Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.575
Peer-review started: November 5, 2023
First decision: January 6, 2024
Revised: January 8, 2024
Accepted: March 1, 2024
Article in press: March 1, 2024
Published online: April 15, 2024
Processing time: 159 Days and 0 Hours
This editorial refers to the article “Comparative analysis of Nε-carboxymethyl-lysine and inflammatory markers in diabetic and non-diabetic coronary artery disease patients”, published in the recent issue of the World Journal of Diabetes 2023 is based on glucose metabolism, advanced glycation end products (AGEs), inflammation and adiposity on diabetes and coronary artery disease (CAD). This study has included CAD patients who were stratified according to glycosylated he
Core Tip: Coronary artery disease (CAD) is associated with 17.8 million deaths annually and nearly 30% have diabetes with insulin resistance. This metabolic disorder increases the circulating glucose levels that allow the non-enzymatic modifications of proteins, lipids, nucleic acids, etc. and form advanced glycation end products (AGEs). Glycosylated hemoglobin is considered a diagnostic marker for diabetes and a risk factor for CAD. However, AGEs through its receptor (RAGE) might increase signal transduction and consequently, inflammatory cytokines, and endothelial dysfunction and be markers and mediators of CAD.
- Citation: Eiras S. Nε-carboxymethyl-lysine and inflammatory cytokines, markers and mediators of coronary artery disease progression in diabetes. World J Diabetes 2024; 15(4): 575-578
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/575.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.575
Cardiovascular disease (CVD) is the major cause of mortality and affects 32% of patients with type 2 diabetes mellitus (T2DM)[1]. This disorder is linked to obesity and a reduction of insulin signaling in cells[2]. Obesity is associated with an increment of stored energy on adipocytes that develop hypertrophy[3] and increase the inflammatory cells' attraction.
Computerized tomography (CT) of coronary arteries with suspected coronary artery disease (CAD) determined an accumulation of adipose tissue around them[4]. However, in patients with diabetes type 1 or 2, this association was not so clear[5]. Recently, artificial intelligence allowed us to find improved predictive models for CAD based on multi-variables (clinical, image, biochemical, etc.) such as epicardial fat quantity, measured by CT, and diabetes. Both factors are CAD risk factors[6]. However, this fat tissue also expresses or releases differential molecules in patients with diabetes[7,8]. The failure of the adipocyte's function enhances circulating glucose levels that modify and reduce proteins, lipids, or nucleic acids in a non-enzymatic reaction[9].
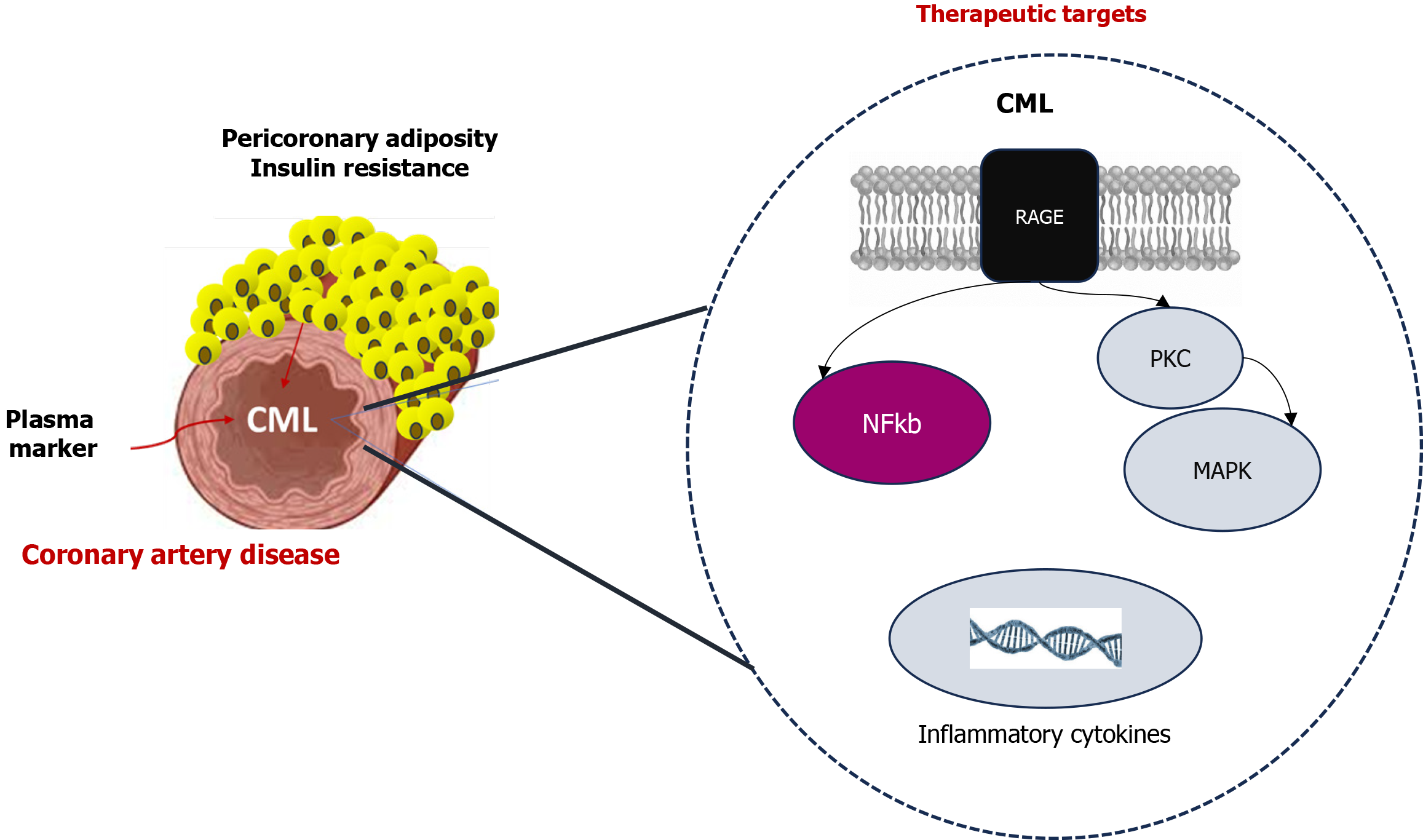
The name of these products is advanced glycation end products (AGEs) and Nε-carboxymethyl-lysine (CML), Nε-carboxyethyl-lysine, pyrraline, crossline, pentosidine, imidazolium cross-link derived from glyoxal and lysine-lysine, and imidazolium cross-link derived from methylglyoxal and lysine-lysine are some of them[10]. CML is one of the most common AGEs and can be processed from food, such as milk, bakery products, and coffee[11]. The study CORDIOPREV showed higher CML levels in those patients with established endothelial dysfunction in comparison with new T2DM[12]. But also circulating levels of AGE were associated with coronary artery calcification[13]. The preclinical atherosclerosis murine models showed that CML might increase the calcification of the plaques through muscle cell effects[14]. The AGE-RAGE signaling can activate secondary messengers (protein kinase C, mitogen-activated protein kinase, and nuclear factor kappa b)[15]. All of them are involved in proliferation or inflammation pathways. But, CML through CD36 can also enhance the macrophage-derived foam cells[16]. These findings suggested that CML can also be a mediator of CAD in patients. The results showed by Shrivastav et al[17] showed the association between CML and inflammatory cytokines in patients with and without diabetes. Thus, the peptides that block the RAGE pathways might be a therapeutic alternative against the proliferation and inflammation effects of CML[18]. Its quantification on patients with high risk for CAD might improve personalized medicine. The knowledge of how adiposity and non-vegetarian diet contribute to CML levels might help us to modify primary preventive strategies with consequences on CAD events.
This study contributes to the knowledge of biomarkers and therapeutic targets for diabetic patients and the identification of the phenotype with a higher risk for CAD events. This is a new avenue of personalized medicine (Figure 1).
Provenance and peer review: Invited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Horowitz M, Australia; Zhang Y, China S-Editor: Qu XL L-Editor: A P-Editor: Zhao S
| 1. | Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1295] [Cited by in RCA: 1341] [Article Influence: 191.6] [Reference Citation Analysis (2)] |
| 2. | Wondmkun YT. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab Syndr Obes. 2020;13:3611-3616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 377] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 3. | Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 457] [Cited by in RCA: 1013] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 4. | Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der Graaf Y, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Zobel EH, Christensen RH, Winther SA, Hasbak P, Hansen CS, von Scholten BJ, Holmvang L, Kjaer A, Rossing P, Hansen TW. Relation of cardiac adipose tissue to coronary calcification and myocardial microvascular function in type 1 and type 2 diabetes. Cardiovasc Diabetol. 2020;19:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Yu W, Yang L, Zhang F, Liu B, Shi Y, Wang J, Shao X, Chen Y, Yang X, Wang Y. Machine learning to predict hemodynamically significant CAD based on traditional risk factors, coronary artery calcium and epicardial fat volume. J Nucl Cardiol. 2023;30:2593-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Couselo-Seijas M, Almengló C, M Agra-Bermejo R, Luis Fernandez Á, Alvarez E, R González-Juanatey J, Eiras S. Higher ACE2 expression levels in epicardial cells than subcutaneous stromal cells from patients with cardiovascular disease: Diabetes and obesity as possible enhancer. Eur J Clin Invest. 2021;51:e13463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Fandiño-Vaquero R, Fernández-Trasancos A, Alvarez E, Ahmad S, Batista-Oliveira AL, Adrio B, Fernández AL, González-Juanatey JR, Eiras S. Orosomucoid secretion levels by epicardial adipose tissue as possible indicator of endothelial dysfunction in diabetes mellitus or inflammation in coronary artery disease. Atherosclerosis. 2014;235:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Pinto-Junior DC, Silva KS, Michalani ML, Yonamine CY, Esteves JV, Fabre NT, Thieme K, Catanozi S, Okamoto MM, Seraphim PM, Corrêa-Giannella ML, Passarelli M, Machado UF. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci Rep. 2018;8:8109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Chuyen NV. Toxicity of the AGEs generated from the Maillard reaction: on the relationship of food-AGEs and biological-AGEs. Mol Nutr Food Res. 2006;50:1140-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Han L, Li L, Li B, Zhao D, Li Y, Xu Z, Liu G. Review of the characteristics of food-derived and endogenous ne-carboxymethyllysine. J Food Prot. 2013;76:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | de la Cruz-Ares S, Cardelo MP, Gutiérrez-Mariscal FM, Torres-Peña JD, García-Rios A, Katsiki N, Malagón MM, López-Miranda J, Pérez-Martínez P, Yubero-Serrano EM. Endothelial Dysfunction and Advanced Glycation End Products in Patients with Newly Diagnosed Versus Established Diabetes: From the CORDIOPREV Study. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | van Eupen MG, Schram MT, Colhoun HM, Scheijen JL, Stehouwer CD, Schalkwijk CG. Plasma levels of advanced glycation endproducts are associated with type 1 diabetes and coronary artery calcification. Cardiovasc Diabetol. 2013;12:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Xu SN, Zhou X, Zhu CJ, Qin W, Zhu J, Zhang KL, Li HJ, Xing L, Lian K, Li CX, Sun Z, Wang ZQ, Zhang AJ, Cao HL. Nϵ-Carboxymethyl-Lysine Deteriorates Vascular Calcification in Diabetic Atherosclerosis Induced by Vascular Smooth Muscle Cell-Derived Foam Cells. Front Pharmacol. 2020;11:626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Tada Y, Yano S, Yamaguchi T, Okazaki K, Ogawa N, Morita M, Sugimoto T. Advanced glycation end products-induced vascular calcification is mediated by oxidative stress: functional roles of NAD(P)H-oxidase. Horm Metab Res. 2013;45:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Xu S, Li L, Yan J, Ye F, Shao C, Sun Z, Bao Z, Dai Z, Zhu J, Jing L, Wang Z. CML/CD36 accelerates atherosclerotic progression via inhibiting foam cell migration. Biomed Pharmacother. 2018;97:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Shrivastav D, Singh DD, Mir R, Mehra P, Mehta V, Dabla PK. Comparative analysis of Nε-carboxymethyl-lysine and inflammatory markers in diabetic and non-diabetic coronary artery disease patients. World J Diabetes. 2023;14:1754-1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (1)] |
| 18. | Dai X, Hou Y, Deng T, Lin G, Cao Y, Yu G, Wei W, Zheng Q, Huang L, Ma S. A specific RAGE-binding peptide inhibits triple negative breast cancer growth through blocking of Erk1/2/NF-κB pathway. Eur J Pharmacol. 2023;954:175861. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |