Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.853
Peer-review started: January 9, 2024
First decision: January 27, 2024
Revised: February 8, 2024
Accepted: March 21, 2024
Article in press: March 21, 2024
Published online: May 15, 2024
Processing time: 122 Days and 2.6 Hours
Tuberculosis (TB) remains a leading cause of death among infectious diseases, particularly in poor countries. Viral infections, multidrug-resistant and ex-tensively drug-resistant TB strains, as well as the coexistence of chronic illnesses such as diabetes mellitus (DM) greatly aggravate TB morbidity and mortality. DM [particularly type 2 DM (T2DM)] and TB have converged making their control even more challenging. Two contemporary global epidemics, TB-DM behaves like a syndemic, a synergistic confluence of two highly prevalent diseases. T2DM is a risk factor for developing more severe forms of multi-drug resistant-TB and TB recurrence after preventive treatment. Since a bidirectional relationship exists between TB and DM, it is necessary to concurrently treat both, and promote recommendations for the joint management of both diseases. There are also some drug-drug interactions resulting in adverse treatment outcomes in TB-DM patients including treatment failure, and reinfection. In addition, autophagy may play a role in these comorbidities. Therefore, the TB-DM comorbidities present several health challenges, requiring a focus on multidisciplinary collaboration and integrated strategies, to effectively deal with this double burden. To effectively manage the comorbidity, further screening in affected countries, more suitable drugs, and better treatment strategies are required.
Core Tip: Tuberculosis (TB)-diabetes mellitus (DM) comorbidities are major health problems due to the increasing number of type 2 DM (T2DM) cases in developing countries, where active TB is prevalent. This can negatively affect the outcomes of TB-DM treatments. T2DM is commonly related to obesity and is being increasingly recognized as a risk factor for TB, whereas TB may worsen glycemic control among DM patients. These bidirectional relationships require more effective drugs, better treatment strategies, and the need to adjust dose schedules for controlling hyperglycemia during active TB infection.
- Citation: Al-Bari MAA, Peake N, Eid N. Tuberculosis-diabetes comorbidities: Mechanistic insights for clinical considerations and treatment challenges. World J Diabetes 2024; 15(5): 853-866
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/853.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.853
Tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb), and type 2 diabetes mellitus (T2DM), is a metabolic disorder characterized by hyperglycemia and insulin resistance. The association between TB and diabetes mellitus (DM) was recognized by Avicenna as early as 1000 AD, and Yugimahamuni, a traditional Indian saint described a cluster of symptoms called ‘meganoikal’ for the TB-DM association[1]. T2DM patients mainly suffer from being overweight, excessive thirst and polyuria[1,2]. Juvenile diabetes was linked with a 10-fold higher risk of pulmonary TB[3,4] and tuberculous diabetic clinics were established in the United Kingdom to improve outcomes for patients with TB-DM[4,5]. Several epidemiological studies indicated that DM is a risk factor for TB in the 1990s based on the increasing prevalence of T2DM in low- and middle-income countries (LMICs) where TB remained endemic[4,6]. This relationship was over-shadowed firstly by the emergence of the acquired immune deficiency syndrome epidemic[7], and secondly by the Coronavirus disease 2019 (COVID-19) pandemic[8]. However, several studies have supported the solid link between TB-DM[9,10]. The global TB Union and the WHO developed a collaborative framework for TB-diabetes issued by the “Bali declaration”[11]; and TANDEM (TB and DM) program, through which the TB Union and the World Diabetes Foundation aimed to prevent the convergence of the TB-DM co-epidemic globally[12,13]. In 2021, it was estimated that 537 million people worldwide had DM. More than 80% of T2DM cases are found in LMICs and in areas where TB remains endemic. Moreover, DM is associated with microvascular and macrovascular complications that predominantly result from hyperglycemia[2,14,15].
TB remains the leading cause of death from a single infectious microorganism, with almost 25% of the human population currently infected with either latent or active TB[16]. DM is an independent risk factor for lower respiratory infections including TB and the two often coexist[1,17,18]. DM-TB comorbidity behaves like a syndemic, in that there is a synergistic confluence of the two conditions[8]. DM is a risk factor for developing severe forms of TB such as active TB (about three fold)[16,19], latent TB infection (LTBI) (approximately two fold)[8], TB recurrence after preventive treatment[16] and worsened TB outcomes after therapeutic treatment[8,16], higher rates of treatment failure, relapse and recurrence of infection and mortality[4,19,20].
Diabetic patients are susceptible to infections and may experience severe illness due to compromised immune system[2]. Various studies have shown that 5%-30% of patients with multi-drug resistant (MDR)-TB also have DM[19]. On the other hand, patients with diabetes have a higher risk of developing MDR-TB, with serious adverse reactions related to the complicated treatment of these diseases[8]. DM may play an important role in the development of resistance to the first-line anti-TB drugs such as rifampin and second-line anti-TB drugs such as linezolid[21-23].
TB itself is an identified factor for glucose intolerance[19] and metabolic alterations[24] that can cause tuberculous pancreatitis with pancreatic endocrine hypofunction resulting in hyperglycemia[8]. Importantly, there is a rising incidence of pre-diabetics worldwide, particularly in TB endemic areas[4]. TB-DM patients may have worse symptoms such as weight loss, dyspnea, and prolonged fever[20], which can worsen in case of poor glycemic control[20,25,26].
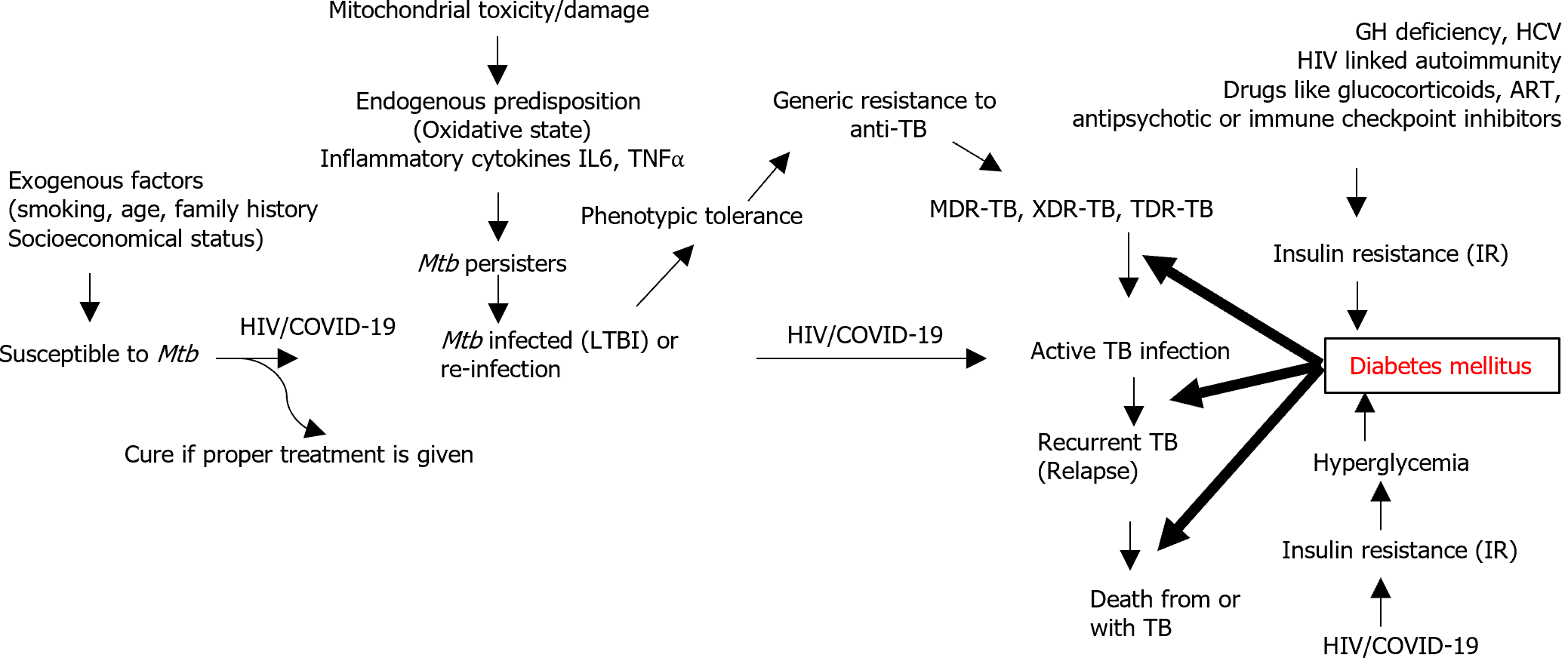
Pathophysiological mechanisms underlying TB-DM interaction: Since the interactions between TB and DM are multifactorial[1], several mechanisms are shown in Figure 1.
It has been shown that the number and function of immune cells such as macrophages and lymphocytes are significantly compromised by metabolic alterations in TB-DM patients[27,28]. The susceptibility of DM patients to TB infection is mainly due to reduced cellular immunity caused by decreasing numbers and function of T-lymphocytes particularly T-helper 1 (TH1) cells[1]. TH1 cytokines such as interleukin (IL)-1 and IL-6 are reduced in patients with concomitant DM-TB compared to non-diabetic individuals. The impaired function of macrophages in DM patients is associated with oxidative stress products including reactive oxygen species (ROS), dysregulated phagocytosis, and chemotactic action[1,2]. This immune dysfunction in DM may play an important role in the reactivation of TB from endogenous LTBI and increase the susceptibility of hosts to exogenous reinfection[1,29]. Hyperglycemia also impairs the force of the respiratory burst that contributes to expelling Mtb, and this stress response to infection may also be associated with dysglycemia, a situation mediated by the effect of IL-1, IL-6, and tumor necrosis factor alpha[1].
Chronic inflammatory diseases such as TB and DM are linked pathogenetically to oxidative stress[27,28], and in DM, intrinsic predispositions including age, family history, and extrinsic factors such as smoking all enhance oxidative stress[30,31]. Increased expression of efflux pumps may be responsible for the phenotypic tolerance of Mtb persisters to anti-TB drugs such as rifampicin in DM patients[32-34].
Oxidative stress in patients with diabetes is linked to the formation of advanced glycation end-products (AGEs), the product of the chemical reactions of proteins with sugars[31,35,36]. An increase in ROS has been positively correlated to enriched AGEs and hyperglycemia, and resveratrol, an antioxidant present in grapes and berries, can ameliorate this effect[31,37]. Excess AGEs in DM patients are involved in the pathophysiology of chronic complications such as diabetic cardiomyopathy via the induced dysfunction of endothelial progenitor cells[38]. AGEs can act in both receptor-dependent and receptor-independent mechanisms. The receptor of AGE (also called RAGE), a Class III MHC protein receptor, has been found on the surface of immune cells, and plays important roles in controlling TB[31]. Macroautophagy (hereafter referred to as autophagy) is a cytoprotective pathway for the clearance of cellular debris upon exposure to various stressors such as oxidative stress. RAGE has been associated with autophagy via its primary ligands, high-mobility group box 1[39,40], and the establishment of neutrophil extracellular traps (NETs)[41]. Mtb has been shown to induce NETs that can activate and trigger the release of pro-inflammatory cytokines by macrophages[31]. Metformin, a prototypical antidiabetic agent, can reduce the impact of AGE production via suppression of soluble RAGE in an AMP-activated protein kinase (AMPK, a key metabolic and energy regulator)-dependent fashion[31,41,42]. The peroxisome proliferator-activated receptor gamma (PPAR) γ agonist rosiglitazone restores AGE-induced dysfunction of endothelial progenitor cells and relieves DM-related vascular complications via activation of the PI3K-AKT-endothelial NO synthase (eNOS) pathway[31,36]. In addition, glucagon-like peptide-1 (GLP-1) and GLP-1 receptor agonists, such as exendin-4, have been proposed as targets for treating diabetes and protecting against diabetic cardiomyopathy[31,43].
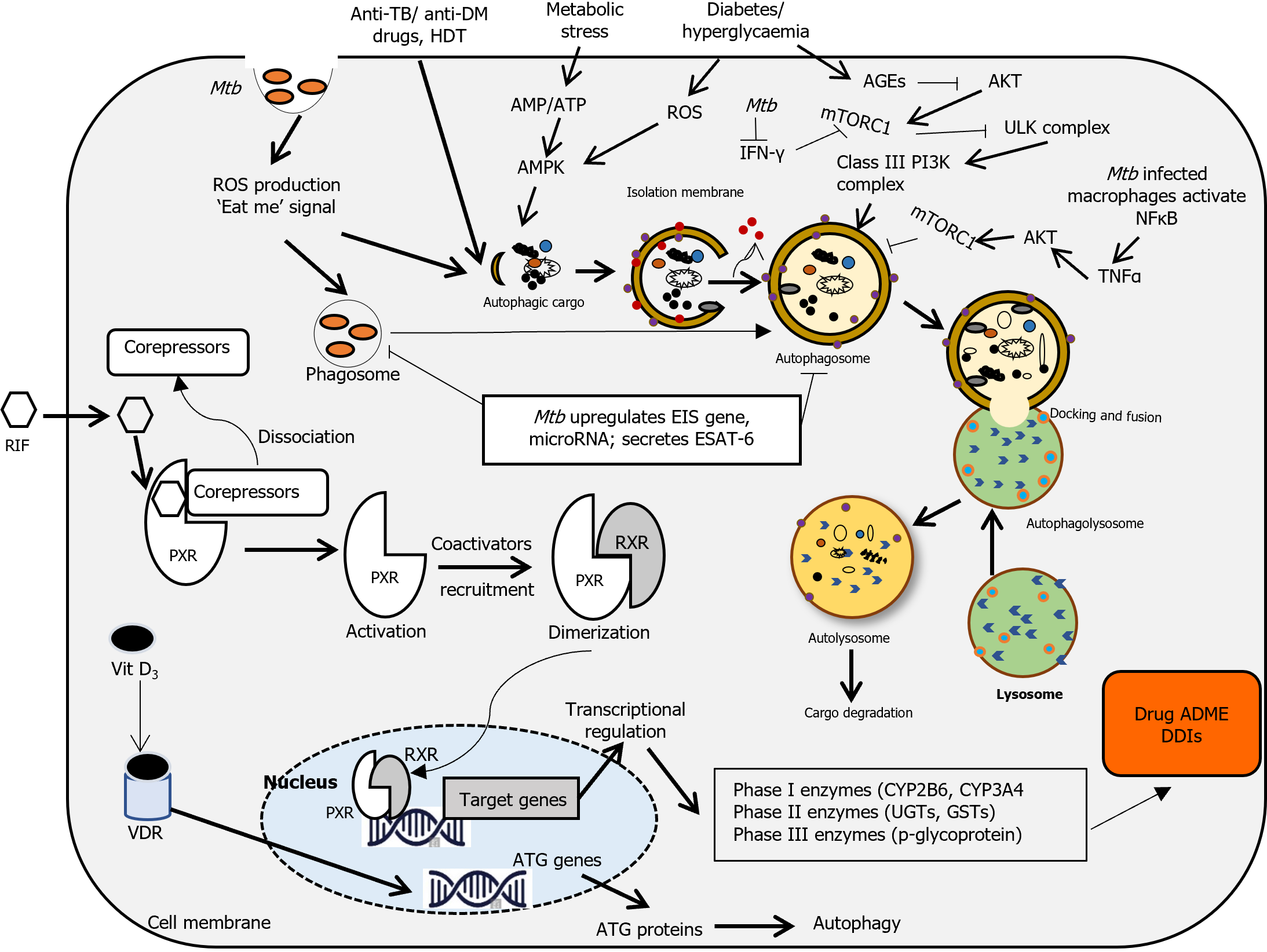
Autophagy-mediated alterations in TB-DM: Autophagy occurs during normal development at the basal level, and selectively targets intracellular pathogens (e.g., mycobacteria called xenophagy) under various stresses, including oxidative stress[44,45-47]. Autophagy functions as a protective process in the pathogenesis of most lung diseases by maintaining cellular homeostasis[48]. Accumulating evidence suggests that any dysregulation of autophagy mechanisms or any mutation in autophagy-related genes (ATGs) may contribute to multiple human diseases including TB[49,50] and DM[51,52]. When mycobacteria gain entry to the cytosol, the rupture of mycobacteria-containing phagosomes stimulates autophagy that selectively degrades or inhibits intracellular mycobacteria survival[53,54]. In addition, activation of mycobacterial killing in infected macrophages via phagosome maturation can be assisted by induction of autophagy using metformin[54-56]. Furthermore, autophagy can contribute to the eradication of Mtb by enhancing antimicrobial activity and modulating inflammation[50,52]. However, Mtb can prevent autolysosome formation and degradation in macrophages by secreting different virulence factors[52]. Mtb has also adopted a mechanism that can combat host autophagy of immune cells by interacting with several essential ATGs so that it can survive within host cells (mac-rophages) for an extended period in the dormant LTBI stage[45,52,57,58]. In most cases, LTBI populations act as an important reservoir for disease reactivation and active TB infection in immunocompromised conditions[45]. Thus, given the importance of autophagy in the maintenance of intracellular homeostasis, autophagy modulators offer host-directed therapeutic (HDT) opportunities to control TB infection and represent promising candidates to combat DM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors such as empagliflozin and dapagliflozin play a critical role in glycemic regulation in T2DM[59,60]. In addition, SGLT2 inhibitors have a beneficial impact on many pathophysiological disturbances including kidney disease[61,62] and atherosclerotic cardiovascular events with and without DM[59,63]. Additional cardiorenal protective mechanisms of SGLT2 inhibitors are linked to their antifibrotic effects that correct inflammation and reduce oxidative stress via modulation of mitochondrial function and autophagy[59]. Persistent hyperglycemia increases intracellular stress, including ROS generation and disruption of normal cellular functioning that leads to chronic inflammation, dysregulated energy metabolism, and insulin resistance in DM. As a cleansing mechanism, autophagy can alleviate these imbalances and restore cellular homeostasis. However, the efficiency of autophagy declines with age and overnutrition, and disrupted autophagy has been linked to the pathogenesis of metabolic disorders, including DM[64]. Several nutrient-sensing pathways, such as downstream of Sirtuin1 (SIRT1), which is involved in the regulation of autophagy, have been proposed as therapeutic targets for reducing the progression of type 1 and T2DM[64-68]. SIRT1 activation can dampen Mtb -mediated persistent inflammatory responses and reduce intracellular growth of Mtb via the induction of phagosome-lysosome fusion and autophagy[69]. SIRT1 also antagonizes oxidative stress in the pathogenesis of DM through several important signal mediators, such as AMPK, NADPH oxidase, and eNOS[70]. As a natural SIRT1 activator, resveratrol induces phagolysosome fusion and autophagy, and restricts Mtb growth in vitro and in vivo[71-73]. SIRT1 also cooperates with AMPK and antagonizes oxidative stress in DM[70,74]. Moreover, forkhead TFs, PPARα, and PPARγ co-activator 1 (PGC-1α) are common targets for SIRT1 and AMPK[70,75]. Therefore, activating AMPK and SIRT1 together can reduce oxidative stress in DM by activating these downstream effectors[70] (Figure 2).
Traditionally, standard TB treatment consists of four anti-TB drugs (rifampicin, isoniazid, pyrazinamide, and ethambutol) for an initial intensive phase of 2 months followed by any two drugs for a further 4-month continuation phase[1,18]. The Food and Drug Administration and the American Diabetes Association have approved several oral hypoglycemic drugs including sulfonylureas (glipizide), biguanides (metformin), thiazolidinediones (rosiglitazone), meglitinides (repaglinide), α-glucosidase inhibitors (acarbose), dipeptidyl peptidase (DPP)-IV inhibitors (the gliptins analogues), SGLT2 inhibitors (dapagliflozin), cycloset (bromocriptine) and bile acid sequestrants (colesevelam) for the treatment of T2DM[76,77].
Metformin remains the first line anti-glycemic agent for T2DM because it activates several mechanisms of controlling hyperglycaemia[18,78]. Metformin can be applied as an adjunct to anti-TB drugs like isoniazid, as it can reduce mycobacterial growth by inducing mitochondrial ROS production[20,78] and suppress excessive inflammation and lung tissue injury[79] via inhibition of the nuclear factor κB pathway irrespective of DM status[80,81]. Due to its efficacy in both prevention and treatment outcomes, metformin is a potential candidate for HDT in TB-DM patients[82,83]. In today's world, rifampin (international nonproprietary name, rifampicin) remains one of the most effective and broad-spectrum cornerstone drugs in TB treatment and an active alternative in treating other infections[84]. The combination of isoniazid with rifampicin is used as a TB treatment regime, however sub-therapeutic plasma concentrations of both drugs have been reported, with levels lower by approximately 50% in TB-DM patients[20,34,81,85]. Pyrazinamide is a prodrug that is metabolized by deamidase and xanthine oxidase (XO), a superoxide-generating enzyme to form its active metabolites[117]. Interestingly, the activity of XO increases in liver and plasma of DM patients significantly and is involved in ROS production in type 1 DM[86,87]. Thus, higher doses of pyrazinamide are required for the treatment of TB in patients with uncontrolled DM[20,86,88]. Due to the well-known adverse effect of peripheral- and optic neuropathy, multiple dose regimens of ethambutol should be lowered when treated TB patients with complicated DM[89-91]. Other anti-tuberculous drugs, such as bedaquiline, pretomanid and delamanid, are on the way towards approval, ready to be used in the TB treatment regime. Due to high binding to plasma proteins, co-administration of bedaquiline and delamanid may compete for plasma protein-binding sites, thereby affecting the free drug concentration[20,85] (Figure 2). Importantly, information related to the current management and treatment gaps for comorbidity is required and needs further study.
Drug efficacy results from the interplay of pharmacokinetic parameters such as drug absorption, distribution, metabolism, and excretion. Drug metabolizing enzymes such as cytochrome P450 enzymes (CYP) located intracellularly, and transport proteins located in distinct membrane domains, are important for drug efficacy. Rifampicin induces several drug-metabolizing enzymes, including CYP3A4 in the liver and small intestine, and drug transporter proteins, such as intestinal and hepatic p-glycoprotein[84]. By promoting the expression of CYP3A4 and CYP2C8/9[20,92], rifampicin reduces the plasma concentrations of their substrates[84,93] including most of the oral hypoglycemic agents[93] and thereby leads to hyperglycemia. Hence, monitoring blood glucose levels and adjusting antidiabetic medications such as sulfonylureas in TB-DM is critical to avoid hypoglycemic episodes[20,84]. On the other hand, isoniazid impairs the CYP2C9 metabolism of sulfonylureas, which worsens glycemic control[1]. Isoniazid also interferes with the release and function of insulin resulting in hyperglycemia even in normal subjects. Therefore, dose adjustment of sulfonylureas or insulin is necessary during the therapeutic usage of these drugs[34].
Metformin is not metabolized by CYP2C to a significant extent[84,89,94], its transport is p-glycoprotein-independent[81] and thus it can be a good alternative for combination with rifampicin, bedaquiline or delamanid due to minimal pharmacokinetic drug-drug interactions (DDIs). However, metformin is a substrate for the solute carrier (SLC) tran-sporters in humans, namely organic cation transporter (OCT1; SLC22 subfamily) and other transporters[89,95], whereas rifampicin increases OCT1 mRNA levels and metformin uptake by the liver, enhancing glucose reduction in healthy subjects[96] and lactic acidosis, the main side effects[89,97]. Rifampicin also alters metformin plasma exposure but not blood glucose levels in DM-TB patients[20,98].
Several anti-TB drugs such as ethambutol are substrates for the SLC transporters in humans namely OCT1/2/3, organic anion transporter (OAT; SLC22 subfamily) families and multidrug and toxin extrusion (MATE), MATE proteins 1 and 2K[22,25,99,100]. These transporters play a major role in the disposition and pharmacology of drugs and thus are important regulators for DDIs[22,25]. Since fluoroquinolones such as moxifloxacin or ciprofloxacin[101] may act as potent inhibitors of OCT1 and MATEs, these agents have shown to reduce the cellular uptake of ethambutol, isoniazid, and metformin in vitro[99], and produce dysglycemia in general[102]. Therefore, additional studies are necessary to focus specific treatment on TB-DM patients[99]. Bedaquiline is metabolized by the CYP3A4 to form N-monodesmethyl metabolite, a major circulating intermediate. Both bedaquiline and its metabolites show bactericidal effects dose dependently and inhibit several transporters, organic anion-transporting polypeptide 1B1 and OCT1/2 essential for the transport of sulfonylureas and SGLT2 inhibitors[85]. Also, concurrent use of bedaquiline and delamanid with hypoglycemic agents including insulin analogues prolong the heart rate and hepatic-related adverse reactions[85]. Further research and post-marketing studies are needed to establish and understand the possible interactions of these new drugs. DPP IV inhibitors (the gliptins) may reduce immunocompetence (immune paresis)[2]; and possibly worsen treatment outcomes of patients with TB[1].
Vitamin A exists in the form of retinol, retinal and retinoic acid (RA) that act as ligands of nuclear receptors such as RA receptor (RAR) and retinoid X receptor (RXR)[102]. Two significant derivatives; 9-cis-RA and all-trans-RA (ATRA) are related to RA. 9-cis RA, an isomerization product of ATRA is found to be a cognate ligand for RXRs. ATRA acts as an endogenous ligand for nuclear RAR that can act as a ligand-inducible transcription factor[103,104]. On the other hand, RXR molecules forming permissive heterodimers with disparate nuclear receptors comprise pregnane X receptor (PXR, NR1I2), constitutive androstane receptor (CAR or NR113), PPARs, liver X receptors and farnesoid X receptor (FXR)[103-105]. The dihydroxyvitamin D3 receptor (VDR) was found to form a nonpermissive heterodimer, while RARs and thyroid hormone receptors form conditional heterodimers[106]. Thus, RXRs are important molecules for controlling various cellular functions under normal conditions and diseases, including DM and TB[106].
In general, PXR and CAR are located largely in the hepatocyte cell membranes, acting as suppressors of transcription by binding with corepressors[107]. As a dimerization partner binding to PXR or CAR, RXRα produces a transcriptionally active heterodimer, this leads to transcription of target genes[108-110]. Hepatic nuclear factor 4α (HNF4α) is recognized as the key transcription factor for PXR. CYP3A4 has also been identified as a specific cis-acting gene enhancer element, conferring HNF4α binding and promoting PXR- and CAR-mediated hepatic gene activation[93,111]. Additionally, many transcription cofactors have been shown to regulate PXR activity, such as members of the p160 family, including steroid receptor coactivators, as well as the PPAR-α[112,113].
Interestingly, activation of PXR and CAR can be regulated by many ligands, inducing the expression of several target genes, including CYP3A4, CYP2C9, and CYP1A2, which are highly expressed in hepatocytes[114]. Accordingly, the accelerated blood clearance activity of PXR and CAR affects the pharmacokinetics (PK) of hepatic enzyme substrates, resulting in increased substrate elimination[115,116]. The human PXR is activated by several molecules including antibiotics, and bile acids, and PXR activation regulates several of xenobiotic-inducible genes including CYP3A4, glutathione S-transferases, sulfotransferases, UDP-glucuronosyltransferases (UGTs) and p-glycoprotein resulting in undesirable effects clinically including harmful DDIs in patients on combination therapy[84,117]. Therefore, detecting PXR activity using various assays can help in the development of safer prescription drugs. In mice, PXR also regulates the expression of the drug transporter genes such as OATP2, MDR protein 1 (MDR1) and MDR-associated proteins 2 and 3[117]. Rifampicin is also involved in DDI by inducing gut (enterocytic) CYP3A4 as well as its own metabolism (autoinduction)[93]. Roscovitine, a cyclin-dependent kinase inhibitor enhances the expressions of UGT1A1, CYP2B6 or CYP3A4, and activates PXR in a ligand-independent manner in HepG2 cells in comparison with the CAR and aryl hydrocarbon receptor[118,119]. UGT1A catalyses the glucuronidation of a wide range of xenobiotics and endogenous substrates. It can be induced by rifampicin through both PXR- and CAR -mediated expression[93,113]. P-glycoprotein is a plasma membrane-bound drug efflux (MDR pump) belonging to the ATP binding cassette superfamily of transport proteins, which are encoded by the MDR genes. Rifampicin induces p-glycoprotein in addition to PXR that induces CYP3A4 and reduction of the intracellular concentration of drug substrates by transmembrane efflux[93]. In addition, rifampicin was found to activate PXR- mediated MDR1 gene expression for the efflux of several drugs and drug conjugates[120]. Furthermore, PXR is involved in the regulation of OATP2, an uptake transporter that uptakes of drugs from blood into the hepatocytes[84]. It is also found that OCT1 (SLC22A1) expression is also upregulated by PXR agonists in chronic myeloid leukemia cells[121]. Another receptor, CAR, is also involved in CYP3A4 transcriptional regulation by binding to PXR, which affects CYP3A4 expression[93]. The liver is the main metabolic organ for glucose homeostasis and the accumulation of bile acids can induce hepatotoxicity. Thus, the activation of multiple bile acid receptors such as CAR, FXR, PXR, VDR is preventive against this toxicity by downregulating the bile acid efflux transporters such as OATP1A1, OATP1A4 and MRP3[82,92].
Due to these multidrug regimens of anti-TB and anti-DM therapies, DDIs, unwanted PK/pharmacodynamics (PD) effects and adverse events including nephrotoxicity, ocular toxicity, neurotoxicity, liver injury and teratogenicity have been reported in the literature[101]. Thus, rational management of combined TB-DM is important but challenging for several reasons: (1) Both DM and active TB may affect the PK and pharmacodynamic parameters of drugs, resulting in lower efficacy and increased toxicity[81,122,123]; (2) compared with nondiabetics, DM reduces the immune responses needed to control TB infection, resulting in a higher rate of therapy failure, death, and recurrence of TB in patients with DM[81,124]; (3) anti-TB drugs such as inhibitors (isoniazid) or inducers (rifampicin) of the CYP enzymes, which are regularly used for TB patients can alter plasma concentrations of hypoglycemic and other anti-TB drugs[81]; and (4) although several anti-TB drugs such as bedaquiline, delamanid, clofazimine, linezolid and carbapenems are concurrently used for the treatment of MDR-TB and extreme drug resistance-TB cases, these drugs cannot show efficacy in DM patients with and active TB[18,125,126]. For properly managing patients with concurrent TB-DM, some key clinical considerations, and recommendations[4,13,89] are included here, as shown below.
Patient education and counselling: Informing patients with TB and DM about the nature of the disease, the duration of treatment, adverse effects of drugs, and disease complications[1]. Intense counselling for promotion of healthy lifestyle choices, dealing with medication adherence and starting insulin therapy as necessary in TB-DM patients.
Consciousness of DDIs: Rifampicin increases metabolism of the major anti-diabetes drugs, and COVID-19/human immunodeficiency virus (HIV) treatment is likely to incur additional DDIs. In these cases, more studies are needed on DDIs between co-administered drugs and between new and existing drugs during the development of new anti-TB drugs and combination regimens[127]. This can help avoid DDIs to ensure therapeutic success and reduce the curve of TB-related mortality.
Therapeutic drug-monitoring: Proper understanding of possible DDIs before designing an anti-TB regimen is mandatory to improve the quality of patient life. Updated knowledge of anti-TB drugs and PK/PD parameters coupled with therapeutic drug-monitoring (TDM)[85] are important for guiding physicians towards effective treatments. For example, chronic hyperglycemia mitigates the efficiency of the anti-TB treatment and affects the elimination of Mtb for optimal immune surveillance. Moreover, a low plasma level of anti-TB drugs in DM patients has been observed compared to non-DM patients. Thus, TDM intervention may establish effective dosing, specifically in uncontrolled DM patients[93,128,129].
Treatment adherence: Perfect adherence to prescribed regimens is a cornerstone for individuals infected with MDR Mtb strains as the therapy typically lasts for around 2 years and involves multiple doses, which can increase the risk of drug-related adverse events. Improper intake of medication by the patient or abandonment of treatment can be for multiple reasons, e.g., quiescent disease symptoms, prior perception of high pill burden[89,98] or adverse effects from the combination of TB and DM drugs[20]. Up to 30% TB-DM patients experience a higher incidence of gastrointestinal adverse effects (nausea and vomiting) when treated with metformin and rifampicin[18] that possibly lead to non-adherence and poor treatment outcome[98]. Thus, TB-DM patients should complete the entire course of TB treatment, while also controlling their DM with diet, lifestyle modifications and specific drugs to avoid possible DDIs[20,130].
Simultaneous TB-DM screening: Although recommendations have been made for screening and diagnosis of combined TB-DM, there is little evidence regarding the efficacy of specific TB testing in individuals with DM and specific DM tests for patients with TB. Because comorbidity represents a risk factor for dangerous TB outcomes, TB clinics provide specific care for this type of illness[18,89,131]. Therefore, further studies are needed to improve the screening of TB patients for diabetes and TB screening in diabetics from a public health perspective, particularly in developing countries where the double burden of TB and diabetes is high. Screening and diagnosing combined TB-DM morbidities according to WHO/IDF recommendations could be a valuable tool.
It is important to pay close attention when constructing the care cascade process for every individual case[132]. Wherever necessary, these patients should be assessed by interdisciplinary specialists, including endocrinologists to confirm the DM diagnosis[20,58], pharmacologists to confirm overlapping toxicities with different types of drugs[72,103], and medical specialists to confirm the risk of coinfection (HIV, COVID-19) or comorbidities (cardiovascular or other diseases)[133]. This is because TB-DM patients vary in terms of frequency of complications such as heart disease, liver and renal problems and other diseases associated with comorbidities[4]. Table 1 summarizes the interactions between anti-TB drugs with anti-DM drugs[134-145].
| Anti-DM drug | Anti-TB drug | Interaction effects | Expected clinical effects |
| Biguanides | |||
| Metformin | Rifampin (RIF, INN, rifampicin) | RIF induces upregulation of metformin effects | Promotes glucose-lowering effect of metformin[98] |
| Sulfonylurea group | |||
| Tolbutamide | Rifampin (INN, rifampicin) | RIF promotes the CYP2C9 and reduces in tolbutamide plasma conc | Hyperglycemia and diminished anti-TB efficacy over time[18,84] |
| Glyburide (INN, glibenclamide) | Rifampin (INN, rifampicin) | RIF promotes the CYP2C9 and reduces 39% glyburide conc | Hyperglycemia and diminished anti-TB efficacy over time[134] |
| Glyburide (INN, glibenclamide) | Rifapentine | Rifapentine induces CYP3A4 and CYP2C8/9 expressions and reduces glyburide plasma conc | Hyperglycemia[81,135] |
| Glyburide (INN, glibenclamide) | Bedaquiline | Glyburide inhibits CYP3A4 in liver | [85,136] |
| Glyburide (INN, glibenclamide) | Delamanid | Glyburide inhibits CYP3A4 in liver | [85,136] |
| Gliclazide | Rifampin (INN, rifampicin) | RIF decreases plasma conc. of gliclazide by 70% by inducing CYP2C9 | Hyperglycemia and diminished anti-TB efficacy over time[18,137] |
| Gliclazide | Rifapentine | Rifapentine induces CYP3A4 and CYP2C8/9 and decreases gliclazide | Hyperglycemia[81] |
| Gliclazide | Bedaquiline | M2 of bedaquiline inhibits effects on CYP3A4 and CYP2C8 | Hypoglycemic episodes[85] |
| Gliclazide | Delamanid | No crossing interaction | [85] |
| Glimepiride | Rifampin (INN, rifampicin) | RIF promotes the expression of CYP2C9 reducing in 34% of glimepiride conc | Hyperglycemia and diminished anti-TB efficacy over time[138] |
| Glipizide | Rifampin (INN, rifampicin) | RIF promotes CYP2C9 and reduces 22% of glipizide conc | Hyperglycemia and diminished anti-TB efficacy over time[134] |
| Gliquidone | Rifapentine | Rifapentine induces CYP3A4 and CYP2C8/9 and decreases gliquidone | Hyperglycemia[81] |
| Gliquidone | Bedaquiline | M2 of bedaquiline inhibits CYP3A4 effects | Hypoglycemic episodes[85] |
| Gliquidone | Delamanid | Delamanid induces CYP3A4 and decreases gliquidone level | [85] |
| Meglitinide analogues | |||
| Repaglinide | Rifampin (INN, rifampicin) | RIF promotes CYP3A4 and reduces repaglinide conc. 57% | Hyperglycemia and diminished anti-TB efficacy over time[18,138-140] |
| Repaglinide | Rifapentine | Rifapentine induces CYP3A4 and CYP2C8 and decreases repaglinide level | Hyperglycemia[81] |
| Repaglinide | Bedaquiline | Interindividual variability | Variable hypoglycemic episodes[85,141] |
| Repaglinide | Delamanid | Interindividual variability | Variable hypoglycemic episodes[85,141] |
| Nateglinide | Rifampin (INN, rifampicin) | RIF promotes CYP3A4 and CYP2C9 and reduces nateglinide conc by 24%. | Hyperglycemia and diminished anti-TB efficacy over time[84] |
| Nateglinide | Rifapentine | Rifapentine induces CYP3A4 and CYP2C9 and decreases nateglinide level | Hyperglycemia[81] |
| Nateglinide | Bedaquiline | M2 of bedaquiline inhibits effects on CYP3A4 | Hypoglycemic episodes[85] |
| Nateglinide | Delamanid | Coadministration of delamanid with nateglinide reduces exposure to delamanid | TB reactivation[85,142] |
| Thiazolidinediones | |||
| Rosiglitazone | Rifampin (INN, rifampicin) | RIF promotes CYP2C8 and reduces of rosiglitazone conc by 65% | Hyperglycemia and diminished anti-TB efficacy over time[18,92] |
| Rosiglitazone | Rifapentine | Rifapentine induces CYP3A4 and CYP2C9 and decreases rosiglitazone level | Hyperglycemia[81] |
| Rosiglitazone | Bedaquiline | M2 of bedaquiline inhibits effects on CYP2A8 | Hypoglycemic episodes[85] |
| Rosiglitazone | Delamanid | No crossing | [116] |
| Pioglitazone | Rifampin (INN, rifampicin) | RIF promotes CYP2C8 and reduces pioglitazone conc by 65% | Hyperglycemia and diminished anti-TB efficacy over time[20,62,143] |
| Pioglitazone | Rifapentine | Rifapentine induces CYP3A4 and CYP2C8 and decreases pioglitazone level | Hyperglycemia[81] |
| Pioglitazone | Bedaquiline | In combination with bedaquiline, pioglitazone may cause severe acute rhabdomyolysis | Dose-independent myalgia[85,144] |
| Pioglitazone | Delamanid | Inhibitory effects on CYP3A4 | Hypoglycemic episodes[85] |
| Dipeptidyl peptidase IV inhibitors | |||
| Sitagliptin | Rifapentine | Rifapentine induces CYP3A4 and CYP2C8 and decreases sitagliptin level | Hyperglycemia[81] |
| Sitagliptin | Bedaquiline | M2 of bedaquiline inhibits effects on CYP3A4 and CYP2A8 | Hypoglycemic episodes[85] |
| Sitagliptin | Delamanid | Inhibitory effects on CYP3A4 | Hypoglycemic episodes[85] |
| Saxagliptin | Rifapentine | Rifapentine induces CYP3A4 and decreases saxagliptin level | Hyperglycemia[81] |
| Saxagliptin | Bedaquiline | M2 of bedaquiline inhibits effects on CYP3A4 | Hypoglycemic episodes[85] |
| Saxagliptin | Delamanid | Inhibitory effects on CYP3A4 | Hypoglycemic episodes[85] |
| Insulin | No effect anticipated | No studies published[85] | |
| Isoniazid | In DM patients | Isoniazid in combination with rifampicin causes hepatotoxicity TB-DM patients (50%) | Low Anti-TB efficacy[34,84,128,129] |
| Isoniazid | HIV-positive patient with type 2 DM | Prophylaxis of TB | Hyperglycaemia induced by isoniazid[20,145] |
| Pyrazinamide | In DM patients | DM patients with higher levels of xanthine oxidase causes low therapeutic targets for pyrazinamide | TB resistance[20,86,88] |
| Ethambutol | In DM patients | Neuritis optica in patients with complicated diabetes. Reduced kidney function in TB-DM patients | Enhances side effects[20,89] |
DM and TB have a bidirectional relationship with epidemiological implications since T2DM is becoming more prevalent in TB-endemic settings. The alarming increase in DM (particularly T2DM) cases in developing countries where active TB is prevalent will negatively influence the outcomes of TB-DM treatments shortly. DM associated with higher age and body weight is considered a risk factor for TB, potentially affecting its presentation, while TB may negatively affect glycemic control in patients with DM. This bidirectional relationship demands more suitable drugs, better treatment strategies, and the need to properly adjust dose schedules for diabetic patients' therapy during active TB infection.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ajijola L, United States; Jain R, India S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Yorke E, Atiase Y, Akpalu J, Sarfo-Kantanka O, Boima V, Dey ID. The Bidirectional Relationship between Tuberculosis and Diabetes. Tuberc Res Treat. 2017;2017:1702578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Niazi AK, Kalra S. Diabetes and tuberculosis: a review of the role of optimal glycemic control. J Diabetes Metab Disord. 2012;11:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Root HF. Diabetic Coma and Pulmonary Tuberculosis. Trans Am Clin Climatol Assoc. 1934;50:210-217. [PubMed] |
| 4. | van Crevel R, Critchley JA. The Interaction of Diabetes and Tuberculosis: Translating Research to Policy and Practice. Trop Med Infect Dis. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Luntz G. Tuberculous diabetics: the Birmingham Regional Service. Lancet. 1954;266:973-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis. 1995;76:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Liu M, Li W, Qiao W, Liang L, Wang Z. Knowledge domain and emerging trends in HIV-MTB co-infection from 2017 to 2022: A scientometric analysis based on VOSviewer and CiteSpace. Front Public Health. 2023;11:1044426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Antonio-Arques V, Franch-Nadal J, Caylà JA. Diabetes and tuberculosis: A syndemic complicated by COVID-19. Med Clin (Engl Ed). 2021;157:288-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Stevenson CR, Critchley JA, Forouhi NG, Roglic G, Williams BG, Dye C, Unwin NC. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn. 2007;3:228-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 895] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 11. | Kapur A, Harries AD, Lönnroth K, Wilson P, Sulistyowati LS. Diabetes and tuberculosis co-epidemic: the Bali Declaration. Lancet Diabetes Endocrinol. 2016;4:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | van Crevel R, Dockrell HM; TANDEM Consortium. TANDEM: understanding diabetes and tuberculosis. Lancet Diabetes Endocrinol. 2014;2:270-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Lin Y, Harries AD, Kumar AMV, Critchley JA, van Crevel R, Owiti P, Dlodlo RA, Kapur A. Tackling diabetes mellitus and tuberculosis: a new Union guide on the management of diabetes-tuberculosis. Int J Tuberc Lung Dis. 2019;23:771-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Magliano DJ, Boyko EJ. IDF Diabetes Atlas. 10th ed. 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK581938/. |
| 15. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4389] [Article Influence: 627.0] [Reference Citation Analysis (0)] |
| 16. | Oglesby W, Kara AM, Granados H, Cervantes JL. Metformin in tuberculosis: beyond control of hyperglycemia. Infection. 2019;47:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Baghaei P, Marjani M, Javanmard P, Tabarsi P, Masjedi MR. Diabetes mellitus and tuberculosis facts and controversies. J Diabetes Metab Disord. 2013;12:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Ruslami R, Aarnoutse RE, Alisjahbana B, van der Ven AJ, van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Esmail A, Sabur NF, Okpechi I, Dheda K. Management of drug-resistant tuberculosis in special sub-populations including those with HIV co-infection, pregnancy, diabetes, organ-specific dysfunction, and in the critically ill. J Thorac Dis. 2018;10:3102-3118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Cáceres G, Calderon R, Ugarte-Gil C. Tuberculosis and comorbidities: treatment challenges in patients with comorbid diabetes mellitus and depression. Ther Adv Infect Dis. 2022;9:20499361221095831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 21. | Meregildo-Rodriguez ED, Asmat-Rubio MG, Zavaleta-Alaya P, Vásquez-Tirado GA. Effect of Oral Antidiabetic Drugs on Tuberculosis Risk and Treatment Outcomes: Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Zhou S, Zeng S, Shu Y. Drug-Drug Interactions at Organic Cation Transporter 1. Front Pharmacol. 2021;12:628705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Sotgiu G, Centis R, D'ambrosio L, Migliori GB. Tuberculosis treatment and drug regimens. Cold Spring Harb Perspect Med. 2015;5:a017822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Pearson F, Huangfu P, McNally R, Pearce M, Unwin N, Critchley JA. Tuberculosis and diabetes: bidirectional association in a UK primary care data set. J Epidemiol Community Health. 2019;73:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Dousa KM, Hamad A, Albirair M, Al Soub H, Elzouki AN, Alwakeel MI, Thiel BA, Johnson JL. Impact of Diabetes Mellitus on the Presentation and Response to Treatment of Adults With Pulmonary Tuberculosis in Qatar. Open Forum Infect Dis. 2019;6:ofy335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic Control and Risk of Infections Among People With Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care. 2018;41:2127-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 27. | Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of Oxidative Stress during Diabetes Mellitus. J Biomark. 2013;2013:378790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 435] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 28. | Yew WW, Leung CC, Zhang Y. Oxidative stress and TB outcomes in patients with diabetes mellitus? J Antimicrob Chemother. 2017;72:1552-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T. Advanced Glycation End Products: A Molecular Target for Vascular Complications in Diabetes. Mol Med. 2015;21 Suppl 1:S32-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 31. | Kiran D, Podell BK, Chambers M, Basaraba RJ. Host-directed therapy targeting the Mycobacterium tuberculosis granuloma: a review. Semin Immunopathol. 2016;38:167-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Ramón-García S, Martín C, Thompson CJ, Aínsa JA. Role of the Mycobacterium tuberculosis P55 efflux pump in intrinsic drug resistance, oxidative stress responses, and growth. Antimicrob Agents Chemother. 2009;53:3675-3682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, van der Ven AJ, Danusantoso H, Aarnoutse RE, van Crevel R. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. 2006;43:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Babalik A, Ulus IH, Bakirci N, Kuyucu T, Arpag H, Dagyildizi L, Capaner E. Plasma concentrations of isoniazid and rifampin are decreased in adult pulmonary tuberculosis patients with diabetes mellitus. Antimicrob Agents Chemother. 2013;57:5740-5742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Nogueira-Machado JA, Chaves MM. From hyperglycemia to AGE-RAGE interaction on the cell surface: a dangerous metabolic route for diabetic patients. Expert Opin Ther Targets. 2008;12:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Liang C, Ren Y, Tan H, He Z, Jiang Q, Wu J, Zhen Y, Fan M, Wu Z. Rosiglitazone via upregulation of Akt/eNOS pathways attenuates dysfunction of endothelial progenitor cells, induced by advanced glycation end products. Br J Pharmacol. 2009;158:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Shemirani F, Yazdanparast R. The interplay between hyperglycemia-induced oxidative stress markers and the level of soluble receptor for advanced glycation end products (sRAGE) in K562 cells. Mol Cell Endocrinol. 2014;393:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomed Pharmacother. 2017;90:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 39. | Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33:567-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 40. | Kang R, Tang D, Lotze MT, Zeh HJ 3rd. AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and bioenergetics through the IL6-pSTAT3 pathway. Autophagy. 2012;8:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, Singhi AD, Kang R, Tang D, Lotze MT, Zeh HJ. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015;22:326-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 42. | Feng X, Chen W, Ni X, Little PJ, Xu S, Tang L, Weng J. Metformin, Macrophage Dysfunction and Atherosclerosis. Front Immunol. 2021;12:682853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 43. | Yi B, Hu X, Wen Z, Zhang T, Cai Y. Exendin-4, a glucagon-like peptide-1 receptor agonist, inhibits hyperglycemia-induced apoptosis in myocytes by suppressing receptor for advanced glycation end products expression. Exp Ther Med. 2014;8:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1204] [Cited by in RCA: 1154] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 45. | Chen DY, Chen YM, Lin CF, Lo CM, Liu HJ, Liao TL. MicroRNA-889 Inhibits Autophagy To Maintain Mycobacterial Survival in Patients with Latent Tuberculosis Infection by Targeting TWEAK. mBio. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2582] [Article Influence: 184.4] [Reference Citation Analysis (1)] |
| 47. | Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 48. | Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5421] [Cited by in RCA: 5293] [Article Influence: 311.4] [Reference Citation Analysis (0)] |
| 49. | Renna M, Schaffner C, Brown K, Shang S, Tamayo MH, Hegyi K, Grimsey NJ, Cusens D, Coulter S, Cooper J, Bowden AR, Newton SM, Kampmann B, Helm J, Jones A, Haworth CS, Basaraba RJ, DeGroote MA, Ordway DJ, Rubinsztein DC, Floto RA. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest. 2011;121:3554-3563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 50. | Liang S, Ma J, Gong H, Shao J, Li J, Zhan Y, Wang Z, Wang C, Li W. Immune regulation and emerging roles of noncoding RNAs in Mycobacterium tuberculosis infection. Front Immunol. 2022;13:987018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Levine B, Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019;176:11-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 2063] [Article Influence: 343.8] [Reference Citation Analysis (0)] |
| 52. | Wang K, Chen Y, Zhang P, Lin P, Xie N, Wu M. Protective Features of Autophagy in Pulmonary Infection and Inflammatory Diseases. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Bao Y, Wang L, Sun J. A Small Protein but with Diverse Roles: A Review of EsxA in Mycobacterium-Host Interaction. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Deretic V, Kimura T, Timmins G, Moseley P, Chauhan S, Mandell M. Immunologic manifestations of autophagy. J Clin Invest. 2015;125:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 55. | Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1521] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 56. | Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 579] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 57. | Huang J, Brumell JH. Bacteria-autophagy interplay: a battle for survival. Nat Rev Microbiol. 2014;12:101-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 449] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 58. | Kumar D, Nath L, Kamal MA, Varshney A, Jain A, Singh S, Rao KV. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell. 2010;140:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 59. | Salvatore T, Galiero R, Caturano A, Rinaldi L, Di Martino A, Albanese G, Di Salvo J, Epifani R, Marfella R, Docimo G, Lettieri M, Sardu C, Sasso FC. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 60. | Salah HM, Verma S, Santos-Gallego CG, Bhatt AS, Vaduganathan M, Khan MS, Lopes RD, Al'Aref SJ, McGuire DK, Fudim M. Sodium-Glucose Cotransporter 2 Inhibitors and Cardiac Remodeling. J Cardiovasc Transl Res. 2022;15:944-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 61. | DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 337] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 62. | Palmer BF, Clegg DJ. Kidney-Protective Effects of SGLT2 Inhibitors. Clin J Am Soc Nephrol. 2023;18:279-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 63. | Pabel S, Hamdani N, Luedde M, Sossalla S. SGLT2 Inhibitors and Their Mode of Action in Heart Failure-Has the Mystery Been Unravelled? Curr Heart Fail Rep. 2021;18:315-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 64. | Kim JY, Mondaca-Ruff D, Singh S, Wang Y. SIRT1 and Autophagy: Implications in Endocrine Disorders. Front Endocrinol (Lausanne). 2022;13:930919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 65. | Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17:647-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 250] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 66. | Muralidharan C, Linnemann AK. β-Cell autophagy in the pathogenesis of type 1 diabetes. Am J Physiol Endocrinol Metab. 2021;321:E410-E416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Shan Z, Fa WH, Tian CR, Yuan CS, Jie N. Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment. Aging (Albany NY). 2022;14:2902-2919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 68. | Parmar UM, Jalgaonkar MP, Kulkarni YA, Oza MJ. Autophagy-nutrient sensing pathways in diabetic complications. Pharmacol Res. 2022;184:106408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 69. | Cheng CY, Gutierrez NM, Marzuki MB, Lu X, Foreman TW, Paleja B, Lee B, Balachander A, Chen J, Tsenova L, Kurepina N, Teng KWW, West K, Mehra S, Zolezzi F, Poidinger M, Kreiswirth B, Kaushal D, Kornfeld H, Newell EW, Singhal A. Host sirtuin 1 regulates mycobacterial immunopathogenesis and represents a therapeutic target against tuberculosis. Sci Immunol. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 70. | Meng T, Qin W, Liu B. SIRT1 Antagonizes Oxidative Stress in Diabetic Vascular Complication. Front Endocrinol (Lausanne). 2020;11:568861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 71. | Bento CF, Empadinhas N, Mendes V. Autophagy in the fight against tuberculosis. DNA Cell Biol. 2015;34:228-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Yu X, Li C, Hong W, Pan W, Xie J. Autophagy during Mycobacterium tuberculosis infection and implications for future tuberculosis medications. Cell Signal. 2013;25:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Abreu R, Giri P, Quinn F. Host-Pathogen Interaction as a Novel Target for Host-Directed Therapies in Tuberculosis. Front Immunol. 2020;11:1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 2738] [Article Influence: 342.3] [Reference Citation Analysis (0)] |
| 75. | Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751-E760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 706] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 76. | Ganesan K, Rana MBM, Sultan S. Oral Hypoglycemic Medications. 2023 May 1. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 77. | Stein SA, Lamos EM, Davis SN. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf. 2013;12:153-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 78. | Novita BD. Metformin: A review of its potential as enhancer for anti tuberculosis efficacy in diabetes mellitus-tuberculosis coinfection patients. Indian J Tuberc. 2019;66:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Padmapriydarsini C, Mamulwar M, Mohan A, Shanmugam P, Gomathy NS, Mane A, Singh UB, Pavankumar N, Kadam A, Kumar H, Suresh C, Reddy D, Devi P, Ramesh PM, Sekar L, Jawahar S, Shandil RK, Singh M, Menon J, Guleria R. Randomized Trial of Metformin With Anti-Tuberculosis Drugs for Early Sputum Conversion in Adults With Pulmonary Tuberculosis. Clin Infect Dis. 2022;75:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJ, Savinko T, Wong AK, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, Rena G. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 2016;119:652-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 497] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 81. | Zheng C, Hu X, Zhao L, Hu M, Gao F. Clinical and pharmacological hallmarks of rifapentine's use in diabetes patients with active and latent tuberculosis: do we know enough? Drug Des Devel Ther. 2017;11:2957-2968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Zhang M, He JQ. Impacts of metformin on tuberculosis incidence and clinical outcomes in patients with diabetes: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Restrepo BI. Metformin: Candidate host-directed therapy for tuberculosis in diabetes and non-diabetes patients. Tuberculosis (Edinb). 2016;101S:S69-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 84. | Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin : clinical relevance. Clin Pharmacokinet. 2003;42:819-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 544] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 85. | Hu M, Zheng C, Gao F. Use of bedaquiline and delamanid in diabetes patients: clinical and pharmacological considerations. Drug Des Devel Ther. 2016;10:3983-3994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Kuppusamy UR, Indran M, Rokiah P. Glycaemic control in relation to xanthine oxidase and antioxidant indices in Malaysian Type 2 diabetes patients. Diabet Med. 2005;22:1343-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Desco MC, Asensi M, Márquez R, Martínez-Valls J, Vento M, Pallardó FV, Sastre J, Viña J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 88. | Alfarisi O, Mave V, Gaikwad S, Sahasrabudhe T, Ramachandran G, Kumar H, Gupte N, Kulkarni V, Deshmukh S, Atre S, Raskar S, Lokhande R, Barthwal M, Kakrani A, Chon S, Gupta A, Golub JE, Dooley KE. Effect of Diabetes Mellitus on the Pharmacokinetics and Pharmacodynamics of Tuberculosis Treatment. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 89. | Riza AL, Pearson F, Ugarte-Gil C, Alisjahbana B, van de Vijver S, Panduru NM, Hill PC, Ruslami R, Moore D, Aarnoutse R, Critchley JA, van Crevel R. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol. 2014;2:740-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 90. | Song W, Si S. The rare ethambutol-induced optic neuropathy: A case-report and literature review. Medicine (Baltimore). 2017;96:e5889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Saxena R, Singh D, Phuljhele S, Kalaiselvan V, Karna S, Gandhi R, Prakash A, Lodha R, Mohan A, Menon V, Garg R. Ethambutol toxicity: Expert panel consensus for the primary prevention, diagnosis and management of ethambutol-induced optic neuropathy. Indian J Ophthalmol. 2021;69:3734-3739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Park JY, Kim KA, Kang MH, Kim SL, Shin JG. Effect of rifampin on the pharmacokinetics of rosiglitazone in healthy subjects. Clin Pharmacol Ther. 2004;75:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Chen J, Raymond K. Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann Clin Microbiol Antimicrob. 2006;5:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 94. | Sun H, Scott DO. Impact of genetic polymorphisms of cytochrome P450 2 C (CYP2C) enzymes on the drug metabolism and design of antidiabetics. Chem Biol Interact. 2011;194:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Tornio A, Niemi M, Neuvonen PJ, Backman JT. Drug interactions with oral antidiabetic agents: pharmacokinetic mechanisms and clinical implications. Trends Pharmacol Sci. 2012;33:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Cho SK, Yoon JS, Lee MG, Lee DH, Lim LA, Park K, Park MS, Chung JY. Rifampin enhances the glucose-lowering effect of metformin and increases OCT1 mRNA levels in healthy participants. Clin Pharmacol Ther. 2011;89:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 97. | Salpeter SR, Greyber E, Pasternak GA, Salpeter Posthumous EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;CD002967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 98. | Te Brake LHM, Yunivita V, Livia R, Soetedjo N, van Ewijk-Beneken Kolmer E, Koenderink JB, Burger DM, Santoso P, van Crevel R, Alisjahbana B, Aarnoutse RE, Ruslami R; TANDEM Consortium. Rifampicin Alters Metformin Plasma Exposure but Not Blood Glucose Levels in Diabetic Tuberculosis Patients. Clin Pharmacol Ther. 2019;105:730-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Te Brake LH, van den Heuvel JJ, Buaben AO, van Crevel R, Bilos A, Russel FG, Aarnoutse RE, Koenderink JB. Moxifloxacin Is a Potent In Vitro Inhibitor of OCT- and MATE-Mediated Transport of Metformin and Ethambutol. Antimicrob Agents Chemother. 2016;60:7105-7114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Nigam SK. What do drug transporters really do? Nat Rev Drug Discov. 2015;14:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 388] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 101. | Parvez MM, Kaisar N, Shin HJ, Lee YJ, Shin JG. Comprehensive Substrate Characterization of 22 Antituberculosis Drugs for Multiple Solute Carrier (SLC) Uptake Transporters In Vitro. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Althaqafi A, Ali M, Alzahrani Y, Ming LC, Hussain Z. How Safe are Fluoroquinolones for Diabetic Patients? A Systematic Review of Dysglycemic and Neuropathic Effects of Fluoroquinolones. Ther Clin Risk Manag. 2021;17:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 569] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 104. | Leal AS, Hung PY, Chowdhury AS, Liby KT. Retinoid X Receptor agonists as selective modulators of the immune system for the treatment of cancer. Pharmacol Ther. 2023;252:108561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 105. | Džopalić T, Božić-Nedeljković B, Jurišić V. The role of vitamin A and vitamin D in modulation of the immune response with a focus on innate lymphoid cells. Cent Eur J Immunol. 2021;46:264-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 106. | Brtko J, Dvorak Z. Natural and synthetic retinoid X receptor ligands and their role in selected nuclear receptor action. Biochimie. 2020;179:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 107. | Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 699] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 108. | Ma X, Idle JR, Gonzalez FJ. The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol. 2008;4:895-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 109. | Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2414] [Cited by in RCA: 2374] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 110. | Hashimoto Y, Miyachi H. Nuclear receptor antagonists designed based on the helix-folding inhibition hypothesis. Bioorg Med Chem. 2005;13:5080-5093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | Kamiya A, Inoue Y, Gonzalez FJ. Role of the hepatocyte nuclear factor 4alpha in control of the pregnane X receptor during fetal liver development. Hepatology. 2003;37:1375-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 112. | Timsit YE, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007;72:231-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 113. | Rakateli L, Huchzermeier R, van der Vorst EPC. AhR, PXR and CAR: From Xenobiotic Receptors to Metabolic Sensors. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Burkina V, Rasmussen MK, Pilipenko N, Zamaratskaia G. Comparison of xenobiotic-metabolising human, porcine, rodent, and piscine cytochrome P450. Toxicology. 2017;375:10-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 115. | Buchman CD, Chai SC, Chen T. A current structural perspective on PXR and CAR in drug metabolism. Expert Opin Drug Metab Toxicol. 2018;14:635-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 116. | Wang F, Wang H, Wu Y, Wang L, Zhang L, Ye X, Peng D, Chen W. Activation of Pregnane X Receptor-Cytochrome P450s Axis: A Possible Reason for the Enhanced Accelerated Blood Clearance Phenomenon of PEGylated Liposomes In Vivo. Drug Metab Dispos. 2019;47:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 117. | Staudinger JL, Ding X, Lichti K. Pregnane X receptor and natural products: beyond drug-drug interactions. Expert Opin Drug Metab Toxicol. 2006;2:847-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 118. | Sugatani J, Uchida T, Kurosawa M, Yamaguchi M, Yamazaki Y, Ikari A, Miwa M. Regulation of pregnane X receptor (PXR) function and UGT1A1 gene expression by posttranslational modification of PXR protein. Drug Metab Dispos. 2012;40:2031-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 119. | Mackowiak B, Wang H. Mechanisms of xenobiotic receptor activation: Direct vs. indirect. Biochim Biophys Acta. 2016;1859:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 120. | Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581-14587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 621] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 121. | Austin G, Holcroft A, Rinne N, Wang L, Clark RE. Evidence that the pregnane X and retinoid receptors PXR, RAR and RXR may regulate transcription of the transporter hOCT1 in chronic myeloid leukaemia cells. Eur J Haematol. 2015;94:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 122. | Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling - mechanisms and research needs. Nat Rev Endocrinol. 2019;15:701-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 123. | Dostalek M, Akhlaghi F, Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs. Clin Pharmacokinet. 2012;51:481-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 124. | Restrepo BI. Diabetes and Tuberculosis. Microbiol Spectr. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 125. | Gualano G, Capone S, Matteelli A, Palmieri F. New Antituberculosis Drugs: From Clinical Trial to Programmatic Use. Infect Dis Rep. 2016;8:6569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 126. | Dheda K, Cox H, Esmail A, Wasserman S, Chang KC, Lange C. Recent controversies about MDR and XDR-TB: Global implementation of the WHO shorter MDR-TB regimen and bedaquiline for all with MDR-TB? Respirology. 2018;23:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 127. | Riccardi N, Canetti D, Rodari P, Besozzi G, Saderi L, Dettori M, Codecasa LR, Sotgiu G. Tuberculosis and pharmacological interactions: A narrative review. Curr Res Pharmacol Drug Discov. 2021;2:100007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 128. | Mtabho CM, Semvua HH, van den Boogaard J, Irongo CF, Boeree MJ, Colbers A, Burger DM, van Crevel R, van der Ven AJAM, Kibiki GS, Tostmann A, Aarnoutse RE. Effect of diabetes mellitus on TB drug concentrations in Tanzanian patients. J Antimicrob Chemother. 2019;74:3537-3545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 129. | Dekkers BGJ, Akkerman OW, Alffenaar JWC. Role of Therapeutic Drug Monitoring in Treatment Optimization in Tuberculosis and Diabetes Mellitus Comorbidity. Antimicrob Agents Chemother. 2019;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 130. | Pizzol D, Di Gennaro F, Chhaganlal KD, Fabrizio C, Monno L, Putoto G, Saracino A. Tuberculosis and diabetes: current state and future perspectives. Trop Med Int Health. 2016;21:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 131. | Washington R, Potty RS, Rajesham A, Seenappa T, Singarajipura A, Swamickan R, Shah A, Prakash KH, Kar A, Kumaraswamy K, Prarthana BS, Maryala BK, Sushma J, Dasari R, Shetty B, Panibatla V, Mohan HL, Becker M. Is a differentiated care model needed for patients with TB? A cohort analysis of risk factors contributing to unfavourable outcomes among TB patients in two states in South India. BMC Public Health. 2020;20:1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 132. | Subbaraman R, Nathavitharana RR, Mayer KH, Satyanarayana S, Chadha VK, Arinaminpathy N, Pai M. Constructing care cascades for active tuberculosis: A strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 2019;16:e1002754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 133. | van Crevel R, Koesoemadinata R, Hill PC, Harries AD. Clinical management of combined tuberculosis and diabetes. Int J Tuberc Lung Dis. 2018;22:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 134. | Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther. 2001;69:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 135. | Zheng HX, Huang Y, Frassetto LA, Benet LZ. Elucidating rifampin's inducing and inhibiting effects on glyburide pharmacokinetics and blood glucose in healthy volunteers: unmasking the differential effects of enzyme induction and transporter inhibition for a drug and its primary metabolite. Clin Pharmacol Ther. 2009;85:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 136. | Kim KA, Park JY. Inhibitory effect of glyburide on human cytochrome p450 isoforms in human liver microsomes. Drug Metab Dispos. 2003;31:1090-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Park JY, Kim KA, Park PW, Park CW, Shin JG. Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide. Clin Pharmacol Ther. 2003;74:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 138. | Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2000;68:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 139. | Bidstrup TB, Stilling N, Damkier P, Scharling B, Thomsen MS, Brøsen K. Rifampicin seems to act as both an inducer and an inhibitor of the metabolism of repaglinide. Eur J Clin Pharmacol. 2004;60:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Hatorp V, Hansen KT, Thomsen MS. Influence of drugs interacting with CYP3A4 on the pharmacokinetics, pharmacodynamics, and safety of the prandial glucose regulator repaglinide. J Clin Pharmacol. 2003;43:649-660. [PubMed] |
| 141. | Ruzilawati AB, Gan SH. CYP3A4 genetic polymorphism influences repaglinide's pharmacokinetics. Pharmacology. 2010;85:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 142. | Lewis JM, Sloan DJ. The role of delamanid in the treatment of drug-resistant tuberculosis. Ther Clin Risk Manag. 2015;11:779-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 143. | Jaakkola T, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ. Effect of rifampicin on the pharmacokinetics of pioglitazone. Br J Clin Pharmacol. 2006;61:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 144. | Slim R, Ben Salem C, Zamy M, Biour M. Pioglitazone-induced acute rhabdomyolysis. Diabetes Care. 2009;32:e84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 145. | Ganguly RJ, Abraham RR, Prasad R, Rao BC. Hyperglycaemia induced by isoniazid preventive therapy. J Family Med Prim Care. 2018;7:1123-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |