Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1060
Peer-review started: December 4, 2023
First decision: February 18, 2024
Revised: February 25, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: June 15, 2024
Processing time: 190 Days and 14.6 Hours
Diabetes is a disease with a high global burden. Current strategies have failed to limit the advancement and impact of the disease. Successful early diagnosis and treatment will require the development of new agents. In this sense, boron-containing compounds have been reported as agents with the ability to reduce glycemia and lipidemia. They have also been used for labeling and measuring carbohydrates and other molecules linked to the initial stages of diabetes and its progression. In addition, certain boron compounds bind to molecules related to diabetes development and their biological activity in the regulation of elevated glycemia. Finally, it should be noted that some boron compounds appear to exert beneficial effects on diabetes complications such as accelerating wound healing while ameliorating pain in diabetic patients.
Core Tip: Diabetes is a high-global burden malady. Boron-containing compounds, from diet or administered as drugs, are promising agents to prevent or reduce progression in diabetic patients. Emerging experimental data offer promise of potential applications in the diagnosis and treatment of diabetes and its complications.
- Citation: Soriano-Ursúa MA, Cordova-Chávez RI, Farfan-García ED, Kabalka G. Boron-containing compounds as labels, drugs, and theranostic agents for diabetes and its complications. World J Diabetes 2024; 15(6): 1060-1069
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1060.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1060
Diabetes is among the diseases with the highest global burden. It is the leading cause of death and disability worldwide[1]. Commissions and research groups around the world have addressed global diabetes inequity to establish concerted approaches for addressing the problem within a larger social context. This includes the development and application of new prevention approaches, early diagnosis, treatment, and minimization of complications. Efforts are currently being focused on providing a sustainable and equitable opportunity for everyone to access a healthy diet to tackle the global diabetes crisis[1,2]. Additional approaches are focused on preventing and limiting the progression of the disease in its early stages[3]. Unfortunately, the cost relating to decreasing diabetes in the global population continues to increase, despite current efforts centered on diet and exercise as prevention, because of the expense of drugs used for weight control and to regain normal glycemia levels[4-6].
Boron-containing compounds (BCC) are emerging as effective drugs in several fields of medicine[7]. In humans, boric acid and borates have been used for medicinal purposes for centuries. The applications of these and other naturally occurring BCC have expanded. Beneficial effects on hormonal, metabolism, and inflammatory systems have recently been reported[8]. BCC have been successfully incorporated into diet supplements in a growing number of countries[7].
In addition, in recent decades, five new BCC have been approved for specific medical applications with certain advantages compared to similar boron-free compounds previously utilized. Thus, bortezomib, tavaborole, crisaborole, ixazomib, and vaborbactam are now FDA-approved and used to treat certain types of cancer, mycosis, inflammatory skin diseases, and some types of urinary infections. Approximately ten other BCC are in clinical trials[9].
Hence, it is clear that medical applications of BCC are expanding in the prevention, diagnosis, and therapy of metabolic disturbances[7,10].
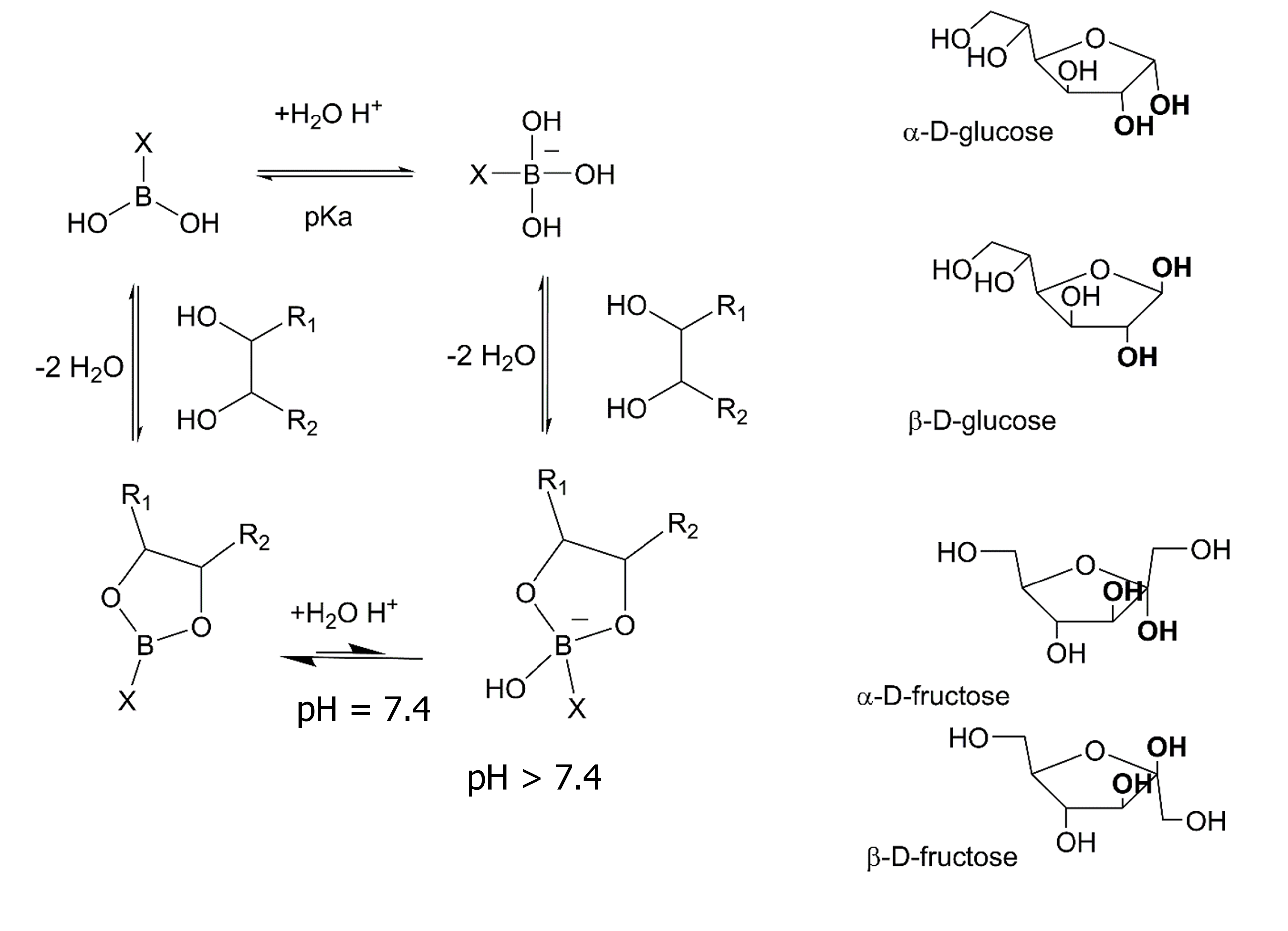
Some BCC react with glucose; this could be useful in the prevention, diagnosis, and control of diabetic patients. Control of glycemia limits the long-term consequences due to the disrupted glycation of vital protein structures in the heart, eyes, kidneys, nerves, and other organs. Arylboronic acids are candidates for application in the determination of glucose concentrations because of their fast and reversible formation of esters with 1,2-cis-diols or 1,3-diols of the glucose molecule[11].
The reactivity of boronic acids with diols has been demonstrated in multiple assays (Figure 1), including those mimicking human physiological conditions[12,13]. Boronic acids readily react with carbohydrates containing adjacent cis (same side)-diols that can achieve a planar arrangement which facilitates boronate ring formation as illustrated for the bolded OH groups in the α-D-glucose, and β-D-fructose furanose isomers illustrated in Figure 1[7,14,15].
Moreover, some BCC have been shown to selectivity form adducts with specific sugars. An example is described by Ramsay et al[16] where a boronic acid attached to a protein selectively formed adducts with α-glucose and β-fructose in the furanose forms which possess cis-diols (Figure 1). While other groups have described fluorescent boronodipyrromethene (BODIPY) conjugates with sugars[17,18].
However, BCC have failed in applications for developing non-invasive methods to measure glycemia because more efficient strategies and compounds have been developed. Despite that, multiple reports support the development of methods with high accuracy for determining glycemia in vitro[19,20], which is the usual procedure in the clinical monitoring of patients for the diagnosis of diabetes and its control by pharmacotherapy. A series of synthetic molecules have been developed to create a glucose sensor that would help to trigger the insulin-releasing systems; some of these are designed from an enzyme-glucose oxidase that oxidizes glucose liberating hydrogen peroxide, which then catalyzes irreversible oxidation of boronates to alcohols or phenols. However, this reaction needs to be reversible to be effective. Some arylboronic acids show a reversible binding to glucose. A boron-based, glucose sensing system might be more sensitive to changes in glucose serum levels and, thus, the release of insulin would be more accurate. Researchers are working on boronate-based materials for insulin delivery to create a more accurate artificial pancreas system[21].
There are BODIPY complexes that have emissions in different colors depending on the chemical structure of both the sugars and BODIPY[22]. It would be interesting to develop sugar-specific systems. Also, specificity among carbohydrates has been improved, including the specific forms of carbohydrates as their pyranose or furanose forms[23]. In addition, there are studies of BODIPY-sugar dyes measuring their ability to enter the cell or measuring a specific sugar in just the vascular compartment[24].
Although there is not a clear role of BCC in human physiology, there are research protocols in which the measurement of boron is used as a potential tool for the assessment and prediction of diabetes evolution in patients. In a cross-sectional observational study, 74 patients between 25 and 75 years old with type 2 diabetes mellitus were divided into two groups: good metabolic control and poor metabolic control. They were also classified by the presence of chronic complications of diabetes mellitus. Saliva and plasma samples were obtained for mass spectrometric analysis of various trace elements, including boron. The results indicated that boron was lower in the saliva and plasma of the group with poor metabolic control. This shows that boron levels in saliva and plasma might be used to assess the control of glucose and predict the possible complications in diabetic patients; the analysis of saliva permits a less invasive and simple method to obtain results identical to the ones observed in blood analyses[25]. Other results show the correlation between the use of metformin with elevated boron levels and low activity of myeloperoxidase in the brain. It supports the importance of boron in the homeostasis of the body and its relation to the prevention of oxidative stress caused by some pathological conditions such as diabetes mellitus. Thus, brain boron levels have been postulated as a possible indicator of reduced toxic and oxidative stress[26].
Determination of boron and other endogenous molecules could improve metabolic dysfunction in diabetic patients. As an example, it should be mentioned that some researchers have found that boron and vitamin D are important in the secretion of insulin and the regulation of glycemia. In this sense, studies have demonstrated that, after administration of dietary boron, the vitamin D3 serum concentration increases; boron was linked to an enhanced response for limiting hyperglycemia[8,10].
BCC also can act as labeling molecules for other processes well-known in the origin and evolution of diabetes; this approach could be useful for the diagnosis and monitoring of diabetic patients.
Currently, the glycated hemoglobin (HbA1c) measurement is considered important in the diagnosis of diabetes and for the patient follow-up, based on the capacity of the heme group in the hemoglobin to bind to the glucose present in the plasma. This glucose is carried on the red blood cells, which have an average life cycle of about 2-3 months, and the value does not change with the daily fluctuations in the glucose serum levels. Studies similar to the closed-loop glucose sensor were carried out for HbA1c. The selectivity between a sensing system based on IgG and a new system using a 4-vinylphenylboronic acid (VPBA) coated nanofilm (using concentrations of 30 mg/mL, 50 mg/mL, and 120 mg/mL HbA1c) was carried out in artificial plasma. It was observed that the VPBA nanofilm had a selectivity coefficient 7.30 times more selective than the IgG-based system, 17.33 times more than the human serum albumin, and 34.66 times more than hemoglobin[27].
Reactions of BODIPY with some membranes as well as intracellular and plasma lipids have been reported as biomarkers of use in diabetes[28-31]. Furthermore, a mix of BODIPYs could be used to determine more than one metabolite linked to diabetes evolution. For example, pyridine-extended BODIPY and BODIPY-cholesterol have been shown to provide reliable and accurate measurements of glucose and cholesterol from plasma samples[20,32].
Other BODIPY dyes have been described for measuring insulin in monomers and oligomers[33], carbohydrate complexes[34], glucagon, and its receptor[35,36]. Also, the design of dyes offers potential methods for using BODIPY targeting to detect the expression and function of glucose transporters among these are SGLT2[37], and GLUT4[38].
The effects of BCC on metabolic parameters in mammals have been recently reviewed[7,8]. Some differences have been observed depending on the administered form of the BCC[39]. It has also been reported that BCC intake exerts ameliorative effects on the metabolism disruption induced by streptozotocin[40-42].
Demirdogen et al[43] observed lower serum boron levels in diabetic patients when compared to healthy patients. They found a negative correlation between serum levels of HbA1c and serum boron levels in normal vs obese diabetic patients. Kuru et al[44] reported that a boron-rich diet induced a decrease in LDL, VLDL cholesterol, triglyceride levels, body weight, body fat, and body mass index. BBC might regulate lipid profile secondary to immunomodulatory effects, as has been suggested for calcium fructoborate[45-47].
In vitro, assays with human adipose-derived stem cells showed that BCC can restrain the expression of adipogenesis-related genes and proteins, like the peroxisome proliferator activated receptor γ. Apparently by regulating decisive growth factors such as β-catenin[48]. Moreover, high concentrations of sodium borate (68 μM to 340 μM) resulted in a progressive decrease in lipid deposition[49,50].
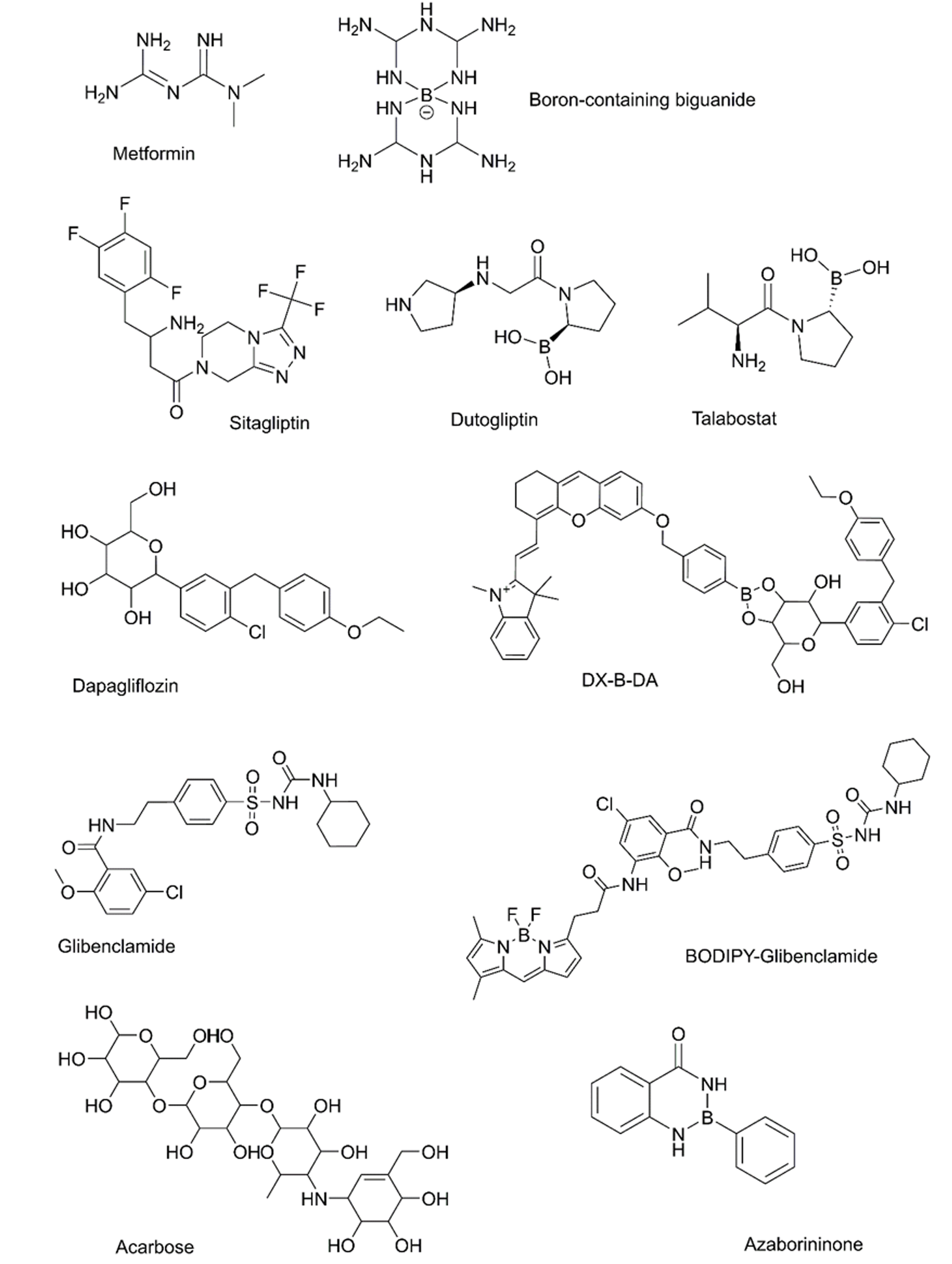
Currently, there are several drugs available for diabetes treatment using modulation of glycemia; and several BCC have shown an ability to act on the same targets (Table 1, Figure 2). Examples are those with action on specific enzymes related to diabetes. Regarding effects on carbohydrate metabolism, dutogliptin is a dipeptidyl peptidase IV inhibitor (the enzyme responsible for the degradation of incretins)[51,52], which is safely used in humans[51,53]. Another BCC, such as talabostat, also acts on dipeptidyl peptidases and other proteins and is an attractive metabolism regulator[54,55]. Some BCC act on glucose transporters, in a similar way to gliflozin. Cakir et al[41] suggested BA could affect glucose transporters. Recently, some BCC have shown an ability to bind the SGLT2[56,57].
| Type of drug | Example in use | BCC acting in a similar or related way | Ref. |
| Biguanides | Metformin | Boron-containing biguanides have been synthesized and some have shown biological effects. Some BCC is being used in biomaterials for metformin release | Anderson et al[59], 1995; Ghosh et al[60], 1998; Lai et al[61], 2023 |
| GLP-1 receptor agonist | Exenatide | No BCC has been designed with a structural analogy to known GLP-1 agonists. However, some BCC seems to be able to modify the incretins serum levels | Das et al[9], 2022; Ri et al[7], 2023 |
| DPP-IV inhibitors | Sitagliptin | Dutogliptin, talabostat, PC06R58, and PC06R108 are potent uncompetitive DPP-IV inhibitors | Wu et al[55], 2021; Prajapati et al[62], 2024 |
| SGLT2 inhibitors | Dapagliflozin | DX-B-DA, a fluorophore-dapagliflozin dyad has been tested as theranostic agent. It can bind the SGLT2 | Yu et al[63], 2021 |
| Sulfonylureas | Glibenclamide | Glibenclamide-BCC acts as a high-affinity blocker of pancreatic β-cell KATP currents. Non-specific binding limited its use | Zünkler et al[64], 2004 |
| α-glucosidase inhibitor | Acarbose | Some azaborininones exhibit moderate to good inhibitory effects against glucosidase to acarbose used as a reference standard | Mphahlele et al[65], 2021 |
| Insulin | Insulin | Several BCC result in changes in insulin serum concentrations. Boric acid and phenylboronic acids diminish the increase of insulin release (and the total weight of visceral fat) in rats exposed to a high-fat diet and streptozotocin. The insulin release could be controlled by complexes with boronic acids | López-Cabrera et al[42], 2018; Banach et al[21], 2021; Kikuchi et al[66], 2021 |
BCC have the potential to act on emerging identified targets, such as AN2898 acting as a PDE4 inhibitor since it could be applied to metabolic modulation in addition to its well-known action on smooth muscle[58] (Table 1[59-66]).
There are a wide variety of diabetes complications such as those related to cardiovascular dysfunction (including amputations and infective diseases), renal failure, retinopathy, and neuropathy[67-69].
Some BCC are effective in the prevention of cardiovascular diseases, mainly modulating chronic inflammation and oxidative stress; there is evidence that BCC modulate cardiac and vascular remodeling[70-73]. Moreover, dietary boron appears to be associated with a healthier diet and seems to be related to lower BMI and a more favorable cardio-metabolic risk profile[74].
Specific BCC are attractive as agents to treat diabetic complications (Figure 3). Significantly, there is evidence regarding their use in the treatment of the diabetic foot. In this sense, it has been observed that the boric acid in a 3% formulation has a great effect on the wound healing process in humans[75,76]; it is associated with important healing mechanisms, such as cell migration, collagen deposition, and superoxide dismutase activity. A study evaluated the effect of boric acid and sodium tetraborate in dermal cell cultures, showing that both compounds, at intermediate concentrations, increased the proliferation and migration of dermal cells[77]. In rats induced with diabetes-like syndromes, BCC (boric acid, sodium tetraborate, phenylboronic-based gel, or hexagonal boron nitrides) improved or accelerated wound healing[78-80]. In addition, a recent research protocol studied the wound-healing effect of BBC; 171 participants with foot ulcers were divided into two groups, one being the control group and the other using a formulated gel with 3% sodium pentaborate twice a day. The treatment, using topical administration, helped to decrease ulceration. Fewer treatments were necessary for the intervention group than the control group and recurrence was not observed in the intervention group; it was suggested that the acidic boron environment promotes angiogenesis and epithelization in the ulceration and increases the antimicrobial activity[81]. The attractiveness of BCC in diabetic foot ulcer treatment goes beyond wound healing acceleration because BCC have also been shown to modulate pain and exert antimicrobial effects[82,83].
Regarding nephropathy, no clear studies exploring BCC in the kidneys of diabetic patients have been reported. However it is well-known that these compounds exert effects in nephron remodeling[84], probably by acting in some enzymes (such as carbonic anhydrase)[85,86]; additionally, they have been used to treat commonly associated maladies, such as lithiasis[87].
No studies have been reported evaluating the retinopathy or neuropathy observed in diabetic patients but the effect of multiple BCC as neuroprotective agents has been described, including the limitation of oxidative or inflammatory damage or maintaining the production of local or systemic neurotrophic agents[88,89]. This is relevant due to the increased interest in neurological disorders and cognitive or motor deficits linked to metabolic disturbance[89].
It should be kept in mind that diabetes not only affects the glucose serum levels, it also predisposes tissues to additional abnormalities. Osteoporosis is a bone health problem associated with diabetes mellitus due to absorption loss and increased secretion of minerals such as calcium, magnesium, phosphorus, and vitamin D3; all of which are required for normal development of bone structure. It has been demonstrated that BCC improve the utilization of these minerals and vitamins in diabetic mice. A proposed mechanism of the action of boron against osteoporosis is the hydroxylation and protection of the steroid hormones from fast degradation while influencing the synthesis of vitamin D[10].
Some compounds are attractive because they can bind molecules that are linked to the onset and evolution of diabetes. However unexpected and unintended biological actions have been reported in the regulation of the metabolism of a cellular system or an organism. Conceptually, the theranostic approach should include simultaneous exploration of the agent as a drug and its diagnostic effect, but also the consideration (by authorities) of the coupled combination in the drug approval process[90]. In fact, to the best of our knowledge, no BCCs are registered to date as theranostic agents for diabetes (some theranostic-BCC have been reported for cancer treatment[91,92]) and some of the recent reports demonstrate the potential role of some BCC in this field.
Examples of BCC with potential as theranostic agents include BCC-labeled enzymes involved in carbohydrate metabolism. Thus, there are chromophoric BODIPY/glycoside systems for use within a specific cellular compartment, designed by chemically modifying the BODIPY skeleton to use them for in vivo imaging with luminescence being shifted to therapeutic purposes[93]. Likewise, BCC labeling of membrane carbohydrate transporters (modifying the tran-sportation rate). These include dyad boronated complexes acting as precursors of near-infrared emitting molecules and prodrug-modulating carbohydrate transport through the membrane which are promising agents in early kidney dysfunction detection[63]. Also, certain BCC reach beta-cells in pancreatic islets and can act as biomarkers and modify the release; thus boronophenylalanine derivatives are efficiently captured in pancreatic cells[94], while structurally related BCC could modulate insulin release[66].
Several BCC affect aspects of metabolism in humans. Moreover, BCC seem to have potential applications for improving the performance of available molecules in the prevention, diagnosis, and treatment of diabetes. This is confirmed by the reactivity of BCC on cis-diols of sugars and other chemical moieties related to the origin and evolution of diabetes and its complications. In this sense, notable effects of some BCC on the concentration of carbohydrates in plasma, the vascular system, inflammation, and wound healing are particularly attractive for studies in and application to diabetic patients.
The authors thank Instituto Politécnico Nacional for supporting projects involving the action of BCC in metabolism.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shalaby MN, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1683] [Cited by in RCA: 1785] [Article Influence: 892.5] [Reference Citation Analysis (18)] |
| 2. | Walker AF, Graham S, Maple-Brown L, Egede LE, Campbell JA, Walker RJ, Wade AN, Mbanya JC, Long JA, Yajnik C, Thomas N, Ebekozien O, Odugbesan O, DiMeglio LA, Agarwal S. Interventions to address global inequity in diabetes: international progress. Lancet. 2023;402:250-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 3. | Rooney MR, Fang M, Ogurtsova K, Ozkan B, Echouffo-Tcheugui JB, Boyko EJ, Magliano DJ, Selvin E. Global Prevalence of Prediabetes. Diabetes Care. 2023;46:1388-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 232] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 4. | Wilding JP. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68:682-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 5. | Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab Vasc Dis Res. 2019;16:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 6. | Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399:394-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 304] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 7. | Ri CC, Mf CR, D RV, T PC, F TC, Ir S, A AG, Ma SU. Boron-Containing Compounds for Prevention, Diagnosis, and Treatment of Human Metabolic Disorders. Biol Trace Elem Res. 2023;201:2222-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Kan F, Kucukkurt I. The effects of boron on some biochemical parameters: A review. J Trace Elem Med Biol. 2023;79:127249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 9. | Das BC, Adil Shareef M, Das S, Nandwana NK, Das Y, Saito M, Weiss LM. Boron-Containing heterocycles as promising pharmacological agents. Bioorg Med Chem. 2022;63:116748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Estevez-Fregoso E, Kilic A, Rodríguez-Vera D, Nicanor-Juárez LE, Romero-Rizo CEM, Farfán-García ED, Soriano-Ursúa MA. Effects of Boron-Containing Compounds on Liposoluble Hormone Functions. Inorganics. 2023;11:84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Hansen JS, Christensen JB, Petersen JF, Hoeg-Jensen T, Norrild JC. Arylboronic acids: A diabetic eye on glucose sensing. Sensors Actuators B Chem. 2012;161:45-79. [DOI] [Full Text] |
| 12. | Williams GT, Kedge JL, Fossey JS. Molecular Boronic Acid-Based Saccharide Sensors. ACS Sens. 2021;6:1508-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Bhavya NR, Mahendra M, Doreswamy BH, Kumar S, Gilandoust M, El-khatatneh NA. Computational and spectroscopic investigations on boronic acid based fluorescent carbohydrate sensor in aqueous solution at physiological pH 7.5. J Mol Struct. 2019;1194:305-319. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Geethanjali HS, Melavanki RM, Nagaraja D, Bhavya P, Kusanur RA. Binding of boronic acids with sugars in aqueous solution at physiological pH-Estimation of association and dissociation constants using spectroscopic method. J Mol Liq. 2017;227:37-43. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Melavanki R, Kusanur R, Sadasivuni KK, Singh D, Patil NR. Investigation of interaction between boronic acids and sugar: effect of structural change of sugars on binding affinity using steady state and time resolved fluorescence spectroscopy and molecular docking. Heliyon. 2020;6:e05081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Ramsay WJ, Bayley H. Single-Molecule Determination of the Isomers of d-Glucose and d-Fructose that Bind to Boronic Acids. Angew Chem Int Ed Engl. 2018;57:2841-2845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Liu B, Novikova N, Simpson MC, Timmer MS, Stocker BL, Söhnel T, Ware DC, Brothers PJ. Lighting up sugars: fluorescent BODIPY-gluco-furanose and -septanose conjugates linked by direct B-O-C bonds. Org Biomol Chem. 2016;14:5205-5209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Silva MP, Saraiva L, Pinto M, Sousa ME. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Liu Y, Zhu J, Xu Y, Qin Y, Jiang D. Boronic Acid Functionalized Aza-Bodipy (azaBDPBA) based Fluorescence Optodes for the Analysis of Glucose in Whole Blood. ACS Appl Mater Interfaces. 2015;7:11141-11145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Namkoong Y, Oh J, Hong JI. Electrochemiluminescent detection of glucose in human serum by BODIPY-based chemodosimeters for hydrogen peroxide using accelerated self-immolation of boronates. Chem Commun (Camb). 2020;56:7577-7580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Banach Ł, Williams GT, Fossey JS. Insulin Delivery Using Dynamic Covalent Boronic Acid/Ester-Controlled Release. Adv Ther. 2021;4:2100118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Gomez AM, Lopez JC. Bringing Color to Sugars: The Chemical Assembly of Carbohydrates to BODIPY Dyes. Chem Rec. 2021;21:3112-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Kanyan D, Horacek-Glading M, Wildervanck MJ, Söhnel T, Ware DC, Brothers PJ. O-BODIPYs as fluorescent labels for sugars: glucose, xylose and ribose. Org Chem Front. 2022;9:720-730. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Papalia T, Siracusano G, Colao I, Barattucci A, Aversa MC, Serroni S, Zappala G, Campagna S, Sciortino MT, Puntoriero F. Cell internalization of BODIPY-based fluorescent dyes bearing carbohydrate residues. Dye Pigment. 2014;110:67-71. [DOI] [Full Text] |
| 25. | Marín-Martínez L, Molino-Pagán D, López-Jornet P. Trace elements in saliva and plasma of patients with type 2 diabetes: Association to metabolic control and complications. Diabetes Res Clin Pract. 2019;157:107871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Ozel AB, Dagsuyu E, Aydın PK, Bugan I, Bulan OK, Yanardag R, Yarat A. Brain Boron Level, DNA Content, and Myeloperoxidase Activity of Metformin-Treated Rats in Diabetes and Prostate Cancer Model. Biol Trace Elem Res. 2022;200:1164-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Çalışır M, Bakhshpour M, Yavuz H, Denizli A. HbA1c detection via high-sensitive boronate based surface plasmon resonance sensor. Sensors Actuators B Chem. 2020;306:127561. [DOI] [Full Text] |
| 28. | Gao YG, My Le LT, Zhai X, Boldyrev IA, Mishra SK, Tischer A, Murayama T, Nishida A, Molotkovsky JG, Alam A, Brown RE. Measuring Lipid Transfer Protein Activity Using Bicelle-Dilution Model Membranes. Anal Chem. 2020;92:3417-3425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Wang X, Bou S, Klymchenko AS, Anton N, Collot M. Ultrabright Green-Emitting Nanoemulsions Based on Natural Lipids-BODIPY Conjugates. Nanomaterials (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Kashirina AS, López-Duarte I, Kubánková M, Gulin AA, Dudenkova VV, Rodimova SA, Torgomyan HG, Zagaynova EV, Meleshina AV, Kuimova MK. Monitoring membrane viscosity in differentiating stem cells using BODIPY-based molecular rotors and FLIM. Sci Rep. 2020;10:14063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Zhang M, Su R, Zhang Q, Hu L, Tian X, Chen Y, Zhou H, Wu J, Tian Y. Ultra-bright intercellular lipids pseudo Di-BODIPY probe with low molecular weight, high quantum yield and large two-photon action cross-sections. Sensors Actuators B Chem. 2018;261:161-168. [DOI] [Full Text] |
| 32. | Bernecic NC, Zhang M, Gadella BM, Brouwers JFHM, Jansen JWA, Arkesteijn GJA, de Graaf SP, Leahy T. BODIPY-cholesterol can be reliably used to monitor cholesterol efflux from capacitating mammalian spermatozoa. Sci Rep. 2019;9:9804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Mora AK, Murudkar S, Shivran N, Mula S, Chattopadhyay S, Nath S. Monitoring the formation of insulin oligomers using a NIR emitting glucose-conjugated BODIPY dye. Int J Biol Macromol. 2021;166:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Antina E, Bumagina N, Marfin Y, Guseva G, Nikitina L, Sbytov D, Telegin F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Tian Y, Fang M, Lin Q. Intracellular bioorthogonal labeling of glucagon receptor via tetrazine ligation. Bioorg Med Chem. 2021;43:116256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Lee JS, Kang NY, Kim YK, Samanta A, Feng S, Kim HK, Vendrell M, Park JH, Chang YT. Synthesis of a BODIPY library and its application to the development of live cell glucagon imaging probe. J Am Chem Soc. 2009;131:10077-10082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Lansdell MI, Burring DJ, Hepworth D, Strawbridge M, Graham E, Guyot T, Betson MS, Hart JD. Design and synthesis of fluorescent SGLT2 inhibitors. Bioorg Med Chem Lett. 2008;18:4944-4947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Hatakeyama H, Kobayashi K, Kanzaki M. Three live-imaging techniques for comprehensively understanding the initial trigger for insulin-responsive intracellular GLUT4 trafficking. iScience. 2022;25:104164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Kucukkurt I, Akbel E, Karabag F, Ince S. The effects of dietary boron compounds in supplemented diet on hormonal activity and some biochemical parameters in rats. Toxicol Ind Health. 2015;31:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Hunt CD, Herbel JL. Boron affects energy metabolism in the streptozotocin-injected, vitamin D3-deprived rat. Magnes Trace Elem. 10:374-386. [PubMed] |
| 41. | Cakir S, Eren M, Senturk M, Sarica ZS. The Effect of Boron on Some Biochemical Parameters in Experimental Diabetic Rats. Biol Trace Elem Res. 2018;184:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | López-Cabrera Y, Castillo-García EL, Altamirano-Espino JA, Pérez-Capistran T, Farfán-García ED, Trujillo-Ferrara JG, Soriano-Ursúa MA. Profile of three boron-containing compounds on the body weight, metabolism and inflammatory markers of diabetic rats. J Trace Elem Med Biol. 2018;50:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Demirdogen RE. Relationship among blood boron level, diabetes mellitus, lipid metabolism, bone metabolism and obesity: Can boron be an efficient indicator for metabolic diseases. Heal Sci J. 2020;14:1-11. [DOI] [Full Text] |
| 44. | Kuru R, Yilmaz S, Balan G, Tuzuner BA, Tasli PN, Akyuz S, Yener Ozturk F, Altuntas Y, Yarat A, Sahin F. Boron-rich diet may regulate blood lipid profile and prevent obesity: A non-drug and self-controlled clinical trial. J Trace Elem Med Biol. 2019;54:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Naghii MR, Mofid M, Asgari AR, Hedayati M, Daneshpour MS. Comparative effects of daily and weekly boron supplementation on plasma steroid hormones and proinflammatory cytokines. J Trace Elem Med Biol. 2011;25:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Hunter JM, Nemzer BV, Rangavajla N, Biţă A, Rogoveanu OC, Neamţu J, Scorei IR, Bejenaru LE, Rău G, Bejenaru C, Mogoşanu GD. The Fructoborates: Part of a Family of Naturally Occurring Sugar-Borate Complexes-Biochemistry, Physiology, and Impact on Human Health: a Review. Biol Trace Elem Res. 2019;188:11-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Nielsen FH. Historical and recent aspects of boron in human and animal health. 2017. [cited 20 March 2024]. Available from: https://dergipark.org.tr/en/pub/boron/issue/33625/373093#article_cite. |
| 48. | Doğan A, Demirci S, Apdik H, Bayrak OF, Gulluoglu S, Tuysuz EC, Gusev O, Rizvanov AA, Nikerel E, Şahin F. A new hope for obesity management: Boron inhibits adipogenesis in progenitor cells through the Wnt/β-catenin pathway. Metabolism. 2017;69:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Akdere ÖE, Shikhaliyeva İ, Gümüşderelioğlu M. Boron mediated 2D and 3D cultures of adipose derived mesenchymal stem cells. Cytotechnology. 2019;71:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Abdik EA, Abdik H, Taşlı PN, Deniz AAH, Şahin F. Suppressive Role of Boron on Adipogenic Differentiation and Fat Deposition in Human Mesenchymal Stem Cells. Biol Trace Elem Res. 2019;188:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Buchtele N, Schwameis M, Schoergenhofer C, Derhaschnig U, Firbas C, Karch R, Nix D, Schenk R, Jilma B. Safety, tolerability, pharmacokinetics and pharmacodynamics of parenterally administered dutogliptin: A prospective dose-escalating trial. Br J Clin Pharmacol. 2020;86:979-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab. 2014;16:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 53. | Dogan EE. Computational bioactivity analysis and bioisosteric investigation of the approved breast cancer drugs proposed new design drug compounds: increased bioactivity coming with silicon and boron. Lett Drug Des Discov. 2021;18:551-561. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Panaro BL, Coppage AL, Beaudry JL, Varin EM, Kaur K, Lai JH, Wu W, Liu Y, Bachovchin WW, Drucker DJ. Fibroblast activation protein is dispensable for control of glucose homeostasis and body weight in mice. Mol Metab. 2019;19:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Wu Y, Shi T, Wang J, He R. Talabostat Alleviates Obesity and Associated Metabolic Dysfunction via Suppression of Macrophage-Driven Adipose Inflammation. Obesity (Silver Spring). 2021;29:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Imperio D, Panza L. Sweet Boron: Boron-Containing Sugar Derivatives as Potential Agents for Boron Neutron Capture Therapy. Symmetry (Basel). 2022;14:182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Kong YK, Song KS, Jung ME, Kang M, Kim HJ, Kim MJ. Discovery of GCC5694A: A potent and selective sodium glucose co-transporter 2 inhibitor for the treatment of type 2 diabetes. Bioorg Med Chem Lett. 2022;56:128466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Nocentini A, Supuran CT, Winum JY. Benzoxaborole compounds for therapeutic uses: a patent review (2010- 2018). Expert Opin Ther Pat. 2018;28:493-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 59. | Anderson KB, Franich RA, Kroese HW, Meder R, Rickard CEF. The structure of biguanide complexes of boron. Polyhedron. 1995;14:1149-1153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Ghosh P, Bag SP, Sur B, Sur P. Antitumor properties of boron complexes with hydroxy biguanide and salicyl hydroxamic acid against Ehrlich ascites carcinoma. Neoplasma. 1998;45:68-72. [PubMed] |
| 61. | Lai Y, Al-Musawi TJ, Hussein UA-R, Waleed I, Ahmed HH, Khallawi AQ, Alsaraf KM, Asiri M, Abosaooda M, Alsaab HO. A first-principal study of pure and encapsulation boron nitride cluster with alkaline metals as the metformin drug carrier. J Mol Liq. 2023;384:122260. [DOI] [Full Text] |
| 62. | Prajapati N, Sharma D, Ashok Bidve P; Akhilesh, Chouhan D, Allani M, Kumar Patel S, Ghosh Chowdhury M, Shard A, Tiwari V. Glucose regulation by newly synthesized boronic acid functionalized molecules as dipeptidyl peptidase IV inhibitor: a potential compound for therapeutic intervention in hyperglycaemia. J Biomol Struct Dyn. 2024;42:2859-2871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Yu W, Huang J, Lin M, Wei G, Yang F, Tang Z, Zeng F, Wu S. Fluorophore-Dapagliflozin Dyad for Detecting Diabetic Liver/Kidney Damages via Fluorescent Imaging and Treating Diabetes via Inhibiting SGLT2. Anal Chem. 2021;93:4647-4656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Zünkler BJ, Wos-Maganga M, Panten U. Fluorescence microscopy studies with a fluorescent glibenclamide derivative, a high-affinity blocker of pancreatic beta-cell ATP-sensitive K+ currents. Biochem Pharmacol. 2004;67:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Mphahlele MJ, Magwaza NM, Malindisa ST, Choong YS. Biological evaluation the 2-aryl-2,3-dihydrobenzodiazaborinin-4(1H)-ones as potential dual α-glucosidase and α-amylase inhibitors with antioxidant properties. Chem Biol Drug Des. 2021;98:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Kikuchi H, Nakamura Y, Inoue C, Nojiri S, Koita M, Kojima M, Koyama H, Miki R, Seki T, Egawa Y. Hydrogen Peroxide-Triggered Conversion of Boronic Acid-Appended Insulin into Insulin and Its Application as a Glucose-Responsive Insulin Formulation. Mol Pharm. 2021;18:4224-4230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17 Suppl 1:S3-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 68. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3404] [Article Influence: 486.3] [Reference Citation Analysis (0)] |
| 69. | Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 944] [Article Influence: 157.3] [Reference Citation Analysis (1)] |
| 70. | Donoiu I, Militaru C, Obleagă O, Hunter JM, Neamţu J, Biţă A, Scorei IR, Rogoveanu OC. Effects of boron-containing compounds on cardiovascular disease risk factors - A review. J Trace Elem Med Biol. 2018;50:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | GBD 2019 Viewpoint Collaborators. Five insights from the Global Burden of Disease Study 2019. Lancet. 2020;396:1135-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 72. | Hernández-Gutiérrez S, Roque-Jorge J, López-Torres A, Díaz-Rosas G, García-Chequer AJ, Contreras-Ramos A. Role of sodium tetraborate as a cardioprotective or competitive agent: Modulation of hypertrophic intracellular signals. J Trace Elem Med Biol. 2020;62:126569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 73. | Asadi R, Raouf Sarshoori J, Ghorbani M, Mofid M. Evaluation of the Effect of Boron on Histopathological Changes of Atherosclerotic Plaque in Aortic Arch and Lipid Profiles in Hyperlipidemic New Zealand Male Rabbits. J Adv Med Biomed Res. 2023;31:2676-6264. [DOI] [Full Text] |
| 74. | Weber KS, Ratjen I, Enderle J, Seidel U, Rimbach G, Lieb W. Plasma boron concentrations in the general population: a cross-sectional analysis of cardio-metabolic and dietary correlates. Eur J Nutr. 2022;61:1363-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Coskun M. Success in treating wounds with local boric acid: a case study. J Wound Care. 2023;32:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 76. | Kanza Gül D, Mercan Y. Effect of boron-based gel on postpartum episiotomy wound healing in primiparous pregnant women. Ann Clin Anal Med. 2023;14:326-331. [DOI] [Full Text] |
| 77. | Demirci S, Doğan A, Aydın S, Dülger EÇ, Şahin F. Boron promotes streptozotocin-induced diabetic wound healing: roles in cell proliferation and migration, growth factor expression, and inflammation. Mol Cell Biochem. 2016;417:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 78. | Şen Ö, Emanet M, Çulha M. Stimulatory Effect of Hexagonal Boron Nitrides in Wound Healing. ACS Appl Bio Mater. 2019;2:5582-5596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Gundogdu G, Nalci KA, Ugur Kaplan AB, Gundogdu K, Demirci T, Demirkaya Miloglu F, Hacımuftuoglu A, Cetin M. The Evaluation of the Effects of Nanoemulsion Formulations Containing Boron and/or Zinc on the Wound Healing in Diabetic Rats. Int J Low Extrem Wounds. 2022;21:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Türkez H, Yıldırım ÖÇ, Öner S, Kadı A, Mete A, Arslan ME, Şahin İO, Yapça ÖE, Mardinoğlu A. Lipoic Acid Conjugated Boron Hybrids Enhance Wound Healing and Antimicrobial Processes. Pharmaceutics. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 81. | Şahin F, Pirouzpanah MB, Farshbaf-Khalili A, Ayşan E, Doğan A, Demirci S, Ostadrahimi A, Mobasseri M. The effect of the boron-based gel on the treatment of diabetic foot ulcers: A prospective, randomized controlled trial. J Trace Elem Med Biol. 2023;79:127261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 82. | Grams RJ, Santos WL, Scorei IR, Abad-García A, Rosenblum CA, Bita A, Cerecetto H, Viñas C, Soriano-Ursúa MA. The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology. Chem Rev. 2024;124:2441-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 83. | Abid HMU, Hanif M, Mahmood K, Aziz M, Abbas G, Latif H. Wound-Healing and Antibacterial Activity of the Quercetin-4-Formyl Phenyl Boronic Acid Complex against Bacterial Pathogens of Diabetic Foot Ulcer. ACS Omega. 2022;7:24415-24422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Çoban FK, İnce S, Demirel HH, İslam İ, Aytuğ H. Acetaminophen-Induced Nephrotoxicity: Suppression of Apoptosis and Endoplasmic Reticulum Stress Using Boric Acid. Biol Trace Elem Res. 2023;201:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 85. | Bagnis C, Marshansky V, Breton S, Brown D. Remodeling the cellular profile of collecting ducts by chronic carbonic anhydrase inhibition. Am J Physiol Renal Physiol. 2001;280:F437-F448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Giovannuzzi S, Nikitjuka A, Pereira Resende BR, Smietana M, Nocentini A, Supuran CT, Winum JY. Boron-containing carbonic anhydrases inhibitors. Bioorg Chem. 2024;143:106976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 87. | Jalali S, Borumandnia N, Basiri A, Nagiee M, Amiri FB, Tavasoli S, Kheirolahkhani Y, Taheri M. A Comparison of Boron Supplement and Tamsulosin as Medical Expulsive Therapy for Urinary Stones After Extracorporeal Shock Wave Lithotripsy: a Randomized Controlled Clinical Trial. Biol Trace Elem Res. 2023;201:5126-5133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 88. | Huang W, Huang L, Wen Z, Honkanen RA, Rigas B. The Antiangiogenic Effect and Ocular Pharmacology of Novel Modified Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Oxygen-Induced Retinopathy. J Ocul Pharmacol Ther. 2023;39:279-289. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 89. | Barrón-González M, Montes-Aparicio AV, Cuevas-Galindo ME, Orozco-Suárez S, Barrientos R, Alatorre A, Querejeta E, Trujillo-Ferrara JG, Farfán-García ED, Soriano-Ursúa MA. Boron-containing compounds on neurons: Actions and potential applications for treating neurodegenerative diseases. J Inorg Biochem. 2023;238:112027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 90. | Landais P, Méresse V, Ghislain JC. Evaluation and validation of diagnostic tests for guiding therapeutic decisions. Therapie. 2009;64:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Mishiro K, Imai S, Ematsu Y, Hirose K, Fuchigami T, Munekane M, Kinuya S, Ogawa K. RGD Peptide-Conjugated Dodecaborate with the Ga-DOTA Complex: A Preliminary Study for the Development of Theranostic Agents for Boron Neutron Capture Therapy and Its Companion Diagnostics. J Med Chem. 2022;65:16741-16753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 92. | Coghi P, Fazal T, Hosmane NS, Zhu Y. Diagnostic and Theranostic Technologies Used in Boron Neutron Capture Therapy-A Brief Review. Inorg Chem Commun. 2023;159:111698. [DOI] [Full Text] |
| 93. | Barattucci A, Gangemi CMA, Santoro A, Campagna S, Puntoriero F, Bonaccorsi P. Bodipy-carbohydrate systems: synthesis and bio-applications. Org Biomol Chem. 2022;20:2742-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Ciardiello A, Altieri S, Ballarini F, Bocci V, Bortolussi S, Cansolino L, Carlotti D, Ciocca M, Faccini R, Facoetti A, Ferrari C, Ficcadenti L, Furfaro E, Giagu S, Iacoangeli F, Macioce G, Mancini-Terracciano C, Messina A, Milazzo L, Pacifico S, Piccolella S, Postuma I, Rotili D, Vercesi V, Voena C, Vulcano F, Capuani S. Multimodal evaluation of (19)F-BPA internalization in pancreatic cancer cells for boron capture and proton therapy potential applications. Phys Med. 2022;94:75-84. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |