Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.935
Peer-review started: January 27, 2024
First decision: February 5, 2024
Revised: February 7, 2024
Accepted: March 11, 2024
Article in press: March 11, 2024
Published online: May 15, 2024
Processing time: 104 Days and 10.4 Hours
In recent years, the emergence of multiplex technology that can simultaneously measure multiple anti-islet autoantibodies has become particularly valuable for the staging and early diagnosis of immune-mediated type 1 diabetes (T1D). While it has been established that 20%-30% of T1D patients suffer from autoimmune thyroid disease (AITD), there is limited available data regarding the presence of anti-islet autoantibodies in AITD patients. Among commercially available anti-islet autoantibodies, glutamic acid decarboxylase 65 autoantibodies (GADAs) are often the first marker measured in general clinical practice.
To investigate the frequency of anti-islet autoantibodies in AITD patients.
Our study involved four hundred ninety-five AITD patients, categorized into three distinct groups: AITD with T1D (n = 18), AITD with phenotypic type 2 diabetes (T2D) (n = 81), and AITD without diabetes (n = 396), and the enzyme-linked immunosorbent assay (ELISA) was employed to determine the frequencies of 3 Screen Islet Cell Autoantibody (3 Screen ICA), GADA, insulinoma-associated antigen-2 autoantibodies (IA-2As), and zinc transporter 8 autoantibodies (ZnT8As) within these groups.
The frequency of 3 Screen ICA in AITD patients with T1D, T2D, and those without diabetes were 88.9%, 6.2%, and 5.1%, respectively, with no significant difference seen between the latter two groups. Notably, the frequency of 3 Screen ICA was 11.1% higher in AITD patients with T1D, 1.3% higher in AITD patients with T2D, and 1.1% higher in AITD patients without diabetes compared to GADA, respectively. Furthermore, 12.5%, 20.0%, and 20.0% of the 3 Screen ICA-positive patients were negative for GADA. Additionally, 1.3% of the AITD patients who tested negative for 3 Screen ICA in both the AITD with T2D and non-diabetic AITD groups were found to be positive for individual autoantibodies. Among the 3 Screen ICA-positive patients, there was a significantly higher proportion of individuals with multiple autoantibodies in AITD patients with T1D compared to those without diabetes (37.5% vs 5.0%, P < 0.05). However, this proportion was similar to that in AITD patients with T2D (20.0%). Nevertheless, there was no significant difference in 3 Screen ICA titers between AITD patients with T1D and those without diabetes (436.8 ± 66.4 vs 308.1 ± 66.4 index). Additionally, no significant difference in 3 Screen ICA titers was observed between Graves’ disease and Hashimoto’s thyroiditis in any of the groups.
Our findings reveal that some AITD patients without diabetes exhibit 3 Screen ICA titers comparable to those in AITD patients with T1D. Thus, 3 Screen ICA outperforms GADA in identifying latent anti-islet autoantibody-positive individuals among AITD patients.
Core Tip: This study presents the clinical utility of 3 Screen Islet Cell Autoantibody enzyme-linked immunosorbent assay (3 Screen ICA ELISA) in identifying anti-islet autoantibody-positive individuals among patients with autoimmune thyroid disease (AITD). Our findings demonstrate that 5.1% of AITD patients test positive for 3 Screen ICA with titers comparable to those of patients with type 1 diabetes (T1D) despite not having diabetes themselves. These results have the potential to enhance the staging and early diagnosis of T1D in AITD patients in clinical practice.
- Citation: Kawasaki E, Tamai H, Fukuyama T, Sagara Y, Hidaka R, Uchida A, Tojikubo M, Tatsumoto N, Akehi Y, Hiromatsu Y. Enzyme-linked immunosorbent assay of 3 Screen Islet Cell Autoantibody in patients with autoimmune thyroid disease. World J Diabetes 2024; 15(5): 935-944
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/935.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.935
Anti-islet autoantibodies, including autoantibodies targeting insulin (IAA), insulinoma-associated antigen-2 autoantibodies (IA-2As), glutamic acid decarboxylase 65 autoantibodies (GADAs), and zinc transporter 8 autoantibodies (ZnT8As), are important serological markers for prediction and diagnosis of immune-mediated type 1 diabetes (T1D)[1]. However, it has been reported that anti-islet autoantibodies can also be detected in other instances of autoimmune disease, such as stiff-person syndrome and autoimmune polyglandular syndrome[2].
One notable association is the co-occurrence of autoimmune thyroid disease (AITD) in 20%-30% of T1D patients, and conversely, it has been reported that 4%-8% of AITD patients are complicated with T1D[3-6]. Additionally, studies have reported the presence of GADA, typically the first diagnostic marker for T1D in general clinical practice[1], even in non-diabetic AITD patients[7].
In recent years, multiplex technology has emerged, allowing for the simultaneous measurement of multiple anti-islet autoantibodies. Technologies like multiplex electrochemiluminescence assay[8] and multiplex antibody detection by agglutination-PCR[9] have been developed to facilitate the staging and early diagnosis of T1D. In Japan, a 3 Screen Islet Cell Autoantibody enzyme-linked immunosorbent assay (3 Screen ICA ELISA), capable of concurrently measuring GADA, IA-2A, and ZnT8A, received manufacturing and sales approval in 2023 and is expected for application in general clinical practice. While there are existing reports on the use of 3 Screen ICA ELISA in patients with T1D[10-13], studies on anti-islet autoantibodies beyond GADA in AITD patients remain limited. Therefore, this study was conducted, employing the 3 Screen ICA ELISA, to investigate the prevalence of anti-islet autoantibodies in AITD patients.
Sera samples were collected from 495 Japanese patients diagnosed with AITD. This cohort comprised 298 patients with Graves’ disease and 197 patients with Hashimoto’s thyroiditis, who had sought medical care at our outpatient clinic of the Diabetes, Thyroid, and Endocrine Center at Shin-Koga Hospital between March 2022 and December 2022. The AITD patients were subsequently categorized into three distinct groups according to their diabetes status: AITD with T1D (n = 18), AITD with phenotypic type 2 diabetes (T2D; n = 81), and AITD without diabetes (n = 396).
AITD diagnoses were established through clinical evaluations conducted by endocrinologists. The confirmation of AITD involved clinical manifestations, abnormal levels of thyroid hormones, and the presence of autoantibodies against thyroid peroxidase, thyroglobulin, and/or thyrotropin receptors, along with ultrasound examinations. Diagnosing T1D and T2D adhered to the classification and diagnostic criteria stipulated by the American Diabetes Association[14].
Table 1 outlines the clinical characteristics of the AITD patient cohort. Sera samples utilized in this study were stored at -20℃ until autoantibody measurement.
| All patients, n = 495 | AITD with T1D, n = 18 | AITD with T2D, n = 81 | AITD without diabetes, n = 396 | |
| Gender (M:F) | 104:391 | 4:14 | 27:54 | 73:323 |
| Age (yr) | 59.8 ± 0.7 | 60.4 ± 3.8 | 66.6 ± 1.2 | 58.3 ± 0.8 |
| Graves/ Hashimoto | 298/197 | 5/13 | 40/41 | 253/143 |
This study’s protocol has been approved by the ethics committee of Shin-Koga Hospital.
The 3 Screen ICA kit, designed to simultaneously measure GADA, IA-2A, and ZnT8A, is a commercially available bridging-type ELISA developed by RSR Ltd. (Cardiff, United Kingdom). This kit utilizes full-length GAD65, the intracellular domain of IA-2 (amino acids 604-979), and dimeric carboxy-terminal domains of ZnT8 (aa275-369) carrying either 325Trp or 325Arg as antigens[13].
In brief, 25 μL of serum sample was applied to an ELISA plate well coated with unlabeled recombinant GAD65, IA-2, and ZnT8 proteins. The samples were subsequently incubated at 4°C overnight before washing the wells 3 times with wash buffer. Then, 100 μL of a biotinylated mixture of GAD65, IA-2, and ZnT8 was added to each well, and the antigen-antibody mixture was incubated at 4°C for 1 h. After removing any unbound biotinylated antigens by washing, 100 μL of streptavidin-peroxidase conjugate was added to each well and further incubated at room temperature for 20 min. After a final aspiration and wash step, 100 μL of peroxidase substrate (tetramethylbenzidine) was added. The reaction was allowed to proceed for 20 min at room temperature before it was stopped by adding 100 μL of stop solution (0.25 mol/L H2SO4). The optical density (OD) of the plate wells was then measured at both 450 nm and 405 nm using an ELISA plate reader. Autoantibody levels were expressed as an index defined as (OD of the test sample/OD of the reference preparation) × 100. Samples were considered positive when the index exceeded 20. The inter-assay and intra-assay coefficient variation (CV) values, using GADA-single-positive, IA-2A-single-positive, and ZnT8A-single-positive sera, were as follows: inter-assay CV values of 2.2%, 3.0%, 3.8% (n = 7) and intra-assay CV of 3.4%, 1.5%, and 4.0% (n = 5), respectively. In the IASP 2020 workshop (Lab ID 1801), the assay demonstrated sensitivities and specificities of 96.0% and 100%, respectively.
Additionally, we measured individual anti-islet autoantibodies, namely GADA, IA-2A, and ZnT8A, using bivalent ELISA kits (RSR Ltd.) in conjunction with corresponding biotinylated proteins, following the previously described methods[13]. Individual autoantibody levels were determined in the same sera samples used for the 3 Screen ICA measurement. Results were read from a calibration curve constructed in the same run as the calibrators and expressed in U/mL. The cut-off value for GADA was 5.0 U/mL, 0.6 U/mL for the IA-2A, and 10 U/mL for the ZnT8A, respectively. In the IASP 2020 workshop, the assay demonstrated sensitivities and specificities of 90.0% and 97.8% for GADA, 72.0% and 97.8% for IA-2A, 76.0% and 98.9% for ZnT8A, respectively.
The data are presented as either mean ± SE or median (range) with frequencies displayed as n (%) unless otherwise specified. To compare prevalence where applicable, we employed the Chi-square test or Fisher's exact test. Differences in nonparametric data were assessed using the Mann-Whitney U test or Kruskal-Wallis test, followed by Bonferroni’s multiple comparison test. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using both StatView statistical software (version 5.0; SAS Institute, Cary, NC, United States) and SigmaPlot software (version 14.0, Systat Software Inc., San Jose, CA, United States).
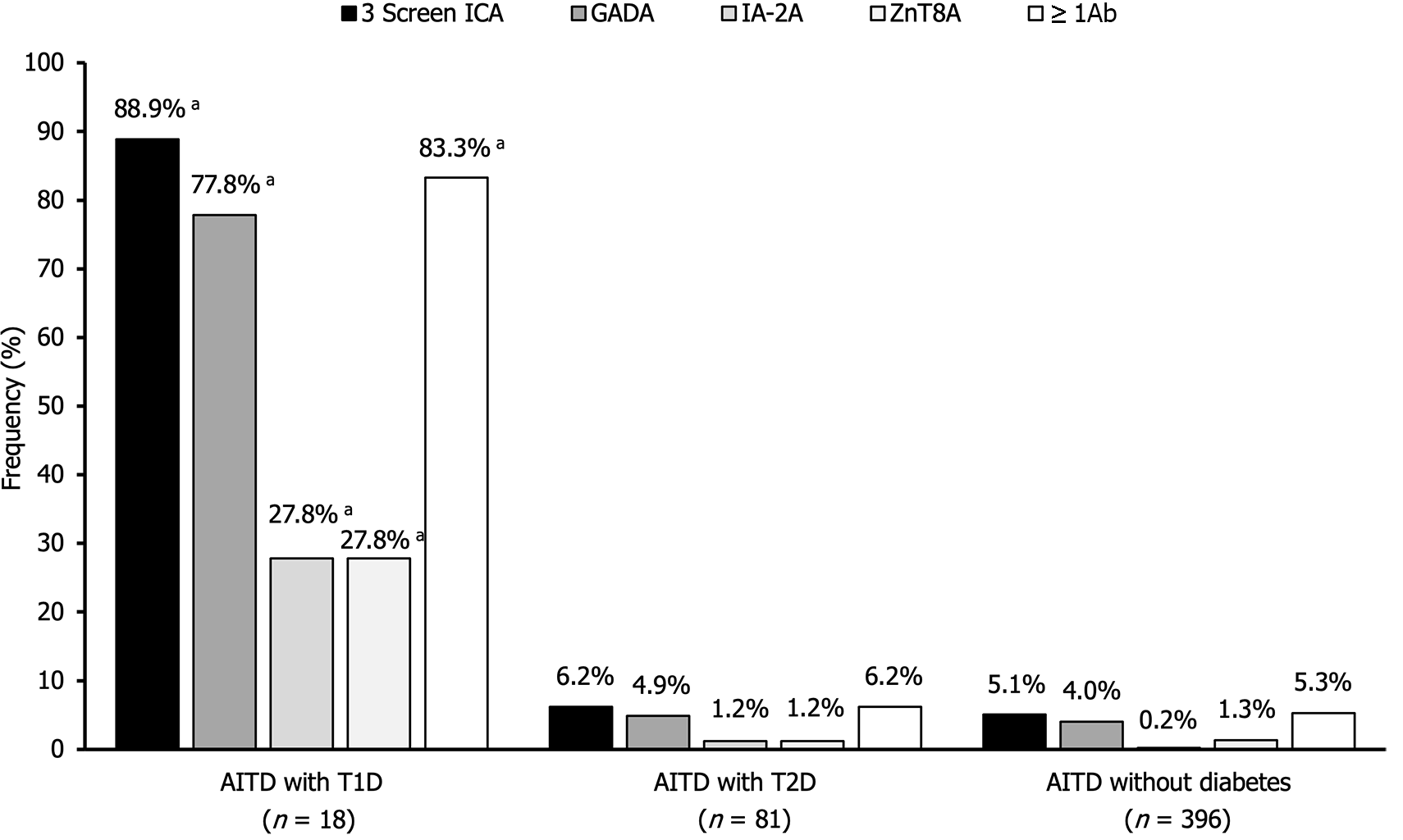
Figure 1 illustrates the prevalence of 3 Screen ICA, GADA, IA-2A, and ZnT8A in the three AITD groups. The frequencies of 3 Screen ICA in AITD with T1D, AITD with T2D, and AITD without diabetes were 88.9%, 6.2%, and 5.1%, respectively. Notably, while the frequency of 3 Screen ICA was significantly higher in AITD with T1D compared to the other two groups (P < 0.0001), no significant difference in frequency was observed between the latter two groups. When compared to GADA, the frequency of 3 Screen ICA was 11.1% higher in AITD patients with T1D, 1.3% higher in AITD patients with T2D, and 1.1% higher in AITD patients without diabetes.
The frequencies of IA-2A and ZnT8A in AITD patients with T1D were also significantly higher than those in the other two groups (P < 0.0001). Combinatorial analysis of individual autoantibodies revealed that 83.3% of AITD patients with T1D, 6.2% of AITD patients with T2D, and 5.3% of AITD patients without diabetes tested positive for one or more of these autoantibodies.
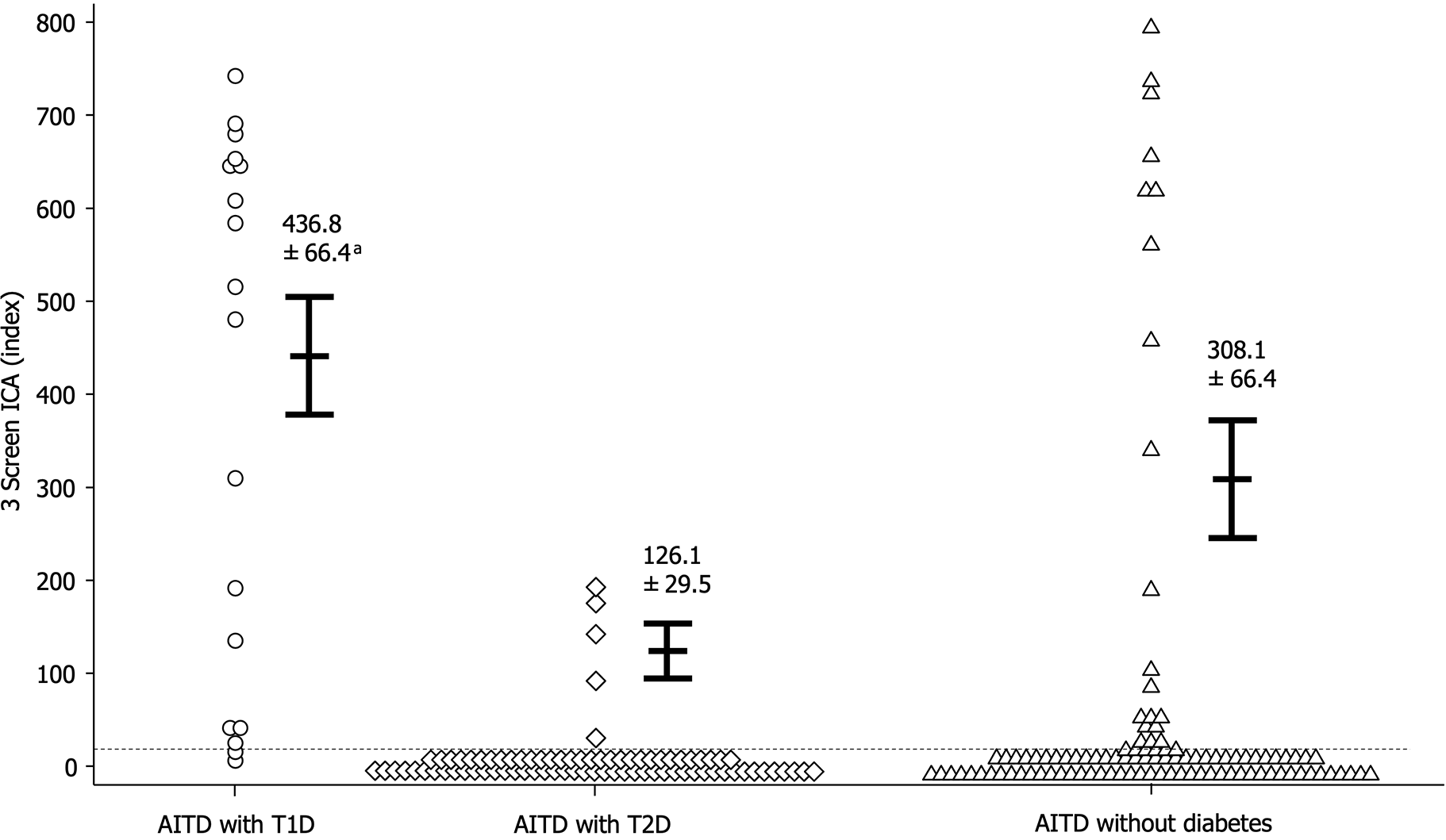
Figure 2 illustrates the comparison of 3 Screen ICA titers among the three groups. While the 3 Screen ICA titer in AITD patients with T1D was significantly higher than that in AITD patients with T2D (436.8 ± 66.4 vs 126.1 ± 29.5 index, P < 0.05), there was no significant difference in 3 Screen ICA titers between AITD patients with T1D and those without diabetes (308.1 ± 66.4 index).
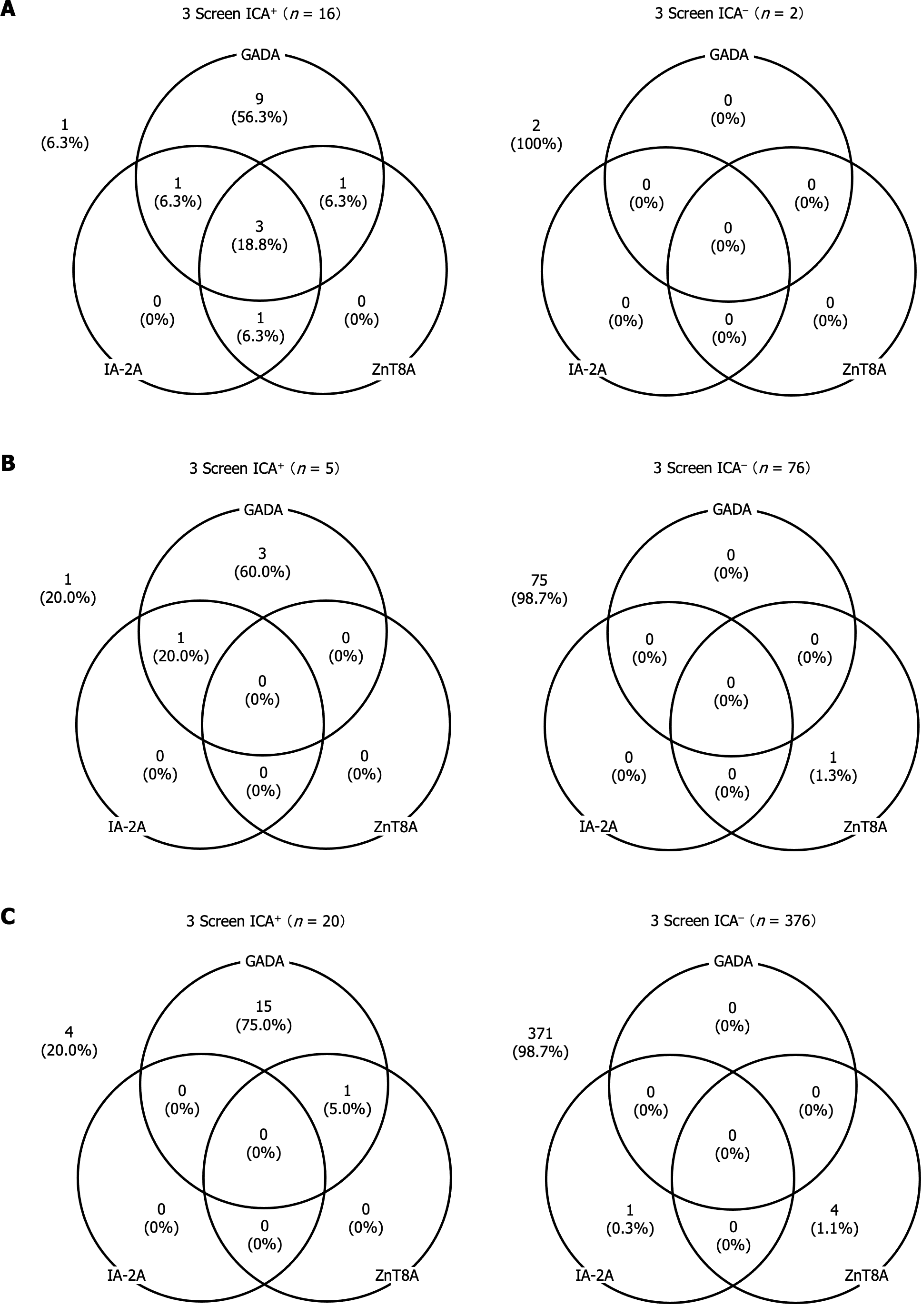
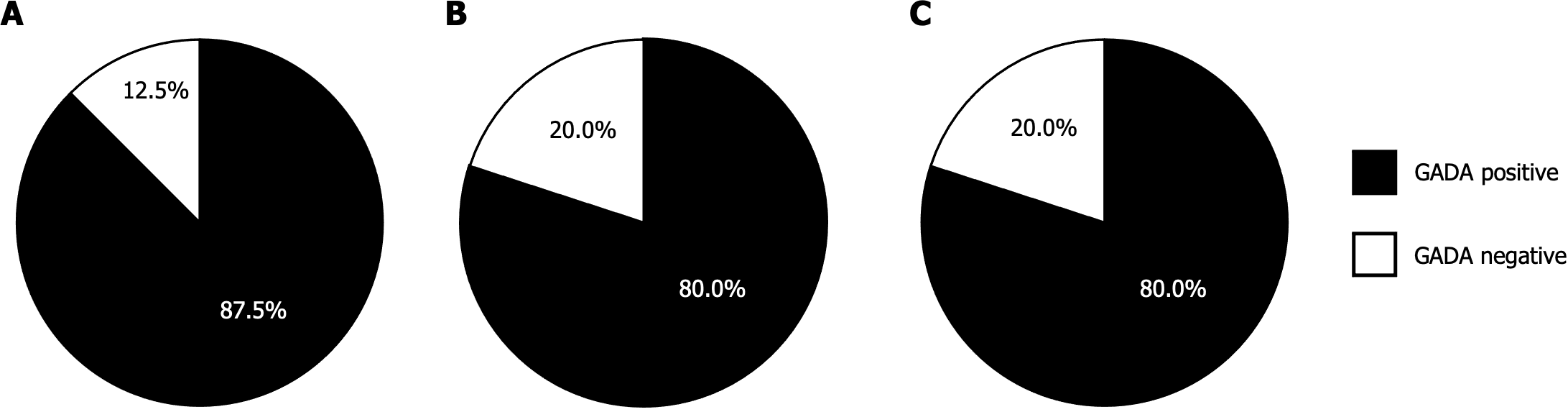
Figure 3A reveals that in AITD patients with T1D, 56.3% of those who tested positive for 3 Screen ICA were positive for GADA alone, while 37.5% (6 out of 16) exhibited positivity for two or more individual autoantibodies. Additionally, one patient out of 16 (6.3%) who tested positive for 3 Screen ICA was negative for individual autoantibodies. In contrast, all patients who tested negative for 3 Screen ICA were also negative for individual autoantibodies.
In AITD patients with T2D and those without diabetes, the distribution of individual autoantibodies was quite similar. Approximately 60%-75% of 3 Screen ICA-positive patients in both groups were positive for GADA alone. Moreover, only 1.3% of 3 Screen ICA-negative patients exhibited positive solely for either IA-2A or ZnT8A (Figure 3B and C). It is noteworthy that among 3 Screen ICA-positive patients, the proportion of individuals positive for multiple individual autoantibodies was significantly higher in AITD patients with T1D than in those without diabetes (37.5% vs 5.0%, P < 0.05). However, this proportion was comparable to that in AITD patients with T2D (20.0%).
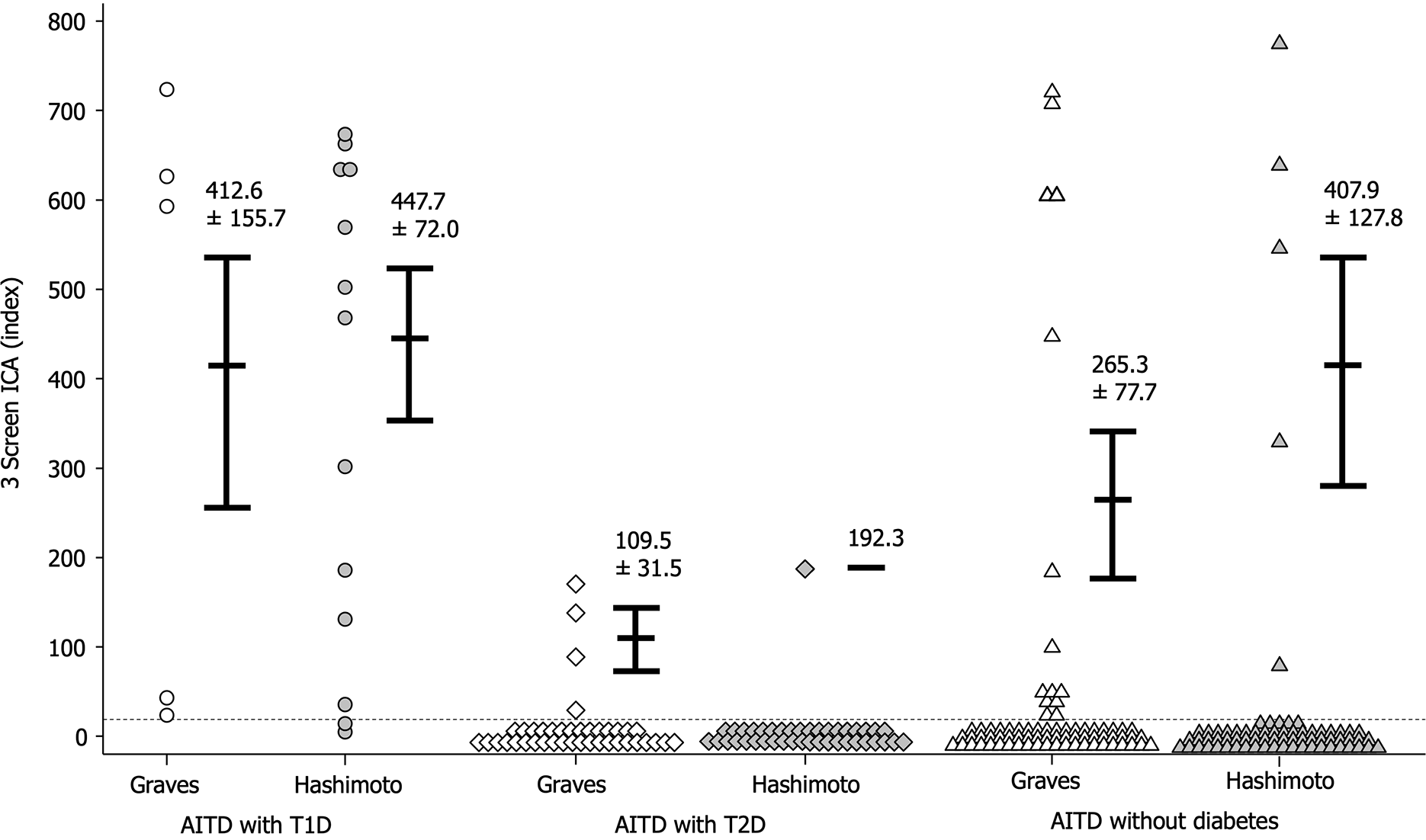
The frequencies of 3 Screen ICA and individual autoantibodies were similar between Graves’ disease and Hashimoto’s thyroiditis across all 3 groups (Table 2). Moreover, there was no significant difference in 3 Screen ICA titers between Graves’ disease and Hashimoto’s thyroiditis in any of the groups (Figure 4).
| AITD with T1D | AITD with T2D | AITD without diabetes | ||||
| Graves, n = 5 | Hashimoto, n = 13 | Graves, n =40 | Hashimoto, n = 41 | Graves, n = 253 | Hashimoto, n = 143 | |
| 3 Screen ICA | 5 (100.0) | 11 (84.6) | 4 (10.0) | 1 (2.4) | 14 (5.5) | 6 (4.2) |
| GADA | 4 (80.0) | 10 (76.9) | 3 (7.5) | 1 (2.4) | 11 (4.3) | 5 (3.5) |
| IA-2A | 1 (20.0) | 3 (23.1) | 1 (2.5) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| ZnT8A | 1 (20.0) | 4 (30.8) | 0 (0.0) | 1 (2.4) | 1 (0.4) | 4 (2.8) |
| 1Ab (+) | 3 (60.0) | 6 (46.2) | 2 (5.0) | 2 (4.9) | 13 (5.1) | 7 (4.9) |
| 2Abs (+) | 0 (0.0) | 3 (23.1) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| 3Abs (+) | 1 (20.0) | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
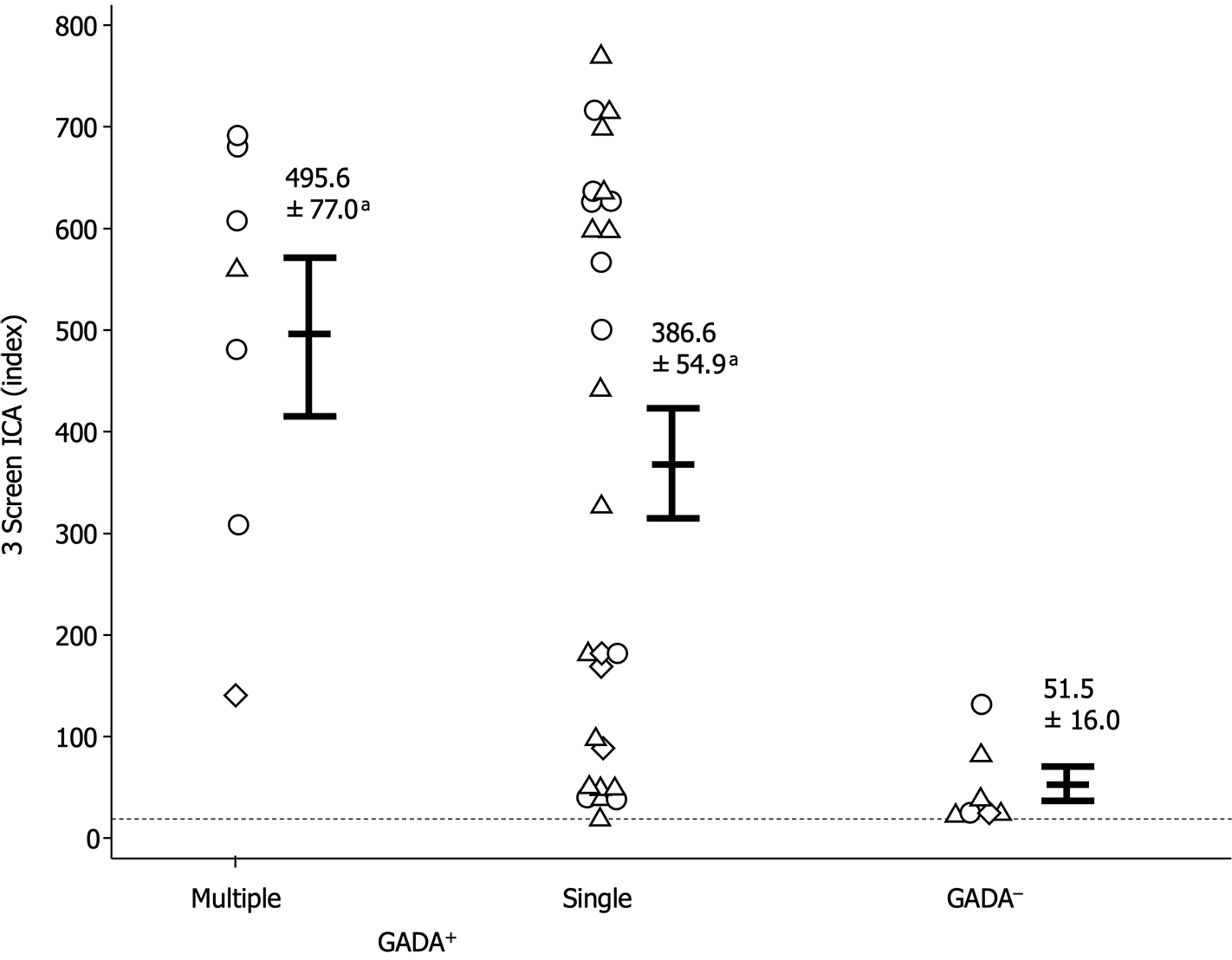
Given that GADA is the anti-islet autoantibody most frequently detected in T1D patients and is typically the initial marker assessed in general clinical practice, we sought to compare the clinical utility of 3 Screen ICA with GADA. As depicted in Figure 5, among the 3 Screen ICA-positive patients, 12.5% of AITD patients with T1D, 20.0% of AITD patients with T2D, and 20.0% of those without diabetes were negative for GADA. Furthermore, 3 Screen ICA titers were significantly higher in GADA-positive patients than in GADA-negative patients (394.7 ± 46.8 vs 51.5 ± 16.0 index, P < 0.001). Within the subset of GADA-positive patients, there was no significant difference in 3 Screen ICA titers between those with multiple individual autoantibodies and those with GADA alone (495.6 ± 77.0 vs 386 ± 54.9 index; Figure 6).
In the present study, we have made several key observations: (1) 3 Screen ICA Detection in AITD Patients: Our findings indicate that 3 Screen ICA is not exclusive to AITD patients with T1D but is also present in AITD patients with T2D and even those without diabetes; (2) Enhanced Sensitivity of 3 Screen ICA: We have demonstrated that 3 Screen ICA exhibits superior sensitivity in identifying individuals with anti-islet autoantibodies compared to GADA; and (3) Comparable 3 Screen ICA Titers: Remarkably, non-diabetic AITD patients displayed 3 Screen ICA titers comparable to those observed in T1D patients.
T1D and AITD are both organ-specific autoimmune diseases characterized by autoimmune responses targeting pancreatic β cells and the thyroid gland, respectively. Several studies have documented an increased prevalence of T1D in individuals with AITD and vice versa[15,16]. It is well-established that AITD is present in 20%-30% of T1D patients, with its frequency estimated at > 90% among T1D patients with coexisting autoimmune diseases[17,18]. Additionally, cross-sectional studies have reported a T1D prevalence of approximately 8% in patients with AITD[5].
Anti-islet autoantibodies represent a hallmark of T1D and serve as important markers for predicting and diagnosing the onset of the disease[1]. In a previous study, we reported that approximately 80% of Japanese patients with T1D tested positive for 3 Screen ICA, with significantly higher titers observed in patients with autoimmune diseases than those without such conditions[13]. Moreover, we found that in AITD patients without diabetes, GADA was detected in 6%-8% and IAA in approximately 4% using the radioimmunoassay (RIA) method. Interestingly, the GADA titers in these patients were lower than those observed in T1D patients, and this phenomenon was unrelated to the expression of IAA[7]. Notably, we also reported a case of an AITD patient who tested positive for anti-islet autoantibodies and was subsequently diagnosed with T1D a decade later[19].
In this study, we detected 3 Screen ICA in approximately 5%-6% of AITD patients without T1D. Intriguingly, among the individuals who tested positive for 3 Screen ICA, 20% were negative for GADA (Figure 5). Furthermore, we demonstrated that 3 Screen ICA titers in AITD patients without diabetes were comparable to those observed in AITD patients with T1D (Figure 2). The 3 Screen ICA assay employs a modified ELISA format, which relies on the ability of autoantibodies to form a bridge between recombinant autoantigens coated on the ELISA plate and biotin-labeled corresponding autoantigens. This approach allows for the detection of autoantibody-bound antigens rather than the immunoglobulins themselves. Notably, this ELISA method is known for its exclusive detection of high-affinity autoantibodies associated with the onset of T1D, in contrast to conventional RIA and radioligand binding assay methods[20,21]. Consequently, AITD patients who test positive for 3 Screen ICA may be at a heightened risk of developing T1D, and 3 Screen ICA is believed to possess greater clinical utility than GADA. To establish this, prospective follow-up studies tracking glucose tolerance and endogenous insulin secretion in 3 Screen ICA-positive patients will be necessary.
This study has several limitations to report. First, the number of patients in each study group, especially AITD patients with T1D and those with T2D was limited. Second, this study was cross-sectional and lacked data on insulin secretory capacity. Therefore, these findings must be validated in larger independent prospective studies that include assessments of glucose tolerance and endogenous insulin secretion in AITD patients with T2D and those without diabetes.
In conclusion, this study highlights that approximately 5% of AITD patients without diabetes exhibit 3 Screen ICA titers comparable to those observed in AITD patients with T1D. 3 Screen ICA proves to be superior to GADA in identifying latent anti-islet autoantibody-positive individuals among AITD patients.
Anti-islet autoantibodies serve as valuable markers for predicting and diagnosing type 1 diabetes (T1D). It is well-established that 20%-30% of T1D patients also have autoimmune thyroid disease (AITD). However, data regarding anti-islet autoantibodies in AITD patients remain limited. The 3 Screen Islet Cell Autoantibody enzyme-linked immunosorbent assay (3 Screen ICA ELISA) is a multiplex anti-islet autoantibody assay capable of simultaneously measuring autoantibodies against glutamic acid decarboxylase (GADA), insulinoma-associated antigen-2 (IA-2A) and zinc transporter 8 (ZnT8A).
The motivation behind this research is to investigate the frequency and titer of anti-islet autoantibodies in patients with AITD, aiming to facilitate the staging and early diagnosis of T1D.
The primary objectives of this research are as follows: (1) To determine the frequency and titer of anti-islet autoantibodies in AITD patients using the 3 Screen ICA ELISA method; and (2) To compare these findings to those of individual autoantibodies, particularly GADA, which are commonly the first markers assessed in general clinical practice.
The research methodology involved the following steps: (1) Selection of a cohort of 495 patients with AITD, categorized into three groups: AITD with T1D (n = 18), AITD with phenotypic type 2 diabetes (T2D) (n = 81), and AITD without diabetes (n = 396); and (2) Examination of the frequency of anti-islet autoantibodies, including 3 Screen ICA, GADA, IA-2A, and ZnT8A using the bridging-type ELISA method.
The key findings of the study include: (1) Frequencies of 3 Screen ICA in AITD patients with T1D, AITD patients with T2D, and those without diabetes being 88.9%, 6.2%, and 5.1%, with no significant difference between the latter two groups; (2) The frequency of 3 Screen ICA was 11.1% higher in AITD patients with T1D, 1.3% higher in AITD patients with T2D, and 1.1% higher in those without diabetes compared to GADA, respectively, and a significant proportion of 3 Screen ICA-positive patients were negative for GADA (12.5%, 20.0%, and 20.0%); and (3) There was no significant difference in 3 Screen ICA titers between AITD patients with T1D and those without diabetes (436.8 ± 66.4 vs 308.1 ± 66.4 index).
This study concludes that some AITD patients without diabetes exhibit comparable 3 Screen ICA titers to those observed in AITD patients with T1D. These findings suggest that 3 Screen ICA is outperforms GADA in identifying latent anti-islet autoantibody-positive individuals within the AITD patient population.
To further investigate the predictive potential of 3 Screen ICA for the development of T1D, it is recommended that prospective studies be conducted in the future. These studies should focus on tracking changes in glucose tolerance and endogenous insulin secretion in AITD patients with T2D and those without diabetes. Such investigations will provide valuable insights into the clinical utility of 3 Screen ICA in identifying individuals at risk of transitioning to T1D.
The authors extend their gratitude to Ms. Fumi Yamaguchi for her invaluable technical assistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Duan W, China S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Kawasaki E. Anti-Islet Autoantibodies in Type 1 Diabetes. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 2. | Hansen MP, Matheis N, Kahaly GJ. Type 1 diabetes and polyglandular autoimmune syndrome: A review. World J Diabetes. 2015;6:67-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in Type 1 diabetes: systematic review and meta-analysis. Diabet Med. 2014;31:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Jin P, Huang G, Lin J, Yang L, Xiang B, Zhou W, Zhou Z. High titre of antiglutamic acid decarboxylase autoantibody is a strong predictor of the development of thyroid autoimmunity in patients with type 1 diabetes and latent autoimmune diabetes in adults. Clin Endocrinol (Oxf). 2011;74:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Sharma H, Sahlot R, Purwar N, Garg U, Saran S, Sharma B, Mathur SK. Co-existence of type 1 diabetes and other autoimmune ailments in subjects with autoimmune thyroid disorders. Diabetes Metab Syndr. 2022;16:102405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Putarek NR, Krnic N, Knezevic-Cuca J, Kusec V, Baretic M, Dumic M. Relative Frequency of Islet Autoimmunity in Children and Adolescents with Autoimmune Thyroid Disease. J Clin Res Pediatr Endocrinol. 2023;15:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Kawasaki E, Abiru N, Yano M, Uotani S, Matsumoto K, Matsuo H, Yamasaki H, Yamamoto H, Yamaguchi Y, Akazawa S. Autoantibodies to glutamic acid decarboxylase in patients with autoimmune thyroid disease: relation to competitive insulin autoantibodies. J Autoimmun. 1995;8:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | He L, Jia X, Rasmussen CG, Waugh K, Miao D, Dong F, Frohnert B, Steck AK, Simmons KM, Rewers M, Yu L. High-Throughput Multiplex Electrochemiluminescence Assay Applicable to General Population Screening for Type 1 Diabetes and Celiac Disease. Diabetes Technol Ther. 2022;24:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Lind A, de Jesus Cortez F, Ramelius A, Bennet R, Robinson PV, Seftel D, Gebhart D, Tandel D, Maziarz M, Agardh D, Larsson HE, Lundgren M, Tsai CT, Lernmark Å. Multiplex agglutination-PCR (ADAP) autoantibody assays compared to radiobinding autoantibodies in type 1 diabetes and celiac disease. J Immunol Methods. 2022;506:113265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 10. | Törn C, Vaziri-Sani F, Ramelius A, Elding Larsson H, Ivarsson SA, Amoroso M, Furmaniak J, Powell M, Smith BR. Evaluation of the RSR 3 screen ICA™ and 2 screen ICA™ as screening assays for type 1 diabetes in Sweden. Acta Diabetol. 2022;59:773-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 11. | Amoroso M, Achenbach P, Powell M, Coles R, Chlebowska M, Carr L, Furmaniak J, Scholz M, Bonifacio E, Ziegler AG, Rees Smith B. 3 Screen islet cell autoantibody ELISA: A sensitive and specific ELISA for the combined measurement of autoantibodies to GAD(65), to IA-2 and to ZnT8. Clin Chim Acta. 2016;462:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Atapattu N, Amoroso M, Powell M, de Silva DGH, de Silva KSH, Furmaniak J, Rees Smith B, Premawardhana LD. The prevalence of diabetes and thyroid related autoantibodies in Sri Lankan children with type 1 diabetes and their unaffected siblings - The utility of a new screening assay. Front Endocrinol (Lausanne). 2023;14:1028285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Kawasaki E, Jinnouchi H, Maeda Y, Okada A, Ito Y, Kawai K. Improving diagnostic accuracy of 3 Screen ICA ELISA kit in the identification of Japanese type 1 diabetes. J Diabetes Investig. 2023;14:1081-1091. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S20-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 680] [Article Influence: 680.0] [Reference Citation Analysis (1)] |
| 15. | Weetman AP. Non-thyroid autoantibodies in autoimmune thyroid disease. Best Pract Res Clin Endocrinol Metab. 2005;19:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, Manji N, Allahabadia A, Armitage M, Chatterjee KV, Lazarus JH, Pearce SH, Vaidya B, Gough SC, Franklyn JA. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-183.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 17. | Kakleas K, Soldatou A, Karachaliou F, Karavanaki K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun Rev. 2015;14:781-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Kawasaki E. Type 1 diabetes and autoimmunity. Clin Pediatr Endocrinol. 2014;23:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Horie I, Kawasaki E, Shimomura A, Satoh T, Ueki I, Kuwahara H, Ando T, Abiru N, Usa T, Eguchi K. Emergence of anti-islet autoantibodies in Japanese patients with type 1 diabetes. Endocr J. 2010;57:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Kawasaki E, Okada A, Uchida A, Fukuyama T, Sagara Y, Nakano Y, Tamai H, Tojikubo M, Koga N. Discrepancy of glutamic acid decarboxylase 65 autoantibody results between RSR radioimmunoassay and enzyme-linked immunosorbent assay in patients with type 1 diabetes is related to autoantibody affinity. J Diabetes Investig. 2019;10:990-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Kawasaki E, Shimada A, Imagawa A, Abiru N, Awata T, Oikawa Y, Osawa H, Kawabata Y, Kozawa J, Kobayashi T, Takahashi K, Chujo D, Fukui T, Miura J, Yasuda K, Yasuda H, Kajio H, Hanafusa T, Ikegami H; Committee of type 1 diabetes, Japan Diabetes Society. Bivalent GAD autoantibody ELISA improves clinical utility and risk prediction for adult autoimmune diabetes. J Diabetes Investig. 2023;14:570-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |