J Cardiovasc Imaging. 2019;35:1989-1999. [PubMed] [DOI] [Full Text] |
| 14. | Huang J, Yang C, Yan ZN, Fan L, Ni CF. Global myocardial work: A new way to detect subclinical myocardial dysfunction with normal left ventricle ejection fraction in essential hypertension patients: Compared with myocardial layer-specific strain analysis. Echocardiography. 2021;38:850-860. [PubMed] [DOI] [Full Text] |
| 15. | Yang QM, Fang JX, Chen XY, Lv H, Kang CS. The Systolic and Diastolic Cardiac Function of Patients With Type 2 Diabetes Mellitus: An Evaluation of Left Ventricular Strain and Torsion Using Conventional and Speckle Tracking Echocardiography. Front Physiol. 2021;12:726719. [PubMed] [DOI] [Full Text] |
| 16. | Wu T, Gong L, Zhang C, Zhang D, Li X. Three-dimensional echocardiography and strain cardiac imaging in patients with prediabetes and type 2 diabetes mellitus. Quant Imaging Med Surg. 2023;13:7753-7764. [PubMed] [DOI] [Full Text] |
| 17. | Wang T, Li L, Huang J, Fan L. Assessment of subclinical left ventricle myocardial dysfunction using global myocardial work in type 2 diabetes mellitus patients with preserved left ventricle ejection fraction. Diabetol Metab Syndr. 2022;14:17. [PubMed] [DOI] [Full Text] |
| 18. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62-S69. [PubMed] [DOI] [Full Text] |
| 19. | Viigimaa M, Sachinidis A, Toumpourleka M, Koutsampasopoulos K, Alliksoo S, Titma T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:110-116. [PubMed] [DOI] [Full Text] |
| 20. | Huang J, Yan ZN, Rui YF, Fan L, Shen D, Chen DL. Left Ventricular Systolic Function Changes in Primary Hypertension Patients Detected by the Strain of Different Myocardium Layers. Medicine (Baltimore). 2016;95:e2440. [PubMed] [DOI] [Full Text] |
| 21. | Derumeaux G, Ternacle J. Layer-specific strain in acute coronary syndrome: back to the future! Eur Heart J Cardiovasc Imaging. 2018;19:1325-1326. [PubMed] [DOI] [Full Text] |
| 22. | Esposito R, Santoro C, Sorrentino R, Riccio E, Citro R, Buonauro A, Di Risi T, Imbriaco M, Trimarco B, Pisani A, Galderisi M; Anderson-Fabry Federico II Naples, ITalY (AFFINIITY) Group. Layer-specific longitudinal strain in Anderson-Fabry disease at diagnosis: A speckle tracking echocardiography analysis. Echocardiography. 2019;36:1273-1281. [PubMed] [DOI] [Full Text] |
| 23. | Ji X, Zhang X. Assessment of left ventricular systolic synchrony by peak strain dispersion in patients with systemic lupus erythematosus. Eur Rev Med Pharmacol Sci. 2022;26:2840-2846. [PubMed] [DOI] [Full Text] |
| 24. | Su Y, Peng Q, Yin L, Li C. Evaluation of Exercise Tolerance in Non-obstructive Hypertrophic Cardiomyopathy With Myocardial Work and Peak Strain Dispersion by Speckle-Tracking Echocardiography. Front Cardiovasc Med. 2022;9:927671. [PubMed] [DOI] [Full Text] |
| 25. | Roemer S, Jaglan A, Santos D, Umland M, Jain R, Tajik AJ, Khandheria BK. The Utility of Myocardial Work in Clinical Practice. J Am Soc Echocardiogr. 2021;34:807-818. [PubMed] [DOI] [Full Text] |
| 26. | Li C, Yuan M, Li K, Bai W, Rao L. Value of peak strain dispersion in discovering left ventricular dysfunction in diabetes mellitus. Sci Rep. 2020;10:21437. [PubMed] [DOI] [Full Text] |
| 27. | Tadic M, Cuspidi C, Calicchio F, Grassi G, Mancia G. Diabetic cardiomyopathy: How can cardiac magnetic resonance help? Acta Diabetol. 2020;57:1027-1034. [PubMed] [DOI] [Full Text] |
| 28. | Zhang Y, Yan WF, Jiang L, Shen MT, Li Y, Huang S, Shi K, Yang ZG. Aggravation of functional mitral regurgitation on left ventricle stiffness in type 2 diabetes mellitus patients evaluated by CMR tissue tracking. Cardiovasc Diabetol. 2021;20:158. [PubMed] [DOI] [Full Text] |
| 29. | Chin JA, Sumpio BE. Diabetes mellitus and peripheral vascular disease: diagnosis and management. Clin Podiatr Med Surg. 2014;31:11-26. [PubMed] [DOI] [Full Text] |