Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1142
Revised: February 17, 2024
Accepted: April 12, 2024
Published online: June 15, 2024
Processing time: 133 Days and 22.2 Hours
Diabetes is a heterogeneous metabolic disease characterized by elevated blood glucose levels resulting from the destruction or malfunction of pancreatic β cells, insulin resistance in peripheral tissues, or both, and results in a non-sufficient production of insulin. To adjust blood glucose levels, diabetic patients need exogenous insulin administration together with medical nutrition therapy and physical activity. With the aim of improving insulin availability in diabetic patients as well as ameliorating diabetes comorbidities, different strategies have been investigated. The first approaches included enhancing endogenous β cell activity or transplanting new islets. The protocol for this kind of intervention has recently been optimized, leading to standardized procedures. It is indicated for diabetic patients with severe hypoglycemia, complicated by impaired hypogly
Core Tip: Common management issues are associated with insulin administration for the treatment of diabetes, a heterogeneous metabolic disease characterized by elevated blood glucose levels resulting from the destruction or malfunction of pancreatic β cells. This review focused on alternative therapeutic strategies, such as islet/pancreas implantation and islet/mesenchymal stem cell and induced pluripotent stem cell transplantation. The use of these different approaches is also associated with amelioration of diabetes-related comorbidities.
- Citation: Annicchiarico A, Barile B, Buccoliero C, Nicchia GP, Brunetti G. Alternative therapeutic strategies in diabetes management. World J Diabetes 2024; 15(6): 1142-1161
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1142.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1142
Diabetes mellitus (DM) is a heterogeneous metabolic pathology characterized by elevated blood glucose levels resulting from destruction or malfunction of β cells, insulin resistance in peripheral tissues, or both, and results in an insufficient production of insulin[1]. Prolonged hyperglycemia in diabetes is linked to serious complications that affect the health of patients with DM and even lead to death. Impairment of growth and susceptibility to certain infections may also accompany chronic hyperglycemia[2].
The etiopathology of diabetes, thus the mechanisms leading to the destruction of β cells in the endocrine pancreas, enables diabetes to be classified as type 1 (T1D) or type-2 (T2D)[3]. Himsworth was the first to classify diabetes into T1D and T2D in 1730[4-6]. Tattersall et al[5] expanded this classification to acknowledge the presence of diabetes subtypes inherited in an autosomal dominant manner.
T1D or juvenile diabetes is a chronic autoimmune disease where β cells in the Islets of Langerhans are progressively destroyed by immune cells. Subsequently insulin production is insufficient to control the homeostasis of glucose[7]. The T1D patient population is highly varied. Individuals within this group may experience the condition at different levels of severity. The origins of the disease can also differ, and individuals may possess diverse genetic backgrounds[8].
T2D features the non-response of peripheral tissue to insulin action, which reduces the ability of the tissue to uptake glucose[9]. T2D is influenced by genetic and lifestyle factors such as diet, physical activity (PA), environment, and poll-ution. These factors, unlike genetic predisposition, are modifiable, bringing significant benefits in complications of T2D[10].
In both T1D and T2D, inflammation plays a crucial role in the destruction of insulin-producing β cells. In T1D, dam
Moreover, there are other types of diabetes, such as gestational diabetes, a condition in which women, who did not have diabetes prior to pregnancy, experience abnormal levels of blood glucose during their pregnancy. In a typical pregnancy, there is an increase in the number of pancreatic β cells due to the stimulation of human placental lactogen and prolactin. This leads to elevated levels of insulin[14].
It is expected that there will be 693 million DM patients worldwide by 2045, thus the increasing prevalence of DM is a significant focal point in public health, imposing unmanageable pressures on individuals, their professional pursuits, healthcare infrastructures, and broader society[15].
T2D represents the majority (> 85%) of the overall prevalence of diabetes. One of the reasons behind this trend can be found in the increasing incidence of obesity as well as unhealthy and sedentary lifestyles[16]. Another factor contributing to the rising prevalence is the improved survival of individuals with diabetes in certain populations. This is attributed to early detection and enhanced diabetes management, leading to a consequent decrease in premature mortality. In recent years, the increase in the prevalence of T2D is linked to the growing number of cases of T2D observed in young indi
Both variants of diabetes can result in complications affecting multiple bodily systems, including bone disease[17-19], microvascular outcomes such as retinopathy, nephropathy, and neuropathy, as well as macrovascular outcomes such as ischemic heart disease, stroke, peripheral vascular disease, and impaired wound healing. The early onset of illness, increased mortality rates, diminished life expectancy, and the associated economic burdens of diabetes underscore its significance as a critical public health issue[16].
Diabetes is a complex metabolic disorder with a multifactorial etiology, involving both genetic and environmental factors. It is essential to understand that T1D and T2D each have a distinct pathophysiology[20,21]. It is important to note that while insulin resistance is a common feature in T2D. It can also be observed in certain conditions, including some cases of T1D. However, in the latter the immune system mistakenly attacks and destroys insulin-producing β cells in the pancreas, leading to a lack of insulin. In some cases though individuals with T1D may also develop insulin resistance over time[22]. Overall, the interplay of genetic and environmental factors contributes to the development of T2D. Lifestyle factors such as diet, PA, and obesity also play crucial roles in the manifestation of the disease. Understanding the genetic basis of T2D can aid in the development of personalized treatment approaches and preventive strategies[22,23].
The frequency of both types of diabetes is increasing globally at a rate that surpasses genetic variation, despite the hereditary roots of the disease, suggesting that environmental variables may play a significant role in both types of diabetes[24]. These environmental factors include diet, endocrine disruptors, various environmental pollutants, and the composition of the gut microbiota. Most of the heritability of T2D is likely attributed to a range of factors, such as the diversity in the disease, interactions between genes, and epigenetic influences.
To adjust blood glucose levels, T1D patients need exogenous insulin administration in the form of subcutaneous injections. Over time, evidence has strongly supported the benefits of more intensive insulin replacement therapies, such as multiple daily injections or continuous subcutaneous infusion via an insulin pump, as they offer the optimal combination of effectiveness and safety for T1D patients[18]. The Diabetes Control and Complications Trial demonstrated that intensive therapy, including multiple daily injections or continuous subcutaneous insulin infusion not only reduced glycated hemoglobin (HbA1c) levels but also led to improved long-term outcomes[25-27].
The integration of continuous glucose monitors (CGMs) into clinical practice has significantly enhanced diabetes management for patients on insulin therapy[28]. CGM usage is now considered standard of care for T1D patients. Notably, the reduction of nocturnal hypoglycemia in patients using insulin pumps with CGM is further improved by the automatic suspension of insulin delivery at a predetermined glucose level[29-31].
The United States’ Food and Drug Administration has approved several hybrid closed-loop pump systems. Literature data support the safety and efficacy of these hybrid closed-loop systems in T1D adolescents and adults[32,33]. Addi
Injectable and oral drugs have been evaluated for their effectiveness in addition to insulin treatment in T1D patients. Pramlintide, an amylin analog, is approved for T1D adults. Clinical trials showed both modest HbA1c decrease and weight loss with pramlintide[36-39]. The trial with liraglutide, a glucagon-like peptide 1 receptor agonists, demonstrated modest HbA1c decrease, weight loss, and insulin dose reductions[40,41]. Likewise, clinical trials testing sodium-glucose cotransporter 2 inhibitors demonstrated HbA1c improvements and weight loss[42-44]. In T1D, sodium-glucose cotransporter 2 inhibitor administration has been associated with diabetic ketoacidosis.
These drugs are used for T2D management, and insulin becomes effective when other agents are not. They should be used in combination in cases of severe hyperglycemia, particularly if catabolic features (weight loss, hypertriglyceridemia, ketosis) are evident. It is common practice to start with insulin therapy for patients with blood glucose levels ≥ 300 mg/dL or HbA1c > 10% or if the patient has symptoms of hyperglycemia (i.e., polyuria or polydipsia) or catabolism evidence. As the emergency resolves, the use of noninsulin agents is possible[28].
It is a common practice before initiating pharmacotherapy in the management of diabetes to prioritize lifestyle inter
Nutrition therapy has an essential role in diabetes management, and diabetes patients should be actively involved in self-management, education, and treatment planning with the health care team, leading to the development of an individualized eating plan[45,46]. All health care professionals should discuss individualized MNT provided by a specialized registered dietitian nutritionist at diagnosis and throughout treatment. MNT determines a HbA1c absolute decrease of 1.0%-1.9% for T1D patients and 0.3%-2.0% for T2D patients[47]. Due to the progressive characteristics of T2D, after medication is initiated, MNT should be modified in relation to disease evolution[45,46].
It has been reported that there is not an ideal calories percentage arising from carbohydrates, protein, and fat for diabetic patients. Consequently, macronutrient distribution should consider a personalized evaluation of preferences, current eating patterns, and metabolic goals. It is important to highlight non-starchy vegetables, minimize added sugars as well as refined grains, and select whole foods over highly processed foods. The Mediterranean, vegetarian, low-carbohydrate, and plant-based eating diets represent healthful eating patterns that have demonstrated positive results in T2D. In contrast, there is insufficient data to establish one eating diet over another for pediatric and adult T1D patients. In particular, the literature gap relates to the efficacy and long-term management implications of nutrition interventions for T1D children[48]. T2D patients not meeting glycemic control should reduce their carbohydrate intake with a low- or very-low-carbohydrate diet[49-51]. However, the recommended approach is to personalize meal plans with a macronutrient distribution that is more coherent with personal preference and usual intake to augment the probability for long-term maintenance. An important challenge of MNT is represented by weight management.
Weight reduction and management is important for T1D, T2D, or prediabetic patients with overweight or obesity. To sustain these patients, MNT together with diabetes self-management education and support services should include a personalized eating plan characterized by an energy deficit associated with increased PA[45]. Lifestyle intervention programs should be intensive with frequent follow-up to realize a significant decrease in extra body weight and ameliorate clinical indicators. Consistently, it has been reported that weight loss can slow down the progression from prediabetes to T2D and is helpful for T2D management. In prediabetes, weight loss of 7%-10% has been shown to prevent the progression to T2D[52]. People with prediabetes at a healthy weight should also be considered for behavioral interventions to help establish routine aerobic and resistance exercise[52-54] and to establish healthy eating patterns.
T2D patients with overweight and obesity needed to achieve a 5% weight loss[55]. However, if the positive outcomes of weight loss are continuing and important (i.e., 15%) the benefits may increase[56,57]. Overweight and obesity are also important in T1D patients. Sustaining weight loss can be difficult[55,58], although it has lasting benefits; interestingly weight loss is associated with HbA1C and lipid level improvements[59].
Different reports have shown that several eating plans, assorted in macronutrient composition, can be utilized successfully and safely over the short term (1-2 years) to accomplish weight loss in diabetic patients (e.g., Mediterranean eating diet[60], structured low-calorie meal plans with meal replacements[57,59,61], and low-carbohydrate meal plans with additional support[62,63]). However, it is important to use meal plans containing nutrient-dense foods, including vegetables, legumes, fruits, dairy, lean sources of protein, seeds, nuts, and whole grains. Any approach to meal planning should be personalized, considering the health status, personal preferences, and ability of the patient to sustain the plan recommendations.
PA, as defined by the World Health Organization, includes any movement involving the skeletal muscles that consumes energy. This includes activities undertaken during leisure, for transport to get to and from places, or as part of work. Both moderate and vigorous levels of PA are beneficial for health. During any type of PA, glucose levels increase in active muscles through insulin-independent pathways, whereas their circulating levels are maintained by hepatic glucose production and mobilization of free fatty acids, which may be impaired by insulin resistance or diabetes[64,65]. More
Regular PA can help diabetes patients achieve a variety of aims including increased cardiorespiratory fitness and vigor, improved glycemic control, decreased insulin resistance, improved lipid profile, reduced blood pressure, maintenance of a healthy body mass after weight loss, less depression and anxiety, less medication use, and overall improved quality of life[71].
Although Bazargan-Hejazi et al[72] reported that less than 50% of the 871 individuals with T2D analyzed met the recommended threshold for exercise, specific suggestions and precautions can be modified based on the type of diabetes, age, activity, and presence of diabetes-related health complications. Females with preexisting familial history of diabetes and those with gestational diabetes should begin moderate PA during their pregnancies as tolerated[73].
In particular, the American Diabetes Association in “Standards of Care in Diabetes”, published in 2023, emphasize that PA plays a key role in the prevention and management of diabetes especially in T2D[71,74]. Recommendations should be personalized for each individual[73], thus according to American Diabetes Association guidelines T1D and T2D adults should perform[73]: (1) 150 min or more of moderate- to vigorous-intensity aerobic activity per week spread over at least 3 d/wk, with no more than 2 consecutive days without activity; (2) 2-3 d/wk of resistance exercise on non-consecutive days with each session consisting of at least one set of five or more different resistance exercises involving the large muscle groups[71]; (3) interrupt prolonged sitting every 30 min to gain blood glucose benefits; and (4) 2-3 times/week of flexibility and balance training.
However, PA in diabetic patients might cause hypoglycemia if the MNT does not contain the appropriate amount of carbohydrates for an exercise session. If glucose levels pretraining are less than 90 mg/dL, people need to eat additional carbohydrates according to their metabolism, intensity, and duration of PA[75]. In some patients, hypoglycemia following exercise may appear and persist for several hours due to heightened insulin sensitivity.
In individuals not treated with insulin or insulin secretagogues, hypoglycemia is less common, and routine preventive measures for hypoglycemia are typically not recommended in such cases. Intense activities may, in fact, elevate blood glucose levels instead of reducing them, particularly if pre-exercise glucose levels are elevated[75]. However, due to the variability in glycemic response to exercise sessions, individuals with diabetes should be instructed to monitor blood glucose levels before and after PA and be aware of potential prolonged effects based on intensity and duration.
Aerobic activities are linked with lower cardiovascular disease and overall mortality risks[76]. Moreover, this type of exercise training increases insulin sensitivity in individuals with diabetes and improved mitochondrial function in muscle fibers from vastus lateralis muscle[77]. Vigorous-intensity aerobic exercise training for 7 d may improve glycemia without reducing body weight through enhanced insulin-stimulated glucose clearance and inhibition of hepatic glucose production[67].
Meta-analyses and systematic reviews determined that regular aerobic exercise training improved glycemia in adults with diabetes including a 0.5%-0.7% reduction in HbA1c[78-83]. A meta-analysis published in 2019 examined the importance of resistance exercise in the management of diabetes and proposed that high-intensity training is more helpful than low-to-moderate-intensity training for overall glucose management and attenuation of insulin levels in diabetics patients. However, they noted that it is important to improve strength, balance, and the ability to engage in activities of daily living during the life span[84].
In 2010, a trial on T2D patients demonstrated that, combined aerobic and resistance training improved HbA1c levels compared with non-exercising controls, although neither resistance nor aerobic training alone resulted in significant changes[85]. Moreover, the patient group doing combined training lost more weight and improved aerobic fitness more so than controls[85].
Another important consideration is the significance of PA for diabetic children. They should perform at least 60 min of aerobic activity every day, with strength training 3 d/wk[86]. T1D children and adolescents benefit from physical activity[87] and thus may have better health outcomes and health-related quality of life[88,89].
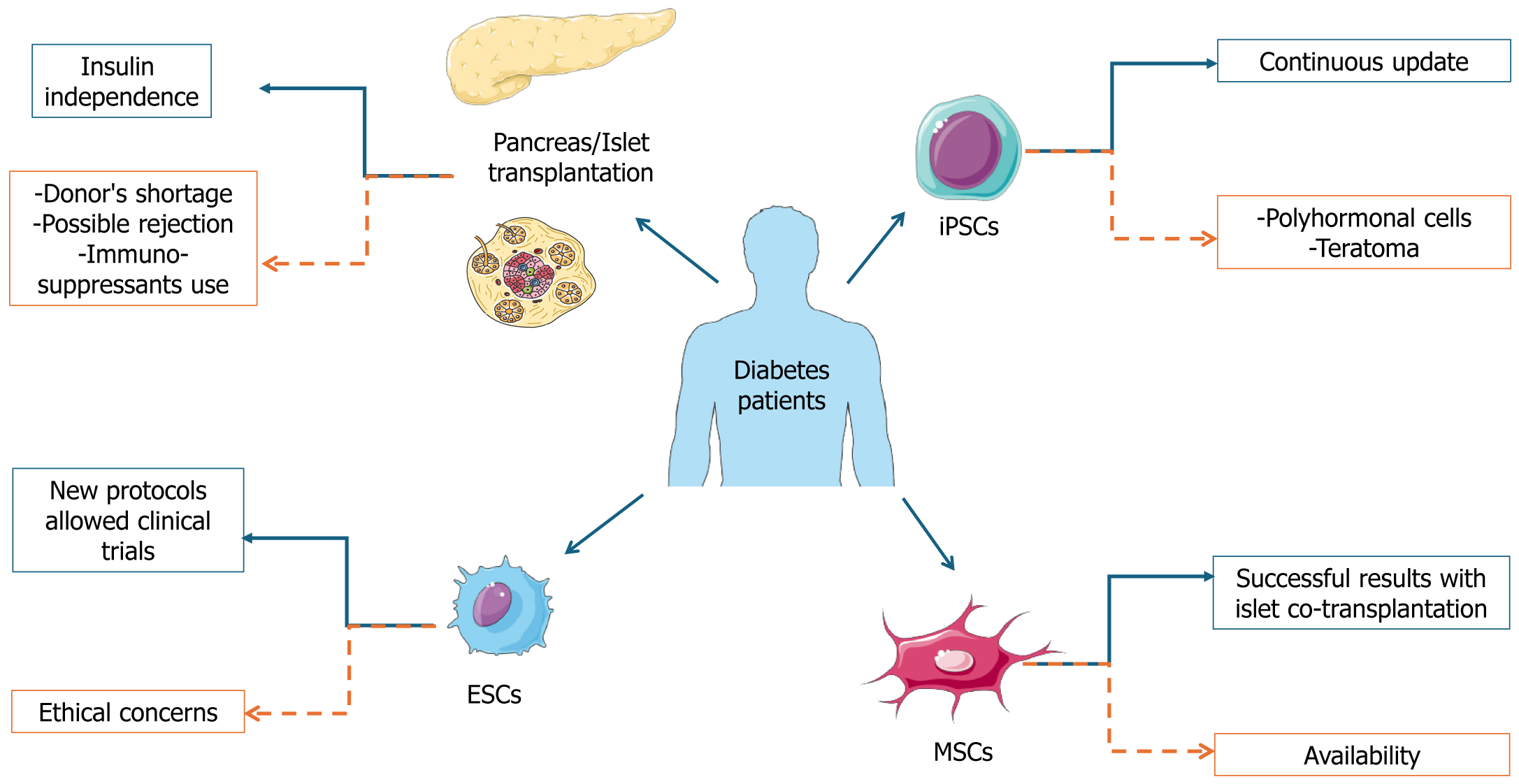
Although new technological devices are available and led to amelioration of metabolic control and life quality, challenges for T1D management are abundant. In detail, insulin administration remains a therapy and not a cure for DM. It requires compliance of the patients, which is particularly difficult for pediatric patients. Furthermore, the risk of severe hypo-glycemia persists. Therefore, with the aim of improving insulin availability in T1D patients, different strategies have been employed, including enhancing endogenous β cell activity and transplanting new islets (Figure 1).
A different approach can arise from the use of plant extracts that are rich of polyphenols, characterized by an anti-diabetic effects. Promising in vitro results have been obtained using extracts from Coronopus didymus[90], Molineria capitulata, Trichosanthes tricuspidate, Amorphophallus campanulatus[91], Datura metel L[92]. Furthermore, in alloxan induced diabetic rats the anti-hyperglycemic and anti-oxidant roles of the 1,3,4-oxadiazole derivative[93] was found, whereas extracts of Centella asiatica leaf[94] affected weight, insulin level, and antioxidant factors compared to the control group. Also, Mitra et al[95] investigated the biological effects of leaf extract of Avicennia alba in alloxan induced diabetic rats, the dosages of 200, 400, and 500 mg/kg reduced glucose levels of blood, increased body weight and have anti-inflammatory activity. However, some polyphenols needed to be included in nanoformulations to overcome pharmacokinetic barriers resulting in the improvement of their anti-diabetic activity[96]. Despite the positive restorative consequences of phytochemicals, inconsistent or contradictory data from clinical trials emerged, although beneficial effects can be observed from the combination of the classic therapy and phytochemicals[97].
The first strategy showing interesting results arose from preclinical models. In detail, in 2022, it was reported that prolonged blocking of the death receptor TMEM219 determined a strong β cell expansion simultaneously with the islet area and insulin levels in non-obese diabetic mice[98]. Furthermore, in 2007, 2019, and 2021, the use of rapamycin, an mTOR inhibitor, was linked to different results[99-101]. For example, in islet xenograft the simultaneous use of rapamycin and anti-CD154 mAb determined graft survival[99]. Whereas in a non-human primate study, rapamycin administration was linked to long-term islet allograft survival and the disappearance of alloantibodies[100]. It has also been reported that rapamycin affects autoimmune response, β cell proliferation, and survival[101]. Interestingly, in long-standing T1D a phase II trial in 2007 showed that rapamycin was not able to improve β cell activity[102]. In contrast, pancreas/islet transplantation has excellent results.
The protocol for this kind of intervention has been recently optimized, leading to standardized procedures. It is indicated for T1D patients with severe hypoglycemia, complicated by impaired hypoglycemia awareness or exacerbated glycemic lability, as defined by the current recommendation[103,104]. Islet/pancreas transplantation can always be associated with kidney transplant; however, the absence of cardiovascular disease is imperative in this case and is typical for patients aged up to 55-years-old. Pancreas transplantation has been associated with improvement of all comorbidities associated to diabetes, quality of life, and survival[105].
Allogenic islet transplantation is fundamental to restore the right levels of insulin, glucagon, and other hormones produced by these cells. Allogenic islets are isolated from the pancreas of a deceased donor, using a standard protocol requiring enzymatic and mechanical digestion, followed by density gradient purification[106]. It is possible to continue with transplantation only if more than 200000 viable pure sterile islets are obtained[103]. Only a few institutions routinely carry out this kind of transplantation due to the complexity of the intervention. Consistently, it requires an islet-isolation team available 24 h/d and 7 d/wk, and there are often difficulties due to the transplantation itself. Islets can be infused through the portal vein for delivery to the liver under immunosuppression. Islets can either be transplanted fresh or after culture that can cover a period of 12-72 h after immunosuppression induction. Normally, two/three islet preparations need to be injected to achieve the right insulin level. Immunosuppression is also fundamental in this case, and it includes an induction phase at each islet infusion, followed by a maintenance period during islet transplantation.
The best-known successful protocol for the transplantation of islets is the Edmonton protocol, which requires an anti-IL2 receptor infusion before each islet administration combined with an mTOR inhibitor (sirolimus)[107]. However, alternative protocols have been used with different immunosuppressive approaches including T-cell depleting agents, steroids, anti-TNFα, or anti-IL1β antibody. A maintenance therapy is also required[108-112].
The CIT Consortium Protocol 07 (CIT-07) trial showed islet transplantation to be an effective treatment for subjects with impaired awareness of hypoglycemia and intractable severe hypoglycemic events[113]. This was a multicenter phase 3 study published in 2016 involving 48 T1D adults with successful results 1 year after transplantation. In 2018, an additional study on the same patients reported that 87.5% of patients reached the first endpoint of freedom from severe hypoglycemic events together with attainment of glycemic control 1 year following islet transplantation and amelioration of health-related quality of life[114].
In 2023, primary graft function was evaluated as an independent predictor of 5-year clinical islet transplantation outcomes[115]. In detail, the authors referred to the Collaborative Islet Transplant Registry, which is a comprehensive global registry that reports all data from most islet transplant programs in North America, Eurasia, and Australia. They demonstrated an inverse, independent, and linear relationship between primary graft function evaluated 28 d after the last islet infusion together with the cumulative 5-year incidence of unfavorable events after islet transplantation such as unsuccessful islet transplantation, graft exhaustion, insufficient glucose control, and the need for exogenous insulin treatment.
Researchers also investigated the omentum as another potential site for islet injection. Its great surface area, vas-cularity, and portal venous drainage system led to its identification as an opportune transplant site[116]. Consistently, a 2021 clinical trial (NCT02213003) is ongoing in Miami with transplantation of islets onto the omentum in T1D patients. This is performed laparoscopically, and the islets are mixed with human thrombin to generate a biological mesh that adheres to the omentum (ClinicalTrials.gov. Allogeneic Islet Cells Transplanted onto the Omentum. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02213003).
In 2017, a preliminary case report described the case of a 43-year-old female patient with a 25-year history of diabetes who had 600000 islet equivalents laparoscopically transplanted onto her omentum. She discontinued insulin infusion after the transplant and maintained insulin independence 12 months after her procedure with stable glycemic control[117]. A partner study is ongoing at the University of Alberta (NCT02821026), in collaboration with the University of Miami, with the same method of omental transplantation and immunosuppression (ClinicalTrials.gov. Omental Islet Transplant. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02821026). Recruitment is ongoing and updates are required.
Despite the beneficial effects of islet/pancreas transplantation, some limitations occur that limit its application on a large scale. In detail, the supply of donor tissues is limited, thus the transplantation can be restricted to only a few patients. Consistently, data from the Eurotransplant foundation showed that in 2020 in North Europe only 163 donors were available for a waiting list of 385 patients and an estimated mean of 50000 T1D patients (Monthly statistics. Eurotransplant. https://www.eurotransplant.org/statistics/monthly-statistics/).
Furthermore, up to 80% of the transplanted islets were lost before becoming included into tissue due to acute inflammatory responses and release of the proinflammatory cytokines IL-1β, TNFα, and IFNγ[118-123]. One year after implantation some β cells were lost due to problems during the engraftment, the always active autoimmunity, and infla
MSCs represent a varied group of multipotent precursor cells found in the supportive tissues of numerous adult tissues. The distinctive features of MSCs, including their ability to renew themselves, their capacity to differentiate into multiple cell types, and their ready availability, coupled with their immunomodulatory properties and minimal ethical concerns, underscore their significance in the field of regenerative medicine. The characteristic of multipotency is defined as the ability to differentiate into different cytotypes under in vivo and in vitro conditions[124,125].
Dominici et al[126] proposed three criteria to define MSCs: Adherence to plastic of the dish in standard types of culture; specific surface antigen expression (CD105, CD73, and CD90); and multipotent differentiation in osteoblasts, adipocires, and chondroblasts. Furthermore, MSCs do not have expression of hematopoietic markers CD45, CD34, CD14, or CD11b, CD79a or CD19, HLA-DR[127], and endothelial marker CD31. Likewise, in vitro MSCs can function as alloantigen presenting cells (antigen-presenting cells). In fact, these cells suppress lymphocyte T proliferation and activation.
Consistently, MSCs have unique immunomodulating properties, which is very important during the procedure of transplantation[128]. MSCs are found in both embryonal, fetal tissues and numerous adult tissues with some exceptions. Embryonic stem cells (ESCs), derived from the inner cell mass of blastocyst-stage human embryos, are a potentially unlimited renewable source for cell transplantation aimed at treating numerous diseases[129]. Well-organized populations of MSCs have been isolated from bone marrow[130]. Furthermore, cells showing MSC properties have been isolated from adipose tissue[131], dental pulps[132], endometrium[133], peripheral blood[134], skin[135], placenta[136], umbilical cord[137], and synovial fluid[138]. It has been proposed that the functions of MSCs may vary depending on the specific tissue they are in, with their adaptability influenced by the surrounding microenvironment[139]. Among the different applications, stem cell therapy is currently the most investigated modality to treat DM[140].
One interesting promising strategy is the cotransplantation of MSCs with islets, thus providing protection against proinflammatory cytokines and hypoxia[141]. In 2021, a pilot study demonstrated that autologous MSCs and islet cotransplantation was safe and increased islet engraftment in patients with chronic pancreatitis[142]. A different approach has been realized by using MSCs as a potential source of β cells, even if the results are not as excellent as expected. Different studies have been performed starting in 2005. D’Amour et al[143] differentiated ESCs toward pancreatic progenitors using different growth factors. They obtained functional multihormonal cells, but following transplantation they became unresponsive to glucose and producing insulin. Kroon et al[144], generated glucose responsive endocrine cells from human ESCs that following transplantation in diabetic mice differentiated into active β cells with the right balance between glucose and insulin levels. Pagliuca et al[145] obtained active β cells in vitro from ESCs.
Other authors have tried to ameliorate the protocol working on the activation of specific pathways (WNT, FGF, BMPs, and Notch) and using suspension cultures leading to the decrease in multihormonal cells and the increase in glucose sensitive cells[146]. All these findings led to the development of two clinical trials by Vertex and ViaCyte (both, Vertex Pharmaceuticals, Boston, MA, United States)[147].
In 2022, Vertex announced the findings of the first trial on a T1D patient receiving an intraportal infusion of ESC-derived islets. They reported an augment in both fasting and post-prandial C-peptide levels. Overall, they reported that the patient reached complete independence from insulin injection (Vertex Pharmaceuticals Incorporated. Vertex Announces Positive Day 90 Data for the First Patient in the Phase 1/2 Clinical Trial DosedWith VX-880, a Novel Investigational Stem Cell-Derived Therapy for the Treatment of Type 1 Diabetes. BusinessWire, 18 October 2021). However, a high chronic dose of immunosuppressant was required to avoid cell rejection.
To overcome the problem of immunosuppressor use, ViaCyte encapsulated ESC-derived pancreatic cells in a biological membrane that was subcutaneously implanted. The device is known as PEC-Encap. The implanted cells further differentiated into active β cells. This first approach was superseded by the same ViaCyte using more mature and functional cells, and preliminary results arising from 17 T1D patients showed that implanted ESCs created a renewable source of islet cells[148].
Encapsulation can be differentiated into two groups: microencapsulation and macroencapsulation. In the former, system islets were coated in a thin natural polymer[149]. The preferred polymers are alginate hydrogels, as they can be adjusted for permeability and firmness. The mix of islet and alginate led to the formation of a fibrin gel that appears as a porous nest for the islets[150]. The major limit associated with microencapsulation is the inflammatory response against the polymer. Researchers moved on to develop macroencapsulation techniques, implanting devices over 1 mm in size, which are easily implantable, monitored, and removed if necessary[149].
Although further improvements have been obtained in β cell differentiation[151], stem cell-derived β cells are not completely like pancreatic β cells, thus they do not reach optimal glucose control. This has prompted different researchers to use induced pluripotent stem cells (iPSCs). This approach overcomes some limitations associated to the use of ESCs, such as the alloimmune response but led to other complications such as polyhormonal cells and teratomas[152]. Recently, the improvement of the protocol for using iPSCs with specific growth factors and a planar technology cultivation for long periods seems to offer good prospectives[153]. Islet-like aggregates have also been created with iPSCs using different biomaterials (e.g., fibronectin, matrigel, decellularized scaffolds) transplanted into diabetic mice as organoids with interesting but still limited results. However, all other protocols based on the use of iPSCs as a source of β cells led to rejection and prolonged immunosuppression. Therefore, the evaluation of the long-term therapeutic effects is difficult and further investigation is required[154].
Additional limitations include low reprogramming efficiency, tumorigenesis, low survival and engraftment, cell phenotype loss following transplantation, and genetic and epigenetic instability. Very recently, a clinical case has been described. A T1D patient developed a teratoma after iPSC-derived β cell transplantation. It is important to underline that in this patient the β cells arose from autologous iPSCs. They were injected into the deltoid and developed a mass there 2 months following implantation. The tumor showed high growth and metastasis to the lymph node and was chemo-therapy resistant[155].
Several studies have investigated the role of the complex secretome released from MSCs[156-162]. In recent years, interest has increasingly been directed toward the study of small extracellular vesicles, called exosomes[163-169]. The interest in these small 30-100 nm vesicles comes from their role in cellular physiology. Indeed, exosomes not only modify the cells from which they originate but also those with which they interact, the target cells[170-172]. Exosomes are studied for their ability to act on intercellular communication, carrying proteins, RNA (mRNA, miRNA, and non-coding RNA), and DNA sequences. Moreover, exosomes interact with target cells, binding receptors, and surface enzymes, activating them for triggering of intracellular signaling[173].
Exosome interaction with the target cells causes the activation of signal transduction mechanisms and consequently gene expression through specific enzymes, transcription factors, and proteins[173,174]. The therapeutic potential of MSC-derived exosomes in different disease including T1D and T2D is of great interest[175,176].
In diabetes patients, neovasculogenesis, certainly one of the most important factors influencing wound repair, is compromised, and this leads to a delay in wound healing[177]. Yu et al[178] investigated the role of MSC-derived exosomes in enhancing angiogenesis during wound repair after making full-thickness skin defects in streptozotocin-induced diabetic rats. In that study, exosomes were isolated from human bone marrow MSCs and treated or not treated with atorvastatin (ATV). A 2-cm diabetic rat lesion was treated with phosphate-buffered saline, exosomes, or ATV-pretreated exosomes. Treatment with exosomes and ATV-treated exosomes accelerated wound closure compared with the control group. Moreover, wounds treated with exosomes and ATV-exosomes had significantly more blood vessels compared to the control group, which was highlighted by immunohistochemistry assays for CD31 and immunofluorescence for CD31 and α-SMA. To establish the mechanism underlying ATV-exosome action in diabetic rats, the AKT/eNOS pathway and 10 candidate microRNAs (miRNAs) were selected for investigation because of their ability to enhance angiogenesis. Among the 19 candidate miRNAs, miR-221-3p levels were upregulated in ATV-exosomes. The authors hypothesized that ATV-exosomes may promote angiogenesis processes through miR-221-3p release and via AKT/eNOS pathway activation[178].
More recently, Tang et al[179] published a study showing the ability of MSC-derived exosomes to increase skin wound healing in diabetic mice through the release of circ-Snhg11. Specifically, by luciferase assay, the involvement of SLC7A11 and miR-144-3p, downstream targets of circ-Snhg11, was confirmed.
Another noteworthy diabetes health complication is diabetic peripheral neuropathy (DPN)[180]. DPN affects the peripheral nervous system and is characterized by sensory axonal loss. DPN begins in the lower extremities and is characterized by pain and morbidity. Hyperglycemia is the main cause of DPN in T1D, while dyslipidemia is a contributory factor in etiopathogenesis of DPN in T2D[181-183].
Fan et al[184] used 20-wk diabetic mice as a DPN model, and MSC-derived exosomes were injected weekly into the tail vein for 8 wk. Neurophysiological, thermal, and mechanical sensitivity measurements were examined. Moreover, toluidine blue-staining and immunohistochemistry for protein gene product 9.5, myelin basic protein, and hypophosphorylated neurofilament H were performed on sciatic nerves of the diabetic mice. MSC-exosome treatment significantly increased motor nerve conduction velocity and sensory nerve conduction velocity at weeks 4 and 8 post-injection in the diabetic mice. In addition, exosome treatment decreased the mechanical response threshold and thermal response time latency at weeks 4, 6, and 8. Moreover, morphological analysis revealed that MSC-derived exosomes significantly improved myelination as well as nerve fiber density and diameter of diabetic mice. MiRNA profiling within exosomes derived from MSCs was examined, and a total of 215 miRNAs were detected. Let-7a, miR-23a, and miR-125b miRNAs, which synergically target the TLR4/NF-κB signaling pathway, were upregulated. Notably, there is some evidence for involvement of the TLR/NF-κB signaling pathway in DPN disease[184].
Notably, Fan et al[185] performed a study to evaluate the therapeutic effects of engineered MSC-derived exosomes with miR-146a (exo-146a) on DPN of diabetic mice. Two-week treatment with exo-146a in diabetic mice compared with non-engineered exosomes significantly increased nerve conduction velocity and decreased the threshold of thermal and mechanical stimuli. In addition, exo-146a significantly reduced inflammatory peripheral blood monocytes and endothelial cell activation targeting the TLR-4/NF-κB signaling pathway.
The role of treatment with MSC-derived exosomes has also been investigated in erectile dysfunction arising in 50% of diabetic men because of corpus cavernosum smooth muscle cell dysfunction[186-188]. Having established that MSC-derived exosomes could improve erectile function, Huo et al[188] investigated the ability of exosomes to communicate intercellularly with corpus cavernosum smooth muscle cells through miR-21-5p release in a rat model of DM-induced erectile dysfunction. The authors hypothesized that miR-21-5p could be released from exosomes in corpus cavernosum smooth muscle cells, enhancing proliferation and inhibiting smooth muscle cell apoptosis, in turn alleviating erectile dysfunction in DM rats[188]. It has been demonstrated that miRNA-21 enhances pulmonary artery smooth muscle cell proliferation and migration and prevents T1D, caused by pancreatic β cell apoptosis through blocking programmed cell death 4 protein production[189,190].
Recently, in 2024, the therapeutic role of MSC-derived exosomes, administered by intracavernous injection, was investigated in erectile dysfunction in a cavernous nerve injury rat model[191]. To examine the beneficial effect of exosomes in erectile dysfunction, intracavernosal pressure/mean arterial pressure ratio, immunohistochemical assay and molecular analysis were performed. Erectile dysfunction, evaluated by intracavernosal pressure/mean arterial pressure ratio, was ameliorated in the exosome group. Moreover, the smooth muscle/collagen ratio was increased after exosome-repeat injection, inhibiting corpus cavernosum fibrosis and atrophy. Molecular analysis identified three genes, significantly expressed in the exosome-treated group, including Ras homolog family member B, which can enhance cell proliferation and angiogenesis of HUVECs[191].
The advantages of MSC implantation are seemingly not restricted to the restoration of pancreatic functionality. An MSC implant has also been shown to be a potential new and valid approach in the treatment of diabetic wound healing through the modulation of fibrosis, tissue regeneration, immune suppression[119,192], and re-epithelization[193]. One common complication of diabetes is impaired wound healing, which leads to prolonged periods of tissue repair and deposition of scars with elevated collagen density and decreased tensile strength[194,195]. In the absence of healthy scarring, diabetic patients present increased risks for infections, sepsis, surgery, and reoperation[195], as well as developing diabetic foot ulcers, a serious and potentially debilitating condition expected to occur in 25% of people diagnosed with diabetes in their lifetime[193].
There currently exists no successful clinical strategies for repairing damaged blood vessels and nerves[193] or enhancing the healing process, apart from amputation for the treatment of the diabetic foot ulcers[196] or conventional treatments that ameliorate the symptoms (e.g., by reducing the risk of infections or keeping the wound bed moist[197]). This lack of approach options emphasizes the importance of proper diabetes management to prevent and mitigate these issues.
MSCs have been successfully tested and administered by local and systemic delivery for the treatment of cutaneous wound healing and diabetic foot ulcers[193,198,199]. For local delivery, nonvascular injections into tissue are reported to be the most widely used route of administration, followed by topical administrations[198]. For systemic cell delivery, it has been performed as an endovascular injection via the intraarterial femoral route in clinical studies, and the intravenous tail vein route in preclinical studies[198]. Despite the number of advantages in the direct injection of MSCs, several limitations to its therapeutical potential have been revealed for this administration route, including reduced cell viability, impaired localization of cells at the bed of diabetic wounds[200], and higher infection risks[198]. For these reasons, new strategies involving biomaterials and scaffold have been developed to improve the efficacy of mesenchymal cell delivery[200].
Hydrogel scaffolds are mesh-like networks of polymer chains that can be either naturally derived or synthetic[201]. Despite reduced water retention capacity, limited biomechanical features, and higher variability in properties between natural hydrogel batches, they both possess the ability to preserve cell viability for resident and engrafted cells at the wound site[201], thus overcoming one of the major issues related with MSC injection.
The advantages of employing hydrogel-based scaffolds also rely on their ability of being functionalized with customizable properties. Hydrogels can be tailored by incorporating specific crosslinkers, such as the RGD-like motif[202], utilizing multiple components (i.e., sodium alginate/gelatin[203]), or adjusting their size, shape, and biodegradability[201]. This customization imparts mechanical and biological cues that not only enhance engraftment adhesion but also restore other physiological functions, including epidermal regeneration, angiogenesis, and collagen recovery[203]. Additionally, hydrogels exhibit great cytocompatibility and antibacterial properties[201], which collectively establish these matrices as suitable and valuable options in the field of regenerative medicine.
Recent advancements in tissue engineering have expanded the application of matrices beyond their traditional use as bulks. They are now utilized in various forms and geometries, including hydrogel sheets, in situ forming hydrogels, and hydrogel microspheres, enhancing their versatility in the field[204,205].
Several types of films or sheets of hydrogels have been successfully developed for treating various types of ulcers and wounds in different pathophysiological contexts[206-208]. The surface of diabetic ulcer wounds, however, typically exhibits a complex and uneven topography, making the hydrogel dressing more prone to detachment due to weak adhesion to the wound surface, and is susceptible to bacterial infections[209]. Therefore, thin hydrogel nanosheets that display low risk of trauma to the wound bed, greater antibacterial performance[210], and inhibition of chronic inflammation[202] are of particular interest in diabetic bacterially-infected tissue damage.
A full list of preclinical and clinical studies of hydrogels combined with different MSCs lines tested for the treatment of diabetic wound healing is available in the paper by Li et al[211].
Sponge scaffolds retain a low percentage of water in their process, which requires longer manufacturing times and additional surface and structural modifications (i.e., pore size network) depending on the type of cells to be delivered[201] compared to non-porous scaffolds.
Sponges have proven to be effective devices in tissue regeneration of diabetic wounds. On one hand, due to their ability in retaining fluids, they are useful in absorbing exudates from the wound, thus creating a conducive environment for cell proliferation and migration[201]. On the other hand, they can be embedded with media enriched with growth factors that can be released once implanted in the wound bed and effectively promote skin regeneration and wound healing[212]. Due to their caveolar structure, sponge-like scaffolds (SLS) are exceptionally suitable for hosting MSC cells, whose adhesion and numerosity can be augmented in functionalized SLS (i.e., soy protein and β-chitin[213], platelet-rich plasma[214]).
Collagen and chitosan-based sponges are the most commonly used scaffolds for MSC delivery[200]. As for the usage of SLS in diabetic wound treatment, collagen scaffold derived from bovine skin has been shown to result in improved biocompatibility, re-epithelization, anti-inflammatory effects, and neovascularization of chronic wounds in diabetic mice[215,216]. Studies performed on MSCs and exosomes have further showed that the combination of gingival MSC-derived exosomes and hydrogel sponges accelerates skin wound healing and the re-epithelization process[217].
Nanofibrous scaffolds are produced from natural or synthetic polymers[218]. The nano-geometry and three-dimensional structures of fibers represent a critical factor in tissue regeneration including elevated pore size together with the higher ratio between pore and cell size, which are reported to be associated with enhanced cell migration and invasion[219] as well as the alignment of nanofibers in multiple directions (i.e., radial, vertical) that confers mechanical resistance to compressive force[220]. Nanofibrous scaffolds exhibit greater potential in the context of wound healing of diabetic ulcers compared to other conventional scaffold matrices. They not only greatly support cell adhesion and proliferation due to their high surface-to-volume ratio but also safeguard the wounded region against dehydration, impede the infiltration and proliferation of microorganisms, and topically deliver drugs and therapeutics that can be incorporated in the matrix design[218].
A relatively fast and safe scaffold technology in wound repair is represented by the spray delivery of stem cells em
Currently, no cure is available for DM, and it can be managed using available medication (primarily insulin) together with MNT, PA, weight loss, and smoking cessation. Consistently, MNT and PA represent the first approach for prediabetes management leading to a retardation of the disease manifestation. However, it is important to consider that the therapeutic approach together with MNT and PA could lead to side effects, such as hypoglycemia and glycemic instability. Furthermore, diabetes can aggravate over time with the consequent development of common comorbidity, such as bone disease, neuropathy, retinopathy, nephropathy, and cardiovascular disease, with consequent augment of mortality and morbidity. A beneficial effect was demonstrated from the use in combination of standard therapy with polyphenols arising from plant extracts. In addition, the alternative pharmacological approach for DM management can manifest adverse effects, leading to an unmet need to develop novel and safe anti-diabetic drugs. Different approaches focused on improvement of β cell/pancreas activity were developed; thus crucial advantages are associated with transplantation. In detail, the major advantage of β cell transplantation is that it leads to normalized glucose levels. Transplantation patients become insulin-independent for long periods. At present, however some issues remain to be resolved including the major availability of β cells/islets from donors to allow transplantation in all patients. However, the possibility of supporting the differentiation of ESCs and iPSCs could represent an alternative in cases of organ shortage. All the engineering approaches associated with the use of these cells are promising for the development of active β cells without the simultaneous use of immunosuppressants. Thus, these hopeful new approaches together with the commitment of the scientific community will lead to further amelioration resulting in the improvement of diabetes management.
| 1. | Ikle JM, Gloyn AL. 100 YEARS OF INSULIN: A brief history of diabetes genetics: insights for pancreatic beta-cell development and function. J Endocrinol. 2021;250:R23-R35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32 Suppl 1:S62-S67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1139] [Cited by in RCA: 1282] [Article Influence: 80.1] [Reference Citation Analysis (1)] |
| 3. | Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 945] [Article Influence: 189.0] [Reference Citation Analysis (0)] |
| 4. | Bryder L, Harper C. Commentary: more than 'tentative opinions': Harry Himsworth and defining diabetes. Int J Epidemiol. 2013;42:1599-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Tattersall RB, Fajans SS. A difference between the inheritance of classical juvenile-onset and maturity-onset type diabetes of young people. Diabetes. 1975;24:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 209] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5:1809-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 281] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Eizirik DL, Szymczak F, Mallone R. Why does the immune system destroy pancreatic β-cells but not α-cells in type 1 diabetes? Nat Rev Endocrinol. 2023;19:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Akil AA, Yassin E, Al-Maraghi A, Aliyev E, Al-Malki K, Fakhro KA. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J Transl Med. 2021;19:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Tinajero MG, Malik VS. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol Metab Clin North Am. 2021;50:337-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 226] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 10. | Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 11. | Montane J, Cadavez L, Novials A. Stress and the inflammatory process: a major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Collier JJ, Sparer TE, Karlstad MD, Burke SJ. Pancreatic islet inflammation: an emerging role for chemokines. J Mol Endocrinol. 2017;59:R33-R46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Cernea S, Dobreanu M. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med (Zagreb). 2013;23:266-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Francis EC, Powe CE, Lowe WL Jr, White SL, Scholtens DM, Yang J, Zhu Y, Zhang C, Hivert MF, Kwak SH, Sweeting A; ADA/EASD PMDI. Refining the diagnosis of gestational diabetes mellitus: a systematic review and meta-analysis. Commun Med (Lond). 2023;3:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 15. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4386] [Article Influence: 626.6] [Reference Citation Analysis (0)] |
| 16. | Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon). 2014;42:698-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 17. | Faienza MF, Pontrelli P, Brunetti G. Type 2 diabetes and bone fragility in children and adults. World J Diabetes. 2022;13:900-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (61)] |
| 18. | Urbano F, Farella I, Brunetti G, Faienza MF. Pediatric Type 1 Diabetes: Mechanisms and Impact of Technologies on Comorbidities and Life Expectancy. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Brunetti G, D'Amato G, De Santis S, Grano M, Faienza MF. Mechanisms of altered bone remodeling in children with type 1 diabetes. World J Diabetes. 2021;12:997-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Yki-Järvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med. 1986;315:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 222] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is 'double diabetes' and what are the risks? Diabetologia. 2013;56:1462-1470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Müller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJ, Vedantam S, Chen H, Florez JC, Fox C, Liu CT, Rybin D, Couper DJ, Kao WH, Li M, Cornelis MC, Kraft P, Sun Q, van Dam RM, Stringham HM, Chines PS, Fischer K, Fontanillas P, Holmen OL, Hunt SE, Jackson AU, Kong A, Lawrence R, Meyer J, Perry JR, Platou CG, Potter S, Rehnberg E, Robertson N, Sivapalaratnam S, Stančáková A, Stirrups K, Thorleifsson G, Tikkanen E, Wood AR, Almgren P, Atalay M, Benediktsson R, Bonnycastle LL, Burtt N, Carey J, Charpentier G, Crenshaw AT, Doney AS, Dorkhan M, Edkins S, Emilsson V, Eury E, Forsen T, Gertow K, Gigante B, Grant GB, Groves CJ, Guiducci C, Herder C, Hreidarsson AB, Hui J, James A, Jonsson A, Rathmann W, Klopp N, Kravic J, Krjutškov K, Langford C, Leander K, Lindholm E, Lobbens S, Männistö S, Mirza G, Mühleisen TW, Musk B, Parkin M, Rallidis L, Saramies J, Sennblad B, Shah S, Sigurðsson G, Silveira A, Steinbach G, Thorand B, Trakalo J, Veglia F, Wennauer R, Winckler W, Zabaneh D, Campbell H, van Duijn C, Uitterlinden AG, Hofman A, Sijbrands E, Abecasis GR, Owen KR, Zeggini E, Trip MD, Forouhi NG, Syvänen AC, Eriksson JG, Peltonen L, Nöthen MM, Balkau B, Palmer CN, Lyssenko V, Tuomi T, Isomaa B, Hunter DJ, Qi L; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium, Shuldiner AR, Roden M, Barroso I, Wilsgaard T, Beilby J, Hovingh K, Price JF, Wilson JF, Rauramaa R, Lakka TA, Lind L, Dedoussis G, Njølstad I, Pedersen NL, Khaw KT, Wareham NJ, Keinanen-Kiukaanniemi SM, Saaristo TE, Korpi-Hyövälti E, Saltevo J, Laakso M, Kuusisto J, Metspalu A, Collins FS, Mohlke KL, Bergman RN, Tuomilehto J, Boehm BO, Gieger C, Hveem K, Cauchi S, Froguel P, Baldassarre D, Tremoli E, Humphries SE, Saleheen D, Danesh J, Ingelsson E, Ripatti S, Salomaa V, Erbel R, Jöckel KH, Moebus S, Peters A, Illig T, de Faire U, Hamsten A, Morris AD, Donnelly PJ, Frayling TM, Hattersley AT, Boerwinkle E, Melander O, Kathiresan S, Nilsson PM, Deloukas P, Thorsteinsdottir U, Groop LC, Stefansson K, Hu F, Pankow JS, Dupuis J, Meigs JB, Altshuler D, Boehnke M, McCarthy MI; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1703] [Cited by in RCA: 1477] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 23. | Gaulton KJ, Ferreira T, Lee Y, Raimondo A, Mägi R, Reschen ME, Mahajan A, Locke A, Rayner NW, Robertson N, Scott RA, Prokopenko I, Scott LJ, Green T, Sparso T, Thuillier D, Yengo L, Grallert H, Wahl S, Frånberg M, Strawbridge RJ, Kestler H, Chheda H, Eisele L, Gustafsson S, Steinthorsdottir V, Thorleifsson G, Qi L, Karssen LC, van Leeuwen EM, Willems SM, Li M, Chen H, Fuchsberger C, Kwan P, Ma C, Linderman M, Lu Y, Thomsen SK, Rundle JK, Beer NL, van de Bunt M, Chalisey A, Kang HM, Voight BF, Abecasis GR, Almgren P, Baldassarre D, Balkau B, Benediktsson R, Blüher M, Boeing H, Bonnycastle LL, Bottinger EP, Burtt NP, Carey J, Charpentier G, Chines PS, Cornelis MC, Couper DJ, Crenshaw AT, van Dam RM, Doney AS, Dorkhan M, Edkins S, Eriksson JG, Esko T, Eury E, Fadista J, Flannick J, Fontanillas P, Fox C, Franks PW, Gertow K, Gieger C, Gigante B, Gottesman O, Grant GB, Grarup N, Groves CJ, Hassinen M, Have CT, Herder C, Holmen OL, Hreidarsson AB, Humphries SE, Hunter DJ, Jackson AU, Jonsson A, Jørgensen ME, Jørgensen T, Kao WH, Kerrison ND, Kinnunen L, Klopp N, Kong A, Kovacs P, Kraft P, Kravic J, Langford C, Leander K, Liang L, Lichtner P, Lindgren CM, Lindholm E, Linneberg A, Liu CT, Lobbens S, Luan J, Lyssenko V, Männistö S, McLeod O, Meyer J, Mihailov E, Mirza G, Mühleisen TW, Müller-Nurasyid M, Navarro C, Nöthen MM, Oskolkov NN, Owen KR, Palli D, Pechlivanis S, Peltonen L, Perry JR, Platou CG, Roden M, Ruderfer D, Rybin D, van der Schouw YT, Sennblad B, Sigurðsson G, Stančáková A, Steinbach G, Storm P, Strauch K, Stringham HM, Sun Q, Thorand B, Tikkanen E, Tonjes A, Trakalo J, Tremoli E, Tuomi T, Wennauer R, Wiltshire S, Wood AR, Zeggini E, Dunham I, Birney E, Pasquali L, Ferrer J, Loos RJ, Dupuis J, Florez JC, Boerwinkle E, Pankow JS, van Duijn C, Sijbrands E, Meigs JB, Hu FB, Thorsteinsdottir U, Stefansson K, Lakka TA, Rauramaa R, Stumvoll M, Pedersen NL, Lind L, Keinanen-Kiukaanniemi SM, Korpi-Hyövälti E, Saaristo TE, Saltevo J, Kuusisto J, Laakso M, Metspalu A, Erbel R, Jöcke KH, Moebus S, Ripatti S, Salomaa V, Ingelsson E, Boehm BO, Bergman RN, Collins FS, Mohlke KL, Koistinen H, Tuomilehto J, Hveem K, Njølstad I, Deloukas P, Donnelly PJ, Frayling TM, Hattersley AT, de Faire U, Hamsten A, Illig T, Peters A, Cauchi S, Sladek R, Froguel P, Hansen T, Pedersen O, Morris AD, Palmer CN, Kathiresan S, Melander O, Nilsson PM, Groop LC, Barroso I, Langenberg C, Wareham NJ, O'Callaghan CA, Gloyn AL, Altshuler D, Boehnke M, Teslovich TM, McCarthy MI, Morris AP; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47:1415-1425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 311] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 24. | Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes. 2017;66:241-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 25. | Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in Type 1 Diabetes in the DCCT/EDIC Versus the General Population. Diabetes Care. 2016;39:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 26. | Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3867] [Cited by in RCA: 3436] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 27. | Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, Zinman B, Jacobson A, Sun W, Lachin JM, Nathan DM; DCCT/EDIC Research Group. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55:3556-3565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S140-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 536] [Article Influence: 268.0] [Reference Citation Analysis (1)] |
| 29. | Pickup JC. The evidence base for diabetes technology: appropriate and inappropriate meta-analysis. J Diabetes Sci Technol. 2013;7:1567-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, Ahmann AJ, Welsh JB, Lee SW, Kaufman FR; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 445] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 31. | Buckingham BA, Raghinaru D, Cameron F, Bequette BW, Chase HP, Maahs DM, Slover R, Wadwa RP, Wilson DM, Ly T, Aye T, Hramiak I, Clarson C, Stein R, Gallego PH, Lum J, Sibayan J, Kollman C, Beck RW; In Home Closed Loop Study Group. Predictive Low-Glucose Insulin Suspension Reduces Duration of Nocturnal Hypoglycemia in Children Without Increasing Ketosis. Diabetes Care. 2015;38:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, Kaufman FR. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA. 2016;316:1407-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 460] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 33. | Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, Brazg RL, Ilany J, Slover RH, Anderson SM, Bergenstal RM, Grosman B, Roy A, Cordero TL, Shin J, Lee SW, Kaufman FR. Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther. 2017;19:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 434] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 34. | Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, Ruan Y, Sibayan J, Kollman C, Cheng P, Beck RW, Acerini CL, Evans ML, Dunger DB, Elleri D, Campbell F, Bergenstal RM, Criego A, Shah VN, Leelarathna L, Hovorka R; APCam11 Consortium. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 306] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 35. | Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, Laffel LM, Levy CJ, Pinsker JE, Wadwa RP, Dassau E, Doyle FJ 3rd, Anderson SM, Church MM, Dadlani V, Ekhlaspour L, Forlenza GP, Isganaitis E, Lam DW, Kollman C, Beck RW; iDCL Trial Research Group. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med. 2019;381:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 675] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 36. | Whitehouse F, Kruger DF, Fineman M, Shen L, Ruggles JA, Maggs DG, Weyer C, Kolterman OG. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Ratner RE, Want LL, Fineman MS, Velte MJ, Ruggles JA, Gottlieb A, Weyer C, Kolterman OG. Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with type 2 diabetes. Diabetes Technol Ther. 2002;4:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Hollander PA, Levy P, Fineman MS, Maggs DG, Shen LZ, Strobel SA, Weyer C, Kolterman OG. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, Weyer C, Kolterman OG. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med. 2004;21:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 40. | Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, Linder M, Bode B; ADJUNCT ONE Investigators. Efficacy and Safety of Liraglutide Added to Insulin Treatment in Type 1 Diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care. 2016;39:1702-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 41. | Ahrén B, Hirsch IB, Pieber TR, Mathieu C, Gómez-Peralta F, Hansen TK, Philotheou A, Birch S, Christiansen E, Jensen TJ, Buse JB; ADJUNCT TWO Investigators. Efficacy and Safety of Liraglutide Added to Capped Insulin Treatment in Subjects With Type 1 Diabetes: The ADJUNCT TWO Randomized Trial. Diabetes Care. 2016;39:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 42. | Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, Zinman B, Skyler JS, George J, Soleymanlou N, Perkins BA. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care. 2018;41:2560-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 43. | Snaith JR, Holmes-Walker DJ, Greenfield JR. Reducing Type 1 Diabetes Mortality: Role for Adjunctive Therapies? Trends Endocrinol Metab. 2020;31:150-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschöpe D, Thorén F, Xu J, Langkilde AM; DEPICT-1 Investigators. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:864-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 45. | Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, Mitri J, Pereira RF, Rawlings K, Robinson S, Saslow L, Uelmen S, Urbanski PB, Yancy WS Jr. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 708] [Cited by in RCA: 758] [Article Influence: 126.3] [Reference Citation Analysis (0)] |
| 46. | Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1622] [Cited by in RCA: 1821] [Article Influence: 260.1] [Reference Citation Analysis (0)] |
| 47. | Franz MJ, MacLeod J, Evert A, Brown C, Gradwell E, Handu D, Reppert A, Robinson M. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Systematic Review of Evidence for Medical Nutrition Therapy Effectiveness and Recommendations for Integration into the Nutrition Care Process. J Acad Nutr Diet. 2017;117:1659-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 48. | Handu D, Piotrowski M. Nutrition Interventions in Pediatric Patients with Type 1 Diabetes: An Evidence Analysis Center Scoping Review. J Acad Nutr Diet. 2022;122:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;139:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 50. | van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. Am J Clin Nutr. 2018;108:300-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 51. | Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5:e000354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 52. | Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 898] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 53. | Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 460] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 54. | Duncan GE, Perri MG, Theriaque DW, Hutson AD, Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003;26:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 55. | Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115:1447-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 424] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 56. | Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L; Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 1248] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 57. | Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Stefanetti R, Trenell M, Welsh P, Kean S, Ford I, McConnachie A, Sattar N, Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1234] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 58. | Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 946] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 59. | Hamdy O, Mottalib A, Morsi A, El-Sayed N, Goebel-Fabbri A, Arathuzik G, Shahar J, Kirpitch A, Zrebiec J. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 5-year longitudinal study. BMJ Open Diabetes Res Care. 2017;5:e000259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 60. | Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, Martínez-González MA; PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378:e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2350] [Cited by in RCA: 2061] [Article Influence: 294.4] [Reference Citation Analysis (1)] |
| 61. | Mottalib A, Salsberg V, Mohd-Yusof BN, Mohamed W, Carolan P, Pober DM, Mitri J, Hamdy O. Effects of nutrition therapy on HbA1c and cardiovascular disease risk factors in overweight and obese patients with type 2 diabetes. Nutr J. 2018;17:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 62. | Yancy WS Jr, Crowley MJ, Dar MS, Coffman CJ, Jeffreys AS, Maciejewski ML, Voils CI, Bradley AB, Edelman D. Comparison of Group Medical Visits Combined With Intensive Weight Management vs Group Medical Visits Alone for Glycemia in Patients With Type 2 Diabetes: A Noninferiority Randomized Clinical Trial. JAMA Intern Med. 2020;180:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Saslow LR, Daubenmier JJ, Moskowitz JT, Kim S, Murphy EJ, Phinney SD, Ploutz-Snyder R, Goldman V, Cox RM, Mason AE, Moran P, Hecht FM. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr Diabetes. 2017;7:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 64. | Suh SH, Paik IY, Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol Cells. 2007;23:272-279. [PubMed] |
| 65. | Zierath JR, He L, Gumà A, Odegoard Wahlström E, Klip A, Wallberg-Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39:1180-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 267] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 66. | Heiskanen MA, Motiani KK, Mari A, Saunavaara V, Eskelinen JJ, Virtanen KA, Koivumäki M, Löyttyniemi E, Nuutila P, Kalliokoski KK, Hannukainen JC. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: a randomised controlled trial. Diabetologia. 2018;61:1817-1828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 67. | Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 68. | Naylor LH, Davis EA, Kalic RJ, Paramalingam N, Abraham MB, Jones TW, Green DJ. Exercise training improves vascular function in adolescents with type 2 diabetes. Physiol Rep. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Magalhães JP, Melo X, Correia IR, Ribeiro RT, Raposo J, Dores H, Bicho M, Sardinha LB. Effects of combined training with different intensities on vascular health in patients with type 2 diabetes: a 1-year randomized controlled trial. Cardiovasc Diabetol. 2019;18:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 70. | Motiani KK, Collado MC, Eskelinen JJ, Virtanen KA, Löyttyniemi E, Salminen S, Nuutila P, Kalliokoski KK, Hannukainen JC. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med Sci Sports Exerc. 2020;52:94-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 71. | Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, Kirwan JP, Zierath JR. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2022;54:353-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 381] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 72. | Bazargan-Hejazi S, Arroyo JS, Hsia S, Brojeni NR, Pan D. A Racial Comparison of Differences between Self-Reported and Objectively Measured Physical Activity among US Adults with Diabetes. Ethn Dis. 2017;27:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1625] [Article Influence: 180.6] [Reference Citation Analysis (1)] |
| 74. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Young-Hyman D, Gabbay RA; on behalf of the American Diabetes Association. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S68-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 210] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 75. | Peters A, Laffel L, Chiang JL. American Diabetes Association/JDRF Type 1 Diabetes Sourcebook. 1st ed. American Diabetes Association, 2013. |
| 76. | Sluik D, Buijsse B, Muckelbauer R, Kaaks R, Teucher B, Johnsen NF, Tjønneland A, Overvad K, Ostergaard JN, Amiano P, Ardanaz E, Bendinelli B, Pala V, Tumino R, Ricceri F, Mattiello A, Spijkerman AM, Monninkhof EM, May AM, Franks PW, Nilsson PM, Wennberg P, Rolandsson O, Fagherazzi G, Boutron-Ruault MC, Clavel-Chapelon F, Castaño JM, Gallo V, Boeing H, Nöthlings U. Physical Activity and Mortality in Individuals With Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch Intern Med. 2012;172:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 77. | Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714-1721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 78. | Umpierre D, Ribeiro PA, Kramer CK, Leitão CB, Zucatti AT, Azevedo MJ, Gross JL, Ribeiro JP, Schaan BD. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 865] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 79. | Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1201] [Cited by in RCA: 1148] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 80. | Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006;29:2518-2527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 513] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 81. | Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 82. | Borror A, Zieff G, Battaglini C, Stoner L. The Effects of Postprandial Exercise on Glucose Control in Individuals with Type 2 Diabetes: A Systematic Review. Sports Med. 2018;48:1479-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 83. | Ostman C, Jewiss D, King N, Smart NA. Clinical outcomes to exercise training in type 1 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;139:380-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | Liu Y, Ye W, Chen Q, Zhang Y, Kuo CH, Korivi M. Resistance Exercise Intensity is Correlated with Attenuation of HbA1c and Insulin in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 85. | Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253-2262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 647] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 86. | Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2341] [Cited by in RCA: 2502] [Article Influence: 166.8] [Reference Citation Analysis (0)] |
| 87. | Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, Kowalski A, Rabasa-Lhoret R, McCrimmon RJ, Hume C, Annan F, Fournier PA, Graham C, Bode B, Galassetti P, Jones TW, Millán IS, Heise T, Peters AL, Petz A, Laffel LM. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 552] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 88. | Adolfsson P, Riddell MC, Taplin CE, Davis EA, Fournier PA, Annan F, Scaramuzza AE, Hasnani D, Hofer SE. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr Diabetes. 2018;19 Suppl 27:205-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 89. | Anderson BJ, Laffel LM, Domenger C, Danne T, Phillip M, Mazza C, Hanas R, Waldron S, Beck RW, Calvi-Gries F, Mathieu C. Factors Associated With Diabetes-Specific Health-Related Quality of Life in Youth With Type 1 Diabetes: The Global TEENs Study. Diabetes Care. 2017;40:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 90. | Muzammil S, Wang Y, Siddique MH, Zubair E, Hayat S, Zubair M, Roy A, Mumtaz R, Azeem M, Emran TB, Shahid MQ. Polyphenolic Composition, Antioxidant, Antiproliferative and Antidiabetic Activities of Coronopus didymus Leaf Extracts. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Reference Citation Analysis (0)] |
| 91. | Shah MS, Talukder MSH, Uddin AMK, Hasan MN, Sayem SAJ, Mostafa-Hedeab G, Rahman MM, Sharma R, Swelum AA, Mohamed AA, Emran TB. Comparative Assessment of Three Medicinal Plants against Diabetes and Oxidative Stress Using Experimental and Computational Approaches. Evid Based Complement Alternat Med. 2023;2023:6359818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Prasathkumar M, Anisha S, Khusro A, Essa MM, Chidambaram SB, Qoronfleh MW, Sadhasivam S, Khayam Sahibzada MU, Alghamdi S, Almehmadi M, Abdulaziz O, Khandaker MU, Iqbal Faruque MR, Emran TB. Anti-pathogenic, anti-diabetic, anti-inflammatory, antioxidant, and wound healing efficacy of Datura metel L. leaves. 2022. [cited 5 March 2024]. Avaliable from: https://www.semanticscholar.org/paper/Anti-pathogenic%2C-anti-diabetic%2C-anti-inflammatory%2C-Prasathkumar-Anisha/35fa0226712099484a7d1ad661fe2648534f0b4f. |
| 93. | Qazi AI, Ahmad B, Sahibzada MUK, Anwar F, Khusro A, Alhumaydhi FA, Mohamed AA, Mostafa-Hedeab G, Emran TB. Evaluation of Antidiabetic Activity of Oxadiazole Derivative in Rats. Evid Based Complement Alternat Med. 2023;2023:1141554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Emran TB, Dutta M, Uddin MMN, Nath AK, Uddin MZ. Antidiabetic potential of the leaf extract of Centella asiatica in alloxan induced diabetic rats. Jahangirnagar University J Bio Sci. 2015;4:51-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Mitra S, Islam F, Das R, Urmee H, Akter A, Idris AM, Khandaker MU, Almikhlafi MA, Sharma R, Emran TB. Pharmacological Potential of Avicennia alba Leaf Extract: An Experimental Analysis Focusing on Antidiabetic, Anti-inflammatory, Analgesic, and Antidiarrheal Activity. Biomed Res Int. 2022;2022:7624189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 96. | Islam F, Khadija JF, Islam MR, Shohag S, Mitra S, Alghamdi S, Babalghith AO, Theyab A, Rahman MT, Akter A, Al Mamun A, Alhumaydhi FA, Emran TB. Investigating Polyphenol Nanoformulations for Therapeutic Targets against Diabetes Mellitus. Evid Based Complement Alternat Med. 2022;2022:5649156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 97. | Emran TB, Haque MA, Guntaka PR, Pratap L. A Renewed Concept on Diabetic Retinopathy: Polyphenols as a Choice of Solution. Biointerface Res Appl Chem. 2023;13:588. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 98. | D'Addio F, Maestroni A, Assi E, Ben Nasr M, Amabile G, Usuelli V, Loretelli C, Bertuzzi F, Antonioli B, Cardarelli F, El Essawy B, Solini A, Gerling IC, Bianchi C, Becchi G, Mazzucchelli S, Corradi D, Fadini GP, Foschi D, Markmann JF, Orsi E, Škrha J Jr, Camboni MG, Abdi R, James Shapiro AM, Folli F, Ludvigsson J, Del Prato S, Zuccotti G, Fiorina P. The IGFBP3/TMEM219 pathway regulates beta cell homeostasis. Nat Commun. 2022;13:684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 99. | Pan H, Lu HM, Hu WM, Tian BL, Liu XB, Zhang ZD, Mai G. Anti-CD25 mAb, anti-IL2 mAb, and IL2 block tolerance induction through anti-CD154 mAb and rapamycin in xenogeneic islet transplantation. Transplant Proc. 2007;39:3452-3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Tuo Y, Xiang M. mTOR: A double-edged sword for diabetes. J Leukoc Biol. 2019;106:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Bolla AM, Gandolfi A, Borgonovo E, Laurenzi A, Caretto A, Molinari C, Catalano RS, Bianconi E, Monti P, Sordi V, Pellegrini S, Lampasona V, Costa S, Scavini M, Bosi E, Piemonti L. Rapamycin Plus Vildagliptin to Recover β-Cell Function in Long-Standing Type 1 Diabetes: A Double-Blind, Randomized Trial. J Clin Endocrinol Metab. 2021;106:e507-e519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, Green T, Rickels MR, Tomaszewski JE, Koeberlein B, Wang Z, Paessler ME, Velidedeoglu E, Rostami SY, Yu M, Barker CF, Naji A. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13:1295-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 103. | Vantyghem MC, de Koning EJP, Pattou F, Rickels MR. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet. 2019;394:1274-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 104. | Holt RIG, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, Klupa T, Ludwig B, Nørgaard K, Pettus J, Renard E, Skyler JS, Snoek FJ, Weinstock RS, Peters AL. The Management of Type 1 Diabetes in Adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2021;44:2589-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 310] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 105. | Bolla AM, Montefusco L, Pastore I, Lunati ME, Ben Nasr M, Fiorina P. Benefits and Hurdles of Pancreatic β-Cell Replacement. Stem Cells Transl Med. 2022;11:1029-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 106. | Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, Clarke WR, Eggerman TL, Hunsicker LG, Kaufman DB, Khan A, Lafontant DE, Linetsky E, Luo X, Markmann JF, Naji A, Korsgren O, Oberholzer J, Turgeon NA, Brandhorst D, Chen X, Friberg AS, Lei J, Wang LJ, Wilhelm JJ, Willits J, Zhang X, Hering BJ, Posselt AM, Stock PG, Shapiro AM. National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities. Diabetes. 2016;65:3418-3428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 107. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3835] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 108. | Turgeon NA, Avila JG, Cano JA, Hutchinson JJ, Badell IR, Page AJ, Adams AB, Sears MH, Bowen PH, Kirk AD, Pearson TC, Larsen CP. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant. 2010;10:2082-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Brooks AM, Walker N, Aldibbiat A, Hughes S, Jones G, de Havilland J, Choudhary P, Huang GC, Parrott N, McGowan NW, Casey J, Mumford L, Barker P, Burling K, Hovorka R, Walker M, Smith RM, Forbes S, Rutter MK, Amiel S, Rosenthal MJ, Johnson P, Shaw JA. Attainment of metabolic goals in the integrated UK islet transplant program with locally isolated and transported preparations. Am J Transplant. 2013;13:3236-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Nijhoff MF, Engelse MA, Dubbeld J, Braat AE, Ringers J, Roelen DL, van Erkel AR, Spijker HS, Bouwsma H, van der Boog PJ, de Fijter JW, Rabelink TJ, de Koning EJ. Glycemic Stability Through Islet-After-Kidney Transplantation Using an Alemtuzumab-Based Induction Regimen and Long-Term Triple-Maintenance Immunosuppression. Am J Transplant. 2016;16:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, Gitelman SE, Greenbaum CJ, Gottlieb PA, Hagopian W, Woodwyk A, Dziura J, Herold KC; Immune Tolerance Network. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia. 2019;62:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 112. | Takita M, Matsumoto S, Shimoda M, Chujo D, Itoh T, Sorelle JA, Purcell K, Onaca N, Naziruddin B, Levy MF. Safety and tolerability of the T-cell depletion protocol coupled with anakinra and etanercept for clinical islet cell transplantation. Clin Transplant. 2012;26:E471-E484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA; Clinical Islet Transplantation Consortium. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care. 2016;39:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 453] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 114. | Foster ED, Bridges ND, Feurer ID, Eggerman TL, Hunsicker LG, Alejandro R; Clinical Islet Transplantation Consortium. Improved Health-Related Quality of Life in a Phase 3 Islet Transplantation Trial in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care. 2018;41:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 115. | Chetboun M, Drumez E, Ballou C, Maanaoui M, Payne E, Barton F, Kerr-Conte J, Vantyghem MC, Piemonti L, Rickels MR, Labreuche J, Pattou F; Collaborative Islet Transplant Registry (CITR) Investigators study group. Association between primary graft function and 5-year outcomes of islet allogeneic transplantation in type 1 diabetes: a retrospective, multicentre, observational cohort study in 1210 patients from the Collaborative Islet Transplant Registry. Lancet Diabetes Endocrinol. 2023;11:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 116. | Damyar K, Farahmand V, Whaley D, Alexander M, Lakey JRT. An overview of current advancements in pancreatic islet transplantation into the omentum. Islets. 2021;13:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 117. | Baidal DA, Ricordi C, Berman DM, Alvarez A, Padilla N, Ciancio G, Linetsky E, Pileggi A, Alejandro R. Bioengineering of an Intraabdominal Endocrine Pancreas. N Engl J Med. 2017;376:1887-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 118. | Dalle S, Abderrahmani A, Renard E. Pharmacological inhibitors of β-cell dysfunction and death as therapeutics for diabetes. Front Endocrinol (Lausanne). 2023;14:1076343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 119. | Jayasinghe M, Prathiraja O, Perera PB, Jena R, Silva MS, Weerawarna PSH, Singhal M, Kayani AMA, Karnakoti S, Jain S. The Role of Mesenchymal Stem Cells in the Treatment of Type 1 Diabetes. Cureus. 2022;14:e27337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 120. | Yan LL, Ye LP, Chen YH, He SQ, Zhang CY, Mao XL, Li SW. The Influence of Microenvironment on Survival of Intraportal Transplanted Islets. Front Immunol. 2022;13:849580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 121. | Llacua LA, Faas MM, de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 2018;61:1261-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 122. | Chen C, Rong P, Yang M, Ma X, Feng Z, Wang W. The Role of Interleukin-1β in Destruction of Transplanted Islets. Cell Transplant. 2020;29:963689720934413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Marfil-Garza BA, Imes S, Verhoeff K, Hefler J, Lam A, Dajani K, Anderson B, O'Gorman D, Kin T, Bigam D, Senior PA, Shapiro AMJ. Pancreatic islet transplantation in type 1 diabetes: 20-year experience from a single-centre cohort in Canada. Lancet Diabetes Endocrinol. 2022;10:519-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (33)] |
| 124. | Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 489] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 125. | Tavakoli S, Ghaderi Jafarbeigloo HR, Shariati A, Jahangiryan A, Jadidi F, Jadidi Kouhbanani MA, Hassanzadeh A, Zamani M, Javidi K, Naimi A. Mesenchymal stromal cells; a new horizon in regenerative medicine. J Cell Physiol. 2020;235:9185-9210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 126. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12686] [Article Influence: 704.8] [Reference Citation Analysis (2)] |
| 127. | Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 746] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 128. | Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1088] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 129. | Liew CG, Moore H, Ruban L, Shah N, Cosgrove K, Dunne M, Andrews P. Human embryonic stem cells: possibilities for human cell transplantation. Ann Med. 2005;37:521-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 130. | Marofi F, Vahedi G, Hasanzadeh A, Salarinasab S, Arzhanga P, Khademi B, Farshdousti Hagh M. Mesenchymal stem cells as the game-changing tools in the treatment of various organs disorders: Mirage or reality? J Cell Physiol. 2019;234:1268-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 131. | Kraskiewicz H, Paprocka M, Bielawska-Pohl A, Krawczenko A, Panek K, Kaczyńska J, Szyposzyńska A, Psurski M, Kuropka P, Klimczak A. Can supernatant from immortalized adipose tissue MSC replace cell therapy? An in vitro study in chronic wounds model. Stem Cell Res Ther. 2020;11:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 132. | Tsutsui TW. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning. 2020;13:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 133. | Zhu X, Péault B, Yan G, Sun H, Hu Y, Ding L. Stem Cells and Endometrial Regeneration: From Basic Research to Clinical Trial. Curr Stem Cell Res Ther. 2019;14:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 134. | Jain A, Khadwal A, Sachdeva MUS, Bose P, Lad D, Bhattacharya S, Prakash G, Malhotra P, Varma N, Varma S. Variables affecting the presence of mesenchymal stromal cells in peripheral blood and their relationship with apheresis products. Br J Haematol. 2020;189:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 135. | Hartwig V, Dewidar B, Lin T, Dropmann A, Ganss C, Kluth MA, Tappenbeck N, Tietze L, Christ B, Frank M, Vogelmann R, Ebert MPA, Dooley S. Human skin-derived ABCB5(+) stem cell injection improves liver disease parameters in Mdr2KO mice. Arch Toxicol. 2019;93:2645-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Silini AR, Cancelli S, Signoroni PB, Cargnoni A, Magatti M, Parolini O. The dichotomy of placenta-derived cells in cancer growth. Placenta. 2017;59:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Yang D, Hou X, Qian K, Li Y, Hu L, Li L, Han M, Yao C, Liu D. Efficacy and safety of human umbilical cord-derived mesenchymal stem cells (hUC-MSC PLEB001) for the treatment of grade II-IV steroid-refractory acute graft-versus-host disease: a study protocol for a multicenter, randomized, double-blind, placebo-controlled, phase II trial. Trials. 2023;24:306. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 138. | Baboolal TG, Mastbergen SC, Jones E, Calder SJ, Lafeber FP, McGonagle D. Synovial fluid hyaluronan mediates MSC attachment to cartilage, a potential novel mechanism contributing to cartilage repair in osteoarthritis using knee joint distraction. Ann Rheum Dis. 2016;75:908-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 139. | Tan L, Liu X, Dou H, Hou Y. Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment - specific factors involved in the regulation of MSC plasticity. Genes Dis. 2022;9:296-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 140. | McCall MD, Toso C, Baetge EE, Shapiro AM. Are stem cells a cure for diabetes? Clin Sci (Lond). 2009;118:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 141. | Hubber EL, Rackham CL, Jones PM. Protecting islet functional viability using mesenchymal stromal cells. Stem Cells Transl Med. 2021;10:674-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 142. | Wang H, Strange C, Nietert PJ, Wang J, Turnbull TL, Cloud C, Owczarski S, Shuford B, Duke T, Gilkeson G, Luttrell L, Hermayer K, Fernandes J, Adams DB, Morgan KA. Autologous Mesenchymal Stem Cell and Islet Cotransplantation: Safety and Efficacy. Stem Cells Transl Med. 2018;7:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 143. | D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1447] [Cited by in RCA: 1413] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 144. | Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1304] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 145. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1526] [Article Influence: 152.6] [Reference Citation Analysis (1)] |
| 146. | Loretelli C, Assi E, Seelam AJ, Ben Nasr M, Fiorina P. Cell therapy for type 1 diabetes. Expert Opin Biol Ther. 2020;20:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 147. | Butler PC, Gale EA. Reversing type 1 diabetes with stem cell-derived islets: a step closer to the dream? J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 148. | Shapiro AMJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J, Wilensky J, Daniels M, Wang RM, Brandon EP, Jaiman MS, Kroon EJ, D'Amour KA, Foyt HL. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2:100466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 149. | Zhang Q, Gonelle-Gispert C, Li Y, Geng Z, Gerber-Lemaire S, Wang Y, Buhler L. Islet Encapsulation: New Developments for the Treatment of Type 1 Diabetes. Front Immunol. 2022;13:869984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 150. | Kogawa R, Nakamura K, Mochizuki Y. A New Islet Transplantation Method Combining Mesenchymal Stem Cells with Recombinant Peptide Pieces, Microencapsulated Islets, and Mesh Bags. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 151. | Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JH, Harb G, Poh YC, Sintov E, Gürtler M, Pagliuca FW, Peterson QP, Melton DA. Charting cellular identity during human in vitro β-cell differentiation. Nature. 2019;569:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 370] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 152. | Kondo Y, Toyoda T, Inagaki N, Osafune K. iPSC technology-based regenerative therapy for diabetes. J Diabetes Investig. 2018;9:234-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 153. | Hogrebe NJ, Maxwell KG, Augsornworawat P, Millman JR. Generation of insulin-producing pancreatic β cells from multiple human stem cell lines. Nat Protoc. 2021;16:4109-4143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 154. | Du Y, Liang Z, Wang S, Sun D, Wang X, Liew SY, Lu S, Wu S, Jiang Y, Wang Y, Zhang B, Yu W, Lu Z, Pu Y, Zhang Y, Long H, Xiao S, Liang R, Zhang Z, Guan J, Wang J, Ren H, Wei Y, Zhao J, Sun S, Liu T, Meng G, Wang L, Gu J, Wang T, Liu Y, Li C, Tang C, Shen Z, Peng X, Deng H. Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat Med. 2022;28:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (1)] |
| 155. | Han L, He H, Yang Y, Meng Q, Ye F, Chen G, Zhang J. Distinctive Clinical and Pathologic Features of Immature Teratomas Arising from Induced Pluripotent Stem Cell-Derived Beta Cell Injection in a Diabetes Patient. Stem Cells Dev. 2022;31:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 156. | Scuteri A, Donzelli E, Rodriguez-Menendez V, Ravasi M, Monfrini M, Bonandrini B, Figliuzzi M, Remuzzi A, Tredici G. A double mechanism for the mesenchymal stem cells' positive effect on pancreatic islets. PLoS One. 2014;9:e84309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 157. | Yang G, Fan X, Liu Y, Jie P, Mazhar M, Dechsupa N, Wang L. Immunomodulatory Mechanisms and Therapeutic Potential of Mesenchymal Stem Cells. Stem Cell Rev Rep. 2023;19:1214-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 158. | Műzes G, Sipos F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 159. | Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 160. | Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, Park JW, Kim TW, An SY, Prockop DJ, Lee RH. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Reports. 2017;8:1214-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 161. | Brandhorst H, Brandhorst D, Abraham A, Acreman S, Schive SW, Scholz H, Johnson PRV. Proteomic Profiling Reveals the Ambivalent Character of the Mesenchymal Stem Cell Secretome: Assessing the Effect of Preconditioned Media on Isolated Human Islets. Cell Transplant. 2020;29:963689720952332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 162. | Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 163. | Mondal J, Pillarisetti S, Junnuthula V, Saha M, Hwang SR, Park IK, Lee YK. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J Control Release. 2023;353:1127-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 168] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 164. | Zhang M, Hu S, Liu L, Dang P, Liu Y, Sun Z, Qiao B, Wang C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 217] [Reference Citation Analysis (0)] |
| 165. | Yao J, Chen Y, Lin Z. Exosomes: Mediators in microenvironment of colorectal cancer. Int J Cancer. 2023;153:904-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 166. | Ye H, Tan L, Tu C, Min L. Exosomes in sarcoma: Prospects for clinical applications. Crit Rev Oncol Hematol. 2023;181:103895. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 167. | Saravanan PB, Kalivarathan J, Khan F, Shah R, Levy MF, Kanak MA. Exosomes in transplantation: Role in allograft rejection, diagnostic biomarker, and therapeutic potential. Life Sci. 2023;324:121722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 168. | Mishra A, Bharti PS, Rani N, Nikolajeff F, Kumar S. A tale of exosomes and their implication in cancer. Biochim Biophys Acta Rev Cancer. 2023;1878:188908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 169. | Mani S, Gurusamy N, Ulaganathan T, Paluck AJ, Ramalingam S, Rajasingh J. Therapeutic potentials of stem cell-derived exosomes in cardiovascular diseases. Exp Biol Med (Maywood). 2023;248:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 170. | Malgundkar SH, Tamimi Y. Exosomes as crucial emerging tools for intercellular communication with therapeutic potential in ovarian cancer. Future Sci OA. 2023;9:FSO833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 171. | Wu Y, Li J, Zeng Y, Pu W, Mu X, Sun K, Peng Y, Shen B. Exosomes rewire the cartilage microenvironment in osteoarthritis: from intercellular communication to therapeutic strategies. Int J Oral Sci. 2022;14:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 172. | Bischoff JP, Schulz A, Morrison H. The role of exosomes in intercellular and inter-organ communication of the peripheral nervous system. FEBS Lett. 2022;596:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 173. | Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr Biol. 2018;28:R435-R444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 665] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 174. | Xu Z, Chen Y, Ma L, Liu J, Guo Y, Yu T, Zhang L, Zhu L, Shu Y. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol Ther. 2022;30:3133-3154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 199] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 175. | Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 176. | Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front Immunol. 2021;12:749192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 181] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 177. | Kant V, Gopal A, Kumar D, Pathak NN, Ram M, Jangir BL, Tandan SK. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015;193:978-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 178. | Yu M, Liu W, Li J, Lu J, Lu H, Jia W, Liu F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 179. | Tang T, Chen L, Zhang M, Wang C, Du X, Ye S, Li X, Chen H, Hu N. Exosomes derived from BMSCs enhance diabetic wound healing through circ-Snhg11 delivery. Diabetol Metab Syndr. 2024;16:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 180. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 862] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 181. | Al-Ani FS, Al-Nimer MS, Ali FS. Dyslipidemia as a contributory factor in etiopathogenesis of diabetic neuropathy. Indian J Endocrinol Metab. 2011;15:110-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 182. | Andersen ST, Witte DR, Andersen H, Bjerg L, Bruun NH, Jørgensen ME, Finnerup NB, Lauritzen T, Jensen TS, Tankisi H, Charles M. Risk-Factor Trajectories Preceding Diabetic Polyneuropathy: ADDITION-Denmark. Diabetes Care. 2018;41:1955-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 183. | Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 184. | Fan B, Li C, Szalad A, Wang L, Pan W, Zhang R, Chopp M, Zhang ZG, Liu XS. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63:431-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 185. | Fan B, Chopp M, Zhang ZG, Liu XS. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp Neurol. 2021;341:113694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 186. | Zhu LL, Huang X, Yu W, Chen H, Chen Y, Dai YT. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018;50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 187. | Ouyang X, Han X, Chen Z, Fang J, Huang X, Wei H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther. 2018;9:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 188. | Huo W, Li Y, Zhang Y, Li H. Mesenchymal stem cells-derived exosomal microRNA-21-5p downregulates PDCD4 and ameliorates erectile dysfunction in a rat model of diabetes mellitus. FASEB J. 2020;34:13345-13360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 189. | Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol. 2010;299:L861-L871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 190. | Ruan Q, Wang T, Kameswaran V, Wei Q, Johnson DS, Matschinsky F, Shi W, Chen YH. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc Natl Acad Sci USA. 2011;108:12030-12035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 191. | Kim MY, Jo MS, Choi SG, Moon HW, Park J, Lee JY. Repeated Injections of Mesenchymal Stem Cell-Derived Exosomes Ameliorate Erectile Dysfunction in a Cavernous Nerve Injury Rat Model. World J Mens Health. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 192. | Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 193. | Yu X, Liu P, Li Z, Zhang Z. Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front Endocrinol (Lausanne). 2023;14:1099310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 194. | Mieczkowski M, Mrozikiewicz-Rakowska B, Kowara M, Kleibert M, Czupryniak L. The Problem of Wound Healing in Diabetes-From Molecular Pathways to the Design of an Animal Model. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 195. | Dasari N, Jiang A, Skochdopole A, Chung J, Reece EM, Vorstenbosch J, Winocour S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin Plast Surg. 2021;35:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 196. | Liao X, Li SH, El Akkawi MM, Fu XB, Liu HW, Huang YS. Surgical amputation for patients with diabetic foot ulcers: A Chinese expert panel consensus treatment guide. Front Surg. 2022;9:1003339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 197. | Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 230] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 198. | Lopes L, Setia O, Aurshina A, Liu S, Hu H, Isaji T, Liu H, Wang T, Ono S, Guo X, Yatsula B, Guo J, Gu Y, Navarro T, Dardik A. Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res Ther. 2018;9:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 199. | Martin KE, Hunckler MD, Chee E, Caplin JD, Barber GF, Kalelkar PP, Schneider RS, García AJ. Hydrolytic hydrogels tune mesenchymal stem cell persistence and immunomodulation for enhanced diabetic cutaneous wound healing. Biomaterials. 2023;301:122256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 200. | Du S, Zeugolis DI, O'Brien T. Scaffold-based delivery of mesenchymal stromal cells to diabetic wounds. Stem Cell Res Ther. 2022;13:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 201. | Zhang HM, Yang ML, Xi JZ, Yang GY, Wu QN. Mesenchymal stem cells-based drug delivery systems for diabetic foot ulcer: A review. World J Diabetes. 2023;14:1585-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 202. | Chen S, Shi J, Zhang M, Chen Y, Wang X, Zhang L, Tian Z, Yan Y, Li Q, Zhong W, Xing M. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:18104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 203. | Sheng W, Song Q, Su X, Lu Y, Bai Y, Ji F, Zhang L, Yang R, Fu X. Sodium alginate/gelatin hydrogels loaded with adipose-derived mesenchymal stem cells promote wound healing in diabetic rats. J Cosmet Dermatol. 2023;22:1670-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 204. | Shi M, Gao Y, Lee L, Song T, Zhou J, Yan L, Li Y. Adaptive Gelatin Microspheres Enhanced Stem Cell Delivery and Integration With Diabetic Wounds to Activate Skin Tissue Regeneration. Front Bioeng Biotechnol. 2022;10:813805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 205. | Huang JN, Cao H, Liang KY, Cui LP, Li Y. Combination therapy of hydrogel and stem cells for diabetic wound healing. World J Diabetes. 2022;13:949-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 206. | Westby MJ, Dumville JC, Soares MO, Stubbs N, Norman G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst Rev. 2017;6:CD011947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 207. | Chiangnoon R, Karawak P, Eamsiri J, Nuchdang S, Thamrongsiripak N, Neramitmansook N, Pummarin S, Pimton P, Nilgumhang K, Uttayarat P. Antibacterial Hydrogel Sheet Dressings Composed of Poly(vinyl alcohol) and Silver Nanoparticles by Electron Beam Irradiation. Gels. 2023;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 208. | Miner SA, Lee J, Protzman NM, Brigido SA. The effect of a silver hydrogel sheet dressing on postsurgical incision healing after foot and ankle surgery. Scars Burn Heal. 2022;8:20595131221122303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 209. | Ko A, Liao C. Hydrogel wound dressings for diabetic foot ulcer treatment: Status-quo, challenges, and future perspectives. BMEMat. 2023;1. [DOI] [Full Text] |
| 210. | Wang S, Zheng H, Zhou L, Cheng F, Liu Z, Zhang H, Wang L, Zhang Q. Nanoenzyme-Reinforced Injectable Hydrogel for Healing Diabetic Wounds Infected with Multidrug Resistant Bacteria. Nano Lett. 2020;20:5149-5158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 328] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 211. | Li Q, Wang D, Jiang Z, Li R, Xue T, Lin C, Deng Y, Jin Y, Sun B. Advances of hydrogel combined with stem cells in promoting chronic wound healing. Front Chem. 2022;10:1038839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 212. | Chen L, Huang C, Zhong Y, Chen Y, Zhang H, Zheng Z, Jiang Z, Wei X, Peng Y, Huang L, Niu L, Gao Y, Ma J, Yang L. Multifunctional sponge scaffold loaded with concentrated growth factors for promoting wound healing. iScience. 2023;26:105835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 213. | Las Heras K, Garcia-Orue I, Aguirre JJ, de la Caba K, Guerrero P, Igartua M, Santos-Vizcaino E, Hernandez RM. Soy protein/β-chitin sponge-like scaffolds laden with human mesenchymal stromal cells from hair follicle or adipose tissue promote diabetic chronic wound healing. Biomater Adv. 2023;155:213682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 214. | Murphy MB, Blashki D, Buchanan RM, Fan D, De Rosa E, Shah RN, Stupp SI, Weiner BK, Simmons PJ, Ferrari M, Tasciotti E. Multi-composite bioactive osteogenic sponges featuring mesenchymal stem cells, platelet-rich plasma, nanoporous silicon enclosures, and Peptide amphiphiles for rapid bone regeneration. J Funct Biomater. 2011;2:39-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 215. | Liu H, Yang R, Zhao S, Zhou F, Liu Y, Zhou Z, Chen L, Xie J. Collagen scaffolds derived from bovine skin loaded with MSC optimized M1 macrophages remodeling and chronic diabetic wounds healing. Bioeng Transl Med. 2023;8:e10467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 216. | Li H, Chen X, Ren K, Wu L, Chen G, Xu L. Qualitative study on diabetic cutaneous wound healing with radiation crosslinked bilayer collagen scaffold in rat model. Sci Rep. 2023;13:6399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 217. | Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, Xu J, Guo X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front Physiol. 2017;8:904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 218. | Yusuf Aliyu A, Adeleke OA. Nanofibrous Scaffolds for Diabetic Wound Healing. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 219. | Bružauskaitė I, Bironaitė D, Bagdonas E, Bernotienė E. Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology. 2016;68:355-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 459] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 220. | Chen S, Wang H, Su Y, John JV, McCarthy A, Wong SL, Xie J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020;108:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 221. | Ter Horst B, Chouhan G, Moiemen NS, Grover LM. Advances in keratinocyte delivery in burn wound care. Adv Drug Deliv Rev. 2018;123:18-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 222. | Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 520] [Article Influence: 28.9] [Reference Citation Analysis (0)] |