Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1234
Revised: March 5, 2024
Accepted: April 17, 2024
Published online: June 15, 2024
Processing time: 144 Days and 7 Hours
Dry eye syndrome (DES) after diabetic cataract surgery can seriously affect the patient’s quality of life. Therefore, effective alleviation of symptoms in patients with this disease has important clinical significance.
To explore the clinical effect of recombinant human epidermal growth factor (rhEGF) plus sodium hyaluronate (SH) eye drops on DES after cataract surgery in patients with diabetes.
We retrospectively evaluated 82 patients with diabetes who experienced DES after cataract surgery at Tianjin Beichen Hospital, Affiliated Hospital of Nankai University between April 2021 and April 2023. They were classified into an observation group (42 cases, rhEGF + SH eye drops) and a control group (40 cases, SH eye drops alone), depending on the different treatment schemes. The thera-peutic efficacy, dry eye symptom score, tear film breakup time (TFBUT), basic tear secretion score [assessed using Schirmer I test (SIt)], corneal fluorescein staining (FL) score, tear inflammatory markers, adverse reactions during treat
Therapeutic efficacy was higher in the observation group compared with the control group. Both groups showed improved TFBUT and dry eye, as well as improved SIt and FL scores after treatment, with a more pronounced improve
rhEGF + SH eye drops rendered clinical benefits to patients by effectively ameliorating dry eye and visual impairment with favorable efficacy, fewer adverse reactions, and high safety levels. Thus, this treatment should be promoted in clinical practice.
Core Tip: Cataracts are a common clinical blinding eye disease with a high incidence among the older population. Phacoemulsification followed by intraocular lens implantation is currently the mainstream surgical method for treating cataracts and restoring visual function. However, patients with diabetes often have a higher incidence of dry eye syndrome (DES) after cataract surgery compared with nondiabetic patients because of factors such as less basic tear secretion and poor tear film stability, which seriously affect the patient’s quality of life. Therefore, the effective improvement of DES after cataract surgery in these patients is of great clinical significance. Considering the specific clinical application scenarios, the effect of recombinant human epidermal growth factor combined with sodium hyaluronate eye drops on DES in patients with diabetes after cataract surgery warrants exploration.
- Citation: Li JL, Zhao J, Guo ZF, Xiao C, Liu X. Efficacy of recombinant human epidermal growth factor plus sodium hyaluronate eye drops in diabetic dry eye post-cataract surgery. World J Diabetes 2024; 15(6): 1234-1241
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1234.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1234
The incidence of cataracts, a common clinical blinding eye disease, is high in the elderly population[1]. Cataracts are associated with many pathogenic factors, such as heredity, aging, immune and nutritional disorders, poisoning, and diabetes[2]. In patients with diabetes, the decrease in galactokinase activity and the increase in blood sugar concentration increases the osmotic pressure of the aqueous humor in the eye, causing the crystal fibers to swell, break, and dis-integrate, eventually resulting in complete lenticular opacity. Diabetic cataracts are considered a unique cataract type[3]. Conventional surgery to treat cataracts and restore visual function involves phacoemulsification followed by intraocular lens implantation[4]. However, patients with diabetes have a higher incidence of dry eye syndrome (DES) after cataract surgery compared with nondiabetic patients due to less basic tear secretion and poor tear film (TF) stability[5]. DES causes discomfort, visual impairment, and TF instability, accompanied by increased TF permeability and ocular surface inflammation, which impairs visual function and seriously affects patients’ quality of life[6]. Thus, an effective treatment to alleviate DES following cataract surgery in such patients has important clinical significance.
Currently, DES is primarily treated using anti-inflammatory drugs and artificial tears, including eye drops containing sodium hyaluronate (SH), which produce high-quality artificial tears with good water retention, effectively alleviating DES symptoms and exerting an antioxidant role. However, some patients suffer from repeated illness and symptoms and a protracted disease course due to corneal endothelial cell damage following treatment. Therefore, the current treatment approach needs to be clinically addressed to make up for the shortcomings of these therapies[7,8]. Recombinant human epidermal growth factor (rhEGF), a multifunctional growth factor that promotes tear secretion and lacrimal gland cell proliferation, improves the regeneration ability of corneal epithelial cells and shortens the repair time of damaged corneal tissues, thereby promoting the functional recovery of corneal endothelial cells[9].
Motivated by this, our study investigated the combined use of rhEGF + SH eye drops to treat DES after cataract surgery in patients with diabetes, aiming to explore its impact on therapeutic efficacy and visual recovery.
We retrospectively evaluated 82 patients with diabetes who developed DES after cataract surgery and were treated in Tianjin Beichen Hospital, Affiliated Hospital of Nankai University between April 2021 and April 2023. They were classified into an observation group (42 cases, rhEGF + SH eye drops) and a control group (40 cases, SH eye drops alone). Patients were included in our study if they successfully underwent cataract surgery and met the diagnostic criteria for DES, including: (1) Tear secretion test < 5 mm; (2) intimal rupture time < 10 s; (3) tear fern reduction or disappearance; (4) corneal epithelium defect; (5) corneal conjunctival inactivation, and (6) combined eyes dryness, foreign body sensation, tingling, itching, and other uncomfortable symptoms. The exclusion criteria were: (1) Oral corticosteroid administration within the past 6 months; (2) hematological diseases; (3) allergies to related drugs used in our study; (4) severe liver and kidney dysfunction; (5) speech disorders or mental illness, and (6) incomplete clinical data.
The control group was treated with SH eye drops [Qilu Pharmaceutical Co., Ltd; Saudi Food and Drug Authority (SFDA) Approval No.: H20133263], 1 drop/application, three times daily for 8 wk. Patients in the observation group were administered rhEGF eye drops (Shenzhen Watsin Genetech Ltd., SFDA Approval No. S20040006) and the same SH eye drops as the control group, 1 drop/application, three times daily for 8 wk for both.
Therapeutic efficacy was classified as follows: (1) Cured: Complete disappearance of clinical symptoms and normal slit-lamp examination (SLE) results; (2) markedly effective: almost complete disappearance of clinical symptoms and improved SLE results; (3) effective: Improved clinical symptoms and improved SLE results; and (4) ineffective: No improvement in clinical symptoms and SLE results. The total effective rate was calculated as follows: Total effective rate = (cured cases + markedly effective cases + effective cases)/ total number of cases × 100 and expressed as a percentage.
To compare pre- and posttreatment DES symptoms, the degree of burning sensation, foreign body sensation, and dryness sensation of the affected eyes was evaluated[10] using a three-point scale (3, significant and persistent symptoms; 2, intermittent occurrence of symptoms; 1, sporadic symptoms, and 0, no symptoms). We also compared the TF breakup time (TFBUT), basic tear secretion score Schirmer I test (SIt), and corneal fluorescein staining (FL) score between the two groups[11]. For FL scoring, fluorescein-stained test strips were placed in contact with the conjunctival sac of the lower eyelid, and then cobalt blue light was used to observe corneal epithelium staining under a slit lamp. The FL score was based on a scale from 0 to 3 (0, no staining; 1, punctate staining; 2, diffuse staining; and 3, flaky staining). We also determined the levels of inflammatory markers in tears, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), before and after treatment for comparative analysis between the two groups. All adverse reactions during the treatment period, including conjunctival hyperemia, aggravated lacrimal gland injury, local inflammation, and corneal erosion, were recorded for both groups for comparison. The level of treatment satisfaction of the patients and their families (very satisfied, satisfied, or dissatisfied) was investigated and recorded to determine the percentage rate of treatment satisfaction as follows: Treatment satisfaction = (very satisfied cases + satisfied cases)/total number of cases × 100.
SPSS v18. 0 statistical software and GraphPad Prism v6 (GraphPad Inc.) were employed for statistical analysis and image rendering of the collected data, respectively. Chi-square tests were performed for categorical variables, and independent sample t-tests and paired t-tests were performed for continuous variables to identify statistically significant intergroup and intragroup differences (before and after treatment), respectively. P values < 0.05 were considered statistically significant.
The two patient cohorts were comparable; there were no notable differences in sex, age, smoking history, and other baseline data (P > 0.05; Table 1).
| Factors | Observation group (n = 42) | Control group (n = 40) | t/χ2 | P value |
| Sex | 0.001 | 0.991 | ||
| Male | 22 (52.38) | 21 (52.50) | ||
| Female | 20 (47.62) | 19 (47.50) | ||
| Age (yr) | 0.002 | 0.965 | ||
| ≤ 61 | 17 (40.48) | 16 (40.00) | ||
| > 61 | 25 (59.52) | 24 (60.00) | ||
| BMI (kg/m2) | 0.001 | 0.983 | ||
| ≤ 23 | 23 (54.76) | 22 (55.00) | ||
| > 23 | 19 (45.24) | 18 (45.00) | ||
| Mean course of disease (months) | 4.65 ± 0.85 | 4.82 ± 0.81 | 0.926 | 0.357 |
| Hypertension | 0.001 | 0.991 | ||
| Yes | 20 (47.62) | 19 (47.50) | ||
| No | 22 (52.38) | 21 (52.50) | ||
| Smoking history | 0.002 | 0.965 | ||
| Yes | 25 (59.52) | 24 (60.00) | ||
| No | 17 (40.48) | 16 (40.00) |
The number of patients who were classified as cured, markedly effective, effective, and ineffective in the observation group was 24, 10, 6, and 2, respectively, whereas the number of patients in the control group was 14, 10, 4, and 12, respectively. The total effective rate in the observation group was 95.24%, which was 25.24% higher than the total effective rate in the control group (P < 0.05; Table 2).
| Therapeutic effect | Observation group (n = 42) | Control group (n = 40) | χ2 | P value |
| Cure | 24 (57.14) | 14 (35.00) | ||
| Marked effectiveness | 10 (23.81) | 10 (25.00) | - | - |
| Effectiveness | 6 (14.29) | 4 (10.00) | - | - |
| Ineffectiveness | 2 (4.76) | 12 (30.00) | - | - |
| Total effective rate | 40 (95.24) | 28 (70.00) | 9.217 | 0.002 |
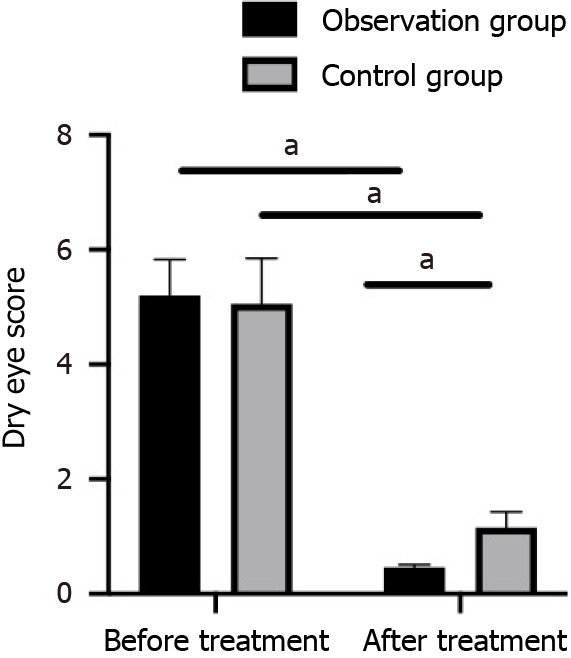
The dry eye symptom scores of the two patient cohorts were similar before treatment (P > 0.05). After 8 wk of treatment, the dry eye symptom score was statistically reduced in both groups and was lower in the observation group compared with the control group (P < 0.05; Figure 1).
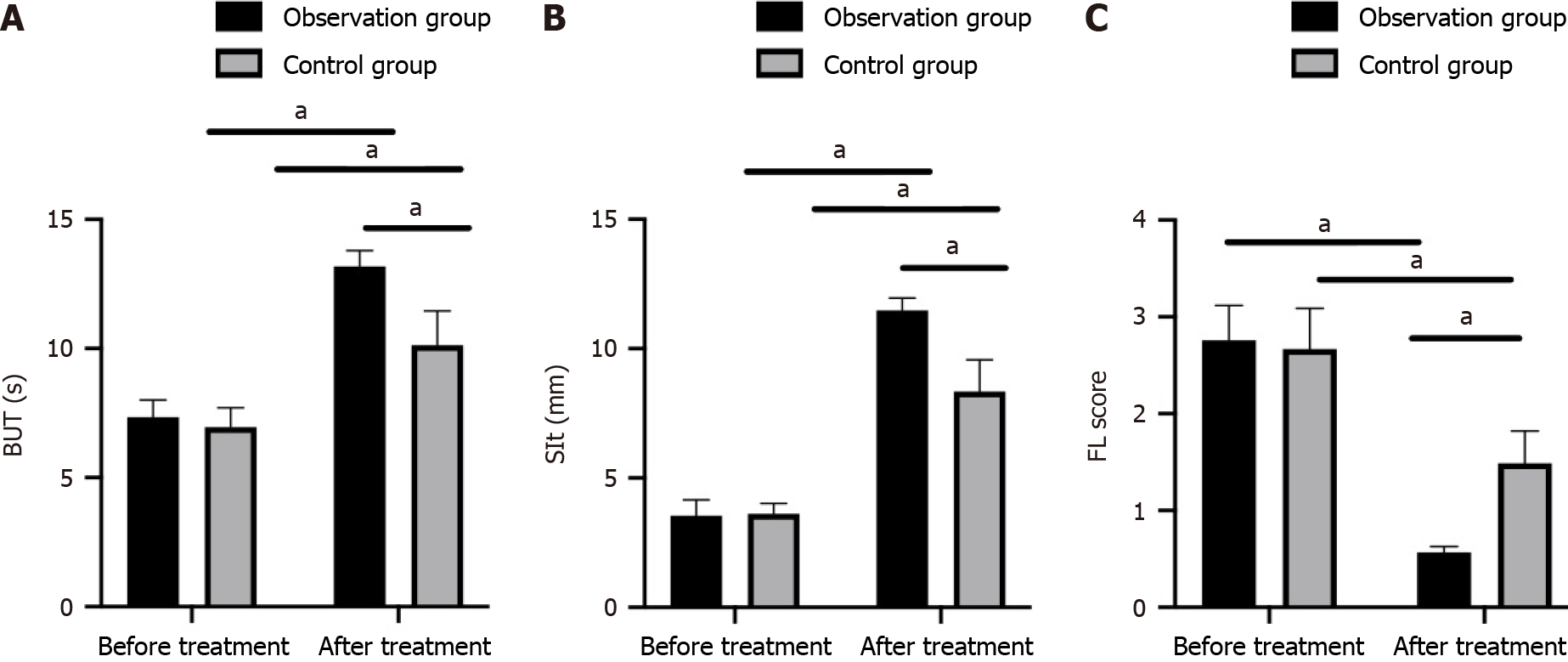
Likewise, the pretreatment TFBUT, SIt, and FL scores were similar between the observation and control groups (P > 0.05). Furthermore, the TFBUT, SIt, and FL scores were improved in both groups after treatment (P < 0.05), with even higher TFBUT and SIt scores and lower FL scores in the observation group compared with the control group (P < 0.05; Figure 2).
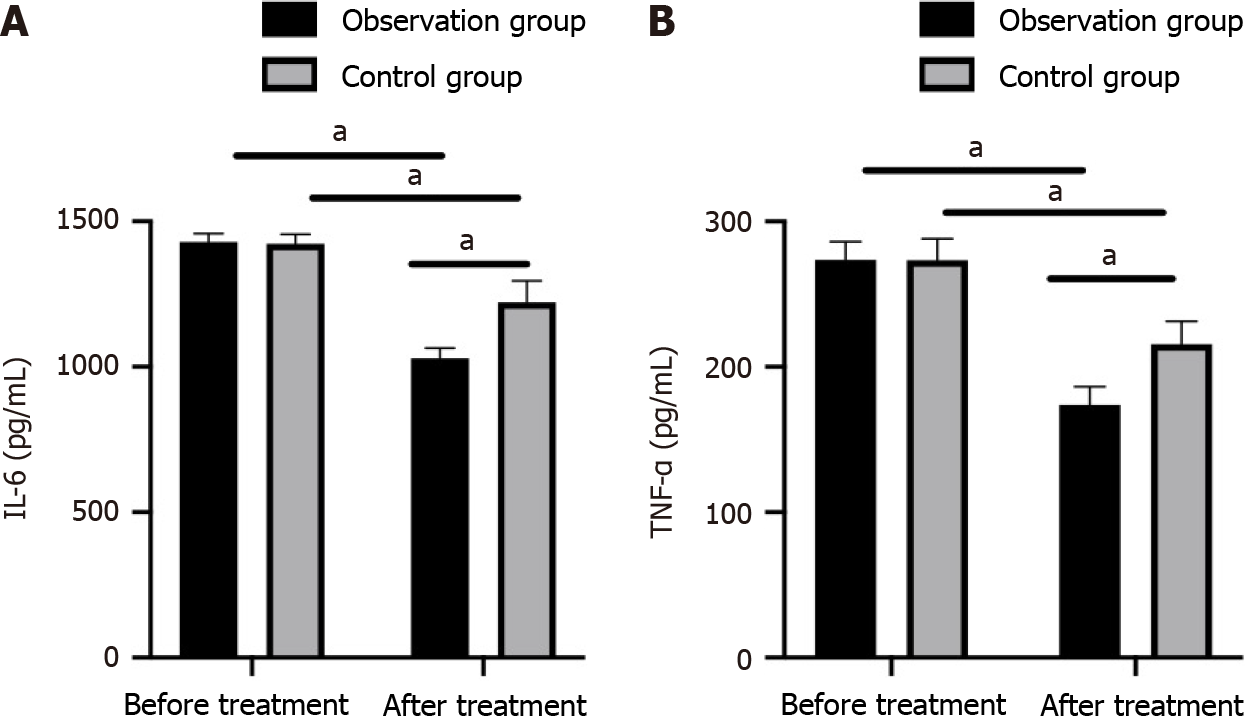
The pretreatment levels of IL-6 and TNF-α in tears were similar between the two groups (P > 0.05). After treatment, the levels of both IL-6 and TNF-α decreased (P < 0.05), with lower levels in the observation group compared with the control group (P < 0.05; Figure 3).
The adverse reaction rate in the observation and control groups was 11.90% and 12.50%, respectively, but these differences were not statistically significant (P > 0.05; Table 3).
| Adverse reactions | Observation group (n = 42) | Control group (n = 40) | χ2 | P value |
| Conjunctival hyperemia | 2 (4.76) | 1 (2.50) | - | - |
| Aggravated lacrimal gland injury | 1 (2.38) | 1 (2.50) | - | - |
| Local inflammation | 0 | 1 (2.50) | - | - |
| Corneal erosion | 2 (4.76) | 2 (5.00) | - | - |
| Adverse reaction rate | 5 (11.90) | 5 (12.50) | 0.007 | 0.934 |
Treatment satisfaction was 97.62% and 72.50% in the observation and control groups, respectively, and this was statistically significant (P < 0.05; Table 4).
| Satisfaction | Observation group (n = 42) | Control group (n = 40) | χ2 | P value |
| Very satisfied | 30 (71.43) | 19 (47.50) | - | - |
| Satisfied | 11 (26.19) | 10 (25.00) | - | - |
| Dissatisfied | 1 (2.38) | 11 (27.50) | - | - |
| Treatment satisfaction | 41 (97.62) | 29 (72.50) | 10.35 | 0.001 |
DES is a common complication following cataract surgery. It mainly manifests as dry and itchy eyes and can be caused by many factors, such as age, medication, trauma, and eye surgery[12]. Patients with diabetes have a high incidence of cataracts. Although conventional treatment involving phacoemulsification and subsequent intraocular lens implantation is effective in quickly restoring vision, it often causes DES[13]. Pathologically, DES in diabetic patients after diabetic cataract surgery is attributed to the destruction of goblet cells of the bulbar conjunctiva, changes in tear quality, a decline in TF stability, and partial detachment of the corneal epithelium[14]. However, because there is currently no better treatment to replace phacoemulsification and lens implantation, postoperative DES is a clinical concern.
We compared the therapeutic efficacy of rhEGF + SH eye drops for treating patients with diabetes who developed DES following cataract surgery. Our findings revealed higher efficacy of the combination therapy vs SH eye drops alone. SH eye drops are a polysaccharide biomaterial with high biocompatibility, strong plasticity, potent hydrophilicity, and favorable water retention capacity, and these properties prevent water loss, enhance the moisture retention capacity of the cornea, and help maintain TF integrity[15]. Additionally, SH eye drops form a covering film on the corneal surface and promote the repair and healing of corneal cells. Despite its higher efficacy for mild DES, SH has limited efficacy for moderate and severe cases of DES, especially those with corneal epithelial injury, warranting the combined use of other drugs to achieve better therapeutic efficacy[16]. rhEGF eye drops are a form of exogenous epidermal growth factor (EGF) that promotes new capillary formation, improves ocular microcirculation, and provides sufficient nutrients and oxygen for corneal repair, thereby accelerating wound healing and restoring TF stability[17]. We found that rhEGF + SH eye drops effectively alleviated DES symptoms and promoted visual recovery, consistent with previous studies.
The immunoinflammatory response is the most recognized pathogenic process of DES. Inflammatory cell infiltration and the release of inflammatory markers in DES activate cell signal transduction pathways, regulate the expression of related genes or proteins, and affect the growth cycle of conjunctival cells, resulting in morphological and functional changes[18]. TNF-α and IL-6 are inflammatory makers and cytokines that regulate inflammatory responses by downregulating neurotransmitter release and controlling tear secretion, leading to DES[19]. Our study revealed that rhEGF + SH eye drops were effective in lowering the content of TNF-α and IL-6 in tears, indicating that the administration of this treatment combination following cataract surgery effectively relieves inflammation and delays disease progression. Furthermore, a comparison of the pre- and posttreatment TFBUT, SIt, and FL scores found that they were more effectively reduced in the observation group compared with the control group after treatment. TFBUT mainly reflects TF stability, which is of great significance to DES evaluation; SIt detects DES-induced tear secretion disorders, which reflect the tear function of patients; and FL reflects corneal surface defects and indirectly evaluates TF stability[20,21]. In our study, the observation group exhibited significantly higher TFBUT and SIt and lower FL and dry eye symptom scores compared with the control group after treatment, confirming the ability of rhEGF + SH eye drops to improve clinical efficacy and alleviate the clinical symptoms of DES after cataract surgery. rhEGF is a structurally modified EGF that actively and specifically binds to EGF receptors on the cell membranes of the damaged corneal endothelium. This results in a series of biochemical chain reactions in the cell that promote the synthesis of large amounts of RNA and DNA to facilitate rapid differentiation and proliferation, thereby playing a role in repairing and shortening the healing time of the corneal endothelium. This may explain why SH eye drops combined with rhEGF improve lacrimal secretion[22]. Our comparison of the adverse reaction rate between the two groups did not reveal any significant intergroup differences, indicating that the combination therapy is safe and does not increase the risk of adverse reactions. Additionally, higher patient treatment satisfaction was recorded in the observation group, implying that the combined treatment significantly improved patient satisfaction.
Treatment with rhEGF + SH eye drops effectively improves DES and visual acuity, with high efficacy, fewer adverse reactions, and a favorable safety profile. The clinical application of this combined treatment benefits patients and deserves clinical promotion.
| 1. | Naderi K, Gormley J, O'Brart D. Cataract surgery and dry eye disease: A review. Eur J Ophthalmol. 2020;30:840-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Garg P, Gupta A, Tandon N, Raj P. Dry Eye Disease after Cataract Surgery: Study of its Determinants and Risk Factors. Turk J Ophthalmol. 2020;50:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Kelkar A, Kelkar J, Mehta H, Amoaku W. Cataract surgery in diabetes mellitus: A systematic review. Indian J Ophthalmol. 2018;66:1401-1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Vergroesen JE, Thee EF, Ahmadizar F, van Duijn CM, Stricker BH, Kavousi M, Klaver CCW, Ramdas WD. Association of Diabetes Medication With Open-Angle Glaucoma, Age-Related Macular Degeneration, and Cataract in the Rotterdam Study. JAMA Ophthalmol. 2022;140:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Alabdulwahhab KM. Senile Cataract in Patients with Diabetes with and Without Diabetic Retinopathy: A Community-Based Comparative Study. J Epidemiol Glob Health. 2022;12:56-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 6. | Becker C, Schneider C, Aballéa S, Bailey C, Bourne R, Jick S, Meier C. Cataract in patients with diabetes mellitus-incidence rates in the UK and risk factors. Eye (Lond). 2018;32:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Costanian C, Aubin MJ, Buhrmann R, Freeman EE. Interaction between postmenopausal hormone therapy and diabetes on cataract. Menopause. 2020;27:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Cagini C, Torroni G, Mariniello M, Di Lascio G, Martone G, Balestrazzi A. Trehalose/sodium hyaluronate eye drops in post-cataract ocular surface disorders. Int Ophthalmol. 2021;41:3065-3071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Gong X, Yao H, Wu J. Sodium hyaluronate combined with rhEGF contributes to alleviate clinical symptoms and Inflammation in patients with Xerophthalmia after cataract surgery. BMC Ophthalmol. 2022;22:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Yu K, Bunya V, Maguire M, Asbell P, Ying GS; Dry Eye Assessment and Management Study Research Group. Systemic Conditions Associated with Severity of Dry Eye Signs and Symptoms in the Dry Eye Assessment and Management Study. Ophthalmology. 2021;128:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Yang YJ, Lee WY, Kim YJ, Hong YP. A Meta-Analysis of the Efficacy of Hyaluronic Acid Eye Drops for the Treatment of Dry Eye Syndrome. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Posa A, Sel S, Dietz R, Sander R, Paulsen F, Bräuer L, Hammer C. Historical Profiling of Dry Eye Patients - Potential Trigger Factors and Comorbidities. Klin Monbl Augenheilkd. 2024;241:110-118. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Li L, Wan XH, Zhao GH. Meta-analysis of the risk of cataract in type 2 diabetes. BMC Ophthalmol. 2014;14:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Soria J, Acera A, Durán JA, Boto-de-Los-Bueis A, Del-Hierro-Zarzuelo A, González N, Reigada R, Suárez T. The analysis of human conjunctival epithelium proteome in ocular surface diseases using impression cytology and 2D-DIGE. Exp Eye Res. 2018;167:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Duan ZH, Tang YF. The clinical effects of sodium hyaluronate, polyethylene glycol, and dextran-70 eye drops in relieving dry eye after phacoemulsification. Medicine (Baltimore). 2021;100:e26358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Miura M, Inomata T, Nojiri S, Sung J, Nagao M, Shimazaki J, Midorikawa-Inomata A, Okumura Y, Fujio K, Akasaki Y, Kuwahara M, Huang T, Nakamura M, Iwagami M, Hirosawa K, Fujimoto K, Murakami A. Clinical efficacy of diquafosol sodium 3% versus hyaluronic acid 0.1% in patients with dry eye disease after cataract surgery: a protocol for a single-centre, randomised controlled trial. BMJ Open. 2022;12:e052488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Ren W, Chen T, Dong W, Tong Q. Effects of sodium hyaluronate combined with rhEGF eye drops in patients with dry eye. Pak J Pharm Sci. 2021;34:2461-2465. [PubMed] |
| 18. | Yang L, Zhang L, Jian Hu R, Yu PP, Jin X. The influence of overnight orthokeratology on ocular surface and dry eye-related cytokines IL-17A, IL-6, and PGE2 in children. Cont Lens Anterior Eye. 2021;44:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Yu H, Zeng W, Zhao G, Hong J, Feng Y. Response of tear cytokines following intense pulsed light combined with meibomian gland expression for treating meibomian gland dysfunction-related dry eye. Front Endocrinol (Lausanne). 2022;13:973962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Qian L, Wei W. Identified risk factors for dry eye syndrome: A systematic review and meta-analysis. PLoS One. 2022;17:e0271267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 21. | Wen Y, Zhang X, Chen M, Han D. Sodium hyaluronate in the treatment of dry eye after cataract surgery: a meta-analysis. Ann Palliat Med. 2020;9:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kim EY, Gao ZG, Park JS, Li H, Han K. rhEGF/HP-beta-CD complex in poloxamer gel for ophthalmic delivery. Int J Pharm. 2002;233:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |