Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1272
Revised: March 6, 2024
Accepted: April 23, 2024
Published online: June 15, 2024
Processing time: 167 Days and 23.7 Hours
Cardiovascular disease has been the leading cause of morbidity and mortality for type 2 diabetes mellitus (T2DM) patients over the last decade.
To determine whether layer-specific global longitudinal strain (GLS) combined with peak strain dispersion (PSD) can be used to assess left ventricle (LV) myocar
We enrolled 97 T2DM patients, 70 T2DM + HP patients and 101 healthy subjects. Layer-specific GLS and PSD were calculated by EchoPAC software in apical three-, four- and two-chamber views. GLS of the epimyocardial, middle-layer and endo
There were significant differences in GLSepi, GLSmid, GLSendo, and PSD bet
Layer-specific GLS and PSD were associated with LV myocardium systolic dysfunction in T2DM patients, T2DM patients with HP. T2DM patients with HP have more severe LV myocardium systolic dysfunction than T2DM patients without HP and normal control patients. The combination of layer-specific GLS and PSD may provide additional prognostic information for T2DM patients with or without HP.
Core Tip: Left ventricle (LV) myocardium systolic dysfunction was found in type 2 diabetes mellitus (T2DM) patients, T2DM patients with hypertension (HP) by layer-specific global longitudinal strain (GLS) and peak strain dispersion (PSD). T2DM patients with HP have more serious LV myocardium systolic dysfunction than T2DM patients without HP and normal control patients. Combined layer-specific GLS and PSD may provide additional prognostic information for T2DM patients with or without HP.
- Citation: Chen ZG, Li GA, Huang J, Fan L. Subclinical impairment of left ventricular myocardium function in type 2 diabetes mellitus patients with or without hypertension. World J Diabetes 2024; 15(6): 1272-1279
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1272.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1272
Type 2 diabetes mellitus (T2DM) is a common metabolic disease whose complications are mainly macro- and microcirculatory disorders[1]. Over the last decade, cardiovascular disease has been the leading cause of morbidity and mortality for T2DM patients[2]. Thus, early identification of myocardial systolic dysfunction in T2DM patients could facilitate earlier intervention and improve patient prognosis.
Recently, many techniques have been used to detect myocardial dysfunction in T2DM patients. For example, cardiac magnetic resonance imaging (MRI)[3] and echocardiography[4,5] have proven impaired cardiac function [including of the left ventricle (LV), left atrium (LA) and right ventricle] in these patients due to strain, strain rate and torsion[6-8]. Al
The aim of this research is to evaluate LV myocardium dysfunction and synchrony in T2DM with or without HP by layer-specific GLS and PSD and to determine whether layer-specific GLS and PSD, alone or in combination, could assess LV myocardium systolic dysfunction in T2DM patients with or without HP.
Ninety-seven T2DM patients and 70 T2DM patients with HP were included. The diagnoses of T2DM patients were determined according to the American Diabetes Association[18], and the diagnoses of HP were determined according to the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) Guidelines for the Management of Arterial HP[19]. Subjects with a history of congenital heart disease, coronary artery disease, valvular disease, car
A total of 101 normal subjects of similar age and sex were enrolled as controls.
The weight, height, heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were recorded when the patients were in hospital, and then body mass index (BMI), and body surface area (BSA) were calculated. La
Echocardiography was performed with a GE Vivid E9 (GE Vingmed Ultrasound, Horten, Norway), cardiac probe was M5s with a frequency of 3.5-5.0 MHz. Left atrial diameter, interventricular septum thickness, LV posterior wall thickness, LV diameter and mitral annular plane systolic excursion (LAd, IVSd, LVPWd, LVDd, and MAPSE) were measured by M-mode. Left ventricular ejection fraction (LVEF) was obtained via the biplane Simpson’s method. Pulsed wave Doppler of the mitral valve and tissue Doppler of the anterior and posterior annulus of the mitral valve were also evaluated, and then the E/A and E/e’ were calculated.
Three consecutive cardiac cycles of apical three-, four- and two-chamber views were recorded for off-line analyses. Layer-specific GLS and PSD were measured by EchoPAC (version: 203).
The normality of all values was assessed by the Kolmogorov-Smirnov test or Shapiro-Wilk test. Differences between the T2DM patients, T2DM patients with HP and healthy subjects were compared with one-way analysis of variance (ANOVA) for normally distributed continuous variables, while the Kruskal-Wallis rank sum test was used for non
Twenty randomly patients among all enrolled subjects were selected for interobserver and interobserver variabilities analysis in GLSepi, GLSmid, GLSendo, and PSD.
Significant differences were detected in weight, BMI, BSA, SBP, DBP, HR, FPG, HbA1c, TG, HDL-C, BUN, and SCR between the healthy subjects, T2DM patients and T2DM + HP patients (P < 0.001). No significant differences were found in age, sex, height, TC, LDL-C or LPA between the healthy subjects, T2DM and T2DM + HP (P > 0.05) (Table 1).
| Clinical parameters | Healthy subjects (n = 101) | T2DM (n = 97) | T2DM + HP (n = 70) | P value |
| Age, yr | 48.06 ± 9.90 | 49.04 ± 12.80 | 52.00 ± 11.50 | 0.068 |
| Male, n (%) | 48 (48) | 61 (63) | 39 (56) | 0.094 |
| Hight, cm | 165.10 ± 7.50 | 166.87 ± 9.25 | 164.66 ± 7.53 | 0.199 |
| Wight, kg | 64.14 ± 10.58 | 72.50 ± 15.341 | 72.23 ± 13.801 | < 0.001 |
| BMI, kg/m2 | 23.44 ± 2.84 | 25.85 ± 3.901 | 26.51 ± 4.021 | < 0.001 |
| BSA, m2 | 1.68 ± 0.17 | 1.79 ± 0.241 | 1.78 ± 0.211 | < 0.001 |
| SBP, mmHg | 123.26 ± 10.82 | 127.65 ± 14.701 | 137.07 ± 17.341,2 | < 0.001 |
| DBP, mmHg | 77.85 ± 7.65 | 79.25 ± 10.59 | 86.64 ± 10.201,2 | < 0.001 |
| HR, bpm | 69.85 ± 9.76 | 75.22 ± 9.601 | 79.10 ± 12.721,2 | <0.001 |
| FPG, mmol/L | 4.95 (4.59, 5.25) | 10.53 (7.87, 14.07)1 | 8.91 (7.57, 10.97)1 | < 0.001 |
| HbA1c, % | 5.45 ± 0.38 | 9.73 ± 2.271 | 8.85 ± 2.491,2 | < 0.001 |
| TC, mmol/L | 4.54 ± 0.85 | 4.37 ± 0.90 | 4.70 ± 1.14 | 0.102 |
| TG, mmol/L | 1.21 (0.88,1.78) | 1.53 (0.97, 2.15)1 | 2.00 (1.38, 2.83)1,2 | < 0.001 |
| HDL-C, mmol/L | 1.27 ± 0.30 | 1.09 ± 0.291 | 1.04 ± 0.281 | < 0.001 |
| LDL-C, mmol/L | 2.68 ± 0.70 | 2.65 ± 0.79 | 2.81 ± 0.99 | 0.556 |
| LPA, g/L | 0.17 (0.11, 0.28) | 0.14 (0.06, 0.24) | 0.14 (0.08, 0.25) | 0.231 |
| BUN, mmol/L | 4.75 (3.60, 5.70) | 5.50 (4.60, 6.30)1 | 5.50 (4.50, 7.40)1 | 0.001 |
| SCR, μmol/L | 61.00 (55.00, 76.00) | 59.00 (49.30, 71.10) | 64.00 (54.00, 87.00)2 | 0.030 |
| Medication, n (%) | ||||
| ACEI/ARB | - | - | 32 (46) | |
| Calcium channel blocker | - | - | 37 (53) | |
| β-blocker | - | - | 4 (6) | |
| SGLT-2 inhibitor | - | 18 (19) | 28 (40) | |
| Metformin | - | 59 (61) | 38 (54) | |
| Insulin | - | 55 (57) | 35 (50) |
Significant differences were found in LAd, LAV index, IVSd, LVPWd, LVEDV, MAPSE, E, A, E/A, and E/e’ between the healthy subjects, T2DM patients and T2DM + HP patients (P < 0.05). No significant differences were found in the LVd, LVESV, LVEF or e’ between the healthy subjects, T2DM patients and T2DM with HP patients (P > 0.05) (Tables 2 and 3).
| Echocardiographic parameters | Healthy subjects (n = 101) | T2DM (n = 97) | T2DM + HP (n = 70) | P value |
| LAd, mm | 34.00 ± 2.97 | 35.03 ± 3.041 | 36.69 ± 3.911,2 | < 0.001 |
| LAV index, mL/m2 | 29.17 ± 7.19 | 32.20 ± 6.801 | 30.92 ± 8.10 | 0.016 |
| IVSd, mm | 9.21 ± 0.79 | 9.01 ± 0.98 | 9.71 ± 0.951,2 | < 0.001 |
| LVPWd, mm | 9.01 ± 0.80 | 8.81 ± 0.93 | 9.53 ± 1.001,2 | < 0.001 |
| LVDd, mm | 46.39 ± 2.95 | 45.85 ± 3.49 | 46.89 ± 3.40 | 0.124 |
| LVEDV, mL | 76.61 ± 15.17 | 69.57 ± 20.541 | 76.77 ± 22.262 | 0.018 |
| LVESV, mL | 27.19 ± 6.65 | 25.01 ± 8.14 | 27.93 ± 8.762 | 0.037 |
| LVEF, % | 64.56 ± 3.74 | 64.35 ± 2.89 | 63.54 ± 2.71 | 0.080 |
| MAPSE, mm | 14.47 ± 1.44 | 14.23 ± 1.75 | 13.42 ± 2.071,2 | 0.002 |
| E, m/s | 0.83 ± 0.13 | 0.78 ± 0.141 | 0.78 ± 0.161 | 0.008 |
| A, m/s | 0.68 ± 0.16 | 0.69 ± 0.15 | 0.82 ± 0.181,2 | < 0.001 |
| E/A | 1.27 ± 0.29 | 1.16 ± 0.291 | 1.00 ± 0.361,2 | < 0.001 |
| e’, m/s | 0.11 ± 0.02 | 0.10 ± 0.02 | 0.10 ± 0.11 | 0.117 |
| E/e’ | 7.62 ± 1.54 | 8.30 ± 1.631 | 9.00 ± 2.471,2 | < 0.001 |
| Healthy subjects (n = 101) | T2DM (n = 97) | T2DM + HP (n = 70) | P1 value | Ptrend value | |
| GLSepi, % | -18.66 ± 1.58 | -17.33 ± 1.931 | -16.74 ± 1.931,2 | < 0.001 | < 0.001 |
| GLSmid, % | -21.58 ± 1.843 | -19.87 ± 2.211,3 | -19.28 ± 2.161,3 | < 0.001 | < 0.001 |
| GLSendo, % | -25.03 ± 2.243,4 | -22.97 ± 2.561,3,4 | -22.48 ± 2.501,3,4 | < 0.001 | < 0.001 |
| PSD, msec | 28.79 ± 7.25 | 34.92 ± 11.191 | 39.23 ± 11.471,2 | < 0.001 | < 0.001 |
| P2 value | < 0.001 | < 0.001 | < 0.001 |
There were significant differences in GLSepi, GLSmid, and GLSendo within the normal control, T2DM and T2DM with HP groups (P < 0.001), and trend tests showed a ranking of healthy subjects > T2DM patients > T2DM with HP patients in the absolute values of GLSepi, GLSmid and GLSendo (P < 0.001). There was a significant difference in PSD between the three groups, and the trend test results were as follows: Healthy subjects < T2DM < T2DM with HP (P < 0.001).
In each of the three groups, there were significant differences between GLSepi, GLSmid and GLSendo (P < 0.001), the trend tests showing an order of GLSepi < GLSmid < GLSendo (P < 0.001).
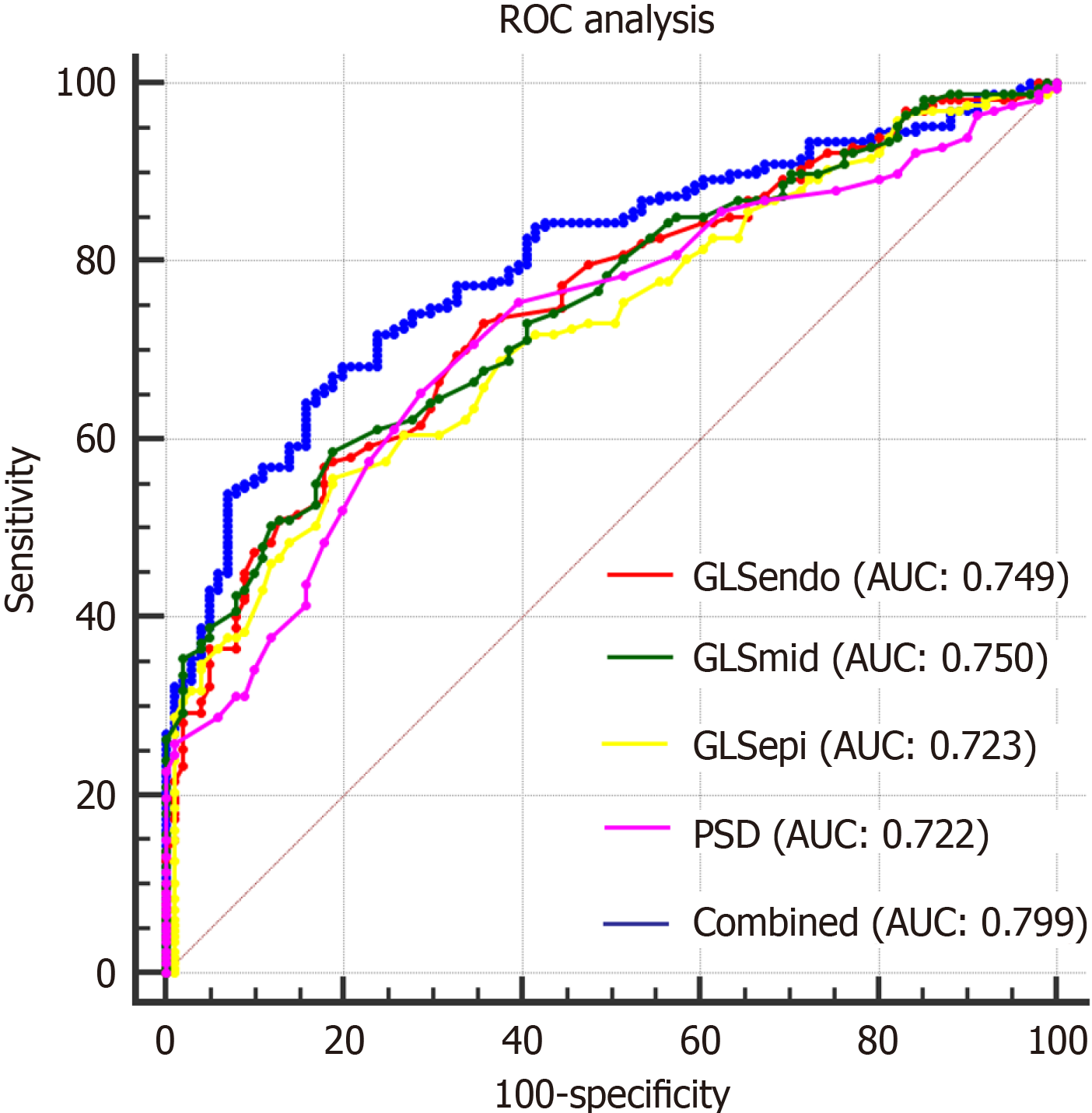
The area under the curve (AUC) of combined layer-specific GLS and PSD was significantly larger than the individual variables (P < 0.05).
There were no significant differences between the AUCs of layer-specific LV GLS and PSD (P > 0.05) (Table 4 and Figure 1).
| Variable | Interobserver variability | Intraobserver variability | ||
| ICC | 95%CI | ICC | 95%CI | |
| GLSendo | 0.959 | 0.896-0.984 | 0.964 | 0.909-0.986 |
| GLSmid | 0.965 | 0.911-0.986 | 0.973 | 0.933-0.989 |
| GLSepi | 0.959 | 0.898-0.984 | 0.975 | 0.936-0.990 |
| PSD | 0.955 | 0.887-0.982 | 0.976 | 0.939-0.990 |
The intraclass correlation coefficient values of Layer-specific GLS and PSD were larger than 0.95 (Table 4).
The study found that LV myocardium systolic dysfunction was impaired and that systolic asynchrony was increased in T2DM patients with or without HP and was more severe in T2DM patients with HP.
Systolic dysfunction is impaired in T2DM patients. Ng et al[8] used the GLS, GCS and GRS to evaluate systolic dys
Compared with GLS, PSD is more accurate in evaluating early lesions of LV function[14]. Previous studies also found that PSD was increased in patients with normal GLS and preserved LVEF[23]. PSD is used to evaluate early systolic dysfunction of the LV by combining the coordination and synchronization of cardiac mechanical movement[14]. PSD can be used as a new reliable index to evaluate LV systolic synchrony in many diseases. Ji et al[17] used PSD to evaluate LV systolic synchrony in patients with RA and found that LV systolic synchrony in patients with RA gradually decreases as the disease course progresses. PSD has also been used in hypertrophic cardiomyopathy (HCM)[16], systemic lupus ery
Due to insulin resistance, microvascular circulation disorders, increased afterload, and other reasons, with the oc
In T2DM patients with HP, LV hypertrophy may also lead to myocardial fibrosis, the sequence of the longitudinal and circumferential myocardium may change under these conditions, and the combined results indicated that the layer-specific GLS was lower in T2DM patients with HP. Layer-specific GLS analysis revealed no difference in GLSmid or GLSendo between the T2DM and T2DM with HP groups, except for GLSepi; however, the trend analysis revealed a decreasing trend. However, the PSD between the three groups decreased in the order of healthy subjects < T2DM patients < T2DM patients with HP, and the difference was also significant. This means that PSD and GLSepi are more sensitive in distinguishing subclinical LV myocardium systolic dysfunction in T2DM patients, T2DM patients with HP.
ROC analysis showed that GLSepi, GLSmid, GLSendo, PSD, and the combination of these indices had high AUCs for evaluating LV myocardium systolic dysfunction in T2DM patients, T2DM patients with HP, and the combined values also had the best predictive value for detecting LV myocardium systolic dysfunction in T2DM patients.
Limitations: First, the sample of the study was relatively small. A larger sample size could improve the robustness and generalizability of the findings. Second, the study was conducted at a single centre, which may limit the generalizability of the findings to broader populations. Multicentre studies involving diverse demographic and geographic populations could enhance the external validity of the results. Third, long-term follow-up data on clinical outcomes such as car
Layer-specific GLS and PSD can find LV myocardium systolic dysfunction in T2DM patients, T2DM patients with HP. T2DM patients with HP have more severe LV myocardium systolic dysfunction than T2DM patients without HP and healthy subjects. The combination of layer-specific GLS and PSD may provide additional prognostic information for T2DM patients with or without HP.
| 1. | Su Y, Liu W, Wang D, Tian J. Evaluation of abdominal aortic elasticity by strain rate imaging in patients with type 2 diabetes mellitus. J Clin Ultrasound. 2014;42:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Cao Y, Zeng W, Cui Y, Kong X, Wang M, Yu J, Zhang S, Song J, Yan X, Greiser A, Shi H. Increased myocardial extracellular volume assessed by cardiovascular magnetic resonance T1 mapping and its determinants in type 2 diabetes mellitus patients with normal myocardial systolic strain. Cardiovasc Diabetol. 2018;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Wang J, Li Y, Guo YK, Huang S, Shi R, Yan WF, Qian WL, He GX, Yang ZG. The adverse impact of coronary artery disease on left ventricle systolic and diastolic function in patients with type 2 diabetes mellitus: a 3.0T CMR study. Cardiovasc Diabetol. 2022;21:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Yang QM, Fang JX, Chen XY, Lv H, Kang CS. The Systolic and Diastolic Cardiac Function of Patients With Type 2 Diabetes Mellitus: An Evaluation of Left Ventricular Strain and Torsion Using Conventional and Speckle Tracking Echocardiography. Front Physiol. 2021;12:726719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Ernande L, Bergerot C, Girerd N, Thibault H, Davidsen ES, Gautier Pignon-Blanc P, Amaz C, Croisille P, De Buyzere ML, Rietzschel ER, Gillebert TC, Moulin P, Altman M, Derumeaux G. Longitudinal myocardial strain alteration is associated with left ventricular remodeling in asymptomatic patients with type 2 diabetes mellitus. J Am Soc Echocardiogr. 2014;27:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Huang J, Li L, Fan L, Chen DL. Evaluation of right ventricular systolic and diastolic dysfunctions in patients with type 2 diabetes mellitus with poor glycemic control by layer specific global longitudinal strain and strain rate. Diabetol Metab Syndr. 2022;14:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Doggrell SA. Oral fingolimod for relapsing-remitting multiple sclerosis Evaluation of: Kappos L, Radue E-M, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387-401; and Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402-15. Expert Opin Pharmacother. 2010;11:1777-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Grund FF, Kristensen CB, Bahrami HSZ, Mogelvang R, Hassager C. Layer-specific longitudinal strain detects transmural dysfunction in chronic severe aortic regurgitation before and after aortic valve surgery. Int J Cardiovasc Imaging. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 10. | Tsugu T, Nagatomo Y, Dulgheru R, Lancellotti P. Layer-specific longitudinal strain predicts left ventricular maximum wall thickness in patients with hypertrophic cardiomyopathy. Echocardiography. 2021;38:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Tsai WC, Lee WH, Liu YW. P1270Effects of blood pressure variability on layer-specific longitudinal strain in hypertension. Eur Heart J Cardiovasc Imaging. 2016;17:ii270-ii276. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Huang J, Yan ZN, Rui YF, Fan L, Shen D, Chen DL. Left Ventricular Systolic Function Changes in Primary Hypertension Patients Detected by the Strain of Different Myocardium Layers. Medicine (Baltimore). 2016;95:e2440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 13. | Shi F, Feng S, Zhu J, Wu Y, Chen J. Left Ventricular Strain and Dyssynchrony in Young and Middle-Aged Peritoneal Dialysis Patients and Healthy Controls: A Case-Matched Study. Cardiorenal Med. 2018;8:271-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Li C, Yuan M, Li K, Bai W, Rao L. Value of peak strain dispersion in discovering left ventricular dysfunction in diabetes mellitus. Sci Rep. 2020;10:21437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Liu C, Yan ZN, Fan L, Huang J, Shen D, Song XT. Layer-specific speckle tracking analysis of left ventricular systolic function and synchrony in maintenance hemodialysis patients. BMC Cardiovasc Disord. 2020;20:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Su Y, Peng Q, Yin L, Li C. Evaluation of Exercise Tolerance in Non-obstructive Hypertrophic Cardiomyopathy With Myocardial Work and Peak Strain Dispersion by Speckle-Tracking Echocardiography. Front Cardiovasc Med. 2022;9:927671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Ji X, Zhang X, Feng H. Evaluation of left ventricular systolic synchrony by peak strain dispersion in patients with rheumatoid arthritis. J Int Med Res. 2021;49:3000605211007737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67-S74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1255] [Cited by in RCA: 1546] [Article Influence: 128.8] [Reference Citation Analysis (5)] |
| 19. | 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension: Erratum. J Hypertens. 2019;37:456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Liu JH, Chen Y, Yuen M, Zhen Z, Chan CW, Lam KS, Tse HF, Yiu KH. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 700] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 22. | Braşoveanu AM, Mogoantă L, Mălăescu GD, Predescu OI, Cotoi BV, Ifrim Chen F. Hypertensive cardiomyopathy - histopathological and immunohistochemical aspects. Rom J Morphol Embryol. 2019;60:487-494. [PubMed] |
| 23. | Ermakov S, Gulhar R, Lim L, Bibby D, Fang Q, Nah G, Abraham TP, Schiller NB, Delling FN. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart. 2019;105:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Li C, Li K, Yuan M, Bai W, Rao L. Peak strain dispersion within the left ventricle detected by two-dimensional speckle tracking in patients with uncomplicated systemic lupus erythematosus. Int J Cardiovasc Imaging. 2021;37:2197-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |