Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.645
Peer-review started: November 21, 2023
First decision: December 5, 2023
Revised: December 15, 2023
Accepted: February 21, 2024
Article in press: February 21, 2024
Published online: April 15, 2024
Processing time: 142 Days and 16.3 Hours
Patients with type 2 diabetes mellitus (T2DM) have large fluctuations in blood glucose (BG), abnormal metabolic function and low immunity to varying degrees, which increases the risk of malignant tumor diseases and affects the efficacy of tumor chemotherapy. Controlling hyperglycemia may have important therapeutic implications for cancer patients.
To clarify the influence of BG fluctuations on chemotherapy efficacy and safety in T2DM patients complicated with lung carcinoma (LC).
The clinical data of 60 T2DM + LC patients who presented to the First Affiliated Hospital of Ningbo University between January 2019 and January 2021 were retrospectively analyzed. All patients underwent chemotherapy and were grouped as a control group (CG; normal BG fluctuation with a mean fluctuation < 3.9 mmol/L) and an observation group (OG; high BG fluctuation with a mean fluctuation ≥ 3.9 mmol/L) based on their BG fluctuations, with 30 cases each. BG-related indices, tumor markers, serum inflammatory cytokines and adverse reactions were comparatively analyzed. Pearson correlation analysis was performed to analyze the correlation between BG fluctuations and tumor markers.
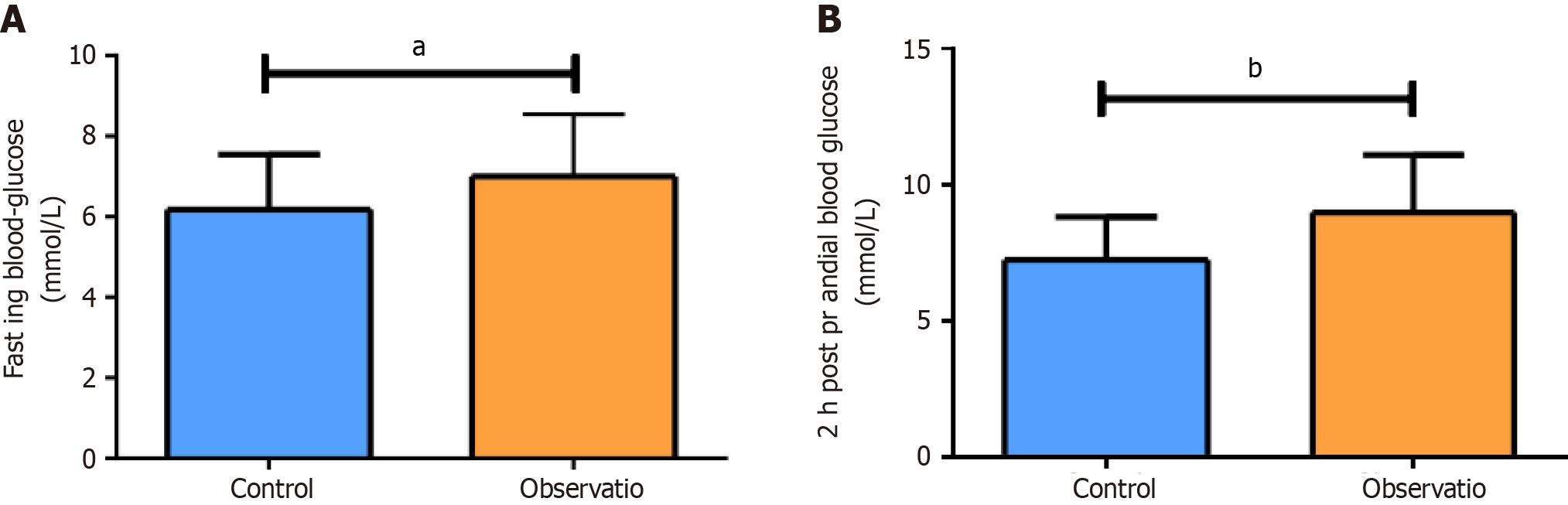
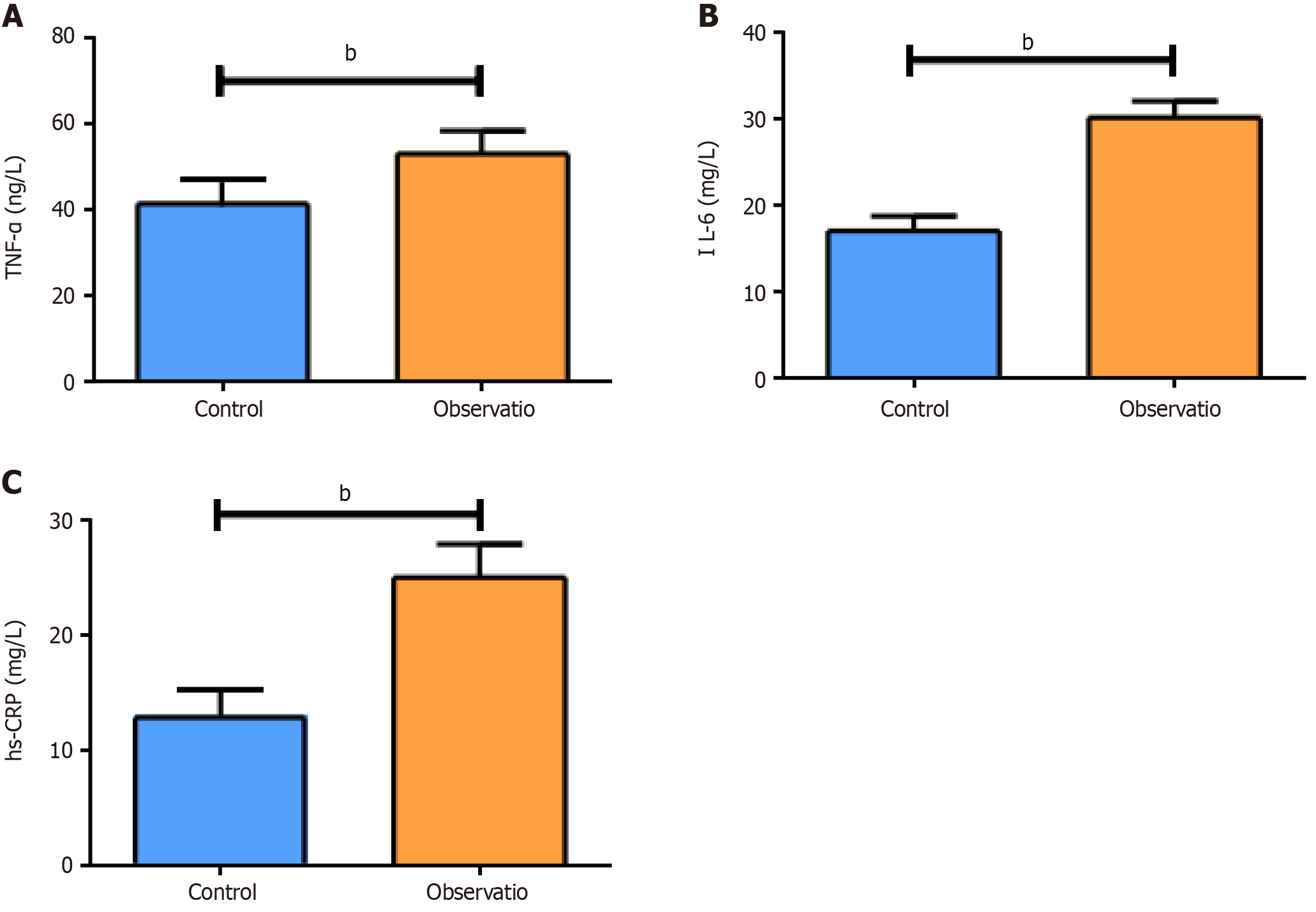
The fasting blood glucose and 2-hour postprandial blood glucose levels in the OG were notably elevated compared with those in the CG, together with markedly higher mean amplitude of glycemic excursions (MAGE), mean of daily differences, largest amplitude of glycemic excursions and standard deviation of blood glucose (P < 0.05). In addition, the OG exhibited evidently higher levels of carbohydrate antigen 19-9, carbohydrate antigen 125, carcinoembryonic antigen, neuron-specific enolase, cytokeratin 19, tumor necrosis factor-α, interleukin-6, and high-sensitivity C-reactive protein than the CG (P < 0.05). Pearson analysis revealed a positive association of MAGE with serum tumor markers. The incidence of adverse reactions was significantly higher in the OG than in the CG (P < 0.05).
The greater the BG fluctuation in LC patients after chemotherapy, the more unfavorable the therapeutic effect of chemotherapy; the higher the level of tumor markers and inflammatory cytokines, the more adverse reactions the patient experiences.
Core Tip: Controlling hyperglycemia may have important therapeutic implications for cancer patients. In this study, we seek to clarify the influence of blood glucose fluctuations on chemotherapy efficacy and safety in type 2 diabetes mellitus patients complicated with lung carcinoma.
- Citation: Fang TZ, Wu XQ, Zhao TQ, Wang SS, Fu GMZ, Wu QL, Zhou CW. Influence of blood glucose fluctuations on chemotherapy efficacy and safety in type 2 diabetes mellitus patients complicated with lung carcinoma. World J Diabetes 2024; 15(4): 645-653
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/645.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.645
Lung carcinoma (LC) is the most common malignant tumor in clinical practice that seriously harms human health, with the distinct characteristics of high incidence, high fatality rate and difficult treatment[1,2]. Diabetes mellitus (DM) is a common chronic metabolic disorder, with type 2 DM (T2DM) being the most prevalent. The prevalence of DM com-plicated with LC is increasing worldwide[3,4]. Epidemiological studies have shown that people with DM have a higher risk of LC[5,6], possibly because of common risk factors between the two diseases[7]. For example, smoking, a major external factor of LC, has also been indicated to have a certain relationship with DM[8,9]. In addition, the incidence of both diseases is correlated with age, with the highest prevalence found in middle-aged and elderly people among all age groups[10]. An estimated 8% to 18% of patients with non-small cell LC (NSCLC) have been reported to have DM[11]. DM may contribute to LC progression through mechanisms such as hyperinsulinemia, hyperglycemia and chronic inflammation, which are related to cell proliferation and cancer progression[12]. LC complicated with DM can obviously involve elevated blood glucose (BG) levels in patients, which causes the patients' body to be always in a state of injury. The immunity of such patients with large BG fluctuations will be reduced to a certain extent, resulting in abnormal metabolic function[13]. In addition, diabetes is associated with a 42 percent increased risk of death, a 21 percent elevated risk of recurrence, and significantly lower 5-year overall and cancer-specific survival[14-16]. Therefore, proper management of comorbid DM is essential for cancer treatment.
Chemotherapy is most commonly used clinically for LC with concomitant T2DM but no dominant locus mutations[17]. Although chemotherapy can partially control the tumor, DM and cancer patients undergoing chemotherapy are at an increased risk of developing BG problems due to the influence of metabolism and blood glucose, resulting in poor overall clinical efficacy and adverse prognosis[18,19]. Hyperglycemia in cancer patients receiving chemotherapy is related to the risk of nonhematological toxicity[20]. The combination of chemotherapy and corticosteroids, commonly used in cancer treatment, puts patients at risk of developing hyperglycemia, a clinical toxicity that may affect the reduction, interruption or cessation of chemotherapy doses[21]. Therefore, BG fluctuation is definitely a factor affecting the chemotherapy efficacy and prognosis of patients with DM complicated with cancer. However, whether the BG level can be a predictor of chemotherapy efficacy in LC patients has rarely been studied, and epidemiological evidence is limited. Accordingly, this study focuses on the influence of BG fluctuations on chemotherapy efficacy in T2DM + LC patients.
The clinical data of 60 T2DM + LC patients treated in the First Affiliated Hospital of Ningbo University from January 2019 to January 2021 were retrospectively analyzed. All patients were treated with chemotherapy. The inclusion criteria were as follows: (1) Patients meeting the diagnostic criteria for T2DM and pathologically confirmed with LC; (2) Patients with an estimated life expectancy exceeding 6 months; (3) Patients with no other vital organ function diseases; (4) Patients with no drug interactions; (5) Patients with normal heart, liver and kidney function; and (6) patients with complete clinical and follow-up data. The exclusion criteria were as follows: (1) Type 1 DM or secondary DM; (2) Renal and liver failure; (3) Mental illness or infectious diseases; and (4) Incomplete clinical and follow-up data. According to BG fluctuations, patients were assigned to normal [control group (CG); mean BG fluctuation < 3.9 mmol/L] and high blood BG range groups [observation group (OG); mean BG fluctuation ≥ 3.9 mmol/L], each with 30 cases. The male-to-female ratio, average age, and mean duration of DM in the CG were 16:14, 59.40 ± 3.00 years, and 2.27 ± 1.10 years, respectively, while those in the OG were 18:12, 59.17 ± 2.57 years, and 2.75 ± 1.22 years, respectively. Patients in the OG and CG were not significantly different in general data and were clinically comparable (P > 0.05).
All patients completed chemotherapy and were continuously monitored for BG fluctuations through a dynamic BG monitoring system. Forty-eight hours later, glycemic fluctuation indices, including the mean amplitude of glycemic excursions (MAGE), mean of daily differences (MODD), largest amplitude of glycemic excursions (LAGE), and standard deviation of BG (SDBG), were read. All patients remained on an empty stomach for more than 10 h prior to the examination and had elbow venous blood drawn early the next morning for testing. BG-related indices [fasting BG (FBG) and 2-h postprandial BG (2Hpg)] were detected using an automatic biochemical analyzer. Tumor markers, including carbohydrate antigen 19-9 (CA19-9), CA125, carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and cytokeratin 19 (CYFRA21-1), were determined with the use of an automatic chemiluminescence immunoassay. Turbidimetric immunoassay was also performed for the measurement of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP). The occurrence of adverse reactions in the two groups of patients, including phlebitis, gastrointestinal reactions, oral ulcers, liver and kidney damage, etc., was observed.
The primary endpoints were the changes in serum tumor markers in the two groups, while the secondary endpoints were glycemic fluctuation indices, changes in inflammatory factors, and adverse reactions.
The data were statistically analyzed by SPSS 25.0. Continuous (expressed by mean and standard deviation) and categorical variables (represented by percentages) were analyzed by the t test and χ2 test, respectively. The correlation between serum tumor markers and MAGE was identified by Pearson analysis at an α = 0.05 Level of significance. A P value < 0.05 was considered significant for all tests.
FBG and 2hPG in the OG were significantly higher than those in the CG (P < 0.05), as shown in Figure 1. In addition, the OG exhibited markedly higher MAGE, MODD, LAGE, and SDBG than the CG (P < 0.05; Table 1). These results indicated that in patients with high fluctuating blood glucose ranges have relatively weaker glycemic control and greater blood glucose fluctuations after chemotherapy.
| MAGE (mmol/L) | MODD (mmol/L) | LAGE (mmol/L) | SDBG (mmol/L) | |
| Control group (n = 30) | 3.75 ± 1.07 | 2.06 ± 0.36 | 4.25 ± 0.90 | 2.13 ± 0.33 |
| Observation group (n = 30) | 5.61 ± 1.15 | 2.59 ± 0.51 | 6.31 ± 1.21 | 2.72 ± 0.40 |
| t value | 6.451 | 4.721 | 7.472 | 6.195 |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
After chemotherapy, patients in both groups were tested to compare the levels of tumor markers. CA19-9, CA125, CEA, NSE and CYFRA21-1 Levels were found to be significantly higher in the RG than in the CG (P < 0.05), as shown in Table 2. The results similarly implied that patients with blood glucose in the normal range of fluctuation have more stable tumor marker levels after chemotherapy.
| CA19-9 (U/mL) | CA125 (U/mL) | CEA (mg/L) | NSE (mg/L) | CYFRA21-1 (mg/L) | |
| Control group (n = 30) | 64.26 ± 5.20 | 65.27 ± 6.60 | 12.12 ± 2.11 | 15.97 ± 1.34 | 4.98 ± 0.82 |
| Observation group (n = 30) | 75.00 ± 9.48 | 83.72 ± 6.06 | 17.83 ± 2.28 | 19.86 ± 1.42 | 7.54 ± 0.96 |
| t value | 5.444 | 11.28 | 10.08 | 10.88 | 11.11 |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
Statistical significance was determined in serum TNF-α, IL-6 and hs-CRP levels between groups, with even higher levels of these inflammatory cytokines in the OG (P < 0.05; Figure 2). These results indicated that high levels of blood glucose fluctuations impair the body's immune function, resulting in abnormalities in the body's immune defense system, various types of inflammation, and a decrease in the ability of white blood cells to act, producing large amounts of inflammatory factors.
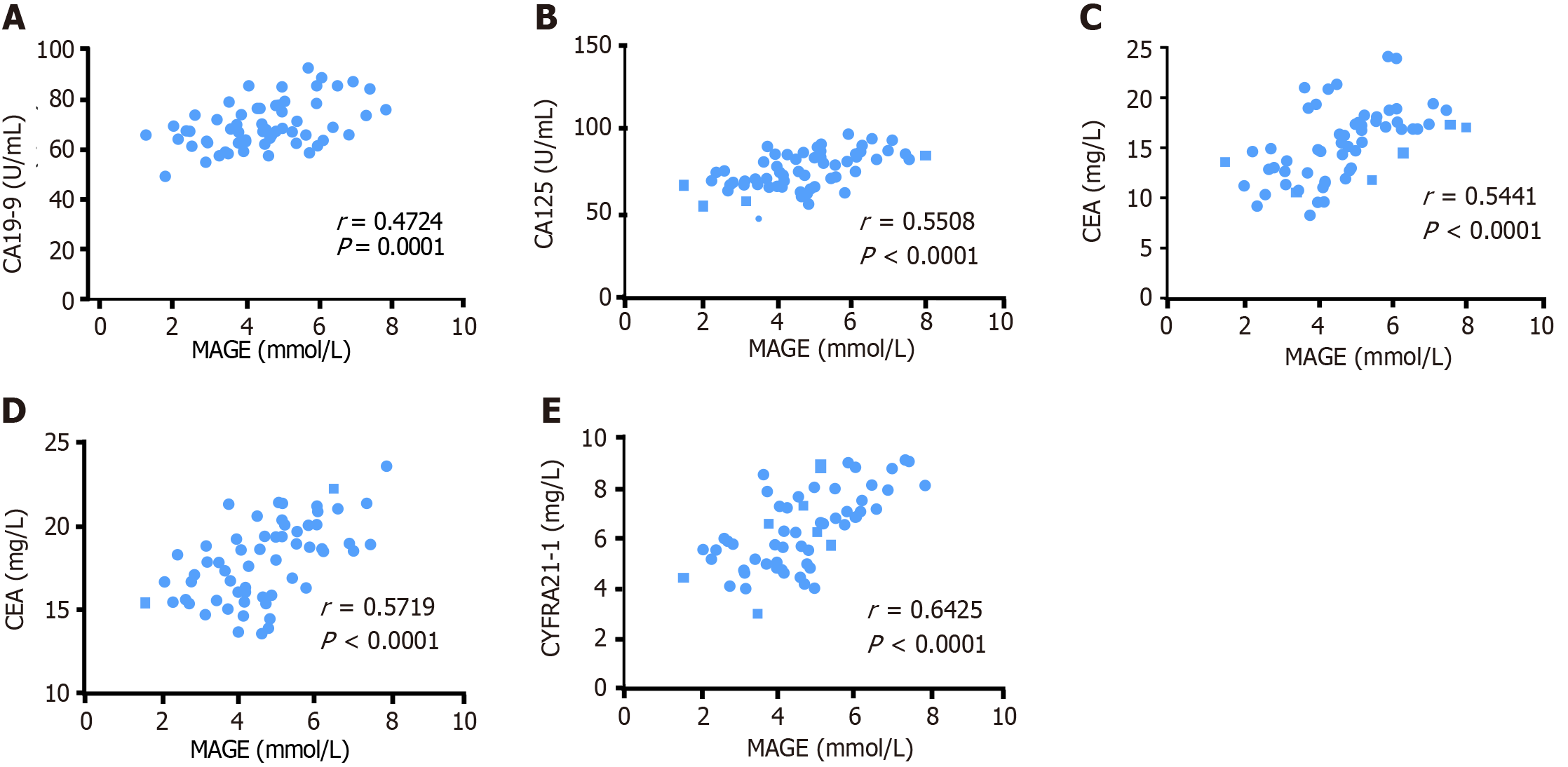
According to Pearson analysis (Figure 3), MAGE was positively associated with serum CA19-9 (r = 0.4724, P = 0.0001), CA125 (r = 0.5508, P < 0.0001), CEA (r = 0.5441, P < 0.0001), NSE (r = 0.5719, P < 0.0001), and CYFRA21-1 (r = 0.6425, P < 0.0001).
The incidence of phlebitis, gastrointestinal reactions, oral ulcers, and liver and kidney dysfunction in the OG was 33.3%, 50.0%, 36.7%, and 26.7%, respectively, the values of which were significantly higher than those in the CG (10.0%, 20.0%, 13.3%, and 6.7%, respectively) (P < 0.05; Table 3). These results indicated that hyperglycemia can also cause adverse reactions such as gastrointestinal reactions, liver damage, kidney damage, and oral ulcers, resulting in reduced safety.
| Phlebitis | Gastrointestinal reactions | Oral ulcers | Liver and kidney dysfunction | |
| Control group (n = 30) | 3 (10.0) | 6 (20.0) | 4 (13.3) | 2 (6.7) |
| Observation group (n = 30) | 10 (33.3) | 15 (50.0) | 11 (36.7) | 8 (26.7) |
| t value | 4.8121 | 5.9341 | 4.3561 | 4.3201 |
| P value | 0.0283 | 0.0149 | 0.0369 | 0.0377 |
There are abnormalities in insulin secretion in patients with T2DM, which leads to a compensatory increase in insulin and a gradual increase in insulin content in blood[22]. Research has linked the occurrence of LC to the specific and nonspecific immunity of diabetic patients, so many experts believe that T2DM will lead to an increase in the incidence of LC[23]. At the same time, in clinical LC research, the frequency of comorbidities in LC patients is very high, of which DM is the most common[24]. During chemotherapy for T2DM with LC, poor glycemic control is often associated with a more clinically aggressive cancer course and occurrence of adverse events such as neutropenia, infection, and death[25,26]. However, chemotherapy itself can lead to abnormal glycolipid metabolism in cancer patients[27]. Glucose metabolism disorders occur after chemotherapy, leading to a significant increase in blood glucose values and even diabetes. It will suspend chemotherapy and affect the quality of life of patients[28]. Therefore, the control of BG fluctuations occupies an important position in T2DM + LC patients undergoing chemotherapy, which can affect the curative effect of chemotherapy.
BG fluctuations refer to the amplitude of glycemic excursions between the highest and the lowest BG levels over time. There are also BG fluctuations in healthy people, but the amplitude is small, being mostly due to invalid fluctuations[29]. Abnormal BG fluctuations in diabetic patients can easily aggravate abnormalities in islet B cells, which affects physiological processes such as insulin cell apoptosis and causes abnormal endothelial cell proliferation, triggering vascular endothelial dysfunction[30]. MAGE is a "golden indicator" reflecting BG fluctuations[31]. In this study, the patients were assigned to normal (CG) and high (OG) BG fluctuation groups according to BG fluctuation amplitude. The results showed that MAGE, MODD, LAGE, SDBG, FBG and 2hPG in the OG were significantly higher than those in the CG. High-level BG indices indicate the provision of sufficient nutrients and other necessary conditions for the infinite division and proliferation of tumor cells. BG fluctuation measurements in NSCLC patients showed that patients with large glycemic variability and high BG levels had poor prognosis and increased mortality and disability[32]. Also, Hyperglycemia reduces the response to chemotherapeutic drugs, directly affects tumor cell growth, and induces drug resistance in tumor cells[18]. Meanwhile, long-term abnormal BG fluctuations or hyperglycemia in LC patients can aggravate the degree of oxidative stress in vivo, activate the protein kinase C pathway, and further promote vascular endothelial cell apoptosis and endothelial cell DNA oxidative damage, leading to an increase in tumor markers such as CA128, CA19-9 and CEA. In this study, markedly higher CA19-9, CA125, CEA, NSE and CYFRA21-1 Levels were determined in the OG than in the CG. In addition, the levels of inflammatory factors in the OG also increased significantly. Of these, hs-CRP is an acute-phase protein, and its level can be significantly increased when the body is disturbed by inflammation[33]. TNF-α is an important inflammatory index closely related to vascular endothelial injury and coagulation state[34]. The decrease in insulin secretion in T2DM + LC patients can lead to increased levels of insulin antibodies and abnormal metabolism of proteins, fats and sugars, increasing blood viscosity and BG, causing microcirculation disorder and affecting tissue defense function. As such, the body's humoral and cellular immunity is reduced, leukocyte function is weakened, and inflammatory reactions are induced, damaging the pulmonary vascular barrier, generating oxygen free radicals, and accelerating cancer cell proliferation[35]. Through Pearson correlation analysis, MAGE was determined to be positively correlated with serum tumor markers, further confirming the influence of BG fluctuation amplitude on the chemotherapy efficacy of patients. Finally, we observed the adverse reactions of both groups of patients. Long-term hyperglycemia in the body will also promote the generation of oxidative stress, which will lead to gastrointestinal reactions, liver damage, kidney damage and oral ulcers, resulting in reduced safety. The results also confirmed an obviously higher incidence of adverse reactions in patients with larger BG fluctuations than in patients with normal BG fluctuations. Therefore, it is of great importance to control hyperglycemia in cancer patients to control disease progression.
However, this study still has some limitations. Limitations of this study include the small clinical sample size and retrospective nature, so case selection bias may be encountered. Also, survival information after chemotherapy in oncology patients at risk for high magnitude of glycemic fluctuations has not been analyzed, and further research is needed to investigate the relationship between glycemic control and adverse outcomes. Thus, a multiple center, large sample size and prospective study is need to further investigate the relationship between blood glucose levels and cancer treatment efficacy.
Taken together, large BG fluctuations can enhance the levels of tumor markers and inflammatory factors in T2DM + LC patients and inhibit chemotherapy efficacy, with low safety. Therefore, in such patients, the BG indicators should be strictly controlled clinically to ensure prognosis.
Lung carcinoma (LC) is the most common malignant tumor in clinical practice that seriously harms human health. Diabetes mellitus (DM) is a common chronic metabolic disorder, with type 2 DM (T2DM) being the most prevalent. The prevalence of DM complicated with LC is increasing worldwide.
Blood glucose (BG) fluctuation is definitely a factor affecting the chemotherapy efficacy and prognosis of patients with DM complicated with cancer. However, whether the BG level can be a predictor of chemotherapy efficacy in LC patients has rarely been studied, and epidemiological evidence is limited.
This study focuses on the influence of BG fluctuations on chemotherapy efficacy in T2DM + LC patients.
The clinical data of 60 T2DM + LC patients were retrospectively analyzed. All patients underwent chemotherapy and were grouped as a control group and an observation group based on their BG fluctuations, with 30 cases each. BG-related indices, tumor markers, serum inflammatory cytokines and adverse reactions were comparatively analyzed.
After chemotherapy, fasting BG and 2-h postprandial BG in the observation group were significantly higher than those in the control group. In addition, the observation group exhibited markedly higher mean amplitude of glycemic excursions, mean of daily differences, largest amplitude of glycemic excursions, and standard deviation of BG than the control group patients with high fluctuating blood glucose ranges have relatively weaker glycemic control and greater blood glucose fluctuations after chemotherapy. The observation group has higher levels of tumor markers and inflammatory indicators than the control group, as well as adverse event rate.
Large BG fluctuations can enhance the levels of tumor markers and inflammatory factors in T2DM + LC patients and inhibit chemotherapy efficacy, with low safety.
The control of BG fluctuations occupies an important position in T2DM + LC patients undergoing chemotherapy, which can affect the curative effect of chemotherapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Groop PH, Finland; Lickert H, Germany S-Editor: Wang JL L-Editor: A P-Editor: Guo X
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9795] [Article Influence: 4897.5] [Reference Citation Analysis (1)] |
| 2. | Maomao C, He L, Dianqin S, Siyi H, Xinxin Y, Fan Y, Shaoli Z, Changfa X, Lin L, Ji P, Wanqing C. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. 2022;19:1121-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 150] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 3. | Suryasa I W, Rodríguez-Gámez M, Koldoris T. Health and treatment of diabetes mellitus. Int J Health Sci. 2021;5. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med. 2020;41:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 1149] [Article Influence: 229.8] [Reference Citation Analysis (0)] |
| 5. | Luo J, Hendryx M, Qi L, Ho GY, Margolis KL. Pre-existing diabetes and lung cancer prognosis. Br J Cancer. 2016;115:76-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Khateeb J, Fuchs E, Khamaisi M. Diabetes and Lung Disease: A Neglected Relationship. Rev Diabet Stud. 2019;15:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Leiter A, Charokopos A, Bailey S, Gallagher EJ, Hirsch FR, LeRoith D, Wisnivesky JP. Assessing the association of diabetes with lung cancer risk. Transl Lung Cancer Res. 2021;10:4200-4208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Sheikh M, Mukeriya A, Shangina O, Brennan P, Zaridze D. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality : A Prospective Cohort Study. Ann Intern Med. 2021;174:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Chang SA. Smoking and type 2 diabetes mellitus. Diabetes Metab J. 2012;36:399-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Hatlen P, Grønberg BH, Langhammer A, Carlsen SM, Amundsen T. Prolonged survival in patients with lung cancer with diabetes mellitus. J Thorac Oncol. 2011;6:1810-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8100] [Article Influence: 506.3] [Reference Citation Analysis (2)] |
| 12. | Szablewski L. Diabetes mellitus: influences on cancer risk. Diabetes Metab Res Rev. 2014;30:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Li X, Fang H, Zhang D, Xia L, Wang X, Yang J, Zhang S, Su Y, Zhu Y. Long-term survival analysis of patients with stage IIIB-IV non-small cell lung cancer complicated by type 2 diabetes mellitus: A retrospective propensity score matching analysis. Thorac Cancer. 2022;13:3268-3273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care. 2010;33:931-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754-2764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 693] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 16. | Yeh HC, Platz EA, Wang NY, Visvanathan K, Helzlsouer KJ, Brancati FL. A prospective study of the associations between treated diabetes and cancer outcomes. Diabetes Care. 2012;35:113-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Tan BX, Yao WX, Ge J, Peng XC, Du XB, Zhang R, Yao B, Xie K, Li LH, Dong H, Gao F, Zhao F, Hou JM, Su JM, Liu JY. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117:5103-5111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Hershey DS, Bryant AL, Olausson J, Davis ED, Brady VJ, Hammer M. Hyperglycemic-inducing neoadjuvant agents used in treatment of solid tumors: a review of the literature. Oncol Nurs Forum. 2014;41:E343-E354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Hammer MJ, Voss JG. Malglycemia and cancer: introduction to a conceptual model. Oncol Nurs Forum. 2012;39:E275-E287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Brunello A, Kapoor R, Extermann M. Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol. 2011;34:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120:1986-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 22. | Kim MJ, Lee EY, You YH, Yang HK, Yoon KH, Kim JW. Generation of iPSC-derived insulin-producing cells from patients with type 1 and type 2 diabetes compared with healthy control. Stem Cell Res. 2020;48:101958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18:525-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 441] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 24. | Leduc C, Antoni D, Charloux A, Falcoz PE, Quoix E. Comorbidities in the management of patients with lung cancer. Eur Respir J. 2017;49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Fuji S, Kim SW, Mori S, Fukuda T, Kamiya S, Yamasaki S, Morita-Hoshi Y, Ohara-Waki F, Honda O, Kuwahara S, Tanosaki R, Heike Y, Tobinai K, Takaue Y. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation. 2007;84:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol. 2003;157:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Qi A, Li Y, Yan S, Sun H, Zhao M, Chen Y. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg. 2021;10:1470-1477. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Yang J, Jia B, Qiao Y, Chen W, Qi X. Variations of blood glucose in cancer patients during chemotherapy. Niger J Clin Pract. 2016;19:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Zhang ZY, Miao LF, Qian LL, Wang N, Qi MM, Zhang YM, Dang SP, Wu Y, Wang RX. Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications. Front Endocrinol (Lausanne). 2019;10:640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Juan-Mateu J, Bajew S, Miret-Cuesta M, Íñiguez LP, Lopez-Pascual A, Bonnal S, Atla G, Bonàs-Guarch S, Ferrer J, Valcárcel J, Irimia M. Pancreatic microexons regulate islet function and glucose homeostasis. Nat Metab. 2023;5:219-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Wang BR, Yao JT, Zheng H, Li QM. Association of Glycated Albumin/Glycosylated Hemoglobin Ratio with Blood Glucose Fluctuation and Long-Term Blood Glucose Control in Patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2021;14:1809-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Luo J, Chen YJ, Chang LJ. Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012;76:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Liu X, Guo X, Zhang Z. Preoperative Serum Hypersensitive-c-Reactive-Protein (Hs-CRP) to Albumin Ratio Predicts Survival in Patients with Luminal B Subtype Breast Cancer. Onco Targets Ther. 2021;14:4137-4148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Tan Z, Xue H, Sun Y, Zhang C, Song Y, Qi Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front Pharmacol. 2021;12:688625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 35. | Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, Gong Z, Gao J, Mu Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. 2019;10:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |