Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.876
Peer-review started: December 13, 2023
First decision: January 24, 2024
Revised: February 4, 2024
Accepted: April 1, 2024
Article in press: April 1, 2024
Published online: May 15, 2024
Processing time: 148 Days and 23.9 Hours
The incidence and prevalence of youth-onset type 2 diabetes mellitus (T2DM) are increasing. The rise in frequency and severity of childhood obesity, inclination to sedentary lifestyle, and epigenetic risks related to prenatal hyperglycemia exposure are important drivers of the youth-onset T2DM epidemic and might as well be responsible for the early onset of diabetes complications. Indeed, youth-onset T2DM has a more extreme metabolic phenotype than adult-onset T2DM, with greater insulin resistance and more rapid deterioration of beta cell function. Therefore, intermediate complications such as microalbuminuria develop in late childhood or early adulthood, while end-stage complications develop in mid-life. Due to the lack of efficacy and safety data, several drugs available for the treatment of adults with T2DM have not been approved in youth, reducing the pharmacological treatment options. In this mini review, we will try to address the present challenges and pitfalls related to youth-onset T2DM and summarize the available interventions to mitigate the risk of microvascular and macrovascular complications.
Core Tip: Youth-onset type 2 diabetes mellitus (T2DM) is a growing medical challenge, affecting children and adolescents worldwide. Its clinical course is often more aggressive than in type 1 or adult-onset type 2 diabetes, with earlier development of complications. Results of lifestyle interventions have been disappointing, yet the available pharmacotherapy options for children and adolescents with T2DM are still more limited than for adult patients. The introduction of newer agents that target glycemic control, but also promote weight loss and offer cardiovascular and renal benefits, has the potential to significantly improve the health trajectory of youth with T2DM.
- Citation: La Grasta Sabolic L, Marusic S, Cigrovski Berkovic M. Challenges and pitfalls of youth-onset type 2 diabetes. World J Diabetes 2024; 15(5): 876-885
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/876.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.876
Type 2 diabetes mellitus (T2DM) has been generally considered a disease of adulthood, appearing in middle-aged or older people, due to longstanding insulin resistance accompanied by impaired insulin secretion. However, about 50 years ago, the first reports of asymptomatic glucose intolerance and hyperinsulinemia in obese youth were published[1-3]. In the 90s of the last century, the rising incidence of non-insulin-dependent diabetes mellitus among children and adolescents of different ethnic backgrounds was observed, with obesity and a family history of T2DM being identified as important risk factors[4-9]. Soon after that, T2DM was recognized as a health problem of international scope in children and adolescents[10], with disproportionally high incidence and prevalence in Native American, Canadian First Nation, Indigenous Australian, African-American, Hispanic, East and South Asian, Middle Eastern, and Pacific Islander populations[11]. A growing number of children and adolescents with T2DM, as well as its aggressive phenotype in young people, are causing a reasonable concern. Namely, limited longitudinal data in youth with T2DM indicate a relatively fast deterioration of beta cell function, suggesting that T2DM in young individuals is a rapidly progressive condition, ultimately leading to the early appearance of microvascular and macrovascular complications[12,13]. The high burden of cardiometabolic risk in youth with T2DM imposes the need for earlier aggressive management of both, hyperglycemia and associated cardiovascular risk factors[13,14]. However, compared to adults, fewer therapeutic options are available for youth with T2DM, mostly due to the challenges of implementing clinical trials for those vulnerable, often socioeconomically disadvantaged patients[15]. Having in mind the urgent unmet need for optimal management of T2DM in children and adolescents, alongside treatments regulating blood glucose levels, faster access to new disease-modifying therapies is required, as well as support to obtain medication and promote treatment adherence[15].
Diabetes already represents a significant global health challenge for individuals, their families, and societies. Moreover, according to International Diabetes Federation (IDF) data, a continued global increase in diabetes prevalence is expected. An estimated 537 million adults were living with diabetes in 2021, with a predicted rise to 643 million by 2030, and 783 million by 2045[16]. Also, diabetes was responsible for 6.7 million deaths in 2021 and caused a significant increase in health expenditure over the last 15 years[16].
Over 90% of people with diabetes have T2DM, which was traditionally considered the disease of adulthood. T2DM complications are a major cause of morbidity and mortality and contribute substantially to healthcare costs. In the last decades, however, youth-onset T2DM has become increasingly common, especially in middle-income countries[14,17]. Obesity has been the main attributable risk factor for T2DM development in children and adolescents globally. Besides, youth from racial/ethnic minorities and families with lower socioeconomic status were more likely to have T2DM[18]. Worth mentioning, the incidence of youth-onset T2DM increased significantly during the coronavirus disease 2019 pandemic, but it is yet unclear whether the increase was caused by the coronavirus disease itself or associated with environmental changes during the pandemic[19]. In a recent systematic review of the literature on T2DM incidence among children and adolescents, it was estimated that there were approximately 41600 children and adolescents with newly diagnosed T2DM in 2021 worldwide[20]. Around 30% of the total incident cases were in IDF Western Pacific region, and 40% in World Bank upper-middle-income countries. The highest estimated number of incident cases was in China, India, and the United States of America[20]. Worldwide, T2DM prevalence is highest among adolescents in Brazil[21], the Ontario First Nations People[22], and youth in Mexico[23], followed by Black youth and American Indian youth in United States of America[24] (Table 1). However, direct comparisons of country-specific statistics should be made with caution given the lack of universal diagnostic criteria for youth-onset T2DM. Besides, it should be taken into account that data on prevalence for many regions and countries is missing.
| Rank | Country/ethnicity | Prevalence (per 1000) |
| 1 | Brazil | 33 |
| 2 | Canada/First Nations People | 5.7 |
| 3 | Mexico | 4 |
| 4 | United States/Black | 1.8 |
| 5 | United States/Indian | 1.6 |
With the increasing incidence and prevalence of youth-onset T2DM, the disease burden is expected to become much more prevalent, and the gap in racial and ethnic disparities is anticipated to widen[25], with increasing numbers of people living and struggling with their condition throughout most of their lives. In such a context, it should be particularly emphasized that youth-onset T2DM is associated with more rapid disease progression than adult-onset diabetes. Moreover, T2DM in children and adolescents leads to the earlier and more severe appearance of microvascular and macrovascular complications compared to both adult-onset T2DM and youth-onset type 2 diabetes mellitus (T1DM)[12,13,26]. Results from the TODAY2 follow-up study of youth with T2DM found that the risk of microvascular complications increased steadily over time, with most participants being affected by the time of young adulthood[13]. Also, among people with T2DM onset at different ages, the risk of cardiovascular disease and death was highest in those diagnosed with T2DM at a younger age[27,28], and survival is more than a decade shorter if T2DM was diagnosed in adolescence[29].
Even though youth-onset T2DM has an aggressive phenotype with the earlier appearance of complications and a substantial increase in the number of children and adolescents with T2DM over the next decades is expected[30], there is a lack of data on the socioeconomic cost of youth-onset T2DM. Based on extrapolation from analyses of the socioeconomic burden of T2DM in adults and T1DM in children and adolescents, it is anticipated that youth-onset T2DM would have higher direct and indirect costs[31]. As early-onset T2DM impairs quality of life, affects work ability, and leads to unfavorable long-term outcomes[32], it is extremely important to understand its drivers, to design interventions for its successful prevention and effective management, and to treat comorbidities including obesity, dyslipidemia, and hypertension more actively [13,14,31].
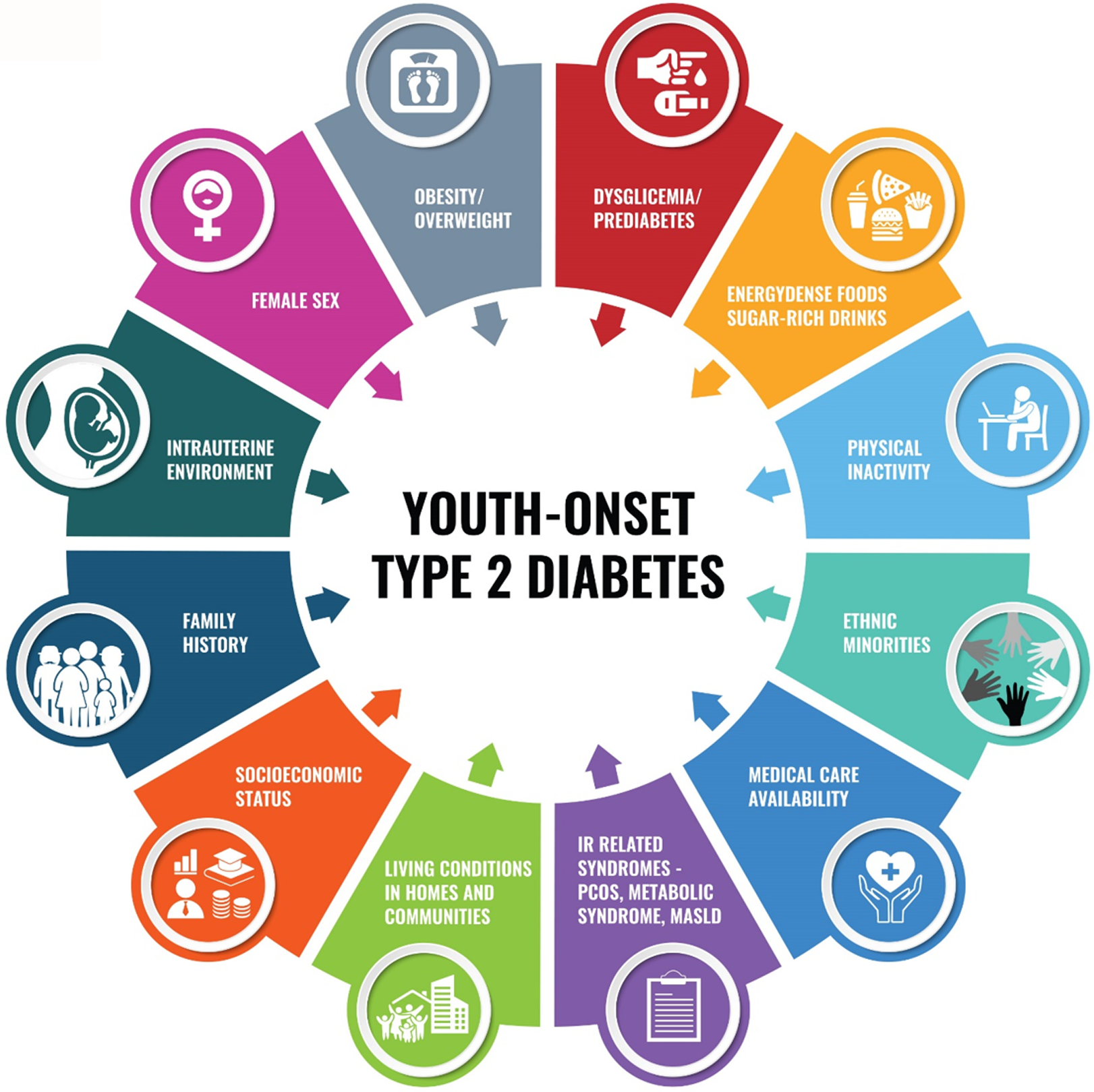
The main driver for the development of T2DM among children and adolescents is obesity/overweight. The projection of a fourfold increase in the prevalence of youth-onset T2DM by 2050 parallels the obesity epidemic rise, having a special impact on white youth in Europe and the United States[30,33]. However, according to the globally gathered data from IDF and data from the population-based study SEARCH For Diabetes in Youth from the United States, the incidence of youth-onset T2DM is much higher in racial and ethnic minority populations. Irrespective of racial and ethnic background, the prevalence of youth-onset T2DM increases with age and is consistently higher among females than males, especially females with associated insulin-resistance syndromes such as polycystic ovary syndrome[21,24,34-36]. Moreover, although dysglycemia (impaired fasting or impaired glucose tolerance), as seen in the case of prediabetes in adults, would be expected to pose a significant risk for further progression to overt T2DM also in children and youth, the link is not straightforward. Namely, a high proportion of male youth with prediabetes revert to normoglycemia after puberty. On the other hand, females with prediabetes are more likely to progress to T2DM, and the reasons behind this are still not fully explained. However, due to as of yet confusing results regarding this topic, further longitudinal trials are needed to better evaluate prediabetes to T2DM progression in youth. On the other hand, there is no doubt that the harmful effects of puberty on metabolic health are significantly aggravated by obesity and inactivity, a pattern nowadays observable from an early age[37-39]. Indeed, obesity and accompanying metabolic derangements such as high circulating levels of free fatty acids and proinflammatory cytokines are much more pronounced in young people with T2DM than in healthy age and sex-matched controls or older individuals with T2DM[32]. In addition to mentioned, the literature suggests the important role of epigenetics and in utero exposition to a high-fat diet, maternal hyperglycemia, and maternal obesity, as well as maternal undernutrition or birth history of small-for-gestational-age, which all influence neonatal and fetal programming and add risk to child's obesity and dysglycemia/diabetes development by still not fully elucidated mechanisms[40-44]. Moreover, drivers for early-onset T2DM are also found in other determinants such as socioeconomic status and health care (un)availability as well as cultural differences which might alter feeding patterns, favoring consumption of energy-dense foods, and sweetened beverages and also change physical activity patterns leading to unattainment of proposed level of physical activity for children and adolescents[45,46]. The drivers of youth-onset T2DM are presented in Figure 1.
T2DM is pathophysiologically a heterogeneous disease, with a spectrum of phenotypical and metabolic characteristics. Genetic susceptibility plays an important role in the pathophysiology of T2DM, with environmental and epigenetic factors influencing diabetes expression and progression[47]. A better understanding of genetic background may be helpful in the accurate characterization of disease subtypes, prediction of clinical course, and selection of targeted pharmacological interventions in youth-onset T2DM[48].
Insulin resistance has an important role in T2DM development, leading to compensatory hyperinsulinemia, and eventually to nonautoimmune beta cell failure with a relative deficiency of insulin secretion. The contribution of insulin resistance to the pathophysiology of T2DM explains the clinical association of diabetes and obesity, as well as the coincidence of increasing T2DM prevalence with increasing prevalence and severity of childhood obesity. However, not all obese children develop T2DM, and some children develop T2DM at a relatively lower body mass index (BMI) for age and sex than others, suggesting the involvement of additional factors in the disease pathogenesis[49]. According to available research, insulin resistance with obesity predominates in the young-onset T2DM population in Europe, whereas insulin deficiency is a key pathophysiological driver and a feature of type 2 diabetes among the young Asian population[50,51]. Data comparison from two pediatric diabetes registries, SEARCH (United States) and YDR (India), revealed significantly higher BMI z-scores in SEARCH than in YDR youth with T2DM[52]. Already published studies and cluster-based phenotypic analyses confirm a higher frequency of severe insulin-deficient diabetes mellitus, as well as younger ages at diagnosis, lower beta cell function, lower insulin resistance, and lower BMI in India's and China's populations than in European people[53,54]. Differences in body composition between Asians and other race/ethnic groups represent a hypothetical mechanism underlying the disproportionate prevalence of T2DM in Asian people. However, a recent study of Asian adults did not find strong evidence that accounting for body composition explains differences in the risk for T2DM[55], while another study revealed that Asian Indians with youth-onset T2DM had a greater genetic risk of poor beta cell function[50]. It seems that patients with newly diagnosed T2DM can be pathophysiologically heterogeneous and may have classical (low insulin sensitivity and low beta cell function), insulinopenic (high insulin sensitivity and low beta cell function), or hyperinsulinemic (low insulin sensitivity and high beta cell function) phenotype[56]. Regardless of the phenotype, a shared feature of early-onset T2DM, when compared to adult type 2 diabetes, is an accelerated decline of beta cell function, seen as a rapid failure of nutrient-stimulated insulin secretion[57,58]. Although the relationship between insulin sensitivity and β-cell function is a hyperbolic function regardless of age, based on the data from the Restoring Insulin Secretion study, greater insulin resistance for any degree of adiposity and greater insulin secretion for any degree of insulin resistance, with similar degrees of dysglycemia, are registered in youth compared with adults[59,60]. Also, early beta-cell function deterioration is found to be 20% to 35% in children with T2DM compared with 7% to 11% in adults with T2DM, despite similar disease durations. Progression to treatment failure is, as well, faster in youth-onset compared to adult-onset T2DM[61], and, unfortunately, none of the existing therapies have been shown to preserve beta cell function in T2DM, particularly during adolescence[62]. Besides, while upregulation of α-cell function has been implicated in the pathophysiology of T2DM in adults, limited data in youth with T2DM exists, with studies showing either hyperglucagonemia or no difference from control subjects without diabetes[63,64].
From the clinical perspective, T2DM is typically considered in pubertal youth with obesity, a family history of T2DM, features of the metabolic syndrome, and absent islet autoantibodies[65]. Data from the Pediatric Diabetes Consortium T2DM Clinic Registry showed that most of the newly diagnosed youth with T2DM presented with symptoms of diabetes and confirming laboratory data, a minority were identified by testing at-risk children, 11% presented with diabetic ketoacidosis (DKA), and 2% with the hyperglycemic hyperosmolar state[66]. Although islet autoantibody positivity supports the diagnosis of type 1 diabetes, it is found in < 10% of children with a clinical diagnosis of T2DM. Positive islet autoantibodies in youth with phenotypically T2DM predict higher rates of ketosis and faster progression to insulin dependence[67].
Individuals diagnosed earliest in life have the most aggressive course of the disease and the highest risk of complications[54]. Children develop insulin deficiency faster than adults, and their rate of chronic complications is also higher[68].
Evidence of microvascular complications and risk markers for macrovascular complications is frequently present at the time of diagnosis in youth-onset T2DM. Rapid progression of complications is also described[69-72]. In First Nations youth with T2DM, renal and neurological complications begin to appear within 5 years of diagnosis, and major complications start to manifest 10 years after the diagnosis[73].
Targeted therapies based on pathophysiological characteristics rather than the currently used "one size fits all" model might improve patient prognosis and T2DM outcomes[56].
Challenges of choosing the right treatment for children and adolescents with T2DM partly arise from the difficulties of making the proper diagnosis, while the extent and early loss of beta cell function are suggestive of T1DM, and features of metabolic syndrome and insulin resistance are pointing toward T2DM. Moreover, patients with early-onset T2DM might have an unfavorable clinical presentation, with the development of vascular complications much earlier than seen in patients with T1DM, together with accompanying comorbidities such as arterial hypertension (especially diastolic) or dyslipidemia (with increased apolipoprotein B and triglycerides despite the statin therapy), making the treatment options additionally challenging. Therefore, management decisions are currently guided by the evidence-based treatment protocols used for older adults with T2DM, and the American Academy of Pediatrics and the American Diabetes Association jointly agree upon the treatment goal being the normalization of glycemia and reduction of complication risk[66,74]. Addressing the social and psychological barriers to care and glycemic control is of utmost importance, as well as close monitoring and appropriate treatment of comorbidities and complications.
Lifestyle interventions are the first ones advised for addressing the hyperglycemia of youth-onset T2DM. Although physical inactivity among young people confers the risk of insulin resistance and associated disorders such as metabolic syndrome and arterial hypertension, employment of physical activity (mainly investigated through engagement in aerobic exercises) in treatment is much less efficient than seen among older T2DM patients[75,76]. Moreover, current evidence, coming from the limited number of studies, failed to show lasting or substantial benefits of aerobic exercise alone in patients with youth-onset T2DM, while dietary interventions based on a very low calorie input (< 800 kcal per day) were efficient either alone or combined with exercise, but only for a limited period time-related to dieting[77].
Decisions on pharmacotherapy of youth-onset T2DM are clouded by the paucity of evidence coming from randomized clinical trials (RCTs), mostly due to the challenges of implementing clinical trials for the population of youth with T2DM while many are vulnerable, and often socioeconomically disadvantaged. Existing data support the use of metformin alone or in combination with sulphonylurea, with an expected LOWERING potential for HbA1c of over 1% and fasting blood glucose by 1-2 mmol/L[78,79]. The durability of monotherapy (usually metformin) cannot be expected long-term in case of initially high HbA1c levels (> 8%) and low insulin secretory capacity. Moreover, treatment response differences observed among different ethnicities can additionally influence therapeutic options[80,81]. Treatment intensification is mainly achieved by (basal) insulin introduction. Thereafter a third of patients can subsequently, for a short period (usually a few months), stop insulin but insulin reinitiation is usually inevitable and related to higher doses and complex regimens upon repeated initiation, which accentuates the risk of hypoglycemia and leads to (additional) weight gain[82]. Although evidence from RCT would be superior in decision making, slow and difficult recruitment leads to off-label use of antidiabetic agents, such as thiazolidinedione, which also efficaciously reduces HbA1c and addresses the metabolic syndrome-related derangements, but eventually leads to weight gain[83].
Recent publications support the use of GLP-1RA for the treatment of overweight/obese children and adolescents with T2DM. Namely, data coming from studies involving children and young adults with T2DM supports the use of liraglutide up to the dose of 1.8 mg daily concomitantly to metformin and/or basal insulin in case of poor glucose re-gulation, from the age of 10 years[84]. Higher liraglutide doses (up to 3 mg sc) can be applied in case of obesity, irrespective of T2DM from the age of 12 years[85]. To date, exenatide and dulaglutide, another two GLP-1RAs used once weekly, received United States Food & Drug Administration (FDA) approval for use in youth-onset T2DM due to the glucose-lowering effect, while only liraglutide adds benefits to weight loss on top of glycemic control[86]. Similarly, after a pivotal trial of the SGLT-2i empagliflozin for the treatment of youth-onset T2DM, it recently received FDA approval for this indication in children 10 years and older. It can be initiated at the dose of 10 mg daily and, in case of need for better glycemic management, up titrated to 25 mg[87-89]. The safety profile of pediatric patients treated with empagliflozin was like that observed in adults with T2DM, except for the risk of hypoglycemia, which was higher in pediatric patients, regardless of concomitant insulin use. Due to the possibility of earlier loss of beta cell function described in youth-onset T2DM, caution would be warranted with the use of empagliflozin and the risk of euglycemic DKA. Although large-scale studies and longer follow-ups are currently not available, bariatric surgery as a treatment option reserved for obese T2DM youth with poorly controlled diabetes and comorbidities, is a promising option, with efficacy and safety comparable to that seen in the adult population[90].
Youth-onset T2DM is an increasing problem, primarily related to growing obesity epidemics among children and adolescents. It seems to have a rapidly progressive course due to a combination of insulin resistance and accelerated loss of beta cell function, responsible for the early appearance of microvascular and macrovascular complications. Knowledge of the optimal treatment options primarily comes from the extrapolations of data available for T2DM in adults. En-couraged are lifestyle interventions and during the last few years use of pharmacotherapy such as GLP-1RAs (liraglutide, exenatide, and dulaglutide) and most recently SGLT-2i empagliflozin in combination with backbone therapy consisting of metformin and/or insulin. Re-evaluation of treatment algorithms with a preference for novel therapeutics with extraglycemic benefits, especially those with cardio- and renoprotective properties and those which help with weight reduction, might be of accentuated importance for this population of patients, while their exposure to hyperglycemia would be longer and risk of earlier development of complications higher than in adult T2DM. Also, recent options include bariatric surgery in case of obese youth with T2DM and associated comorbidities, after failure of previously mentioned pharmacotherapy and lifestyle interventions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Horowitz M, Australia; Ng HY, China; Zeng Y, China S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Drash A. Relationship between diabetes mellitus and obesity in the child. Metabolism. 1973;22:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Martin MM, Martin AL. Obesity, hyperinsulinism, and diabetes mellitus in childhood. J Pediatr. 1973;82:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Deschamps I, Giron BJ, Lestradet H. Blood glucose, insulin, and free fatty acid levels during oral glucose tolerance tests in 158 obese children. Diabetes. 1977;26:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Dean HJ, Mundy RL, Moffatt M. Non-insulin-dependent diabetes mellitus in Indian children in Manitoba. CMAJ. 1992;147:52-57. [PubMed] |
| 5. | Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 653] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 6. | Harris SB, Perkins BA, Whalen-Brough E. Non-insulin-dependent diabetes mellitus among First Nations children. New entity among First Nations people of north western Ontario. Can Fam Physician. 1996;42:869-876. [PubMed] |
| 7. | Kitagawa T, Owada M, Urakami T, Yamauchi K. Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr (Phila). 1998;37:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 174] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Pihoker C, Scott CR, Lensing SY, Cradock MM, Smith J. Non-insulin dependent diabetes mellitus in African-American youths of Arkansas. Clin Pediatr (Phila). 1998;37:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Neufeld ND, Raffel LJ, Landon C, Chen YD, Vadheim CM. Early presentation of type 2 diabetes in Mexican-American youth. Diabetes Care. 1998;21:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 400] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 11. | Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, Chang N, Fu J, Dabadghao P, Pinhas-Hamiel O, Urakami T, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022;23:872-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 12. | Dabelea D, Stafford JM, Mayer-Davis EJ, D'Agostino R Jr, Dolan L, Imperatore G, Linder B, Lawrence JM, Marcovina SM, Mottl AK, Black MH, Pop-Busui R, Saydah S, Hamman RF, Pihoker C; SEARCH for Diabetes in Youth Research Group. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. 2017;317:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 476] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 13. | TODAY Study Group; Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 317] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 14. | Perng W, Conway R, Mayer-Davis E, Dabelea D. Youth-Onset Type 2 Diabetes: The Epidemiology of an Awakening Epidemic. Diabetes Care. 2023;46:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (1)] |
| 15. | Tamborlane W, Shehadeh N. Unmet Needs in the Treatment of Childhood Type 2 Diabetes: A Narrative Review. Adv Ther. 2023;40:4711-4720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. [cited 3 March 2024]. Available from: https://www.diabetesatlas.org. |
| 17. | Xie J, Wang M, Long Z, Ning H, Li J, Cao Y, Liao Y, Liu G, Wang F, Pan A. Global burden of type 2 diabetes in adolescents and young adults, 1990-2019: systematic analysis of the Global Burden of Disease Study 2019. BMJ. 2022;379:e072385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 18. | Butler AM. Social Determinants of Health and Racial/Ethnic Disparities in Type 2 Diabetes in Youth. Curr Diab Rep. 2017;17:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Magge SN, Wolf RM, Pyle L, Brown EA, Benavides VC, Bianco ME, Chao LC, Cymbaluk A, Balikcioglu PG, Halpin K, Hsia DS, Huerta-Saenz L, Kim JJ, Kumar S, Levitt Katz LE, Marks BE, Neyman A, O'Sullivan KL, Pillai SS, Shah AS, Shoemaker AH, Siddiqui JAW, Srinivasan S, Thomas IH, Tryggestad JB, Yousif MF, Kelsey MM; COVID-19 and Type 2 Diabetes Consortium. The Coronavirus Disease 2019 Pandemic is Associated with a Substantial Rise in Frequency and Severity of Presentation of Youth-Onset Type 2 Diabetes. J Pediatr. 2022;251:51-59.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Wu H, Patterson CC, Zhang X, Ghani RBA, Magliano DJ, Boyko EJ, Ogle GD, Luk AOY. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract. 2022;185:109785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 21. | Telo GH, Cureau FV, Szklo M, Bloch KV, Schaan BD. Prevalence of type 2 diabetes among adolescents in Brazil: Findings from Study of Cardiovascular Risk in Adolescents (ERICA). Pediatr Diabetes. 2019;20:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Shulman R, Slater M, Khan S, Jones C, Walker JD, Jacklin K, Green ME, Frymire E, Shah BR. Prevalence, incidence and outcomes of diabetes in Ontario First Nations children: a longitudinal population-based cohort study. CMAJ Open. 2020;8:E48-E55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Simental-Mendía LE, Gamboa-Gómez CI, Aradillas-García C, Rodríguez-Morán M, Guerrero-Romero F. The triglyceride and glucose index is a useful biomarker to recognize glucose disorders in apparently healthy children and adolescents. Eur J Pediatr. 2020;179:953-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Lawrence JM, Divers J, Isom S, Saydah S, Imperatore G, Pihoker C, Marcovina SM, Mayer-Davis EJ, Hamman RF, Dolan L, Dabelea D, Pettitt DJ, Liese AD; SEARCH for Diabetes in Youth Study Group. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001-2017. JAMA. 2021;326:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 366] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 25. | Tönnies T, Brinks R, Isom S, Dabelea D, Divers J, Mayer-Davis EJ, Lawrence JM, Pihoker C, Dolan L, Liese AD, Saydah SH, D'Agostino RB, Hoyer A, Imperatore G. Projections of Type 1 and Type 2 Diabetes Burden in the U.S. Population Aged <20 Years Through 2060: The SEARCH for Diabetes in Youth Study. Diabetes Care. 2023;46:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 26. | Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, Khunti K, Magliano DJ, Luk A. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2023;11:768-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 27. | Huo L, Magliano DJ, Rancière F, Harding JL, Nanayakkara N, Shaw JE, Carstensen B. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997-2011. Diabetologia. 2018;61:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Zhao M, Song L, Sun L, Wang M, Wang C, Yao S, Li Y, Yun C, Zhang S, Sun Y, Hou Z, Wu S, Xue H. Associations of Type 2 Diabetes Onset Age With Cardiovascular Disease and Mortality: The Kailuan Study. Diabetes Care. 2021;44:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 29. | Sattar N, Rawshani A, Franzén S, Svensson AM, Rosengren A, McGuire DK, Eliasson B, Gudbjörnsdottir S. Age at Diagnosis of Type 2 Diabetes Mellitus and Associations With Cardiovascular and Mortality Risks. Circulation. 2019;139:2228-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 346] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 30. | Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ, Rodriguez BL, Standiford D; SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35:2515-2520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 365] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 31. | Rodriquez IM, O'Sullivan KL. Youth-Onset Type 2 Diabetes: Burden of Complications and Socioeconomic Cost. Curr Diab Rep. 2023;23:59-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 32. | Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 531] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 33. | Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL; GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5047] [Article Influence: 630.9] [Reference Citation Analysis (2)] |
| 34. | Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4:270-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 201] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (3)] |
| 35. | Khanolkar AR, Amin R, Taylor-Robinson D, Viner R, Warner J, Stephenson T. Ethnic Minorities Are at Greater Risk for Childhood-Onset Type 2 Diabetes and Poorer Glycemic Control in England and Wales. J Adolesc Health. 2016;59:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Joham AE, Ranasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:E447-E452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Mehreen TS, Kamalesh R, Pandiyan D, Kumar DS, Anjana RM, Mohan V, Ranjani H. Incidence and Predictors of Dysglycemia and Regression to Normoglycemia in Indian Adolescents and Young Adults: 10-Year Follow-Up of the ORANGE Study. Diabetes Technol Ther. 2020;22:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Ishii K, Shibata A, Adachi M, Nonoue K, Oka K. Gender and grade differences in objectively measured physical activity and sedentary behavior patterns among Japanese children and adolescents: a cross-sectional study. BMC Public Health. 2015;15:1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Vrachnis N, Antonakopoulos N, Iliodromiti Z, Dafopoulos K, Siristatidis C, Pappa KI, Deligeoroglou E, Vitoratos N. Impact of maternal diabetes on epigenetic modifications leading to diseases in the offspring. Exp Diabetes Res. 2012;2012:538474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Jiang X, Ma H, Wang Y, Liu Y. Early life factors and type 2 diabetes mellitus. J Diabetes Res. 2013;2013:485082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Kadayifci FZ, Haggard S, Jeon S, Ranard K, Tao D, Pan YX. Early-life Programming of Type 2 Diabetes Mellitus: Understanding the Association between Epigenetics/Genetics and Environmental Factors. Curr Genomics. 2019;20:453-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB Jr, Liese AD, Vehik KS, Narayan KM, Zeitler P, Hamman RF. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31:1422-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 44. | Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 856] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 45. | Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010;100:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 631] [Cited by in RCA: 569] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 46. | Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med Sci Sports Exerc. 2000;32:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 569] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 47. | Redondo MJ, Hagopian WA, Oram R, Steck AK, Vehik K, Weedon M, Balasubramanyam A, Dabelea D. The clinical consequences of heterogeneity within and between different diabetes types. Diabetologia. 2020;63:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 48. | Srinivasan S, Todd J. The Genetics of Type 2 Diabetes in Youth: Where We Are and the Road Ahead. J Pediatr. 2022;247:17-21. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Shah AS, Nadeau KJ, Dabelea D, Redondo MJ. Spectrum of Phenotypes and Causes of Type 2 Diabetes in Children. Annu Rev Med. 2022;73:501-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Siddiqui MK, Anjana RM, Dawed AY, Martoeau C, Srinivasan S, Saravanan J, Madanagopal SK, Taylor A, Bell S, Veluchamy A, Pradeepa R, Sattar N, Venkatesan R, Palmer CNA, Pearson ER, Mohan V. Young-onset diabetes in Asian Indians is associated with lower measured and genetically determined beta cell function. Diabetologia. 2022;65:973-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 51. | Prasad RB, Asplund O, Shukla SR, Wagh R, Kunte P, Bhat D, Parekh M, Shah M, Phatak S, Käräjämäki A, Datta A, Kakati S, Tuomi T, Saboo B, Ahlqvist E, Groop L, Yajnik CS. Subgroups of patients with young-onset type 2 diabetes in India reveal insulin deficiency as a major driver. Diabetologia. 2022;65:65-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 52. | Hockett CW, Praveen PA, Ong TC, Amutha A, Isom SP, Jensen ET, D'Agostino RB Jr, Hamman RF, Mayer-Davis EJ, Lawrence JM, Pihoker C, Kahn MG, Mohan V, Tandon N, Dabelea D. Clinical profile at diagnosis with youth-onset type 1 and type 2 diabetes in two pediatric diabetes registries: SEARCH (United States) and YDR (India). Pediatr Diabetes. 2021;22:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1486] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 54. | Ke C, Narayan KMV, Chan JCN, Jha P, Shah BR. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol. 2022;18:413-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 55. | Flowers E, Lin F, Kandula NR, Allison M, Carr JJ, Ding J, Shah R, Liu K, Herrington D, Kanaya AM. Body Composition and Diabetes Risk in South Asians: Findings From the MASALA and MESA Studies. Diabetes Care. 2019;42:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Stidsen JV, Henriksen JE, Olsen MH, Thomsen RW, Nielsen JS, Rungby J, Ulrichsen SP, Berencsi K, Kahlert JA, Friborg SG, Brandslund I, Nielsen AA, Christiansen JS, Sørensen HT, Olesen TB, Beck-Nielsen H. Pathophysiology-based phenotyping in type 2 diabetes: A clinical classification tool. Diabetes Metab Res Rev. 2018;34:e3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Taha D, Umpaichitra V, Banerji MA, Castells S. Type 2 diabetes mellitus in African-American adolescents: impaired beta-cell function in the face of severe insulin resistance. J Pediatr Endocrinol Metab. 2006;19:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Druet C, Tubiana-Rufi N, Chevenne D, Rigal O, Polak M, Levy-Marchal C. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J Clin Endocrinol Metab. 2006;91:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care. 2018;41:1696-1706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 60. | RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: II. Observations Using the Oral Glucose Tolerance Test. Diabetes Care. 2018;41:1707-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 61. | Barrett T, Jalaludin MY, Turan S, Hafez M, Shehadeh N; Novo Nordisk Pediatric Type 2 Diabetes Global Expert Panel. Rapid progression of type 2 diabetes and related complications in children and young people-A literature review. Pediatr Diabetes. 2020;21:158-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 62. | RISE Consortium. Impact of Insulin and Metformin Versus Metformin Alone on β-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care. 2018;41:1717-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 63. | Kahn SE, Mather KJ, Arslanian SA, Barengolts E, Buchanan TA, Caprio S, Ehrmann DA, Hannon TS, Marcovina S, Nadeau KJ, Utzschneider KM, Xiang AH, Edelstein SL; RISE Consortium; Rise Consortium Investigators:. Hyperglucagonemia Does Not Explain the β-Cell Hyperresponsiveness and Insulin Resistance in Dysglycemic Youth Compared With Adults: Lessons From the RISE Study. Diabetes Care. 2021;44:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Lat J, Caprio S. Understanding the Pathophysiology of Youth-Onset Type 2 Diabetes (T2D): Importance of Alpha-Cell Function. J Clin Endocrinol Metab. 2022;107:e3957-e3958. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 65. | Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, Kaufman FR, Linder B, Marcovina S, McGuigan P, Pyle L, Tamborlane W, Willi S; TODAY Study Group. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96:159-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 66. | Klingensmith GJ, Connor CG, Ruedy KJ, Beck RW, Kollman C, Haro H, Wood JR, Lee JM, Willi SM, Cengiz E, Tamborlane WV; Pediatric Diabetes Consortium. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes. 2016;17:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 67. | Rivera-Vega MY, Flint A, Winger DG, Libman I, Arslanian S. Obesity and youth diabetes: distinguishing characteristics between islet cell antibody positive vs. negative patients over time. Pediatr Diabetes. 2015;16:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Nadeau KJ, Anderson BJ, Berg EG, Chiang JL, Chou H, Copeland KC, Hannon TS, Huang TT, Lynch JL, Powell J, Sellers E, Tamborlane WV, Zeitler P. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care. 2016;39:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 280] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 69. | TODAY Study Group. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1758-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 70. | TODAY Study Group. Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36:1772-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 71. | TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1735-1741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 72. | Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. 2015;1353:113-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 73. | Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 74. | Copeland KC, Silverstein J, Moore KR, Prazar GE, Raymer T, Shiffman RN, Springer SC, Thaker VV, Anderson M, Spann SJ, Flinn SK; American Academy of Pediatrics. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131:364-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 75. | McGavock J, Sellers E, Dean H. Physical activity for the prevention and management of youth-onset type 2 diabetes mellitus: focus on cardiovascular complications. Diab Vasc Dis Res. 2007;4:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | McGavock J, Dart A, Wicklow B. Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr Diab Rep. 2015;15:568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Willi SM, Martin K, Datko FM, Brant BP. Treatment of type 2 diabetes in childhood using a very-low-calorie diet. Diabetes Care. 2004;27:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 79. | Gaylor AS, Condren ME. Type 2 diabetes mellitus in the pediatric population. Pharmacotherapy. 2004;24:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | George MM, Copeland KC. Current treatment options for type 2 diabetes mellitus in youth: today's realities and lessons from the TODAY study. Curr Diab Rep. 2013;13:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, Kaufman F; TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 726] [Article Influence: 55.8] [Reference Citation Analysis (2)] |
| 82. | Kelsey MM, Geffner ME, Guandalini C, Pyle L, Tamborlane WV, Zeitler PS, White NH; Treatment Options for Type 2 Diabetes in Adolescents and Youth Study Group. Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatr Diabetes. 2016;17:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Kelsey MM, Zeitler PS, Nadeau KJ, Shah AS. Type 2 diabetes in youth: Rationale for use of off-label antidiabetic agents. Pediatr Diabetes. 2022;23:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, Jalaludin MY, Kovarenko M, Libman I, Lynch JL, Rao P, Shehadeh N, Turan S, Weghuber D, Barrett T; Ellipse Trial Investigators. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med. 2019;381:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 85. | Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, Mastrandrea LD, Prabhu N, Arslanian S; NN8022-4180 Trial Investigators. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N Engl J Med. 2020;382:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 86. | Hitt TA, Hannon TS, Magge SN. Approach to the Patient: Youth-Onset Type 2 Diabetes. J Clin Endocrinol Metab. 2023;109:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Laffel LM, Danne T, Klingensmith GJ, Tamborlane WV, Willi S, Zeitler P, Neubacher D, Marquard J; DINAMO Study Group. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023;11:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 88. | United Stated food & drug administration. FDA approves new class of medicines to treat pediatric type 2 diabetes. Jun 20, 2023. [cited 22 November 2023]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-class-medicines-treat-pediatric-type-2-diabetes. |
| 89. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 14. Children and Adolescents: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S230-S253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 90. | Janson A, Järvholm K, Sjögren L, Dahlgren J, Beamish AJ, Gronowitz E, Olbers T. Metabolic and Bariatric Surgery in Adolescents: For Whom, When, and How? Horm Res Paediatr. 2023;96:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |