Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.686
Peer-review started: December 20, 2023
First decision: January 10, 2024
Revised: January 19, 2024
Accepted: March 1, 2024
Article in press: March 1, 2024
Published online: April 15, 2024
Processing time: 113 Days and 20.6 Hours
The two-way relationship between periodontitis and type 2 diabetes mellitus (T2DM) is well established. Prolonged hyperglycemia contributes to increased periodontal destruction and severe periodontitis, accentuating diabetic complications. An inflammatory link exists between diabetic retinopathy (DR) and perio
To determine the correlation of periodontal inflamed surface area (PISA) with glycated Hb (HbA1c), serum IL-6 and Lp(a) in T2DM subjects with retinopathy.
This cross-sectional study comprised 40 T2DM subjects with DR and 40 T2DM subjects without DR. All subjects were assessed for periodontal parameters [bleeding on probing (BOP), probing pocket depth, clinical attachment loss (CAL), oral hygiene index-simplified, plaque index (PI) and PISA], and systemic parameters [HbA1c, fasting plasma glucose and postprandial plasma glucose, fasting lipid profile, serum IL-6 and serum Lp(a)].
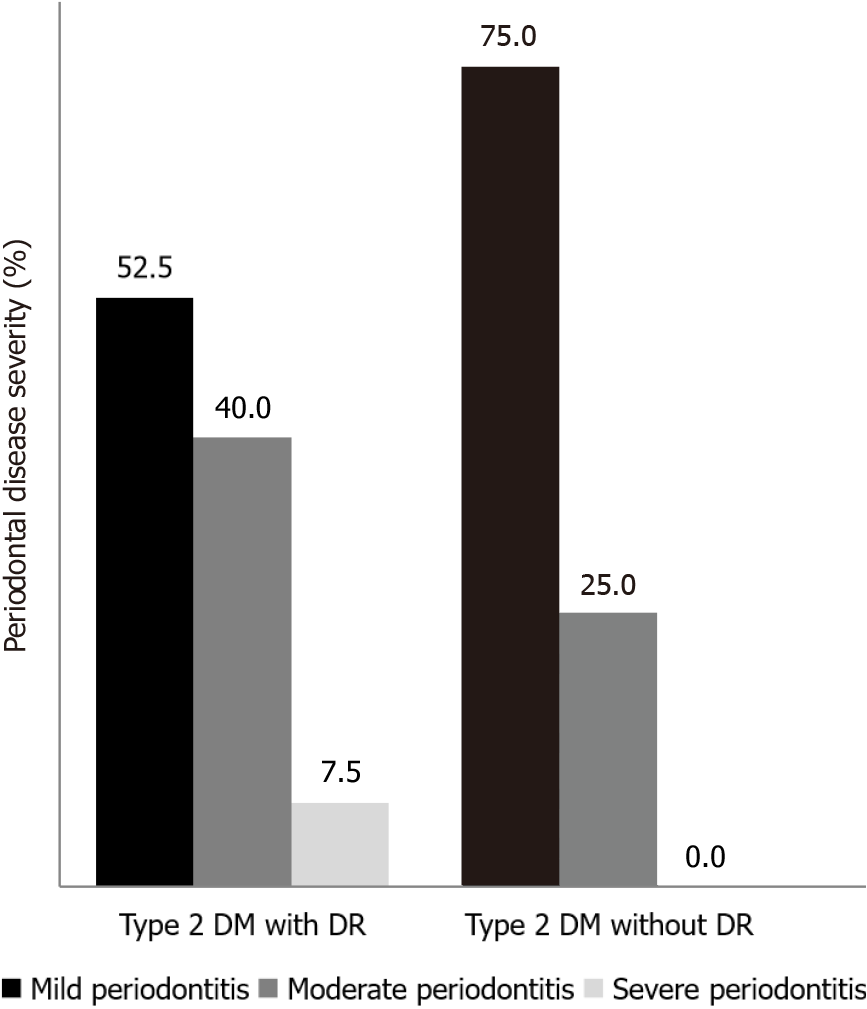
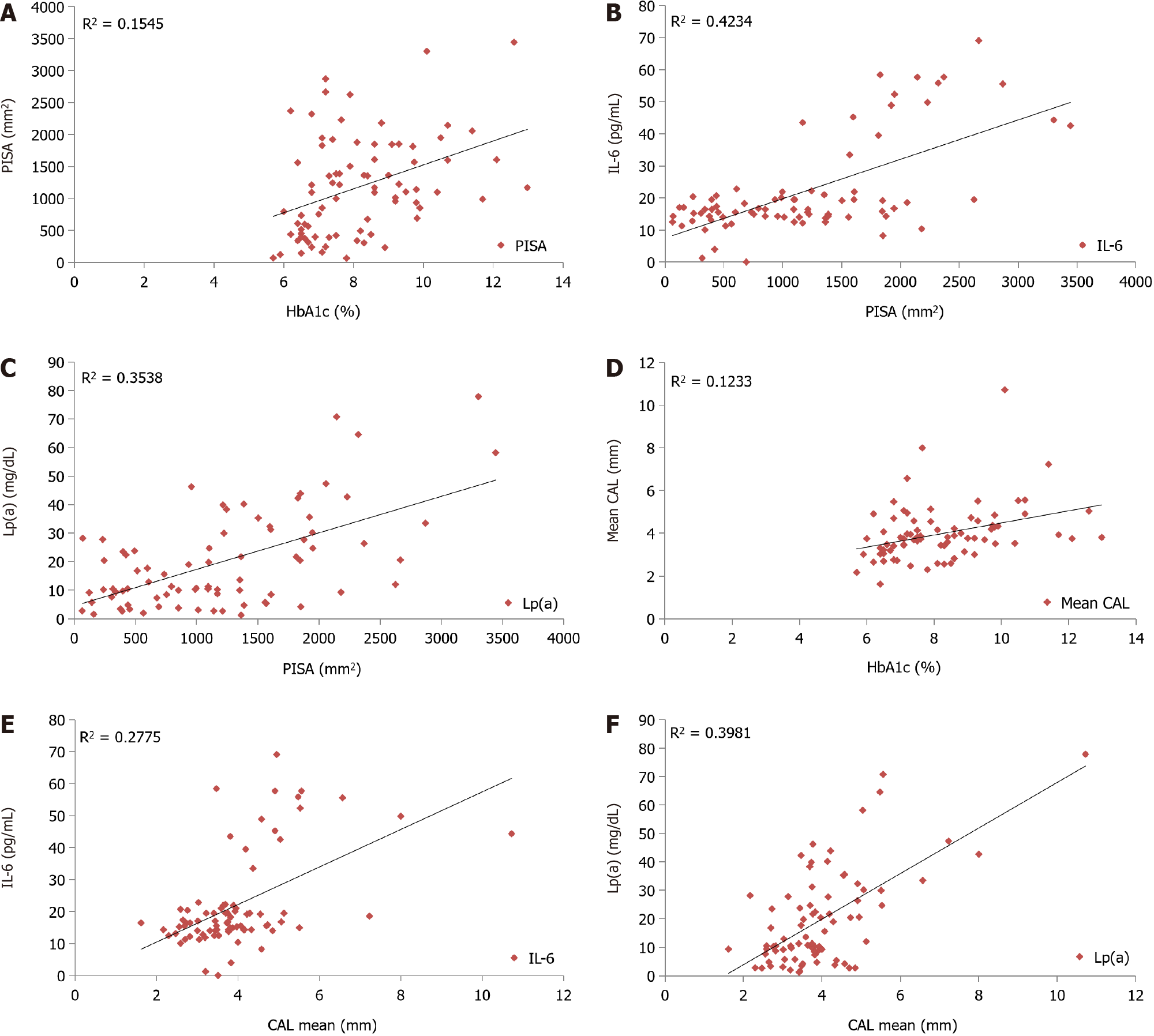
The proportion of periodontitis in T2DM with and without DR was 47.5% and 27.5% respectively. Severity of periodontitis, CAL, PISA, IL-6 and Lp(a) were higher in T2DM with DR group compared to T2DM without DR group. Sig-nificant difference was observed in the mean percentage of sites with BOP between T2DM with DR (69%) and T2DM without DR (41%), but there was no significant difference in PI (P > 0.05). HbA1c was positively correlated with CAL (r = 0.351, P = 0.001), and PISA (r = 0.393, P ≤ 0.001) in study subjects. A positive correlation was found between PISA and IL-6 (r = 0.651, P < 0.0001); PISA and Lp(a) (r = 0.59, P < 0.001); CAL and IL-6 (r = 0.527, P < 0.0001) and CAL and Lp(a) (r = 0.631, P < 0.001) among study subjects.
Despite both groups having poor glycemic control and comparable plaque scores, the periodontal parameters were higher in DR as compared to T2DM without DR. Since a bidirectional link exists between periodontitis and DM, the presence of DR may have contributed to the severity of periodontal destruction and periodontitis may have influenced the progression of DR.
Core Tip: Periodontal inflamed surface area (PISA) estimates the periodontal inflammatory burden. Prolonged hyperglycemia contributes to increased periodontal destruction and accentuates diabetic complications. An inflammatory link may exist between diabetic retinopathy (DR) and periodontitis. This study assessed correlation between PISA with glycated Hb (HbA1c), interleukin-6 (IL-6) and lipoprotein(a) [Lp(a)] in type 2 diabetes mellitus (T2DM) subjects with and without DR. Significant positive correlation between PISA with HbA1C, IL-6 and Lp(a) were observed. Proportion and severity of periodontitis, PISA, IL-6 and Lp(a) were higher in DR compared to T2DM without DR. Presence of DR may have contributed to the severity of periodontitis and periodontitis may have influenced the progression of DR.
- Citation: Thazhe Poyil NJ, Vadakkekuttical RJ, Radhakrishnan C. Correlation of periodontal inflamed surface area with glycated hemoglobin, interleukin-6 and lipoprotein(a) in type 2 diabetes with retinopathy. World J Diabetes 2024; 15(4): 686-696
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/686.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.686
Diabetes mellitus (DM) is a multifaceted metabolic disorder characterized by impaired glucose tolerance and hyperglycemia. Currently, 537 million people are living with diabetes, which is predicted to rise to 643 million by 2030 and 784 million by 2045[1]. Type 2 DM (T2DM) occurs when there is a progressive loss of insulin secretion on the background of insulin resistance. Secondary pathophysiologic changes in diabetes lead to the development of microvascular (retinopathy, neuropathy, and nephropathy) and macrovascular (ischemic heart disease, peripheral vascular disease, and cerebrovascular disease) complications. Periodontitis has been recognized as the “sixth com-plication” of diabetes[2].
Periodontitis is a microbially-associated, host-mediated inflammation of the supporting tissues of teeth characterized by the presence of periodontal pockets, clinical attachment loss (CAL), gingival recession, and progressive destruction of periodontal structures[3]. The periodontal pocket acts as a portal for the entry of microorganisms into the systemic circulation, leading to subclinical systemic inflammation. Periodontitis induced bacteremia and inflammatory response depends on the area of inflamed periodontal tissue. Periodontal inflamed surface area (PISA) counts the surface area of bleeding periodontal pocket epithelium and assesses the inflammatory burden[4]. A two-way relationship exists between periodontitis and diabetes. Poor glycemic control, longer duration and complications of DM lead to periodontal disease (PD) severity[5]. Systemic inflammation reduces insulin sensitivity, increases insulin resistance, and thus adversely affects the glycemic status, which in turn increases the risk of complications of DM[6].
Diabetic retinopathy (DR) is one of the most common microvascular complications of DM, and it is the progressive dysfunction of the retinal blood vessels caused by chronic hyperglycemia[7]. It is one of the leading causes of blindness. Approximately one in three people with diabetes have DR and one in ten will develop a vision – threatening form of the disease[8]. DR is perceived as a vascular and neurodegenerative disease. Inflammation plays a crucial role in the development of the early and late stages of DR[9].
Lipoprotein(a) [Lp(a)] is involved in the development of atherothrombosis and the activation of acute inflammation, exerting a proatherogenic and hypofibrinolytic effect[10]. Since capillary occlusion is a frequent finding in DR, the factor Lp(a) has an important role in the development and progression of DR. Lp(a) is susceptible to oxidative modifications, leading to the formation of pro-inflammatory and pro-atherogenic oxidized phospholipids. It has been reported that PD leads to elevated levels of lipoproteins and inflammatory mediators in the serum and gingival crevicular fluid[11]. Elevated inflammatory mediators and lipoproteins in diabetic patients with PD may contribute to retinal blood vessel damage, leading to DR.
Studies regarding the relationship between DR and PD and the role of Lp(a) and interleukin-6 (IL-6) in these conditions are scarce in the literature. Therefore, the objectives of the present study were: (1) To compare the proportion and severity of periodontitis; and (2) To correlate CAL and PISA with glycemic status, serum IL-6, and Lp(a) in T2DM subjects with and without DR.
This cross-sectional study was carried out by the Department of Periodontics, Government Dental College, Calicut in association with the diabetic clinic and Dept of Ophthalmology, Government Medical College, Calicut. The participants were T2DM patients attending the diabetic clinic of Government Medical College, Calicut. T2DM subjects with and without DR in the age group between 30-75 years were included in the study. Exclusion criteria were: History of intraocular surgery or previous laser photocoagulation, patients who were already on lipid-lowering drugs, known systemic diseases and conditions, pregnant and lactating mothers, psychiatric illness, systemic antibiotics within six months, periodontal therapy (scaling and root planing or surgery) within the past one year.
A total of 80 T2DM subjects (40 with DR and 40 without DR) were randomly selected. This study was approved by the Institutional Ethics Committee, Government Dental College Calicut (IEC No: 149/2019/DCC dated 14-11-2019). Informed consent was obtained from all subjects and the study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. The duration of the study was 18 months (from 30/05/2020 to 30/11/2021).
All subjects were evaluated using a detailed questionnaire including personal information, socio-demographic characteristics, medical history, oral hygiene practices, history of diabetes, duration of diabetes and diabetic complications, and drug allergy. Diagnosis of DR was done by dilated fundoscopy, performed by an ophthalmologist and its severity (mild, moderate, and severe) was assessed based on the International Clinical Diabetic Retinopathy Disease Severity Scale[12].
Biochemical variables were assessed using peripheral blood samples collected by venipuncture from the ante cubital fossa in the same period of clinical examination. The parameters included glycated Hb (HbA1c), fasting plasma glucose, postprandial plasma glucose, fasting lipid profile (FLP), IL-6, and Lp(a).
Plaque index (PI), oral hygiene index-simplified (OHI-S), percentage of sites with bleeding on probing (BOP), gingival recession (GR), probing pocket depth (PPD), CAL, and PISA were recorded. All periodontal assessments were carried out by a qualified examiner (Nusreen Jamal Thazhe Poyil).
William’s periodontal probe was used to assess PPD, GR, and CAL at six sites per tooth. The periodontal status was recorded as no/mild, moderate, and severe periodontitis, based on the CDC criteria (CDC 2012 update)[13].
PISA was calculated using a Microsoft Excel spreadsheet available from the website: www.parsprototo.info. CAL, GR, and BOP on six sites for each tooth were entered in this spreadsheet. Mean CAL and GR for each tooth were computed and converted into periodontal epithelial surface area (PESA). PISA for a particular tooth was measured by multiplying PESA for that tooth with the percentage of sites with BOP. PISA (mm2) per subject was estimated by adding PISA around each tooth.
mean ± SD and frequency were computed for quantitative and qualitative data respectively. Unpaired t-test was done to evaluate the quantitative variables [age, duration of diabetes, BOP, OHI-S, PI, PPD, CAL, HbA1c, fasting blood glucose, postprandial blood glucose, FLP, IL-6, Lp(a) and PISA] between T2DM patients with and without DR. The χ2 test analyzed qualitative data such as oral hygiene practices, past smoking status, severity of DR, proportion, and severity of periodontitis. Mean CAL and PISA were analyzed between nonproliferative, proliferative type 2 DR and T2DM without DR by one-way ANOVA test with post-hoc adjustment (Bonferroni test). Correlation between PISA and HbA1c, PISA and IL-6, PISA and Lp(a), CAL and HbA1c, CAL and IL-6, and CAL and Lp(a) were done by Pearson correlation test.
Socio-demographic, behavioral, and clinical characteristics of the study subjects are given in Table 1. No significant difference was observed regarding the age, educational level and smoking status between T2DM subjects with and without DR. A significant difference in the percentage distribution of gender between the two groups with a male predominance in DR group (P = 0.001) was observed. The mean duration of T2DM (P = 0.005), mean debris score (P < 0.001), OHI-S score (P = 0.02) and Decay, missing and filled Teeth score (P = 0.04) were significantly higher in DR group, but there was no difference in PI (P = 0.19) and calculus index (P = 0.36).
| Variables | T2DM with DR (n = 40) | T2DM without DR (n = 40) | P value | |
| Age, yr (mean ± SD) | 53.85 ± 6.86 | 54.03 ± 10.75 | 0.093 | |
| Gender frequency, n (%) | Male | 31 (77.5) | 17 (42.5) | 0.001 |
| Female | 9 (22.5) | 23 (57.5) | ||
| Education frequency, n (%) | Illiterate | 0 | 1 (2.5) | 0.172 |
| Primary school | 5 (12.50) | 4 (10) | ||
| Middle school | 21 (52.50) | 12 (30.0) | ||
| High school | 11 (27.50) | 21 (52.5) | ||
| Diploma and above | 3 (7.5) | 2 (5.0) | ||
| Socioeconomic status frequency, n (%) | APL | 25 (62.5) | 19 (47.5) | 0.178 |
| BPL | 15 (37.5) | 21 (52.5) | ||
| Behavioural | ||||
| Smoking status, n (%) | Current smoker | 1 (2.5) | 1 (2.5) | 0.49 |
| Ex-smoker | 5 (12.5) | 2 (5.0) | ||
| Non-smoker | 34 (85.0) | 37 (92.5) | ||
| Frequency of teeth cleaning (daily), n (%) | Once | 23 (57.5) | 19 (47.5) | 0.44 |
| Twice | 17 (42.5) | 20 (50.0) | ||
| After every meal | 0 (0) | 1 (2.5) | ||
| Clinical (mean ± SD) | ||||
| Duration of DM (yr) | 11.94 ± 6.83 | 8.07 ± 4.87 | 0.005a | |
| DI-S | 1.78 ± 0.69 | 1.22 ± 0.54 | < 0.001a | |
| CI-S | 1.15 ± 0.79 | 1.00 ± 0.67 | 0.36 | |
| OHI-S | 2.90 ± 1.38 | 2.22 ± 1.12 | 0.02a | |
| PI | 1.72 ± 0.57 | 1.54 ± 0.62 | 0.19 | |
| DMFT | 7.48 ± 4.08 | 5.53 ± 4.45 | 0.04a | |
The distribution of biochemical and periodontal variables between T2DM with and without DR group are displayed in Table 2. A significant difference was observed in the HbA1c (P < 0.005), serum IL-6 (P < 0.001), Lp(a) (P < 0.001), HDL (P < 0.001), low-density lipoprotein (LDL; P = 0.025), very LDL (P = 0.005) and triglyceride (P = 0.004) levels between the groups, but no significant difference was seen in the total cholesterol levels (P = 0.254).
| Variables | T2DM with DR (n = 40) | T2DM without DR (n = 40) | P value |
| HbA1c (%) | 8.67 ± 1.81 | 7.64 ± 1.28 | 0.004a |
| FPG (mmol/L) | 8.66 ± 2.67 | 8.62 ± 3.58 | 0.95 |
| PPG (mmol/L) | 12.22 ± 4.21 | 11.95 ± 4.05 | 0.77 |
| IL-6 (pg/mL) | 30.52 ± 16.57 | 13.47 ± 4.15 | < 0.001a |
| Lp(a) (mg/dL) | 27.76 ± 18.82 | 11.45 ± 9.08 | < 0.001a |
| Total cholesterol (mmol/L) | 4.93 ± 0.90 | 4.70 ± 0.89 | 0.254 |
| HDL (mmol/L) | 1.41 ± 0.34 | 1.78 ± 0.36 | < 0.001a |
| LDL (mmol/L) | 2.80 ± 0.93 | 2.31 ± 0.97 | 0.025 a |
| VLDL (mmol/L) | 0.76 ± 0.28 | 0.61 ± 0.19 | 0.005 a |
| TG (mmol/L) | 1.67 ± 0.61 | 1.32 ± 0.41 | 0.004 a |
| Periodontal variables | |||
| PPD (mm) | 3.87 ± 0.93 | 3.08 ± 0.66 | < 0.001a |
| CAL (mm) | 4.50 ± 1.49 | 3.43 ± 0.85 | < 0.001a |
| CAL, ≤ 3 mm | 36.14 ± 22.81 | 63.88 ± 26.52 | < 0.001a |
| CAL, 4 to 5 mm | 40.62 ± 15.56 | 28.91 ± 18.55 | 0.003a |
| CAL, ≥ 6 mm | 23.42 ± 24.29 | 8.44 ± 11.99 | 0.001a |
| BOP (% of sites) | 69.33 ± 23.36 | 40.95 ± 25.47 | < 0.001a |
| PISA (mm2) | 1570.559 ± 759.89 | 789.79 ± 589.34 | < 0.001a |
| Periodontitis, n (%) | 19 (47.5) | 11 (27.5) | 0.065 |
The mean difference in PPD (P < 0.001), CAL (P < 0.001), PISA (P < 0.001) and percentage of sites with BOP (P < 0.001) between T2DM with DR group and T2DM without DR group was significant.
The proportion of periodontitis in T2DM with DR and in T2DM without DR was 47.5% and 27.5% respectively. Significant difference was observed in the severity of PD among the groups (P = 0.05; Figure 1). The proportion of mild periodontitis was higher in T2DM without DR (75%) as compared to T2DM with DR (52.5%) whereas moderate periodontitis was significantly higher among DR group (40%) than T2DM without DR (25%). No subjects had severe periodontitis in T2DM without retinopathy.
The mean difference in CAL and PISA between T2DM with nonproliferative, proliferative retinopathy group and T2DM without retinopathy groups was significant (P < 0.001). Bonferroni post-hoc adjustment revealed a significant difference between T2DM without retinopathy and nonproliferative retinopathy, and proliferative retinopathy groups (P < 0.001; Tables 3 and 4).
A statistically significant positive correlation was observed between PISA and HbA1c (r = 0.393, P < 0.001; Figure 2A), PISA and IL-6 (r = 0.651, P < 0.001; Figure 2B), PISA and Lp(a) (r = 0.59, P < 0.001; Figure 2C) among all study subjects. A statistically significant positive correlation was seen between CAL and HbA1c (r = 0.351, P = 0.001; Figure 2D), CAL and IL-6 (r = 0.527, P < 0.001; Figure 2E), CAL and Lp(a) (r = 0.631, P < 0.001; Figure 2F) in all study subjects.
In the present study, the percentage of males was higher in DR group as compared to T2DM without DR and it is in accordance with the findings of Deshpande et al[14]. DR is often preceded by neurodegenerative changes and females may have some protection from, or resistance to, these changes relative to males[15]. This may be attributed to the low frequency of females in the current study.
The duration of diabetes was significantly longer in DR group (11.94 ± 6.83 years) in contrast to T2DM without DR (8.07 ± 4.87 years). Even though both the study groups had poor glycemic control, the HbA1c values were significantly higher in the DR group (8.67 ± 1.8) as compared to T2DM without DR (7.64 ± 1.2). Longer duration of diabetes and increased glycemic burden are positively associated with the incidence of DR[16].
It is interesting to note that plaque score was similar in both groups, but the mean DI-S score, OHI-S score and DMFT scores were significantly higher in DR group as compared to T2DM group without DR. This may be due to the poor glycemic control and associated periodontal inflammation in DR which in turn could have adversely affected the ability to maintain good oral hygiene. Sadzeviciene et al[17] reported that a correlation exists between complications inherent to DM, such as DR or nephropathy, and an increased degree of periodontal inflammation.
T2DM with DR showed a higher percentage of periodontitis (47.5%) as compared to T2DM without DR (27.5%) in the present study. Although Amiri et al[18] in 2014 indicated a probable relationship between retinal microvascular complications in diabetes and PD, to the best of our knowledge, no studies have been conducted to compare the percentage of periodontitis between T2DM with and without DR. In this study, T2DM with DR group had a higher mean value of PPD and CAL. This observation was in accordance with the findings of Sadzeviciene et al[17] in 2005 who reported increased periodontal breakdown in the presence of microvascular complications of T2DM. Similar to this, Adhenkavil Radhakrishnan et al[19] in 2022 reported a higher frequency of periodontitis in diabetic foot patients. In contrast, Bridges et al[20] in 1996 opined that there was no association between glycemic control and periodontal variables.
DR group showed a higher percentage of sites with CAL ≥ 6 mm and a higher percentage of sites with moderate/severe periodontitis as compared to T2DM without DR. This showed that the severity of periodontitis was more in T2DM with DR. Anil et al[21] reported that the uncontrolled T2DM group with microvascular complications had the highest percentage of sites with CAL ≥ 6 mm than the uncontrolled T2DM group without microvascular complications and controlled T2DM group. Inflammatory cytokines and Advanced Glycation End products in hyperglycemic state induce neutrophils to create oxidative stress that accentuates alveolar bone loss by acting on osteoclasts, resulting in increased attachment loss and severity of periodontitis in diabetic complications[22].
The link between DR and periodontitis may be possibly related to the presence of inflammation. NLRP3 (nucleotide-binding domain and leucine-rich repeat receptor containing a pyrin domain 3), is a protein responsible for several intracellular signalling events in the inflammatory mechanism. NLRP3 is not only involved in the pathogenesis of periodontitis but is also present in the retina of patients with progressive DR[23,24].
It is evident from this study that as the severity of DR increases the severity of periodontal breakdown increases. Proliferative DR had higher CAL compared to non-proliferative DR. A positive correlation was observed between CAL and HbA1c in T2DM with and without DR and these observations are comparable to the report of H R et al[25] in 2018. They reported that the severity of PD strongly correlated with HbA1c in T2DM with DR patients.
BOP is the earliest indicator of periodontal inflammation. The percentage of sites with BOP was 69% and 40.95% respectively in T2DM with DR and T2DM without DR. Severe periodontal inflammation in T2DM with DR may be accounted for increased bleeding sites. Consistent with this, Anil et al[21] reported 79%of sites with BOP in uncontrolled T2DM group with microvascular complications. Zoellner et al[26] reported that histologically, the microvascular pathological conditions of gingivitis and retinopathies are similar; both are described as microvascular angiopathies with oedema, vascular proliferation and tortuosity, haemorrhaging, and membrane thickening. Lp(a) exerts antifibrinolytic and prothrombotic effects, which may contribute to the increased BOP in DR patients. Microvascular pathological conditions of retinopathy might have influenced the increased BOP and further studies are needed to confirm this.
Periodontitis induces systemic inflammatory burden by the ingress of inflammatory mediators. Tools such as CAL and PPD for grading periodontitis are linear measurements that do not adequately estimate the inflammatory load induced by periodontitis. So, in this study inflammatory burden of periodontitis was assessed by PISA. PISA shows the surface area of bleeding pocket epithelium[4], used to assess the periodontal inflammation. In this study T2DM with DR group had a higher mean PISA (1570 mm2) than T2DM without DR (789 mm2). This is in accordance with studies by Anil et al[21] and Lindner et al[27]. Nesse et al[4] in 2008 observed a higher PISA score with upper and lower limits of 0 and 1087 mm2 respectively, in T2DM with periodontitis. Estimated PISA values in this study corroborate with the inflammatory link between periodontitis and DR. A positive correlation had been obtained between PISA and HbA1c in T2DM with DR and without DR which is similar to the report of Adhenkavil Radhakrishnan et al[19] in 2022. They reported a dose-response relationship exists between PISA and HbA1c and showed that an increase in PISA of 50.77 mm2 was associated with a 1% increase in HbA1c. Nesse et al[4] in 2008 opined that a change in PISA of 333 mm2 was associated with a 1% increase in HbA1c independent of other factors. From this, it is evident that periodontal inflammation could have influenced the HbA1c level in T2DM with and without DR.
The inflammatory response in periodontitis is characterized by dysregulated secretion of host-derived inflammatory mediators which may provoke systemic inflammation, enhance hyperglycemia, and insulin resistance and exacerbate complications in T2DM. In this study, serum levels were significantly high in retinopathy (30.52 pg/mL) when compared to T2DM without DR (13.47 pg/mL). Similar findings are reported by Quevedo-Martínez et al[28]. In this study, the presence of a higher percentage of periodontitis in T2DM with DR as compared to without DR contributed to the higher IL-6 level in DR. A significant positive correlation between serum IL-6 and PISA and also between IL-6 and CAL was obtained among the study subjects. DR is a low-grade inflammatory disease and inflammation specifically leukocyte adhesion to the retinal vasculature triggers the disease in a hyperglycemic environment[29]. This study reveals that elevated IL-6 concentrations may contribute to the disease activity in DR. A bidirectional link may exist between DR and PD via IL-6 since it might have been involved in the pathophysiology of both conditions.
Lp(a) is an independent risk factor for developing vascular disease. It has the potential to cause vessel damage through lipoprotein oxidation to exert antifibrinolytic and prothrombotic effects. In this study, it is interesting to note that T2DM with DR subjects showed a significantly higher Lp(a) level (approximately 27.76 mg/dL) as compared to the group without DR (11.45 mg/dL). Reports are there in the literature pointing to the relation between serum Lp(a) concentrations and DR[30]. Conflicting reports are also available in the literature. Paige et al[31] in 2017 reported an inverse association between Lp(a) concentration and risk of T2DM. A significant positive correlation between serum Lp(a) and PISA and also between Lp(a) and CAL were obtained in the present study.
Lp(a) is susceptible to oxidative modifications, leading to the formation of ‘oxidation-specific epitopes’ (OSEs). Different OSEs are present on Lp(a) as ‘danger-associated molecular patterns’, triggering innate immunity[32]. Modified Lp(a) binds and carries MCP-1/CCL2 (pro-inflammatory molecules such as the monocyte chemoattractant protein-1), which induces and maintains vascular inflammation.
Inflammation plays an important role in the relationship between periodontitis and DR. Despite both groups having poor glycemic control and comparable plaque scores, the periodontal parameters were higher in DR as compared to T2DM without DR. Since a bidirectional link exists between periodontitis and DM, the presence of DR may have contributed to the severity of periodontal destruction and periodontitis may have influenced the progression of DR.
One of the limitations of this study was its small sample size. Periodontal parameters like PPD and CAL were measured manually using a William’s graduated periodontal probe. More accurate results can be obtained with new-generation computerized probes. In this study it was unfeasible to corroborate the causality and the direction of the relationship between periodontitis and type 2 DR, due to its cross-sectional study design. Large multicentric clinical studies with a proper longitudinal study design and appropriate adjustments for confounders are needed to ascertain whether PD affects the progression of DR and DR contributes to the severity of PD.
Despite both groups having poor glycemic control and comparable plaque scores, the periodontal and inflammatory parameters were higher in the DR group compared to T2DM without DR. The presence of DR may have contributed to the severity of periodontal destruction and periodontitis may have influenced the progression of DR. Proper periodontal care can help in improving glycemic control and prevent the progression of DR to some extent. A better understanding of the association between type 2 DR and periodontitis will help create awareness among the public and improve their overall quality of life.
The two-way relationship between periodontitis and type 2 diabetes mellitus (T2DM) is well established. Prolonged hyperglycemia contributes to increased periodontal destruction and severe periodontitis, accentuating diabetic complications. An inflammatory link exists between diabetic retinopathy (DR) and periodontitis.
Studies regarding this relation and the role of lipoprotein(a) [Lp(a)] and interleukin-6 (IL-6) in these conditions are scarce in the literature. This study assessed the proportion and severity of periodontitis and the correlation between periodontal inflamed surface area (PISA), and clinical attachment loss (CAL) with glycated hemoglobin (HbA1c), serum IL-6 and Lp(a).
(1) To determine and compare the proportion and severity of periodontitis in T2DM subjects with and without DR; (2) To assess the correlation between PISA and HbA1c, serum IL-6, and Lp(a) in T2DM subjects with and without DR; and (3) To assess the correlation between CAL and HbA1c, serum IL-6, and Lp(a) in T2DM subjects with and without DR.
The duration of the study was 18 months. In this study, 80 T2DM subjects (40 with DR and 40 without DR) were selected from the diabetic clinic of Department of Internal Medicine, Government Medical College, Calicut. They were divided into two groups based on the presence of DR as follows: Group I- T2DM with DR and Group II- T2DM without DR. Subjects were assessed with a detailed questionnaire regarding their socio-demographic characteristics, medical history, oral hygiene practice, history of diabetes and drug allergy. HbA1c, fasting plasma glucose and postprandial plasma glucose, serum IL-6, and Lp(a) were evaluated. Probing pocket depth, CAL, bleeding on probing, oral hygiene index-simplified, PISA and periodontal disease severity were determined. Diagnosis of DR was done by dilated fundoscopy.
The proportion of periodontitis in T2DM with DR and in T2DM without DR was 47.5% and 27.5% respectively. Severity of periodontitis, CAL, PISA, serum IL-6 and Lp(a) were higher in T2DM with DR group compared to T2DM without DR group. HbA1c was positively correlated with CAL (r = 0.351, P = 0.001), and PISA (r = 0.393, P ≤ 0.001) in study subjects. A positive correlation was found between PISA and IL6 (r = 0.651, P < 0.0001); PISA and Lp(a) (r = 0.59, P < 0.001); CAL and IL6 (r = 0.527, P < 0.0001) and CAL and Lp(a) (r = 0.631, P < 0.001) among study subjects.
The presence of DR may have contributed to the severity of periodontal destruction and periodontitis may have influenced the progression of DR.
Since a bidirectional link exists between periodontitis and diabetes mellitus, periodontal therapy should be included in the diabetes management. Proper periodontal care can help in improving glycemic control and prevent the progression of DR to some extent. A better understanding of the association between type 2 DR and periodontitis will help to create awareness among the public and to improve their overall quality of life.
We are grateful to Dr. Jyothi P T, Professor and Head, Dept. of Ophthalmology, Govt. Medical College, Calicut for her support in conducting this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dąbrowski M, Poland; Ghusn W, United States S-Editor: Lin C L-Editor: A P-Editor: Yuan YY
| 1. | Wagenknecht LE, Lawrence JM, Isom S, Jensen ET, Dabelea D, Liese AD, Dolan LM, Shah AS, Bellatorre A, Sauder K, Marcovina S, Reynolds K, Pihoker C, Imperatore G, Divers J; SEARCH for Diabetes in Youth study. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: results from the population-based SEARCH for Diabetes in Youth study. Lancet Diabetes Endocrinol. 2023;11:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 2. | Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329-334. [PubMed] |
| 3. | Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018;89 Suppl 1:S159-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 1304] [Article Influence: 217.3] [Reference Citation Analysis (0)] |
| 4. | Nesse W, Abbas F, van der Ploeg I, Spijkervet FK, Dijkstra PU, Vissink A. Periodontal inflamed surface area: quantifying inflammatory burden. J Clin Periodontol. 2008;35:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 358] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 5. | Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 278] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 6. | Southerland JH, Taylor GW, Offenbacher S. Diabetes and Periodontal Infection: Making the Connection. Clin Diabetes. 2005;23:171-178. [DOI] [Full Text] |
| 7. | Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol. 2008;126:1740-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, Wong IY, Ting DSW, Tan GSW, Jonas JB, Sabanayagam C, Wong TY, Cheng CY. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 1003] [Article Influence: 250.8] [Reference Citation Analysis (1)] |
| 9. | Rübsam A, Parikh S, Fort PE. Role of Inflammation in Diabetic Retinopathy. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 509] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 10. | Malaguarnera G, Gagliano C, Bucolo C, Vacante M, Salomone S, Malaguarnera M, Leonardi DG, Motta M, Drago F, Avitabile T. Lipoprotein(a) serum levels in diabetic patients with retinopathy. Biomed Res Int. 2013;2013:943505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Bostanci N, Belibasakis GN. Gingival crevicular fluid and its immune mediators in the proteomic era. Periodontol 2000. 2018;76:68-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT; Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1980] [Cited by in RCA: 2277] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 13. | Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 959] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 14. | Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1015] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 15. | Ozawa GY, Bearse MA Jr, Bronson-Castain KW, Harrison WW, Schneck ME, Barez S, Adams AJ. Neurodegenerative differences in the retinas of male and female patients with type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:3040-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Simó-Servat O, Hernández C, Simó R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019;62:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 17. | Sadzeviciene R, Paipaliene P, Zekonis G, Zilinskas J. The influence of microvascular complications caused by diabetes mellitus on the inflammatory pathology of periodontal tissues. Stomatologija. 2005;7:121-124. [PubMed] |
| 18. | Amiri AA, Maboudi A, Bahar A, Farokhfar A, Daneshvar F, Khoshgoeian HR, Nasohi M, Khalilian A. Relationship between Type 2 Diabetic Retinopathy and Periodontal Disease in Iranian Adults. N Am J Med Sci. 2014;6:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Adhenkavil Radhakrishnan R, Joseph Vadakkekuttical R, Radhakrishnan C. Proportion and severity of periodontitis and correlation of periodontal inflamed surface area with glycemic status in patients with type 2 diabetic neuropathy with and without diabetic foot. J Periodontol. 2022;93:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Bridges RB, Anderson JW, Saxe SR, Gregory K, Bridges SR. Periodontal status of diabetic and non-diabetic men: effects of smoking, glycemic control, and socioeconomic factors. J Periodontol. 1996;67:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Anil K, Vadakkekuttical RJ, Radhakrishnan C, Parambath FC. Correlation of periodontal inflamed surface area with glycemic status in controlled and uncontrolled type 2 diabetes mellitus. World J Clin Cases. 2021;9:11300-11310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Wu YY, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 2015;7:63-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 23. | Marchesan JT, Girnary MS, Moss K, Monaghan ET, Egnatz GJ, Jiao Y, Zhang S, Beck J, Swanson KV. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontol 2000. 2020;82:93-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 24. | Loukovaara S, Piippo N, Kinnunen K, Hytti M, Kaarniranta K, Kauppinen A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017;95:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | H R V, Natesh S, Patil SR. Association between Diabetic Retinopathy and Chronic Periodontitis-A Cross-Sectional Study. Med Sci (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Zoellner H, Chapple CC, Hunter N. Microvasculature in gingivitis and chronic periodontitis: disruption of vascular networks with protracted inflammation. Microsc Res Tech. 2002;56:15-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Lindner M, Arefnia B, Ivastinovic D, Sourij H, Lindner E, Wimmer G. Association of periodontitis and diabetic macular edema in various stages of diabetic retinopathy. Clin Oral Investig. 2022;26:505-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Quevedo-Martínez JU, Garfias Y, Jimenez J, Garcia O, Venegas D, Bautista de Lucio VM. Pro-inflammatory cytokine profile is present in the serum of Mexican patients with different stages of diabetic retinopathy secondary to type 2 diabetes. BMJ Open Ophthalmol. 2021;6:e000717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 30. | Kim CH, Park HJ, Park JY, Hong SK, Yoon YH, Lee KU. High serum lipoprotein(a) levels in Korean type 2 diabetic patients with proliferative diabetic retinopathy. Diabetes Care. 1998;21:2149-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Paige E, Masconi KL, Tsimikas S, Kronenberg F, Santer P, Weger S, Willeit J, Kiechl S, Willeit P. Lipoprotein(a) and incident type-2 diabetes: results from the prospective Bruneck study and a meta-analysis of published literature. Cardiovasc Diabetol. 2017;16:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, Yang X, Dennis E, Witztum JL, Koschinsky ML, Tsimikas S. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J Lipid Res. 2013;54:2815-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |