Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.429
Peer-review started: November 2, 2023
First decision: December 6, 2023
Revised: December 19, 2023
Accepted: February 20, 2024
Article in press: February 20, 2024
Published online: March 15, 2024
Processing time: 134 Days and 4.3 Hours
Myosteatosis, rather than low muscle mass, is the primary etiologic factor of sarcopenia in patients with type 2 diabetes mellitus (T2DM). Myosteatosis may lead to a series of metabolic dysfunctions, such as insulin resistance, systematic inflammation, and oxidative stress, and all these dysfunctions are closely associated with the acceleration of T2DM and atherosclerosis.
To investigate the association between myosteatosis and coronary artery calcification (CAC) in patients with T2DM.
Patients with T2DM, who had not experienced major cardiovascular events and had undergone both abdominal and thoracic computed tomography (CT) scans, were included. The mean skeletal muscle attenuation was assessed using abdominal CT images at the L3 level. The CAC score was determined from thoracic CT images using the Agatston scoring method. Myosteatosis was diagnosed according to Martin’s criteria. Severe CAC (SCAC) was defined when the CAC score exceeded 300. Logistic regression and decision tree analyses were performed.
A total of 652 patients with T2DM were enrolled. Among them, 167 (25.6%) patients had SCAC. Logistic regression analysis demonstrated that myosteatosis, age, duration of diabetes, cigarette smoking, and alcohol consumption were independent risk factors of SCAC. Myosteatosis was significantly associated with an increased risk of SCAC (OR = 2.381, P = 0.003). The association between myosteatosis and SCAC was significant in the younger patients (OR = 2.672, 95%CI: 1.477-4.834, P = 0.002), but not the older patients (OR = 1.456, 95%CI: 0.863-2.455, P = 0.188), and was more prominent in the population with lower risks of atherosclerosis. The decision tree analyses prioritized older age as the primary variable for SCAC. In older patients, cigarette smoking was the main contributing factor for SCAC, while in younger patients, it was myosteatosis.
Myosteatosis is a novel risk factor for atherosclerosis in patients with T2DM, especially in the population with younger ages and fewer traditional risk factors.
Core Tip: Myosteatosis, rather than low muscle mass, is the primary etiologic factor of sarcopenia in patients with type 2 diabetes mellitus (T2DM). Myosteatosis may lead to a series of metabolic dysfunctions that are closely associated with the acceleration of T2DM and atherosclerosis. This study demonstrated that myosteatosis was a novel risk factor for atherosclerosis in patients with T2DM, especially in the population with younger ages and fewer traditional risk factors. Therefore, this indicates the potential benefit of initiating muscle-strengthening exercises and improving muscle quality at a younger age.
- Citation: Liu FP, Guo MJ, Yang Q, Li YY, Wang YG, Zhang M. Myosteatosis is associated with coronary artery calcification in patients with type 2 diabetes. World J Diabetes 2024; 15(3): 429-439
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/429.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.429
The prevalence of diabetes, especially type 2 diabetes mellitus (T2DM), has been dramatically increasing in China, from 10.9% in 2013 to 12.4% in 2018, and atherosclerotic cardiovascular disease is the leading cause of mortality in these patients[1,2]. Myosteatosis, a marker of muscle quality, has been proposed as a novel risk factor for atherosclerotic cardiovascular diseases, independent of muscle mass[3-6]. Myosteatosis may lead to a series of metabolic dysfunctions, such as insulin resistance, systematic inflammation, and oxidative stress, and all these dysfunctions are closely associated with the acceleration of T2DM and atherosclerosis (Supplementary Figure 1)[3,7,8].
Computed tomography (CT) is considered the gold standard for myosteatosis measurement, and lower muscle radiodensity indicates higher fat infiltration (i.e., myosteatosis)[9]. Recently, a large-sample study involving 20986 participants indicated that the patients with T2DM had significantly higher values of muscle mass but significantly lower values of muscle quality[10,11]. Therefore, low muscle quality rather than low muscle mass is the major characteristic change of skeletal muscle in patients with T2DM. Patients with T2DM have high risks of myosteatosis and atherosclerosis. However, the association between myosteatosis and coronary artery calcification (CAC) in this population has not been reported yet.
CAC score (CACS), which can be calculated with the Agatston scoring method, is considered a useful tool for identifying coronary atherosclerosis. The risk of coronary events in patients with CACS > 300 across various ethnic groups has a nearly 10-fold increase[12-14]. In Australia, CACS is used to help define the risk in the primary prevention of cardiovascular diseases[15]. The long-term (> 10 years) prognostic value of CACS in cardiovascular diseases has also been validated in patients with T2DM[16].
Herein, we performed this cross-sectional study to analyze the association of myosteatosis with CAC in patients with T2DM. The myosteatosis and CACS were evaluated with abdominal and thoracic CT, respectively.
Patients with T2DM who were hospitalized in the Department of Endocrinology, Affiliated Hospital of Jining Medical University between January 2017 and December 2021 were included in this study. They all underwent abdominal and thoracic CT scans. The exclusion criteria included: (1) Patients with age < 30 or > 80 years old; (2) patients with a history of major cardiovascular events (i.e., myocardial infarction, congestive heart failure, coronary stent implantation, and cerebrovascular accidents); and (3) patients with consumptive or critical diseases (i.e., malignant tumors, abnormal thyroid function, and stage V diabetic nephropathy). At admission, all patients were informed that their medical records may be used for research purposes unless they indicate their opposition. For the present study, no patient indicated opposition. This study was approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University (No. 2021-08-C001).
All biochemical and immune indexes were measured in the laboratory of our hospital. Fasting glucose and C-peptide were measured for calculating homeostasis model assessment 2 of insulin resistance and homeostasis model assessment 2 of beta-cell function (HOMA2-β).
Both abdominal and thoracic CT scans were performed using a Dual-Source Flash CT scanner (Siemens, Erlangen, Germany). The body composition was assessed using abdominal axial CT images at the L3 level and the Slice-O-Matic software (V.5.0, TomoVision, Montreal, Quebec, Canada), as described in our previous study[17]. The CT attenuation thresholds were from -29 to 150 Hounsfield Unit (HU) for skeletal muscle, from -150 to -30 HU for visceral adipose tissue, and from -190 to -30 HU for intramuscular and subcutaneous adipose tissue[18]. The mean skeletal muscle attenuation (MMA), which was automatically calculated by the software, was shown as the mean radiation attenuation of skeletal muscle in HU. Myosteatosis was diagnosed according to Martin’s criteria, i.e. MMA < 33 HU with body mass index (BMI) ≥ 25 kg/m2 or MMA < 41 HU with BMI < 25 kg/m2[19]. The skeletal muscle index (SMI) (cm2/m2) was calculated by normalizing the L3 cross-sectional skeletal muscle area in cm2 to height in m2[20]. The fat mass index (kg/m2), which is proposed by VanItallie et al[21] and is an indicator of nutritional status, was calculated by normalizing fat mass in kg to height in m2[21,22]. The fat mass was calculated with the following formula: fat mass (kg) = 0.042 × (total adipose area at L3 in cm2) + 11.2[22]. The CACS was calculated based on the thoracic CT images by the automated software of syngo via and with the Agatston method. Severe CAC (SCAC) was defined when the CACS was > 300[14].
Coronary heart disease (CHD) was defined as a suspected history of CHD confirmed through CT coronary angiography. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, and/or use of antihypertensive medications. Dyslipidemia was defined as disorders of lipoprotein metabolism and/or the use of lipid medications. Alcohol consumption was defined as consuming at least 30 g of alcohol per week for at least a year. Cigarette smoking was defined as smoking at least 100 cigarettes in a lifetime[23]. Diabetic complications were assessed systematically according to the guidelines for the prevention and control of T2DM in China[24]. Diabetic nephropathy was diagnosed when there was elevated urinary albumin excretion and reduced estimated glomerular filtration rate in the absence of other primary causes of kidney damage. Diabetic peripheral neuropathy referred to the symptoms or signs of peripheral nerve dysfunction in diabetic patients that cannot be attributed to other causes. Asymptomatic patients must be diagnosed by physical examination or neuro-electrophysiological examination. Diabetic retinopathy was diagnosed by an ophthalmologist who specialized in diabetic retinopathy, according to the international clinical grading standard for diabetic retinopathy. Lower-extremity arterial disease was diagnosed if the patients had a resting ankle-brachial index (ABI) ≤ 0.90. For patients who experienced discomfort upon moving and had a resting ABI ≥ 0.90, lower-extremity arterial disease was also diagnosed if the ABI decreased by 15%-20% after a treadmill test.
Continuous variables with normal distribution are presented as mean ± standard deviation, whereas those with non-normal distribution are presented as median and interquartile range. Categorical variables are described by the number and percentage. The characteristics of the study population were compared using independent samples t-test, Mann-Whitney U test, or χ2 test, as appropriate. The variables with statistical significance between the two groups were enrolled in the logistic regression analysis to identify independent factors for SCAC. Receiver operating characteristic (ROC) curves were plotted and the area under the curve (AUC) of independent factors for SCAC was compared using the z-test. The Youden index was calculated to determine the cut-off points of age in distinguishing SCAC. Subgroups were stratified based on the risk factors of atherosclerosis. The Chi-squared Automatic Interaction Detection (CHAID) decision tree analysis was further performed based on the identified independent factors. The minimum parent and child nodes were determined as 100 and 50, respectively. Statistical analysis was performed using SPSS software (V.26.0). The two-sided P value less than 0.05 was considered statistically significant.
A total of 652 patients with T2DM were enrolled in this study. The characteristics of the study population are presented in Table 1. There were 425 (65.2%) males and 227 (34.8%) females. Among the 652 patients, 167 (25.6%) had SCAC and were classified into the T2DM + SCAC group. The remaining patients were classified into the T2DM group. Patients in the T2DM + SCAC group had higher values of age, diabetes duration, fasting glucose, creatinine, blood urea nitrogen, and cystatin C; had higher percentages of myosteatosis, CHD, cigarette smoking, alcohol consumption, aspirin usage, hypertension, diabetic nephropathy, and diabetic retinopathy; and received more types of antidiabetics, lipid-lowering, and antihypertensive drugs. However, they had lower values of hemoglobin, alanine transaminase, low-density lipoprotein, free triiodothyronine, and SMI. The comparison of clinical characteristics of patients with and without myosteatosis is presented in the Supplementary Table 1.
| Variables | All patients (n = 652) | T2DM group (n = 485) | T2DM + SCAC group (n = 167) | P value |
| Male (%) | 425 (65.2) | 312 (64.3) | 113 (67.7) | 0.453 |
| Age (yr) | 55.95 ± 10.87 | 53.75 ± 10.62 | 62.34 ± 8.94 | < 0.001 |
| Diabetes duration (yr) | 8.97 ± 7.13 | 7.87 ± 6.36 | 12.15 ± 8.22 | < 0.001 |
| Body mass index (kg/m2) | 25.79 ± 3.66 | 25.87 ± 3.61 | 25.56 ± 3.79 | 0.358 |
| Fasting glucose (mmol/L) | 7.67 ± 2.23 | 7.53 ± 2.19 | 8.09 ± 2.31 | 0.018 |
| Fasting C peptide (ng/mL) | 2.27 ± 1.06 | 2.25 ± 0.97 | 2.32 ± 1.30 | 0.581 |
| Hemoglobin A1c (%) | 8.71 ± 2.19 | 8.76 ± 2.23 | 8.59 ± 2.06 | 0.384 |
| HOMA2-β | 166.51 ± 93.75 | 169.89 ± 92.31 | 155.97 ± 97.78 | 0.170 |
| HOMA2-IR | 5.62 ± 2.67 | 5.55 ± 2.48 | 5.84 ± 3.20 | 0.314 |
| Hemoglobin (g/L) | 138.69 ± 21.98 | 139.77 ± 22.44 | 135.52 ± 20.30 | 0.032 |
| Albumin (g/L) | 43.14 ± 4.51 | 43.34 ± 4.31 | 42.55 ± 5.01 | 0.071 |
| Alanine transaminase (U/L) | 18.20 (13.30, 27.80) | 19.35 (13.93, 28.90) | 15.90 (12.20, 21.90) | < 0.001 |
| Creatinine (mg/L) | 61.38 ± 16.65 | 60.30 ± 15.78 | 64.49 ± 18.66 | 0.010 |
| Blood urea nitrogen (mg/dL) | 5.65 ± 1.59 | 5.52 ± 1.45 | 6.04 ± 1.90 | < 0.001 |
| Cystatin C (mg/L) | 1.00 ± 0.29 | 0.97 ± 0.25 | 1.11 ± 0.35 | < 0.001 |
| Triglycerides (mmol/L) | 1.44 (0.98, 2.24) | 1.44 (0.98, 2.32) | 1.39 (0.95, 1.99) | 0.199 |
| Total cholesterol (mmol/L) | 4.63 ± 1.56 | 4.70 ± 1.33 | 4.44 ± 2.09 | 0.059 |
| HDL (mmol/L) | 1.18 ± 0.38 | 1.18 ± 0.40 | 1.16 ± 0.33 | 0.506 |
| LDL (mmol/L) | 2.77 ± 1.18 | 2.86 ± 1.22 | 2.53 ± 1.05 | 0.002 |
| FT3 (pmol/L) | 4.53 ± 1.40 | 4.61 ± 1.57 | 4.30 ± 0.71 | 0.016 |
| FT4 (pmol/L) | 16.58 ± 3.04 | 16.57 ± 3.08 | 16.59 ± 2.91 | 0.943 |
| TSH (pmol/L) | 2.25 ± 1.41 | 2.24 ± 1.37 | 2.30 ± 1.54 | 0.597 |
| SBP (mmHg) | 136.23 ± 19.06 | 136.10 ± 19.82 | 136.63 ± 16.72 | 0.736 |
| DBP (mmHg) | 81.13 ± 13.00 | 81.19 ± 13.43 | 80.95 ± 11.68 | 0.840 |
| MMA (HU) | 36.41 ± 7.29 | 37.24 ± 7.23 | 34.02 ± 6.95 | < 0.001 |
| Myosteatosis (%) | 309 (47.4) | 199 (41.0) | 110 (65.9) | < 0.001 |
| CHD (%) | 166 (25.5) | 96 (19.8) | 70 (41.9) | < 0.001 |
| SMI (cm2/m2) | 46.71 ± 9.31 | 47.18 ± 9.30 | 45.34 ± 9.23 | 0.027 |
| FMI (kg/m2) | 8.56 ± 1.79 | 8.51 ± 1.79 | 8.68 ± 1.79 | 0.290 |
| Cigarette smoking (%) | 261 (40.0) | 170 (35.1) | 91 (54.5) | < 0.001 |
| Alcohol intake (%) | 295 (45.2) | 204 (42.1) | 91 (54.5) | 0.007 |
| Dyslipidemia (%) | 369 (56.6) | 282 (58.1) | 87 (52.1) | 0.176 |
| Hypertension (%) | 308 (47.2) | 215 (44.3) | 93 (55.7) | 0.012 |
| Diabetic complications (%) | 564 (86.5) | 410 (84.5) | 154 (92.2) | 0.012 |
| DN (%) | 247 (37.9) | 169 (34.8) | 78 (46.7) | 0.007 |
| DPN (%) | 498 (76.4) | 362 (74.6) | 136 (81.4) | 0.091 |
| LEAD (%) | 105 (16.1) | 73 (15.1) | 32 (19.2) | 0.223 |
| DR (%) | 185 (28.4) | 118 (24.3) | 67 (40.1) | < 0.001 |
| Antidiabetics (%) | 569 (87.3) | 411 (84.7) | 158 (94.6) | 0.001 |
| Insulin (%) | 249 (38.2) | 172 (35.5) | 77 (46.1) | 0.016 |
| Metformin (%) | 434 (66.6) | 310 (63.9) | 124 (74.3) | 0.017 |
| Sulphonylureas (%) | 312 (47.9) | 230 (47.7) | 82 (49.1) | 0.720 |
| Acarbose (%) | 257 (39.4) | 180 (37.1) | 77 (46.1) | 0.044 |
| Others (%) | 167 (25.6) | 120 (24.7) | 47 (28.1) | 0.411 |
| Lipid-lowering drugs (%) | 140 (21.5) | 84 (17.3) | 56 (33.5) | < 0.001 |
| Statins (%) | 131 (20.1) | 75 (15.5) | 56 (33.5) | < 0.001 |
| Fibrates (%) | 9 (1.4) | 9 (1.9) | 0 (0.0) | 0.121 |
| Antihypertensive drugs (%) | 237 (36.3) | 158 (32.6) | 79 (47.3) | 0.001 |
| ACE inhibitors (%) | 33 (5.1) | 20 (4.1) | 13 (7.8) | 0.068 |
| ARBs (%) | 105 (16.1) | 71 (14.6) | 34 (20.4) | 0.088 |
| Calcium antagonists (%) | 134 (20.6) | 89 (18.4) | 45 (26.9) | 0.020 |
| β-Blockers (%) | 64 (9.8) | 36 (7.4) | 28 (16.8) | 0.001 |
| Diuretics (%) | 32 (4.9) | 22 (4.5) | 10 (6.0) | 0.533 |
| Aspirin (%) | 145 (22.2) | 84 (17.3) | 61 (36.5) | < 0.001 |
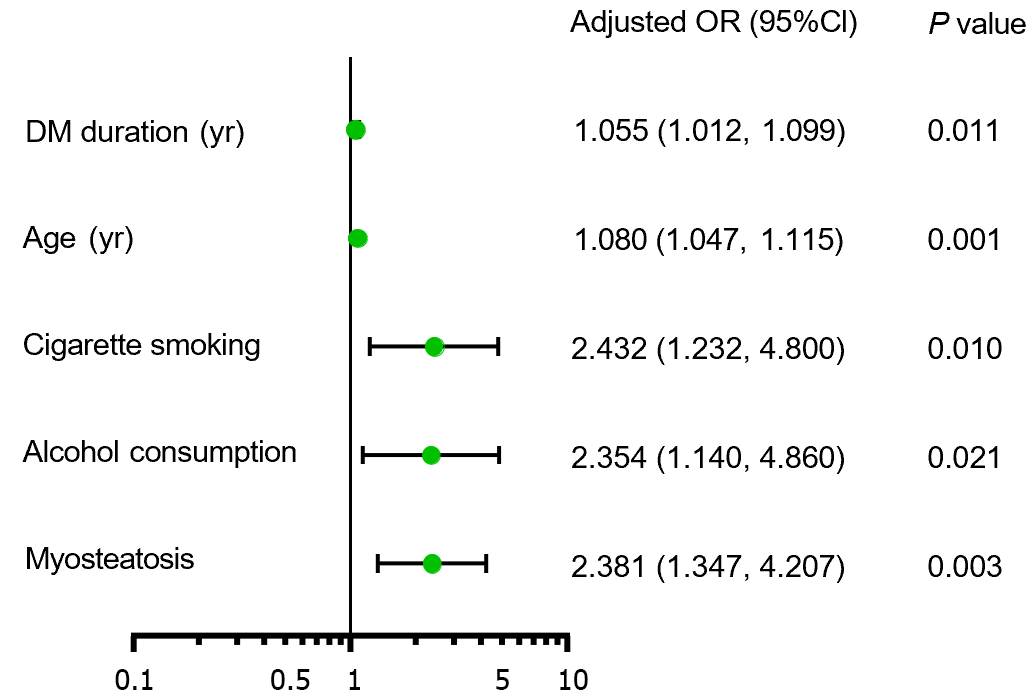
The patients with myosteatosis exhibited significantly higher percentages of SCAC compared with those without myosteatosis (35.6% vs 16.6%). Logistic regression analysis revealed that myosteatosis, age, duration of diabetes, cigarette smoking, and alcohol consumption were independent risk factors for SCAC (Figure 1). Patients with myosteatosis showed an increased risk of SCAC (OR = 2.381, 95%CI: 1.347-4.207, P = 0.003) after adjustment for age, diabetes duration, cigarette smoking, and alcohol consumption.
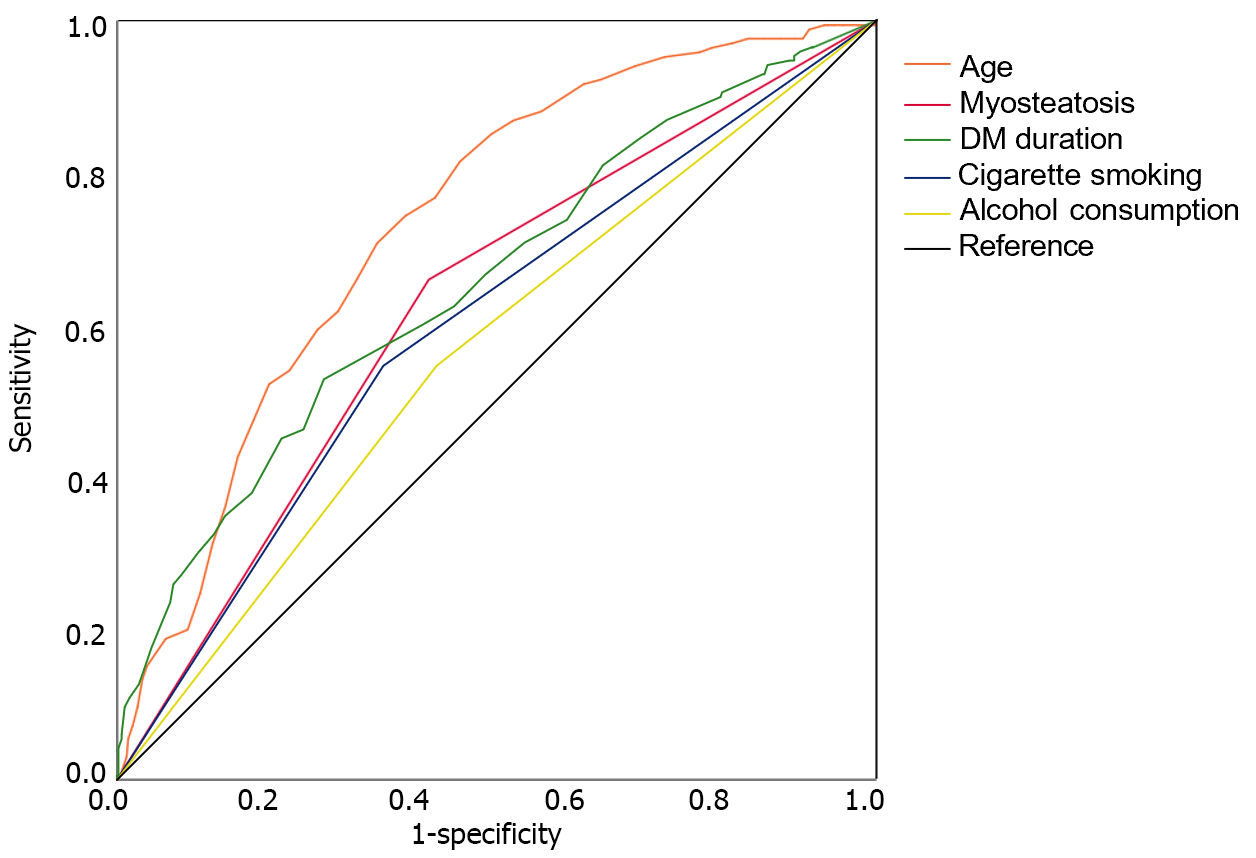
The predictive abilities of the aforementioned five factors for SCAC were evaluated using ROC curve analysis (Figure 2). Age had the highest AUC, followed by duration of diabetes, myosteatosis, cigarette smoking, and drinking. The combined model of the five independent risk factors yielded a higher AUC than age alone (0.794 vs 0.734, P = 0.034).
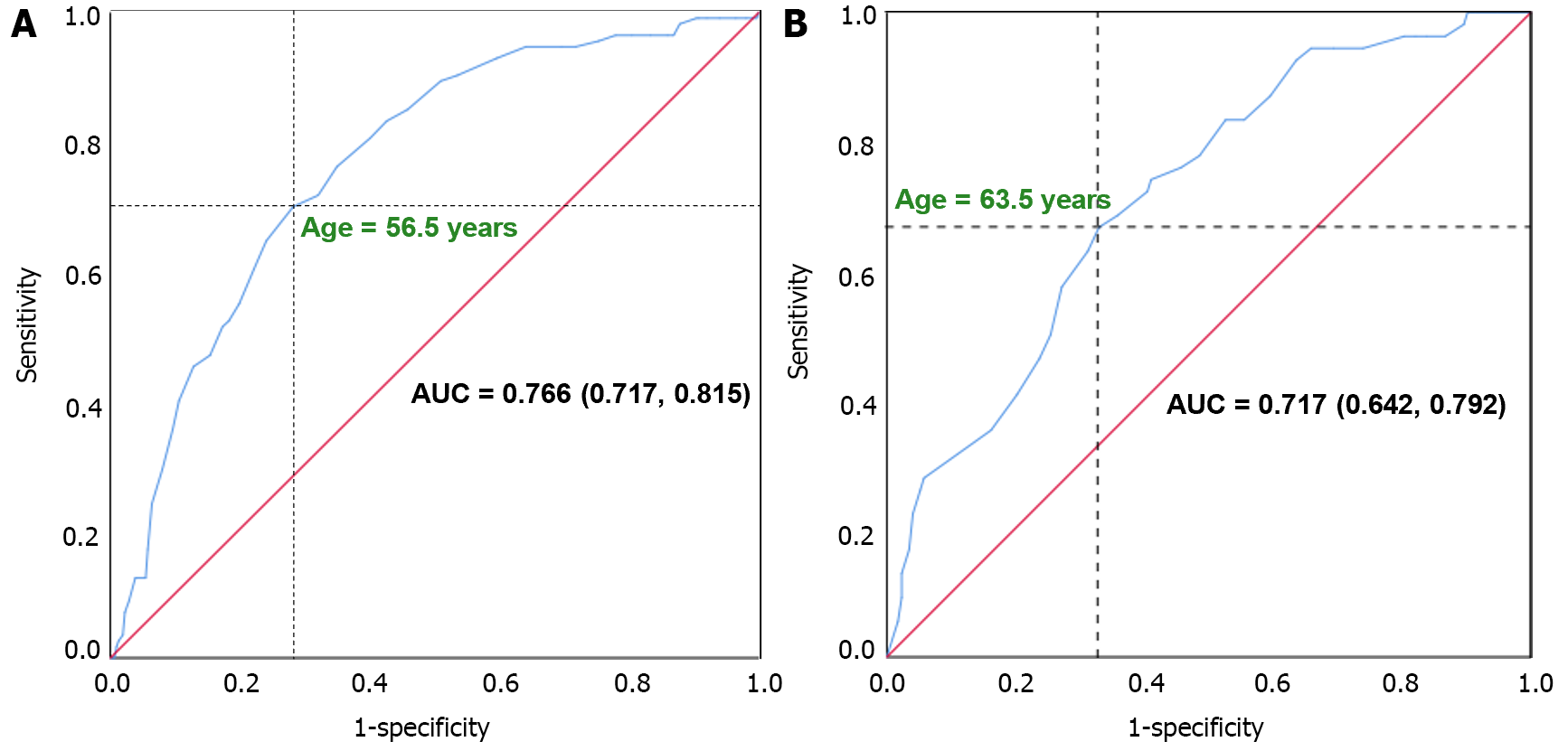
Given the variation in age-specific risk of cardiovascular disease by gender, ROC curve analyses were conducted to determine the cut-off points of age in predicting SCAC. The cut-off points for older age were identified as age > 56.5 years in males and age > 63.5 years in females (Figure 3). Patients in the older age group exhibited significantly higher percentages of SCAC compared to those in the younger age group (47.3% vs 13.2% in males and 38.7% vs 13.4% in females).
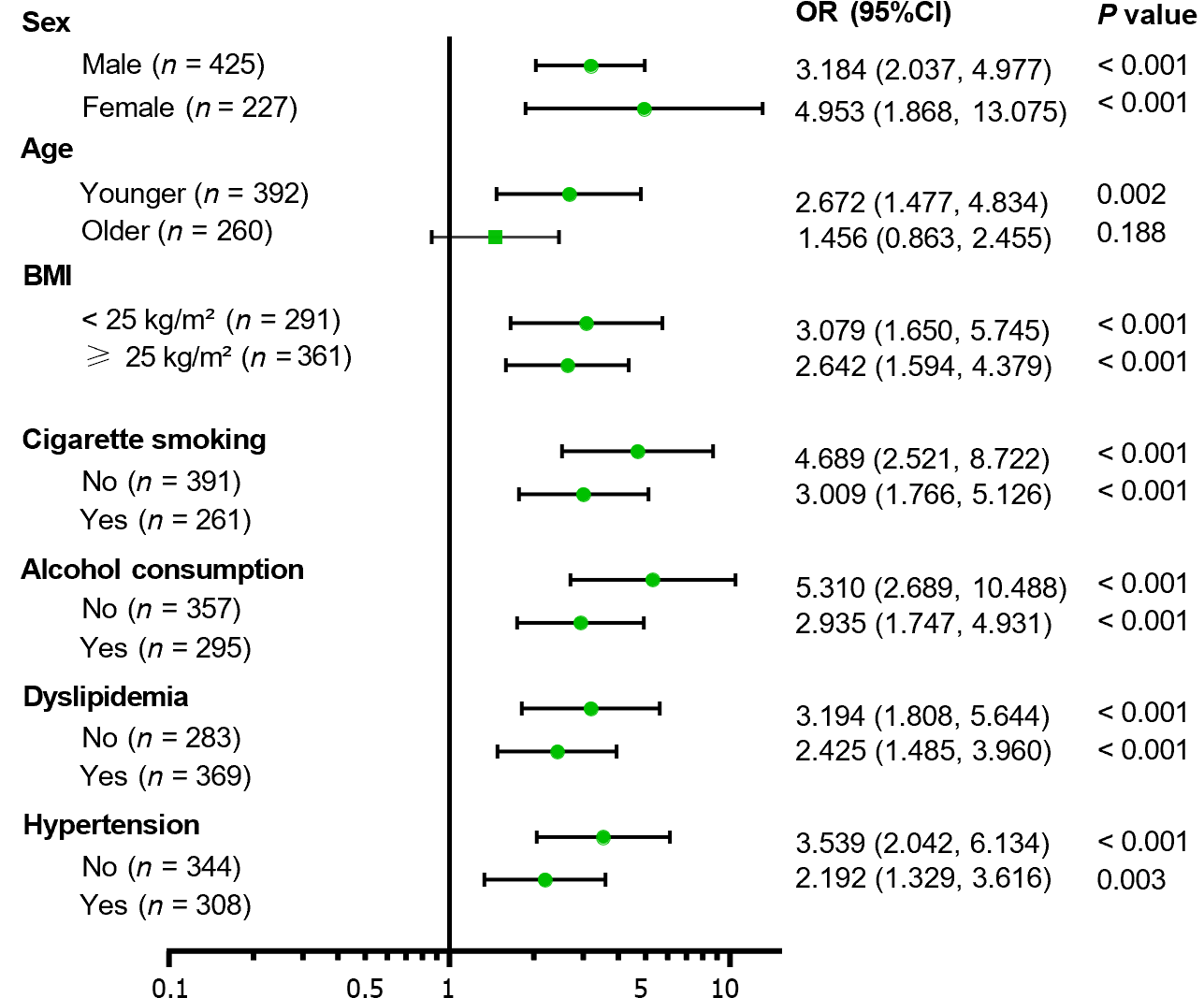
Subgroup stratification based on sex, age, BMI, cigarette smoking, alcohol consumption, dyslipidemia, and hyper-tension was performed (Figure 4). The association between myosteatosis and SCAC was found to be significant in younger patients (OR = 2.672, 95%CI: 1.477-4.834, P = 0.002) rather than in older patients (OR = 1.456, 95%CI: 0.863-2.455, P = 0.188), and was more prominent in patients with a lower risk of atherosclerosis, such as BMI < 25 kg/m2, without cigarette smoking, alcohol consumption, dyslipidemia, and hypertension.
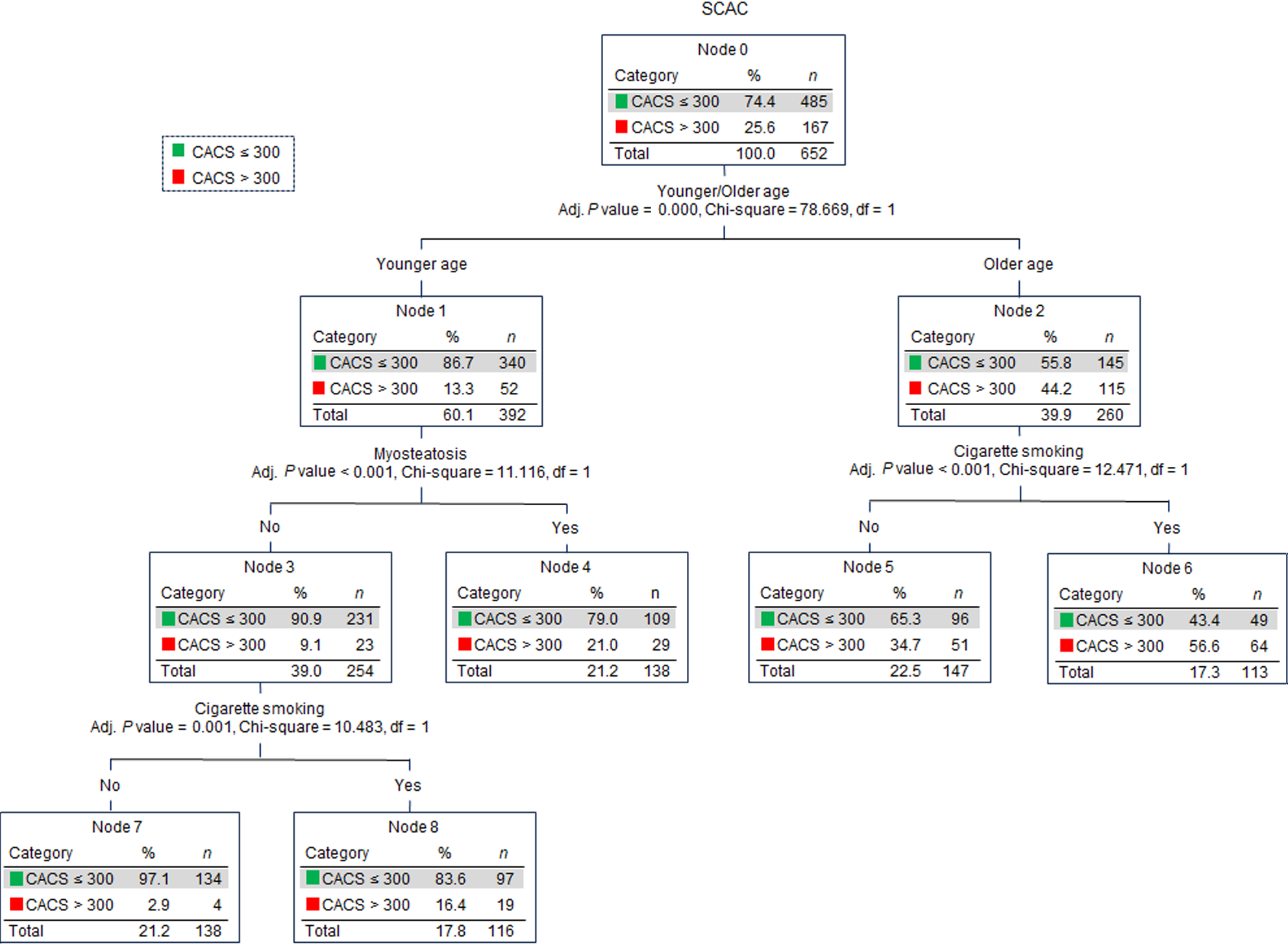
CHAID decision tree analysis was conducted using the older age, myosteatosis, and other significantly different factors between the T2DM + SCAC and T2DM groups. Older age, myosteatosis, and cigarette smoking were determined as critical variables and were included in the construction of the CHAID decision tree (Figure 5). The primary variable for SCAC was older age (OR = 5.186, 95%CI: 3.543-7.590, P < 0.001). Among patients of older age, the primary factor was cigarette smoking (OR = 2.459, 95%CI: 1.486-4.069, P < 0.001), while among younger patients, the primary factor was myosteatosis (OR = 2.672, 95%CI: 1.477-4.834, P = 0.001).
To the best of our knowledge, this is the first study to investigate the relationship of myosteatosis with CAC in patients with T2DM. Logistic regression and CHAID decision tree analyses confirmed that myosteatosis, age, cigarette smoking, and alcohol consumption were independent factors of SCAC. Moreover, the association between myosteatosis and CAC might be more prominent in the younger population.
Two large-sample cross-sectional studies have investigated the relationship of muscle quality with CAC in populations other than T2DM[25]. The Coronary Artery Risk Development in Young Adults study enrolled 3051 participants aged 43 to 55 years and defined CAC with CACS > 0[25]. Compared with those with the lowest quartile, the young adults with the upper quartile of abdominal intermuscular adipose tissue volume had a higher risk of CAC [OR 1.6 (1.2-2.1)] after adjusting for cardiovascular disease risk factors[25]. In another study by Lee et al[4], a total of 4068 subjects without cardiovascular diseases were included and significant CAC was defined if CACS was > 100. They found that the higher ratio of the muscle area with normal attenuation to the total abdominal muscle area was strongly associated with a lower prevalence of significant CAC after adjustment[4]. Different from these two studies, our study focused on patients with T2DM, and this population is associated with high risks of both myosteatosis and CAC. We demonstrated that myosteatosis was significantly associated with SCAC in patients with T2DM, independent of traditional cardiovascular disease risk factors.
In our study, we found that in addition to myosteatosis, factors such as age, duration of diabetes, smoking, and drinking[26-28] were identified as independent risk factors for SCAC. It is worth noting that the age-specific risk of cardiovascular disease varies by gender, being significantly lower in women before menopause[29,30]. We determined the cut-off age values for predicting SCAC to be > 56.5 years in males and > 63.5 years in females. This finding aligns with a previous study, which revealed that the prevalence of CACS > 0 exceeded 25% in young males with at least one risk factor by the age of 40, and in young females with at least one traditional risk factor by the age of 50[31].
Muscle mass has been regarded as a predictor for coronary atherosclerosis in previous studies[32,33]. However, these studies are limited by the use of dual-energy X-ray absorptiometry or bioelectrical impedance analysis, which are not allowed to be used to evaluate muscle quality. In our study, both logistic regression and CHAID decision tree analyses showed no significant association between SMI and SCAC, even when SMI was transferred into a binary variable according to the diagnostic criteria of low muscle mass (data not shown)[9]. This result is consistent with the study by Lee et al[4], which assessed the association between muscle quality and CAC in the general population. Therefore, myosteatosis might play a more important role than low muscle mass in the development of CAC, especially in the population with T2DM.
CHAID algorithm for decision tree analysis was used to visualize the relationship between SCAC and related factors in an easy-to-interpret tree image. Myosteatosis was a primary factor for SCAC in younger patients and was associated with a more than two-fold increased risk of SCAC. Therefore, the occurrence of severe atherosclerosis in certain younger individuals might be attributed to myosteatosis. It is important to note that while myosteatosis was not included in the CHAID decision tree analysis for the older age subgroup, the quality of muscle in elderly patients remains significant. Our study revealed that approximately 65.8% of older patients were diagnosed with myosteatosis (data not shown), and therefore, myosteatosis cannot truly reflect the difference in their muscle quality. Thus, large epidemiological studies are needed to establish an improved criterion for myosteatosis based on age, especially for myosteatosis in elderly individuals.
In addition to CAC, we also assessed the associations of myosteatosis with diabetes complications, hormonal status, and medication usage. Although no difference was found in the risk of diabetes complications, patients with myosteatosis exhibited a higher risk of CHD. This finding supports our conclusion regarding the association between myosteatosis with SCAC. Hormonal status plays a crucial role in maintaining muscle health. In this cross-sectional study, patients with myosteatosis showed no significant differences in the levels of thyroid hormones. Further research is necessary to evaluate the association of myosteatosis with other hormones, including growth hormone, estrogen, testosterone, and adrenal hormones. Patients with myosteatosis had a higher prevalence of insulin, statins, and aspirin usage. However, this does not imply that these medications induce myosteatosis, as patients with myosteatosis require these medications due to their elevated risk of CHD and lower levels of HOMA2-β.
Our study has several limitations. First, the characteristics of the cross-sectional study limited the further exploration of the causal inference and the clarification of the underlying pathophysiological mechanism between myosteatosis and coronary atherosclerosis. Second, we did not assess the muscle function (e.g., handgrip strength and gait speed), which is highly associated with muscle quality[34]. Third, some information that may be associated with CAC, such as the family history of premature cardiovascular disease and the physical activity of patients, was missing. Fourth, our study did not analyze the association of myosteatosis with the features of plaque vulnerability, such as volume and density, which may have opposite relationships with cardiovascular events[35]. Lastly, because our study was conducted in Chinese adults with T2DM, the findings may not be readily generalizable to other populations or ethnicities.
However, our study also has several strengths. First, this study included a large sample of 652 individuals and used CT-derived measures of both myosteatosis and SCAC. Second, our study focused on patients with T2DM and this population has a high prevalence of both myosteatosis and atherosclerotic cardiovascular diseases. Third, most of the important biochemical variables were available and all the diabetic complications were assessed by professional clinicians. Fourth, the CHAID decision tree analysis highlighted that the association between myosteatosis and SCAC might be more prominent in individuals with younger ages and lower risks of atherosclerosis. This is a novel finding of our study.
In conclusion, myosteatosis was a novel risk factor for atherosclerosis in patients with T2DM, especially in the population with younger ages or fewer traditional risk factors. This suggests the potential benefit of initiating muscle-strengthening exercises and improving muscle quality at a younger age. Further follow-up studies are warranted to validate the role of myosteatosis in cardiovascular events or mortality in patients with T2DM.
Myosteatosis rather than low muscle mass is the major etiologic factor of sarcopenia in patients with type 2 diabetes mellitus (T2DM). Myosteatosis may lead to a series of metabolic dysfunctions which are closely associated with acceleration of T2DM and atherosclerosis.
The association between myosteatosis and coronary atherosclerosis in patients with T2DM has not been reported yet.
To investigate the association between myosteatosis and coronary artery calcification (CAC) in patients with T2DM.
Severe CAC (SCAC) was defined when the CAC score was > 300. Logistic regression and decision tree analyses were performed to assess the association between myosteatosis and SCAC.
Myosteatosis was significantly associated with increased risk of SCAC. The association between myosteatosis and SCAC was significant in the younger, rather than older patients, and was more prominent in the population with lower risks of atherosclerosis.
In the patients with older age, the main factor for SCAC was cigarette smoking, while in the patients with younger age, the main factor was myosteatosis.
Myosteatosis was a novel risk factor of atherosclerosis in patients with T2DM, especially in the population with younger age or lower traditional risk factors.
Follow-up studies are warranted to confirm the role of myosteatosis in cardiovascular events or mortality in patients with T2DM.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai L, United States; Kotlyarov S, Russia; Wu QN, China S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, Zhang X, Li C, Huang Z, Sun X, Wang L, Zhou M, Wu J, Wang Y. Prevalence and Treatment of Diabetes in China, 2013-2018. JAMA. 2021;326:2498-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 561] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 2. | Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 366] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 3. | Miljkovic I, Kuipers AL, Cauley JA, Prasad T, Lee CG, Ensrud KE, Cawthon PM, Hoffman AR, Dam TT, Gordon CL, Zmuda JM; Osteoporotic Fractures in Men Study Group. Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. J Gerontol A Biol Sci Med Sci. 2015;70:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Lee MJ, Kim HK, Kim EH, Bae SJ, Kim KW, Kim MJ, Choe J. Association Between Muscle Quality Measured by Abdominal Computed Tomography and Subclinical Coronary Atherosclerosis. Arterioscler Thromb Vasc Biol. 2021;41:e128-e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Keddar M, Muylle T, Carrie E, Trefois P, Nachit M, Crott R, Christiaens C, Bammens B, Jadoul M, Goffin E, Morelle J. Non-invasive Quantification of Fat Deposits in Skeletal Muscle Predicts Cardiovascular Outcome in Kidney Failure. Front Physiol. 2020;11:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Morel A, Ouamri Y, Canouï-Poitrine F, Mulé S, Champy CM, Ingels A, Audard V, Luciani A, Grimbert P, Matignon M, Pigneur F, Stehlé T. Myosteatosis as an independent risk factor for mortality after kidney allograft transplantation: a retrospective cohort study. J Cachexia Sarcopenia Muscle. 2022;13:386-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 7. | Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018;98:2133-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1764] [Article Influence: 252.0] [Reference Citation Analysis (0)] |
| 8. | Neeland IJ, Poirier P, Després JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137:1391-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 9. | Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography: A Systematic Review. J Gerontol A Biol Sci Med Sci. 2019;74:1671-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 10. | Kim EH, Kim HK, Lee MJ, Bae SJ, Kim KW, Choe J. Association between type 2 diabetes and skeletal muscle quality assessed by abdominal computed tomography scan. Diabetes Metab Res Rev. 2022;38:e3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Kim HK, Kim CH. Quality Matters as Much as Quantity of Skeletal Muscle: Clinical Implications of Myosteatosis in Cardiometabolic Health. Endocrinol Metab (Seoul). 2021;36:1161-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5194] [Cited by in RCA: 5843] [Article Influence: 166.9] [Reference Citation Analysis (0)] |
| 13. | Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1777] [Article Influence: 296.2] [Reference Citation Analysis (0)] |
| 14. | Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2093] [Cited by in RCA: 2210] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 15. | Jennings GL, Audehm R, Bishop W, Chow CK, Liaw ST, Liew D, Linton SM. National Heart Foundation of Australia: position statement on coronary artery calcium scoring for the primary prevention of cardiovascular disease in Australia. Med J Aust. 2021;214:434-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Malik S, Zhao Y, Budoff M, Nasir K, Blumenthal RS, Bertoni AG, Wong ND. Coronary Artery Calcium Score for Long-term Risk Classification in Individuals With Type 2 Diabetes and Metabolic Syndrome From the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol. 2017;2:1332-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 17. | Yang Q, Zhang M, Sun P, Li Y, Xu H, Wang K, Shen H, Ban B, Liu F. Cre/CysC ratio may predict muscle composition and is associated with glucose disposal ability and macrovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Albersheim J, Sathianathen NJ, Zabell J, Renier J, Bailey T, Hanna P, Konety BR, Weight CJ. Skeletal Muscle and Fat Mass Indexes Predict Discharge Disposition after Radical Cystectomy. J Urol. 2019;202:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1512] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 20. | Loosen SH, Schulze-Hagen M, Püngel T, Bündgens L, Wirtz T, Kather JN, Vucur M, Paffenholz P, Demir M, Bruners P, Kuhl C, Trautwein C, Tacke F, Luedde T, Koch A, Roderburg C. Skeletal Muscle Composition Predicts Outcome in Critically Ill Patients. Crit Care Explor. 2020;2:e0171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 672] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 22. | Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1653] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 23. | Xu S, Ming J, Jia A, Yu X, Cai J, Jing C, Liu C, Ji Q. Normal weight obesity and the risk of diabetes in Chinese people: a 9-year population-based cohort study. Sci Rep. 2021;11:6090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Weng J, Ji L, Jia W, Lu J, Zhou Z, Zou D, Zhu D, Chen L, Guo L, Guo X, Ji Q, Li Q, Li X, Liu J, Ran X, Shan Z, Shi L, Song G, Yang L, Yang Y, Yang W; Chinese Diabetes Society. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32:442-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 25. | Terry JG, Shay CM, Schreiner PJ, Jacobs DR Jr, Sanchez OA, Reis JP, Goff DC Jr, Gidding SS, Steffen LM, Carr JJ. Intermuscular Adipose Tissue and Subclinical Coronary Artery Calcification in Midlife: The CARDIA Study (Coronary Artery Risk Development in Young Adults). Arterioscler Thromb Vasc Biol. 2017;37:2370-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234:16812-16823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 593] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 27. | Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 28. | Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 404] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 29. | Gao Z, Chen Z, Sun A, Deng X. Gender differences in cardiovascular disease. Med Nov Technol Devices. 2019;4:100025. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 30. | Kane AE, Howlett SE. Differences in Cardiovascular Aging in Men and Women. Adv Exp Med Biol. 2018;1065:389-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Dzaye O, Razavi AC, Dardari ZA, Shaw LJ, Berman DS, Budoff MJ, Miedema MD, Nasir K, Rozanski A, Rumberger JA, Orringer CE, Smith SC Jr, Blankstein R, Whelton SP, Mortensen MB, Blaha MJ. Modeling the Recommended Age for Initiating Coronary Artery Calcium Testing Among At-Risk Young Adults. J Am Coll Cardiol. 2021;78:1573-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 32. | Jun JE, Kang M, Jin SM, Kim K, Hwang YC, Jeong IK, Kim JH. Additive effect of low skeletal muscle mass and abdominal obesity on coronary artery calcification. Eur J Endocrinol. 2021;184:867-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Knowles R, Carter J, Jebb SA, Bennett D, Lewington S, Piernas C. Associations of Skeletal Muscle Mass and Fat Mass With Incident Cardiovascular Disease and All-Cause Mortality: A Prospective Cohort Study of UK Biobank Participants. J Am Heart Assoc. 2021;10:e019337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7810] [Article Influence: 1301.7] [Reference Citation Analysis (1)] |
| 35. | Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 478] [Article Influence: 43.5] [Reference Citation Analysis (0)] |