Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.488
Peer-review started: August 31, 2023
First decision: December 25, 2023
Revised: January 6, 2024
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: March 15, 2024
Processing time: 197 Days and 5.3 Hours
Diabetic kidney disease (DKD) is a major complication of diabetes mellitus. Renal tubular epithelial cell (TEC) damage, which is strongly associated with the inflammatory response and mesenchymal trans-differentiation, plays a significant role in DKD; However, the precise molecular mechanism is unknown. The recently identified microRNA-630 (miR-630) has been hypothesized to be closely asso
To investigate how miR-630 affects TEC injury and the inflammatory response in DKD rats.
Streptozotocin was administered to six-week-old male rats to create a hypergly
The expression level of miR-630 was reduced in the kidney tissue of rats with DKD (P < 0.05). The miR-630 and TLR4 expressions in rat renal TECs (NRK-52E) were measured using quantitative reverse transcription polymerase chain reaction. The mRNA expression level of miR-630 was significantly lower in the high-glucose (HG) and HG + mimic negative control (NC) groups than in the normal glucose (NG) group (P < 0.05). In contrast, the mRNA expression level of TLR4 was significantly higher in these groups (P < 0.05). However, miR-630 mRNA expression increased and TLR4 mRNA expression significantly decreased in the HG + miR-630 mimic group than in the HG + mimic NC group (P < 0.05). Furthermore, the levels of tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 were significantly higher in the HG and HG + mimic NC groups than in NG group (P < 0.05). However, the levels of these cytokines were significantly lower in the HG + miR-630 mimic group than in the HG + mimic NC group (P < 0.05). Notably, changes in protein expression were observed. The HG and HG + mimic NC groups showed a significant decrease in E-cadherin protein expression, whereas TLR4, α-smooth muscle actin (SMA), and collagen IV protein expression increased (P < 0.05). Conversely, the HG + miR-630 mimic group exhibited a significant increase in E-cadherin protein expression and a notable decrease in TLR4, α-SMA, and collagen IV protein expression than in the HG + mimic NC group (P < 0.05). The miR-630 targets TLR4 gene expression. In vivo experiments demonstrated that DKD rats treated with miR-630 agomir exhibited significantly higher miR-630 mRNA expression than DKD rats injected with agomir NC. Additionally, rats treated with miR-630 agomir showed significant reductions in urinary albumin, blood glucose, TLR4, and proinflammatory markers (TNF-α, IL-1β, and IL-6) expression levels (P < 0.05). Moreover, these rats exhibited fewer kidney lesions and reduced infiltration of inflammatory cells.
MiR-630 may inhibit the inflammatory reaction of DKD by targeting TLR4, and has a protective effect on DKD.
Core Tip: This study revealed that microRNA-630 (miR-630) expression in the renal tissue was significantly lower in diabetic kidney disease (DKD) rats than in normal rats. The miR-630 alleviates renal injury and inflammatory reactions in DKD rats by targeting toll-like receptor 4. Our findings provide new insights into the pathogenesis of DKD indicating that miR-630 may be a potential noninvasive biomarker for diagnosing and predicting the prognosis of DKD.
- Citation: Wu QS, Zheng DN, Ji C, Qian H, Jin J, He Q. MicroRNA-630 alleviates inflammatory reactions in rats with diabetic kidney disease by targeting toll-like receptor 4. World J Diabetes 2024; 15(3): 488-501
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/488.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.488
Diabetic kidney disease (DKD), a chronic kidney condition due to diabetes, is characterized by a steady decline in glomerular filtration rate and the onset and progression of albuminuria. End-stage renal failure is more common in patients with DKD than with chronic glomerulonephritis[1-4]. The primary early morphological alterations in DKD include glomerular and tubular hypertrophy, thickening of the glomerular basement membrane, fusion of foot processes, and growth of the mesangial matrix. These alterations progress to various degrees of tubulointerstitial fibrosis, which ultimately causes the loss of renal function[5]. However, the molecular mechanisms underlying DKD remain unclear.
Renal tubular epithelial cell (TEC) damage has been linked to DKD[6]. The TEC epithelial mesenchymal transition (EMT), a key mechanism in renal interstitial fibrosis, has gained recent attention[7]. EMT is caused by a complex interaction between several variables, including the inflammatory response, hypoxia, oxidative stress, growth factors, signaling pathways, microRNA (miRNA), and transcription factors Snail, Slug and Twist[8]. Therefore, focusing on these pathways may help comprehend the molecular mechanisms underlying damage to TECs in DKD.
The miRNAs are small noncoding RNAs consisting of 19-23 nucleotides that negatively regulate posttranscriptional gene expression by targeting the 3’-untranslated regions (3’UTR) of protein-coding messenger RNA (mRNA) transcripts; which play important roles in different physiological and pathological processes[9,10]. Because miRNA imbalance is directly associated with pathological processes in DKD, miRNAs may serve as diagnostic biomarkers and therapeutic targets. For instance, DKD is promoted by increased expression of toll-like receptor 4 (TLR4) when miR-203 is expressed at low levels[11]. The overexpression of miR-92b can minimize renal fibrosis and restore miR-92b expression to normal levels in the kidneys of mice with DKD[12].
The miR-630, a recently identified miRNA, is closely linked to tumor cell development and apoptosis and demonstrates aberrant expression in various malignancies, including liver cancer, colon cancer, gastric cancer, and other tumors[13,14]. Liu et al[15] reported that miR-630 targets TLR4 in immunoglobulin A (IgA) nephropathy to control the production of glycosylated IgA1 in tonsils. Studies on the expression and function of miR-630 in DKD-related renal tissue and the underlying pathophysiological mechanisms are lacking.
In this study, we assessed the relative expression of miR-630 mRNA in the kidney tissue of DKD rats and found that its expression was considerably lower than that in normal rats. Mechanistic studies have indicated TLR4 as the target gene for miR-630; therefore, miR-630 can be considered a possible pharmacological target for DKD treatment and a non-invasive biomarker for diagnosing DKD and determining its prognosis.
Sixty specific pathogen-free male Sprague-Dawley rats (6-wk-old and weighing 200 ± 20 g), were provided by the Experimental Animal Center of Jiangsu University. The rats were housed in the experimental animal feeding room at a temperature of 21 °C, humidity of 55 °C, and a 12-h light/dark period. Prior to the experiments, all rats were fed the same diet for one week. The rat renal TECs (NRK-52E) cell line was purchased from the Treasure Cell Bank of the China Academy of Sciences. The rat renal TECs (NRK-52E) cell line was purchased from the TreasureCell Bank of the China Academy of Sciences.
Streptozotocin (STZ) was purchased from Shanghai Aiyan Biotechnology Co., Ltd. The citrate buffer was purchased from Beijing Noble Food Technology Co., Ltd. Lentiviral negative control (LV-NC), LV-miR630 and primers were all purchased from ABclonal. RIPA lysis buffer and BCA kits were purchased from Beyotime. The TLR4 antibody was purchased from Affinity. Enzyme-linked immunosorbent assay (ELISA) kits for interleukin-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) were purchased from Mlbio.
The BK-200 automatic biochemical analyser was purchased from BIOBASE, the DR3518G enzyme-labelled instrument was purchased from Wuxi HiwellDiatek, the FluorChem HD2 gel imaging system was purchased from protein simple, and the CytoFLEXS flow cytometry was purchased from Beckman.
Model construction and processing: The experimental rats were randomly divided into a control group, model (DKD), model + negative control (NC) agomir (LV-NC), and model + miR-630 agomir (LV-miR-630) groups (n = 15 in each group). Six-week-old rats were fed adaptively for one week. Following a 12-h fast, the rats in the model and experimental groups received intraperitoneal injections of 60 mg/kg STZ solution to establish the DKD model, whereas the control group was injected with the same volume of sodium citrate buffer (0.1 mmol/L)[16]. After 72 h, blood was collected from the tail vein for analyzing glucose levels, and serum glucose ≥ 16.7 mmol/L was used for establishing the diabetic model. Urine was collected for 24 h, and the urine protein content was > 30 mg/24 h, for successful DKD modelling[17]. The rats in each experimental group were administered 100 μL of agomir NC and miR-630 agomir (50 nM) intravenously at weeks 2 and 5 after STZ injection, whereas rats in the model group received intravenous injections of equal volumes of normal saline. After 8 wk, the rats in each group were fasted for 12 h before blood samples were collected from the tail vein to detect fasting blood glucose. Urine was collected in a metabolic cage for 24 h 1 d before execution. The rats were injected intraperitoneally with 3% pentobarbital sodium (30 mg/kg), blood was collected from the abdominal aorta and kidneys, and the rats were sacrificed. The renal tissue was collected for further analysis.
Indicator monitoring: The mental state, color change of the hair and nails, activity, urine volume, and weight of rats were observed during administration. On the last day of the experiment, 24-h urine samples of the rats in a metabolic cage. After mixing, the samples were centrifuged at 3000 rpm (centrifugal radius: 16.5 cm) for 10 min, and the supernatant was collected to detect the 24-h urine protein quantification. Random blood glucose levels were measured, and the rats were anesthetized using an intraperitoneal injection of 3% pentobarbital sodium (30 mg/kg). Blood was collected from the abdominal aorta and centrifuged at 4 °C and 3500 rpm (centrifugal radius: 16.5 cm) for 15 min. Serum blood urea nitrogen (BUN) and creatinine levels were measured using a kit.
Calculation of renal weight index: The kidneys were washed with precooled normal saline, dried using filter paper and weighed, and the rat kidney index was calculated using the formula: Kidney index (%) = (total weight of bilateral kidneys/weight of rats) × 100.
The TRIzol method was used to extract total RNA from each group. The expression of miR-630 and TLR4 mRNA was detected using a one-step reverse transcription fluorescence quantitative kit (Table 1 for primers). The reaction system and quantitative reverse transcription polymerase chain reaction (qRT-PCR) procedures were performed according to the manufacturer’s instructions, and 2-ΔΔCt method was used for relative quantitative analysis, with U6 and GAPDH as internal references.
| Primers | Sequence (5’-3’) |
| TLR4 forward | TAGCCATTGCTGCCAACATC |
| TLR4 reverse | ACACCAACGGCTCTGGATAA |
| miR-630 forward | TTGAGCTGGATTGGCGGGAT |
| miR-630 reverse | TTGACGGATGCGGAGGCT |
| GAPDH forward | TATGTCGTGGAGTCTACTGTGT |
| GAPDH reverse | GAGTTGTCATATTTCTCGTGG |
| U6 forward | CATCACCATCAGGAGAGTCG |
| U6 reverse | TGACGCTTGCCCACAGCCTT |
For the construction of wild-type and mutant TLR4 3’-UTR double-fluorescent reporter plasmids, 293T cells in the logarithmic growth period were inoculated in a 12-well cell plate at a density of 1 × 105 cells/well. Negative controls of TLR4-WT, TLR4-MUT, and miR-630 mimics or mimic NC were transfected into 293T cells according to the manu-facturer’s instructions for LipofectamineTM2000. Three replicates were performed for each group of experiments. After 48 h of incubation, luciferase activity was detected using a double luciferase reporter gene detection kit.
RIPA lysate was added to the tissues of each group, placed on ice for 20 min, and centrifuged at 4 °C and 13000 rpm at 4 °C for 20 min, and the protein content in the supernatant was determined using a BCA kit. The sodium-dodecyl sulfate gel electrophoresis was performed with a 35-μg protein solution, transferred to the polyvinylidene fluoride membrane, and sealed with a 2% BSA sealing solution. The primary antibodies were added at 4 °C and left overnight. The GAPDH antibody was used as the reference, and secondary antibodies were added and incubated for 1 h at room temperature. Enhanced chemiluminescence exposure imaging was performed using an Alpha Imager HP gel imaging system to analyze the results.
The kidneys were fixed with 4% paraformaldehyde, routinely dehydrated, and embedded in paraffin. After dewaxing, the tissues were stained with hematoxylin and eosin (HE), followed by 1% hydrochloric acid ethanol differentiation, 0.6% ammonia return to blue, 0.5% eosin staining, conventional dehydration, xylene transparency, and neutral gum sealing. Renal injury was observed under a microscope (400 × magnification).
The slices were dewaxed in water. Sections were stained with the prepared Weigert hematoxylin staining solution for 5-10 min, differentiated with an acidic ethanol differentiation solution for 5-15 s, and washed with water. The Masson blue-stained solution returned to blue after 3-5 min and was washed with water. After washing with distilled water for l min, the sections were stained with Ponceau magenta dye solution for 5-10 min. The weak acid working solution used in this procedure was prepared using a 2:1 ratio of distilled water to the weak acid solution. The sample was washed with the weak acid working solution for 1 min, then washed with the phosphomolybdic acid solution for 1-2 min, and washed again with the prepared weak acid working solution for l min. The sample was placed directly in the aniline blue dye solution for 1-2 min and then washed with the prepared weak-acid working solution for 1 min. The sample was quickly dehydrated using 95% ethanol for 2-3 s and anhydrous ethanol three times for 5-10 s each time. The sections were transparentized with xylene three times for 1-2 min each and sealed with neutral gum.
Renal tissue was ground on ice and transformed into a 10% tissue homogenate. The levels of IL-6, IL-1β and TNF-α in renal tissue were detected according to the instructions of the TNF-α ELISA kit.
All statistical analyses were performed using SPSS 17.0 (IBM Corp., Armonk, NY, United States) and GraphPad Prism 9.0 software, and the results are expressed as the mean ± standard error (mean ± SEM) or mean ± SD. The T-test, one-way ANOVA, and SNK-q tests were used for intergroup and intragroup mean comparisons. A P value of 0.05 indicated statistical significance. Correlation analyses were performed using Pearson correlation analysis and linear regression.
Rats in the normal group were in a good mental state, lively and active, with bright eyes, sensitive reactions, and white and shiny fur. The model rats were depressed; their fur was yellow, dry, and dirty; their movements were slow; and they exhibited symptoms such as excessive drinking, excessive eating, excessive urination, and thin feces. Over time, some rats with DKD exhibited varying degrees of abdominal distension. Rats in the miR-630 agomir group had better general conditions than those in the model group.
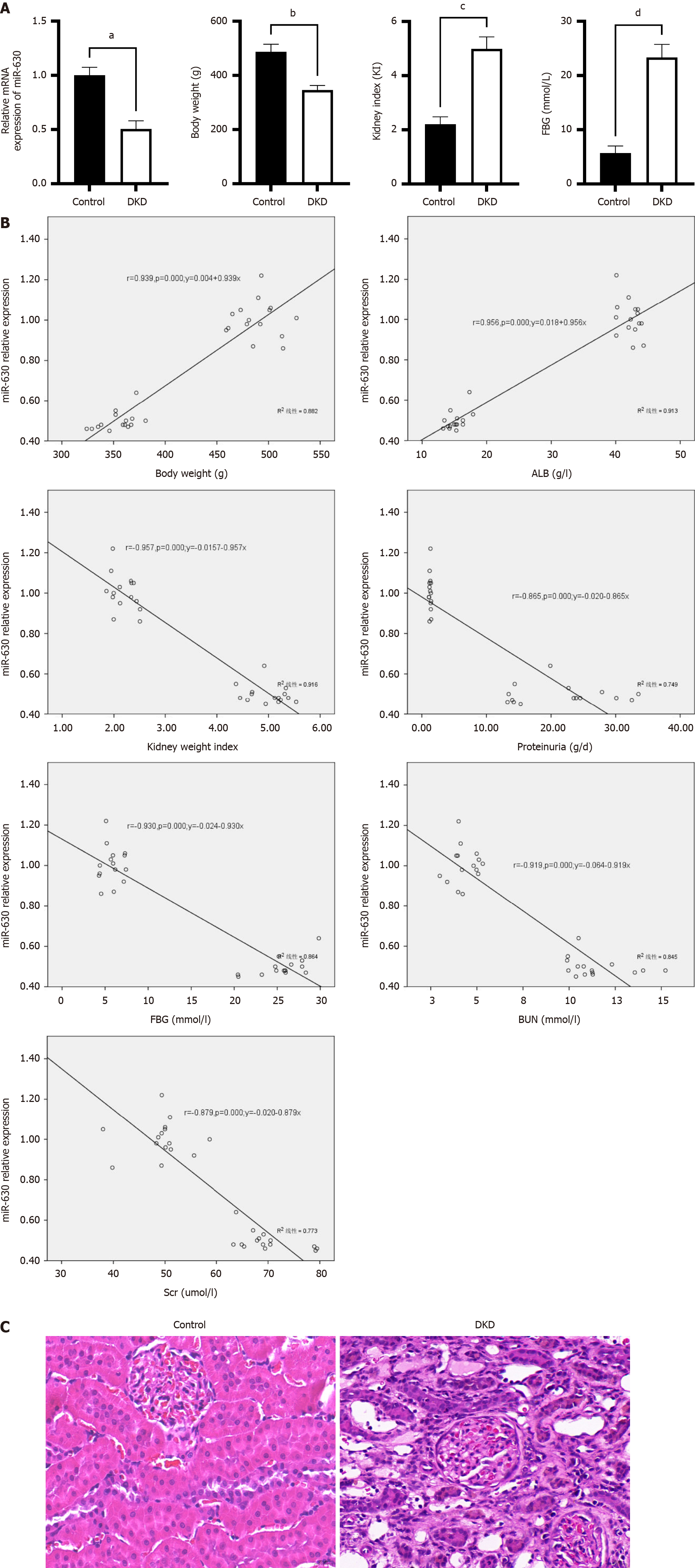
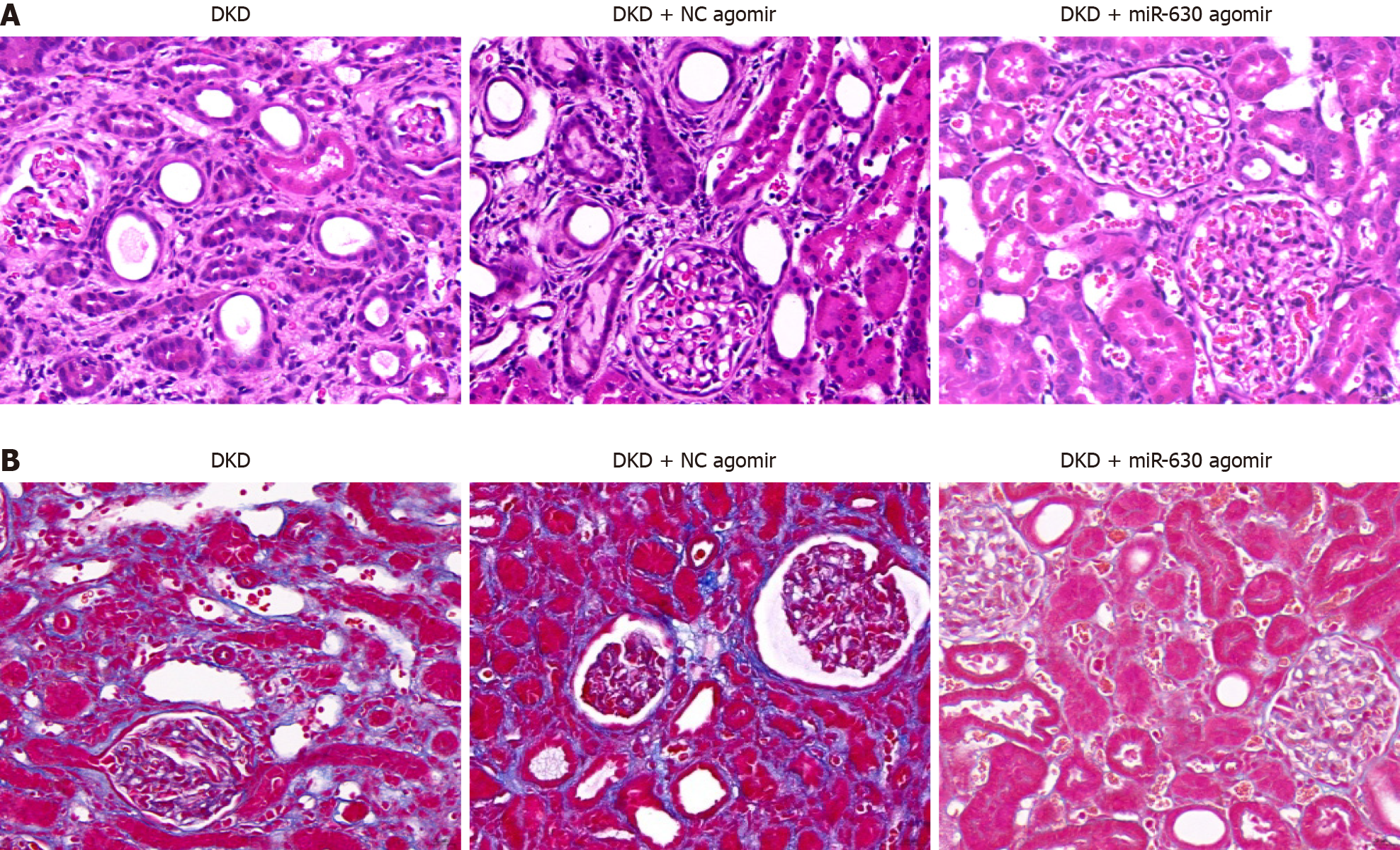
As demonstrated in Figure 1A, compared with the control group, miR-630 mRNA expression in the DKD group declined considerably, as did body weight, whereas blood glucose levels and the kidney weight index increased significantly (all P < 0.01). Pearson correlation analysis indicated that the level of miR-630 in rats was positively correlated with body weight and albumin but negatively correlated with renal weight index, urea nitrogen, serum creatinine (SCr), 24-h urine protein quantification, blood glucose, and other variables (Figure 1B). These findings imply that miR-630 expression in renal tissues is associated with clinical variables and may be associated with DKD. The results of the HE staining are shown in Figure 1C. The renal tissue cell structure remained unaltered in the control group, and no overt pathological alterations were observed. Enlarged or detached TECs, renal interstitial cell infiltration, mesangial hyperplasia, interstitial fibrosis, and glomerular edema were detected in the DKD group.
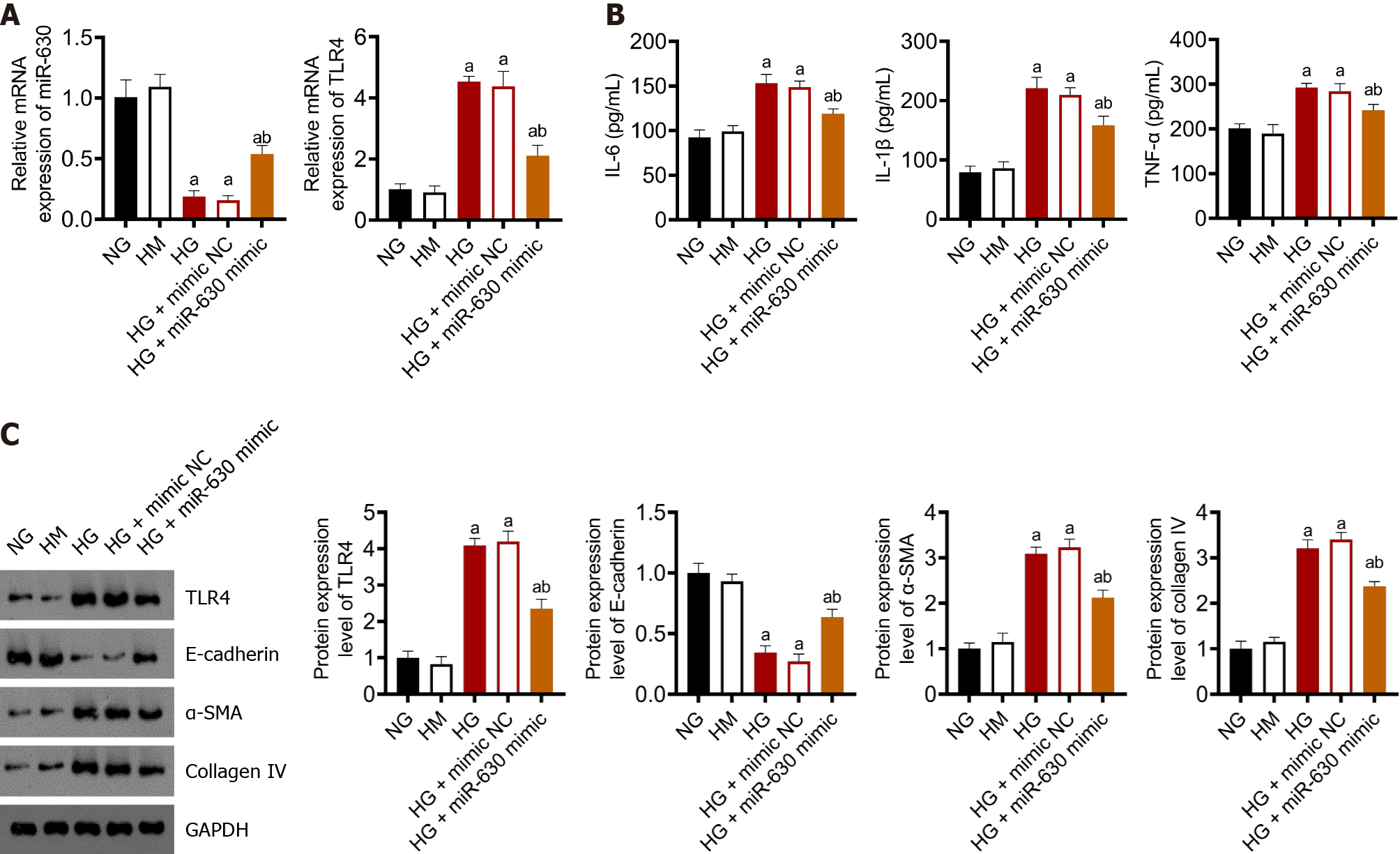
Figure 2A shows the expression levels of miR-630 and TLR4 in NRK-52E determined using qRT-PCR. The TLR4 and miR-630 mRNA expression levels in the normal glucose (NG) and high mannitol (HM) groups were not significantly different. Although miR-630 and TLR4 mRNA expression levels were significantly decreased in the high glucose (HG) and HG + mimic NC groups compared with those in the NG group, TLR4 mRNA expression levels considerably increased. Compared with the HG + miR-630 mimic NC group, the mRNA expression levels of miR-630 and TLR4 in the NG group substantially increased and decreased, respectively.
ELISA was used to determine the levels of TNF-α, IL-1β, and IL-6 in each group. The results are shown in Figure 2B. TNF-α, IL-1β, and IL-6 levels in the NG and HM groups were not altered considerably; however, the HG and HG + mimic NC groups demonstrated a significant increase than that observed in the NG group. The levels of TNF-α, IL-1β, and IL-6 in the HG + miR-630 mimic group were considerably lower than those in the HG + mimic NC group.
Figure 2C displays the results of western blot analysis used to determine the protein expression levels of TLR4, E-cadherin, E-smooth muscle actin (SMA), and collagen IV in each group. The expression levels of TLR4, E-cadherin, α-SMA, and collagen IV were not significantly altered in the NG and HM groups. E-cadherin expression levels in the HG group and HG + mimic NC group were significantly lower than those in the NG group, although TLR4, α-SMA, and collagen IV expression levels were significantly increased. Compared with the HG + mimic NC group, the expression levels of E-cadherin protein in the HG + miR-630 mimic group increased substantially, whereas those of TLR4, α-SMA, and collagen IV protein decreased significantly.
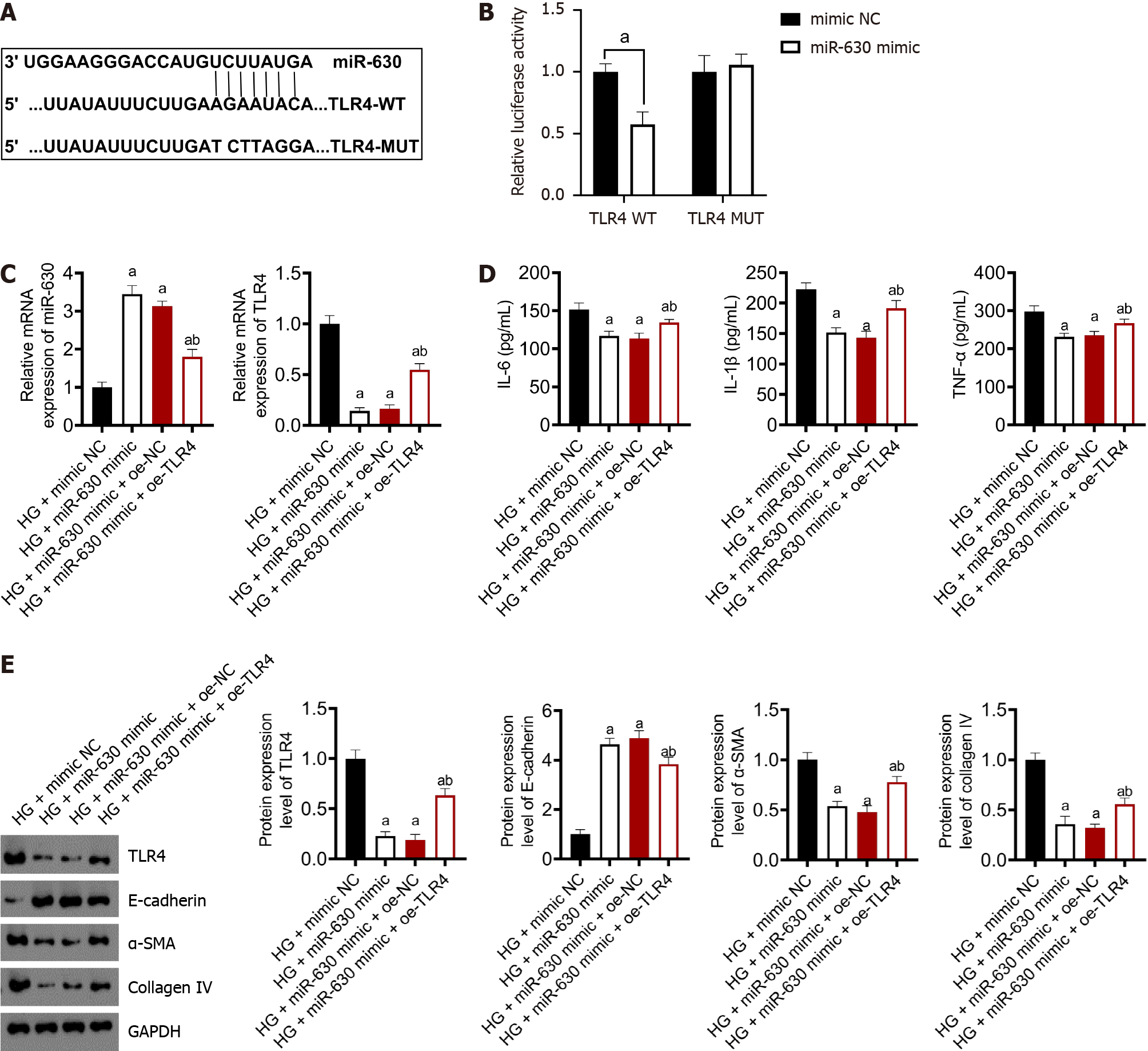
TargetScan and other databases predicted that miR-630 has a binding site in the 3’-UTR of TLR4 (Supplementary Table 1 and Supplementary Figure 1) (Figure 3A). Based on the experimental findings using a double luciferase reporter gene, high levels of miR-630 substantially reduced the luciferase activity of the wild-type TLR4 plasmid (P < 0.01) but had no effect on the mutant TLR4 plasmid (Figure 3B).
Figure 3C shows the levels of miR-630 and TLR4 mRNA expression determined using qRT-PCR. Compared with the HG + mimic NC group, the mRNA expression of miR-630 increased significantly in the HG + miR-630 mimic group, whereas the mRNA expression of TLR4 was significantly decreased. The mRNA expression of miR-630 in the HG + miR-630 mimic + oe-TLR4 group was considerably lower than that in the HG + miR-630 mimic + oe-NC group; however, the mRNA expression of TLR4 was dramatically higher.
The ELISA results showed that miR-630 downregulated the levels of INF-α, IL-1 β, and IL-6 in TLR4 (Figure 3D). The levels of INF-α,IL-1 β, and IL-6 in the HG + miR-630 mimic group were considerably lower than those in the HG + mimic NC group. Compared with the HG + miR-630 mimic + oe-NC group, the TNF-α, IL-1β, and IL-6 levels were significantly increased in the HG + miR-630 mimic + oe-TLR4 group.
Western blotting was used to detect the protein expression levels of TLR4, α-SMA, collagen IV and E-cadherin, which were downregulated by miR-630 (Figure 3E). Compared with the HG + mimic NC group, the protein expression levels of TLR4, α-SMA and collagen IV in the HG + miR-630 mimic group decreased significantly, whereas the protein expression level of E-cadherin increased significantly. Compared with the HG + miR-630 mimic + oe-NC group, the expression levels of TLR4, α-SMA and collagen IV proteins in the HG + miR-630 mimic + oe-TLR4 group were significantly increased, whereas the expression level of E-cadherin protein was significantly decreased.
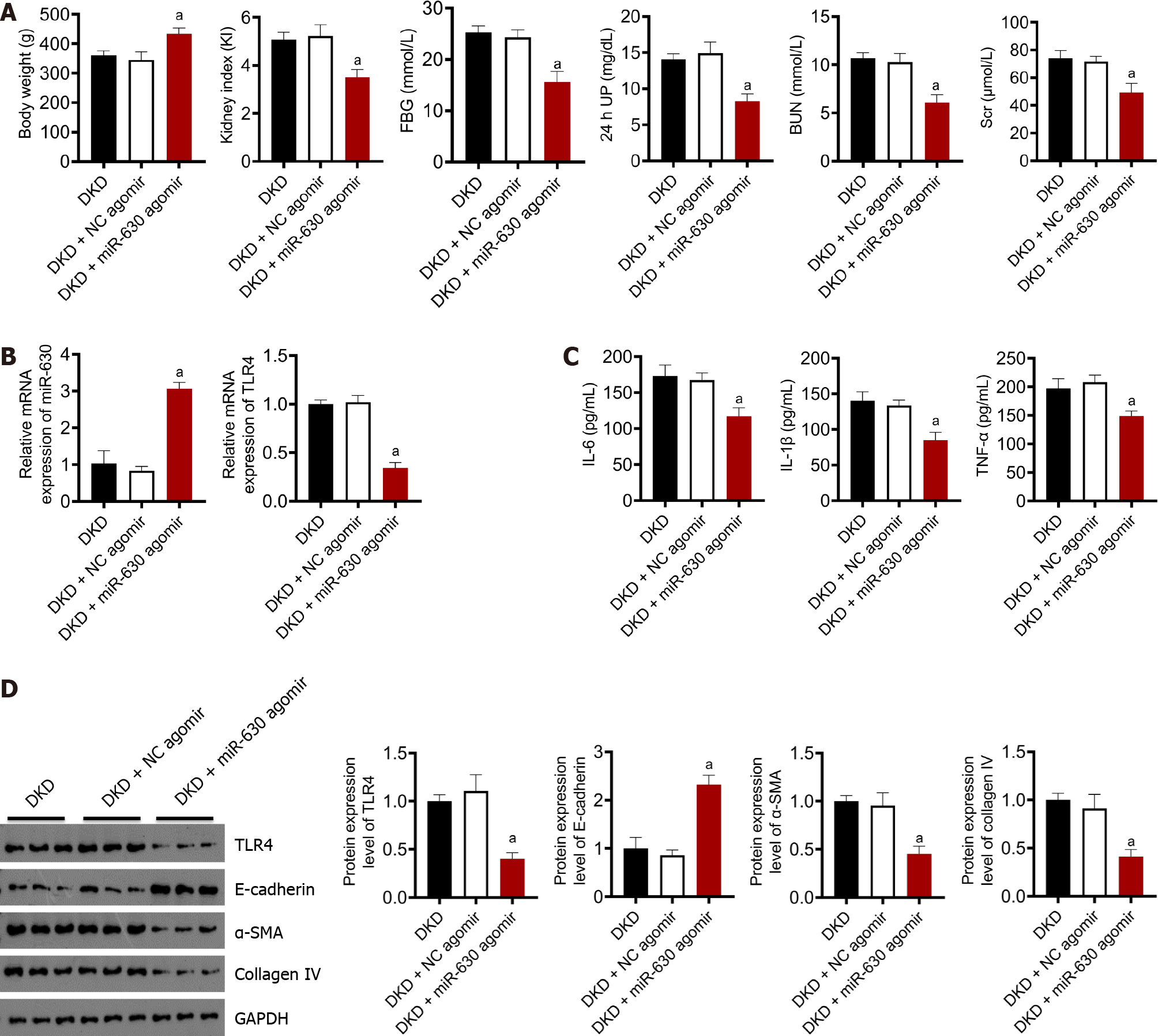
Figure 4A summarizes the body weight, renal weight index, blood sugar, 24-h urinary protein, BUN, and SCr. No significant differences in body weight, renal weight index, blood glucose, 24-h urinary protein, BUN, or SCr levels was observed between the DKD and DKD + NC agomir groups. Compared with the DKD + NC agomir group, the body weight increased significantly in the DKD + miR-630 agomir group, and the renal index decreased significantly. As shown in Figure 4A, no significant differences in blood glucose levels were observed between the DKD and DKD + NC agomir groups. Compared with the DKD + NC agomir group, blood glucose levels in the DKD + miR-630 agomir group decreased significantly. Figure 4C summarizes the results of the automatic biochemical analyzer; no significant difference in 24-h urine protein, BUN, and SCr levels were observed between the DKD and DKD + NC agomir groups. Compared with the DKD + NC agomir group, the contents of 24-h urine protein, BUN, and SCr levels in the DKD + miR-630 agomir group decreased significantly.
The mRNA expression levels of miR-630 and TLR4 were measured using qRT-PCR. As shown in Figure 4B, no significant differences in the mRNA expression levels of miR-630 and TLR4 were observed between the DKD and the DKD + NC agomir groups. Compared with the DKD + NC agomir group, the mRNA expression of miR-630 in the DKD + miR-630 agomir group increased significantly, whereas the mRNA expression of TLR4 decreased significantly. The protein levels of IL-6, IL-1β, and TNF-α were detected by ELISA. As shown in Figure 4C, no significant difference in the contents of IL-6, IL-1β, and TNF-α was observed between the DKD group and the DKD + NC agomir group. Compared with the DKD + NC agomir group, the contents of IL-6, IL-1β, and TNF-α in the DKD + miR-630 agomir group decreased significantly. The expression levels of TLR4, E-cadherin, α-SMA, and collagen IV were detected by western blotting. As shown in Figure 4D, no significant difference in the expression levels of TLR4, E-cadherin, α-SMA and collagen IV was observed between the DKD group and the DKD + NC agomir group. Compared with the DKD + NC agomir group, the expression of E-cadherin protein in the DKD + miR-630 agomir group was significantly increased, whereas the expression of TLR4, α-SMA, and collagen IV protein was significantly decreased.
The results of the HE test are shown in Figure 5A. In the DKD and DKD + NC agomir groups, glomerular swelling, TEC swelling or falling off, renal interstitial cells infiltrating inflammatory cells, and some mesangial hyperplasia and interstitial fibrosis were observed. Renal pathological changes were alleviated and were accompanied by a small amount of inflammatory cell infiltration in the DKD + miR-630 agomir group. The Masson test results are shown in Figure 5B, and many blue-stained collagen fibers appeared in the renal glomeruli of rats in the DKD and DKD + NC agomir groups. Few blue-stained collagen fibers in the kidney tissue of rats were observed in the DKD + miR-630 agomir group.
The prevalence of DKD has been increasing globally, with significant morbidity and mortality. The pathophysiology of DKD is complex and has not been elucidated to date. Numerous studies have now established the role of miRNA in the occurrence and progression of diabetic nephropathy[18]. The expression levels of miR-21, miR-146a-5p, miR-10a-5p, miR-874, and miR-192 are significantly increased in diabetic nephropathy, whereas miR-26a-5p, miR-451, and miR-155 are expressed at low levels[17,19-21]. Among them, miR-21 functions by targeting the PTEN gene, thereby promoting the activation of the Akt kinase signaling pathway, which in turn increases the production of the renal fibrosis proteins type I collagen a2 and mucin and glomerular hypertrophy[17]. By targeting the ZEB1/2 gene, miR-192 activates the transforming growth factor-beta signaling pathway, increasing the transcription of the renal fibrosis protein Coll2 and the amount of albumin in urine[21].
The recently identified miRNA, miR-630, is a noncoding single-stranded RNA fragment with a length of 21-23 nucleotides that regulates gene expression at the translational level and participates in several pathophysiological processes, including cell proliferation and differentiation[22], apoptosis[23], and immune response[24]. Aberrant expression of the miR-630 gene has been reported in numerous malignancies, such as liver, colon, and gastric cancers[14,25]. Moreover, miR-630 is strongly associated with autophagy, cell proliferation, migration, and apoptosis[26-28]. However, the expression and functions of miR-630 in the renal tissues of patients with DKD are unknown. A rat model of DKD was created by intraperitoneally injecting STZ. The 24-h urinary total protein, SCr, and BUN levels increased and the model rats manifested clear signs of diabetes. The model was successful because it revealed the characteristic renal pathological abnormalities of DKD when stained with Masson’s trichrome and HE. Furthermore, the expression of miR-630 was investigated, which was much lower in DKD renal tissue than in normal renal tissue, indicating that miR-630 may play a role in the pathogenesis of DKD.
Inflammation is crucial for the pathogenesis of DKD[29]. TLR4, the first confirmed member of the toll-like family in humans, can activate signaling pathways such as the nuclear factor-kappa B and mitogen-activated protein kinase family pathways after interacting with ligands in vivo, which increases the production of inflammatory markers and activates an inflammatory response[30]. Activation of the TLR4 signaling pathway is closely related to the pathogenesis of diabetic nephropathy, and the expression of TLR4 and related inflammatory factors TNF-α, IL-6, and IL-1β increases during the occurrence and development of diabetic nephropathy[31,32]. This work used a bioinformatics website to predict that miR-630 may combine with the 3’-UTR of TLR4 and a twofold luciferase assay to confirm that TLR4 was the direct target of miR-630. Overexpression of miR-630 in DKD rats caused a decrease in TLR4 expression and the levels of the proinflammatory molecules TNF-α, IL-6, and IL-1β, demonstrating a negative regulatory link between miR-630 and TLR4. To prevent TLR4 from being translated and transcribed, miR-630 attaches to its 3’-UTR on the mRNA, which inhibits the synthesis of the proinflammatory protein TNF-α. In addition, overexpression of miR-630 in DKD rats led to an improvement in general health, an increase in weight, a drop in renal index, an improvement in urine protein and renal function, and a reduction in renal pathological damage. Consequently, miR-630 overexpression could reduce the inflammatory response and mesenchymal trans-differentiation of diabetic nephropathy. Moreover, the contents of TNF-α, IL-6, and IL-1β significantly decreased, and the expression of the renal tubular epithelial marker protein E-cadherin increased, whereas the expression of the mesenchymal marker proteins α-SMA and collagen IV decreased.
In addition, as shown in Figure 4A, overexpression of miR-630 can significantly reduce blood glucose levels, which may prevent the progression of DKD. However, the role of miR-630 in promoting insulin secretion has not been demonstrated, which requires further study and exploration. In conclusion, this study showed that miR-630 targets TLR4, and inhibits the inflammatory response that results in DKD, and exerts protective effects on the kidney under diabetic conditions.
To our knowledge, our study was the first to report that the expression of miR-630 in renal tissue is significantly lower in DKD rats than in normal rats. These results revealed the underlying mechanism by which miR-630 alleviates renal injury and inflammatory reactions in rats with DKD by targeting TLR4. Taken together, our findings provide new insights into the pathogenesis of DKD and show that miR-630 may be a non-invasive biomarker for the diagnosis and prediction of the prognosis of DKD.
Diabetic kidney disease (DKD) is a major complication of diabetes mellitus. Numerous studies have demonstrated that tubular epithelial cell (TEC) damage, which is strongly associated with the inflammatory response and mesenchymal trans-differentiation, plays a significant role in DKD; however, the precise molecular mechanism is unknown. The recently identified microRNA-630 (miR-630) has been hypothesized to be closely associated with cell migration, apoptosis, and autophagy.
The relationship between miR-630 and DKD and the underlying mechanism remains unknown.
The object of this study is to investigate how miR-630 affects TEC injury and the inflammatory response in DKD rats.
Streptozotocin was administered to six-week-old male rats to create a hyperglycemic diabetic model, and in the second week of modeling, the rats were divided into control, DKD, negative control lentivirus, and miR-630 overexpression groups. After eight weeks, urine and blood samples were collected for the kidney injury assay, and renal tissues were removed for further molecular assays, such as real-time polymerase chain reaction, western blotting, enzyme-linked immunosorbent assay, and immunohistochemistry. The target gene for miR-630 was predicted using bioinformatics, and in vitro investigations and double luciferase reporter gene assays confirmed the association between miR-630 and toll-like receptor 4 (TLR4).
The expression level of miR-630 was decreased in the kidney tissue of rats with DKD (P < 0.05). In vitro experiments, the mRNA expression level of miR-630 was significantly lower in the high glucose (HG) and HG + mimic negative control (NC) groups than in the normal glucose group (P < 0.05). In contrast, the mRNA expression level of TLR4 was significantly higher in these groups (P < 0.05). The HG and HG + mimic NC groups showed a significant decrease in E-cadherin protein expression, whereas TLR4, α-smooth muscle actin (SMA), and collagen IV protein expression increased (P < 0.05). Conversely, compared with the HG + mimic NC group, a significant increase in E-cadherin protein expression and a notable decrease in TLR4, α-SMA, and collagen IV protein expression were observed in the HG + miR-630 mimic group (P < 0.05). In vivo experiments, DKD rats treated with miR-630 agomir exhibited significantly higher miR-630 mRNA expression than DKD rats injected with agomir NC. Additionally, rats treated with miR-630 agomir showed significant reductions in urinary albumin, blood glucose, TLR4, and proinflammatory markers (TNF-α, IL-1β, and IL-6) expression levels (P < 0.05). Moreover, these rats exhibited fewer kidney lesions and reduced infiltration of inflammatory cells.
The miR-630 may inhibit the inflammatory reaction in DKD by targeting TLR4, and has a protective effect on DKD.
The follow-up study needs to further confirm the expression difference of clinical human blood, urine or kidney tissue in diabetic nephropathy patients and its relationship with DKD staging, and further clarify the regulatory mechanism of its upstream signal pathway.
I am really grateful to Professor Qiang He, my tutor, for giving me with a platform, funds, and essential help. I am particularly grateful to Professor Juan Jin for her constant supervision and assistance throughout the project’s execution. In addition, I’d like to thank Jiangsu University’s Dean Hui Qian and Dr. Cheng Ji for their excellent laboratory resources and expert guidance. Finally, I’d like to express my profound gratitude to Yi-Wen Li and Bo Lin, the Directors of Zhejiang Provincial People’s Hospital, for their tremendous guidance and support during my clinical and scientific study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ankrah AO, Netherlands; Shao JQ, China; Sultana N, Bangladesh; Wu QN, China S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Zhang P, Colagiuri S. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 507] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 2. | Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1784] [Article Influence: 223.0] [Reference Citation Analysis (0)] |
| 3. | Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14:361-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 504] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 4. | Navaneethan SD, Schold JD, Jolly SE, Arrigain S, Winkelmayer WC, Nally JV Jr. Diabetes Control and the Risks of ESRD and Mortality in Patients With CKD. Am J Kidney Dis. 2017;70:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, Park J, Nair V, Schlafly A, Saulnier PJ, Satake E, Simeone CA, Shah H, Qiu C, Looker HC, Fiorina P, Ware CF, Sun JK, Doria A, Kretzler M, Susztak K, Duffin KL, Nelson RG, Krolewski AS. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25:805-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 295] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 6. | Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease. J Nephrol. 2017;30:701-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 7. | Bai X, Hou X, Tian J, Geng J, Li X. CDK5 promotes renal tubulointerstitial fibrosis in diabetic nephropathy via ERK1/2/PPARγ pathway. Oncotarget. 2016;7:36510-36528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Jing Y, Han Z, Zhang S, Liu Y, Wei L. Epithelial-Mesenchymal Transition in tumor microenvironment. Cell Biosci. 2011;1:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 9. | Yao X, Zhai Y, An H, Gao J, Chen Y, Zhang W, Zhao Z. MicroRNAs in IgA nephropathy. Ren Fail. 2021;43:1298-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1596] [Article Influence: 266.0] [Reference Citation Analysis (0)] |
| 11. | Liu ZM, Zheng HY, Chen LH, Li YL, Wang Q, Liao CF, Li XW. Low expression of miR-203 promoted diabetic nephropathy via increasing TLR4. Eur Rev Med Pharmacol Sci. 2018;22:5627-5634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Wang LP, Geng JN, Sun B, Sun CB, Shi Y, Yu XY. MiR-92b-3p is Induced by Advanced Glycation End Products and Involved in the Pathogenesis of Diabetic Nephropathy. Evid Based Complement Alternat Med. 2020;2020:6050874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Torban E, Braun F, Wanner N, Takano T, Goodyer PR, Lennon R, Ronco P, Cybulsky AV, Huber TB. From podocyte biology to novel cures for glomerular disease. Kidney Int. 2019;96:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Zhang S, Zhang JY, Lu LJ, Wang CH, Wang LH. MiR-630 promotes epithelial ovarian cancer proliferation and invasion via targeting KLF6. Eur Rev Med Pharmacol Sci. 2017;21:4542-4547. [PubMed] |
| 15. | Liu C, Ye MY, Yan WZ, Peng XF, He LY, Peng YM. microRNA-630 Regulates Underglycosylated IgA1 Production in the Tonsils by Targeting TLR4 in IgA Nephropathy. Front Immunol. 2020;11:563699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Zhong WL, Xie T, Hu W, Ran LJ, Gan HF, Liu WL, Wei HM, Xiang SW. Protective mechanism of Tangshenbao on kidney of diabetic nephropathy rats. Inter J Traditional Chinese Med. 2023;45:174-180. [DOI] [Full Text] |
| 17. | Chen X, Zhao L, Xing Y, Lin B. Down-regulation of microRNA-21 reduces inflammation and podocyte apoptosis in diabetic nephropathy by relieving the repression of TIMP3 expression. Biomed Pharmacother. 2018;108:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Wang LP, Gao YZ, Song B, Yu G, Chen H, Zhang ZW, Yan CF, Pan YL, Yu XY. MicroRNAs in the Progress of Diabetic Nephropathy: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2019;2019:3513179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Sun Y, Peng R, Peng H, Liu H, Wen L, Wu T, Yi H, Li A, Zhang Z. miR-451 suppresses the NF-kappaB-mediated proinflammatory molecules expression through inhibiting LMP7 in diabetic nephropathy. Mol Cell Endocrinol. 2016;433:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Gholaminejad A, Abdul Tehrani H, Gholami Fesharaki M. Identification of candidate microRNA biomarkers in diabetic nephropathy: a meta-analysis of profiling studies. J Nephrol. 2018;31:813-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Yu S, Zhao H, Yang W, Amat R, Peng J, Li Y, Deng K, Mao X, Jiao Y. The Alcohol Extract of Coreopsis tinctoria Nutt Ameliorates Diabetes and Diabetic Nephropathy in db/db Mice through miR-192/miR-200b and PTEN/AKT and ZEB2/ECM Pathways. Biomed Res Int. 2019;2019:5280514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Wang M, Liu C, Su Y, Zhang K, Zhang Y, Chen M, Ge M, Gu L, Lu T, Li N, Yu Z, Meng Q. miRNA-34c inhibits myoblasts proliferation by targeting YY1. Cell Cycle. 2017;16:1661-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Xu X, Wang Y, Mojumdar K, Zhou Z, Jeong KJ, Mangala LS, Yu S, Tsang YH, Rodriguez-Aguayo C, Lu Y, Lopez-Berestein G, Sood AK, Mills GB, Liang H. A-to-I-edited miRNA-379-5p inhibits cancer cell proliferation through CD97-induced apoptosis. J Clin Invest. 2019;129:5343-5356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Liu H, Lei C, He Q, Pan Z, Xiao D, Tao Y. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Mol Cancer. 2018;17:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 25. | Liu F, Chen J, Luo C, Meng X. Pathogenic Role of MicroRNA Dysregulation in Podocytopathies. Front Physiol. 2022;13:948094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 26. | Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 1127] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 27. | Akin D, Wang SK, Habibzadegah-Tari P, Law B, Ostrov D, Li M, Yin XM, Kim JS, Horenstein N, Dunn WA Jr. A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy. 2014;10:2021-2035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 28. | Zhi X, Zhong Q. Autophagy in cancer. F1000Prime Rep. 2015;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Pérez-Morales RE, Del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C, Navarro-González JF. Inflammation in Diabetic Kidney Disease. Nephron. 2019;143:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 30. | Liu HS, Jin X, Zhang N, He QS. Research progress of sTLR2 and sTLR4 in infectious diseases and autoimmune diseases. Int J Immunol. 2020;. |
| 31. | Liu S, Wang X, Kai Y, Tian C, Guo S, He L, Zhai D, Song X. Clinical significance of high mobility group box 1/toll-like receptor 4 in obese diabetic patients. Endocr J. 2022;69:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Wang HQ, Wang SS, Chiufai K, Wang Q, Cheng XL. Umbelliferone ameliorates renal function in diabetic nephropathy rats through regulating inflammation and TLR/NF-κB pathway. Chin J Nat Med. 2019;17:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |