Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.977
Peer-review started: December 19, 2023
First decision: January 9, 2024
Revised: January 22, 2024
Accepted: March 15, 2024
Article in press: March 15, 2024
Published online: May 15, 2024
Processing time: 143 Days and 10.1 Hours
Recently, type 2 diabetic osteoporosis (T2DOP) has become a research hotspot for the complications of diabetes, but the specific mechanism of its occurrence and development remains unknown. Ferroptosis caused by iron overload is con-sidered an important cause of T2DOP. Polycytosine RNA-binding protein 1 (PCBP1), an iron ion chaperone, is considered a protector of ferroptosis.
To investigate the existence of ferroptosis and specific role of PCBP1 in the development of type 2 diabetes.
A cell counting kit-8 assay was used to detect changes in osteoblast viability under high glucose (HG) and/or ferroptosis inhibitors at different concentrations and times. Transmission electron microscopy was used to examine the morphological changes in the mitochondria of osteoblasts under HG, and western blotting was used to detect the expression levels of PCBP1, ferritin, and the ferroptosis-related protein glutathione peroxidase 4 (GPX4). A lentivirus silenced and overexpressed PCBP1. Western blotting was used to detect the expression levels of the osteoblast functional proteins osteoprotegerin (OPG) and osteocalcin (OCN), whereas flow cytometry was used to detect changes in reactive oxygen species (ROS) levels in each group.
Under HG, the viability of osteoblasts was considerably decreased, the number of mitochondria undergoing atrophy was considerably increased, PCBP1 and ferritin expression levels were increased, and GPX4 expression was decreased. Western blotting results demonstrated that infection with lentivirus overexpressing PCBP1, increased the expression levels of ferritin, GPX4, OPG, and OCN, compared with the HG group. Flow cytometry results showed a reduction in ROS, and an opposite result was obtained after silencing PCBP1.
PCBP1 may protect osteoblasts and reduce the harm caused by ferroptosis by promoting ferritin expression under a HG environment. Moreover, PCBP1 may be a potential therapeutic target for T2DOP.
Core Tip: Type 2 diabetic osteoporosis (T2DOP) is one of the most common complications of type 2 diabetes and its incidence has increased annually to seriously affect human health. T2DOP has a complicated environment and pathogenesis, including high glucose (HG) toxicity, inflammatory response, and iron metabolism disorders. However, the molecular mechanisms underlying T2DOP remain unclear. This study demonstrated that polycytosine RNA-binding protein 1 (PCBP1) protects osteoblasts and reduces the damage caused by ferroptosis by promoting ferritin expression in a HG environment in T2DOP, suggesting the promising therapeutic potential of PCBP1 in T2DOP.
- Citation: Ma HD, Shi L, Li HT, Wang XD, Yang MW. Polycytosine RNA-binding protein 1 regulates osteoblast function via a ferroptosis pathway in type 2 diabetic osteoporosis. World J Diabetes 2024; 15(5): 977-987
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/977.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.977
Recently, the incidence of diabetes, especially type 2 diabetes, has increased annually, posing a serious threat to human health[1]. There are > 100 complications of diabetes, including diabetic osteoporosis, diabetic nephropathy, diabetic foot, diabetic heart disease, diabetic vascular disease, and diabetic peripheral neuropathy[2]. Type 2 diabetic osteoporosis (T2DOP) is particularly common, especially in the elderly population. As individuals age, they have a considerably higher risk of fractures, which seriously affects their quality of life and imposes a heavy burden on society[3]. Several types of osteoporosis exist. Compared with type 1 diabetic osteoporosis and other types of osteoporosis, T2DOP has a more complicated environment and pathogenesis, including high glucose (HG) toxicity, inflammatory response, advanced glycation end products aggregation, and iron ion metabolism disorders[4]. However, the exact cause of T2DOP has not been fully elucidated.
Iron is a crucial trace element that sustains the life of organisms. The metabolic process of iron has a complete control system in advanced animals, including humans, to ensure that the body maintains a steady state. Moreover, iron homeostasis is essential for cells to perform physiological functions[5]. Ferritin, the main intracellular iron storage protein, is widely expressed in animals, plants, and microorganisms. Ferritin can protect cells from the toxic effects of excessive iron and release iron ions to meet the functional requirements of cells when iron is deficient[6]. Thus, it is essential for the maintenance of iron homeostasis.
Ferroptosis is a non-apoptotic programmed death pathway that is dependent on iron ions and reactive oxygen species (ROS). When iron ions are excessively accumulated in cells, the large amount of ROS produced by the Fenton reaction attacks biological macromolecules, resulting in an imbalance in iron homeostasis in the cell, ultimately leading to cell death[7]. Numerous studies have investigated ferroptosis; however, there have been few studies on bone metabolism. For example, Lu et al[8] reported that ferroptosis may occur during the pathogenesis of osteoporosis, as detected using a mouse model of osteoporosis and bioinformatics methods. Seibt et al[9] found that glutathione peroxidase 4 (GXP4) is a key regular of ferroptosis. Our previous studies revealed that iron overload caused by HG is an important factor causing osteoblast injury, and ROS levels are considerably increased during this period[10]. These findings suggest that ferroptosis is closely associated with the pathogenesis of diabetic osteoporosis; however, the specific occurrence process and whether ferritin is involved in this process require further examination.
Polycytosine RNA-binding protein 1 (PCBP1) is an iron ion chaperone protein widely present in cells that can bind iron ions and transfer iron ions to ferritin, thereby increasing the load of iron ions in ferritin[11]. Among the PCBP family, PCBP1 is one of the most studied and has a wide range of functions, including regulating gene transcription, maintaining mRNA stability, promoting translation, and acting as an iron ion partner[12-14]. Ryu et al[15] reported that mice lacking PCBP1 exhibited small cell iron deficiency anemia, which was compensated by regulating erythropoietin. Protchenko et al[16] found that unchaperoned iron in PCBP1-delated mouse hepatocytes produced ROS, indicating that PCBP1 may be a key factor in preventing ferroptosis. To the best of our knowledge, no study has confirmed whether PCBP1 is involved in the occurrence and development of T2DOP, and the relationship between changes in PCBP1 levels, ferritin, and ferroptosis in osteoblasts remains unknown.
Therefore, this study aimed to determine the existence of ferroptosis and the role of PCBP1 in the pathogenesis of T2DOP. We hypothesized that the compensatory increase of PCBP1 in T2DOP may lead to increased ferritin levels in osteoblasts, as well as reduce the excessive accumulation of iron ions and slow the occurrence and development of ferroptosis. This study first detected the expression levels of PCBP1 and ferritin in a HG environment, and then tested the relevant indicators, such as ferritin, after lentiviral silencing and overexpression of PCBP1. The current findings will help elucidate the pathogenesis of osteoporosis in type 2 diabetes and provide a theoretical basis for the treatment of metabolic diseases that target PCBP1 to regulate ferroptosis.
The human osteoblast-like cell line, hFOB1.19, was purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences, cultured in DMEM/F12 medium (glucose concentration 17.5 mmol/L; Hyclone; Cytiva) supplemented with 10% FBS (Hyclone; Cytiva) and 1% penicillin (Invitrogen; Thermo Fisher Scientific, Inc.), and cultured at 34 ˚C in a 5% CO2 incubator. The solution was changed every two days. When the cells were 80% confluent, they were subcultured with trypsin-EDTA (Sigma, America).
PCBP1 Lentiviral reagents were purchased from GeneChem Co., Ltd (Shanghai, China). PCBP1 (EPR11055, 1:500), ferritin (EPR3005Y, 1:500), and GPX4 (EPNCIR144, 1:500) antibodies were purchased from Abcam. Osteoprotegerin (OPG, sc-390518, 1:500) and osteocalcin (OCN, sc-390877, 1:500) antibodies were purchased from Santa Cruz Biotechnology, Inc. The human bone mineralization induction culture base was purchased from Cyagen Biosciences, Inc. β-actin (bsm-33036M, 1:1000) and all secondary antibodies (goat anti-rabbit IgG, bs-40295G-HRP, 1:5000 and goat anti-mouse IgG, bs-40296G-HRP, 1:5000) were purchased from BIOSS.
Cell viability was detected using a cell counting kit (CCK)-8 (Abcam) assay, according to the manufacturer’s instructions. Cells from each group treated with different glucose concentrations (17.5, 26.25, 35, 43.75, and 52.5 mmol/L, Sigma, America) and/or ferroptosis inhibitor (Ferrostatin-1, Fer-1, Sigma, America) were seeded in 96-well plates at a density of 6 × 103 cells/well, and cultured with 10% serum-containing medium. When the percentage of cell growth reached 70%–90%, the serum concentration of the medium was reduced to 1%, and the culture was continued to maintain the cells in the same division state as much as possible. After 24, 48, and 72 h, 10 µL CCK-8 solution was added to each well, and the reaction was continued in the incubator for 4 h. The optical density (OD) value of the cells in each well was measured using a microplate reader at a wavelength of 490 nm. Relative cell activity (%) = (OD value of wells in treatment group-OD value of blank group)/(OD value of control group-OD value of blank group).
Cells in the logarithmic growth phase were collected and inoculated into 6-well plates at a density of 5 × 106 cells/mL and cultured in an incubator at 37.5 ˚C for 72 h. We used 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma, America) to evaluate the ROS level. Briefly, DCFH-DA was diluted (1:1,000) with serum-free medium to a final concentration of 10 μmol/L. After the cells were collected, they were suspended in diluted DCFH-DA, incubated in a cell incubator for 20 min, inverted, and mixed every 3–5 min. After washing the cells thrice with serum-free cell culture solution, the ROS level in the cells in each group was measured using a flow cytometer at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
After the cells were digested with trypsin-EDTA, a lysis buffer (Abcam) containing protease and phosphatase inhibitor cocktail was added, placed on ice for lysis for 30 min, and then centrifuged at 12000 × g at 4 ˚C for 30 min, after which the supernatant containing total protein was collected. After the total protein was extracted, the protein concentration was determined using the BCA method. The protein sample (50 μg protein) was separated using 12% sodium lauryl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (MerckMillipore, America) at 60 V for 2 h. The membrane was blocked with a blocking buffer containing 5% skim milk for 2 h before being incubated overnight at 4 ˚C with primary antibodies (diluted at 1:100 to 1:1000). Subsequently, the membrane was incubated with a HRP-conjugated secondary antibody (anti-mouse or anti-rabbit; IgG was diluted at 1:6000 or 1:10000) for 2 h at room temperature. The bands were visualized with an EC3 imaging system (Analytik Jena AG), and the ratio of the OD of each band to β-actin, the internal reference protein, was measured using ImageJ 1.48V software (National Institutes of Health).
The cells were seeded at a density of 4 × 104 cells/mL in a 96-well culture plate, with a volume of 90 μL per well, and cultured in a 37.5 ˚C incubator for 24 h. An appropriate amount of the virus stock solution was diluted with enhanced infection solution to a titer of 1 × 108 TU/mL, which was added to the osteoblast-like cells for virus infection, and then the culture plate was returned to the incubator for incubation. After cells were infected for 3-4 d, the fluorescence expression was observed using a fluorescence microscope. Cell clones with positive expression were selected and expanded to obtain cell with stable expression. At a later stage of infection, the cells were exchanged and passaged according to their growth, to obtain stable expressing cell lines.
After the cells were digested with trypsin-EDTA, the suspended cells were collected via centrifugation and washed three times with pre-chilled phosphate buffer solution (Gibco, America). The cells were then fixed with 5% glutaraldehyde (Sigma, America) fixing solution for 1 h. After washing the cells again, they were routinely dehydrated, embedded, sectioned, and stained. Morphological changes in mitochondria were observed using a transmission electron microscope.
Cells were seeded at a density of 2 × 104 cells/cm2 in a 6-well plate coated with 0.1% gelatin, and 2 mL of medium was added to each well, which was then cultured in a 37.5 ˚C, 5% CO2 incubator. When the degree of cell fusion reached 60%–70%, the medium in the 6-well plate was replaced with complete osteogenic induction differentiation medium (Cyagen Biosciences, Inc.), which was replaced with fresh osteogenic induction differentiation medium every three days. After 2–4 wk of induction, the cells were stained with Alizarin Red (Shanghai Beyotime Co., Ltd), and the cell mor-phology was observed under an inverted fluorescence microscope.
Each experiment was repeated ≥ 3 times, and the data are presented as mean ± SD. Data were analyzed using the SPSS software (version 22.0; IBM Corp.). An unpaired t-test was used to compare two groups, whereas a one-way ANOVA was used to compare multiple groups. P < 0.05 was considered statistically significant.
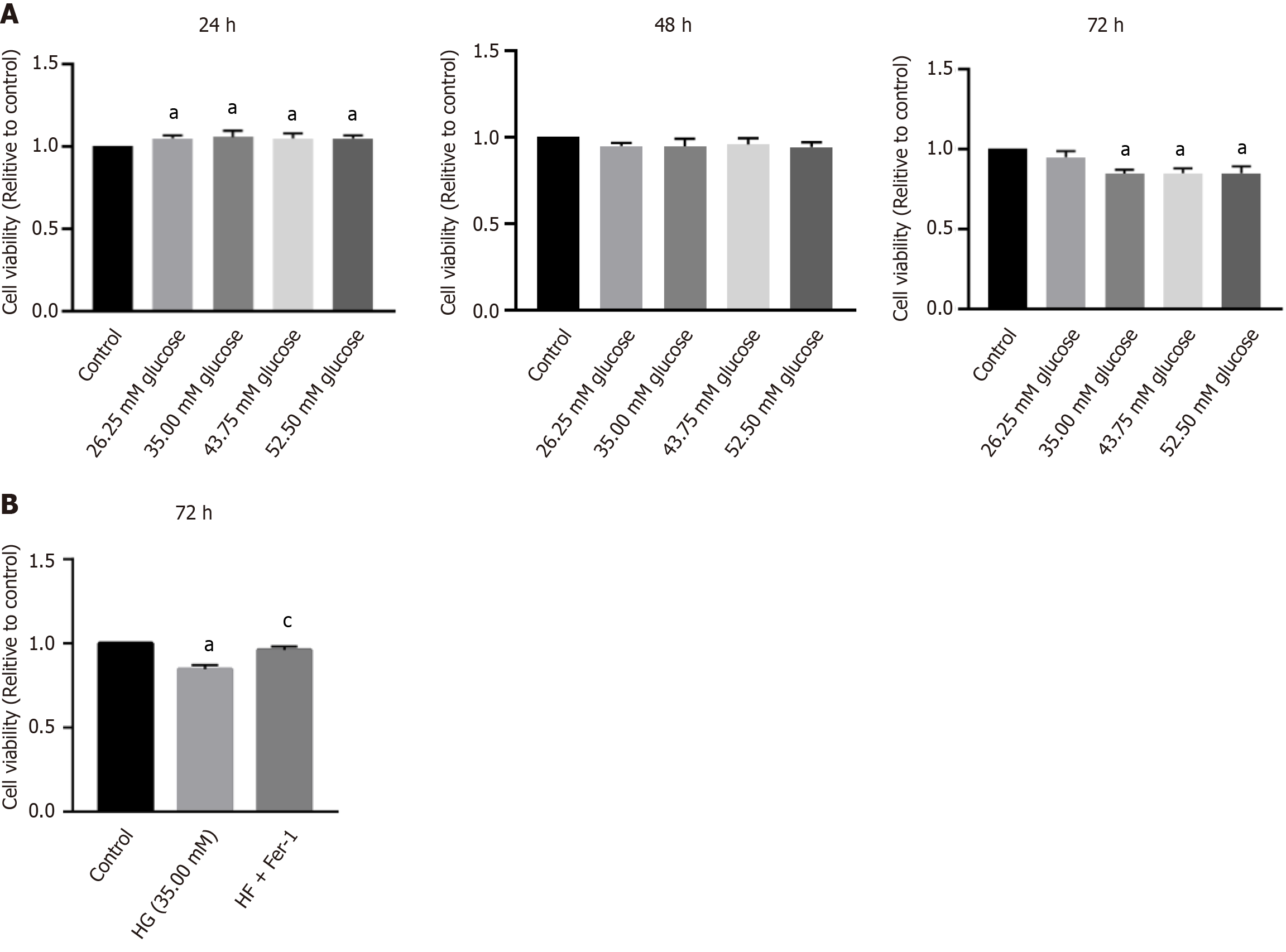
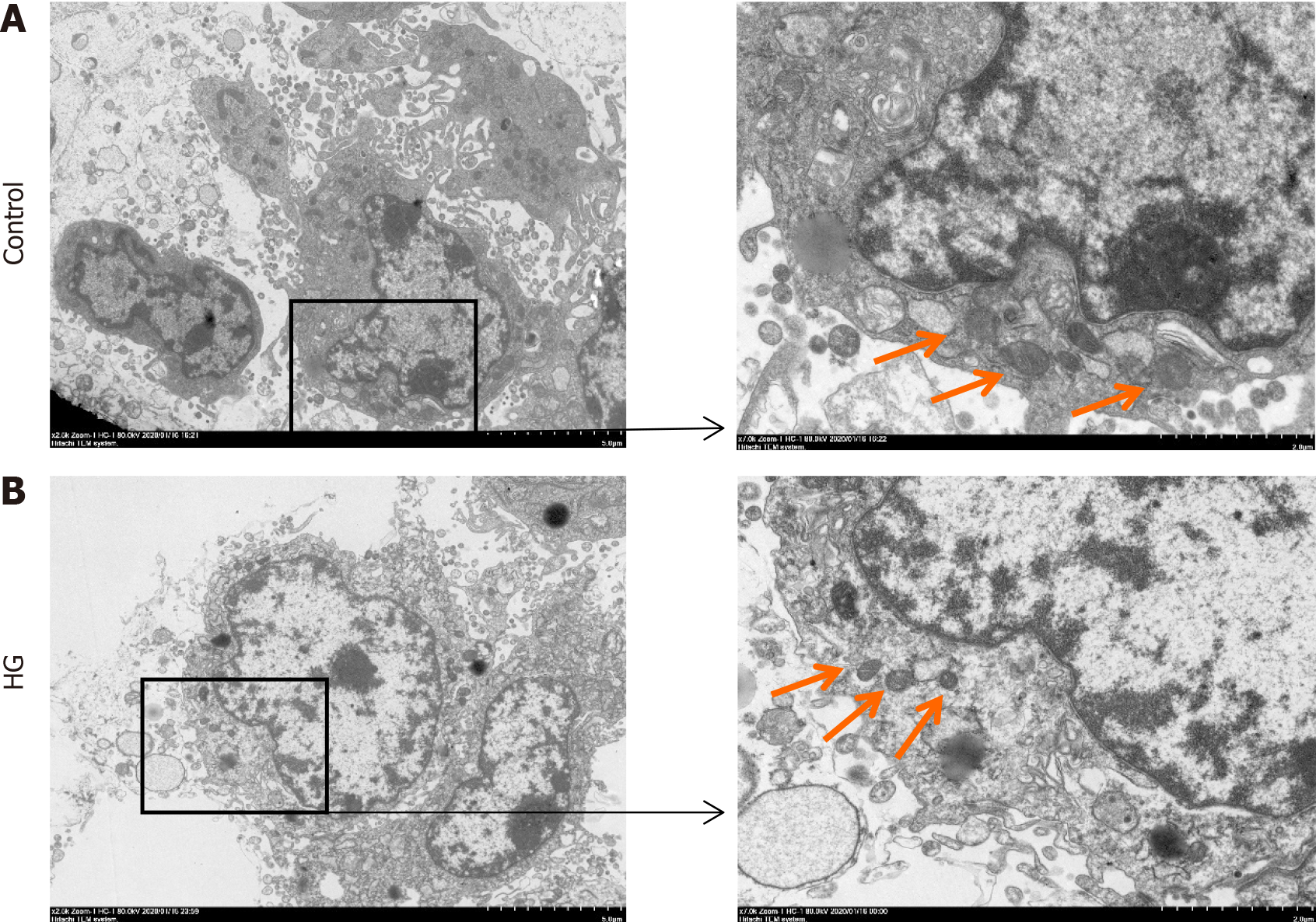
After the cells were treated for 24 h, groups with glucose concentrations of 35 mmol/L or higher showed an osteoblast promotion effect, but there was no major difference between the four groups. After 48 h of treatment, the cell viability of each group was slightly reduced compared with that of the control group, but there was no major difference between all groups. After 72 h of treatment, the viability of the three groups of cells with glucose concentrations greater than 35.00 mmol/L was considerably reduced, but there was no statistical difference between the three groups (Figure 1A). Compared with the control group, the HG group had lower osteoblast viability, and after the addition of the ferroptosis inhibitor, osteoblast viability increased compared with the HG group (Figure 1B). Electron microscopy revealed that the mitochondria in the HG group were fused and shrunken, and the staining intensity was deeper (Figure 2). These results suggest that osteoblast activity decreases in HG environments and may be associated with ferroptosis.
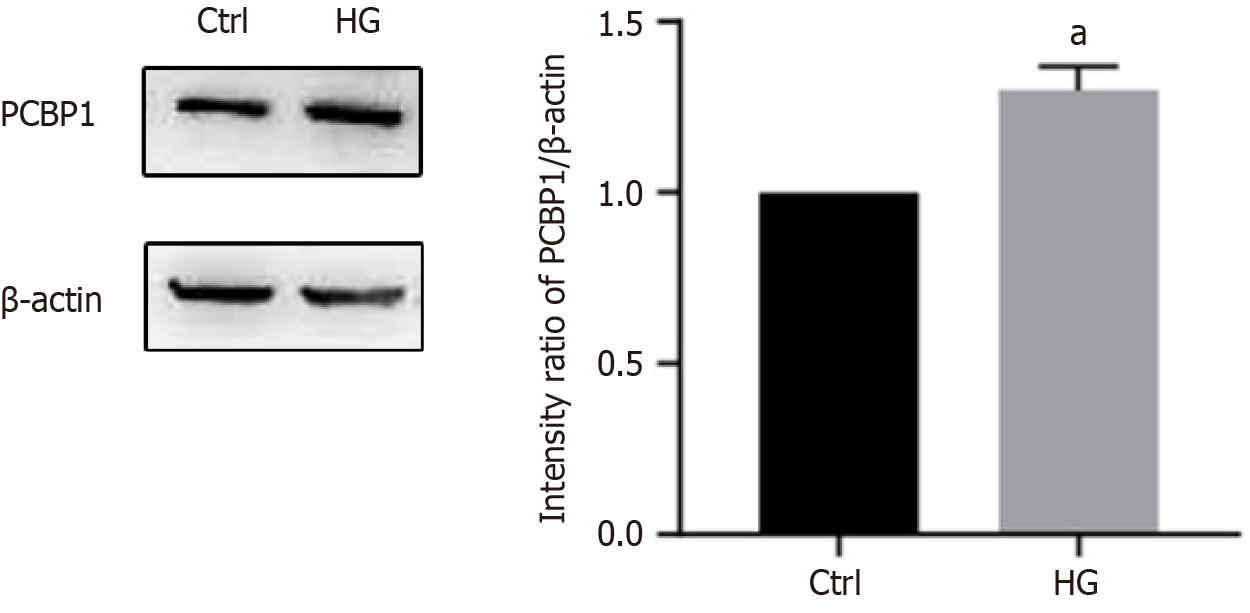
This study determined the expression level of PCBP1 in osteoblasts in a HG environment using western blotting (Figure 3). PCBP1 expression was higher in osteoblasts under HG environment compared with that in the control group. This indicates that a HG environment can promote the expression level of PCBP1 in osteoblasts.
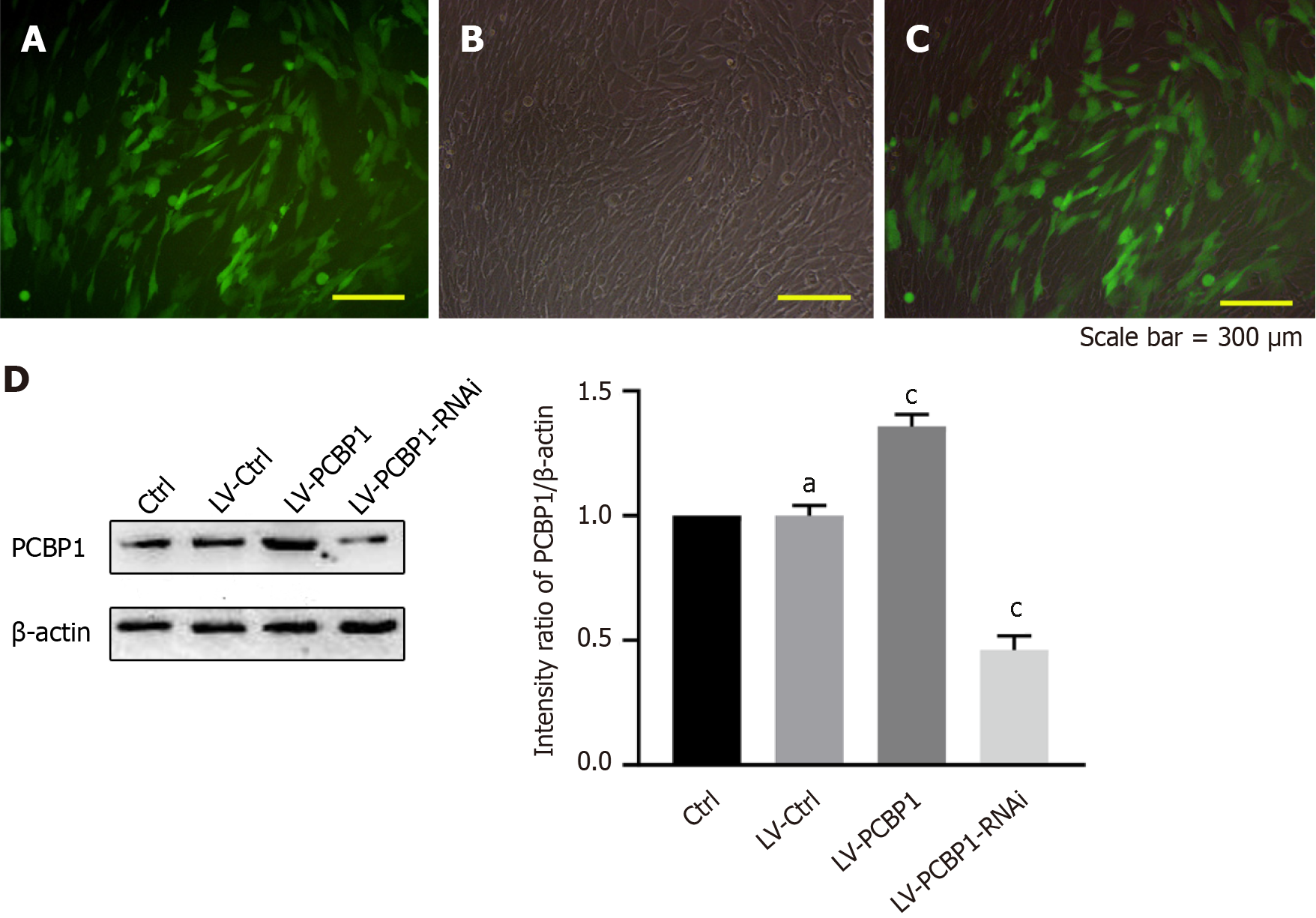
To confirm the role of PCBP1 in diabetic osteoporosis, the expression level of PCBP1 in hFOB1.19 cells was altered. In total, > 80% of the cells expressed green fluorescent protein (Figure 4A-C). Subsequently, western blotting was used to detect the expression level of PCBP1 after PCBP1-encoded lentivirus infection (Figure 4D), and the results confirmed that the cells were successfully transfected with PCBP1 lentivirus.
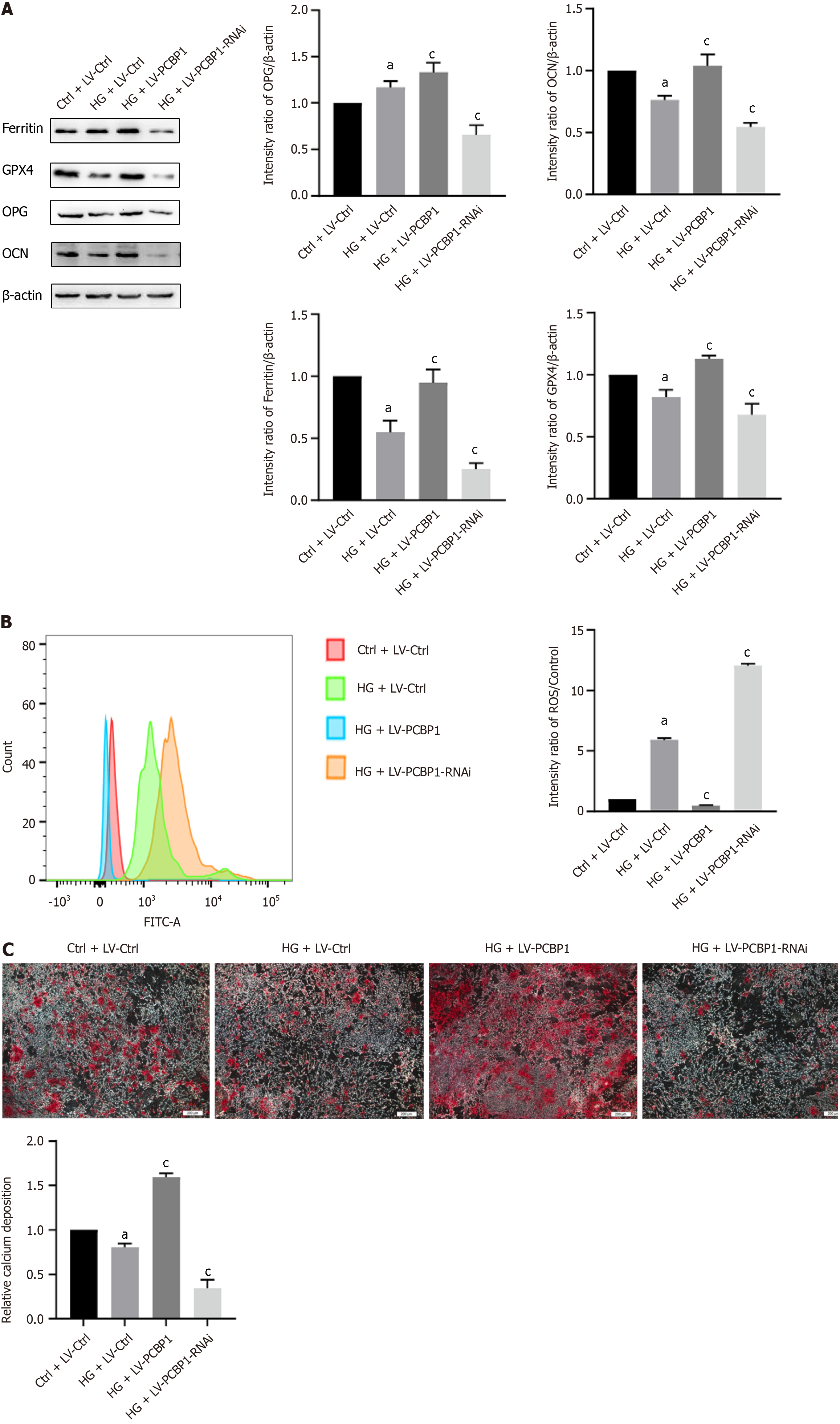
Ferritin, the ferroptosis marker protein GPX4, the osteogenic marker proteins OPG and OCN (Figure 5A), cellular ROS levels (Figure 5B), and alizarin red-stained calcium nodules (Figure 5C) were detected in osteoblasts under HG conditions alone or in combination with the infection of PCBP1 lentivirus via western blotting, flow cytometry, and induction of mineralization.
Western blotting results demonstrated that, compared with the normal group, ferritin and PCBP1 expression levels were increased in the HG group, while the expression levels of the ferroptosis marker protein GPX4 and the functional indicator proteins OPG and OCN were decreased. Under the same conditions of HG concentration, the expression levels of ferritin, GPX4, OPG, and OCN in the PCBP1 overexpression group were considerably increased compared with those in the HG alone group, while these expression levels were decreased in the knockdown group.
The flow cytometry results indicated that the ROS production was higher in the HG group compared with that in the normal group. Moreover, compared with the HG group, ROS production in the PCBP1 overexpression group was considerably reduced, whereas the knockdown group demonstrated the opposite result.
The osteogenic mineralization induction results showed that calcium nodule deposition was reduced in the HG group compared with that in the normal group. Compared with the HG group, calcium nodule deposition in the PCBP1 overexpression group was considerably increased, while the opposite result was found in the knockdown group. These results indicate that PCBP1 could reduce the toxic effect of ferroptosis on osteoblasts by promoting ferritin expression in a HG environment.
This study demonstrated that ferroptosis occurred in osteoblasts after HG treatment, and that PCBP1 and ferritin expression levels were higher than those in normal cells.
Ferroptosis, an iron-dependent lipid peroxide aggregation process, is a recently discovered mechanism of cell death[7]. Ferroptosis has become a research hotspot in several fields. For example, epidemiology and animal studies have reported that iron affects cell proliferation[17,18]. Moreover, abnormal iron metabolism and high intracellular iron content are characteristics of most cancer cells[19]. It has been suggested that oxidative stress may be the main cause of cancer, and that ferroptosis is involved in the pathogenesis of various cancer types[20]. Ferroptosis can lead to numerous diseases, such as liver and kidney damage, iron overload cardiomyopathy, and neurodegenerative diseases[21-23]. Studies have shown that ferroptosis may be a potential mechanism of endothelial cell damage caused by particulate matter 2.5[24]. Moreover, ferroptosis, a novel death mechanism different from that of apoptosis and necrosis, has a wide range of effects on diseases and cell damage. For example, Zhang et al[25] revealed that the upregulation of GPX4 reduces the incidence of ferroptosis and helps decrease secondary brain injury after cerebral hemorrhage. Nguyen et al[26] summarized the basic information of ferroptosis discovery and further explained the role of ferroptosis in human cancer, whereas Zhu et al[27] performed a literature search and analysis to compare the anticancer activity of artemisinin and its derivatives, and reported that ferroptosis was a new mechanism for treating cancer. These studies indicate that ferroptosis is widely studied in the fields of tumors and nerve injury than it is in the field of bone metabolism. These findings suggested that ferroptosis may be involved in the pathogenesis of T2DOP.
Ferritin is a natural reservoir of iron ions in the body, and plays a crucial role in iron storage and the control of intracellular iron distribution[28]. Bu et al[29] reported that ferritin can reduce free iron in cells, thereby inhibiting lipid peroxidation, oxygen free radical damage, and pro-inflammatory cascades that are induced when cells are stressed. Furthermore, Liu et al[30] examined the detoxification and utilization mechanisms of high-concentration heavy metals in the body using blind shrimp from a deep-sea hydrothermal area and found that the heavy chain subunit of ferritin had iron oxidase activity, which could convert divalent iron into non-toxic trivalent iron and store the iron. Santiago González et al[31] studied the iron metabolism of Schwann cells in myelinated nerve fibers, and found that doublesex and mab-3 related transcription factor 1, ferritin, and transferrin receptor 1 were key proteins for early iron uptake and storage in Schwann cells, as well as the normal myelination of the peripheral nervous system. Thus, based on the aforementioned findings, it is suggested that ferritin exerts a detoxification effect on iron overload and a protective effect on cells.
A previous study found that intracellular ferritin heavy peptide 1 and transferrin receptor 1 were upregulated in mice fed a high iron diet[32]. Furthermore, Li et al[33] showed that ferroportin1 protein is localized in the cytoplasm of bone cells of rats and ferroportin1 may be involved in the pathological process of iron overload-induced bone lesions. Additionally, Zhou et al[34] revealed that at the same time as the bone iron content was decreased in iron responsive element binding protein 2-knockout mice, the gene transcription of ferritin was correspondingly reduced. Thomson et al[35] pointed out that when the concentration of iron ions in the cell is low, iron-regulatory proteins /iron-responsive elements (IRP/IRE) prevents the assembly of ribosomes, thereby inhibiting the translation of ferritin. When the concentration of iron ions increases, IRP/IRE decreases, thereby increasing the translation of ferritin to store excess iron. These studies indicate that changes in intracellular iron ions are closely associated with ferritin, which is an important protector against iron overload and ferroptosis in human body.
PCBP1, an iron ion partner, can transfer iron ions to ferritin. Halon-Golabek et al[36] reported that, in the skeletal muscle of rats with amyotrophic lateral sclerosis, an increase in PCBP1 protein expression was accompanied by an increase in ferritin light and heavy chains. Moreover, Ryu et al[37] revealed the role of PCBP1 in iron flux via ferritin in developing erythroid cells, and showed that mice lacking PCBP1 could develop small cell anemia. Therefore, PCBP1 is important for ferritin formation and function. However, the effect of PCBP1 on ferritin in osteoblasts remains unknown.
Studies by Zarjou et al[38] showed that ferritin regulates osteoblast activity and osteogenesis. However, to the best of our knowledge, no studies have yet elucidated the regulatory mechanism of ferritin in T2DOP or its effect on T2DOP. Our previous study showed that osteoblasts from T2DOP bone tissues are overloaded with iron, causing the cells to produce an oxidative stress response, which ultimately affects the osteogenic function of the osteoblasts[4,39]. However, the specific processes and mechanisms of iron overload are yet to be fully elucidated.
This study demonstrated that the mitochondria exhibited typical morphological changes[7], and that the expression levels of PCBP1 and ferritin were considerably increased after HG treatment in osteoblasts. After lentiviral silencing and overexpression of PCBP1, ferritin was downregulated and upregulated, respectively. This study identified a key role of PCBP1 in the regulation of ferritin in T2DOP. Ferritin is the main storage protein of intracellular iron and an important component of intracellular iron homeostasis[28]. Thus, it was suggested that the increase in ferritin in the HG environment was due to the regulation of PCBP1, which compensated for the intracellular iron overload.
Ferritin regulated by PCBP1 affects osteoblast function via ferroptosis. Park et al[40] reported that ferroptosis is regulated by ferritin and transferrin receptors. Moreover, Mumbauer et al[41] showed that ferritin can protect cells from harmful effects caused by active oxygen accumulation and ferroptosis. Our previous study revealed that under HG conditions, ROS levels are considerably increased in osteoblasts, which could cause osteoblast function to decline. This study suggests that PCBP1 can reduce the occurrence of ferroptosis by promoting the formation of ferritin, which may be a potential target for the treatment of T2DOP. Future studies will attempt to prepare targeted drugs containing PCBP1, which can target the patient’s osteoblasts via oral administration, intravenous drip, or local injection, to inhibit the occurrence and development of osteoporosis, thereby improving or even reversing osteoporosis.
In conclusion, our findings revealed novel regulatory mechanisms involving PCBP1-associated ferroptosis as a promising therapeutic target for clinical therapy of T2DOP.
Type 2 diabetic osteoporosis (T2DOP) has become the most common complication of type 2 diabetes and its incidence has increased annually to seriously threaten human health. Exploring the molecular mechanisms underlying T2DOP is critical for its treatment.
The molecular mechanisms underlying T2DOP are poorly understood.
This study aimed to determine the involvement of ferroptosis and the specific role of polycytosine RNA-binding protein 1 (PCBP1) in the development of T2DOP.
A high glucose (HG)-induced in vitro model was established and treated with PCBP1 overexpression lentivirus vectors. The levels of the osteoblast functional proteins osteoprotegerin and osteocalcin, and the ferroptosis-related markers reactive oxygen species and glutathione peroxidase 4 (GPX4) were evaluated.
HG environment considerably decreased the viability of osteoblasts and enhanced mitochondria atrophy, along with increased PCBP1 and decreased GPX4 expression, whereas treatment with PCBP1 repressed ferroptosis and protected osteoblast function.
PCBP1 protects osteoblasts from ferroptosis by promoting ferritin expression under the HG stimulation in T2DOP, suggesting the promising therapeutic potential of PCBP1 in T2DOP.
Our findings revealed a novel regulatory mechanism involved in T2DOP and provided a promising therapeutic approach for clinical use.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cho IJ, South Korea; Gholizadeh P, Iran; Horowitz M, Australia; Sun XD, China S-Editor: Gong ZM L-Editor: A P-Editor: Guo X
| 1. | Magliano DJ, Islam RM, Barr ELM, Gregg EW, Pavkov ME, Harding JL, Tabesh M, Koye DN, Shaw JE. Trends in incidence of total or type 2 diabetes: systematic review. BMJ. 2019;366:l5003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 2. | Shirmohammadi N, Soltanian AR, Borzouei S. Public Awareness of Early and Late Complications of Type 2 Diabetes - Application of Latent Profile Analysis in Determining Questionnaire Cut-Off Points. Osong Public Health Res Perspect. 2018;9:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Abdulameer SA, Sahib MN, Sulaiman SAS. The Prevalence of Osteopenia and Osteoporosis Among Malaysian Type 2 Diabetic Patients Using Quantitative Ultrasound Densitometer. Open Rheumatol J. 2018;12:50-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Wang X, Ma H, Sun J, Zheng T, Zhao P, Li H, Yang M. Mitochondrial Ferritin Deficiency Promotes Osteoblastic Ferroptosis Via Mitophagy in Type 2 Diabetic Osteoporosis. Biol Trace Elem Res. 2022;200:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 5. | Yaskolka Meir A, Tsaban G, Zelicha H, Rinott E, Kaplan A, Youngster I, Rudich A, Shelef I, Tirosh A, Brikner D, Pupkin E, Sarusi B, Blüher M, Stümvoll M, Thiery J, Ceglarek U, Stampfer MJ, Shai I. A Green-Mediterranean Diet, Supplemented with Mankai Duckweed, Preserves Iron-Homeostasis in Humans and Is Efficient in Reversal of Anemia in Rats. J Nutr. 2019;149:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 1851] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 7. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11645] [Article Influence: 895.8] [Reference Citation Analysis (1)] |
| 8. | Lu J, Yang J, Zheng Y, Chen X, Fang S. Extracellular vesicles from endothelial progenitor cells prevent steroid-induced osteoporosis by suppressing the ferroptotic pathway in mouse osteoblasts based on bioinformatics evidence. Sci Rep. 2019;9:16130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 999] [Article Influence: 166.5] [Reference Citation Analysis (0)] |
| 10. | Zhang WL, Meng HZ, Yang MW. Regulation of DMT1 on Bone Microstructure in Type 2 Diabetes. Int J Med Sci. 2015;12:441-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 390] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 12. | Huo LR, Ju W, Yan M, Zou JH, Yan W, He B, Zhao XL, Jenkins EC, Brown WT, Zhong N. Identification of differentially expressed transcripts and translatants targeted by knock-down of endogenous PCBP1. Biochim Biophys Acta. 2010;1804:1954-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Thiele BJ, Doller A, Kähne T, Pregla R, Hetzer R, Regitz-Zagrosek V. RNA-binding proteins heterogeneous nuclear ribonucleoprotein A1, E1, and K are involved in post-transcriptional control of collagen I and III synthesis. Circ Res. 2004;95:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Kosturko LD, Maggipinto MJ, Korza G, Lee JW, Carson JH, Barbarese E. Heterogeneous nuclear ribonucleoprotein (hnRNP) E1 binds to hnRNP A2 and inhibits translation of A2 response element mRNAs. Mol Biol Cell. 2006;17:3521-3533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Ryu MS, Zhang D, Protchenko O, Shakoury-Elizeh M, Philpott CC. PCBP1 and NCOA4 regulate erythroid iron storage and heme biosynthesis. J Clin Invest. 2017;127:1786-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Protchenko O, Baratz E, Jadhav S, Li F, Shakoury-Elizeh M, Gavrilova O, Ghosh MC, Cox JE, Maschek JA, Tyurin VA, Tyurina YY, Bayir H, Aron AT, Chang CJ, Kagan VE, Philpott CC. Iron Chaperone Poly rC Binding Protein 1 Protects Mouse Liver From Lipid Peroxidation and Steatosis. Hepatology. 2021;73:1176-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 17. | Weber RA, Yen FS, Nicholson SPV, Alwaseem H, Bayraktar EC, Alam M, Timson RC, La K, Abu-Remaileh M, Molina H, Birsoy K. Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation. Mol Cell. 2020;77:645-655.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 18. | Phiwchai I, Thongtem T, Thongtem S, Pilapong C. Liver Cancer Cells Uptake Labile Iron via L-type Calcium Channel to Facilitate the Cancer Cell Proliferation. Cell Biochem Biophys. 2021;79:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Du Y, Yang C, Li F, Liao H, Chen Z, Lin P, Wang N, Zhou Y, Lee JY, Ding Q, Ling D. Core-Shell-Satellite Nanomaces as Remotely Controlled Self-Fueling Fenton Reagents for Imaging-Guided Triple-Negative Breast Cancer-Specific Therapy. Small. 2020;16:e2002537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Toyokuni S, Ito F, Yamashita K, Okazaki Y, Akatsuka S. Iron and thiol redox signaling in cancer: An exquisite balance to escape ferroptosis. Free Radic Biol Med. 2017;108:610-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 21. | Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res. 2018;341:154-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Park SJ, Cho SS, Kim KM, Yang JH, Kim JH, Jeong EH, Yang JW, Han CY, Ku SK, Cho IJ, Ki SH. Protective effect of sestrin2 against iron overload and ferroptosis-induced liver injury. Toxicol Appl Pharmacol. 2019;379:114665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Sumneang N, Siri-Angkul N, Kumfu S, Chattipakorn SC, Chattipakorn N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch Biochem Biophys. 2020;680:108241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Tang M. PM2.5 induces ferroptosis in human endothelial cells through iron overload and redox imbalance. Environ Pollut. 2019;254:112937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 25. | Zhang Z, Wu Y, Yuan S, Zhang P, Zhang J, Li H, Li X, Shen H, Wang Z, Chen G. Glutathione peroxidase 4 participates in secondary brain injury through mediating ferroptosis in a rat model of intracerebral hemorrhage. Brain Res. 2018;1701:112-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 26. | Nguyen THP, Mahalakshmi B, Velmurugan BK. Functional role of ferroptosis on cancers, activation and deactivation by various therapeutic candidates-an update. Chem Biol Interact. 2020;317:108930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Zhu S, Yu Q, Huo C, Li Y, He L, Ran B, Chen J, Liu W. Ferroptosis: A Novel Mechanism of Artemisinin and its Derivatives in Cancer Therapy. Curr Med Chem. 2021;28:329-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | GRANICK S. Ferritin; its properties and significance for iron metabolism. Chem Rev. 1946;38:379-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 173] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Bu W, Liu R, Cheung-Lau JC, Dmochowski IJ, Loll PJ, Eckenhoff RG. Ferritin couples iron and fatty acid metabolism. FASEB J. 2012;26:2394-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Liu XL, Ye S, Li HW, Lu B, Yu YQ, Fan YP, Yang WJ, Yang JS. An H-ferritin from the hydrothermal vent shrimp Rimicaris exoculata and its potential role in iron metabolism. Biometals. 2019;32:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Santiago González DA, Cheli VT, Wan R, Paez PM. Iron Metabolism in the Peripheral Nervous System: The Role of DMT1, Ferritin, and Transferrin Receptor in Schwann Cell Maturation and Myelination. J Neurosci. 2019;39:9940-9953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Simão M, Camacho A, Ostertag A, Cohen-Solal M, Pinto IJ, Porto G, Hang Korng E, Cancela ML. Iron-enriched diet contributes to early onset of osteoporotic phenotype in a mouse model of hereditary hemochromatosis. PLoS One. 2018;13:e0207441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Li Y, Bai B, Zhang Y. Expression of iron-regulators in the bone tissue of rats with and without iron overload. Biometals. 2018;31:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Zhou Y, Yang Y, Liu Y, Chang H, Liu K, Zhang X, Chang Y. Irp2 Knockout Causes Osteoporosis by Inhibition of Bone Remodeling. Calcif Tissue Int. 2019;104:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Thomson AM, Rogers JT, Leedman PJ. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. Int J Biochem Cell Biol. 1999;31:1139-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Halon-Golabek M, Borkowska A, Kaczor JJ, Ziolkowski W, Flis DJ, Knap N, Kasperuk K, Antosiewicz J. hmSOD1 gene mutation-induced disturbance in iron metabolism is mediated by impairment of Akt signalling pathway. J Cachexia Sarcopenia Muscle. 2018;9:557-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Ryu MS, Duck KA, Philpott CC. Ferritin iron regulators, PCBP1 and NCOA4, respond to cellular iron status in developing red cells. Blood Cells Mol Dis. 2018;69:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Zarjou A, Jeney V, Arosio P, Poli M, Zavaczki E, Balla G, Balla J. Ferritin ferroxidase activity: a potent inhibitor of osteogenesis. J Bone Miner Res. 2010;25:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Liu F, Zhang WL, Meng HZ, Cai ZY, Yang MW. Regulation of DMT1 on autophagy and apoptosis in osteoblast. Int J Med Sci. 2017;14:275-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 621] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 41. | Mumbauer S, Pascual J, Kolotuev I, Hamaratoglu F. Ferritin heavy chain protects the developing wing from reactive oxygen species and ferroptosis. PLoS Genet. 2019;15:e1008396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |