Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.945
Peer-review started: December 22, 2023
First decision: January 9, 2024
Revised: February 7, 2024
Accepted: March 13, 2024
Article in press: March 13, 2024
Published online: May 15, 2024
Processing time: 140 Days and 10 Hours
Diabetic peripheral neuropathy (DPN) is a debilitating complication of diabetes mellitus with limited available treatment options. Radix Salviae, a traditional Chinese herb, has shown promise in treating DPN, but its therapeutic mech-anisms have not been systematically investigated.
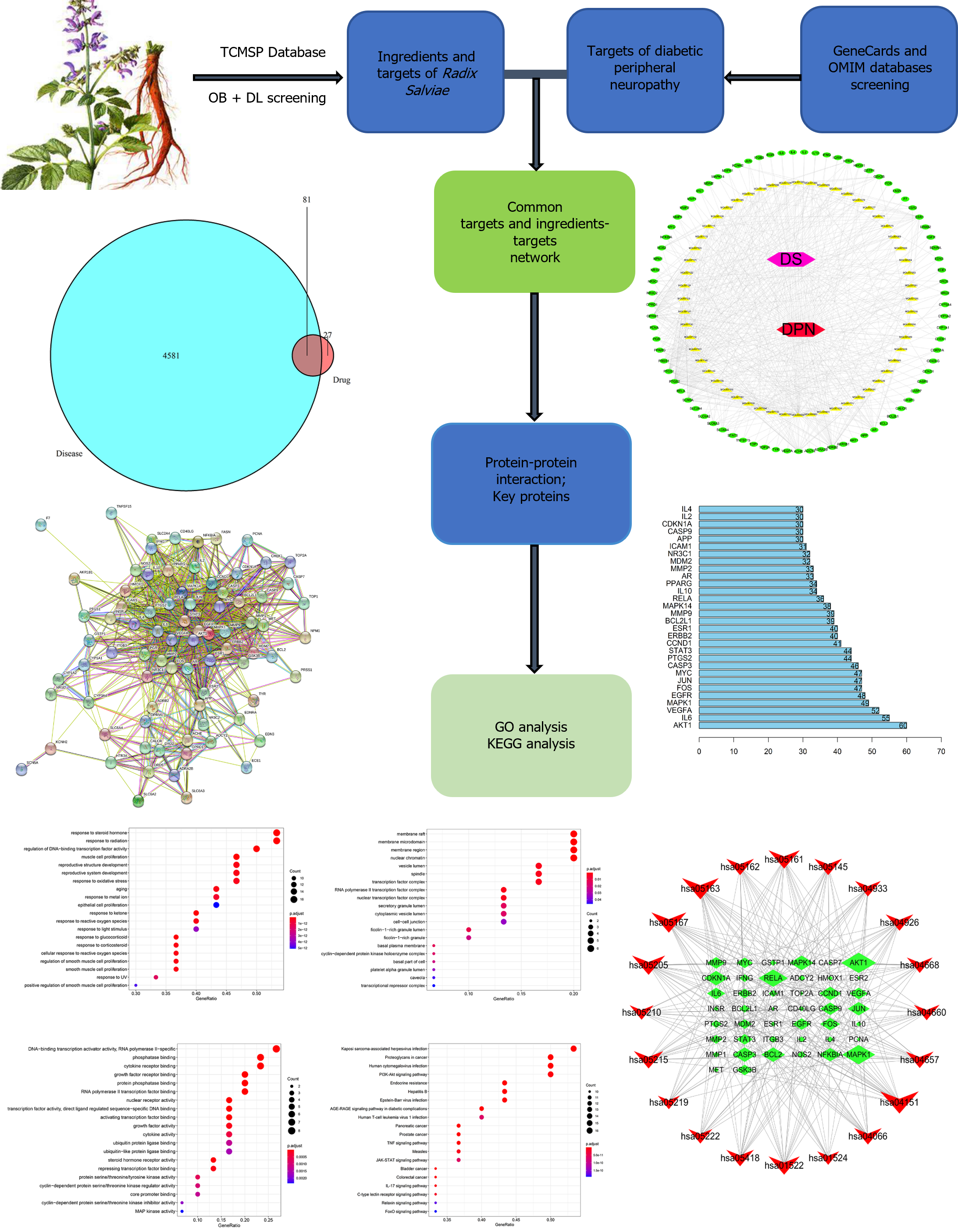
Radix Salviae (Danshen in pinin), a traditional Chinese medicine (TCM), is widely used to treat DPN in China. However, the mechanism through which Radix Salviae treats DPN remains unclear. Therefore, we aimed to explore the mechanism of action of Radix Salviae against DPN using network pharmacology.
The active ingredients and target genes of Radix Salviae were screened using the TCM pharmacology database and analysis platform. The genes associated with DPN were obtained from the Gene Cards and OMIM databases, a drug-com-position-target-disease network was constructed, and a protein–protein inter-action network was subsequently constructed to screen the main targets. Gene Ontology (GO) functional annotation and pathway enrichment analysis were performed via the Kyoto Encyclopedia of Genes and Genomes (KEGG) using Bioconductor.
A total of 56 effective components, 108 targets and 4581 DPN-related target genes of Radix Salviae were screened. Intervention with Radix Salviae for DPN mainly involved 81 target genes. The top 30 major targets were selected for enrichment analysis of GO and KEGG pathways.
These results suggested that Radix Salviae could treat DPN by regulating the AGE-RAGE signaling pathway and the PI3K-Akt signaling pathway. Therefore, Danshen may affect DPN by regulating inflammation and apoptosis.
Core Tip: This study utilizes network pharmacology to explore the potential therapeutic mechanism of Radix Salviae in treating diabetic peripheral neuropathy (DPN), revealing its regulation of the AGE-RAGE and PI3K-Akt signaling pathways. Through identification of active ingredients, target genes, and interaction networks, this research provides a comprehensive understanding of the molecular basis for Radix Salviae's effects on DPN, offering valuable insights for further investigation and potential drug development.
- Citation: Kang T, Qin X, Chen Y, Yang Q. Systematic investigation of Radix Salviae for treating diabetic peripheral neuropathy disease based on network Pharmacology. World J Diabetes 2024; 15(5): 945-957
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/945.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.945
Diabetic peripheral neuropathy (DPN) is a troublesome complication of diabetic mellitus that leads to a reduced quality of life as a result of neuropathic pain, sensory loss, foot ulceration and amputation[1,2]. According to the World Health Organization, 422 million people were diagnosed with diabetes worldwide in 2014, and this number is expected to increase to 590 million by 2035[3]. In fact, as the duration of diabetes progresses, the incidence of neuropathy in diabetic patients will eventually reach 50%[4]. However, there are scarcely available drugs available for treating DPN. Treatment for symptomatic patients, such as controlling blood sugar and relieving pain and numbness caused by neuropathy, is the current main intervention strategy for DPN[5]. However, these treatment strategies have unsatisfactory results in preventing DPN[6]. Therefore, investigating DPN as a possible medicinal focus and finding novel treatments for this disease are crucial.
For more than two thousand years, traditional herbs have been generally accepted and used in China for the treatment of DPN. Traditional herbs have been shown in relevant research to significantly increase blood glucose levels and clinical marker levels in DPN patients and to prevent the development of this disease[7]. Salviae Bunge's desiccated root, known as Radix Salviae (Danshen in Chinese), is a conventional whole grass herb that is effective at treating DPN. Clinical trials have shown that the herb Radix Salvia can reduce the severity of sensory deficits, paralysis, and nerve pain associated with DPN. Modern chemical research has confirmed the in vivo and in vitro efficacy of Radix Salviae and its active components in treating diabetes mellitus. For instance, Radix Salviae complete preparations and components such as tanshinone IIA, salvianolic acid A, and salvianolic acid B have been shown to have beneficial effects on diabetic diabetes and associated complications through anti-inflammatory, antioxidative stress, and antiapoptotic mechanisms[8-15]. However, the curative effects and fundamental processes of treatment for DPN have not yet been systematically investigated.
In recent years, network pharmacology based on the concept of "disease-gene-target-medicine" has been introduced to explain the complicated process of traditional Chinese medicine (TCM) as a consequence of the continual development of pharmacological research on TCM[16]. In the present study, we used the TCM pharmacology database and analysis platform (TCMSP) database to first filter the related components and targets of Radix Salviae. By compiling data from multiple sources, we identified targets linked to DPN. Based on the matching results between the Radix Salviae targets and DPN targets, we further screened their intersection targets and constructed the “Radix Salviae-ingredient-target-DPN” network. Subsequently, a protein-protein interaction (PPI) network was constructed to evaluate the relationships between the targets, and the top 30 targets were chosen for analysis via the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) databases.
We used a special system pharmacology tool developed for Chinese plant remedies called the Traditional Chinese Medicine System Pharmacology Library (TCMSP, http://Lsp.nwu.edu.cn) to compile the ingredients of Radix Salviae. The active ingredients of Radix Salviae were isolated by applying the following criteria: An oral bioavailability (OB) ≥ 30% and a drug likeness (DL) ≥ 0.18. OB represents the percentage of an orally administered dose of drug that reaches systemic circulation. High OB is often a key indicator of the drug-like property of bioactive molecules as therapeutic agents. DL is a qualitative concept used in drug design for estimating how “drug like” a prospective compound is, which helps to optimize pharmacokinetic and pharmaceutical properties, such as solubility and chemical stability. The “drug-like” level of the compounds was 0.18, which is used as a criterion for selecting “drug-like” compounds in traditional Chinese herbs. Ultimately, 65 ingredients related to Radix Salviae were obtained.
We first examined the Radix Salviae targets in the TCMSP database to identify the possible targets of the active ingredients in Radix Salviae. The UniProt database was subsequently used to determine the gene name of each ingredient. Finally, 667 targets of Radix Salvia were identified.
In this study, we utilized the keywords "Diabetic peripheral neuropathy" to screen for DPN-related disease target gene information through the human gene database (Gene Cards: https://www.genecards.org) and the Online Mendelian Inheritance in Man database (OMIM; http://www.ncbi.nlm.nih.gov/omim). After removing duplicate genes in Excel and merging the lists, the DPN target genes were identified. The target genes of Radix Salviae were also collected and matched with those of DPN. Through this comparison, commonly shared target genes were identified and are believed to be potential therapeutic targets for Radix Salviae in treating DPN.
The effective components of Radix Salviae were mapped to corresponding target genes and DPN target genes using R software to construct a Venn diagram. The intersection of the two sets of target genes was input into the String database to conduct protein-protein interaction analysis, limited to H. sapiens, and a confidence level > 0.4 was used to indicate a significant interaction between two proteins. A PPI network diagram was constructed accordingly. Using the node connectivity value (degree) of each target gene in the network as an indicator, the core protein targets were screened, and the top 30 target maps were retained via Perl software.
GO function and KEGG pathway analyses were performed using the Bioconductor library in R software to identify the main targets that interact with the active components of Radix Salviae in terms of gene function and signaling pathways, with a significance level of P < 0.05. The GO functional results included cellular component (CC), molecular function (MF), and biological process (BP) terms. Eventually, the top 20 metabolic pathways were exhibited in the network.
All substances that satisfied the screening standards of DL ≥ 0.18 and OB ≥ 30% were regarded as active substances. Sixty-five distinctive components of Radix Salviae were found in the TCMSP database (Figure 1).
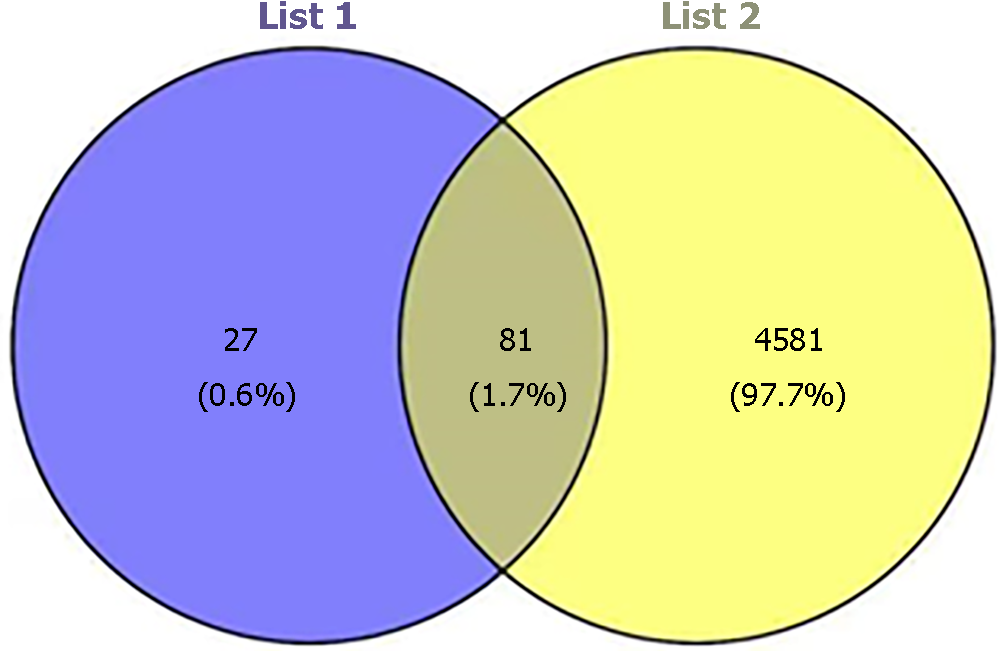
By exploring the TCMSP database, we were able to identify 918 targets for 65 active ingredients. After querying from UniProt, 667 targets were identified for 58 ingredients of Radix Salviae. Moreover, using the Gene Cards and OMIM databases, 5472 related disease genes were acquired. The intersection targets of Radix Salviae and DPN were screened from a Venn diagram constructed with R software. Among the duplicate targets, Radix Salviae included 108 related targets, and DPN contained 4581 targets. Consequently, Radix Salviae and DPN were found to share 81 similar targets (Figure 2).
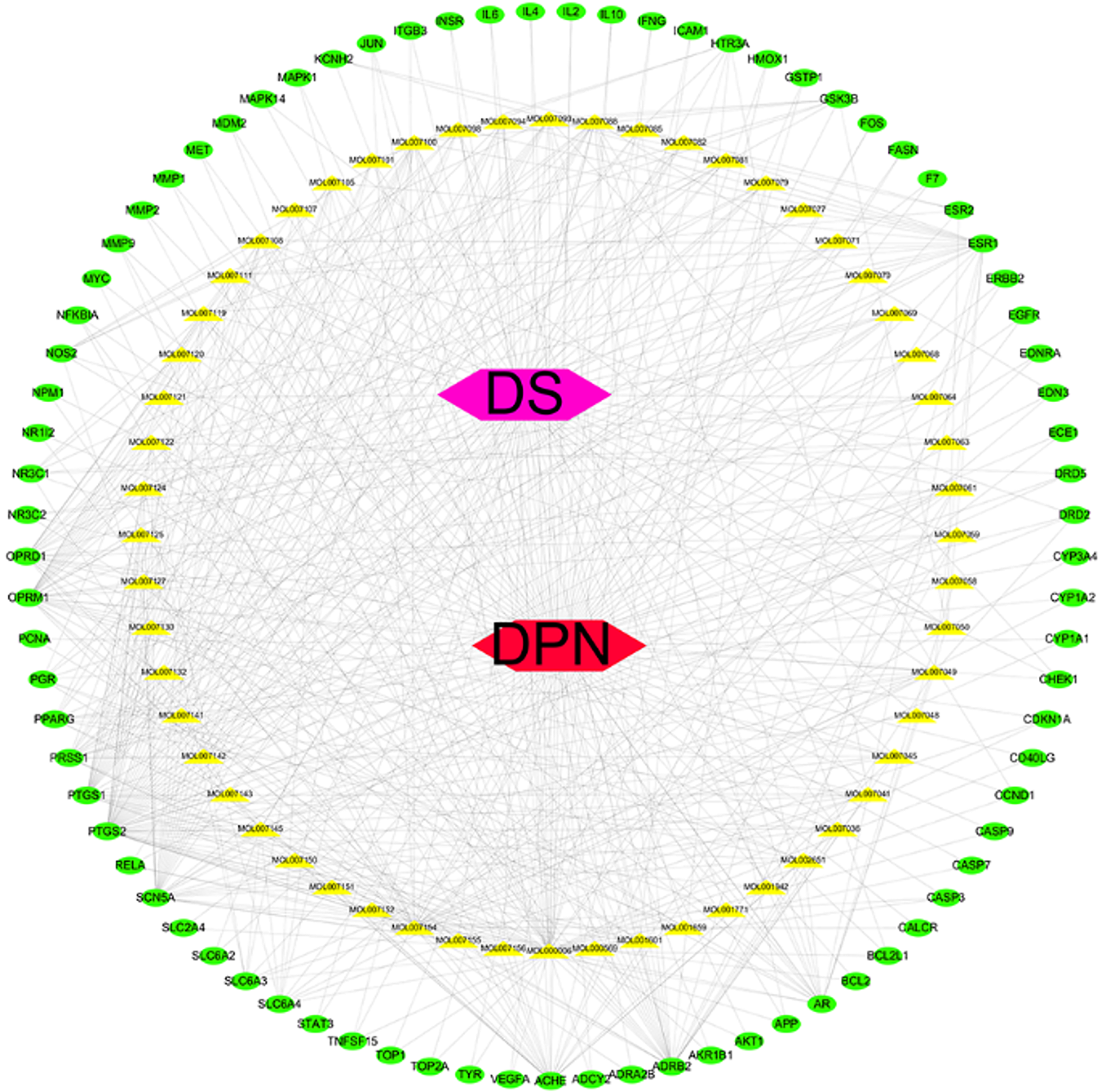
Using Perl software, we found 108 targets of Radix Salvia that respond to 56 ingredients. Thus, a network of Radix Salviae-ingredients-targets-DPN was constructed that included 56 ingredients and 81 common targets (137 nodes and 553 edges; Figure 3).
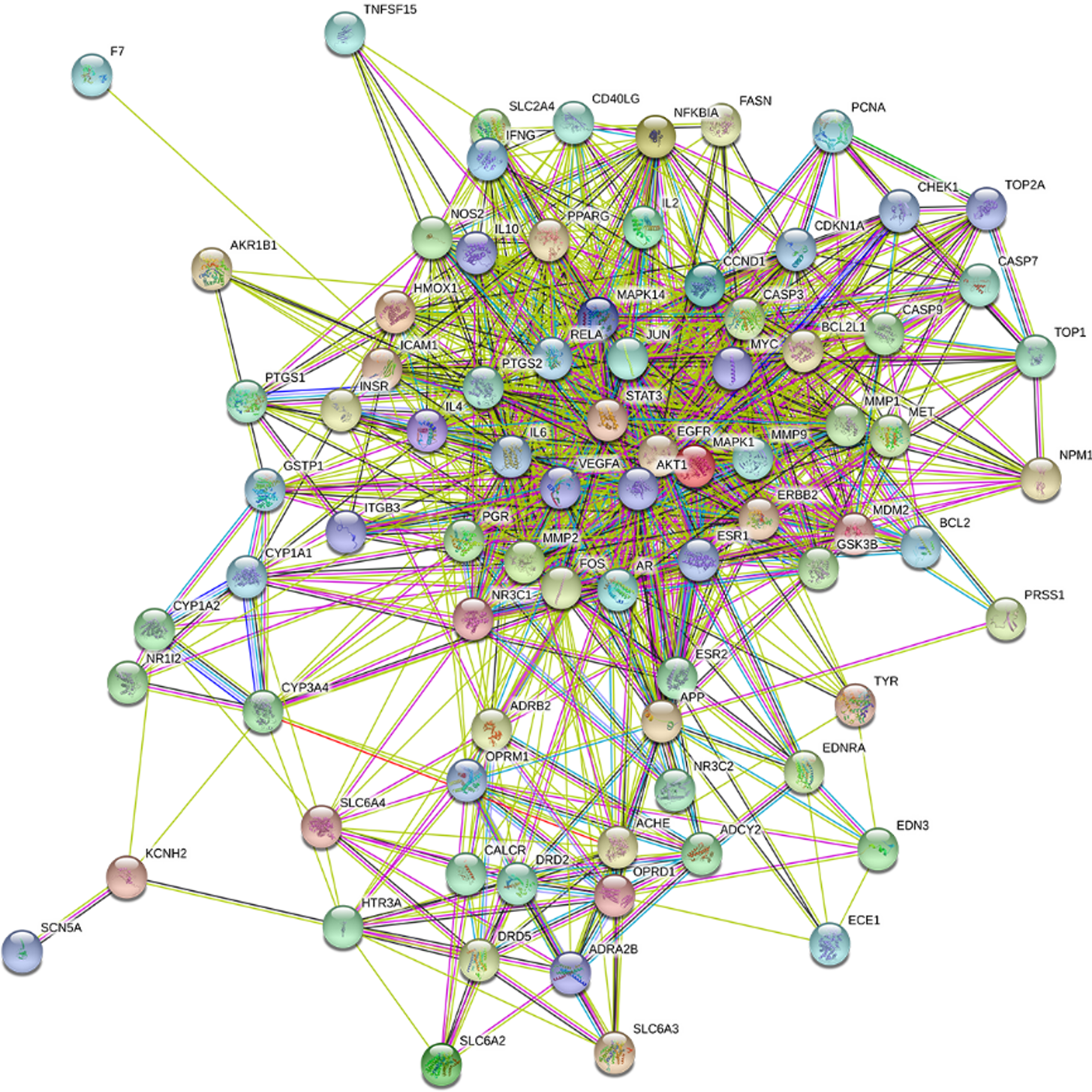
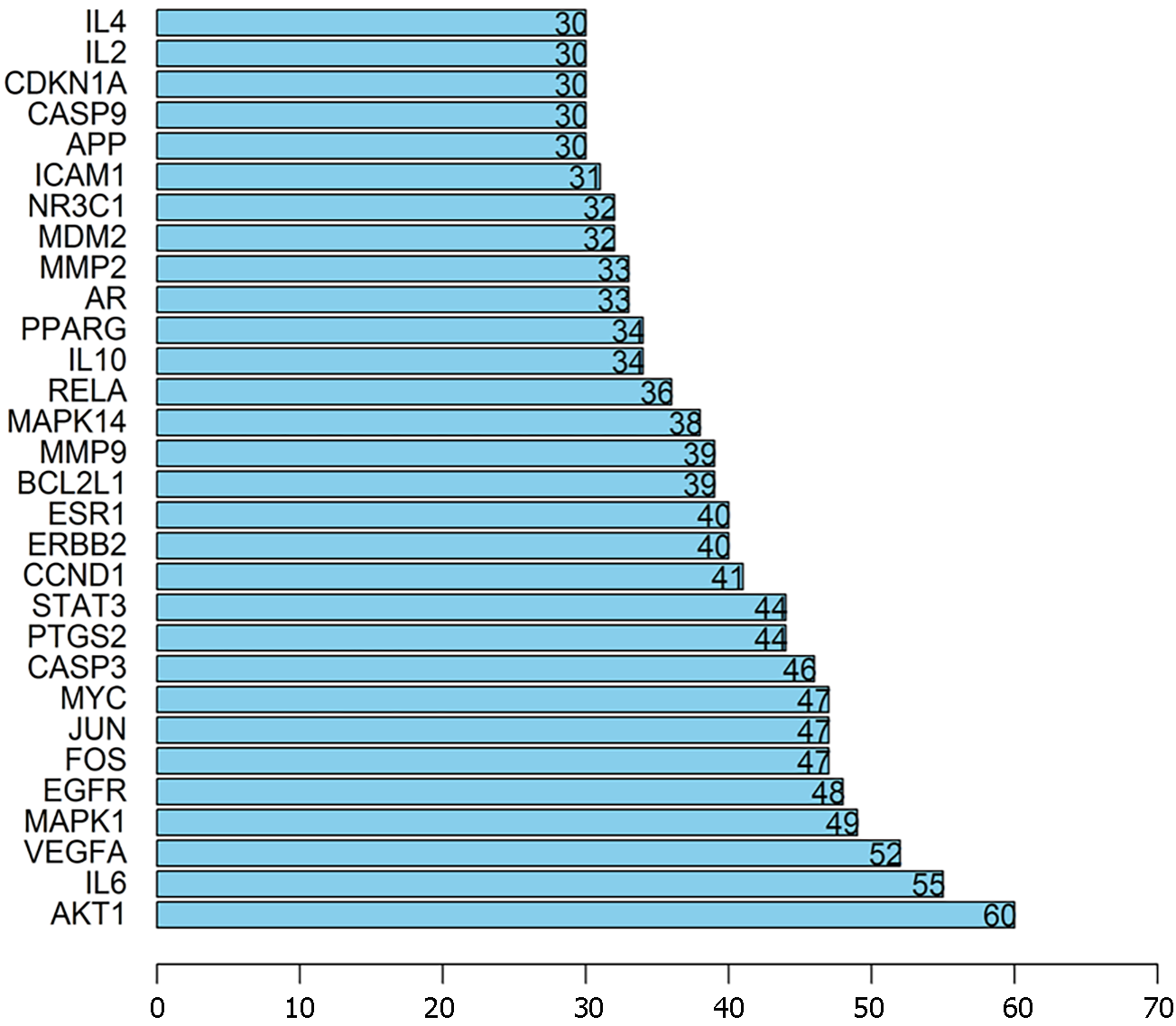
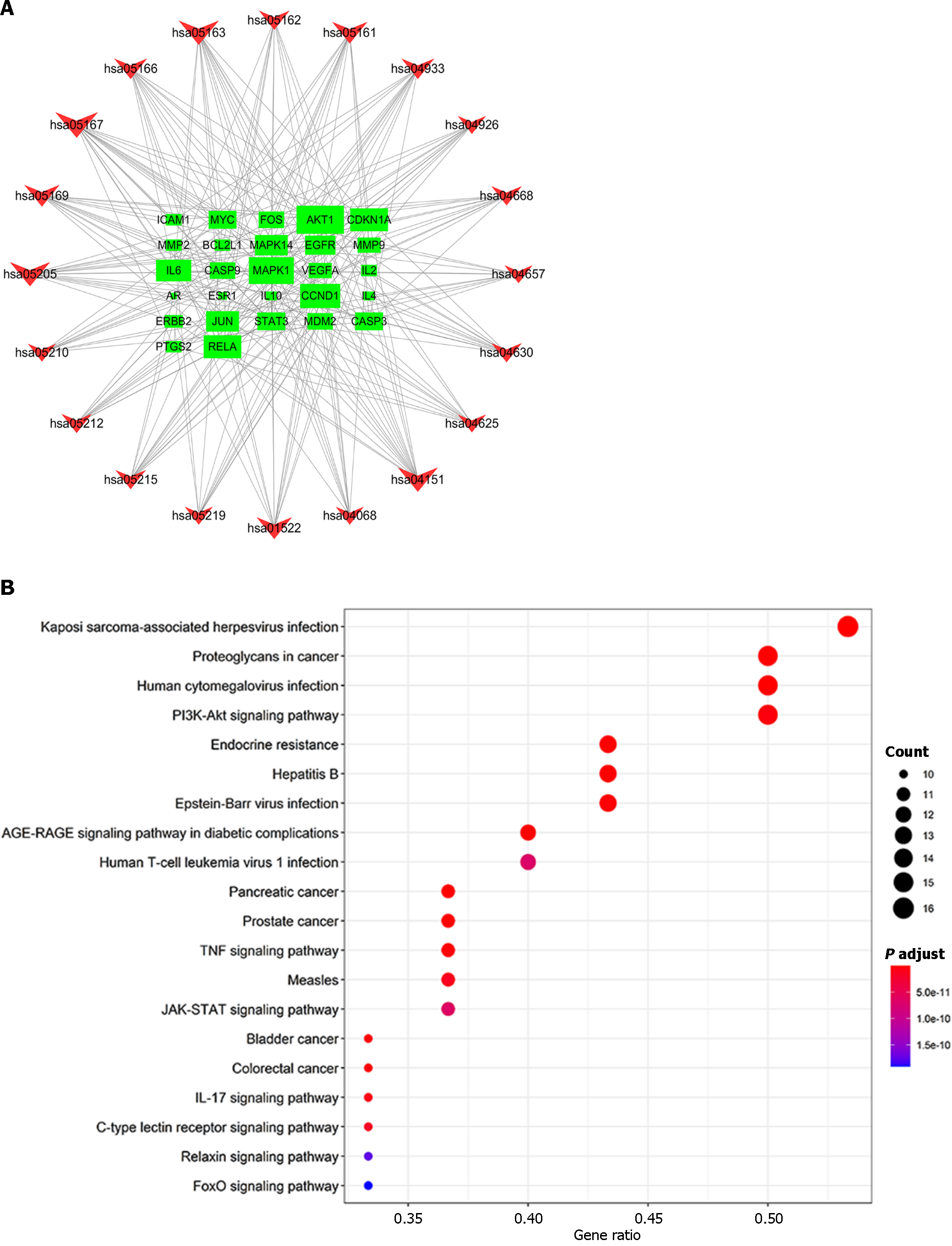
The intersection targets between Radix Salviae and DPN were first uploaded to STRING to clarify target interactions. The interaction results were subsequently arranged according to interaction nodes, and the preliminary major nodes (30 nodes) were selected; these nodes included AKT1, IL6, VEGFA, MAPK1, EGFR, FOS, JUN, MYC, CASP3, PTGS2, STAT3, CCND1, ERBB2, ESR1, BCL2L1, MMP9, MAPK14, RELA, IL10, PPARG, AR, MMP2, MDM2, NR3C1, ICAM1, APP, CASP9, CDKN1A, IL2, and IL4 (Figures 4 and 5).
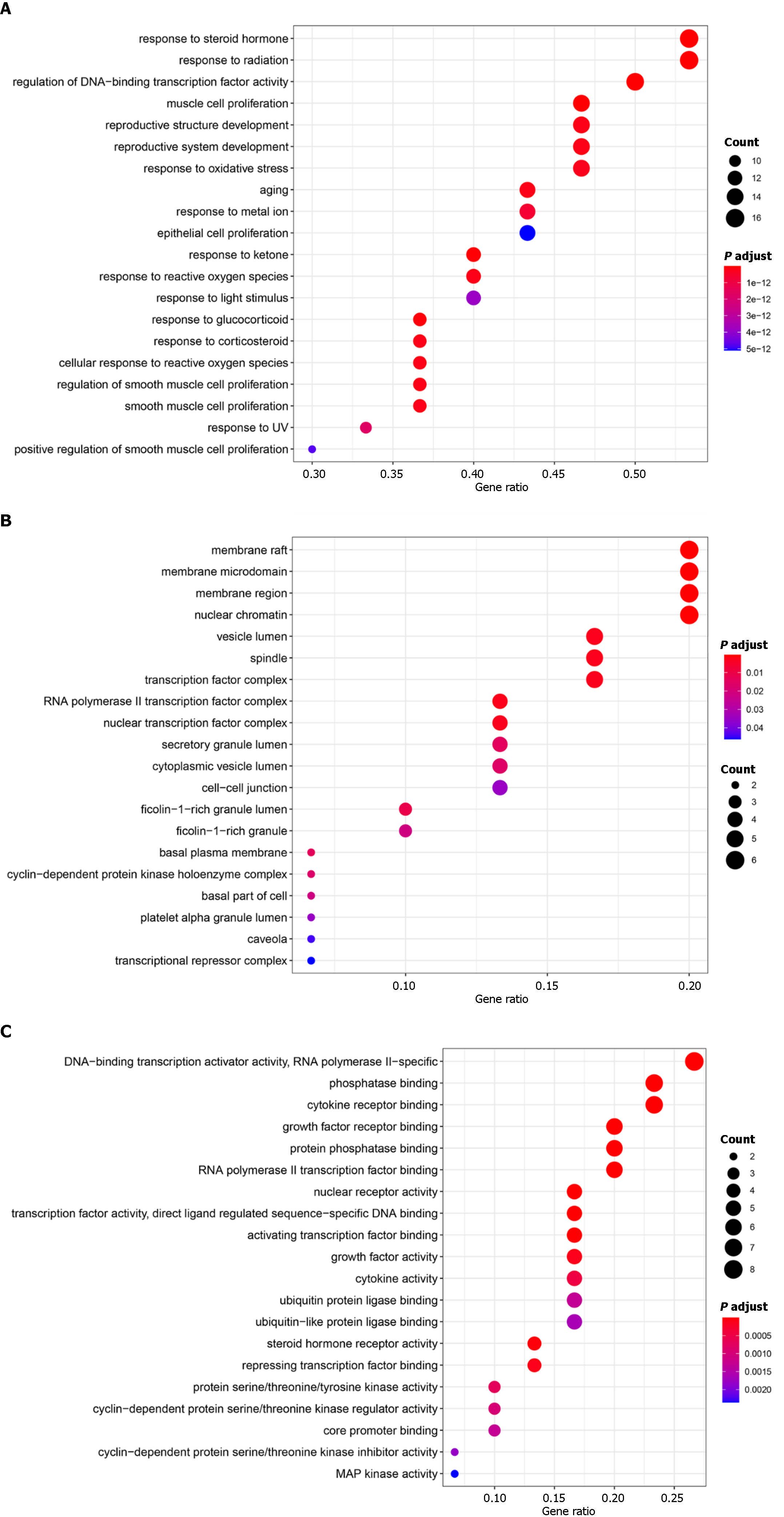
Bioconductor, a library compatible with R software, was used for the GO enrichment analysis of the top targets. Figure 6 shows the results of a screening of the 20 best enrichment results for the three groups (MF, BP, and CC): MF (66 records), BP (1534 records), and CC (22 records). The proteins classified as BP were primarily involved in processes such as responding to a steroid hormone, radiation response, DNA-binding transcription factor activity regulation, reproductive structure development, muscle cell proliferation, reproductive system development, and responding to oxidative stress. The target proteins in the MF category were involved in DNA-binding transcription activator activity, phosphatase binding, cytokine receptor binding, RNA polymerase II-specific binding, growth factor receptor binding, nuclear receptor activity, protein phosphatase binding, RNA polymerase II transcription factor binding, and so on. Proteins associated with the nucleus genome and the plasma membrane were classified based on the CC category. Based on the GO enrichment analysis, the active ingredients of Radix Salviae can bind kinase in the plasma membrane or nuclear chromatin of cells during the processes of oxidative stress, cell proliferation, angiogenesis, inflammatory response, and apoptosis, thereby exerting antioxidative stress, anti-inflammatory, and antiapoptotic effects.
To more thoroughly elucidate the relationship between targets and pathways, we constructed a target pathway interaction network in R using data retrieved from the Bioconductor database. The 20 pathways related to the genes exhibiting the most significant changes (Figure 7). The results showed that the targets were involved in Kaposi sarcoma-associated herpesvirus infection, proteoglycans in cancer, the TNF signaling pathway, the AGE-RAGE signaling pathway in diabetic complications, human cytomegalovirus infection, Epstein–Barr virus infection, the PI3K-Akt signaling pathway, the IL-17 signaling pathway and other pathways. Among these pathways, the largest number of targets pathway is Kaposi sarcoma-associated herpesvirus infection involving RAC-AKT1, IL-6, MAPK1, FOS, VEGFA, PTGS2, JUN, CASP3, MYC, STAT3, MAPK14, RELA, CCND1, ICAM1, CASP9, CDKN1A; The targets included PI3K-Akt signaling pathway involving RAC-AKT1, MYC, IL-6, MAPK1, EGFR, BCL2L1, CCND1, CDKN1A, VEGFA, RELA, ERBB2, MDM2, CASP9, IL-2, IL-4. This results in multiple proteins serving as targets in a single pathway and the presence of the same target protein in multiple pathways. These findings identify these signaling pathways as potential targets for the anti-inflammatory and antiapoptotic effects of the potent pharmaceutical active ingredient identified in Radix Salviae.
DPN, a troublesome chronic disease, is classified as part of the Xiao Ke category in traditional Chinese medicine (numbness, soreness, and coolness of limbs) and is intimately associated with blood stasis. Radix Salviae has been used extensively to treat DPN and has been demonstrated to be effective at increasing blood circulation and removing blood stagnation. However, the underlying mechanism is still vague.
In our work, pharmacological analysis of potential ingredients and network-based research methodologies were used to provide insight into the process of Radix Salviae across various levels of data. Radix Salviae's 81 DPN-related targets and 56 active ingredients were chosen and examined. Moreover, after screening the top 30 targets and merging the findings of GO and KEGG analyses, the possible mechanism of healing in patients treated with Radix Salviae was shown to likely be related to the processes of growth, angiogenesis, the inflammatory response, apoptosis, and oxidative stress.
β-cell proliferation plays an important role in the progression of diabetes-related complications. EGFR is an ErbB receptor that is vital for β cell proliferation. It has been reported that EGFR can regulate cell proliferation through activating the MAPK and PI3K/AKT signaling pathways[17-20].
In hyperglycemia, new blood vessel development is stimulated by glucose, which increases the activity of MMP9 and 2 and accelerates basement membrane breakdown. Angiogenesis involves new blood vessel formation, and one of the key factors in this process is VEGFA, a member of the VEGF family. The proteins MMP9, MMP2, and VEGFA have been shown to play important roles in DPN. Bhatt discovered that inhibiting MMP9 and MMP2 helped ameliorate hy-perglycemia-induced impairments in sensory and motor nerve conduction velocities (i.e., SNCY and MNCV)[21]. Leinninger et al[22] reported elevated amounts of VEGF in the cell nuclei and nerve bundles of diabetic rat sciatic nerves and dorsal root ganglia. In DPN, Kaur et al[23] discovered that the injection of VEGF could normalize nerve blood flow, the nerve transmission rate, and the nerve artery number.
The inflammatory response is a key process in the progression of DPN. RELA and IL-6 are key inflammatory factors that are intimately associated with nerve damage[24]. Zhu et al[25] reported that DPN patients have markedly greater IL-6 levels than healthy individuals without DPN.
The production of free radicals is the main factor in the progression of DPN. An increase in free radicals damages mitochondrial DNA and induces cell apoptosis[26]. Proteins in the Bcl-2 family, both antiapoptotic and proapoptotic, play a role in controlling the apoptotic pathway, which is a tightly controlled method of cell death. After the breakdown of antiapoptotic proteins and proapoptotic proteins, the levels of MMP9 and MMP2 increase, activating Caspase9 and Caspase3[27]. Zhu et al[28] found that the expression of Bax and cleaved caspase-3 increased while that of Bcl-2 decreased in a DPN mouse model.
As a theoretical foundation for future drug research and design, the network structure of active ingredients and targets is an essential method for describing the physiological process of active ingredients. Using MAPK and PI3K/AKT signaling pathway regulation, we determined that luteolin and tanshinone IIA are the primary active ingredients in Radix Salviae and increase cell growth and alleviate the inflammatory response, apoptosis, and reactive stress[29-34]. Thus, Radix Salviae, a novel therapy for DPN, can safely and effectively halt disease development.
Network pharmacology provides an innovative perspective on the study of the active ingredients of TCMs, which allows us to decipher multi-ingredient, multitargets model mechanisms. However, some caveats should be suggested when using pharmacological techniques to predict active ingredients and targets: (1) It is possible that the active ingredients we screened in the TCMSP are not consistent with the actual assimilated ingredients in DPN patients’ blood; (2) Some variation in the expected outcomes across datasets is possible. As a consequence, these prediction findings require additional experimental verification; and (3) We explored the mechanism of Radix Salviae in treating DPN using bioinformatics analysis techniques. However, no specific in vivo or in vitro experiments were conducted to validate these findings. Future studies must address this challenge to better understand the potential of Radix Salviae as a treatment for DPN.
The potential pathways identified in the present study, such as the AGE-RAGE and PI3K-Akt signaling pathways, play crucial roles in the pathophysiology of DPN. Elucidating the biological significance of these pathways and their relationship with known physiological processes in DPN is essential for comprehending the potential mechanisms of Radix Salviae in treating this condition.
The AGE-RAGE signaling pathway is known to be involved in the development and progression of diabetic complications, including DPN. AGEs interact with their receptor (RAGE), leading to the activation of various downstream signaling pathways that contribute to oxidative stress, inflammation, and endothelial dysfunction, all of which are implicated in the pathogenesis of DPN. By targeting this pathway, Radix Salviae may modulate the detrimental effects of AGE-RAGE signaling, potentially alleviating oxidative stress and inflammation and preserving nerve function in DPN patients.
In addition, the PI3K-Akt signaling pathway has been implicated in regulating neuronal survival, growth, and function. Dysregulation of this pathway has been linked to the development of DPN, particularly in relation to neurovascular dysfunction and impaired nerve regeneration. The potential modulation of the PI3K-Akt pathway by Radix Salviae suggests a mechanism by which this pathway could promote neuronal survival, mitigate neurovascular damage, and support nerve regeneration, all of which are critical in managing DPN.
Further exploration of the biological significance of these pathways in the context of DPN will provide valuable insight into the specific mechanisms through which Radix Salviae exerts its therapeutic effects. This deeper understanding can inform the development of targeted interventions and the identification of specific molecular biomarkers for assessing the efficacy of Radix Salviae in clinical applications.
Moreover, the identification of active ingredients, such as luteolin and tanshinone IIA, which are key components of Radix Salviae, reinforces the potential for clinical translation. Investigating the pharmacokinetics, pharmacodynamics, and safety profiles of these specific compounds, either individually or in combination, would be essential for advancing clinical practice involving Radix Salviae-based treatments for DPN. Conducting controlled clinical trials to validate the efficacy and safety of Radix Salviae in treating DPN is a logical next step in translating these findings into clinical applications.
The findings of this study hold promising implications for the clinical management of DPN. By elucidating the potential molecular mechanisms through which Radix Salviae modulates key signaling pathways, such as AGE-RAGE and PI3K-Akt, this research offers valuable insights into the therapeutic potential of Danshen for DPN. However, further experimental validation and clinical studies are warranted to translate these findings into practical applications. Future research endeavors should aim to bridge the gap between these mechanistic insights and their clinical implementation, ultimately advancing the development of novel therapeutic approaches for DPN management.
Diabetic peripheral neuropathy (DPN) is a common and debilitating complication of diabetes, lacking effective treatment options. Radix Salviae (Danshen in Chinese), a traditional Chinese medicine, has shown promise in treating DPN, but its mechanism of action remains unclear.
Given the growing global prevalence of diabetes and the lack of satisfactory treatments for DPN, there is a critical need to investigate potential medicinal interventions, such as Radix Salviae, for the condition. Understanding the molecular mechanisms underlying the therapeutic effects of Radix Salviae on DPN could lead to the development of novel treatments.
This study aims to explore the mechanism of Radix Salviae in treating DPN using network pharmacology. The specific objectives include identifying the active ingredients and target genes of Radix Salviae, investigating the interactions between Radix Salviae and DPN-related target genes, and elucidating the potential pathways and processes involved in its therapeutic effects on DPN.
The research utilized the Traditional Chinese medicine pharmacology database and analysis platform to identify active ingredients and target genes of Radix Salviae. DPN-related target genes were obtained from public databases, and network pharmacology approaches were employed to construct interaction networks, perform enrichment analyses, and explore potential mechanisms.
The study identified 56 active components, 108 targets, and 4581 DPN-related target genes for Radix Salviae. Eighty-one common targets were discovered between Radix Salviae and DPN. Functional annotation and pathway enrichment analyses highlighted the involvement of the AGE-RAGE and PI3K-Akt signaling pathways in the therapeutic effects of Radix Salviae on DPN.
The findings suggest that Radix Salviae may exert its therapeutic effects on DPN by regulating inflammation, apoptosis, and oxidative stress through the identified pathways. This study provides a theoretical basis for the potential role of Radix Salviae in improving symptoms of DPN and offers valuable insights for future research and drug development in the field.
Further experimental validation and clinical studies are needed to confirm the findings and understand the potential of Radix Salviae as a treatment for DPN. Subsequent research should focus on elucidating the specific biological processes and molecular targets influenced by Radix Salviae, as well as exploring its clinical applicability and safety in the context of DPN treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Desoye G, Austria; Horowitz M, Australia; Papazafiropoulou A, Greece; Serban V, Romania S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Barrell K, Smith AG. Peripheral Neuropathy. Med Clin North Am. 2019;103:383-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. 2017;8:646-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Yonel Z, Sharma P, Gray LJ. Use of Dental Practices for the Identification of Adults With Undiagnosed Type 2 Diabetes Mellitus or Nondiabetic Hyperglycemia: Protocol for a Systematic Review. JMIR Res Protoc. 2018;7:e11843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Archvadze A, Kistauri A, Gongadze N, Makharadze T, Chirakadze K. Medical Basis Of Diabetic Neuropathy Formation (Review). Georgian Med News. 2018;154-162. [PubMed] |
| 5. | Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 728] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 6. | Charnogursky G, Lee H, Lopez N. Diabetic neuropathy. Handb Clin Neurol. 2014;120:773-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Yu M, Song X, Yang W, Li Z, Ma X, Hao C. Identify the Key Active Ingredients and Pharmacological Mechanisms of Compound XiongShao Capsule in Treating Diabetic Peripheral Neuropathy by Network Pharmacology Approach. Evid Based Complement Alternat Med. 2019;2019:5801591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Cheng B, Gong H, Li X, Sun Y, Chen H, Zhang X, Wu Q, Zheng L, Huang K. Salvianolic acid B inhibits the amyloid formation of human islet amyloid polypeptide and protects pancreatic beta-cells against cytotoxicity. Proteins. 2013;81:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Qian S, Huo D, Wang S, Qian Q. Inhibition of glucose-induced vascular endothelial growth factor expression by Salvia miltiorrhiza hydrophilic extract in human microvascular endothelial cells: evidence for mitochondrial oxidative stress. J Ethnopharmacol. 2011;137:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Ren Y, Tao S, Zheng S, Zhao M, Zhu Y, Yang J, Wu Y. Salvianolic acid B improves vascular endothelial function in diabetic rats with blood glucose fluctuations via suppression of endothelial cell apoptosis. Eur J Pharmacol. 2016;791:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Ri-Ge-le A, Guo ZL, Wang Q, Zhang BJ, Kong DW, Yang WQ, Yu YB, Zhang L. Tanshinone IIA Improves Painful Diabetic Neuropathy by Suppressing the Expression and Activity of Voltage-Gated Sodium Channel in Rat Dorsal Root Ganglia. Exp Clin Endocrinol Diabetes. 2018;126:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Yang XY, Qiang GF, Zhang L, Zhu XM, Wang SB, Sun L, Yang HG, Du GH. Salvianolic acid A protects against vascular endothelial dysfunction in high-fat diet fed and streptozotocin-induced diabetic rats. J Asian Nat Prod Res. 2011;13:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Yu X, Zhang L, Yang X, Huang H, Huang Z, Shi L, Zhang H, Du G. Salvianolic acid A protects the peripheral nerve function in diabetic rats through regulation of the AMPK-PGC1α-Sirt3 axis. Molecules. 2012;17:11216-11228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Chen J, Ji H, Xiao ZG, Shen P, Xu LH. Protective effects of Danshen injection against erectile dysfunction via suppression of endoplasmic reticulum stress activation in a streptozotocin-induced diabetic rat model. BMC Complement Altern Med. 2018;18:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Zhao W, Yuan Y, Zhao H, Han Y, Chen X. Aqueous extract of Salvia miltiorrhiza Bunge-Radix Puerariae herb pair ameliorates diabetic vascular injury by inhibiting oxidative stress in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2019;129:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Yang M, Chen J, Xu L, Shi X, Zhou X, An R, Wang X. A Network Pharmacology Approach to Uncover the Molecular Mechanisms of Herbal Formula Ban-Xia-Xie-Xin-Tang. Evid Based Complement Alternat Med. 2018;2018:4050714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4880] [Cited by in RCA: 4814] [Article Influence: 267.4] [Reference Citation Analysis (0)] |
| 19. | Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391-7393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Bhatt LK, Veeranjaneyulu A. Minocycline with aspirin: a therapeutic approach in the treatment of diabetic neuropathy. Neurol Sci. 2010;31:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Leinninger GM, Vincent AM, Feldman EL. The role of growth factors in diabetic peripheral neuropathy. J Peripher Nerv Syst. 2004;9:26-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Kaur S, Pandhi P, Dutta P. Painful diabetic neuropathy: an update. Ann Neurosci. 2011;18:168-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 24. | Zhang HH, Hu J, Zhou YL, Qin X, Song ZY, Yang PP, Hu S, Jiang X, Xu GY. Promoted Interaction of Nuclear Factor-κB With Demethylated Purinergic P2X3 Receptor Gene Contributes to Neuropathic Pain in Rats With Diabetes. Diabetes. 2015;64:4272-4284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Zhu T, Meng Q, Ji J, Zhang L, Lou X. TLR4 and Caveolin-1 in Monocytes Are Associated With Inflammatory Conditions in Diabetic Neuropathy. Clin Transl Sci. 2017;10:178-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid Med Cell Longev. 2013;2013:168039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Inam-U-Llah, Shi X, Zhang M, Li K, Wu P, Suleman R, Shahbaz M, Taj A, Piao F. Protective Effect of Taurine on Apoptosis of Spinal Cord Cells in Diabetic Neuropathy Rats. Adv Exp Med Biol. 2019;1155:875-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Zhu L, Hao J, Cheng M, Zhang C, Huo C, Liu Y, Du W, Zhang X. Hyperglycemia-induced Bcl-2/Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp Cell Res. 2018;367:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Feng FB, Qiu HY. Neuroprotective effect of tanshinone IIA against neuropathic pain in diabetic rats through the Nrf2/ARE and NF-κB signaling pathways. Kaohsiung J Med Sci. 2018;34:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Li M, Li Q, Zhao Q, Zhang J, Lin J. Luteolin improves the impaired nerve functions in diabetic neuropathy: behavioral and biochemical evidences. Int J Clin Exp Pathol. 2015;8:10112-10120. [PubMed] |
| 31. | Liu Y, Wang L, Li X, Lv C, Feng D, Luo Z. Tanshinone IIA improves impaired nerve functions in experimental diabetic rats. Biochem Biophys Res Commun. 2010;399:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Lu M, Luo Y, Hu P, Dou L, Huang S. Tanshinone IIA inhibits AGEs-induced proliferation and migration of cultured vascular smooth muscle cells by suppressing ERK1/2 MAPK signaling. Iran J Basic Med Sci. 2018;21:83-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Nabavi SF, Braidy N, Gortzi O, Sobarzo-Sanchez E, Daglia M, Skalicka-Woźniak K, Nabavi SM. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res Bull. 2015;119:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 34. | Su CC, Chiu TL. Tanshinone IIA decreases the protein expression of EGFR, and IGFR blocking the PI3K/Akt/mTOR pathway in gastric carcinoma AGS cells both in vitro and in vivo. Oncol Rep. 2016;36:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |