Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1242
Revised: March 5, 2024
Accepted: April 25, 2024
Published online: June 15, 2024
Processing time: 138 Days and 5.5 Hours
The birth of large-for-gestational-age (LGA) infants is associated with many short-term adverse pregnancy outcomes. It has been observed that the proportion of LGA infants born to pregnant women with gestational diabetes mellitus (GDM) is significantly higher than that born to healthy pregnant women. However, tradi
To develop and validate a nomogram prediction model of delivering LGA infants among pregnant women with GDM, and provide strategies for the effective pre
The multivariable prediction model was developed by carrying out the following steps. First, the variables that were associated with LGA risk in pregnant women with GDM were screened by univariate analyses, for which the P value was < 0.10. Subsequently, Least Absolute Shrinkage and Selection Operator regression was fit using ten cross-validations, and the optimal combination factors were se
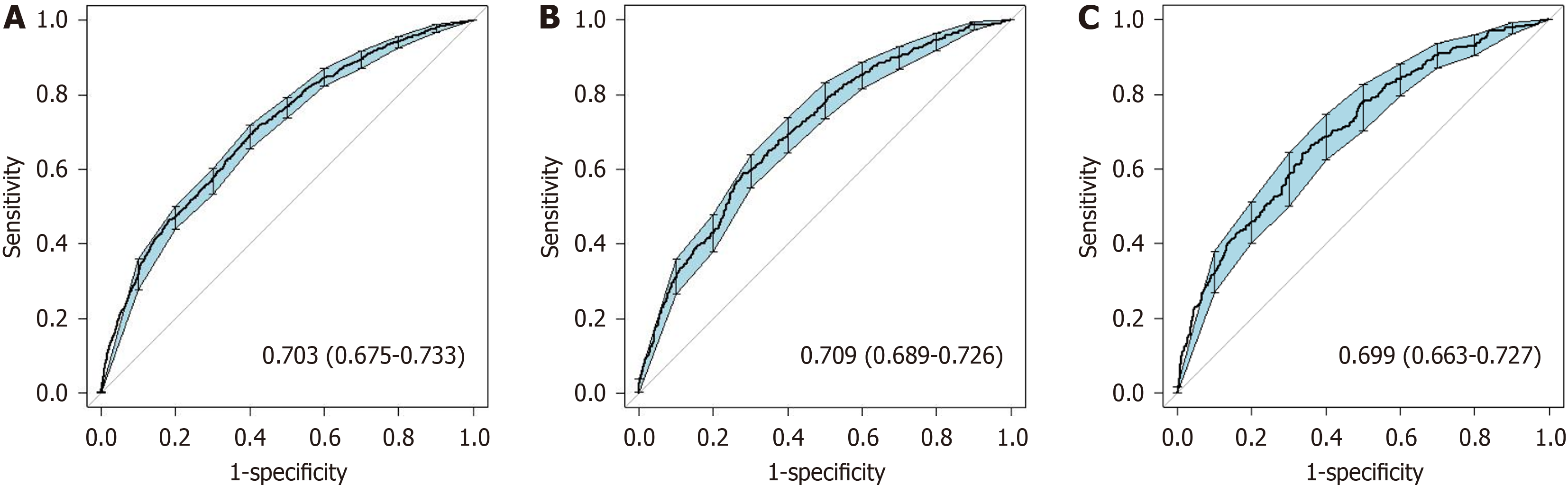
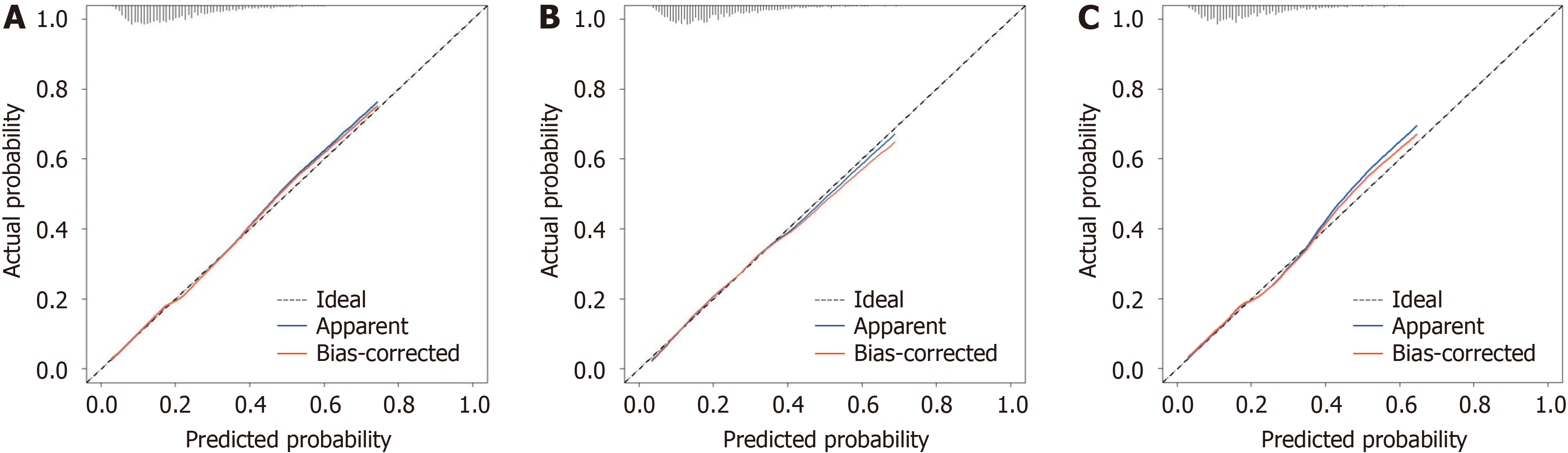
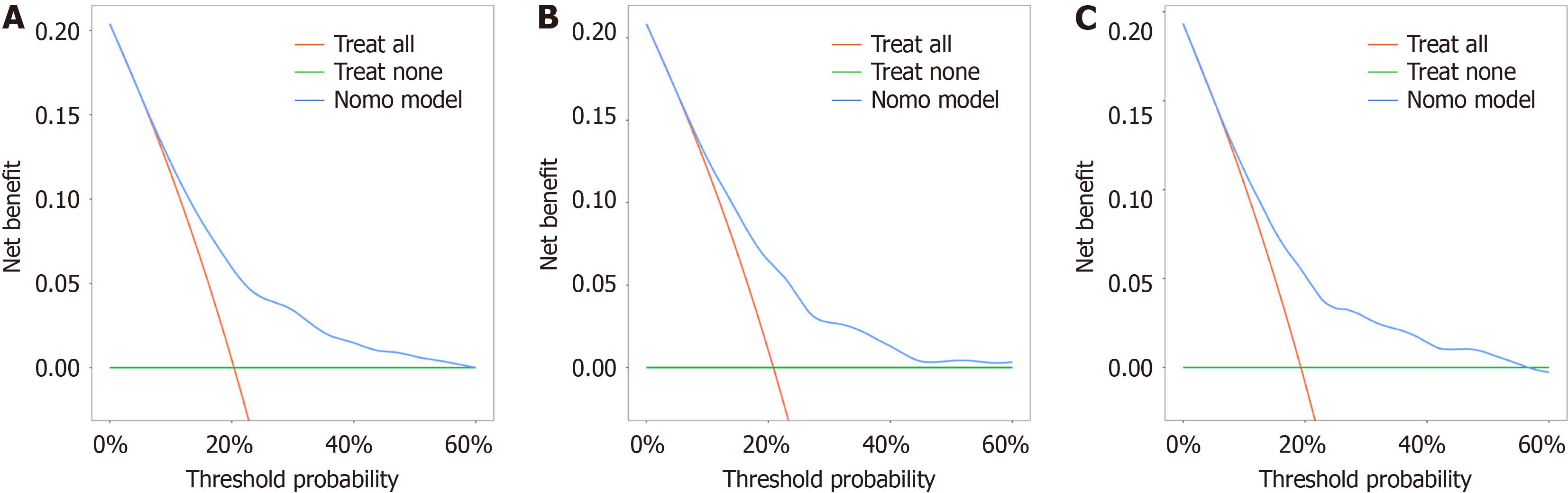
After using a multistep screening method, we establish a predictive model. Several risk factors for delivering an LGA infant were identified (P < 0.01), including weight gain during pregnancy, parity, triglyceride-glucose index, free tetraiodothyronine level, abdominal circumference, alanine transaminase-aspartate aminotransferase ratio and weight at 24 gestational weeks. The nomogram’s prediction ability was supported by the area under the curve (0.703, 0.709, and 0.699 for the training cohort, validation cohort, and test cohort, respectively). The calibration curves of the three cohorts displayed good agreement. The decision curve showed that the use of the 10%-60% threshold for identifying pregnant women with GDM who are at risk of delivering an LGA infant would result in a positive net benefit.
Our nomogram incorporated easily accessible risk factors, facilitating individualized prediction of pregnant women with GDM who are likely to deliver an LGA infant.
Core Tip: Gestational diabetes mellitus (GDM) is a global problem, and the prevalence of large-for-gestational-age (LGA) is increasing. Early prediction of LGA can enable timely intervention and improve pregnancy outcomes. We developed and validated a predictive nomogram for pregnant women with GDM at risk of delivering an LGA infant. Four demographic parameters and second-trimester maternal serum biochemical markers were identified. The nomogram effectively stratified women with GDM in their second trimester based on their risk of delivering LGA infants.
- Citation: Zhu YT, Xiang LL, Chen YJ, Zhong TY, Wang JJ, Zeng Y. Developing and validating a predictive model of delivering large-for-gestational-age infants among women with gestational diabetes mellitus. World J Diabetes 2024; 15(6): 1242-1253
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1242.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1242
A large-for-gestational-age (LGA) infant is defined as an infant with a birth weight equal to or above the 90th percentile based on a given gestational age (GA) and newborn sex and for the particular population[1]. LGA is associated with adverse short-term perinatal outcomes, such as cesarean section, shoulder dystocia, prolonged labor, postpartum he
Currently, the traditional methods for the clinical prediction of LGA in obstetric practice are abdominal examination and evaluation using the Hadlock ultrasound formula[8]. The accuracy of abdominal examination is affected by obesity, uterine fibroids, amniotic fluid volume, and clinical experience; moreover, the ultrasound formula was established in the 1980s in a Western population and mainly depends on bone markers[9]. However, GDM is associated with racial or eth
Several risk factors, including demographic characteristics and laboratory test results, have been well established for susceptibility to LGA, but there are also discrepancies[11,12]. Åmark et al[13] developed a prediction model for LGA in parous women with a body mass index (BMI) ≥ 30 kg/m2. Other unusual indicators (e.g., carnitine metabolism or fetal soft tissue) were selected in some of the previous models, but these indicators are not always available, which is troubling for clinicians. In addition, methods for predicting LGA fetuses are generally applicable in the third trimester[14], which leaves a limited window for early intervention. Therefore, it is necessary to establish an LGA prediction model for the second trimester (20th to 24th weeks of pregnancy) to identify and provide intervention for pregnant women at high risk of giving birth to LGA offspring, with a possible intervention duration of 16 to 20 weeks. The development of LGA is a multifaceted and intricate process that encompasses numerous risk factors, especially in pregnant women with GDM.
The triglyceride-glucose (TyG) index is a simple noninvasive index and has been proposed as a useful surrogate marker of insulin resistance (IR)[15]. Notably, IR is the pathophysiological basis of metabolic syndrome and an important risk factor for cardiovascular diseases and diabetes[16]. and the TyG index was independently associated with IR-related diseases, including atherosclerosis[17], cardiovascular diseases[18], and GDM[19]. The TyG index, which can be easily measured and calculated based only on fasting triglyceride (TG) and glucose levels[20], may be used to assess suscep
Therefore, to fill this knowledge gap and provide more information for future research, we aimed to study the asso
This retrospective study was performed at the Women’s Hospital of Nanjing Medical University, Nanjing Women and Children’s Healthcare Hospital (NJWCHH) in Nanjing, China (this is an “AAA” hospital for Obstetrics and Neonatology, with 18000-22000 births per year). The project was approved by the Medical Ethics Committee of NJWCHH (No. 2022KY-068).
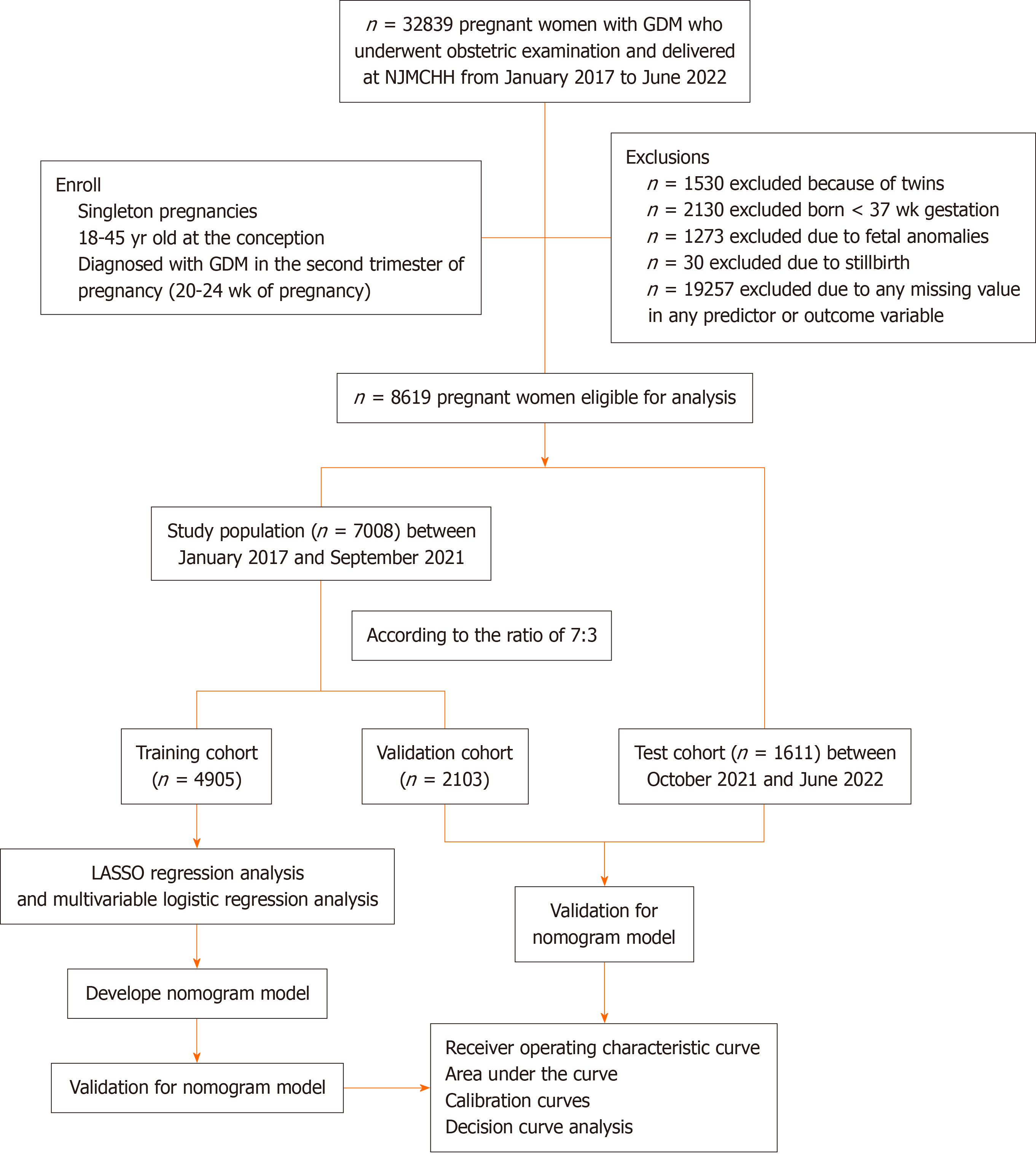
We reviewed the medical records of pregnant women with GDM who underwent obstetric examination and who delivered from January 2017 to June 2022 at NJWCHH. In addition, we included women from the same institution bet
Pregnant women with singleton pregnancies who were aged 18-45 years at the time of conception and were diagnosed with GDM underwent clinical investigation during their first prenatal examination in the second trimester of pregnancy. GDM was diagnosed according to the International Association of the Diabetes and Pregnancy Study Groups criteria using abnormal plasma glucose values during the 2-h, 75-g oral glucose tolerance test at 24-28 wk of gestation[24]. The thresholds for fasting plasma glucose (FPG) and 1-h and 2-h plasma glucose levels were 5.1 mmol/L, 10.0 mmol/L and 8.5 mmol/L, respectively. GDM was diagnosed when one or more of the above thresholds were reached or exceeded. A total of 32839 pregnant women met the inclusion criteria were included. After considering the exclusion criteria and missing data, 8619 eligible pregnant women were included for further analysis (Figure 1). Then, at a ratio of 7:3, all the enrolled participants were randomly divided into a training cohort (n = 4905) and a validation cohort (n = 2103). A total of 1611 pregnant women were included in the test cohort.
From registration to delivery, the NJWCHH electronic medical records system recorded demographic and anthropometric data for mothers and infants. The demographic characteristics included maternal age, self-reported prepregnancy height and weight, prepregnancy BMI, number of pregnancies, parity, assisted reproductive technology, early pregnancy preservation history and GA, which was estimated with self-reported data for the last menstrual period at the first an
For each subject, venous blood samples were collected at the 24th wk of pregnancy for the measurement of laboratory indices, which included fasting plasma glucose (FBG), glycosylated hemoglobin (HbA1c), TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, creatinine (Cr), uric acid, urea, alanine transaminase (ALT), aspartate aminotransferase (AST), albumin (ALB), total protein (TP), thyroid stimulating hormone (TSH), free tetraiodothyronine (FT4), and thyroid peroxidase antibody levels. These biochemical markers were all measured in the clinical laboratory of NJWCHH, which was certified by ISO15189.
Weight gain during pregnancy (WGW) was calculated by subtracting the prepregnancy weight from the weight at 24 gestational weeks. BMI and the TyG index were calculated with the following formulas: BMI = weight (kg)/height2 (m); and TyG index = ln (TG [mg/dL] × FBG [mg/dL]/2).
The occurrence of LGA in newborns was the primary outcome of this study. Typical macrosomia was defined as a birth weight ≥ 4000 g regardless of GA. However, in this study, LGA was defined as a birth weight ≥ 90th percentile adjusted for GA and fetal sex according to China’s percentile grids, and normal-for-gestational-age (NGA) infants (10%-90th percentile) were included as the control group. Immediately after delivery, the newborn infants were weighed (in grams) using an automatic device.
Continuous data with a normal distribution are presented as the mean (standard deviation), and nonnormal data are presented as the median (interquartile range). Categorical data are presented as counts and percentages, n (%). Two independent sample t tests or Mann-Whitney U tests were used to compare the differences between groups for con
The multivariable prediction model was developed by carrying out the following steps. First, the variables that were associated with LGA risk in pregnant women with GDM were screened by univariate analyses, for which the P value was < 0.10. Subsequently, Least Absolute Shrinkage and Selection Operator (LASSO) regression was fit using ten cross-validations, and the optimal combination factors were selected by choosing lambda 1se as the criterion. The final predictors were determined by multiple backward stepwise logistic regression analysis, in which only the independent variables were associated with LGA risk, with a P value < 0.05. The results of logistic models are reported as odds ratios (ORs) with bilateral 95% confidence intervals (CIs), Z values and P values.
To facilitate clinical application, a risk prediction model illustrated as a nomogram was established based on mul
All the statistical analyses were performed using SPSS version 26.0 (SPSS, IBM Corporation, Armonk, NY, United States) and R statistical software version 4.1. All reported P values were based on two-sided tests of significance, and a P value < 0.05 was considered to indicate statistical significance.
Figure 1 shows the flow diagram for inclusion and exclusion of participants in the study. Overall, 7008 eligible pregnant women with GDM were enrolled in this study. A total of 1748 women delivered LGA infants (20.28%). The initial dataset was divided into a training cohort (n = 4905) and a validation cohort (n = 2103) at a ratio of 7:3. In addition, pregnant women with GDM who delivered between October 2021 and June 2022 were included as the test cohort (n = 1611). The incidence of LGA was 20.37% (999/4905), 20.83% (438/2103), and 19.30% (311/1611) in the training cohort, validation cohort and test cohort, respectively. The pregnant women were divided into two groups (the GDM-LGA group and GDM-NGA group) according to the birth weight of their neonates. We compared the intergroup differences between the GDM-LGA group and GDM-NGA group in the training cohort. Age, prepregnancy height, weight and BMI, clinical data at 24 gestational weeks including weight, WGW, BMI, waist-height ratio, and AC, laboratory indices including TG, FBG, lactate dehydrogenase and HbA1c levels, the TyG, TG/HDL-C ratios, the cesarean section rate and infant weight were significantly greater in the LGA group than in the NGA group, while urea, Cr, TP, ALB, ALT, AST, HDL-C, FT4 levels, the ALT/AST ratio and birth week were significantly lower in the LGA group than in the NGA group (Table 1). The number of pregnancies and parity, excluding the current pregnancy, were divided into 1, 2, and ≥ 3. The proportions of multiple pregnancies and births in the LGA group were significantly greater than those in the NGA group (Table 1). The intergroup differences between the LGA group and the NGA group in the validation and test cohorts are shown in Supplementary Tables 1 and 2.
| Variables | GDM-NGA group (n = 3906) | GDM-LGA group (n = 999) | P value |
| Mothers | |||
| Age (yr) | 29.87 ± 3.53 | 30.13 ± 3.73a | 0.041 |
| Prepregnancy height (m) | 1.62 ± 0.05 | 1.63 ± 0.05b | < 0.001 |
| Prepregnancy weight (kg) | 57.94 ± 8.68 | 62.52 ± 10.07b | < 0.001 |
| Prepregnancy BMI (kg/m2) | 22.07 ± 3.03 | 23.41 ± 3.50b | < 0.001 |
| Number of pregnancies | |||
| 1 | 2001 (51.23) | 414 (41.44)b | < 0.001 |
| 2 | 1158 (29.65) | 306 (30.63) | |
| ≥ 3 | 747 (19.12) | 279 (27.93) | |
| Parity | |||
| 1 | 2862 (73.21) | 606 (60.66)b | < 0.001 |
| 2 | 999 (25.58) | 371 (37.14) | |
| ≥ 3 | 45 (1.15) | 22 (2.20) | |
| Clinical data at 24 gestational weeks | |||
| Weight (kg) | 63.86 ± 8.80 | 69.53 ± 10.03b | < 0.001 |
| WGW (kg) | 5.92 ± 3.17 | 7.02 ± 3.65b | < 0.001 |
| BMI (kg/m2) | 24.32 ± 3.04 | 26.04 ± 3.45b | < 0.001 |
| Waist-height ratio | 0.55 ± 0.04 | 0.57 ± 0.04b | < 0.001 |
| SBP (mm/Hg) | 112.11 ± 10.95 | 112.80 ± 11.34 | 0.080 |
| DBP (mm/Hg) | 69.75 ± 8.20 | 69.89 ± 8.34 | 0.632 |
| AC (cm) | 89.72 ± 5.93 | 93.02 ± 7.01b | < 0.001 |
| Laboratory indices | |||
| Urea (mmol/L) | 3.10 ± 0.70 | 3.02 ± 0.67a | 0.001 |
| Cr (μmol/L) | 39.35 ± 6.63 | 38.36 ± 6.65b | < 0.001 |
| UA (μmol/L) | 234.18 ± 49.49 | 235.78 ± 50.02 | 0.364 |
| TP (g/L) | 65.99 ± 3.43 | 65.67 ± 3.40a | 0.009 |
| ALB (g/L) | 39.36 ± 2.23 | 39.14 ± 2.25a | 0.005 |
| TC (mmol/L) | 5.96 ± 0.98 | 5.89 ± 1.05 | 0.051 |
| TG (mmol/L) | 2.26 ± 0.91 | 2.51 ± 1.03b | < 0.001 |
| FBG (mmol/L) | 4.81 ± 0.47 | 4.97 ± 0.59b | < 0.001 |
| ALT (U/L) | 20.79 ± 17.60 | 17.62 ± 13.68b | < 0.001 |
| AST (U/L) | 19.17 ± 9.06 | 17.73 ± 6.73b | < 0.001 |
| ALP (U/L) | 56.82 ± 14.10 | 55.95 ± 13.20 | 0.079 |
| LDH (U/L) | 163.02 ± 25.84 | 164.92 ± 31.08a | 0.048 |
| GGT (U/L) | 16.05 ± 9.91 | 16.19 ± 9.90 | 0.691 |
| HDL-C (mmol/L) | 2.35 ± 0.40 | 2.28 ± 0.37b | < 0.001 |
| LDL-C (mmol/L) | 3.01 ± 0.65 | 3.00 ± 0.65 | 0.758 |
| TSH (mIU/L) | 2.19 ± 1.09 | 2.16 ± 1.02 | 0.487 |
| TPOAb (IU/mL) | 22.74 ± 41.30 | 23.45 ± 46.52 | 0.638 |
| FT4 (pmol/L) | 12.60 ± 1.74 | 12.17 ± 1.74b | < 0.001 |
| HbA1c | 5.09 ± 0.28 | 5.19 ± 0.35b | < 0.001 |
| TyG | 9.00 ± 0.38 | 9.13 ± 0.40b | < 0.001 |
| ALT/AST | 1.01 ± 0.36 | 0.94 ± 0.34b | < 0.001 |
| TG/HDL-C | 0.99 ± 0.53 | 1.10 ± 0.58b | < 0.001 |
| Information about pregnancy | |||
| Cesarean section | 1508 (38.61) | 591 (59.16)b | < 0.001 |
| Birth week (weeks) | 39.09 ± 0.97 | 38.96 ± 0.96b | < 0.001 |
| Infant weight (kg) | 3.32 ± 0.28 | 3.97 ± 0.28b | < 0.001 |
| Infant sex | |||
| Boy | 2019 (51.69) | 505 (50.55) | 0.523 |
| Girl | 1887 (48.31) | 494 (49.45) |
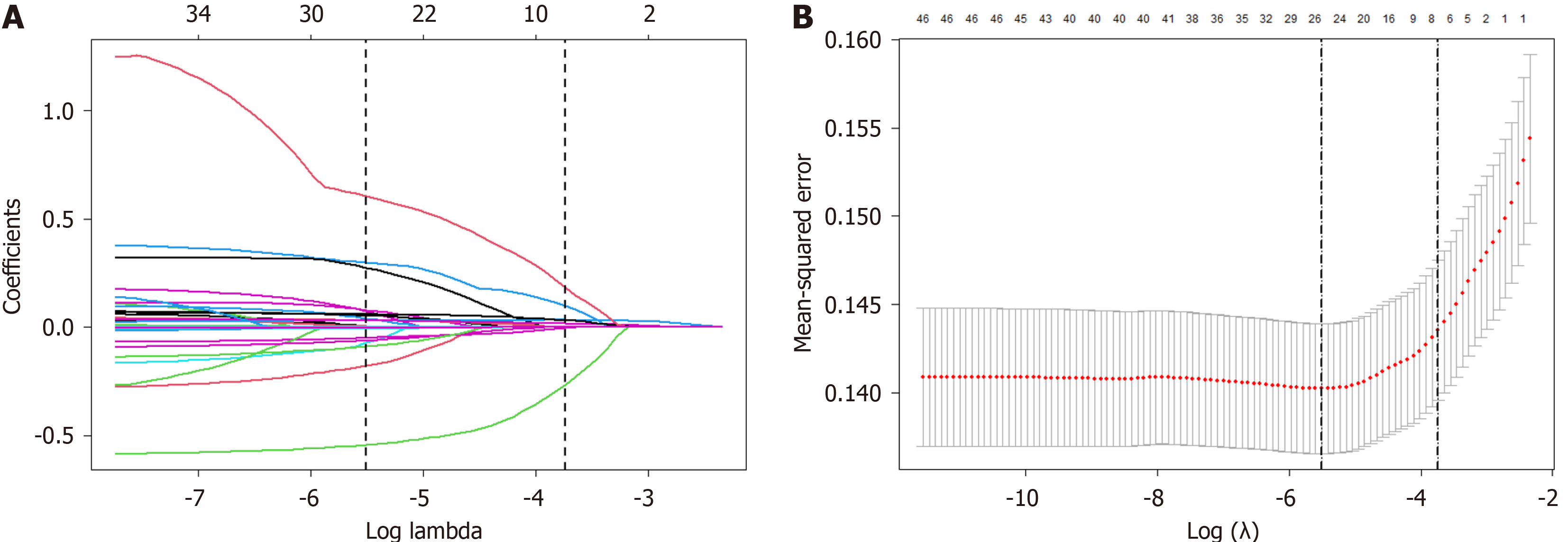
LASSO regression analysis was employed to further select the potential predictors by calculating the value of lambda.1se. The LASSO regression analysis revealed that seven potential predictive coefficients were nonzero, and lambda coefficient was 0.02438 (Figure 2). The results of the LASSO regression analysis, based on 4905 participants (excluding data missing any of the seven aforementioned features), These variables included WGW, parity, TyG index, FT4 level, AC, ALT/AST ratio and weight at 24 gestational weeks.
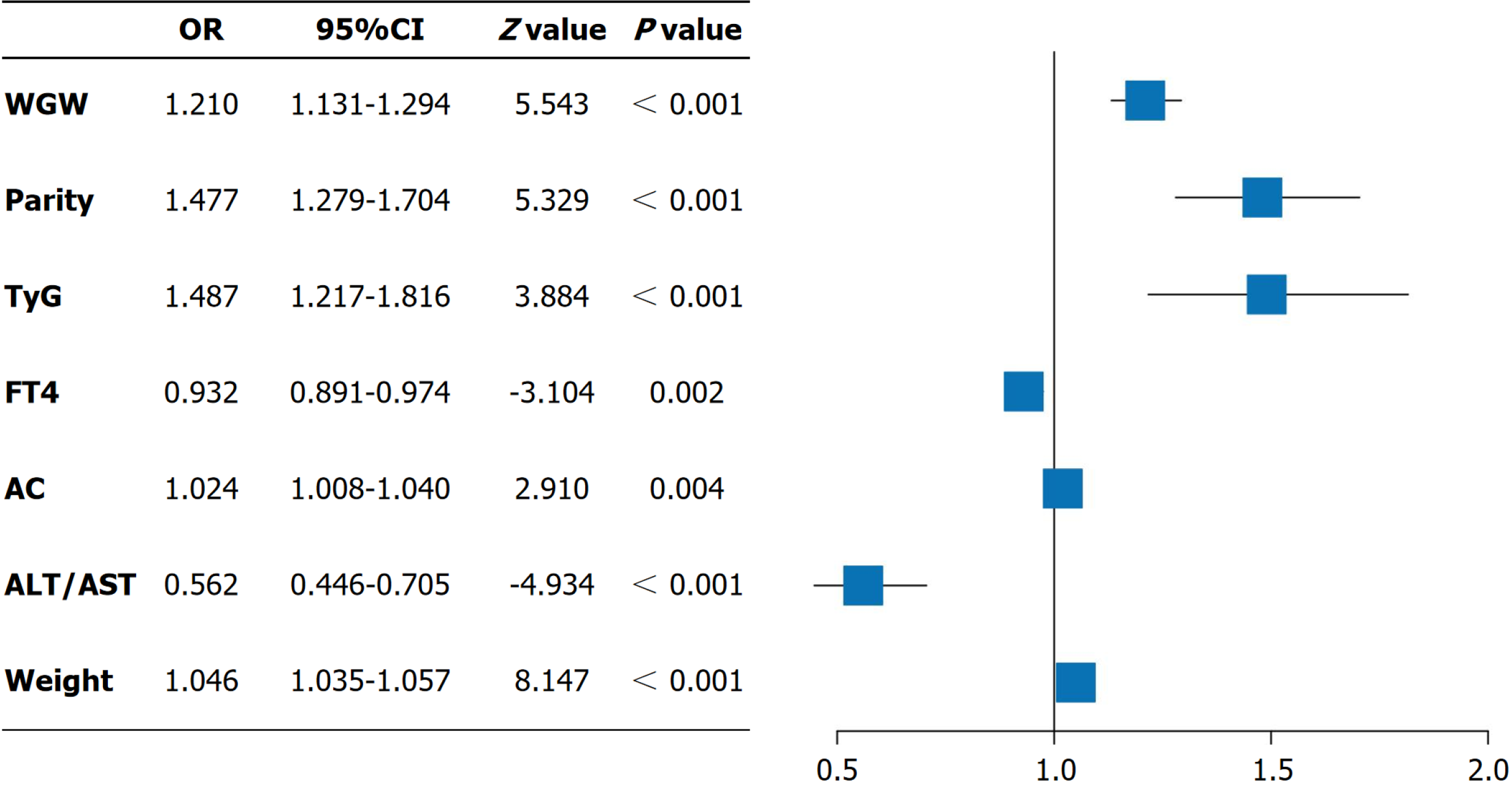
Subsequently, multivariate logistic regression analysis demonstrated that WGW (OR = 1.210, 95%CI: 1.131-1.294, P < 0.001), the number of births (OR = 1.477, 95%CI: 1.297-1.704, P < 0.001), TyG index (OR = 1.487, 95%CI: 1.217-1.816, P < 0.001), FT4 level (OR = 0.932, 95%CI: 0.891-0.974, P = 0.002), AC (OR = 1.024, 95%CI: 1.008-1.040, P = 0.004), ALT/AST ratio (OR = 0.562, 95%CI: 0.446-0.705, P < 0.001) and weight (OR = 1.046, 95%CI: 1.035-1.057, P < 0.001) were independent risk factors for delivering an LGA infant in the training cohort, as shown in Figure 3.
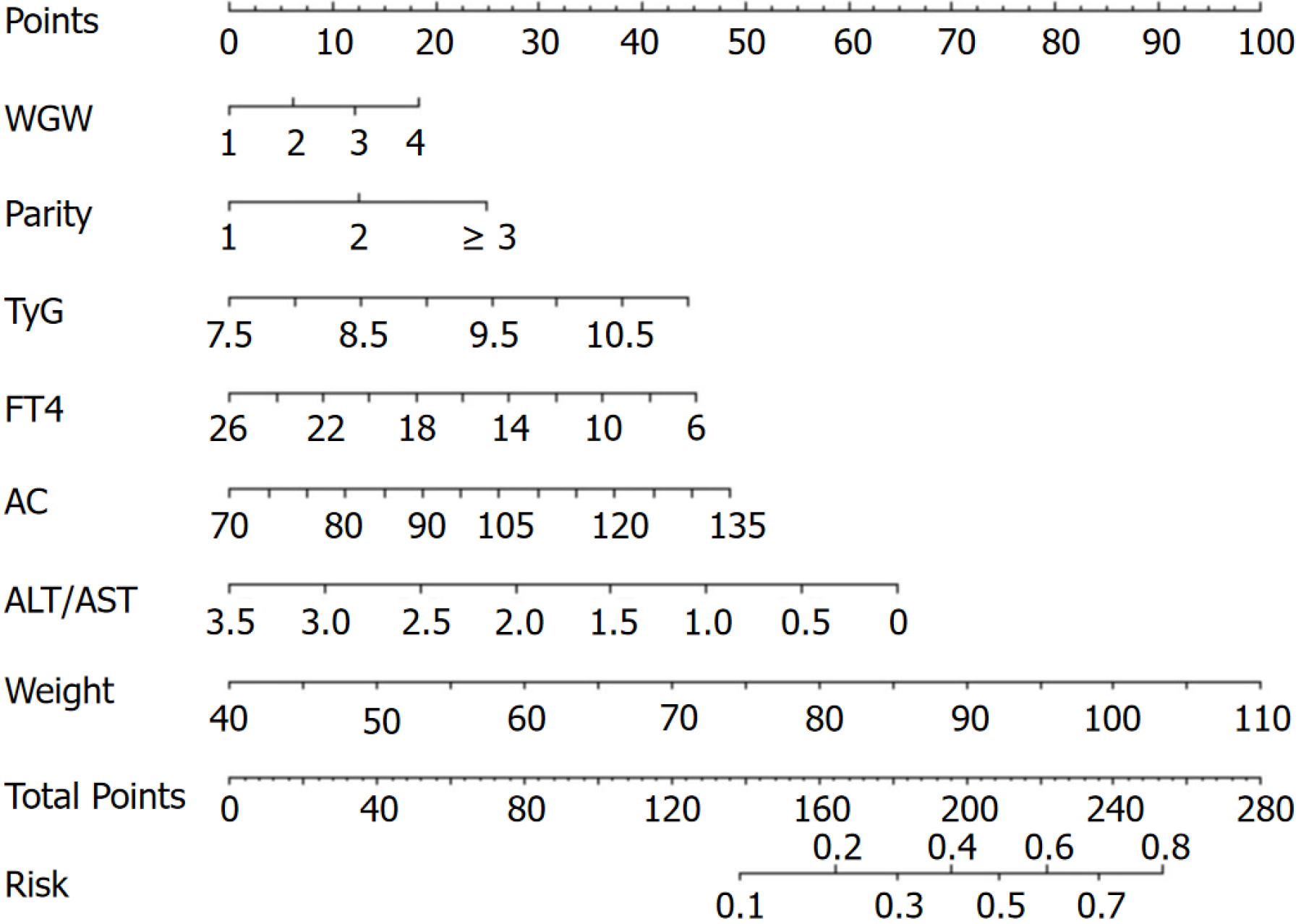
According to the multivariate logistic regression results, a nomogram was developed to predict the occurrence of LGA in pregnant women with GDM. In personalized healthcare, the specific values of WGW, parity, TyG index, FT4, AC, ALT/AST index and weight at 24 gestational weeks can be used to determine the corresponding points on the nomogram. By summarizing these points from each variable, the nomogram estimates the probability of delivering a LGA infant for pregnant women with GDM, as shown in Figure 4.
Random sampling was performed at a ratio of 7:3 between the training cohort (n = 4905) and the validation cohort (n = 2103). In addition, pregnant women with GDM who delivered between October 2021 and June 2022 were included in the test cohort (n = 1611). The discrimination of the nomogram was validated using ROC and calibration curve analyses in these three cohorts. The results showed that the area under the curve (AUC) was 0.703 (95%CI: 0.675-0.733), 0.709 (95%CI: 0.689-0.726), and 0.699 (95%CI: 0.663-0.727) for the training cohort, validation cohort, and test cohort, respectively (Figure 5) The Hosmer-Lemeshow test revealed no statistically significant difference between the predicted and observed probabilities of LGA in the training cohort (R2 = 10.950, P = 0.205 > 0.05), the validation cohort (R2 = 2.830, P = 0.945 > 0.05) and the test cohort (R2 = 8.552, P = 0.479 > 0.05), suggesting good discrimination between the observed and predicted probabilities.
The accuracy of the nomogram was validated using calibration curves in the three cohorts. The probability of de
The clinical utility of the nomogram model was evaluated using decision curve analysis to determine the net benefit based on different threshold probabilities in the three cohorts. The results showed that our model demonstrated a positive net benefit with a threshold between 10% and 60% (Figure 7).
In this study, we developed and validated a diagnostic nomogram for predicting LGA in pregnant patients with GDM. This nomogram incorporated several risk factors, including WGW, parity, the TyG index, the FT4 level, AC, the ALT/AST ratio and weight at 24 gestational weeks. The nomogram effectively stratified women with GDM in their second trimester based on their risk of delivering LGA infants. The prediction performance of the nomogram was confirmed through AUC, calibration curve, and decision curve analyses for the training, validation, and test cohorts, respectively. By incorporating these easily accessible risk factors, our nomogram facilitates individualized prediction of outcomes in pregnant women with GDM who are likely to deliver an LGA infant.
Our research revealed that among the 8619 pregnant women with GDM, 1748 women delivered LGA newborns, resulting in a 20.28% incidence, which was higher than the rate among healthy pregnant women. Kondracki et al[25] conducted a retrospective cross-sectional analysis using a large United States National Vital Statistics Natality File (n = 3229783) and identified a potential mediating role of GDM in the occurrence of LGA in newborns, aligning with our findings. However, variations in ethnicity[25], overweight/obese status[26], and the criteria for LGA or macrosomia[27] could account for the inconsistent incidence rates reported in the literature.
In the medical literature, increasing epidemiological parameters and laboratory biomarkers are being evaluated for their predictive ability for LGA. For instance, Zou et al[10] retrospectively analyzed 783 pregnant Chinese women with GDM at 28-32 wk of gestation and reported that prepregnancy BMI, WGW, the FPG level, the TG level, the biparietal diameter and the amniotic fluid index were significant independent predictors of LGA in pregnant women with GDM. In addition, many laboratory biomarkers in plasma, such as the hepatic steatosis index[28], plasma protein A[29], and beta-human chorionic gonadotrophin[13] have been identified as promising predictors of LGA. Currently, one of the greatest challenges facing obstetricians worldwide is identifying appropriate pregnancy biomarkers and establishing a prediction model for LGA in pregnant women with GDM. However, for only a few well-established risk factors, such as prepreg
To obtain a relatively reliable estimate, we established a large database from a 3-A-Class Specialized Hospital affiliated with a medical university in Nanjing that included 32839 women with GDM between January 2017 and June 2022. To control confounding factors, we employed a multistep screening approach and only identified risk factors significantly associated with increased LGA risk. After excluding factors with strong correlations, we identified seven significant factors independently associated with the risk of delivering an LGA infant in pregnant women with GDM, three of which are laboratory biomarkers. For demographic and anthropometric indices, we confirmed the contribution of WGW and parity to the increased risk of LGA, which was consistent with the results of most previous studies[13,31].
The TyG index has been proposed as a simple alternative surrogate marker of IR[15], and previous studies have shown its potential clinical utility for predicting LGA in Iranian[32] and Chinese[33] pregnant women, whereas it could not predict LGA risk in the study by Song et al[28]. In contrast, we found that the TyG index was a significant predictor of LGA in women with GDM. The reproducibility of an authentic result can be hindered by a range of factors, including variations in genetic backgrounds and insufficient statistical power, which may prevent the replication of the original findings. Nevertheless, these results also indicate that LGA is a multifactorial disease affected by genetic, metabolic, environmental, cultural, and lifestyle factors[26]. The risk of a single indicator or clinical biomarker is minimal, while the presence of other factors may exacerbate these minor effects. Therefore, it is imperative to construct an LGA multivariate prediction model with decent predictive performance.
On the basis of the results from the multistep screening method, we established a nomogram that included several significant factors; this nomogram can transform tedious regression equations into a visually legible graph for convenient and rapid application in practical activities. The nomogram was evaluated by the AUC, Hosmer-Lemeshow test, cali
This study had several strengths. First, our study involved a large cohort, and the definition of LGA was based on the distribution of birth weight according to the gestational week and sex of the newborn. Data for all variables in this study were collected from routine prenatal evaluations and laboratory examinations without any additional tests. Second, the nomogram developed in this study will allow for the early prediction of LGA at 20-24 wk GA by clinicians and conse
This study has several limitations. First, all participants were exclusively enrolled from one “AAA” hospital in Nan
Through big data analysis, we identified four demographic parameters and second-trimester maternal serum biochemical markers that are significantly associated with LGA risk in pregnant women with GDM, and importantly, a nomogram modeling these predictors demonstrated favorable discrimination ability, accurate prediction ability, and clinical usa
| 1. | Nahavandi S, Price S, Sumithran P, Ekinci EI. Exploration of the shared pathophysiological mechanisms of gestational diabetes and large for gestational age offspring. World J Diabetes. 2019;10:333-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Beta J, Khan N, Khalil A, Fiolna M, Ramadan G, Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;54:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Kuciene R, Dulskiene V, Medzioniene J. Associations between high birth weight, being large for gestational age, and high blood pressure among adolescents: a cross-sectional study. Eur J Nutr. 2018;57:373-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2015;10:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 5. | Song X, Shu J, Zhang S, Chen L, Diao J, Li J, Li Y, Wei J, Liu Y, Sun M, Wang T, Qin J. Pre-Pregnancy Body Mass Index and Risk of Macrosomia and Large for Gestational Age Births with Gestational Diabetes Mellitus as a Mediator: A Prospective Cohort Study in Central China. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66 Suppl 2:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 553] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 7. | NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3952] [Cited by in RCA: 3512] [Article Influence: 390.2] [Reference Citation Analysis (0)] |
| 8. | Nguyen MT, Ouzounian JG. Evaluation and Management of Fetal Macrosomia. Obstet Gynecol Clin North Am. 2021;48:387-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Melamed N, Yogev Y, Meizner I, Mashiach R, Pardo J, Ben-Haroush A. Prediction of fetal macrosomia: effect of sonographic fetal weight-estimation model and threshold used. Ultrasound Obstet Gynecol. 2011;38:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Zou Y, Zhang Y, Yin Z, Wei L, Lv B, Wu Y. Establishment of a nomogram model to predict macrosomia in pregnant women with gestational diabetes mellitus. BMC Pregnancy Childbirth. 2021;21:581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Jin WY, Lin SL, Hou RL, Chen XY, Han T, Jin Y, Tang L, Zhu ZW, Zhao ZY. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. 2016;16:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 12. | HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3783] [Cited by in RCA: 3691] [Article Influence: 217.1] [Reference Citation Analysis (0)] |
| 13. | Åmark H, Westgren M, Persson M. Prediction of large-for-gestational-age infants in pregnancies complicated by obesity: A population-based cohort study. Acta Obstet Gynecol Scand. 2019;98:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Sparks TN, Cheng YW, McLaughlin B, Esakoff TF, Caughey AB. Fundal height: a useful screening tool for fetal growth? J Matern Fetal Neonatal Med. 2011;24:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Brito ADM, Hermsdorff HHM, Filgueiras MS, Suhett LG, Vieira-Ribeiro SA, Franceschini SDCC, Novaes JF. Predictive capacity of triglyceride-glucose (TyG) index for insulin resistance and cardiometabolic risk in children and adolescents: a systematic review. Crit Rev Food Sci Nutr. 2021;61:2783-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited. Can J Cardiol. 2020;36:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 17. | Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, Augoulea A, Kaparos G, Alexandrou A, Stamatelopoulos K. The TyG Index as a Marker of Subclinical Atherosclerosis and Arterial Stiffness in Lean and Overweight Postmenopausal Women. Heart Lung Circ. 2018;27:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 225] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 19. | Song T, Su G, Chi Y, Wu T, Xu Y, Chen C. Triglyceride-glucose index predicts the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Gynecol Endocrinol. 2022;38:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Yu X, Wang L, Zhang W, Ming J, Jia A, Xu S, Li Q, Ji Q. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: A nationwide study. J Diabetes Investig. 2019;10:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Li H, Miao C, Liu W, Gao H, Li W, Wu Z, Cao H, Zhu Y. First-Trimester Triglyceride-Glucose Index and Risk of Pregnancy-Related Complications: A Prospective Birth Cohort Study in Southeast China. Diabetes Metab Syndr Obes. 2022;15:3705-3715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2005;70:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Poveda NE, Garcés MF, Darghan AE, Jaimes SAB, Sánchez EP, Díaz-Cruz LA, Garzón-Olivares CD, Parra-Pineda MO, Bautista-Charry AA, Müller EÁ, Alzate HFS, Acosta LMM, Sanchez E, Ruíz-Parra AI, Caminos JE. Triglycerides/Glucose and Triglyceride/High-Density Lipoprotein Cholesterol Indices in Normal and Preeclamptic Pregnancies: A Longitudinal Study. Int J Endocrinol. 2018;2018:8956404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Todi S, Sagili H, Kamalanathan SK. Comparison of criteria of International Association of Diabetes and Pregnancy Study Groups (IADPSG) with National Institute for Health and Care Excellence (NICE) for diagnosis of gestational diabetes mellitus. Arch Gynecol Obstet. 2020;302:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Kondracki AJ, Valente MJ, Ibrahimou B, Bursac Z. Risk of large for gestational age births at early, full and late term in relation to pre-pregnancy body mass index: Mediation by gestational diabetes status. Paediatr Perinat Epidemiol. 2022;36:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | He XJ, Qin FY, Hu CL, Zhu M, Tian CQ, Li L. Is gestational diabetes mellitus an independent risk factor for macrosomia: a meta-analysis? Arch Gynecol Obstet. 2015;291:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Gorban de Lapertosa S, Alvariñas J, Elgart JF, Salzberg S, Gagliardino JJ; EduGest group. The triad macrosomia, obesity, and hypertriglyceridemia in gestational diabetes. Diabetes Metab Res Rev. 2020;36:e3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Song S, Duo Y, Zhang Y, Qiao X, Xu J, Zhang J, Peng Z, Chen Y, Nie X, Sun Q, Yang X, Wang A, Sun W, Fu Y, Dong Y, Lu Z, Yuan T, Zhao W. The Predictive Ability of Hepatic Steatosis Index for Gestational Diabetes Mellitus and Large for Gestational Age Infant Compared with Other Noninvasive Indices Among Chinese Pregnancies: A Preliminary Double-center Cohort Study. Diabetes Metab Syndr Obes. 2021;14:4791-4800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Monari F, Menichini D, Spano' Bascio L, Grandi G, Banchelli F, Neri I, D'Amico R, Facchinetti F. A first trimester prediction model for large for gestational age infants: a preliminary study. BMC Pregnancy Childbirth. 2021;21:654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Hong YH, Lee JE. Large for Gestational Age and Obesity-Related Comorbidities. J Obes Metab Syndr. 2021;30:124-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Lewandowska M. The Role of Maternal Weight in the Hierarchy of Macrosomia Predictors; Overall Effect of Analysis of Three Prediction Indicators. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Liu PJ, Liu Y, Ma L, Yao AM, Chen XY, Hou YX, Wu LP, Xia LY. The Predictive Ability of Two Triglyceride-Associated Indices for Gestational Diabetes Mellitus and Large for Gestational Age Infant Among Chinese Pregnancies: A Preliminary Cohort Study. Diabetes Metab Syndr Obes. 2020;13:2025-2035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Pazhohan A, Rezaee Moradali M, Pazhohan N. Association of first-trimester maternal lipid profiles and triglyceride-glucose index with the risk of gestational diabetes mellitus and large for gestational age newborn. J Matern Fetal Neonatal Med. 2019;32:1167-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Wang N, Guo H, Jing Y, Zhang Y, Sun B, Pan X, Chen H, Xu J, Wang M, Chen X, Song L, Cui W. Development and validation of risk prediction models for large for gestational age infants using logistic regression and two machine learning algorithms. J Diabetes. 2023;15:338-348. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |