Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.867
Peer-review started: October 8, 2023
First decision: December 18, 2023
Revised: December 31, 2023
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: May 15, 2024
Processing time: 215 Days and 10.1 Hours
Diabetes mellitus is a prevalent disorder with multi-system manifestations, causing a significant burden in terms of disability and deaths globally. Angio-tensin receptor-neprilysin inhibitor (ARNI) belongs to a class of medications for treating heart failure, with the benefits of reducing hospitalization rates and mortality. This review mainly focuses on the clinical and basic investigations related to ARNI and diabetic complications, discussing possible physiological and molecular mechanisms, with insights for future applications.
Core Tip: Diabetes mellitus is a prevalent disorder with multi-system manifestations, causing a significant burden in terms of disability and deaths globally. Angiotensin receptor-neprilysin inhibitor (ARNI) belongs to a class of medications for treating heart failure, with the benefits of reducing hospitalization rates and mortality. This review mainly focuses on the clinical and basic investigations related to ARNI and diabetic complications, discussing possible physiological and molecular mechanisms, with insights for future applications.
- Citation: Liu Y, Lu CY, Zheng Y, Zhang YM, Qian LL, Li KL, Tse G, Wang RX, Liu T. Role of angiotensin receptor-neprilysin inhibitor in diabetic complications. World J Diabetes 2024; 15(5): 867-875
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/867.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.867
Over the past 30 years, the number of individuals suffering from diabetes mellitus (DM) has increased nearly 4-fold worldwide, with DM being the ninth leading cause of reduced life expectancy[1]. In 2021, approximately 537 million adults (20-79 years) are living with diabetes, and by 2045, International Diabetes Federation projections show that 1 in 8 adults, approximately 783 million, will be living with diabetes, an increase of 46%[2]. DM-related complications include neuropathy, retinopathy, nephropathy, dementia, osteoporosis, peripheral vascular disease, myocardial infarction, heart failure (HF) and sudden cardiac death[3,4], all of which are associated with higher morbidity and mortality[5]. Patients with severe diabetes-related complications have a poor prognosis, highlighting the need for more effective and early treatment.
LCZ696 is the first clinical application of an angiotensin receptor-neprilysin inhibitor (ARNI), a 1:1 combination of angiotensin receptor blocker (ARB, valsartan) and neprilysin inhibitor (NEPi, Sacubitril, AHU377)[6]. Previous clinical studies have shown that LCZ696 has significant benefits in reducing the rates of hospitalization, mortality and major cardiovascular events[7-9], which are likely attributable to improve cardiac remodeling[10]. Compared with ARB, LCZ696 has been reported to confer cardiovascular and renal protective effects in animal models[11-13].
With increasing recognition of better prognosis in HF patients receiving ARNI, recent studies have explored its possible benefits beyond HF, such as in DM, cancer and renal disease[13-15]. A post-hoc analysis of the PARADIGM-HF trial “Prospective comparison of ARNI with angiotensin-converting enzyme inhibitors (ACEI) to determine impact on global mortality and morbidity in heart failure” found that in patients with DM and HF, the hemoglobin A1c (HbA1c) level in the LCZ696 group was significantly lower than that in patients treated with enalapril over 1-3 years of follow-up[16]. These findings are consistent with the inverse correlation between blood glucose control and urinary atrial natriuretic peptide (ANP) levels. Initial insulin use was significantly lower in DM patients in the LCZ696 group [114 (7%) vs 153 (10%)]. Similarly, ARNI administration resulted in better insulin resistance and metabolic profiles in non-obese HF patients with reduced ejection fraction (HFrEF) and pre-diabetes[17]. In high-fat-fed neprilysin-deficient mice, improved beta cell function was accompanied by elevated active glucagon-like peptide 1 and reduced plasma dipeptidyl peptidase-4 activity[18]. Thus, ARNI plays an important role in the regulation of blood glucose and insulin in diabetic patients, indicating its potential clinical value in diabetes. This review summarizes the evidence supporting the beneficial effects of ARNI on diabetic complications, with discussion on the molecular mechanisms.
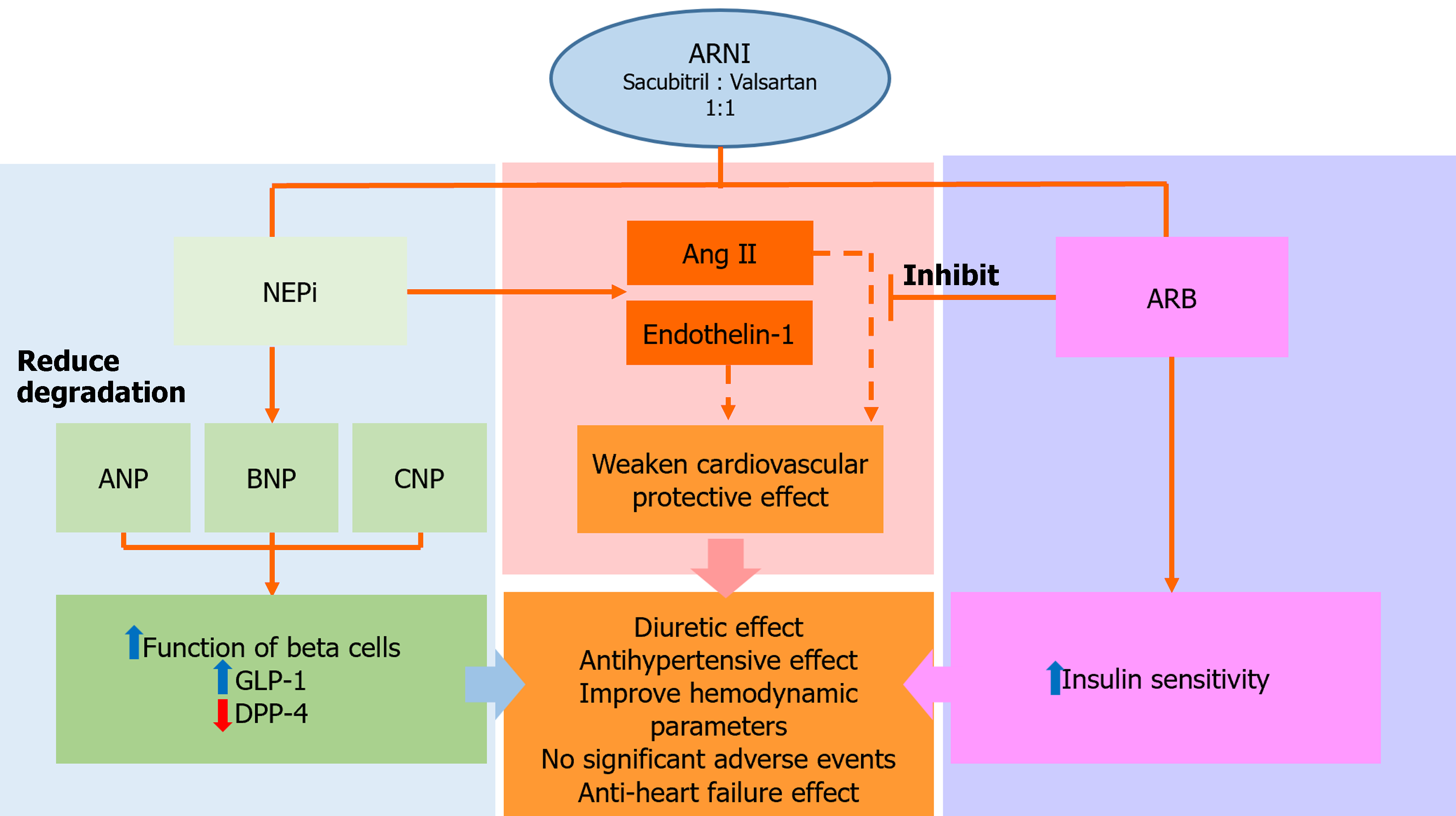
Due to the combined effects of valsartan and sacubitril, LCZ696 not only inhibits over-activation of the renin-angiotensin-aldosterone system (RAAS), but also reduces the over-degradation of NPs (Figure 1). NEPi inhibits the activation of RAAS, with cardiovascular protective effects[19]. NPs can regulate the diuretic, natriuretic and vasodilating functions, but also regulate the reduction of sympathetic drive and anti-proliferation. NEPi can reduce the degradation of NPs by inhibiting the effect of NEP and increasing the biological activity of the NP system, indirectly protecting cardiovascular function[20]. Candoxatril, a NEPi, has diuretic effects and showed a concentration-dependent increase of ANP in patients with mild HF[21]. The increase in angiotensin II and endothelin-1 by NEPi weakens its cardiovascular protective effect. Omapatrilat, a complex composed of ACEI and NEPi, has antihypertensive effects and can significantly improve hemodynamic parameters in patients with HF[22]. Despite its significant effectiveness in hypertension, the combined effects of NEPi and ACEI unfortunately lead to the frequent occurrence of angioedema, restricting their widespread clinical application[23]. By contrast, ARNI use is not associated with angioedema, with distinct anti-HF and hypotensive effects compared to valsartan and enalapril[24,25].
Dysglycemia is often associated with structural and functional damage to the heart[26,27], and accordingly, DM increases the risk of hospitalization in patients with HF by more than 50%[28]. Studies have shown that compared to patients with normal HbA1c, patients with DM had lower left ventricular ejection fraction (LVEF) and significantly higher hospitalization rates due to HF and cardiovascular death[29,30]. Diabetic cardiomyopathy can significantly increase the risk of death in patients with DM[31]. It does not appear to be reversible, and hypoglycemic agents may have additional adverse effects on patients with HF.
LCZ696 has been demonstrated to reduce the risk of cardiovascular death and HF-related hospitalization in patients with DM or pre-diabetes compared with enalapril[29]. The mortality and HF rehospitalization rates were similar between HFrEF patients with and without DM after application of ARNI[32]. However, one study showed that all-cause mortality was higher in patients with diabetes than in those without diabetes (25% vs 8%)[33]. Furthermore, a pooled analysis of PARAGON-HF and PARADIGM-HF suggested that ARNI may increase the risk of hypoglycemia[34]. Compared with valsartan, ARNI can significantly reduce the level of N-terminal pro-brain NP (NT-proBNP) in diabetic rats[35]. Moreover, compared with non-diabetic patients, diabetic patients had a more pronounced decrease in LVEF, a higher degree of myocardial fibrosis, and a higher incidence of ischemic cardiomyopathy[36], which may lead to a higher incidence of ventricular arrhythmia in diabetic patients. Nevertheless, the incidence of ventricular tachyarrhythmia in diabetic patients was similar to that in non-diabetic patients[33], which demonstrates that to some extent, ARNI can reduce the risk of ventricular arrhythmias in diabetic patients.
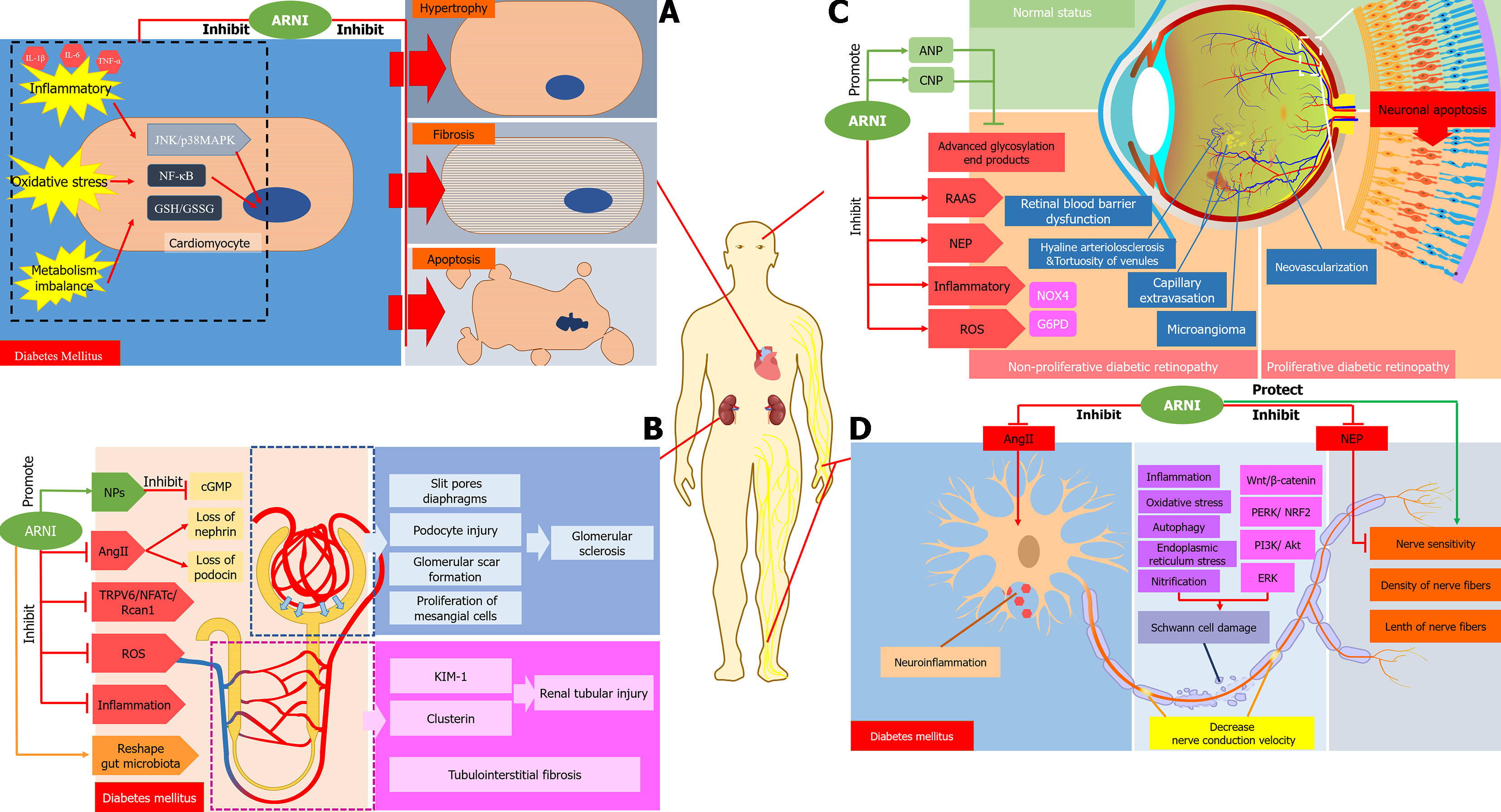
This review mainly discusses the related mechanisms of ARNI in improving diabetic cardiomyopathy in relation to the following aspects: ARNI improves cardiac remodeling and cardiac function in patients with diabetic cardiomyopathy. In comparison with diabetic control rats, treatment with LCZ696 and valsartan significantly reduced the heart weight to body weight ratio and improved LVEF[35]. ARNI can reduce appetite, body weight and normalize insulin and glycosylated hemoglobin in rats fed high-fat high fructose diet-induced DM[37], this may be related to an increase in satiety and decrease in hunger and food intake by NPs through inhibition of the appetite-stimulating hormone ghrelin[38]. Secondly, ARNI reduces myocardial fibrosis and prevents myocardial apoptosis. Animal experiments found a reduction in apoptotic cells and myocardial fibrosis, and down-regulation of the expression level of related landmark proteins[19,39]. ARNI inhibits oxidative stress related indicators and inflammatory factors induced by high glucose or diabetes, including c-Jun N-terminal kinase/p38 mitogen-activated protein kinase, nuclear factor-κB nuclear translocation, glutathione (GSH) contents and GSH/GSH disulfide ratios, and interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α in the serum, etc[19]. Thiorphan monotherapy, which is a NEPi, decreased the expression of cardiac NEP proteins, telmisartan monotherapy significantly reduced the expression of heart-specific ANP, BNP and NEP proteins, and compared with control, ARNI significantly limited the expression of plasma and heart-specific NPs in diabetes [11,35]. In conclusion, ARNI has protective effects on diabetic myocardial tissue through anti-apoptotic, anti-fibrotic, anti-inflammatory, and anti-oxidative actions, providing a theoretical basis for protection against cardiac dysfunction in diabetes (Figure 2A).
Patients with abnormal glucose tolerance and insulin resistance are at high risk of developing chronic kidney disease (CKD). Of note, diabetic nephropathy is an important and independent risk factor for serious cardiovascular events of DM, and eventually develops into end-stage renal disease[40-42]. Compared with enalapril, ARNI has profound effects on protecting renal function in patients with diabetes and CKD[43], likely through a reduction in proteinuria and delayed progression of diabetic nephropathy from RAAS inhibition[44]. ARNI use is associated with better renal outcomes in patients with HFrEF, slowing the rate of estimated glomerular filtration rate (eGFR) reduction more effectively than enalapril[45]. In addition, the index of renal insufficiency was also significantly decreased, independent of the blood glucose status[29]. Whether ARNI is more effective than ARB in albuminuria (especially microalbuminuria) and glycemic control, which play important roles in the progression of diabetic kidney disease (DKD), is still unknown[46].
The mechanisms by which ARNI improves renal function in diabetic patients remain elusive. In the section below, we highlight some of the potential key mechanisms. Firstly, the occurrence of hyperlipidemia in diabetic patients is often associated with oxidized low-density lipoprotein and kidney damage[47]. Total plasma cholesterol in obese rats was significantly reduced after the administration of ARNI[48]. Thus, the improvement in renal function may be explained by improvement in total plasma cholesterol. ARNI improved renal function in rats after partial nephrectomy and in a model of diabetic kidney damage[49,50]. ARNI use is associated with improved renal function independent of blood pressure effects, characterized by reduced proteinuria and glomerulosclerosis[51]. In terms of glomerular filtration function, it mainly showed preservation of renal plasma flow and glomerular filtration rate, and the creatinine clearance rate was higher than that in the non-treated group[50]. Similarly, LCZ696 also restricts the increase in blood urea nitrogen and creatinine level, which indicated that it could maintain renal function in diabetic rats[52]. Importantly, diabetic nephropathy with increased proteinuria and decreased glomerular filtration is closely related to its pathological changes, including hypertrophy of glomerular and tubular components, glomerular and tubule basement membrane thickening, disappearance of podocytes, and eventually glomerular sclerosis and tubulointerstitial fibrosis[53]. Following treatment with ARNI, renal pathology scores, such as focal segmental glomerulosclerosis, glomerulosclerosis score and tubular injury score, were improved[51]. Whilst ARNI had a negative effect on the occurrence of glomerulosclerosis by reducing glomerular scar formation[52], ARNI can preserve the integrity of podocytes by inhibiting the expression of transient receptor potential cation channel, subfamily C, member 6 transient receptor potential-6 (TRPC6) or the role of its downstream Rcan1 promoter[50]. Rcan 1, which is positively related to the activation of TRPC6, was 50% suppressed by ARNI [52]. In addition, ANP reduces the number of TRPC6 channels by lowering blood glucose[50,54]. Furthermore, ARNI can prevent renal tubular injury, as reflected by reductions in the renal injury markers, clusterin and kidney injury molecule-1. ARNI, valsartan and hydralazine inhibited tissue interstitial fibrosis by 35%, 47%, and 19%, respectively[48]. The NP system could play a role in natriuretic, diuretic and vasodilation by reducing the synthesis of cyclic guanosine monophosphate (cGMP), and inhibit the proliferation of mesangial cells and renal fibrosis[55,56]. However, there was no significant change in cGMP in the plasma and urine of diabetic rats after treatment with ARNI[51]. ARNI can increase the gene expression of nephrin and podocin[48]. The renal protective effect of ARNI can be attributed to reduced oxidative stress response in the glomeruli or renal tubules[48]. Lastly, ARNI partially reshaped the composition of gut microbiota, reduced the abundance of some harmful bacteria and increased the abundance of beneficial bacteria. Functional prediction analysis suggested that ARNI can improve kidney function in DKD rats[57]. Thus, ARNI reduces hyper-glycemia, proteinuria and inflammation, improves intestinal flora disorder, retains eGFR, and inhibits the TRPC6/NFATc/Rcan1 pathway, which may improve podocyte integrity, and protects glomerular and renal tubular function and structure (Figure 2B).
Cardiovascular disease, HF, atherosclerosis and cerebrovascular events are mainly caused by dysfunction of the macrovascular system. Diabetic retinopathy is one of the most common diabetic microangiopathies, whose incidence is projected to reach 146 million people by 2050[58]. It is an important cause of acquired blindness in adults[59], and mainly consists of hyaline arteriolosclerosis, thickening of capillary basement membrane, formation of microangioma and tortuosity of venules. Further development may lead to changes such as retinal capillary extravasation and macular edema. Diabetic retinopathy is divided into two stages: Non-proliferative diabetic retinopathy and proliferative diabetic retinopathy[60]. Retinal and iris neovascularization is the hallmark of proliferative retinopathy.
Prasad et al[61] found that RAAS and NEP dual inhibition (irbesartan + thiorphan) could inhibit the further deve-lopment of diabetic retinopathy more effectively than irbesartan alone. The protective mechanisms of ARNI on diabetic retinopathy are shown in Figure 2C. Diabetic retinopathy is associated with local RAAS activation in the eye vasculature, and its retinal angiotensin II level increases[62]. Angiotensin II blockade leads to anti-angiogenesis, anti-inflammatory and improving retinal function. Inhibiting the mineralocorticoid receptor and angiotensin II type 1 receptor, the oxygen-induced retinopathy could be significantly improved through inhibition of the aldosterone induced inflammatory pathway and regulation of factors such as glucose-6-phosphate dehydrogenase and Nicotinamide adenine dinucleotide phosphate NADPH oxidases 4[63]. The level of retinal NEP activity increased after streptozotocin induced diabetes in Ren2 rats[64]. Clinical studies have shown that the expression of NEP in the serum of patients with diabetic retinopathy was markedly higher than those without retinopathy. NEP expression gradually rises with worsening retinopathy[64]. Due to its effects on the RAAS as well as the NEP system, ARNI can significantly prevent or delay diabetic retinopathy[61]. ANP and CNP could reverse the retinal blood barrier dysfunction induced by advanced glycosylation end products of retinal pigment epithelium; thus, inhibiting the activity of retinal NEP and increasing the level of NPs may be beneficial in the treatment of diabetic retinopathy[65]. Increased expression of inflammatory cytokines, excessive accumulation of ROS, loss of capillaries, glial cell proliferation and neuronal apoptotic cell death are all indicators of diabetic retinopathy[61,66]. Long-term treatment with ARNI significantly reduced the damage related to the above diabetic retinopathy in comparison with ARB[61].
Diabetic peripheral neuropathy (DPN) affects the quality of life of approximately 50% of DM patients[67], due to increased pain and the risk of falls[67]. Approximately half of patients with diabetes develop foot ulcers, which can eventually lead to lower limb amputations[68]. At present, the treatments for DPN are very limited, and most patients only have symptomatic treatment, such as neurotrophic agents[69].
ACEI and ARB have benefits on peripheral neuropathy in diabetic rats[70], mainly manifested in improving neurological function and endoneurial blood flow in diabetic rats[71]. Hyperglycemia can increase the level of tissue angiotensin II, leading to endothelial injury, neuroinflammation and vascular dysfunction, and thus induce diabetic neuropathy. The development of diabetic neuropathy could be slowed by ACEI/ARB through inhibition of this pathway[72]. Clinical and preclinical studies showed that the sensory and motor nerve conduction velocity was significantly decreased in diabetes, while valsartan had no effect during early intervention, but ARNI can preserve the conduction velocity of action potentials through motor and sensory nerves. Measurements of sensory nerve density in the skin and cornea and the associated biosensitivity of these nerves have been promoted as possible alternative markers of peripheral neuropathy[73]. ARNI intervention can completely reverse the loss and heat sensitivity of skin sensory nerve fibers[71]. Similarly, NEPis can significantly improve nerve conduction velocity, improve thermal sensitivity, and protect the density of nerve fibers in the epidermis of diabetic sciatic nerve[74]. Calcitonin gene-related peptide promotes the regeneration and elongation of nerves. In NEP knockout mice, the expression of calcitonin gene-related peptide was markedly increased in the corneal nerve, which explains why NEP inhibitors increase the expression of calcitonin gene-related peptide to promote the regeneration of nerve cells, finally delaying the development of DPN[75]. By inhibiting the activity of neprilysin, corneal sensitivity of diabetic animals could be restored[76]. Schwann cells are glial cells in the peripheral nervous system and form the myelin sheath that protects axons, which play a crucial role in the pathogenesis of DPN. Decreased Schwann cell activity leads to demyelination, which aggravates the development of DPN forming a vicious circle[77]. Apoptosis of Schwann cells increases in diabetes, mainly through inflammation, oxidative stress, autophagy, endoplasmic reticulum stress and nitrification, as well as signal pathways such as extracellular signal-regulated kinase, protein kinase RNA-like endoplasmic reticulum kinase/nuclear factor erythroid 2-related factor 2, phosphoinositide 3-kinase/Akt and Wnt/β-catenin[78]. Calcitonin gene-related peptide also plays an important role in peripheral nerve regeneration and Schwann cell proliferation[79]. NEP inhibitors can protect the calcitonin gene-related peptide. Taken together, these results show that ARNI can delay the development of DPN by reducing angiotensin II, improving nerve conduction velocity and sensitivity, and protecting Schwann cells (Figure 2D).
In conclusion, although ARNI is currently indicated for HF, it has demonstrated benefits in a number of diabetes-related complications such as cardiomyopathy, nephropathy, retinopathy and peripheral neuropathy. These data might encourage additional research into the beneficial metabolic properties of drugs of this class. More studies are needed to explore the potential benefits of ARNI in diabetes, especially in diabetic cardiomyopathy and diabetic nephropathy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Islam MS, South Africa; Nayak S, Trinidad and Tobago; Tziomalos K, Greece S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Cai YX
| 1. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3378] [Article Influence: 482.6] [Reference Citation Analysis (0)] |
| 2. | International Diabetes Federation. IDF Diabetes Atlas. 2021. [cited 20 February 2024]. Available from: https://diabetesatlas.org/. |
| 3. | Lee S, Zhou J, Guo CL, Wong WT, Liu T, Wong ICK, Jeevaratnam K, Zhang Q, Tse G. Predictive scores for identifying patients with type 2 diabetes mellitus at risk of acute myocardial infarction and sudden cardiac death. Endocrinol Diabetes Metab. 2021;4:e00240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Lee S, Zhou J, Wong WT, Liu T, Wu WKK, Wong ICK, Zhang Q, Tse G. Glycemic and lipid variability for predicting complications and mortality in diabetes mellitus using machine learning. BMC Endocr Disord. 2021;21:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Lee S, Zhou J, Leung KSK, Wu WKK, Wong WT, Liu T, Wong ICK, Jeevaratnam K, Zhang Q, Tse G. Development of a predictive risk model for all-cause mortality in patients with diabetes in Hong Kong. BMJ Open Diabetes Res Care. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4078] [Cited by in RCA: 4726] [Article Influence: 429.6] [Reference Citation Analysis (0)] |
| 7. | Jaffuel D, Molinari N, Berdague P, Pathak A, Galinier M, Dupuis M, Ricci JE, Mallet JP, Bourdin A, Roubille F. Impact of sacubitril-valsartan combination in patients with chronic heart failure and sleep apnoea syndrome: the ENTRESTO-SAS study design. ESC Heart Fail. 2018;5:222-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Dec GW. LCZ696 (sacubitril/valsartan): can we predict who will benefit? J Am Coll Cardiol. 2015;66:2072-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 893] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 10. | Sun Y, Song S, Zhang Y, Mo W, Zhang X, Wang N, Xia Y, Tse G, Liu Y. Effect of angiotensin receptor neprilysin inhibitors on left atrial remodeling and prognosis in heart failure. ESC Heart Fail. 2022;9:667-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Malek V, Gaikwad AB. Telmisartan and thiorphan combination treatment attenuates fibrosis and apoptosis in preventing diabetic cardiomyopathy. Cardiovasc Res. 2019;115:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Suematsu Y, Jing W, Nunes A, Kashyap ML, Khazaeli M, Vaziri ND, Moradi H. LCZ696 (Sacubitril/Valsartan), an Angiotensin-Receptor Neprilysin Inhibitor, Attenuates Cardiac Hypertrophy, Fibrosis, and Vasculopathy in a Rat Model of Chronic Kidney Disease. J Card Fail. 2018;24:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Li Y, Kang L, Rong K, Zhang Y, Suo Y, Yuan M, Bao Q, Shao S, Tse G, Li R, Liu T, Li G. Renal protective effects and mechanisms of the angiotensin receptor-neprilysin inhibitor LCZ696 in mice with cardiorenal syndrome. Life Sci. 2021;280:119692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Tse G, Roever L, Liu T. Sacubitril/valsartan in the treatment of cancer therapy-related cardiac dysfunction. Int J Cardiol. 2020;318:130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Liu X, Huang L, Tse G, Liu T, Che J. Effects of sacubitril-valsartan in the treatment of chronic heart failure patients with end-stage renal disease undergoing dialysis. Clin Cardiol. 2023;46:930-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 16. | Seferovic JP, Claggett B, Seidelmann SB, Seely EW, Packer M, Zile MR, Rouleau JL, Swedberg K, Lefkowitz M, Shi VC, Desai AS, McMurray JJV, Solomon SD. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 17. | Cloro C, Zaffina I, Sacchetta L, Arturi F, Clausi C, Lucà S, Pelle MC, Giofrè F, Armentaro G, Forte V, De Rosa FM, Sciacqua A. Effects of sacubitril/valsartan on both metabolic parameters and insulin resistance in prediabetic non-obese patients with heart failure and reduced ejection fraction. Front Endocrinol (Lausanne). 2022;13:940654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Willard JR, Barrow BM, Zraika S. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia. 2017;60:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Ge Q, Zhao L, Ren XM, Ye P, Hu ZY. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Exp Biol Med (Maywood). 2019;244:1028-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Díez J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur J Heart Fail. 2017;19:167-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Northridge DB, Newby DE, Rooney E, Norrie J, Dargie HJ. Comparison of the short-term effects of candoxatril, an orally active neutral endopeptidase inhibitor, and frusemide in the treatment of patients with chronic heart failure. Am Heart J. 1999;138:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, Porter CB, Proulx G, Qian C, Block AJ. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 280] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Malek V, Gaikwad AB. Neprilysin inhibitors: A new hope to halt the diabetic cardiovascular and renal complications? Biomed Pharmacother. 2017;90:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1593] [Article Influence: 265.5] [Reference Citation Analysis (0)] |
| 25. | Suzuki K, Claggett B, Minamisawa M, Nochioka K, Mitchell GF, Anand IS, Zannad F, Shah SJ, Lefkowitz M, Shi V, Pfeffer MA, McMurray JJV, Solomon SD. Pulse Pressure, Prognosis, and Influence of Sacubitril/Valsartan in Heart Failure With Preserved Ejection Fraction. Hypertension. 2021;77:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J, Bello N, Aguilar D, Vardeny O, Matsushita K, Selvin E, Solomon S. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ Heart Fail. 2015;8:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Sun DK, Zhang N, Liu Y, Qiu JC, Tse G, Li GP, Roever L, Liu T. Dysglycemia and arrhythmias. World J Diabetes. 2023;14:1163-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (0)] |
| 28. | Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP, Schnaidt S, Ofstad AP, Brueckmann M, Jamal W, Bocchi EA, Ponikowski P, Perrone SV, Januzzi JL, Verma S, Böhm M, Ferreira JP, Pocock SJ, Zannad F, Packer M. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients With Heart Failure by Baseline Diabetes Status: Results From the EMPEROR-Reduced Trial. Circulation. 2021;143:337-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 29. | Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, Martinez F, Starling RC, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray JJ, Packer M; PARADIGM-HF Investigators and Committees. Risk Related to Pre-Diabetes Mellitus and Diabetes Mellitus in Heart Failure With Reduced Ejection Fraction: Insights From Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial. Circ Heart Fail. 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 30. | Vaduganathan M, Fonarow GC, Greene SJ, DeVore AD, Kavati A, Sikirica S, Albert NM, Duffy CI, Hill CL, Patterson JH, Spertus JA, Thomas LE, Williams FB, Hernandez AF, Butler J. Contemporary Treatment Patterns and Clinical Outcomes of Comorbid Diabetes Mellitus and HFrEF: The CHAMP-HF Registry. JACC Heart Fail. 2020;8:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1038] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 32. | Witte KK, Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Fonseca C, Lonn E, Noè A, Schwende H, Butylin D, Chiang Y, Pascual-Figal D; TRANSITION investigators. Influence of diabetes on sacubitril/valsartan titration and clinical outcomes in patients hospitalized for heart failure. ESC Heart Fail. 2023;10:80-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 33. | El-Battrawy I, Demmer J, Abumayyaleh M, Crack C, Pilsinger C, Zhou X, Mügge A, Akin I, Aweimer A. The impact of sacubitril/valsartan on outcome in patients suffering from heart failure with a concomitant diabetes mellitus. ESC Heart Fail. 2023;10:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Wijkman MO, Claggett B, Vaduganathan M, Cunningham JW, Rørth R, Jackson A, Packer M, Zile M, Rouleau J, Swedberg K, Lefkowitz M, Shah SJ, Pfeffer MA, McMurray JJV, Solomon SD. Effects of sacubitril/valsartan on glycemia in patients with diabetes and heart failure: the PARAGON-HF and PARADIGM-HF trials. Cardiovasc Diabetol. 2022;21:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail. 2016;18:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 36. | El-Battrawy I, Borggrefe M, Akin I. The Risk for Sudden Cardiac Death and Effect of Treatment With Sacubitril/Valsartan in Heart Failure. JACC Heart Fail. 2019;7:999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Abo-Khookh AM, Ghoneim HA, Abdelaziz RR, Nader MA, Shawky NM. The dual inhibitor Sacubitril-valsartan ameliorate high-fat high-fructose-induced metabolic disorders in rats superiorly compared to valsartan only. J Pharm Pharmacol. 2023;75:846-858. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Vila G, Grimm G, Resl M, Heinisch B, Einwallner E, Esterbauer H, Dieplinger B, Mueller T, Luger A, Clodi M. B-type natriuretic peptide modulates ghrelin, hunger, and satiety in healthy men. Diabetes. 2012;61:2592-2596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Liu Y, Fan Y, Li J, Chen M, Chen A, Yang D, Guan X, Cao Y. Combination of LCZ696 and ACEI further improves heart failure and myocardial fibrosis after acute myocardial infarction in mice. Biomed Pharmacother. 2021;133:110824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Maqbool M, Cooper ME, Jandeleit-Dahm KAM. Cardiovascular Disease and Diabetic Kidney Disease. Semin Nephrol. 2018;38:217-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Fouli GE, Gnudi L. The Future: Experimental Therapies for Renal Disease in Diabetes. Nephron. 2019;143:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 43. | Packer M, Claggett B, Lefkowitz MP, McMurray JJV, Rouleau JL, Solomon SD, Zile MR. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018;6:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 44. | Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, Wiebe N, Ruospo M, Wheeler DC, Strippoli GF. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 45. | Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, Zile MR, Packer M, Desai AS, Solomon SD, McMurray JJV. Renal Effects and Associated Outcomes During Angiotensin-Neprilysin Inhibition in Heart Failure. JACC Heart Fail. 2018;6:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 288] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 46. | Zhang X, Zhou Y, Ma R. Potential effects and application prospect of angiotensin receptor-neprilysin inhibitor in diabetic kidney disease. J Diabetes Complications. 2022;36:108056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Furukawa S, Suzuki H, Fujihara K, Kobayashi K, Iwasaki H, Sugano Y, Yatoh S, Sekiya M, Yahagi N, Shimano H. Malondialdehyde-modified LDL-related variables are associated with diabetic kidney disease in type 2 diabetes. Diabetes Res Clin Pract. 2018;141:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Habibi J, Aroor AR, Das NA, Manrique-Acevedo CM, Johnson MS, Hayden MR, Nistala R, Wiedmeyer C, Chandrasekar B, DeMarco VG. The combination of a neprilysin inhibitor (sacubitril) and angiotensin-II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker Obese rat. Cardiovasc Diabetol. 2019;18:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Jing W, Vaziri ND, Nunes A, Suematsu Y, Farzaneh T, Khazaeli M, Moradi H. LCZ696 (Sacubitril/valsartan) ameliorates oxidative stress, inflammation, fibrosis and improves renal function beyond angiotensin receptor blockade in CKD. Am J Transl Res. 2017;9:5473-5484. [PubMed] |
| 50. | Uijl E, 't Hart DC, Roksnoer LCW, Groningen MCC, van Veghel R, Garrelds IM, de Vries R, van der Vlag J, Zietse R, Nijenhuis T, Joles JA, Hoorn EJ, Danser AHJ. Angiotensin-neprilysin inhibition confers renoprotection in rats with diabetes and hypertension by limiting podocyte injury. J Hypertens. 2020;38:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 51. | Roksnoer LC, van Veghel R, Clahsen-van Groningen MC, de Vries R, Garrelds IM, Bhaggoe UM, van Gool JM, Friesema EC, Leijten FP, Hoorn EJ, Danser AH, Batenburg WW. Blood pressure-independent renoprotection in diabetic rats treated with AT1 receptor-neprilysin inhibition compared with AT1 receptor blockade alone. Clin Sci (Lond). 2016;130:1209-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Rahman A, Sherajee SJ, Rafiq K, Kobara H, Masaki T, Nakano D, Morikawa T, Konishi Y, Imanishi M, Nishiyama A. The angiotensin II receptor-neprilysin inhibitor LCZ696 attenuates the progression of proteinuria in type 2 diabetic rats. J Pharmacol Sci. 2020;142:124-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, Fort PE. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 291] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 54. | Undank S, Kaiser J, Sikimic J, Düfer M, Krippeit-Drews P, Drews G. Atrial Natriuretic Peptide Affects Stimulus-Secretion Coupling of Pancreatic β-Cells. Diabetes. 2017;66:2840-2848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Judge P, Haynes R, Landray MJ, Baigent C. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant. 2015;30:738-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 56. | Ogawa Y, Mukoyama M, Yokoi H, Kasahara M, Mori K, Kato Y, Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Tokudome T, Kishimoto I, Sugawara A, Nakao K. Natriuretic peptide receptor guanylyl cyclase-A protects podocytes from aldosterone-induced glomerular injury. J Am Soc Nephrol. 2012;23:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Wang P, Guo R, Bai X, Cui W, Zhang Y, Li H, Shang J, Zhao Z. Sacubitril/Valsartan contributes to improving the diabetic kidney disease and regulating the gut microbiota in mice. Front Endocrinol (Lausanne). 2022;13:1034818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Motz CT, Chesler KC, Allen RS, Bales KL, Mees LM, Feola AJ, Maa AY, Olson DE, Thule PM, Iuvone PM, Hendrick AM, Pardue MT. Novel Detection and Restorative Levodopa Treatment for Preclinical Diabetic Retinopathy. Diabetes. 2020;69:1518-1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 698] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 60. | Mrugacz M, Bryl A, Zorena K. Retinal Vascular Endothelial Cell Dysfunction and Neuroretinal Degeneration in Diabetic Patients. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 61. | Prasad T, Roksnoer LC, Zhu P, Verma A, Li Y, Batenburg WW, de Vries R, Danser AH, Li Q. Beneficial Effects of Combined AT1 Receptor/Neprilysin Inhibition (ARNI) Versus AT1 Receptor Blockade Alone in the Diabetic Eye. Invest Ophthalmol Vis Sci. 2016;57:6722-6730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Yamagata R, Nemoto W, Nakagawasai O, Takahashi K, Tan-No K. Downregulation of spinal angiotensin converting enzyme 2 is involved in neuropathic pain associated with type 2 diabetes mellitus in mice. Biochem Pharmacol. 2020;174:113825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 63. | Wilkinson-Berka JL, Tan G, Jaworski K, Harbig J, Miller AG. Identification of a retinal aldosterone system and the protective effects of mineralocorticoid receptor antagonism on retinal vascular pathology. Circ Res. 2009;104:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Li B, Li N, Guo S, Zhang M, Li J, Zhai N, Wang H, Zhang Y. The changing features of serum adropin, copeptin, neprilysin and chitotriosidase which are associated with vascular endothelial function in type 2 diabetic retinopathy patients. J Diabetes Complications. 2020;34:107686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Dahrouj M, Alsarraf O, Liu Y, Crosson CE, Ablonczy Z. C-type natriuretic peptide protects the retinal pigment epithelium against advanced glycation end product-induced barrier dysfunction. J Pharmacol Exp Ther. 2013;344:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 67. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 858] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 68. | Hicks CW, Selvin E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr Diab Rep. 2019;19:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 69. | Tang HY, Jiang AJ, Ma JL, Wang FJ, Shen GM. Understanding the Signaling Pathways Related to the Mechanism and Treatment of Diabetic Peripheral Neuropathy. Endocrinology. 2019;160:2119-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Ogata Y, Nemoto W, Nakagawasai O, Yamagata R, Tadano T, Tan-No K. Involvement of Spinal Angiotensin II System in Streptozotocin-Induced Diabetic Neuropathic Pain in Mice. Mol Pharmacol. 2016;90:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Davidson EP, Coppey LJ, Shevalye H, Obrosov A, Yorek MA. Vascular and Neural Complications in Type 2 Diabetic Rats: Improvement by Sacubitril/Valsartan Greater Than Valsartan Alone. Diabetes. 2018;67:1616-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, Dua TK, Kalita J, Manna P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol. 2018;833:472-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 73. | Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O, Alam U, Marshall A, Fadavi H, Ferdousi M, Azmi S, Tavakoli M, Efron N, Jeziorska M, Malik RA. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38:1138-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 74. | Davidson EP, Coppey LJ, Holmes A, Yorek MA. Effect of inhibition of angiotensin converting enzyme and/or neutral endopeptidase on vascular and neural complications in high fat fed/low dose streptozotocin-diabetic rats. Eur J Pharmacol. 2012;677:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Yorek MS, Obrosov A, Lu B, Gerard C, Kardon RH, Yorek MA. Effect of Inhibition or Deletion of Neutral Endopeptidase on Neuropathic Endpoints in High Fat Fed/Low Dose Streptozotocin-Treated Mice. J Neuropathol Exp Neurol. 2016;75:1072-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | Davidson EP, Coppey LJ, Yorek MA. Early loss of innervation of cornea epithelium in streptozotocin-induced type 1 diabetic rats: improvement with ilepatril treatment. Invest Ophthalmol Vis Sci. 2012;53:8067-8074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 77. | Xi C, Zhang Y, Yan M, Lv Q, Lu H, Zhou J, Wang Y, Li J. Exogenous neuritin treatment improves survivability and functions of Schwann cells with improved outgrowth of neurons in rat diabetic neuropathy. J Cell Mol Med. 2020;24:10166-10176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Liu YP, Shao SJ, Guo HD. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020;248:117459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 79. | Chung AM. Calcitonin gene-related peptide (CGRP): role in peripheral nerve regeneration. Rev Neurosci. 2018;29:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |