Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.712
Peer-review started: December 30, 2023
First decision: January 17, 2024
Revised: January 29, 2024
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: April 15, 2024
Processing time: 103 Days and 10.5 Hours
Dyslipidemia is frequently present in patients with diabetes. The associations of remnant cholesterol and mortality remains unclear in patients with diabetes.
To explore the associations of remnant cholesterol with all-cause and cardiovas
This prospective cohort study included 4740 patients with diabetes who par
During a median follow-up duration of 83 months, 1370 all-cause deaths and 389 cardiovascular deaths were documented. Patients with remnant cholesterol levels in the third quartile had a reduced risk of all-cause mortality [hazard ratio (HR) 95% confidence interval (CI): 0.66 (0.52-0.85)]; however, when remnant cholesterol was modeled as a continuous variable, it was associated with increased risks of all-cause [HR (95%CI): 1.12 (1.02-1.21) per SD] and cardiovascular [HR (95%CI): 1.16 (1.01-1.32), per SD] mortality. The RCS demonstrated nonlinear associations of remnant cholesterol with all-cause and cardiovascular mortality. Subgroup and sensitivity analyses did not reveal significant differences from the above results.
In patients with diabetes, higher remnant cholesterol was associated with increased risks of all-cause and cardiovascular mortality, and diabetes patients with slightly higher remnant cholesterol (0.68-1.04 mmol/L) had a lower risk of all-cause mortality.
Core Tip: This cohort study of 4740 patients with diabetes from the National Health and Nutrition Examination Survey was aimed at evaluating the associations of remnant cholesterol with all-cause and cardiovascular mortality. Diabetes patients with remnant cholesterol levels in the third quartile (0.68-1.04 mmol/L) had a lower risk of all-cause mortality than did nondiabetic patients with remnant cholesterol levels in the other quartiles, and the associations of remnant cholesterol with all-cause and cardiovascular mortality were U-shaped. A per standard deviation increase in remnant cholesterol was associated with a greater risk of all-cause and cardiovascular mortality. A focus should be placed on the level of remnant cholesterol in patients with diabetes.
- Citation: Pan D, Xu L, Zhang LX, Shi DZ, Guo M. Associations between remnant cholesterol levels and mortality in patients with diabetes. World J Diabetes 2024; 15(4): 712-723
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/712.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.712
The prevalence of diabetes is estimated to be 10.9% by 2030[1]. The prevalence of cardiovascular disease is two to four times greater in patients with diabetes than in patients without diabetes[2]. Dyslipidemia is also frequently present in patients with diabetes as a result of changes in lipoprotein levels triggered by insulin dysfunction and hyperglycemia[3]. Among lipid profile, low-density lipoprotein cholesterol (LDL-C) is highly focused in patients with diabetes[4]. Therefore, statins are widely used in clinical practice for patients with diabetes, irrespective of the presence of complications[5-7]. However, the incidence of major adverse cardiovascular events remains high with the use of current LDL-C lowering strategies[4]. Therefore, there is an evidence gap between existing drug therapies and the prevention of adverse events in patients with diabetes, necessitating a focus on lipoproteins other than LDL-C.
Remnant cholesterol, the remaining cholesterol that is not LDL-C or high-density lipoprotein cholesterol (HDL-C), has been found to be associated with a higher risk of peripheral artery disease, ischemic stroke, etc., and the relationship persisted when controlling for other risk factors, including hypertension and high LDL-C[8-10]. Remnant cholesterol and triglycerides are both carried in lipoproteins that are enriched in triglycerides. Moreover, triglyceride-rich lipoproteins can accumulate in the arterial intima, which may further accelerate the progression of atherosclerosis and increase the risk of cardiovascular events[11]. Thus, in clinical practice, remnant cholesterol should be considered a potential predictor of atherosclerosis and cardiovascular events for individuals with diabetes, and it is essential to assess the impact of remnant cholesterol on mortality among those patients.
Therefore, in this study, we aimed to explore the associations of remnant cholesterol with all-cause and cardiovascular mortality in patients with diabetes using data from the population-based National Health and Nutrition Examination Survey (NHANES).
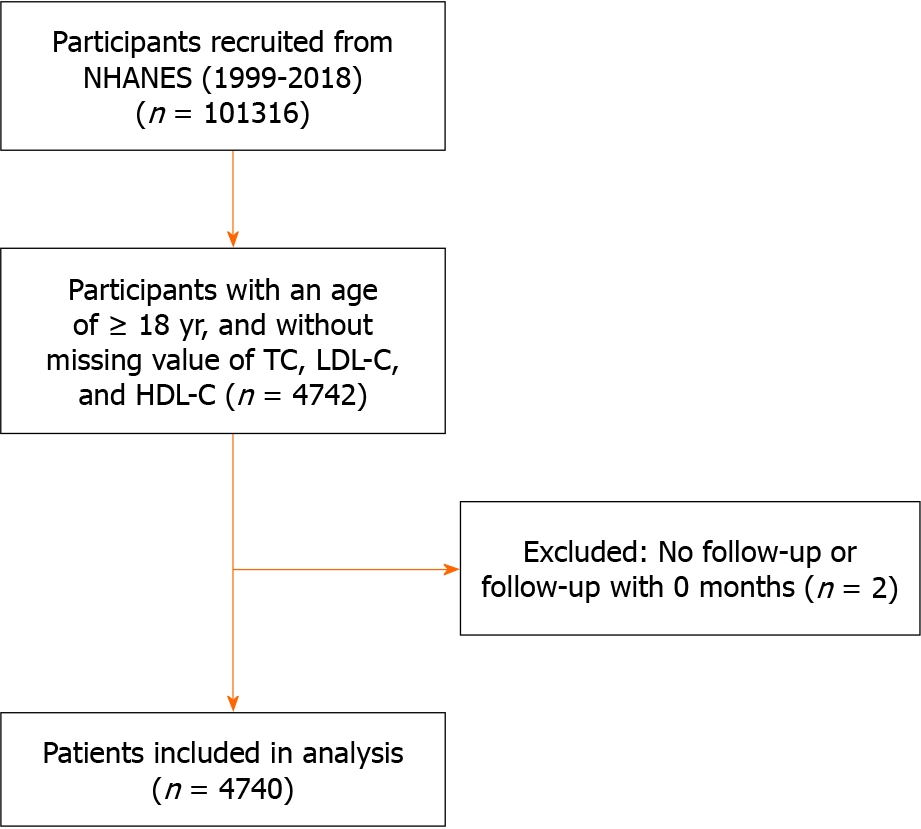
This study included individuals who participated in the NHANES between 1999 and 2018. We included all adults aged ≥ 18 years with diabetes and complete data on total cholesterol (TC), LDL-C, and HDL-C in mmol/L, resulting in a cohort of 4742 patients. We also excluded patients without a follow-up time (or 0 months) (n = 2). Therefore, 4740 patients with diabetes were ultimately included in this study (Figure 1). All procedures were performed in accordance with the Declaration of Helsinki. The NHANES study was reviewed and approved by the NCHS Research Ethics Review Board. Written informed consent was obtained from all participants.
Diabetes status was defined on the basis of the following criteria: Diagnosed with diabetes by a physician, using insulin or oral diabetes medications, hemoglobin A1c ≥ 6.5%, plasma fasting glucose ≥ 126 mg/dL (≥ 7.0 mmol/L) (after at least 8 h of fasting) or 2-h blood glucose ≥ 200 mg/dL (≥ 11.1 mmol/L) during an oral glucose tolerance test[12].
Blood specimens were collected as part of the NHANES, and lipid profile data, including TC, LDL-C, HDL-C and triglyceride levels, were retrieved from the NHANES website. Using that data, remnant cholesterol was calculated by the following equation: remnant cholesterol (mmol/L) = TC - HDL-C - LDL-C[13].
We obtained death data by linking the cohort data with the National Death Index (NDI) through December 31st, 2019. All-cause mortality was defined as death for any reason. Cardiovascular mortality was defined by using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes I00 to I78.
Demographic and lifestyle information was collected via standard questionnaires during in-person interviews. The demographic covariates included age (continuous), sex (male and female), ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic white, and others), poverty-income ratio (< 1, 1-3, ≥ 3 or unknown), education (less than 9th grade, 9-11th grade, high school graduate, college, college graduate or above or unknown), body mass index (BMI) (continuous), and survey periods (1999-2000, 2001-2002, 2003-2004, 2005-2006, 2007-2008, 2009-2010, 2011-2012, 2013-2014, 2015-2016 or 2017-2018). Lifestyle covariates included self-reported smoking status (every day, some days, not at all, or unknown) and self-reported alcohol consumption (nondrinker, 1-3 drinks per day, ≥ 4 drinks per day or unknown). Clinical covariates included self-reported history of hypertension (yes, no or unknown), hypercholesterolemia (yes, no or unknown), heart failure (yes, no or unknown), coronary heart disease (yes, no or unknown), and cancer (yes, no or unknown).
In the present study, all analyses incorporated sample weights, clustering and stratification given the complex sampling design of the NHANES. For normally distributed continuous variables, the data are presented as the mean and SD; for nonnormally distributed continuous variables, the data are presented as the median and interquartile range. Categorical variables are presented as percentages. Differences in age were analyzed using student’s t test. Differences in nonnormally distributed continuous variables were analyzed using the Mann-Whitney U test. Differences among categorical variables were analyzed using Pearson’s χ2 test. Hazard ratios (HRs) and 95% confidence intervals (CIs) of remnant cholesterol for all-cause mortality and cardiovascular mortality were calculated by Cox hazards models, adjusting for age, sex, ethnicity, BMI, poverty-income ratio, education, smoking status, alcohol consumption, survey period, hypercholesterolemia, hypertension, heart failure, coronary heart disease, and cancer. The group with the lowest quartile of remnant cholesterol was set as the reference group, for which the HR was 1. Additionally, the HR and 95%CI were also calculated for each increase in the SD of remnant cholesterol (treated as a continuous variable). For continuous variables, missing values were imputed by the median value, while for categorical variables, missing data were coded as a separate category “missing”.
Stratified analyses were performed by sex (male, female), age (≥ 60 years, < 60 years), BMI (≥ 30 kg/m2, < 30 kg/m2), duration of diabetes (≥ 10 years, < 10 years), and ethnicity (Mexican American, non-Hispanic white, non-Hispanic black and others). Furthermore, we conducted the following sensitivity analyses: (1) Excluded patients who died within 1 year of follow-up; (2) further adjusted for serum triglyceride levels; (3) adjusted for lipid-lowering and antihypertensive drugs; and (4) adjusted for cardiovascular mortality. We performed analyses accounting for all-cause death as a competing event with the Fine-Gray competing risks model.
We constructed a restricted cubic spline (RCS) model (knots were selected according to the Akaike information criterion; Supplementary Table 1 to examine the associations of remnant cholesterol with all-cause mortality and cardiovascular mortality (25th percentile as the reference category), adjusting for age, sex, ethnicity, BMI, poverty-income ratio, education, smoking status, alcohol consumption, survey period, hypercholesterolemia, hypertension, heart failure, coronary heart disease, and cancer. A two-sided P < 0.05 was set as the threshold for statistical significance. All analyses were performed with Stata 17.0 (StataCorp LLC, College Station, Texas).
A total of 4740 patients with diabetes were included in our analysis. During a median follow-up of 83 months, 1370 all-cause deaths and 389 cardiovascular deaths were observed. Table 1 shows the baseline characteristics stratified by sex. Among them, 2447 patients were male (51.6%). Male patients had a lower BMI and a lower incidence of hypertension. In addition, male patients had a higher fasting serum glucose level. Regarding the lipid profile, we observed higher levels of TC, HDL-C, and LDL-C in female patients with diabetes. However, no significant differences were found in the remnant cholesterol or triglyceride levels between female and male patients.
| Male | Female | All participants | P value | |
| Sample size | 2447 | 2293 | 4740 | |
| Age [mean (SD)] | 61.8 (13.7) | 61.3 (14.3) | 61.6 (14.0) | 0.09 |
| Body mass index, median (IQR) | 29.7 (26.2-33.7) | 31.4 (27.4-37.0) | 30.5 (26.7-35.4) | < 0.01 |
| Ethnicity, n (%) | ||||
| Mexican American | 466 (19.0) | 471 (20.5) | 937 (19.8) | < 0.01 |
| Other Hispanic | 228 (9.3) | 230 (10.0) | 458 (9.7) | |
| Non-Hispanic white | 986 (40.3) | 790 (34.5) | 1776 (37.5) | |
| Non-Hispanic black | 539 (22.0) | 611 (26.7) | 1150 (24.3) | |
| Others | 228 (9.3) | 191 (8.3) | 419 (8.8) | |
| Education, n (%) | ||||
| Less than 9th grade | 547 (22.4) | 559 (24.4) | 1106 (23.3) | < 0.01 |
| 9-11th grade | 422 (17.3) | 426 (18.6) | 848 (17.9) | |
| High school graduate | 631 (25.8) | 573 (25.0) | 1204 (25.4) | |
| College | 471 (19.3) | 491 (21.4) | 962 (20.3) | |
| College graduate or above | 365 (14.9) | 232 (10.1) | 597 (12.6) | |
| Unknown | 11 (0.45) | 12 (0.52) | 23 (0.48) | |
| Poverty-income ratio, n (%) | ||||
| < 1 | 439 (17.9) | 536 (23.4) | 975 (20.6) | < 0.01 |
| 1-3 | 1265 (51.7) | 1242 (54.2) | 2507 (52.9) | |
| > 3 | 743 (30.7) | 515 (22.5) | 1258 (26.6) | |
| Smoke, n (%) | ||||
| Every day | 299 (12.2) | 196 (8.6) | 495 (10.4) | < 0.01 |
| Some days | 46 (1.9) | 43 (1.9) | 89 (1.9) | |
| Not at all | 76 (31.7) | 414 (18.1) | 1190 (25.1) | |
| Unknown | 1326 (54.2) | 1640 (71.3) | 2966 (62.6) | |
| Alcohol consumption, n (%) | ||||
| Non-drinker | 358 (14.7) | 437 (19.7) | 795 (16.8) | < 0.01 |
| 1-3 drinks per day | 969 (39.6) | 745 (32.5) | 1714 (36.2) | |
| ≥ 4 drinks per day | 378 (15.5) | 12 (5.3) | 499 (10.1) | |
| Unknown | 742 (30.3) | 990 (43.2) | 1732 (36.6) | |
| Hypertension, n (%) | 1490 (60.9) | 1519 (66.3) | 3009 (63.5) | < 0.01 |
| Hypercholesterolemia, n (%) | 1324 (54.1) | 1300 (56.7) | 2624 (55.4) | 0.3 |
| Plasma fasting glucose (mmol/L), median (IQR) | 9.8 ± 3.5 | 8.5 ± 3.5 | 8.6 ± 3.5 | < 0.01 |
| HbA1c, %, median (IQR) | 7.2 ± 1.8 | 7.1 ± 1.7 | 7.2 ± 1.8 | 0.21 |
| Remnant cholesterol, median (IQR) | 0.68 (0.45-1.07) | 0.68 (0.46-1.03) | 0.68 (0.46-1.04) | 0.53 |
| TC, median (IQR) | 4.53 (3.80-5.38) | 4.86 (4.11-5.69) | 4.68 (3.93-5.53) | < 0.01 |
| HDL-C, median (IQR) | 1.09 (0.93-1.32) | 1.29 (1.06-1.58) | 1.19 (0.98-1.45) | < 0.01 |
| LDL-C, median (IQR) | 2.43 (1.71-3.18) | 2.59 (1.81-3.34) | 2.51 (1.76-3.23) | < 0.01 |
| Triglyceride, median (IQR) | 1.48 (1.02-2.21) | 1.58 (1.05-2.11) | 1.48 (1.04-2.16) | 0.97 |
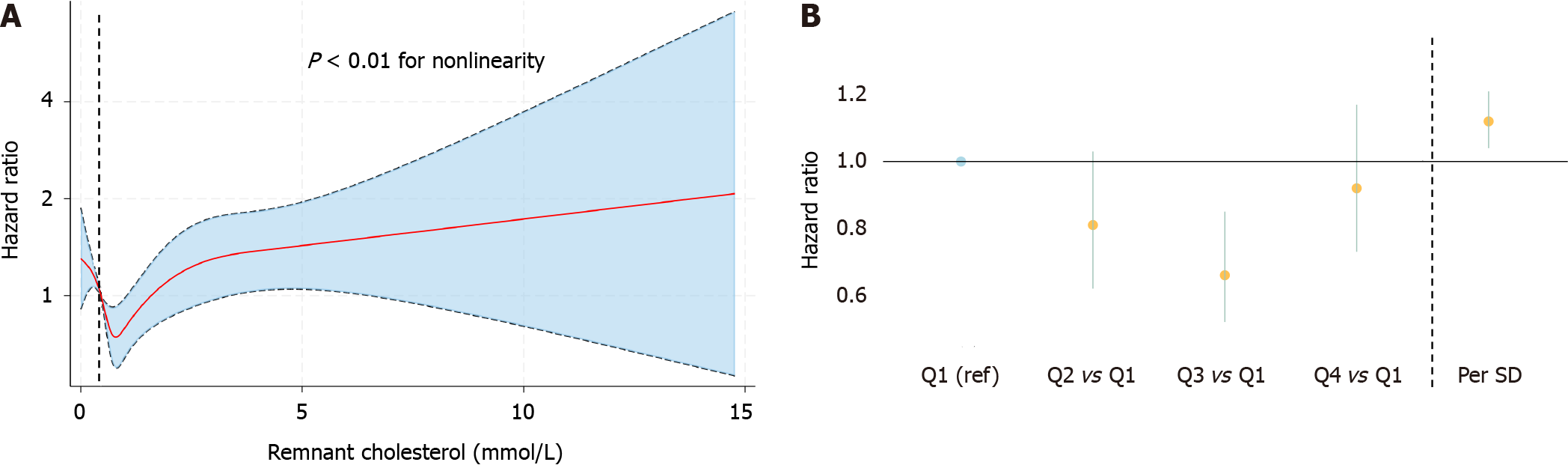
We observed a nonlinear association between remnant cholesterol levels and all-cause mortality according to the RCS (Figure 2A). When we treated remnant cholesterol as a categorical variable, we found a lower risk in patients in the third quartile of remnant cholesterol than in those in the lowest quartile [Q3: HR (95%CI): 0.66 (0.52-0.85), 0.68-1.04 mmol/L]. Moreover, we found a greater risk of all-cause mortality when remnant cholesterol was modeled as a continuous variable per SD increase [HR (95%CI): 1.12 (1.02-1.21)] (Table 2, Figure 2B).
| Hazard ratio (95%CI) | ||||||
| Q1 | Q2 | Q3 | Q4 | Per SD | P value | |
| All-cause mortality | ||||||
| Model 1 | Reference | 0.88 (0.68-1.15) | 0.65 (0.50-0.84) | 0.87 (0.68-1.10) | 1.04 (0.97-1.11) | 0.01 |
| Model 2 | Reference | 0.80 (0.62-1.03) | 0.67 (0.52-0.86) | 0.93 (0.73-1.17) | 1.13 (1.04-1.22) | < 0.01 |
| Model 3 | Reference | 0.80 (0.62-1.02) | 0.66 (0.52-0.85) | 0.92 (0.72-1.17) | 1.13 (1.04-1.22) | < 0.01 |
| Model 4 | Reference | 0.81 (0.62-1.03) | 0.66 (0.52-0.85) | 0.92 (0.73-1.17) | 1.12 (1.02-1.21) | < 0.01 |
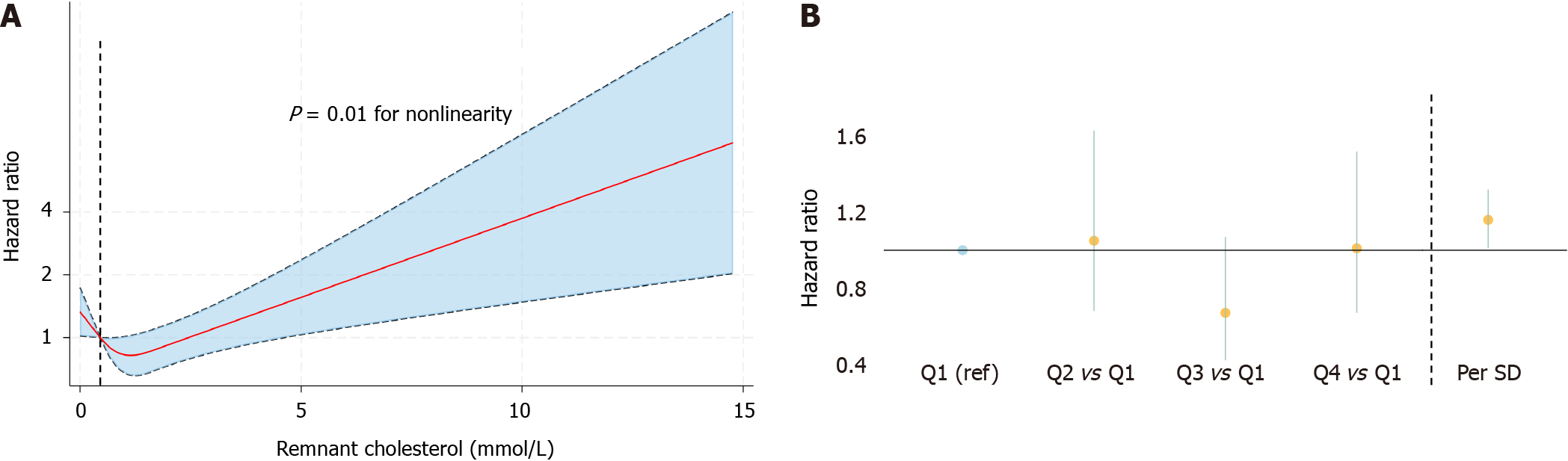
| Cardiovascular mortality | ||||||
| Model 1 | Reference | 1.13 (0.72-1.77) | 0.60 (0.38-0.96) | 0.92 (0.61-1.39) | 1.07 (0.95-1.21) | < 0.01 |
| Model 2 | Reference | 1.01 (0.65-1.55) | 0.63 (0.40-0.99) | 0.99 (0.66-1.50) | 1.19 (1.04-1.37) | < 0.01 |
| Model 3 | Reference | 1.02 (0.66-1.57) | 0.64 (0.41-1.01) | 0.98 (0.65-1.48) | 1.18 (1.03-1.35) | < 0.01 |
| Model 4 | Reference | 1.05 (0.68-1.63) | 0.67 (0.42-1.07) | 1.01 (0.67-1.52) | 1.16 (1.01-1.32) | < 0.01 |
Similarly, the RCS also showed a nonlinear association between remnant cholesterol and cardiovascular mortality (Figure 3A). When remnant cholesterol was modeled as a categorical variable, we also observed a U-shaped association, whereas we did not observe a significantly greater or lower risk in the other three quartiles than in the lowest quartile, despite a favorable trend toward a lower risk in the third quartile [Q3: HR (95%CI): 0.67 (0.42-1.07)]. In addition, we also found a greater risk per SD increase of remnant cholesterol when it was treated as a continuous variable [HR (95%CI): 1.16 (1.01-1.32)] (Table 2, Figure 3B).
We found no interaction between any of the strata and the level of remnant cholesterol (all Pinteraction > 0.05) regarding the association with all-cause mortality. The corresponding trends of the different strata were also similar. However, the lower risk associated with the third quartile of remnant cholesterol was not observed in patients with diabetes with a BMI > 30 kg/m2 and a duration of diabetes > 10 years or in Mexican Americans [BMI > 30 kg/m2, HR (95%CI): 0.77 (0.52-1.12); duration of diabetes over 10 years, HR (95%CI): 0.69 (0.47-1.02); Mexican American ethnicity, HR (95%CI): 0.55 (0.28-1.05)]. Moreover, in non-Hispanic black individuals and individuals of other ethnicities, we observed that there was a trend toward an increase in all-cause mortality risk with elevated remnant cholesterol levels, although no significant increase in risk was associated with any of the individual quartiles of remnant cholesterol.
In addition, there were no significant interactions between any of the strata and remnant cholesterol (all Pinteraction > 0.05), indicating no evidence of a differential effect of remnant cholesterol on cardiovascular mortality across the different strata. However, among patients in the third quartile of remnant cholesterol, female patients, patients with a BMI < 30 kg/m2, patients with a duration of diabetes less than 10 years and patients of other ethnicities had a lower risk of cardiovascular mortality [female, HR (95%CI): 0.39 (0.19-0.81); BMI > 30, HR (95%CI): 0.30 (0.15-0.60); duration of diabetes less than 10 years, HR (95%CI): 0.53 (0.29-0.97); other ethnicities, HR (95%CI): 0.12 (0.03-0.45)] (Table 3).
| Hazard ratio (95%CI) | |||||
| Q1 | Q2 | Q3 | Q4 | P for interaction | |
| All-cause mortality | |||||
| Gender | |||||
| Male | Reference | 0.84 (0.61-1.16) | 0.67 (0.48-0.94) | 0.96 (0.71-1.32) | 0.36 |
| Female | Reference | 0.83 (0.57-1.19) | 0.64 (0.44-0.93) | 0.85 (0.59-1.23) | |
| Age | |||||
| > 60 | Reference | 0.93 (0.65-1.07) | 0.72 (0.55-0.95) | 0.89 (0.68-1.17) | 0.45 |
| < 60 | Reference | 0.76 (0.39-1.51) | 0.44 (0.24-0.81) | 0.91 (0.55-1.51) | |
| BMI | |||||
| > 30 | Reference | 1.13 (0.78-1.63) | 0.77 (0.52-1.12) | 1.09 (0.75-1.57) | 0.57 |
| < 30 | Reference | 0.70 (0.51-0.96) | 0.66 (0.47-0.93) | 0.89 (0.64-1.22) | |
| Duration of diabetes | |||||
| > 10 yr | Reference | 0.97 (0.68-1.38) | 0.69 (0.47-1.02) | 0.78 (0.54-1.12) | 0.53 |
| < 10 yr | Reference | 0.80 (0.57-1.11) | 0.68 (0.49-0.94) | 1.02 (0.73-1.41) | |
| Ethnicities | |||||
| Mexican American | Reference | 0.59 (0.29-1.20) | 0.55 (0.28-1.05) | 0.73 (0.39-1.37) | 0.85 |
| Non-Hispanic white | Reference | 0.85 (0.61-1.19) | 0.62 (0.45-0.86) | 0.86 (0.63-1.18) | |
| Non-Hispanic black | Reference | 0.91 (0.62-1.33) | 0.97 (0.60-1.59) | 1.02 (0.62-1.70) | |
| Others | Reference | 0.54 (0.27-1.07) | 0.57 (0.28-1.15) | 1.37 (0.71-2.63) | |
| Cardiovascular mortality | |||||
| Gender | |||||
| Male | Reference | 1.40 (0.75-2.61) | 1.06 (0.58-1.95) | 1.31 (0.76-2.26) | 0.17 |
| Female | Reference | 0.75 (0.41-1.34) | 0.39 (0.19-0.81) | 0.76 (0.42-1.37) | |
| Age | |||||
| > 60 | Reference | 1.09 (0.70-1.71) | 0.60 (0.36-1.01) | 0.91 (0.58-1.44) | 0.3 |
| < 60 | Reference | 0.78 (0.22-2.81) | 1.11 (0.35-3.49) | 1.43 (0.46-4.49) | |
| BMI | |||||
| > 30 | Reference | 1.20 (0.65-2.19) | 0.96 (0.50-1.85) | 0.87 (0.48-1.58) | 0.16 |
| < 30 | Reference | 1.1 (0.60-2.07) | 0.30 (0.15-0.60) | 1.55 (0.90-2.66) | |
| Duration of diabetes | |||||
| > 10 yr | Reference | 1.03 (0.54-2.00) | 0.88 (0.44-1.77) | 0.93 (0.47-1.80) | 0.4 |
| < 10 yr | Reference | 1.14 (0.62-2.03) | 0.53 (0.29-0.97) | 1.07 (0.62-1.86) | |
| Ethnicities | |||||
| Mexican American | Reference | 5.3 (1.44-19.01) | 0.72 (0.18-2.91) | 4.21 (1.20-14.7) | 0.16 |
| Non-Hispanic white | Reference | 1.15 (0.65-2.04) | 0.70 (0.39-1.26) | 0.96 (0.56-1.67) | |
| Non-Hispanic black | Reference | 1.28 (0.69-2.38) | 1.17 (0.49-2.81) | 1.46 (0.73-2.91) | |
| Others | Reference | 0.35 (0.12-1.06) | 0.12 (0.03-0.45) | 0.57 (0.18-1.84) | |
The results did not change substantially after the following adjustments were implemented: excluding patients who died within 1 year of follow-up; further adjusting for serum triglycerides; adjusting for lipid-lowering and antihypertensive drugs; and treating all-cause mortality as a competing event for cardiovascular mortality. Our results showed that the associations of remnant cholesterol levels with all-cause and cardiovascular mortality were generally robust (Tables 4-7).
| Hazard ratio (95%CI) | ||||||
| Q1 | Q2 | Q3 | Q4 | Per SD | P value | |
| All-cause mortality | ||||||
| Model 1 | Reference | 0.95 (0.72-1.24) | 0.70 (0.54-0.92) | 0.95 (0.74-1.21) | 1.04 (0.97-1.12) | 0.04 |
| Model 2 | Reference | 0.89 (0.69-1.14) | 0.72 (0.56-0.93) | 0.98 (0.76-1.25) | 1.11 (1.02-1.20) | < 0.01 |
| Model 3 | Reference | 0.88 (0.69-1.13) | 0.72 (0.56-0.92) | 0.99 (0.77-1.26) | 1.11 (1.02-1.21) | < 0.01 |
| Model 4 | Reference | 0.88 (0.69-1.12) | 0.70 (0.54-0.91) | 0.97 (0.76-1.25) | 1.10 (1.01-1.20) | < 0.01 |
| Cardiovascular mortality | ||||||
| Model 1 | Reference | 1.30 (0.81-2.09) | 0.65 (0.39-1.07) | 0.96 (0.62-1.49) | 1.04 (0.91-1.19) | 0.05 |
| Model 2 | Reference | 1.19 (0.76-1.87) | 0.66 (0.41-1.07) | 0.98 (0.64-1.51) | 1.12 (0.97-1.29) | < 0.01 |
| Model 3 | Reference | 1.20 (0.77-1.88) | 0.67 (0.42-1.08) | 0.98 (0.64-1.50) | 1.11 (0.97-1.28) | < 0.01 |
| Model 4 | Reference | 1.21 (0.77-1.90) | 0.69 (0.43-1.13) | 0.98 (0.64-1.52) | 1.10 (0.96-1.26) | < 0.01 |
| Hazard ratio (95%CI) | ||||||
| Q1 | Q2 | Q3 | Q4 | Per SD | P value | |
| All-cause mortality | ||||||
| Model 4 | Reference | 1.30 (0.81-2.09) | 0.65 (0.39-1.07) | 0.96 (0.62-1.49) | 1.04 (0.91-1.19) | < 0.01 |
| Hazard ratio (95%CI) | ||||||
| Q1 | Q2 | Q3 | Q4 | Per SD | P value | |
| All-cause mortality | ||||||
| Model 6 | Reference | 1.08 (0.70-1.65) | 0.65 (0.41-1.03) | 0.98 (0.66-1.47) | 1.37 (1.13-1.65) | 0.05 |
| Hazard ratio (95%CI) | ||||||
| Q1 | Q2 | Q3 | Q4 | Per SD | P value | |
| Cardiovascular mortality | ||||||
| Model 4 | Reference | 1.14 (0.74-1.77) | 0.73 (0.46-1.16) | 1.03 (0.69-1.55) | 1.12 (0.99-1.28) | < 0.01 |
To our knowledge, this is the first study on the association between remnant cholesterol and mortality in patients with diabetes. In this study, we analyzed data obtained from a population-based database, NHANES, and explored the association of mortality with the NDI. We found that, in patients with diabetes, patients with a remnant cholesterol level in the third-quartile (0.68-1.04 mmol/L) had a lower risk of all-cause mortality. In addition, a similar trend was observed for cardiovascular mortality, but the association between cardiovascular mortality and a remnant cholesterol level in the third quartile was not statistically significant.
Circulating lipoproteins contain both triglycerides and cholesterol. HDL-C and LDL-C primarily transport cholesterol, while other lipoproteins, such as intermediate-density lipoproteins, chylomicrons, and very low-density lipoproteins (VLDLs), not only transport cholesterol but are also enriched with triglycerides[14]. These lipoproteins vary in size, and particles such as VLDLs and chylomicrons may not be able to enter the arterial wall. However, their remnants are able to penetrate into the arterial wall and become trapped. Subsequently, these proteins interact with apoE and apoC-III before being taken up by macrophages[15]. Finally, the accumulation of these remnants accelerates the progression of atherosclerosis[16,17]. A Danish study recruited patients with ischemic heart disease and revealed that those with elevated remnant cholesterol (> 1 mmol/L) had a greater risk of all-cause mortality[18]. In addition to its significant role in exacerbating the progression of atherosclerosis, remnant cholesterol is related to impaired vasodilation and an aggravated inflammatory response[19,20]. Genetic studies have also reported that elevated remnant cholesterol is a causal risk factor for coronary artery disease[21,22]. Another study demonstrated that remnant cholesterol is associated with hepatic steatosis[23]. Moreover, a different study based on NHANES data indicated that patients with hepatic steatosis had an increased systemic immune-inflammation index, which is also associated with an increased risk of all-cause mortality[24,25]. Additionally, the systemic immune-inflammation index was shown to be associated with a higher risk of abdominal aortic calcification, which is also a strong predictor of cardiovascular mortality[26]. In line with the findings of previous studies, our findings similarly demonstrated a greater risk of both all-cause and cardiovascular mortality when analyzing their association with remnant cholesterol levels as a continuous variable.
In patients with diabetes, remnant cholesterol has recently received much attention because of its association with clinical events. For example, in a Chinese cohort study that included 516 individuals diagnosed with type 2 diabetes mellitus, the results indicated that patients with peripheral artery disease exhibited elevated levels of remnant cholesterol, and patients with higher remnant cholesterol (> 0.64 mmol/L) had an increased risk of developing peripheral artery disease[27]. Another study including 4569 white Danish patients with diabetes reported that patients with elevated remnant cholesterol had increased risks of peripheral artery disease, myocardial infarction, ischemic stroke and any atherosclerotic cardiovascular disease[28]. However, despite the established understanding that a higher level of remnant cholesterol increases the risk of clinical events, which was supported in the present study, the exact level of remnant cholesterol associated with mortality in patients with diabetes is still uncertain due to limited study data. A Chinese study indicated that patients with diabetes and diabetic nephropathy who had elevated remnant cholesterol (over 30 mg/dL, 0.77 mmol/L) had a greater risk of cardiovascular mortality than did those with lower remnant cholesterol levels[29]. Cao et al[30] reported that individuals with coronary artery disease and diabetes or prediabetes who had elevated remnant cholesterol (> 0.54 mmol/L) had a greater risk of major adverse cardiovascular events. Nonetheless, these studies primarily focused on Asian individuals and were limited by relatively shorter follow-up periods. Studies have demonstrated diverse metabolic statuses in different ethnicities[31,32]. The present study included patients from the NHANES, a nationwide population-based database with diverse ethnicities. Furthermore, this study had a longer follow-up duration, allowing us to draw more reliable conclusions.
Our research included a highly representative population and used a long follow-up period. Furthermore, weights were used in our statistical analysis, leading to credible conclusions. However, several limitations should also be noted. First, mortality was ascertained via the NDI, potentially leading to misclassification. However, a prior validation study confirmed the accuracy of the matching method[33]. Second, the estimated remnant cholesterol aligned closely with the measured remnant cholesterol levels at lower LDL-C levels, but a noticeable difference was evident at higher LDL-C levels. However, the measured remnant cholesterol level is not currently provided by the NHANES, which may warrant further investigation. Third, the incidence of cardiovascular mortality was relatively low, which might result in a broad CI and hinder us from examining the true association between remnant cholesterol and cardiovascular mortality. Fourth, dietary inflammation plays an important role in hepatic steatosis and lipid metabolism, especially in patients with diabetes[34,35]. However, the provision of dietary data was not consistent throughout the entire study period, which limited our ability to arrive at a more comprehensive conclusion.
In patients with diabetes, higher remnant cholesterol increased the risk of all-cause and cardiovascular mortality, and diabetes patients with slightly higher remnant cholesterol (0.68-1.04 mmol/L) had a lower risk of all-cause mortality.
Additional research is needed to explore the underlying mechanism of the relationship between remnant cholesterol and mortality.
The optimal remnant cholesterol level for decreasing the risk of all-cause mortality in patients with diabetes was 0.68-1.04 mmol/L. A high level of remnant cholesterol was associated with an increased risk of all-cause and cardiovascular mortality.
The associations of remnant cholesterol with all-cause and cardiovascular mortality were U-shaped. Patients with diabetes in the third quartile of remnant cholesterol (0.68-1.04 mmol/L) had a lower risk of all-cause mortality, and a per standard deviation increase in remnant cholesterol was associated with a higher risk of all-cause and cardiovascular mortality.
This cohort study included 4740 patients with diabetes who participated in the National Health and Nutrition Examination Survey from 1999 through 2018. We divided remnant cholesterol into four quartiles, and all participants were followed from the interview date until death or December 31, 2019. Multivariate proportional Cox regression models were used to calculate hazard ratios and 95% confidence intervals. Additionally, a series of subgroup and sensitivity analyses were performed.
The aim of the present study was to explore the associations of remnant cholesterol with all-cause and cardiovascular mortality in patients with diabetes.
In current clinical practice, the lipid profile is different between patients with diabetes and nondiabetic patients. However, evidence for the association between remnant cholesterol levels and mortality is lacking.
Remnant cholesterol is associated with mortality, but the role of remnant cholesterol in patients with diabetes is unclear.
We would like to appreciate the support by participants involved in the National Health and Nutrition Examination Survey study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dąbrowski M, Poland; Horowitz M, Australia; Zhang Y, China S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5839] [Article Influence: 973.2] [Reference Citation Analysis (8)] |
| 2. | Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 932] [Article Influence: 155.3] [Reference Citation Analysis (1)] |
| 3. | Goldberg IJ. Clinical review 124: Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 341] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA. 2016;316:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 996] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 5. | Duracková Z, Mendiola MA, Sevilla MT, Valent A. Thiohydrazone copper(II) complexes. The relationship between redox properties and superoxide dismutase mimetic activity. Bioelectrochem Bioenerg. 1999;48:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | O'Malley PG, Arnold MJ, Kelley C, Spacek L, Buelt A, Natarajan S, Donahue MP, Vagichev E, Ballard-Hernandez J, Logan A, Thomas L, Ritter J, Neubauer BE, Downs JR. Management of Dyslipidemia for Cardiovascular Disease Risk Reduction: Synopsis of the 2020 Updated U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann Intern Med. 2020;173:822-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Krane V, Schmidt KR, Gutjahr-Lengsfeld LJ, Mann JF, März W, Swoboda F, Wanner C; 4D Study Investigators (the German Diabetes and Dialysis Study Investigators). Long-term effects following 4 years of randomized treatment with atorvastatin in patients with type 2 diabetes mellitus on hemodialysis. Kidney Int. 2016;89:1380-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Chait A, Ginsberg HN, Vaisar T, Heinecke JW, Goldberg IJ, Bornfeldt KE. Remnants of the Triglyceride-Rich Lipoproteins, Diabetes, and Cardiovascular Disease. Diabetes. 2020;69:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 9. | Huh JH, Han KD, Cho YK, Roh E, Kang JG, Lee SJ, Ihm SH. Remnant cholesterol and the risk of cardiovascular disease in type 2 diabetes: a nationwide longitudinal cohort study. Cardiovasc Diabetol. 2022;21:228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 10. | Varbo A, Nordestgaard BG. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol. 2019;85:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Nordestgaard BG. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ Res. 2016;118:547-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 746] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 12. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 1846] [Article Influence: 461.5] [Reference Citation Analysis (0)] |
| 13. | Burnett JR, Hooper AJ, Hegele RA. Remnant Cholesterol and Atherosclerotic Cardiovascular Disease Risk. J Am Coll Cardiol. 2020;76:2736-2739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Toth PP. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc Health Risk Manag. 2016;12:171-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Borén J, Williams KJ. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol. 2016;27:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 16. | Nordestgaard BG, Zilversmit DB. Large lipoproteins are excluded from the arterial wall in diabetic cholesterol-fed rabbits. J Lipid Res. 1988;29:1491-1500. [PubMed] |
| 17. | Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 230] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased Remnant Cholesterol Explains Part of Residual Risk of All-Cause Mortality in 5414 Patients with Ischemic Heart Disease. Clin Chem. 2016;62:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Zheng XY, Liu L. Remnant-like lipoprotein particles impair endothelial function: direct and indirect effects on nitric oxide synthase. J Lipid Res. 2007;48:1673-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Taskinen MR, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 279] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 22. | Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 23. | Chin J, Mori TA, Adams LA, Beilin LJ, Huang RC, Olynyk JK, Ayonrinde OT. Association between remnant lipoprotein cholesterol levels and non-alcoholic fatty liver disease in adolescents. JHEP Rep. 2020;2:100150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Wang H, Nie H, Bu G, Tong X, Bai X. Systemic immune-inflammation index (SII) and the risk of all-cause, cardiovascular, and cardio-cerebrovascular mortality in the general population. Eur J Med Res. 2023;28:575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 25. | Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, Liu Q, Zhang Y. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front Immunol. 2022;13:925690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 141] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 26. | Xie R, Liu X, Wu H, Liu M, Zhang Y. Associations between systemic immune-inflammation index and abdominal aortic calcification: Results of a nationwide survey. Nutr Metab Cardiovasc Dis. 2023;33:1437-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 27. | Song Y, Zhao Y, Bai X, Cheng W, Wang L, Shu M, Shu Y, Zhang L, Jin S. Remnant cholesterol is independently asssociated with an increased risk of peripheral artery disease in type 2 diabetic patients. Front Endocrinol (Lausanne). 2023;14:1111152. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Wadström BN, Pedersen KM, Wulff AB, Nordestgaard BG. Elevated remnant cholesterol and atherosclerotic cardiovascular disease in diabetes: a population-based prospective cohort study. Diabetologia. 2023;66:2238-2249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 29. | Yu D, Wang Z, Zhang X, Qu B, Cai Y, Ma S, Zhao Z, Simmons D. Remnant Cholesterol and Cardiovascular Mortality in Patients With Type 2 Diabetes and Incident Diabetic Nephropathy. J Clin Endocrinol Metab. 2021;106:3546-3554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Cao YX, Zhang HW, Jin JL, Liu HH, Zhang Y, Gao Y, Guo YL, Wu NQ, Hua Q, Li YF, Li XL, Xu RX, Cui CJ, Liu G, Dong Q, Sun J, Zhu CG, Li JJ. The longitudinal association of remnant cholesterol with cardiovascular outcomes in patients with diabetes and pre-diabetes. Cardiovasc Diabetol. 2020;19:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Xie R, Liu Y, Wang J, Zhang C, Xiao M, Liu M, Zhang Y. Race and Gender Differences in the Associations Between Cadmium Exposure and Bone Mineral Density in US Adults. Biol Trace Elem Res. 2023;201:4254-4261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 32. | He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL. Trends in Cardiovascular Risk Factors in US Adults by Race and Ethnicity and Socioeconomic Status, 1999-2018. JAMA. 2021;326:1286-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 33. | Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 34. | Xie R, Zhang Y. Associations between dietary flavonoid intake with hepatic steatosis and fibrosis quantified by VCTE: Evidence from NHANES and FNDDS. Nutr Metab Cardiovasc Dis. 2023;33:1179-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 35. | Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 687] [Article Influence: 52.8] [Reference Citation Analysis (0)] |