Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.886
Peer-review started: December 12, 2023
First decision: December 25, 2023
Revised: January 9, 2024
Accepted: March 6, 2024
Article in press: March 6, 2024
Published online: May 15, 2024
Processing time: 149 Days and 13.1 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) are a growing health burden across a significant portion of the global patient population. However, these conditions seem to have disparate rates and outcomes between different ethnic populations. The combination of MASLD/MASH and type 2 diabetes increases the risk of hepatocellular carcinoma (HCC), and Hispanic patients experience the greatest burden, particularly those in South Texas.
To compare outcomes between Hispanic and non-Hispanic patients in the United States, while further focusing on the Hispanic population within Southeast Texas to determine whether the documented disparity in outcomes is a function of geographical circumstance or if there is a more widespread reason that all clinicians must account for in prognostic consideration.
This cohort analysis was conducted with data obtained from TriNetX, LLC (“TriNetX”), a global federated health research network that provides access to deidentified medical records from healthcare organizations worldwide. Two cohort networks were used: University of Texas Medical Branch (UTMB) hospital and the United States national database collective to determine whether disparities were related to geographic regions, like Southeast Texas.
This study findings revealed Hispanics/Latinos have a statistically significant higher occurrence of HCC, type 2 diabetes mellitus, and liver fibrosis/cirrhosis in both the United States and the UTMB Hispanic/Latino groups. All-cause mortality in Hispanics/Latinos was lower within the United States group and not statistically elevated in the UTMB cohort.
This would appear to support that Hispanic patients in Southeast Texas are not uniquely affected compared to the national Hispanic population.
Core Tip: We reviewed the outcome data between Hispanic and non-Hispanic patients diagnosed with metabolic dysfunction-associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis in both University of Texas Medical Branch (UTMB) and separately in a United States national cohort to determine if disparities in outcomes were confined to a geographically-defined region, i.e., Southeast Texas, or observed nationally. Outcomes included rates of all-cause mortality, hepatocellular carcinoma, type 2 diabetes mellitus, and liver fibrosis/cirrhosis between the Hispanic and non-Hispanic. Outcome disparities between Hispanic and non-Hispanic groups were observed in the UTMB and United States cohorts at similar rates in all outcomes with the exception of all-cause mortality in the UTMB cohort.
- Citation: Gosnell JM, Golovko G, Arroyave E, Moghe A, Kueht ML, Saldarriaga OA, McKinney KH, Stevenson HL, Ferguson MR. Disparate outcomes in Hispanic patients with metabolic dysfunction-associated steatotic liver disease/steatohepatitis and type 2 diabetes: Large cohort study. World J Diabetes 2024; 15(5): 886-897
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/886.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.886
Steatotic, or fatty, liver disease (SLD) has been consistently increasing in incidence worldwide and is projected to become the primary etiology responsible for liver transplantation in the near future[1-3]. In June 2023, the American Association for the Study of Liver Diseases (AASLD) released new nomenclature guidelines outlining the terminology and criteria of these new SLD entities[4,5]. SLD encompasses all conditions that result in abnormal accumulation of lipids within hepatocytes, regardless of etiology. Examples of the various SLD etiologies include metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH), alcohol-associated liver disease (ALD), combined MASLD and ALD, monogenic conditions that present with hepatic steatosis, drug-induced liver injury (DILI) steatotic liver disease, and cryptogenic steatosis/liver disease.
Per the previous AASLD guidelines on nomenclature and practice from 2012, MASLD is characterized by hepatic parenchyma with at least 5% steatosis without hepatocellular injury; this is opposed to MASH, which also is characterized by at least 5% steatosis of hepatic parenchyma with hepatocellular injury, frequently with hepatocellular ballooning degeneration[6,7]. Long-term potential outcomes from MASLD/MASH progression are cirrhosis and hepatocellular carcinoma (HCC).
AASLD guidelines published in June 2023 now refer to SLD as an overarching term for conditions that result in abnormal lipid accumulation within hepatocytes, with further division into metabolic dysfunction-associated conditions, such as MASLD and MASH, when the patient also demonstrates at least one of five cardiometabolic criteria, such as: (1) Body mass index greater than 25 kg/m2 or waist circumference > 94 cm in males or > 80 cm in females (> 23 kg/m2 in South and East Asians), waist circumference > 90 cm in South and East Asian males (no changes for females); (2) Fasting serum glucose ≥ 5.6 mmol/L (100 mg/dL) or hemoglobin A1c ≥ 5.7% or type 2 diabetes mellitus (T2DM) or treatment for T2DM; (3) Blood pressure ≥ 130/85 mmHg or treatment with anti-hypertensive therapeutics; (4) Plasma triglycerides ≥ 1.70 mmol/L (150 mg/dL) or lipid-lowering therapeutics; and (5) Plasma high-density lipoprotein-cholesterol ≤ 1.0 mmol/L (40 mg/dL) in males and ≤ 1.3 mmol/L in females or lipid-lowering therapeutics.
These criteria are meant to differentiate those who are diagnosed with SLD from those who may demonstrate steatotic injury or etiology from ALD, DILI, monogenic metabolic conditions, etc. However, overlap conditions do occur, and patients may benefit from the management of underlying metabolic syndrome/dysfunction with lifestyle modification and endocrinology-based interventions[4].
Non-invasive methods for determining fibrosis in chronic liver disease patients, including those with SLD, generally involve use of elastrography-based methods. Fibroscan is one such example of elastography-based detection for determination of fibrosis stage in MASH patients and is used within the University of Texas Medical Branch (UTMB) healthcare system. Fibroscan and other elastrographic methods use complex algorithms to determine a measure of liver stiffness and extrapolate an appropriate fibrosis stage from this measurement. AASLD and other professional hepatology societies have been moving toward more frequent use of non-invasive methodologies for patient care, to reduce potential complications as well as improving patient experience[4]. Indeed, most healthcare organizations (HCOs) with high volume hepatology centers use elastography for surveillance and staging, rather than relying on liver biopsies.
However, there are limitations to elastography-based methodologies, namely the ability to detect subsinusoidal fibrosis or delicate bridging fibrosis, allowing for greater levels of progression surveillance in such patients. Subsinusoidal fibrosis surrounding areas of ballooning hepatocytes could portend a patient who is likely to progress to cirrhosis[8].
Differentiating MASLD from MASH is critical for prognosis, therapeutic considerations, and surveillance requirements per AASLD guidelines. The confirmation of MASLD vs MASH is ensured via histological examination of liver tissue, often in the form of a trans-cutaneous or transjugular biopsy for evaluation by a trained hepatopathologist or surgical pathologist. MASLD as ≥ 5% of hepatocytes display macrovesicular steatosis with: (1) No alternative explanations for said steatosis, such as medications, alterations in nutritional status, or monogenic disorders; and (2) Abstinence from alcohol or very little imbibement of alcohol. MASH requires these 2 conditions with the addition of: (1) Inflammation; (2) Hepatocellular injury resulting in ballooning hepatocytes; and (3) Some level of fibrosis, ranging from periportal or perivenular fibrosis up to cirrhosis[4]. Those with a fibrosis score between 2-4 (out of a possible total score of 4) are at increased risk of liver-related events or mortality[9,10], while those with bridging fibrosis or cirrhosis (3-4 out of 4) experience a far higher rate of related instances of morbidity and/or mortality[11,12].
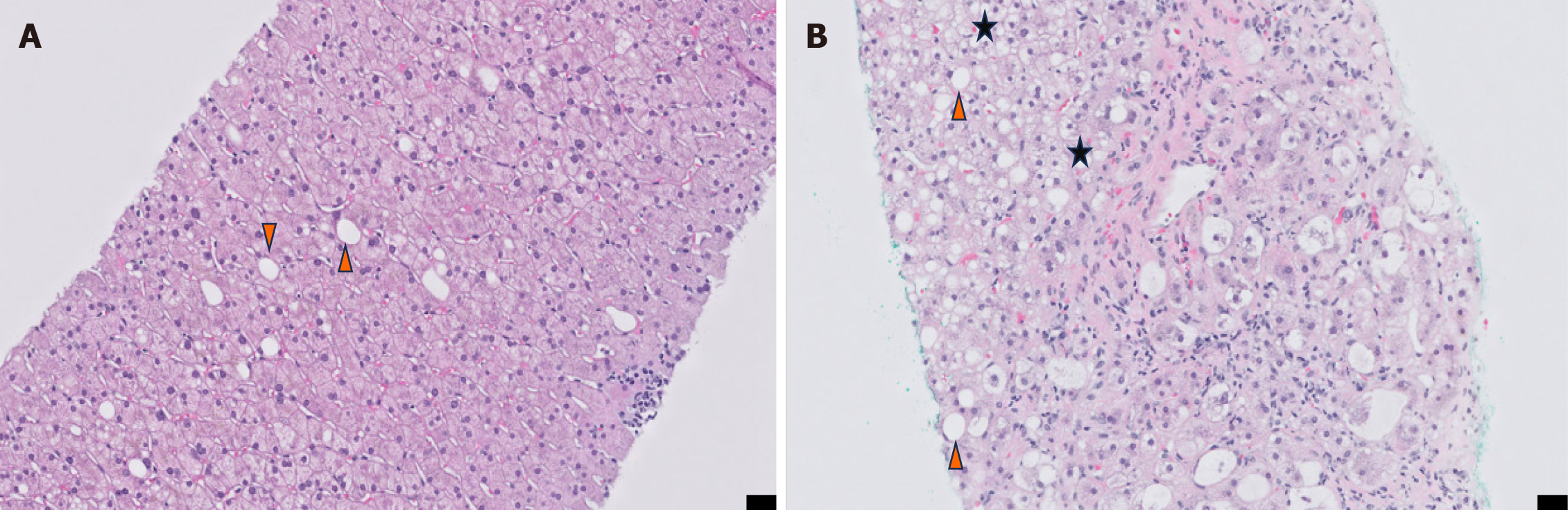
When MASLD or MASH biopsies are histologically evaluated, hepatopathologists use a standardized score to grade the degrees of steatosis, inflammation, and hepatocellular injury, called the non-alcoholic fatty liver disease activity score (NAS)[13]. This generates an unweighted score of these 3 parameters for a total NAS score between 0 and 8, with those scoring ≥ 5 largely considered diagnostic for MASH, while NAS scores between 3-4 are considered borderline or positive MASH, and 2 deemed non-diagnostic to MASH. Additionally, the trained hepatopathologist will thoroughly review the patient’s history and laboratory results to inform the histological diagnosis and present a unified explanation of the pathology to the clinical team. This allows the pathologist to ensure there are no alternative explanations for the histological appearance of the hepatic parenchyma[14]. A comparison of the histological appearance of a MASLD (NAS 3/8) biopsy vs a MASH (NAS 6/8) is included in Figure 1.
Management of MASLD/MASH involves treating liver disease and associated metabolic comorbidities, including diabetes, obesity, and hyperlipidemia. While there are no approved drugs for the treatment of MASLD/MASH, clinical practice guidelines from AASLD recommended that first-line treatment for patients should focus on lifestyle intervention and/or insulin therapy targeting diabetes[15]. Antidiabetic therapy is considered the safest and most effective therapeutic management in patients with chronic liver disease. As such, evidence supports the efficacy of the antidiabetic drug pioglitazone in patients with biopsy-proven MASH[16]. Pioglitazone is a thiazolidinedione, a class of drugs that targets insulin resistance and adipose tissue dysfunction, which in turn reduces the lipid content in the liver by modulating several mediators[17]. Numerous studies, including systemic reviews and meta-analyses of pioglitazone in MASH patients, benefit liver function, reducing liver fat, leading to MASH resolution[15,18-20]. More recently, semaglutide, a long-acting glucagon-like receptor 1 agonist, has been approved for treating type 2 diabetes and obesity and is currently in late-stage clinical trials for MASH. Semaglutide has been shown to improve body weight, glucose, and lipid metabolism and may induce MASH resolution with benefits on inflammation[21]. However, larger trials are needed to characterize better the long-term efficacy, clinical outcomes, and adverse effects of diabetes pharmacotherapy in MASH/MASLD patients.
MASLD and MASH have become tremendous healthcare burdens in most countries worldwide[22,23]. While this was previously seen as a problem for high-income countries, including the United States, where 30% of the population is afflicted with MASLD[24], it has since exploded in incidence worldwide[25]. However, these two entities rarely occur alone; instead, patients often present with concomitant conditions, such as metabolic syndrome, T2DM, obesity, and cardiovascular disease[26]. The combination of these conditions, especially T2DM, complicates the course of MASLD and MASH in many of these patients, especially in Hispanic populations[27]. It is well-established that increased hepatic lipogenesis strongly associates with insulin resistance and increased cardiometabolic risk profiles[28]. Additionally, T2DM increases the risk of different cancers, including HCC in patients with underlying MASLD[29], and Hispanic patients experience the greatest burden, particularly those in South Texas[27]. It is important to note that diabetes and obesity, risk factors for MASLD, are also independently associated with HCC in Hispanics[30]. Studies have shown that the prevalence of MASLD is increased in Hispanics/Latinos compared to African Americans, leading to the highest risk of progression to liver cirrhosis and HCC[31]. The prevalence of obesity in the United States (2005-2014) in Hispanics and African males is similar (38%), while Hispanic females have a low prevalence (47%) compared to African Americans (57%). As such, obesity may contribute to liver cancer deaths in African Americans and Hispanics than in non-Hispanic whites in Texas[32]. Yet, the exact contributions of genetic and environmental factors on these differences in the prevalence rates have not been determined. Given the increased burden of MASLD and HCC, high-risk patients, particularly Hispanics and Latinos with T2DM, should be closely monitored for HCC development. These studies investigate the associations between metabolic conditions and HCC development in Hispanic patients with MASLD and MASH. Our aim in this cohort study was to investigate disparities in outcomes between Hispanic/Latino patients and non-Hispanic/Latino patients with MASH and T2DM, determine if there is a geographical difference in any outcome disparities, and to conduct these analyses on the largest cohort of MASH patients to date.
This study was conducted with data obtained from TriNetX, LLC (“TriNetX”), a global federated health research network that provides access to deidentified medical records from HCOs all over the world. TriNetX data contains diagnoses, procedures, medications, laboratory values, and genomic information for more than 120 million patients from over 100 HCOs. The analyses conducted herein utilized local UTMB (about 2 million) and United States Collaborative Network (56 HCOs, about 92 million) patients to compare outcomes between Hispanic and non-Hispanic patients in the United States as a whole while further focusing on the Hispanic population within Southeast Texas to determine whether the documented disparity in outcomes is a function of geographical circumstance or if there is a more widespread reason that all clinicians must account for in prognostic consideration. It is important to note, that the UTMB network was included within the broader United States Collaborative Network for analyses, with the UTMB network acting as a sub-cohort analysis.
The entire process of data collection, processing, and transmission was carried out in accordance with various Data Protection laws applicable to the contributing HCOs, including the EU Data Protection Law Regulation 2016/679, the General Data Protection Regulation, and the Health Insurance Portability and Accountability Act (HIPAA). It is worth noting that HIPAA is a United States federal law protecting healthcare data’s privacy and security. TriNetX EMEA and Global Collaborative Networks are distributed networks that utilize anonymized or pseudonymized/deidentified (as per HIPAA) data kept at the HCOs, with only aggregated results being sent back to the TriNetX platform. Personal data is not transferred from the HCOs. Additionally, TriNetX is ISO 27001:2013 certified and maintains a robust IT security program that safeguards personal and healthcare data.
Inclusion criteria included diagnosis with International Classification of Diseases (ICD)-10 code K75.81 (nonalcoholic steatohepatitis) with the index event being the diagnosis date. Since multiple HCOs participate in TriNetX without disclosing their criteria for diagnosis, the presence of the K75.81 code does not necessarily indicate biopsy-proven MASH under the new AASLD criteria for SLD or MASH. Additionally, patients were unable to be screened for every cardiometabolic criteria (CMC) per new AASLD guidelines for SLD; however, a large percentage of patients were previously diagnosed with or were diagnosed after the index event with T2DM, one of the five CMCs for MASH diagnosis.
Exclusion criteria was centered around demographic data, specifically to differentiate patients who identified as Hispanic/Latino into the appropriate Hispanic cohorts from those who identified as non-Hispanic/Latino into the appropriate non-Hispanic cohorts in both the United States and UTMB datasets.
The Hispanic MASH United States (HMUS) cohort was designed as Hispanic or Latino patients (HL7) of 18 years or older who were diagnosed with MASH. Alternatively, the non-HMUS (NHMUS) cohort was defined using the same selection criteria but excluding Hispanic or Latino patients. For improved comparison between the two cohorts, propensity score matching (PSM) was performed; this resulted in 17051 patients in the HMUS and NHMUS cohorts.
The Hispanic MASH UTMB (HMUT) cohort was designed identically to the HMUS cohort but focused solely on the University of Texas Medical Branch population. Conversely, the non-HMUT (NHMUT) cohort was also defined with the same selection criteria but excluded all patients with the selected demographic criteria, i.e., Hispanic or Latino identification (HL7). PSM was performed to reduce bias in comparison between the two cohorts.
All analyses were generated with TriNetX platform software (TriNetX, Cambridge, MA) in April of 2023. We compared UTMB and United States nationwide data to estimate the odds and risk ratio of the development of liver cell carcinoma (ICD10:C22.0), T2DM (ICD10:E11), all-cause mortality (ICD10:R99), and fibrosis/cirrhosis of the liver (ICD10:K74).
The number of patients with the outcome between Hispanic and non-Hispanic groups was compared with risk ratios (RRs) and 95% confidence intervals (95%CI). Kaplan-Meier analysis was used to estimate survival probabilities, and the difference between groups was tested using the log-rank test and quantified with hazard ratios (HRs) (95%CI), calculated with TriNetX Analytics features. All the cohorts were propensity score-matched on age and gender using a greedy nearest-neighbor matching algorithm with a caliper of 0.1 pooled standard deviations. The description of the baseline characteristic of the cohorts before and after matching can be found in Supplementary material.
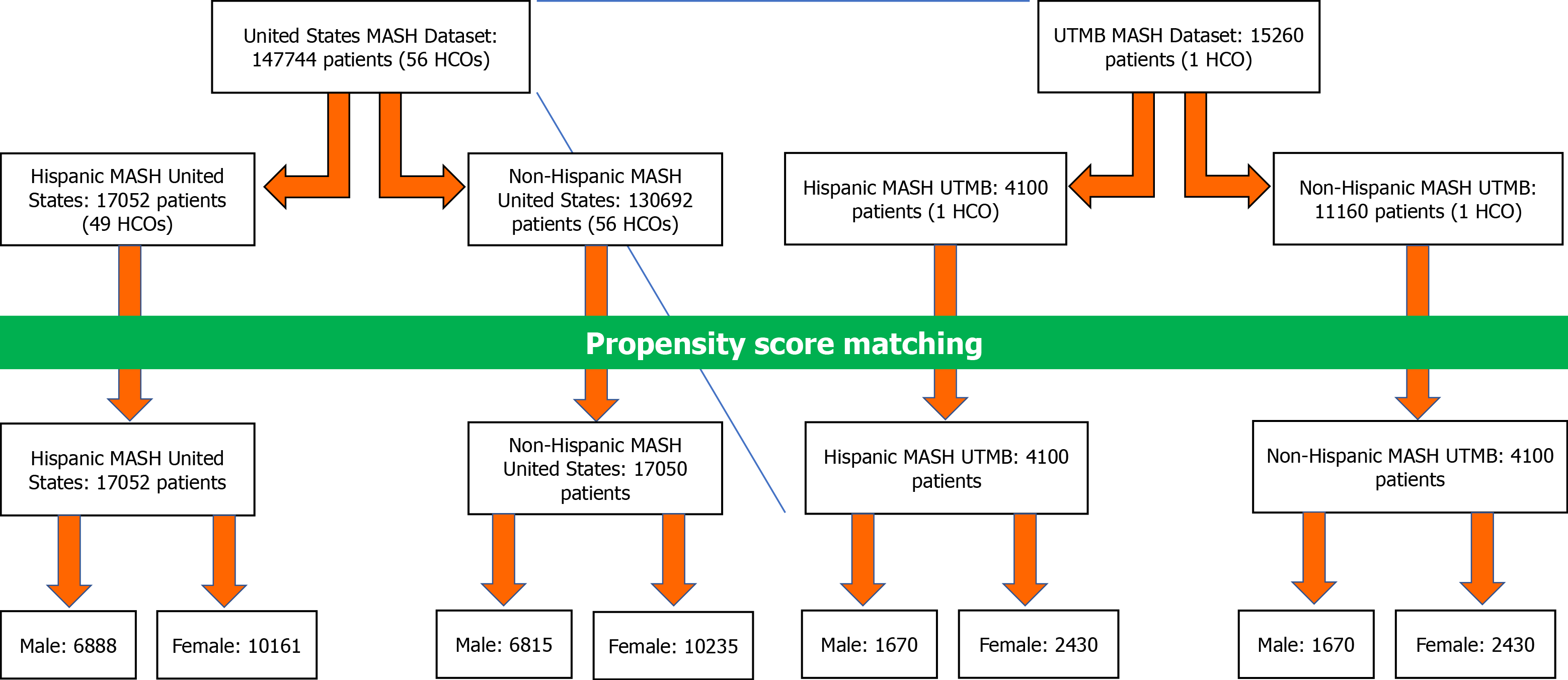
In total, 147744 patients were reported from 56 HCOs in the United States dataset. Out of the 146914 that were diagnosed with MASH, 17052 patients from 49 HCOs were identified as Hispanic/Latino (HMUS) and 130692 patients from 56 HCOs identified as non-Hispanic/Latino (NHMUS). After undergoing PSM, 17052 Hispanic/Latino patients were compared with 17050 non-Hispanic/Latino patients and were further divided by sex, resulting in 6888 Hispanic/Latino males and 10161 females, with an additional 6815 non-Hispanic/Latino males and 10235 females (Table 1 and Figure 2).
| Variable | HMUS (n = 17052) | NHMUS (n = 130692) | HMUS (n = 17052) | NHMUS (n = 17050) | HMUT (n = 4100) | NHMUT (n = 11160) | HMUT (n = 4100) | NHMUT (n = 4100) |
| Age (yr), mean ± SD | 47.2 ± 19.0 | 56.5 ± 15.1 | 47.2 ± 19.0 | 47.2 ± 18.9 | 45.8 ± 16.9 | 52.6 ± 17.0 | 45.8 ± 16.9 | 45.8 ± 16.9 |
| Sex | ||||||||
| Male | 6889 (40.4) | 57116 (43.7) | 6888 (40.4) | 6815 (40.0) | 1670 (40.8) | 5060 (45.4) | 1670 (40.7) | 1670 (40.7) |
| Female | 10163 (59.6) | 73576 (56.3) | 10161 (59.6) | 10235 (60.0) | 2430 (59.4) | 6100 (54.7) | 2430 (59.3) | 2430 (59.3) |
| Ethnicity | ||||||||
| Hispanic/Latino | 17052 (100) | 0 (0) | 17052 (100) | 0 (0) | 4100 (100) | 0 (0) | 4100 (100) | 0 (0) |
| Not Hispanic/Latino | 0 (0) | 130692 (100) | 0 (0) | 17050 (100) | 0 (0) | 11160 (100) | 0 (0) | 4100 (100) |
| HCC (ICD-10: C22.0) | 586 (3.4) | 3174 (2.4) | 586 (3.4) | 263 (1.5) | 30 (0.7) | 60 (0.5) | 30 (0.7) | 30 (0.7) |
| T2DM (ICD-10: E11) | 5943 (34.9) | 45312 (34.7) | 5943 (34.9) | 4894 (28.7) | 2040 (49.9) | 5850 (52.5) | 2040 (49.9) | 1940 (47.4) |
| Fibrosis/cirrhosis of the liver (ICD-10: K74) | 4284 (25.1) | 33338 (25.5) | 4284 (25.1) | 3446 (20.2) | 410 (10.0) | 970 (8.7) | 410 (10.0) | 310 (7.6) |
| All-cause mortality (ICD-10: R99) | 215 (1.3) | 1245 (1.0) | 215 (1.3) | 146 (0.9) | 220 (5.4) | 460 (4.1) | 220 (5.4) | 160 (3.9) |
When narrowed to a stand-in for Southeast Texas, UTMB was used. From this single HCO, 4100 patients were identified as Hispanic/Latino with the required MASH diagnosis (HMUT), while 11160 patients identified as non-Hispanic/Latino (NHMUT) (Table 1). After PSM, this was reduced to a comparison between 4100 Hispanic/Latino patients and 4100 non-Hispanic/Latino patients. Further categorization via sex results in 1670 male patients and 2430 female patients in both the Hispanic/Latino and non-Hispanic/Latino categories (Table 1 and Figure 2).
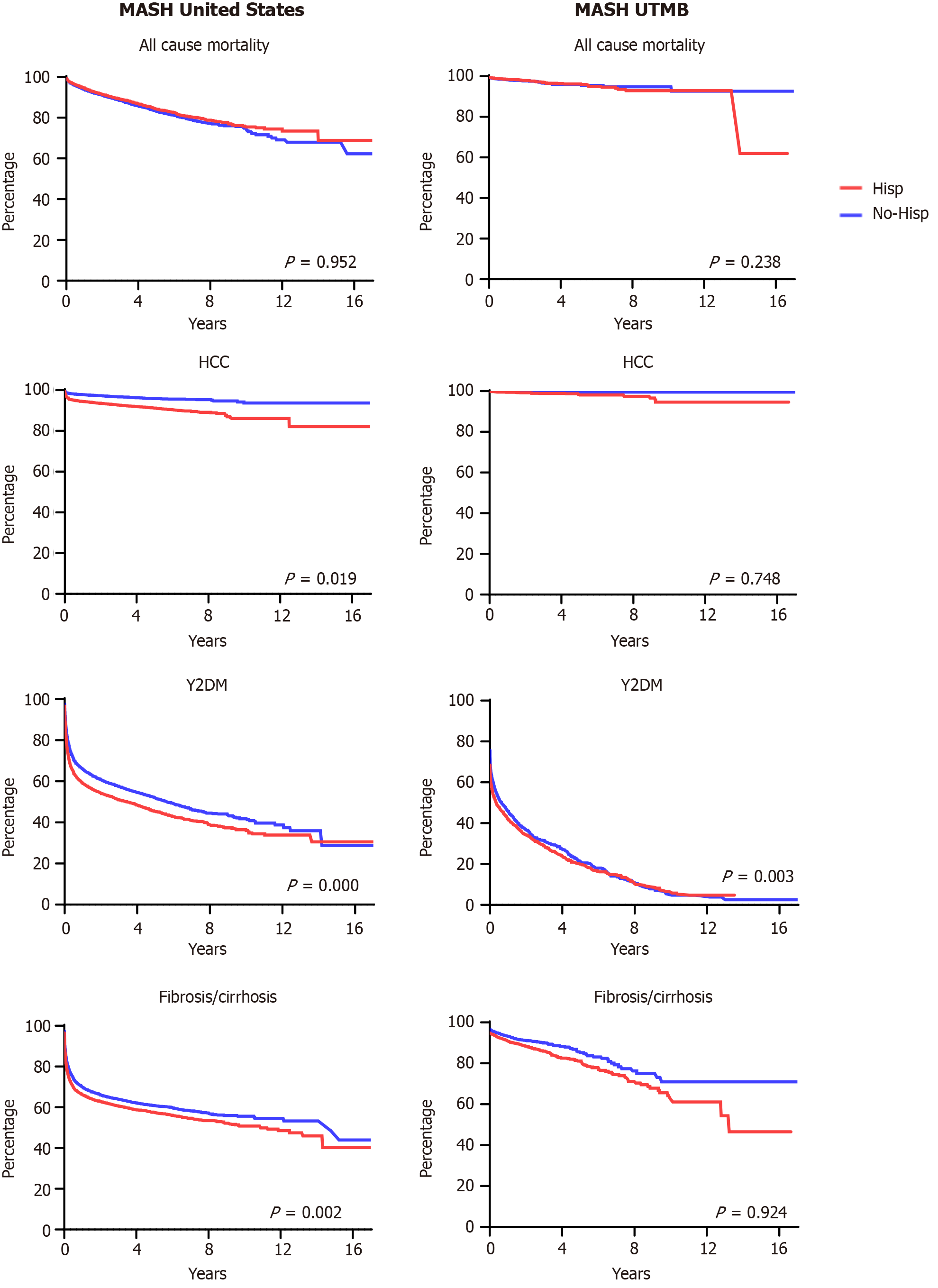
After PSM all 4 cohorts (2 United States cohorts, 2 UTMB-only cohorts, each divided by Hispanic vs non-Hispanic demographic descriptors), we reviewed outcome data amongst them, including risk ratios for developing HCC, T2DM, cirrhosis/hepatic fibrosis, and all-cause mortality. These were calculated using a 95%CI and undergoing Kaplan-Meier survival analysis. Table 2 and Figure 3 contain the visual representation of the results of each outcome’s risk ratios and the Kaplan-Meier survival analysis curves, respectively.
| Outcomes | Risk ratio | 95%CI | P value |
| All-cause mortality (R99.0) | |||
| United States cohort | 0.924 | 0.868-0.983 | 0.013 |
| UTMB cohort | 1.000 | 0.749-1.335 | 1.000 |
| HCC (C22.0) | |||
| United States cohort | 2.183 | 1.964-2.426 | < 0.001 |
| UTMB cohort | 4.010 | 2.008-8.007 | < 0.001 |
| T2DM (E11) | |||
| United States cohort | 1.169 | 1.139-1.200 | < 0.001 |
| UTMB cohort | 1.066 | 1.027-1.106 | 0.001 |
| Fibrosis/cirrhosis of the liver (K74) | |||
| United States cohort | 1.117 | 1.084-1.151 | < 0.001 |
| UTMB cohort | 1.388 | 1.207-1.596 | < 0.001 |
Within the HMUS cohort, 1710 patients died within the examined time period, while 1851 patients within the non-Hispanic MASH cohort died within the same period. This results in an RR of 0.924 (95%CI: 0.868-0.983, P = 0.013). By comparison, 90 patients in the Hispanic and 90 Non-Hispanic MASH cohorts experienced the same outcome, resulting in an RR of 1 (95%CI: 0.749-1.335, P = 1.000) (Table 2). Kaplan-Meier survival analysis of United States patient outcomes demonstrated an HR of 0.920 (95%CI: 0.861-0.982, P = 0.952), while analysis of the UTMB patient outcomes demonstrated an HR of 0.937 (95%CI: 0.692-1.269, P = 0.238) (Figure 3).
The total number of Hispanic patients within the HMUS cohort diagnosed with MASH after PSM developed the HCC outcome is 1052, while non-Hispanic patients within the NHMUS cohort counted 482 with a RR of 2.183 (95%CI: 1.964-2.426, P = 0.000). This is in comparison to the HMUT cohort, which produced 40 Hispanic patients with HCC as an outcome after PSM, while the NHMUT cohort produced 10 non-Hispanic patients with the HCC outcome. This results in an RR of 4.010 (95%CI: 2.008-8.007, P = 0.000) (Table 2). Kaplan-Meier survival analysis of the United States patient HCC outcomes demonstrated a HR of 2.206 (95%CI: 1.980-2.457, P = 0.019), while the UTMB patient HCC outcomes showed an HR of 3.409 (95%CI: 1.629-7.134, P = 0.748) (Figure 3).
The total number of patients within the HMUS cohort diagnosed with MASH PSM who developed the T2DM outcome was 7367, while patients in the NHMUS cohort who developed T2DM was 6302, resulting in an RR of 1.169 (95%CI: 1.139-1.200, P = 0.000). Meanwhile, in the HMUT cohort, a total of 2430 patients developed T2DM, while 2280 developed T2DM within the NHMUT with an RR of 1.066 (95%CI: 1.027-1.106, P = 0.001) (Table 2). Kaplan-Meier survival analysis of United States patient outcomes demonstrated an HR of 1.241 (95%CI: 1.200-1.283, P = 0.000), while the analysis of UTMB patient outcomes demonstrated an HR of 1.126 (95%CI: 1.064-1.193, P = 0.003) (Figure 3).
The total number of patients within the HMUS cohort diagnosed with hepatic fibrosis/cirrhosis after PSM was 6017, while 5389 patients in the NHMUS cohort were diagnosed with hepatic fibrosis/cirrhosis. This results in an RR of 1.117 (95%CI: 1.084-1.151, P = 0.000). In comparison, the HNMUT cohort produced 420 patients with the fibrosis/cirrhosis outcome during the study period, while 310 patients in the NHMUT cohort experienced fibrosis/cirrhosis, resulting in an RR of 1.388 (95%CI: 1.207-1.596, P = 0.000) (Table 2). Kaplan-Meier survival analysis of the United States patient outcomes demonstrated an HR of 1.149 (95%CI: 1.108-1.192, P = 0.002), while the UTMB patient outcomes analysis showed an HR of 1.382 (95%CI: 1.191-1.604, P = 0.924) (Figure 3).
These results demonstrate that Hispanic/Latino patients within the US cohort experienced worse outcomes than their non-Hispanic/Latino counterparts, including greater rates of HCC, T2DM, and fibrosis/cirrhosis of the liver. Hispanic/Latino patients demonstrated a lower rate of all-cause mortality in the US cohort, while the UTMB cohort, these disparities were also seen, except for all-cause mortality, which did not demonstrate a significant p-value. These results support previously published findings that Hispanic/Latino patients with MASH experience a disparity in outcomes from their condition.
MASLD/MASH and its coexistence with T2DM is increasing rapidly worldwide, reaching epidemic proportions, particularly in Hispanic populations. HCC has become one of the fastest-rising causes of cancer-related mortality, with the rising trend of MASLD, MASH, and T2DM. More recent studies demonstrate that MASLD/MASH in combination with T2DM increases the risk of HCC, confirming the direct hepatocarcinogenic effect of diabetes among cirrhosis patients[26,29,33]. Given the global increase in the burden of MASLD and MASH, high-risk patients should be monitored for HCC development.
In South Texas, Hispanics experience more disparate trends in the incidence and prevalence of HCC compared to other ethnic groups[27]. Although Texas accounts for the second-greatest distribution of Hispanics by state, the incidence of HCC among Hispanics in Texas, particularly South Texas, was significantly higher than elsewhere in the United States[27]. This was also seen in the present study, wherein the UTMB Hispanic/Latino population had a 4-fold increase in rates of HCC compared to non-Hispanic/Latino patients, while the US cohort demonstrated only a 2-fold increase in HCC rates. This trend may be caused by the prevalence of unique regional risk factors, such as obesity, T2DM, MASLD, and MASH[27]. However, this conflicts with a reported negative association between T2DM and hepatitis C virus (HCV) infection in Mexican Americans[34]. These data suggest that Mexican American Hispanics may have a decreased risk of HCV compared with non-Mexican Hispanics[34] and that unique risk factors may be involved in the border community. Another cohort analysis investigated the prevalence of MASLD diagnosis at various stages associated with T2DM using the National Health and Nutrition Examination Survey database (2017-2018) by applying transient elastography (FibroScan®)[35]. This cohort analysis shows a high prevalence of MASLD with fibrosis (≥ F2) in the general United States population (25.3%), with greater prevalence in participants with T2DM (54.6%%)[35]. Although Hispanics formed the largest ethnic group among participants with MASLD, the greatest prevalence (35.5%) was observed in Mexican Americans in their analysis[35]. Despite the regional variation, the global prevalence of HCV in T2DM is increasing overall and is higher than in the general population[18,36]. Studies demonstrate that MASLD doubles the risk of T2DM, varying with the fat and fibrosis liver scores, and accelerates the development of cardiovascular disease. Conversely, T2DM increases the risk of MASLD, cirrhosis, and HCC[16,18].
With confirmation of previously disclosed trends in outcomes between Hispanic and non-Hispanic patients in the largest cohort study to date, the question remains why the outcomes demonstrate such a chasm in severity. Is this due to internal bias amongst United States physicians? Is this the result of unequal access to care, therapeutics, and inter-ventions? Unfortunately, these analyses do not elucidate the cause of the outcome disparities, only that they do not show a geographic difference between the United States and Southeast Texas.
During the course of authoring this article, the terminology surrounding the entities formerly termed non-alcoholic fatty liver disease and non-alcoholic steatohepatitis was changed to MASLD and MASH, respectively. While this change in nomenclature is meant to replace outdated or stigmatizing language, both entities require CMC for an official diagnosis, of which the patient must demonstrate at least one. Unfortunately, we could not confirm that all selected MASH patients included in this cohort study possessed at least one of these CMCs, except for those with the demonstrated T2DM outcome or previous T2DM diagnosis prior to the index event.
Additionally, though these analyses worked to exclude alternative etiologies for steatotic liver disease within the examined cohorts, there is no reliable way to exclude alcoholic liver disease or the newly termed metabolic-alcoholic liver disease within the study population.
Medical practitioners should be highly cognizant of the well-established outcome disparity between Hispanic and non-Hispanic patients regarding MASLD/MASH and HCC, especially in settings with concomitant metabolic conditions, such as T2DM, obesity, metabolic syndrome, and cardiovascular disease. In addition, developing and validating accurate predictive models, including age, race/ethnicity, and metabolic syndrome features, may help target MASLD, MASH, and HCC screening to those at the highest risk.
Metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) are part of a growing health burden across the globe with disparate rates and outcomes between ethnic groups within the United States. While these disparities have been presented in the context of specific geographic sub-regions of the United States, no comparison has been made to determine whether these effects are truly geographically limited within the United States via a large cohort study.
To determine if disparities in outcomes associated with MASLD/MASH in Hispanic patient populations were geographically determined or if consistent amongst a United States national cohort of patients.
Given the inclusion of University of Texas Medical Branch (UTMB) within the well-studied Southeast Texas sub-region and the disparity of outcomes experienced by Hispanic patients within this region, we set out to determine if this effect was comparable in the wider United States cohort available through the TriNetX platform.
Data collection was performed exclusively through the TriNetX database system, a global federated healthcare research network with formation of two cohorts, a UTMB-only cohort and a United States national cohort. Selection was made for those diagnosed with MASH and further subdivided into those who identified as Hispanic vs non-Hispanic.
Disparities are seen in outcomes such as rates of liver fibrosis/cirrhosis, incidence of hepatocellular carcinoma, all-cause mortality, and rates of type 2 diabetes mellitus (T2DM) within the national and UTMB Hispanic cohorts when compared to the non-Hispanic cohorts, while all-cause mortality in the US cohort was lower in Hispanic/Latino patients and . all-cause mortality within the UTMB cohort was not statistically significant.
Hispanic patients do have disparity of outcomes associated with MASLD/MASH, especially in connection with rates of T2DM. This disparity is not limited to a single geographic location, like Southeast Texas, but is observed in the United States national population cohort also.
Next step, we will investigate whether cardiometabolic criteria or risk factors as listed in the 2023 American Association for the Study of Liver Diseases guidelines occur with greater frequency in the Southeast Texas Hispanic population in comparison to other ethnic groups and whether this offers a possible explanation for the disparity in outcomes observed in similar studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American Association for the Study of Liver Diseases, 259538.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Horowitz M, Australia; Kotlyarov S, Russia; Li SY, China; Papazafiropoulou A, Greece S-Editor: Wang JJ L-Editor: A P-Editor: Guo X
| 1. | Stepanova M, Kabbara K, Mohess D, Verma M, Roche-Green A, AlQahtani S, Ong J, Burra P, Younossi ZM. Nonalcoholic steatohepatitis is the most common indication for liver transplantation among the elderly: Data from the United States Scientific Registry of Transplant Recipients. Hepatol Commun. 2022;6:1506-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Wang S, Toy M, Hang Pham TT, So S. Causes and trends in liver disease and hepatocellular carcinoma among men and women who received liver transplants in the U.S., 2010-2019. PLoS One. 2020;15:e0239393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, Klein AS, Sundaram V, Nissen NN. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 445] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 4. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1141] [Article Influence: 570.5] [Reference Citation Analysis (1)] |
| 5. | Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80:e54-e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 214] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 6. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 7. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4935] [Article Influence: 705.0] [Reference Citation Analysis (9)] |
| 8. | Heyens LJM, Busschots D, Koek GH, Robaeys G, Francque S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front Med (Lausanne). 2021;8:615978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Hagström H, Nasr P, Ekstedt M, Kechagias S, Stål P, Bedossa P, Hultcrantz R. SAF score and mortality in NAFLD after up to 41 years of follow-up. Scand J Gastroenterol. 2017;52:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-97.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2224] [Article Influence: 222.4] [Reference Citation Analysis (0)] |
| 11. | Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, Ishigami M, Toyoda H, Wai-Sun Wong V, Peleg N, Shlomai A, Sebastiani G, Seko Y, Bhala N, Younossi ZM, Anstee QM, McPherson S, Newsome PN. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611-1625.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 732] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 12. | Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, Loomba R, Chalasani N, Kowdley K, Hameed B, Wilson LA, Yates KP, Belt P, Lazo M, Kleiner DE, Behling C, Tonascia J; NASH Clinical Research Network (CRN). Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385:1559-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 698] [Article Influence: 174.5] [Reference Citation Analysis (0)] |
| 13. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8225] [Article Influence: 411.3] [Reference Citation Analysis (5)] |
| 14. | Boyd A, Cain O, Chauhan A, Webb GJ. Medical liver biopsy: background, indications, procedure and histopathology. Frontline Gastroenterol. 2020;11:40-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Blazina I, Selph S. Diabetes drugs for nonalcoholic fatty liver disease: a systematic review. Syst Rev. 2019;8:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 16. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1492] [Article Influence: 248.7] [Reference Citation Analysis (0)] |
| 17. | Chang E, Park CY, Park SW. Role of thiazolidinediones, insulin sensitizers, in non-alcoholic fatty liver disease. J Diabetes Investig. 2013;4:517-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Diaconu CT, Guja C. Nonalcoholic Fatty Liver Disease and Its Complex Relation with Type 2 Diabetes Mellitus-From Prevalence to Diagnostic Approach and Treatment Strategies. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 19. | He L, Liu X, Wang L, Yang Z. Thiazolidinediones for nonalcoholic steatohepatitis: A meta-analysis of randomized clinical trials. Medicine (Baltimore). 2016;95:e4947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Polyzos SA, Mantzoros CS. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: A systematic review. Metabolism. 2016;65:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, Lawitz E, Ratziu V, Sanyal AJ, Schattenberg JM, Newsome PN; NN9931-4492 investigators. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 236] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 22. | Younossi Z, Aggarwal P, Shrestha I, Fernandes J, Johansen P, Augusto M, Nair S. The burden of non-alcoholic steatohepatitis: A systematic review of health-related quality of life and patient-reported outcomes. JHEP Rep. 2022;4:100525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 23. | Torre E, Di Matteo S, Bruno GM, Martinotti C, Valentino MC, Testino G, Rebora A, Bottaro LC, Colombo GL. Economic Burden of Non-Alcoholic Steatohepatitis (NASH) Among Diabetic Population in Italy: Analysis and Perspectives. Clinicoecon Outcomes Res. 2022;14:607-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Hamid O, Eltelbany A, Mohammed A, Alsabbagh Alchirazi K, Trakroo S, Asaad I. The epidemiology of non-alcoholic steatohepatitis (NASH) in the United States between 2010-2020: a population-based study. Ann Hepatol. 2022;27:100727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 397] [Article Influence: 132.3] [Reference Citation Analysis (2)] |
| 26. | Antwi SO, Craver EC, Nartey YA, Sartorius K, Patel T. Metabolic Risk Factors for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: A Prospective Study. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Ha J, Chaudhri A, Avirineni A, Pan JJ. Burden of hepatocellular carcinoma among hispanics in South Texas: a systematic review. Biomark Res. 2017;5:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Ferguson M, Vel J, Phan V, Ali R, Mabe L, Cherner A, Doan T, Manakatt B, Jose M, Powell AR, McKinney K, Serag H, Sallam HS. Coronavirus Disease 2019, Diabetes, and Inflammation: A Systemic Review. Metab Syndr Relat Disord. 2023;21:177-187. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Teng PC, Huang DQ, Lin TY, Noureddin M, Yang JD. Diabetes and Risk of Hepatocellular Carcinoma in Cirrhosis Patients with Nonalcoholic Fatty Liver Disease. Gut Liver. 2023;17:24-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 31. | Arshad T, Golabi P, Henry L, Younossi ZM. Epidemiology of Non-alcoholic Fatty Liver Disease in North America. Curr Pharm Des. 2020;26:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin. 2017;67:273-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 33. | Gutiérrez-Cuevas J, Lucano-Landeros S, López-Cifuentes D, Santos A, Armendariz-Borunda J. Epidemiologic, Genetic, Pathogenic, Metabolic, Epigenetic Aspects Involved in NASH-HCC: Current Therapeutic Strategies. Cancers (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Watt GP, Vatcheva KP, Beretta L, Pan JJ, Fallon MB, McCormick JB, Fisher-Hoch SP. Hepatitis C virus in Mexican Americans: a population-based study reveals relatively high prevalence and negative association with diabetes. Epidemiol Infect. 2016;144:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Noureddin M, Ntanios F, Malhotra D, Hoover K, Emir B, McLeod E, Alkhouri N. Predicting NAFLD prevalence in the United States using National Health and Nutrition Examination Survey 2017-2018 transient elastography data and application of machine learning. Hepatol Commun. 2022;6:1537-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 36. | Riazi K, Swain MG, Congly SE, Kaplan GG, Shaheen AA. Race and Ethnicity in Non-Alcoholic Fatty Liver Disease (NAFLD): A Narrative Review. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |