Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.629
Peer-review started: October 30, 2023
First decision: December 12, 2023
Revised: December 28, 2023
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: April 15, 2024
Processing time: 164 Days and 19.5 Hours
Diabetic foot (DMF) complications are common and are increasing in incidence. Risk factors related to wound complications are yet to be established after trans-tibial amputation under the diagnosis of DMF infection.
To analyze the prognosis and risk factors related to wound complications after transtibial amputation in patients with diabetes.
This retrospective cohort study included seventy-two patients with DMF complications who underwent transtibial amputation between April 2014 and March 2023. The groups were categorized based on the occurrence of wound complications, and we compared demographic data between the complication group and the non-complication group to analyze risk factors. Moreover, a multivariate logistic regression analysis was performed to identify risk factors.
The average follow-up period was 36.2 months. Among the 72 cases, 31 (43.1%) had wound complications. Of these, 12 cases (16.7%) received further treatment, such as debridement, soft tissue stump revision, and re-amputation at the proximal level. In a group that required further management due to wound complications after transtibial amputation, the hemoglobin A1c (HbA1c) level was 9.32, while the other group that did not require any treatment had a 7.54 HbA1c level. The prevalence of a history of kidney transplantation with wound complications after transtibial amputation surgery in DMF patients was significantly greater than in cases without wound complications (P = 0.02). Other factors did not show significant differences.
Approximately 43.1% of the patients with transtibial amputation surgery experienced wound complications, and 16.7% required additional surgical treatment. High HbA1c levels and kidney transplant history are risk factors for postoperative wound complications.
Core Tip: In this study, 43.1% of the patients with transtibial amputation surgery experienced wound complications, and 16.7% necessitated additional wound revision procedures, such as debridement. High hemoglobin A1c (HbA1c) levels (HbA1c > 7.2) and kidney transplant history are risk factors for postoperative wound complications.
- Citation: Park YU, Eim SH, Seo YW. Prevalence and risk factors of wound complications after transtibial amputation in patients with diabetic foot. World J Diabetes 2024; 15(4): 629-637
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/629.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.629
The World Health Organization reported that the estimated number of patients with diabetes reached nearly 425 million in 2017. Unsurprisingly, this led to an increasing number of diabetes-related complications[1]. Diabetic foot (DMF) is one of the most devastating, if not the most critical, complications of diabetes mellitus (DM)[2]. DM foot infection complications range from 10% to 25% throughout the lifetime of one DM patient[3]. It is also the main reason for non-traumatic lower extremity amputations[4]. Risk factors for amputation include smoking history, kidney transplantation history, high sugar levels, hyperlipidemia, and ischemia[5,6]. Amputation can be subdivided into minor and major amputations, and transtibial amputations are regarded as major. Post-operative 30-d mortality rates have been reported to be 6%-17%; the 1-year mortality rate after major amputation is 69.7%; and the 5-year mortality rate is 34.7%[7-9]. Thorud et al[10] reported the overall 5-year mortality rate to be very high among patients with any amputation, ranging from 53% to 100% and from 52% to 80% for patients with major amputations[10].
Major amputation is considered the final therapeutic option; nevertheless, after major amputation, wound complications may persist, necessitating further surgical interventions. Prognosis and risk factors related to wound complications have yet to be established after transtibial amputation under the diagnosis of DMF complications. The purpose of this study was to analyze the prognosis and risk factors related to wound complications after transtibial amputation in patients with diabetes.
This study was approved by the Institutional Review Board of Ajou University School of Medicine, Suwon, South Korea. Seventy-two patients with DMF infection underwent transtibial amputations between April 2014 and March 2023. The medical records and photographs stored in Picture Archiving and Communication System (PACS) were analyzed to ascertain the presence of wound complications and to categorize the types of wound complications, all of which were then meticulously documented. The Size (area, depth), Sepsis, Arteriopathy, and Denervation system was introduced in 1999 and is primarily designed for clinical audits[11]. The system was initially validated in 2004, and to enhance the classification of ulcers for prospective research, certain criteria that were absent in the UT system were subsequently incorporated[12]. In this study, the criteria for necrosis and infection were defined as grade 2 or higher based on the previously published guidelines. The criterion for wound necrosis was defined as wound necrosis over 1 cm2, and wound infection was defined as suspected local infection, such as pus discharge or cellulitis over 1 cm2[12,13].
Patient details were obtained by analyzing documented electronic medical records and test results. These details included body mass index (BMI), smoking history, kidney transplantation history, dialysis therapy history, lower extremity endovascular intervention history, previous amputation at the same extremity, and the need for stump revision surgery during follow-up period. Additionally, pre-operative erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and hemoglobin A1c (HbA1c) results were collected.
The occurrence of postoperative wound complications (infection, necrosis, etc.) after transtibial amputation surgery was classified into two groups and the contribution of each risk factor was analyzed. Group 1 was defined as cases without wound complications after transtibial amputation surgery in DMF patients, and Group 2 was defined as cases with wound complications.
All statistical analyses were performed using SPSS for Windows, version 22.0 (IBM Corporation, Armonk, NY, United States). Univariate and multivariate logistic regression analyses were performed to identify risk factors. Statistical significance was set at P < 0.05. The optimal HbA1c cutoff for postoperative wound complications was calculated using the receiver operating characteristic (ROC) curve.
A significant sample size was calculated by the Muller and Buttner method, and Walter et al[14] G*Power version 3.1.9.7 software (Franz Faul, Universität Kiel, Germany) was utilized for the calculation, and the sample size was analyzed by setting the odds ratio (OR) to 20.47, R2 other X to 0.25 (moderate association), alpha error probability to 0.05, and power to 0.80[15,16].
Seventy-two patients with DMF complications underwent transtibial amputation between April 2014 and March 2023. Of the 72 patients, 48 (66.7%) were male patients and 24 (33.3%) were female patients. The average age was 64.1 years (39-87), the average BMI was 22.6 (14.3-34.3), and the average period of DM was 18.9 years. Right-side surgery was performed in 30 cases (41.7%), left-side surgery was performed in 39 cases (54.2%), and bilateral surgery was performed in 3 cases (4.2%). Regarding renal function, 43 cases (59.7%) did not undergo dialysis, 19 cases (26.4%) underwent dialysis, and 11 cases (15.3%) received kidney transplants. A total of 19 patients smoked (26.4%). Concerning the history of DMF amputation before this transtibial amputation surgery, there were 19 cases (26.4%) wherein minor amputation (below hindfoot level) was done on the same side, the ankle and hindfoot level amputation cases were 4 (5.6%), the opposite transtibial amputation cases were 6 (8.3%), and the opposite minor amputation cases were 6 (8.3%) (Table 1).
| Group 1 (n = 41) | Group 2 (n = 31) | P value | ||
| Age (years), mean ± SD | 0.66 | |||
| 66.0 (9.9) | 61.4 (10.9) | |||
| Median (Q1 to Q3) | 65.0 (59.0 to 74.0) | 61 (56.0 to 66.5) | ||
| Sex | 0.93 | |||
| Female | 13 (31.7) | 11 (35.5) | ||
| Male | 28 (68.3) | 20 (64.5) | ||
| BMI, mean ± SD | 0.64 | |||
| 22.4 (3.8) | 22.8 (3.8) | |||
| Median (Q1 to Q3) | 22.5 (19.5 to 24.4) | 22.2 (19.5 to 25.7) | ||
| Location | 0.92 | |||
| Right | 16 (39.0) | 14 (45.2) | ||
| Left | 23 (56.1) | 16 (51.6) | ||
| Both | 2 (4.9) | 1 (3.2) | ||
| Dialysis | 0.86 | |||
| No | 31 (75.6) | 22 (71.0) | ||
| Yes | 10 (24.4) | 9 (29.0) | ||
| Kidney transplant history | 0.02 | |||
| No | 40 (97.6) | 21 (67.7) | ||
| Yes | 1 (2.4) | 10 (32.3) | ||
| Smoking | 0.71 | |||
| No | 29 (70.7) | 24 (77.4) | ||
| Yes | 12 (29.3) | 7 (22.6) | ||
| Amputation history | 0.84 | |||
| No | 22 (53.7) | 15 (48.4) | ||
| Yes | 19 (46.3) | 16 (51.6) | ||
| HTN | 0.06 | |||
| No | 17 (41.5) | 4 (12.9) | ||
| Yes | 24 (58.5) | 27 (87.1) | ||
| Endovascular intervention history | 0.38 | |||
| No | 29 (70.7) | 18 (58.1) | ||
| Yes | 12 (29.3) | 13 (41.9) | ||
| HbA1 | 7.54 | 9.32 | 0.01 | |
| HbA1c ≥ 7.2 | 0.01 | |||
| No | 18 (43.9) | 4 (12.9) | ||
| Yes | 23 (56.1) | 27 (87.1) |
The average follow-up period was 36.2 months (confidence interval: 8-72 months). Among the 72 cases, 12 cases (16.7%) were performed with additional wound management (stump revision = 11 cases; transfemoral amputation = 1 case). In 12 cases, wound healing did not progress satisfactorily with a simple dressing alone, necessitating daily debridement to address infection or necrotic tissue. Some cases had to be followed up with delayed suturing after improvement. Among them, one case exhibited infection and soft tissue necrosis extending up to the knee joint, leading to transfemoral amputation. The remaining 11 cases were discharged after achieving wound stabilization and receiving outpatient follow-up observations.
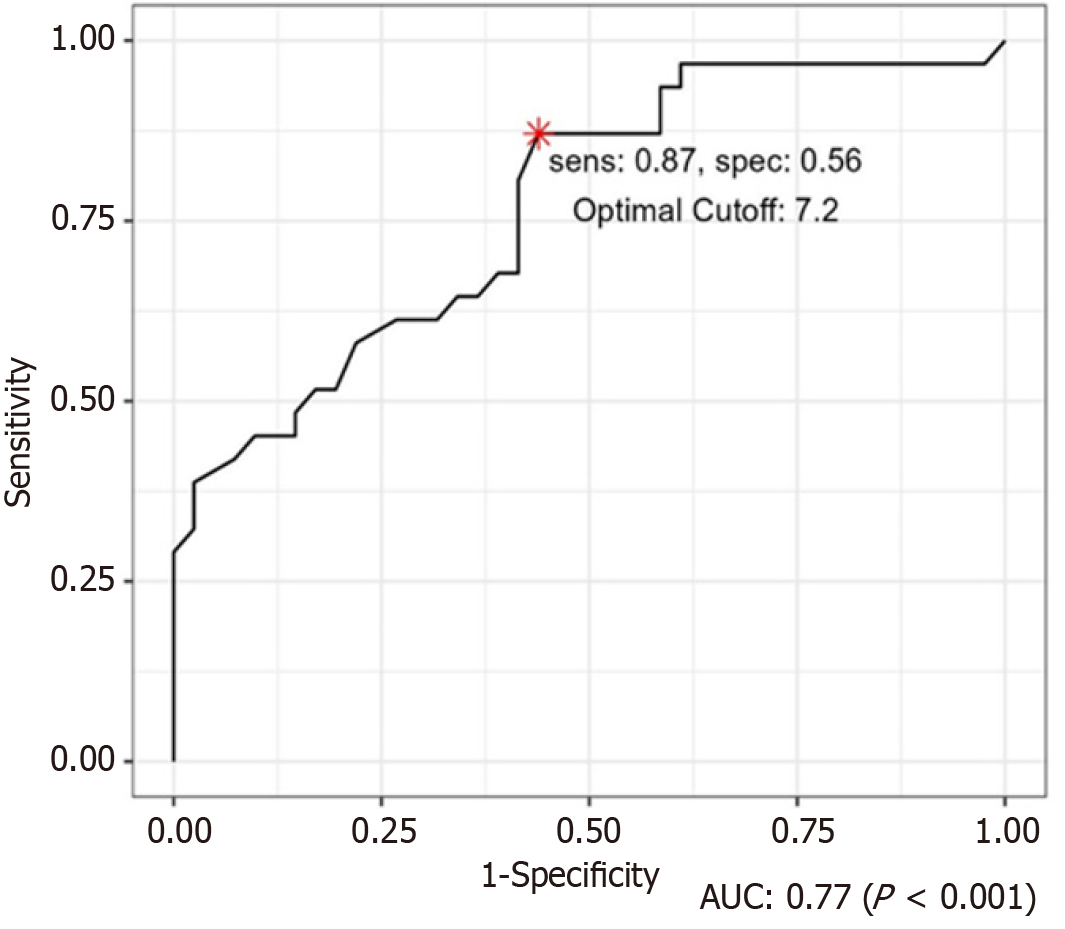
Compared with HbA1c level of Group 1 (7.54), the HbA1c level of Group 2 (9.32) was significantly higher (P = 0.01). The optimal HbA1c cutoff for postoperative wound complications was calculated using the ROC curve, and the result was an HbA1c of 7.2 (Figure 1). In cases with HbA1c levels greater than or equal to 7.2, the probability of postoperative wound complications was 31.28 times higher than in those with lower levels (P < 0.01). The prevalence of a history of kidney transplantation in Group 2 was significantly greater than that in Group 1 (P = 0.02) (OR: 26.22) (Table 2).
| Univariable | Multivariable_all | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age (years) | 0.96 (0.91-1.00) | 0.066 | 1.01 (0.93-1.09) | 0.866 | |
| Sex | |||||
| Female | - | - | |||
| Male | 0.84 (0.31-2.29) | 0.737 | 0.53 (0.08-2.92) | 0.476 | |
| BMI | 1.03 (0.91-1.17) | 0.630 | 1.10 (0.87-1.45) | 0.439 | |
| Location | |||||
| Right | - | - | |||
| Left | 0.80 (0.30-2.08) | 0.640 | 0.62 (0.14-2.65) | 0.525 | |
| Both | 0.57 (0.02-6.60) | 0.662 | 0.11 (0.00-3.08) | 0.239 | |
| Kidney transplant history | |||||
| No | - | - | |||
| Yes | 19.05 (3.32-361.72) | 0.006a | 26.22 (2.36-811.46) | 0.020 | |
| Smoking | |||||
| No | - | - | |||
| Yes | 0.70 (0.23-2.04) | 0.525 | 0.26 (0.04-1.52) | 0.150 | |
| ICU | |||||
| No | - | - | |||
| Yes | 0.64 (0.23-1.71) | 0.379 | 0.20 (0.03-1.09) | 0.085 | |
| HTN | |||||
| No | - | - | |||
| Yes | 4.78 (1.52-18.42) | 0.012 | 6.19 (1.20-44.22) | 0.054 | |
| Endovascular intervention history | |||||
| No | - | - | |||
| Yes | 1.75 (0.66-4.72) | 0.266 | 2.86 (0.63-16.12) | 0.193 | |
| HbA1c ≥ 7.2 | |||||
| No | - | - | |||
| Yes | 5.28 (1.69-20.33) | 0.007a | 31.28 (5.04-355.9) | 0.001 | |
In Group 2, the HbA1c level was significantly higher at 8.77 than the HbA1c level of 7.07 in Group 1 (P = 0.01) (OR: 29.65). The prevalence of a history of kidney transplantation in Group 2 (33.3%) was significantly higher compared to Group 1 (11.7%) (P = 0.03) (OR: 21.24).
No statistically significant difference was observed in the ratio of dialysis in the group comparison related to additional surgery or treatment. Other factors, including CRP, culture results, DM morbidity period, smoking history, and previous amputation history, did not display significant differences.
The most important finding of this study is that postoperative wound complications after transtibial amputation are relatively common, thus requiring close observation. Several of these complications lead to the necessity of wound revisions, emphasizing the significance for physicians to acknowledge this aspect and engage in proactive discussions regarding the potential course of the condition with patients.
In this study, 30-d mortality after transtibial amputation was 5 out of 72 cases (6.9%), and 3-year mortality after transtibial amputation was 14 out of 40 cases (37.5%). Previous studies have also reported the survival rates after transtibial amputation surgery. The range of mortality after transtibial amputation ranged from 40% to 82%, and transtibial amputation ranged from 40% to 90%. The 30-d mortality after major amputations appeared to range from about 5.5% to 13.3%[17-19]. Overall, the 5-year mortality rate was very high among patients with any amputation, ranging from 53 to 100% and from 52% to 80% for patients with major amputations[10]. Increased 5-year mortality was related to old age and kidney function[10,19].
However, there is a lack of reported studies on wound prognosis after transtibial amputation surgery. Among the 72 cases, 31 (43.1%) had wound complications (infection = 8 cases, necrosis = 19 cases, infection and necrosis = 4 cases) in this study. Among the 72 cases, 12 cases (16.7%) were performed with additional wound management. There were 11 cases of stump revision and 1 case of additional surgery with transfemoral amputation (above-knee amputation). The reasons for wound revision were infection in 4 cases (33.3%), necrosis in 1 case (8.4%), and infection with necrosis occurring concomitantly in 7 cases (58.3%). Since wound complications requiring wound revision may occur in 16.7% of patients after transtibial amputation surgery, it is important to fully explain the progress to the patient before transtibial amputation surgery. In addition, proper wound management after surgery is important, which is thought to increase the length of hospital stay and the subsequent occurrence of other complications such as pneumonia. However, additional major amputation was performed in 1 case out of 72 (1.3%), so wound recovery can be expected through appropriate wound management. Various risk factors related to DMF ulcers and DMF amputations are known[1,20-25]. Cervantes-García and Salazar-Schettino[20] found that males and smoking history, which were indicated as risk factors for amputation in diabetes-related foot ulceration, were also identified as risk factors for amputation in DMF infection in a meta-analysis[20]. Diabetic complications, including peripheral Arterial Disease, peripheral neuropathy, nephropathy, and severe infection, were identified as major causes of amputation[1,20,21]. However, Sen et al[23] reported that DM neuropathy was not associated with DMF amputation risk factors. Although nephropathy is important in the development of DMF ulcers, this complication was not found in this meta-analysis to be the cause of amputation in patients with DMF infection (DFI)[22,23]. Based on studies conducted by Aziz et al[24] and Shojaiefard et al[25], elevated average leukocytosis, CRP, ESR, HbA1c levels, and hyperglycemia have been identified as risk factors for amputation. Park et al[26] reported that high glucose levels (> 300 mg/dL) and hypotension at admission are identified as inde-pendent risk factors for limb loss in patients with necrotizing fasciitis.
Furthermore, there is limited understanding of the risk factors associated with wound complications following transtibial amputation surgery. Various risk factors were analyzed in this study. In this study, high HbA1c and kidney transplantation history were analyzed as risk factors for postoperative wound complications after transtibial amputation surgery. Sinacore reported that wound healing is delayed due to the use of immunosuppressive agents after trans-plantation[27]. Seo et al[28] reported that post-pancreas transplantation, 6.9% of individuals developed DMF ulcers, and 3.2% developed Charcot arthropathy. A study by Sharma et al[29], involving 235 kidney transplant patients, revealed a 15% incidence of DMF ulcers. In their multivariate analysis, Uçkay et al[30] observed that the presence of chronic, enhanced immune suppression, compared to its absence, is linked with an increased likelihood of clinical failures in DFI cases, indicated by a hazard ratio of 1.5 and a 95% confidence interval ranging from 1.1 to 2.0. This suggests that a consistently elevated level of immune suppression may act as an independent factor increasing the risk of unsuccessful treatment outcomes in DFI[30]. According to Huang et al[31], the wound healing process in DMF patients as intricate, involving factors such as elevated blood sugar levels, reduced blood flow, low oxygen levels, heightened inflammatory response, and ongoing infections. Based on these prior studies, this study also identifies risk factors for wound complications associated with impaired wound healing, such as immunosuppressive agent usage following kidney trans-plantation. No significant differences (P > 0.05) were found in age, gender, duration of diabetes, BMI, smoking status, whether the patient received treatment in the intensive care unit, or whether they underwent dialysis as risk factors for surgical wound complications.
The present study had limitations. There is no research conducted on the correlation between risk factor adjustment and a reduction in the occurrence of complications. Further research is required to investigate this matter. This study is retrospective in design and carries inherent limitations when compared to prospective studies. Nevertheless, within this study, clinical photographs were serially captured for all patients before and after surgery, as well as during each wound management, and these images were stored in the PACS for documentation. This approach facilitated a precise evaluation of complication presence and wound status, utilizing not only medical records but also PACS clinical photos. Due to the analysis of actual wound status using PACS, which could be missed in medical records, this study concludes that the high incidence of wound complications after transtibial amputation surgery is the reason for its observation.
In this study, 43.1% of the patients with transtibial amputation surgery experienced wound complications, and 16.7% necessitated additional wound revision procedures, such as debridement. High HbA1c levels (HbA1c > 7.2) and kidney transplant history are risk factors for postoperative wound complications.
Diabetic foot (DMF) complications are common and are increasing in incidence. Risk factors related to wound complications are yet to be established after transtibial amputation under the diagnosis of DMF infection.
The purpose of this study was to analyze the prognosis and risk factors related to wound complications after transtibial amputation in patients with diabetes.
Having knowledge of the research findings on the prevalence and risk factors of wound complications after transtibial amputation in patients with DMF, we can utilize this information in a clinical setting for purposes such as predicting patient outcomes and providing explanations to patients.
Seventy-two patients with DMF infection underwent transtibial amputations between April 2014 and March 2023. The medical records and photographs stored in Picture Archiving and Communication System were analyzed to ascertain the presence of wound complications and to categorize the types of wound complications. The occurrence of postoperative wound complications after transtibial amputation surgery was classified into two groups and the contribution of each risk factor was analyzed. Group 1 was defined as cases without wound complications after transtibial amputation surgery in DMF patients, and Group 2 was defined as cases with wound complications.
Among the 72 cases, 12 cases (16.7%) were performed with additional wound management (stump revision = 11 cases; transfemoral amputation = 1 case). In 12 cases, wound healing did not progress satisfactorily with a simple dressing alone, necessitating daily debridement to address infection or necrotic tissue. Compared with hemoglobin A1c (HbA1c) level of Group 1 (7.54), the HbA1c level of Group 2 (9.32) was significantly higher (P = 0.01). The optimal HbA1c cutoff for postoperative wound complications was calculated using the receiver operating characteristic curve, and the result was an HbA1c of 7.2. The prevalence of a history of kidney transplantation in Group 2 was significantly greater than that in Group 1 (P = 0.02) In Group 2, the HbA1c level was significantly higher at 8.77 than the HbA1c level of 7.07 in Group 1 (P = 0.01) [odds ratio (OR): 29.65]. The prevalence of a history of kidney transplantation in Group 2 (33.3%) was significantly higher compared to Group 1 (11.7%) (P = 0.03) (OR: 21.24).
In this study, 43.1% of the patients with transtibial amputation surgery experienced wound complications, and 16.7% necessitated additional wound revision procedures. High HbA1c levels (HbA1c > 7.2) and kidney transplant history are risk factors for postoperative wound complications.
No research has been conducted yet on the correlation between adjusting risk factors and reducing complications, highlighting the need for future studies in this area.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lu X, China S-Editor: Fan JR L-Editor: A P-Editor: Guo X
| 1. | Shin JY, Roh SG, Sharaf B, Lee NH. Risk of major limb amputation in diabetic foot ulcer and accompanying disease: A meta-analysis. J Plast Reconstr Aesthet Surg. 2017;70:1681-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV; American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45:S1-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 469] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 4. | Calle-Pascual AL, Redondo MJ, Ballesteros M, Martinez-Salinas MA, Diaz JA, De Matias P, Calle JR, Gil E, Jimenez M, Serrano FJ, Martin-Alvarez PJ, Maranes JP. Nontraumatic lower extremity amputations in diabetic and non-diabetic subjects in Madrid, Spain. Diabetes Metab. 1997;23:519-523. [PubMed] |
| 5. | Van Olmen J, Marie KG, Christian D, Clovis KJ, Emery B, Maurits VP, Heang H, Kristien VA, Natalie E, François S, Guy K. Content, participants and outcomes of three diabetes care programmes in three low and middle income countries. Prim Care Diabetes. 2015;9:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 6. | Markowitz JS, Gutterman EM, Magee G, Margolis DJ. Risk of amputation in patients with diabetic foot ulcers: a claims-based study. Wound Repair Regen. 2006;14:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Nelson MT, Greenblatt DY, Soma G, Rajimanickam V, Greenberg CC, Kent KC. Preoperative factors predict mortality after major lower-extremity amputation. Surgery. 2012;152:685-94; discussion 694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Aulivola B, Hile CN, Hamdan AD, Sheahan MG, Veraldi JR, Skillman JJ, Campbell DR, Scovell SD, LoGerfo FW, Pomposelli FB Jr. Major lower extremity amputation: outcome of a modern series. Arch Surg. 2004;139:395-9; discussion 399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 323] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality After Nontraumatic Major Amputation Among Patients With Diabetes and Peripheral Vascular Disease: A Systematic Review. J Foot Ankle Surg. 2016;55:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 11. | Macfarlane RM, Jeffcoate WJ. Classification of diabetic foot ulcers: the S (AD) SAD system. Diabet Foot. 1999;2:123-126. |
| 12. | Treece KA, Macfarlane RM, Pound N, Game FL, Jeffcoate WJ. Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med. 2004;21:987-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Wang X, Yuan CX, Xu B, Yu Z. Diabetic foot ulcers: Classification, risk factors and management. World J Diabetes. 2022;13:1049-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (5)] |
| 14. | Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17:101-110. [PubMed] [DOI] [Full Text] |
| 15. | Pemayun TG, Naibaho RM, Novitasari D, Amin N, Minuljo TT. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a hospital-based case-control study. Diabet Foot Ankle. 2015;6:29629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Stern JR, Wong CK, Yerovinkina M, Spindler SJ, See AS, Panjaki S, Loven SL, D'Andrea RF Jr, Nowygrod R. A Meta-analysis of Long-term Mortality and Associated Risk Factors following Lower Extremity Amputation. Ann Vasc Surg. 2017;42:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 17. | Shah SK, Bena JF, Allemang MT, Kelso R, Clair DG, Vargas L, Kashyap VS. Lower extremity amputations: factors associated with mortality or contralateral amputation. Vasc Endovascular Surg. 2013;47:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Scott SW, Bowrey S, Clarke D, Choke E, Bown MJ, Thompson JP. Factors influencing short- and long-term mortality after lower limb amputation. Anaesthesia. 2014;69:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | O'Hare AM, Feinglass J, Reiber GE, Rodriguez RA, Daley J, Khuri S, Henderson WG, Johansen KL. Postoperative mortality after nontraumatic lower extremity amputation in patients with renal insufficiency. J Am Soc Nephrol. 2004;15:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Cervantes-García E, Salazar-Schettino PM. Clinical and surgical characteristics of infected diabetic foot ulcers in a tertiary hospital of Mexico. Diabet Foot Ankle. 2017;8:1367210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Aragón-Sánchez J, Lázaro-Martínez JL, Campillo-Vilorio N, Quintana-Marrero Y, Hernández-Herrero MJ. Controversies regarding radiological changes and variables predicting amputation in a surgical series of diabetic foot osteomyelitis. Foot Ankle Surg. 2012;18:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Quilici MT, Del Fiol Fde S, Vieira AE, Toledo MI. Risk Factors for Foot Amputation in Patients Hospitalized for Diabetic Foot Infection. J Diabetes Res. 2016;2016:8931508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Sen P, Demirdal T, Emir B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab Res Rev. 2019;35:e3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 24. | Aziz Z, Lin WK, Nather A, Huak CY. Predictive factors for lower extremity amputations in diabetic foot infections. Diabet Foot Ankle. 2011;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Shojaiefard A, Khorgami Z, Larijani B. Independent risk factors for amputation in diabetic foot. Int J Diabetes Dev Ctries. 2008;28:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Park HG, Yang JH, Park BH, Yi HS. Necrotizing Soft-Tissue Infections: A Retrospective Review of Predictive Factors for Limb Loss. Clin Orthop Surg. 2022;14:297-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Sinacore DR. Healing times of pedal ulcers in diabetic immunosuppressed patients after transplantation. Arch Phys Med Rehabil. 1999;80:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Seo DK, Lee HS, Park J, Ryu CH, Han DJ, Seo SG. Diabetic Foot Complications Despite Successful Pancreas Transplantation. Foot Ankle Int. 2017;38:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Sharma A, Vas P, Cohen S, Patel T, Thomas S, Fountoulakis N, Karalliedde J. Clinical features and burden of new onset diabetic foot ulcers post simultaneous pancreas kidney transplantation and kidney only transplantation. J Diabetes Complications. 2019;33:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Uçkay I, Schöni M, Berli MC, Niggli F, Noschajew E, Lipsky BA, Waibel FWA. The association of chronic, enhanced immunosuppression with outcomes of diabetic foot infections. Endocrinol Diabetes Metab. 2022;5:e00298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Huang F, Lu X, Yang Y, Li Y, Kuai L, Li B, Dong H, Shi J. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv Sci (Weinh). 2023;10:e2203308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 144] [Article Influence: 72.0] [Reference Citation Analysis (0)] |