Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.418
Peer-review started: October 28, 2023
First decision: December 23, 2023
Revised: January 5, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 15, 2024
Processing time: 138 Days and 17.3 Hours
The prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD) is rapidly increasing, currently affecting approximately 25% of the global popu
To investigate the predictive value of angiopoietin-like protein 8 (ANGPTL8) in MAFLD and its progression.
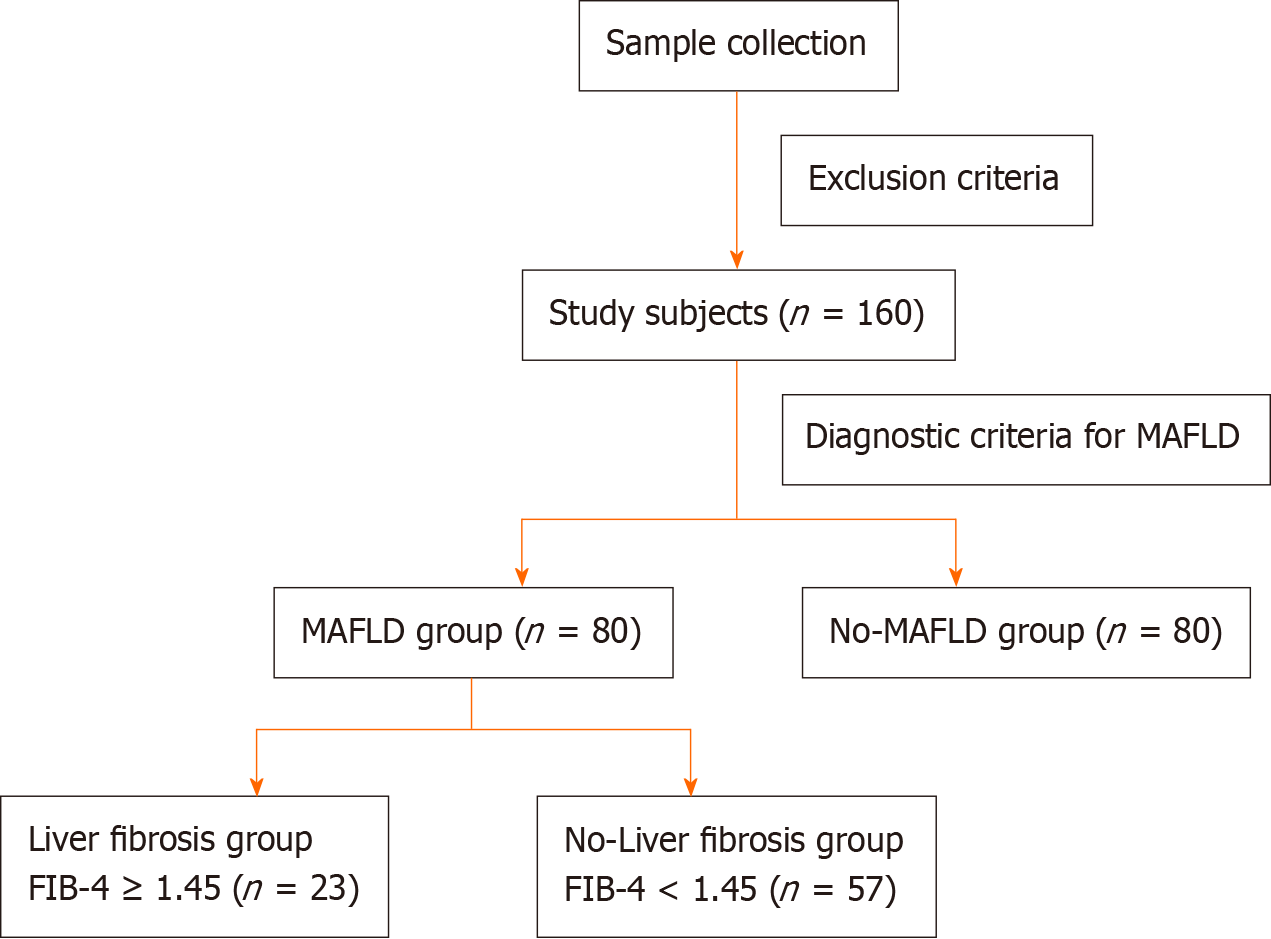
We analyzed 160 patients who underwent abdominal ultrasonography in the Endocrinology Department, Xiaogan Central Hospital affiliated to Wuhan University of Science and Technology, during September 2021-July 2022. Using abdominal ultrasonography and MAFLD diagnostic criteria, among the 160 patients, 80 patients (50%) were diagnosed with MAFLD. The MAFLD group was divided into the liver fibrosis group (n = 23) and non-liver fibrosis group (n = 57) by using a cut-off fibrosis-4 index ≥ 1.45. Logistical regression was used to analyze the risk of MAFLD and the risk factors for its progression. Receiver operating characteristic curves were used to evaluate the predictive value of serum ANGPTL8 in MAFLD and its progression.
Compared with non-MAFLD patients, MAFLD patients had higher serum ANGPTL8 and triglyceride-glucose (TyG) index (both P < 0.05). Serum ANGPTL8 (r = 0.576, P < 0.001) and TyG index (r = 0.473, P < 0.001) were positively correlated with MAFLD. Serum ANGPTL8 was a risk factor for MAFLD [odds ratio (OR): 1.123, 95% confidence interval (CI): 1.066-1.184, P < 0.001). Serum ANGPTL8 and ANGPTL8 + TyG index predicted MAFLD [area under the curve (AUC): 0.832 and 0.886, respectively; both P < 0.05]. Compared with MAFLD patients without fibrosis, those with fibrosis had higher serum ANGPTL8 and TyG index (both P < 0.05), and both parameters were positively correlated with MAFLD-associated fibrosis. Elevated serum ANGPTL8 (OR: 1.093, 95%CI: 1.044-1.144, P < 0.001) and TyG index (OR: 2.383, 95%CI: 1.199-4.736, P < 0.013) were risk factors for MAFLD-associated fibrosis. Serum ANGPTL8 and ANGPTL8 + TyG index predicted MAFLD-associated fibrosis (AUC: 0.812 and 0.835, respectively; both P < 0.05).
The serum levels of ANGPTL8 are elevated and positively correlated with MAFLD. They can serve as predictors for the risk of MAFLD and liver fibrosis, with the ANGPTL8 + TyG index potentially exhibiting even higher predictive value.
Core Tip: This study unveils elevated serum levels of angiopoietin-like protein 8 (ANGPTL8) in individuals with metabolic dysfunction-associated fatty liver disease (MAFLD). Serum ANGPTL8 emerges not only as a predictive factor for MAFLD risk but also as a powerful indicator for the presence of liver fibrosis in MAFLD. The amalgamation of serum ANGPTL8 with the triglyceride-glucose index demonstrates potential for heightened predictive accuracy in both conditions. Further exploration of serum ANGPTL8 holds promise for enhancing clinical strategies in the prevention and treatment of MAFLD.
- Citation: Gan LL, Xia C, Zhu X, Gao Y, Wu WC, Li Q, Li L, Dai Z, Yan YM. Predictive value of angiopoietin-like protein 8 in metabolic dysfunction-associated fatty liver disease and its progression: A case-control study. World J Diabetes 2024; 15(3): 418-428
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/418.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.418
Metabolic dysfunction-associated fatty liver disease (MAFLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is a collective term that encompasses a range of liver diseases from simple steatosis, non-alcoholic steatohepatitis, and liver fibrosis to MAFLD-related cirrhosis and hepatocellular carcinoma[1]. All histological stages of MAFLD are significantly associated with increased overall mortality, and as the disease progresses, the overall mortality increases[2]. The National Health and Nutrition Examination Survey also reported that MAFLD and its associated metabolic disorders are significantly associated with overall and cardiovascular mortality; in addition, the survey found a significant upward trend in the prevalence of MAFLD, which has increased by 14% in the past 3 decades[3]. Nevertheless, there is still a lack of simple and effective methods for the diagnosis of this disease, so it is crucial to find a convenient laboratory index for the early identification of MAFLD and for monitoring its progression.
Angiopoietin-like protein 8 (ANGPTL8) is a 22-kDa protein composed of 198 amino acids; the ANGPTL8 gene is located on chromosome 19p13.2. ANGPTL8 is mainly expressed in the liver, white adipose tissue, and brown adipose tissue, and plays an important role in lipid flux, glucose regulation, and chronic inflammation[4,5]. Many studies have shown that the ANGPTL8 concentration is significantly higher in patients with metabolic diseases such as diabetes[6], obesity[7], and metabolic syndrome[8] than in healthy individuals. Since the pathogenesis of MAFLD is driven by metabolic disorders and changes in glucose-insulin homeostasis, we speculated that ANGPTL8 may be associated with MAFLD. To validate this hypothesis and explore the relationship between MAFLD and ANGPTL8, we evaluated the value of ANGPTL8 in predicting MAFLD and its progression. We hope to provide a simple reference index for the early prediction and prompt management of MAFLD in clinical practice.
We collected the clinical data of 160 patients who underwent abdominal ultrasound examination and were admitted to the Endocrinology Department of Xiaogan Central Hospital, Wuhan University of Science and Technology, between September 2021 and July 2022. The following exclusion criteria were applied: (1) Age < 18 years or > 85 years; (2) patients with mental illness or malignant tumor; (3) patients with autoimmune diseases, active viral hepatitis, drug-induced liver damage, genetic diseases, or chronic schistosomiasis; and (4) patients with acute infection or severe cardiopulmonary, kidney, or cerebrovascular diseases or malnutrition. A total of 160 patients were enrolled according to the above criteria. MAFLD was diagnosed using a combination of abdominal ultrasound findings and relevant diagnostic criteria from the International Expert Consensus on MAFLD[1]: Evidence of fat accumulation in the liver combined with one of the following three criteria, namely, overweight/obesity, presence of type 2 diabetes mellitus, or evidence of metabolic dysregulation (type 2 diabetes was diagnosed using the 1999 World Health Organization diabetes diagnostic criteria). Patients with MAFLD were further divided into those with hepatic fibrosis [defined as fibrosis-4 (FIB-4) index ≥ 1.45] and those without hepatic fibrosis (FIB-4 index < 1.45). The study protocol was approved by the ethics committee of Xiaogan Central Hospital, and all study subjects signed informed consent forms.
The age, gender, medical history, height, weight, waist circumference, hip circumference, and blood pressure of all patients were obtained from their medical records. The levels of the following indicators were collected through the Hospital Information Management System: Platelet count (PLT), alanine transaminase (ALT), aspartate transaminase (AST), triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and C-reactive protein (CRP). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = [FPG (mmol/L) × fasting insulin (FINS) (µU/mL)]/22.5, where FINS stands for fasting insulin level. The homeostasis model assessment of β-cell function (HOMA-β) was calculated using the following formula: HOMA-β = [20 × FINS (µU/mL)]/[FPG (mmol/L) - 3.5]. The TG-glucose (TyG) index was calculated using the formula: TyG = Ln [TG (mg/dL) × FPG (mg/mL)/2]. Liver fibrosis was evaluated using the non-invasive FIB-4 index, which was calculated as follows: FIB-4 = [age (years) × AST (U/L)]/[PLT (× 109/L) × ALT1/2 (U/L)]. FIB-4 index ≥ 1.45 indicated the presence of liver fibrosis[9,10].
Serum ANGPTL8 concentration was measured using an enzyme-linked immunosorbent assay kit (Wuhan Huamei Biological Engineering Co. Ltd., product number: CSB-EL028107HU, detection range: 6.25-400 pg/mL, sensitivity: 1.56 pg/mL).
Data were analyzed and processed using SPSS (version 27.0) statistical software. The normality of the data was tested using the Shapiro-Wilk test. Normally distributed data were expressed as mean ± SD and compared using one-way analysis of variance. Non-normally distributed data were expressed as median and interquartile range, and compared using the Wilcoxon rank-sum test. Count data were expressed as percentages and compared using the chi-square test. Spearman correlation analysis was used to analyze the correlation of MAFLD with various indicators as well as the correlation of MAFLD-associated liver fibrosis with ANGPTL8, TyG index, and CRP. Binary logistic regression analysis was used to analyze the risk factors for MAFLD and MAFLD-associated liver fibrosis. Receiver operating characteristic (ROC) curves were used to evaluate the predictive value of ANGPTL8 for the risk of MAFLD and liver fibrosis (FIB-4 ≥ 1.45). Differences with P < 0.05 were considered statistically significant.
A flow chart of subject selection is presented in Figure 1, and the clinical characteristics of the study subjects are listed in Table 1. A total of 160 patients were enrolled, including 90 men (56.3%) and 70 women (43.7%). The age of the included population ranged from 18 to 85 years. Among the 160 patients, 80 patients (50%) were diagnosed with MAFLD. Among the patients with MAFLD, 23 patients had liver fibrosis, and 57 patients did not have liver fibrosis, based on the FIB-4 index. The following factors did not significantly differ between the MAFLD and non-MAFLD groups: age, gender, systolic blood pressure, diastolic blood pressure, mean arterial pressure, FPG, and HOMA-β (P > 0.05). In contrast, body mass index (BMI), waist-to-hip ratio (WHR), HbA1c, FINS, HOMA-IR, TC, TG, TG/HDL-C ratio, LDL-C/HDL-C ratio, CRP, FIB-4 index, TyG index, and ANGPTL8 level were significantly higher in the MAFLD group than in the non-MAFLD group (P < 0.05 for all), while HDL-C was significantly lower in the MAFLD group than in the non-MAFLD group (P = 0.015). The LDL-C was also higher in the MAFLD group than in the non-MAFLD group (2.28 ± 0.99 vs 2.51 ± 1.05 mmol/L), but the difference was not statistically significant (P = 0.144). Finally, the ALT level [14.00 (10.00, 22.75) vs 18.00 (12.00, 25.75) U/L] was significantly higher in the MAFLD group (P = 0.014), while the AST level did not significantly differ between the 2 groups (P = 0.181).
| Non-MAFLD group (n = 80) | MAFLD group (n = 80) | P valuea | |
| Sex (female/male) | 37/43 | 33/47 | 0.5241 |
| Age (yr) | 55.35 ± 12.77 | 54.70 ± 12.15 | 0.7452 |
| WHR | 0.89 ± 0.09 | 0.92 ± 0.07 | 0.0392 |
| BMI (kg/m2) | 22.06 (20.16, 24.64) | 24.41 (22.89, 27.61) | < 0.0013 |
| SBP (mmHg) | 131.00 (121.75, 147.25) | 136.00 (125.00, 150.00) | 0.1933 |
| DBP (mmHg) | 83.50 (75.75, 94.00) | 87.00 (79.00, 92.00) | 0.2373 |
| MAP (mmHg) | 100.00 (91.00, 112.25) | 104.00 (95.00, 111.00) | 0.1513 |
| ALT (U/L) | 14.00 (10.00, 22.75) | 18.00 (12.00, 25.75) | 0.0143 |
| AST (U/L) | 18.00 (15.75, 22.00) | 19.50 (15.00, 24.00) | 0.1813 |
| TC (mmol/L) | 4.29 (3.55, 4.96) | 4.39 (3.61, 5.54) | 0.2223 |
| TG (mmol/L) | 1.04 (0.62, 1.54) | 2.69 (1.39, 4.35) | < 0.0013 |
| HDL-C (mmol/L) | 1.31 (1.04, 1.60) | 1.17 (0.85, 1.41) | 0.0153 |
| LDL-C (mmol/L) | 2.28 ± 0.99 | 2.51 ± 1.05 | 0.1442 |
| TG/HDL-C | 0.84 (0.46, 1.33) | 2.73 (1.14, 4.24) | < 0.0013 |
| LDL-C/HDL-C | 1.71 (1.26, 2.29) | 2.06 (1.59, 2.92) | 0.0053 |
| HbA1c (%) | 6.00 (5.44, 8.70) | 7.00 (6.13, 8.58) | 0.0183 |
| FPG (mmol/L) | 6.58 (5.63, 8.68) | 6.67 (5.93, 9.28) | 0.3703 |
| FINS (μU∕mL) | 7.01 (4.98, 9.72) | 10.00 (5.97, 13.66) | 0.0043 |
| HOMA-β (%) | 50.10 (23.25, 87.63) | 55.29 (33.96, 94.46) | 0.1563 |
| HOMA-IR | 2.08 (1.40, 3.34) | 2.99 (1.73, 5.50) | 0.0083 |
| CRP (mg/L) | 1.57 (0.98, 2.21) | 1.74 (1.19, 2.90) | 0.0203 |
| ANGPTL8 (pg/mL) | 23.95 (16.52, 34.20) | 44.93 (33.41, 58.01) | < 0.0013 |
| FIB-4 index | 1.18 (0.96, 1.46) | 1.39 (1.23, 1.76) | < 0.0013 |
| TyG index | 8.66 ± 0.71 | 9.56 ± 0.92 | < 0.0012 |
Spearman correlation analysis was performed to assess the correlation of the indicators with significant between-group differences with MAFLD. The results are shown in Table 2. Serum ANGPTL8, TyG index, FIB-4 index, BMI, WHR, TG, ALT, FINS, HbA1c, HOMA-IR, CRP, TG/HDL-C, and LDL-C/HDL-C were positively correlated with MAFLD (P < 0.05), while HDL-C was negatively correlated with MAFLD (r = -0.195, P = 0.014). Further, binary logistic regression analysis of the potential risk factors for MAFLD was performed (Table 3). The results showed that ANGPTL8, BMI, and TG had a statistically significant impact on the risk of MAFLD (P < 0.05). Serum ANGPTL8 was a risk factor for the occurrence of MAFLD.
| r | P valuea | |
| ANGPTL8 | 0.576 | < 0.001 |
| TyG index | 0.473 | < 0.001 |
| FIB-4 index | 0.318 | < 0.001 |
| BMI | 0.394 | < 0.001 |
| WHR | 0.172 | 0.031 |
| TG | 0.581 | < 0.001 |
| HDL-C | -0.195 | 0.014 |
| ALT | 0.195 | 0.014 |
| FINS | 0.231 | 0.003 |
| HbA1c | 0.189 | 0.017 |
| HOMA-IR | 0.211 | 0.008 |
| CRP | 0.189 | 0.018 |
| TG/HDL-C | 0.551 | < 0.001 |
| LDL-C/HDL-C | 0.226 | 0.004 |
| B | S.E. | Wals | P valuea | EXP(B) | 95%CI | |
| ANGPTL8 | 0.116 | 0.027 | 19.118 | < 0.001 | 1.123 | 1.066-1.184 |
| BMI | 0.275 | 0.111 | 6.142 | 0.013 | 1.317 | 1.059-1.637 |
| ALT | 0.032 | 0.021 | 2.394 | 0.122 | 1.032 | 0.992-1.075 |
| HDL-C | -0.386 | 0.804 | 0.231 | 0.631 | 0.680 | 0.141-3.287 |
| FINS | 0.085 | 0.091 | 0.875 | 0.350 | 1.088 | 0.911-1.300 |
| HbA1c | -0.248 | 0.176 | 1.982 | 0.159 | 0.780 | 0.552-1.102 |
| HOMA-IR | -0.426 | 0.251 | 2.869 | 0.090 | 0.653 | 0.399-1.069 |
| CRP | 0.160 | 0.088 | 3.285 | 0.070 | 1.173 | 0.987-1.395 |
| WHR | -3.793 | 4.066 | 0.870 | 0.351 | 0.023 | 0.000-65.098 |
| TG | 2.255 | 0.527 | 18.277 | < 0.001 | 9.532 | 3.391-26.800 |
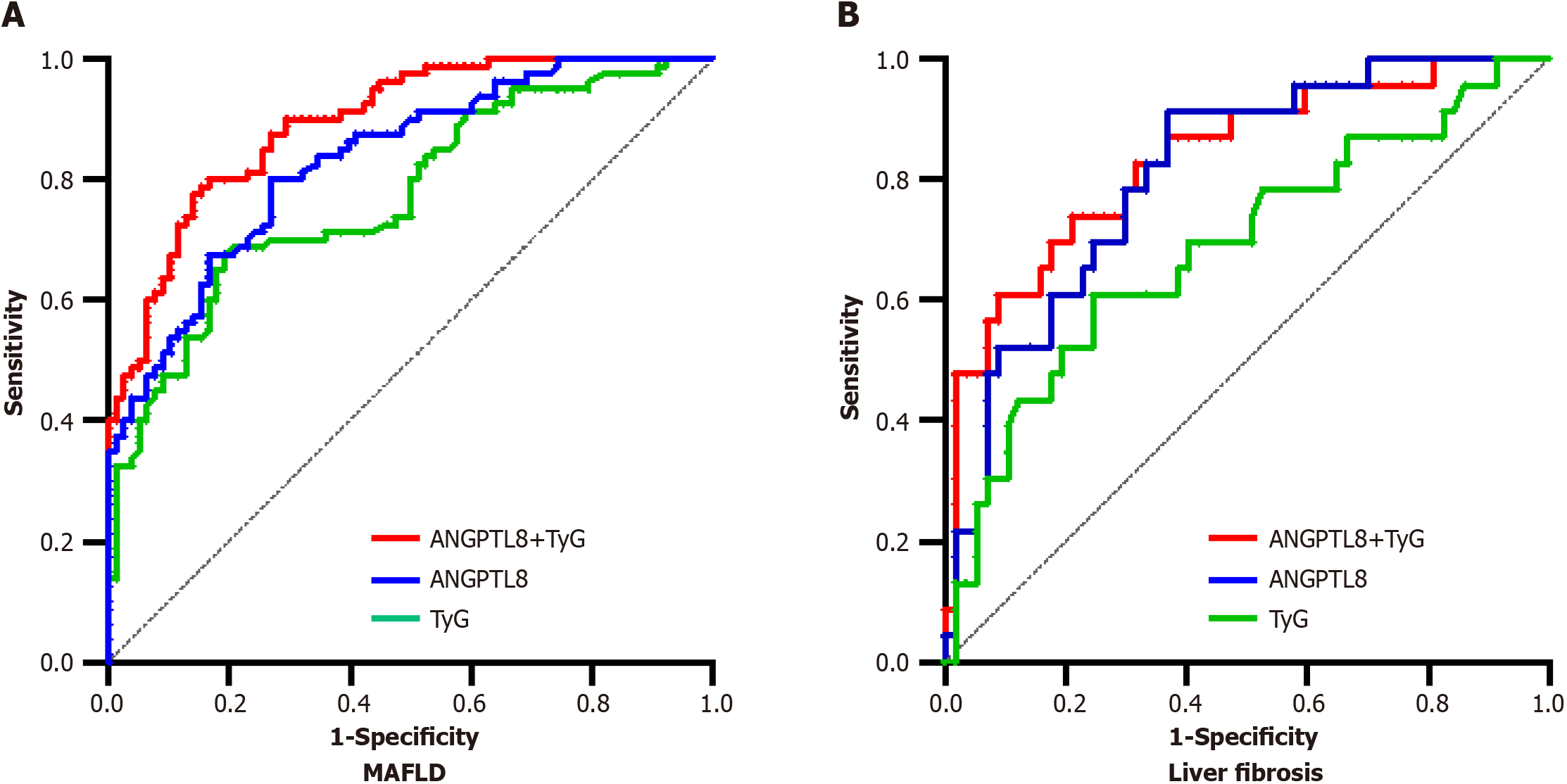
ROC curves were used to evaluate the diagnostic performance of ANGPTL8, the TyG index, and their combination for the risk of MAFLD (Figure 2). The ROC curve of ANGPTL8 had an area under the curve (AUC) of 0.832 [95% confidence interval (CI): 0.771-0.893, P < 0.001], with an optimal cutoff point of 31.18 pg/mL, sensitivity of 80%, and specificity of 73.08%. The ROC curve of the TyG index had an AUC of 0.773 (95%CI: 0.701-0.845, P < 0.001), with an optimal cutoff point of 9.155, sensitivity of 67.50%, and specificity of 80.77%. The combined ROC curve of ANGPTL8 and the TyG index had an AUC of 0.886 (95%CI: 0.846-0.941, P < 0.001), with an optimal cutoff point of 0.546, sensitivity of 75.5%, and specificity of 85.9%.
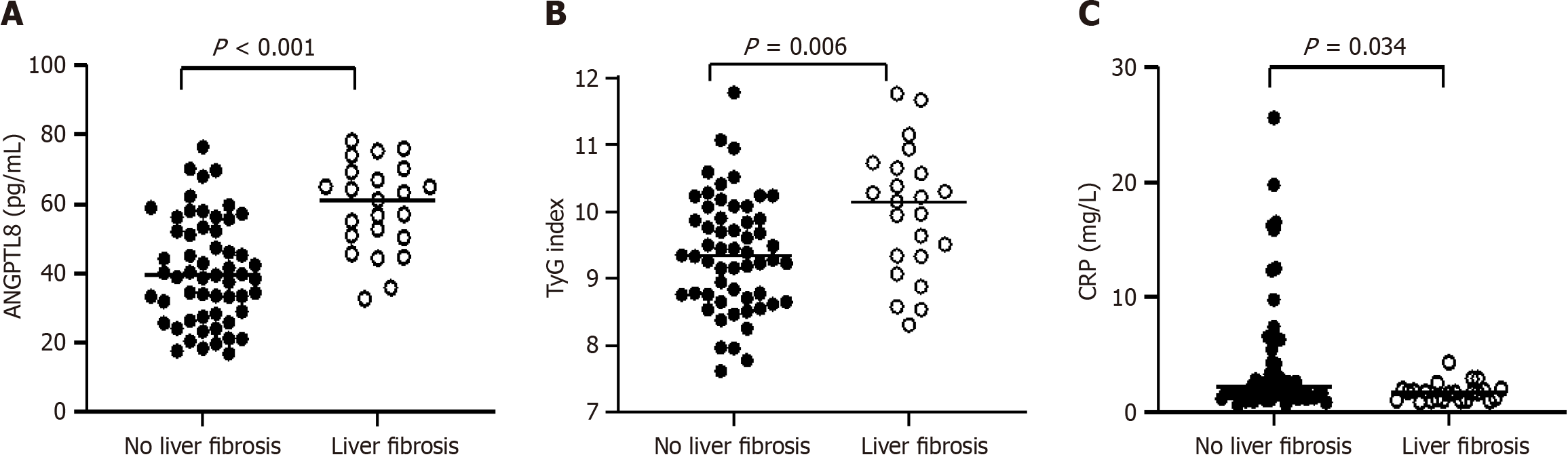
According to the FIB-4 index, patients with MAFLD were divided into the non-fibrosis group (FIB-4 < 1.45) and the liver fibrosis group (FIB-4 ≥ 1.45). The serum ANGPTL8 Level (40.75 ± 15.11 vs 58.94 ± 12.81 pg/mL, P < 0.001) and TyG index (9.38 ± 0.85 vs 9.99 ± 0.96, P = 0.006) were significantly higher in the liver fibrosis group than in the non-fibrosis group, while serum CRP level [2.13 (1.31, 4.23) vs 1.63 (0.98, 1.96) mg/L, P = 0.034] was opposite. However, age, gender, systolic blood pressure, diastolic blood pressure, mean arterial pressure, FPG, HOMA-β, BMI, WHR, HbA1c, FINS, HOMA-IR, TC, TG, HDL-C, LDL-C, TG/HDL-C, LDL-C/HDL-C, ALT, and AST did no significantly differ between the fibrosis and non-fibrosis groups (all P > 0.05, Figure 3).
Considering the above results, we speculated that there exists a correlation between ANGPTL8 and MAFLD complicated with liver fibrosis. Spearman correlation analysis revealed that liver fibrosis in MAFLD was positively correlated with the serum ANGPTL8 Level (r = 0.489, P < 0.001) and TyG index (r = 0.294, P = 0.008), and negatively correlated with the CRP level (r = -0.238, P = 0.033, Table 4). Furthermore, logistic regression analysis revealed that elevated serum ANGPTL8 level and TyG index were risk factors for liver fibrosis in MAFLD (both P < 0.05, Table 5).
ROC curve analysis was performed to evaluate the predictive value of ANGPTL8, TyG index, and their combination for liver fibrosis in MAFLD (Table 6, Figure 2). The results showed that the AUC of ANGPTL8 was 0.812 (95%CI: 0.713-0.910, P < 0.001), with an optimal cutoff point of 44.36 pg/mL, sensitivity of 91.3%, and specificity of 61.36%. The AUC of TyG index was 0.688 (95%CI: 0.553-0.822, P < 0.001), with an optimal cutoff point of 9.925, sensitivity of 60.87%, and specificity of 75.44%. The AUC of their combination was 0.835 (95%CI: 0.735-0.935, P < 0.001), with an optimal cutoff point of 0.3897, sensitivity of 73.91%, and specificity of 78.95%.
| AUC | 95%CI | Cutoff | Sensitivity | Specificity | P valuea | |
| Prediction of MAFLD | ||||||
| ANGPTL8 | 0.832 | 0.771-0.893 | 31.18 | 80.00 | 73.08 | < 0.001 |
| TyG index | 0.773 | 0.701-0.845 | 9.155 | 67.5 | 80.77 | < 0.001 |
| ANGPTL8 + TyG index | 0.886 | 0.846-0.941 | 0.546 | 75.5 | 85.9 | < 0.001 |
| Prediction of MAFLD with liver fibrosis | ||||||
| ANGPTL8 | 0.812 | 0.713-0.910 | 44.36 | 91.30 | 61.36 | < 0.001 |
| TyG index | 0.688 | 0.553-0.822 | 9.925 | 60.87 | 75.44 | < 0.001 |
| ANGPTL8 + TyG index | 0.835 | 0.735-0.935 | 0.3897 | 73.91 | 78.95 | < 0.001 |
The data from this study indicate that serum ANGPTL8 is significantly elevated in MAFLD patients and is closely associated with the risk of MAFLD and MAFLD-associated liver fibrosis. As serum ANGPTL8 levels increase, the risks of MAFLD and liver fibrosis also increase. Hence, serum ANGPTL8 may be a powerful indicator for predicting the risk of MAFLD and MAFLD-associated liver fibrosis. The combination of serum ANGPTL8 and the TyG index may have an even higher predictive value for the risk of MAFLD and the presence of liver fibrosis.
MAFLD is a progressive liver disease that can lead to steatohepatitis, liver fibrosis, cirrhosis, and even hepatocellular carcinoma. Its pathophysiology is complex and involves many interconnected processes, including metabolic dysregulation, lipotoxicity, insulin resistance, chronic inflammation, oxidative stress, mitochondrial autophagy, and gut microbiota[11-13]. Currently, MAFLD has become an increasingly common factor in end-stage liver disease and cardiovascular disease, posing a significant economic burden on public health[14,15].
Studies have suggested that ANGPTL8, as a novel protein secreted by the liver or adipose tissue, plays a dual role in lipid and glucose metabolism. The latest research has proposed a dual mode of ANGPTL8 regulation of lipoprotein lipase (LPL), known as the “ANGPTL3-4-8” model[16]. ANGPTL8 can inhibit LPL activity; control postprandial TG flux, and lipid uptake and storage in brown adipose tissue through synergistic interactions with ANGPTL3 and ANGPTL4; reduce TG clearance; and ultimately lead to a significant increase in serum TG levels[17]. Animal experiments also support a positive correlation between ANGPTL8 and TG levels; knockout of ANGPTL8 leads to decreased lipid content in adipocytes[18], which can significantly improve lipid uptake and reduce lipid deposition[4]. It is worth noting that ANGPTL8 is also associated with TC, HDL-C, and LDL-C[19]. Serum ANGPTL8 can also improve insulin resistance by directly activating insulin-mediated AKT phosphorylation[20] or regulating the PI3K/Akt signaling pathway to enhance insulin sensitivity[21], promote glycogen synthesis, and inhibit gluconeogenesis. All of the above findings indicate that ANGPTL8 is an important link in glucose and lipid metabolism, and may be involved in the development of MAFLD.
Research has shown that the ANGPTL8 level is significantly increased in patients with MAFLD[22], and it is positively correlated with the lipid content in liver cells[23]. In a mouse experiment, it was discovered that inhibiting serum ANGPTL8 levels with antisense oligonucleotides can suppress hepatic steatosis in high-fat-fed mice[4]. This further suggests that ANGPTL8 may play an important role in the occurrence and development of MAFLD. ANGPTL8 expression is increased in the livers of MAFLD patients, which is possibly related to endoplasmic reticulum stress in liver cells[24]. ANGPTL8 can also induce the expression of inflammatory factors by regulating the NF-kB signaling pathway[25], thereby participating in the inflammatory response and promoting the progression of MAFLD to liver fibrosis and cirrhosis. The present study also found similar results by comparing the clinical characteristics of the non-MAFLD and MAFLD populations. Serum ANGPTL8 was positively correlated with the risk of MAFLD, and as serum ANGPTL8 levels increased, the risk of MAFLD also increased. Logistic regression analysis showed that ANGPTL8 is a risk factor for MAFLD. The TyG index, which reflects insulin resistance, has been proven to be of significant value in predicting the risk of MAFLD and cardiovascular risk factors such as hypertension and atherosclerosis. In our study, we also found a close correlation between the TyG index and the risk of MAFLD. We further constructed ROC curves for predicting MAFLD by using ANGPTL8 alone and in combination with the TyG index, and obtained AUCs of 0.832 and 0.886, respectively. This indicates that ANGPTL8 can serve as a predictive factor for the risk of MAFLD, and its combination with the TyG index may have an even higher predictive value for the risk of MAFLD.
FIB-4 is a widely used non-invasive index for assessing liver fibrosis, with diagnostic efficacy comparable to the gold standard liver biopsy[26]. Studies have proposed that a FIB-4 value of < 1.45 can exclude progressive fibrosis, and this has been widely validated[9,10]. ANGPTL8 can promote the occurrence and development of MAFLD by participating in glucose and lipid metabolism, insulin resistance, and energy homeostasis[22,27], but the relationship between ANGPTL8 and liver fibrosis remains controversial. In 2016, Cengiz et al[28] found that serum ANGPTL8 levels were lower in NAFLD patients than in healthy individuals, and higher in NAFLD patients with mild fibrosis than in patients with significant fibrosis. However, some authors have reported contradictory conclusions. Ke et al[22] reported that the circulating level of ANGPTL8 is significantly higher in MAFLD patients than in healthy individuals, and is not affected by race, region, BMI, etc.; they concluded that elevated serum ANGPTL8 is positively correlated with MAFLD. In addition, Zhang et al[29] suggested that ANGPTL8 can act as a pro-inflammatory factor, and promote the development of liver fibrosis. In our study, we found similar results: ANGPTL8 levels were significantly increased in MAFLD patients with liver fibrosis (FIB-4 ≥ 1.45), and were positively correlated with MAFLD combined with liver fibrosis. Hence, we concluded that elevated serum ANGPTL8 levels are associated with an increased risk of liver fibrosis in MAFLD patients.
Studies have also shown a significant correlation between the TyG index and the severity of hepatic steatosis and liver fibrosis in NAFLD patients[30]. Elevated TyG index is an independent predictor of MAFLD and is positively correlated with the MAFLD fibrosis score and FIB-4 index, suggesting that a significant increase in the TyG index may indicate the development of liver fibrosis[31]. In 113 patients with histologically confirmed NAFLD, the TyG index levels were significantly higher in the F3 fibrosis stage than in the F0–F1 stage (P < 0.0001)[32]. However, Guo et al[30] found that the AUC value of the TyG index for predicting NAFLD was 0.761, while its AUC value for predicting liver fibrosis was 0.589, indicating that the TyG index has a relatively low accuracy for predicting NAFLD-associated liver fibrosis. In our study, we constructed ROC curves to analyze whether serum ANGPTL8 as well as the combination of serum ANGPTL8 and the TyG index could predict the coexistence of liver fibrosis with MAFLD, and obtained AUC values of 0.812 and 0.835, respectively. Hence, serum ANGPTL8 is a powerful predictor of the coexistence of liver fibrosis with MAFLD, and the combined use of serum ANGPTL8 and the TyG index may improve the predictive efficacy for liver fibrosis in MAFLD.
This study has the following limitations: First, the gold standard for the diagnosis of MAFLD and liver fibrosis is liver biopsy, but this study only used imaging examinations and the FIB-4 index due to limitations in conditions. Second, this study had a small sample size, and the results may be biased and require further validation with a larger sample size. Third, this study is a cross-sectional study and cannot reflect the specific mechanisms of ANGPTL8 in MAFLD. Further prospective studies are needed to explore and clarify these mechanisms.
In conclusion, the study reveals that serum levels of ANGPTL8 are elevated in patients diagnosed with MAFLD, and notably higher in those with concurrent liver fibrosis. The close correlation of serum ANGPTL8 with the risks associated with MAFLD and the progression of liver fibrosis underscores its significance. Serving as a predictive marker for both MAFLD risks and its associated liver fibrosis, serum ANGPTL8 exhibits enhanced predictive value when combined with the TyG index. Consequently, we posit that serum ANGPTL8 stands as a valuable and straightforward reference marker for the early prediction and timely management of MAFLD, enriching the repertoire of non-invasive assessment methods in clinical settings. Monitoring changes in ANGPTL8 levels facilitates early detection of MAFLD-associated liver fibrosis, enabling prompt intervention and management strategies to mitigate disease progression and reduce the risk of mortality.
Metabolic dysfunction-associated fatty liver disease (MAFLD) is now recognized as a prevalent global chronic liver disorder, standing as the primary contributor to end-stage liver disease and cardiovascular complications. This condition imposes a significant economic burden on public health. Previous research has elucidated the pivotal role of angiopoietin-like protein 8 (ANGPTL8) in the pathogenesis and progression of MAFLD, showcasing variations across different disease stages. Understanding ANGPTL8's involvement in MAFLD development informs early intervention strategies and aids in reducing mortality.
This study was motivated by the need for a convenient laboratory indicator facilitating the early identification of MAFLD and dynamic monitoring of its progression. Providing early intervention strategies for MAFLD patients is crucial to delay disease progression and reduce mortality.
The primary objective of this study was to conduct a comparative analysis of serum ANGPTL8 levels between individuals with and without MAFLD, investigating the predictive value of ANGPTL8 in relation to MAFLD development and progression.
In this cross-sectional study, 160 patients were enrolled, with 80 (50%) diagnosed with MAFLD. MAFLD patients were further categorized into hepatic fibrosis and non-hepatic fibrosis groups based on the fibrosis-4 index. Logistic regression analysis and receiver operating characteristic curves were employed to explore the impact and predictive ability of serum ANGPTL8.
Compared with the non-MAFLD group, serum ANGPTL8 levels and the triglyceride-glucose (TyG) index were significantly elevated in the MAFLD group, positively correlated with MAFLD. The combined ANGPTL8 and TyG index showed high predictive accuracy for MAFLD. Similarly, in the liver fibrosis group, both ANGPTL8 and the TyG index were significantly increased, positively correlated with liver fibrosis, with robust predictive accuracy for MAFLD-associated liver fibrosis.
Serum ANGPTL8 appears pivotal in the pathogenesis and progression of MAFLD, emerging as a potential biomarker for predicting both MAFLD and its associated hepatic fibrosis. The combined assessment of serum ANGPTL8 levels and the TyG index enhances predictive accuracy. Understanding the underlying mechanisms linking ANGPTL8 with MAFLD provides valuable insights for early diagnosis, risk stratification, and timely intervention, ultimately alleviating the burden of this disease.
Future studies should consider expanding sample sizes and incorporating liver biopsy and other methods to distinguish MAFLD-related liver fibrosis. Longitudinal studies are necessary to analyze serum ANGPTL8 changes at each MAFLD stage, confirming the relationship between serum ANGPTL8 and the occurrence and progression of MAFLD.
We express our gratitude to all the medical and technical personnel who willingly agreed to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Horowitz M, Australia S-Editor: Qu XL L-Editor: A P-Editor: Zhao YQ
| 1. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2200] [Article Influence: 440.0] [Reference Citation Analysis (1)] |
| 2. | Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 429] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 3. | Xie ZQ, Li HX, Wang BK, Yang ZM, Zhang ZY, Tan WL, Li WX, Wang QB, Yang L, Zhuang HK, Tang CW, Shang CZ, Chen YJ. Trends in prevalence and all-cause mortality of metabolic dysfunction-associated fatty liver disease among adults in the past three decades: Results from the NHANES study. Eur J Intern Med. 2023;110:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 4. | Vatner DF, Goedeke L, Camporez JG, Lyu K, Nasiri AR, Zhang D, Bhanot S, Murray SF, Still CD, Gerhard GS, Shulman GI, Samuel VT. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia. 2018;61:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, Zheng L, Huang K. A new way to regulate inflammation: selective autophagic degradation of IKKγ mediated by ANGPTL8. Cell Stress. 2018;2:66-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Yin Y, Ding X, Peng L, Hou Y, Ling Y, Gu M, Wang Y, Peng Y, Sun H. Increased Serum ANGPTL8 Concentrations in Patients with Prediabetes and Type 2 Diabetes. J Diabetes Res. 2017;2017:8293207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Ye J, Qin Y, Wang D, Yang L, Yuan G. The Relationship between Circulating ANGPTL8/Betatrophin Concentrations and Adult Obesity: A Meta-Analysis. Dis Markers. 2019;2019:5096860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Wang H, Lai Y, Han C, Liu A, Fan C, Wang H, Zhang H, Ding S, Teng W, Shan Z. The Effects of Serum ANGPTL8/betatrophin on the Risk of Developing the Metabolic Syndrome - A Prospective Study. Sci Rep. 2016;6:28431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1609] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 10. | Chinese Society of Hepatology; Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and treatment of hepatic fibrosis (2019). J Dig Dis. 2020;21:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Nassir F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 215] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 12. | Ji J, Wu L, Wei J, Wu J, Guo C. The Gut Microbiome and Ferroptosis in MAFLD. J Clin Transl Hepatol. 2023;11:174-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Clare K, Dillon JF, Brennan PN. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis of MAFLD. J Clin Transl Hepatol. 2022;10:939-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 14. | Zhang P, Dong X, Zhang W, Wang S, Chen C, Tang J, You Y, Hu S, Zhang S, Wang C, Wen W, Zhou M, Tan T, Qi G, Li L, Wang M. Metabolic-associated fatty liver disease and the risk of cardiovascular disease. Clin Res Hepatol Gastroenterol. 2023;47:102063. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1676] [Article Influence: 419.0] [Reference Citation Analysis (33)] |
| 16. | Abu-Farha M, Ghosh A, Al-Khairi I, Madiraju SRM, Abubaker J, Prentki M. The multi-faces of Angptl8 in health and disease: Novel functions beyond lipoprotein lipase modulation. Prog Lipid Res. 2020;80:101067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Zhang R, Zhang K. An updated ANGPTL3-4-8 model as a mechanism of triglyceride partitioning between fat and oxidative tissues. Prog Lipid Res. 2022;85:101140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 18. | Luo D, Chen X, Yang W, Ran W, Wen Z. Angiopoietin-like 8 Improves Insulin Resistance and Attenuates Adipose Tissue Inflammation in Diet-Induced Obese Mice. Exp Clin Endocrinol Diabetes. 2020;128:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Yang S, Jiao X, Huo X, Zhu M, Wang Y, Fang X, Yang Y, Yue W, Qin Y. Association between circulating full-length angiopoietin-like protein 8 and non-high-density lipoprotein cholesterol levels in Chinese non-diabetic individuals: a cross-sectional study. Lipids Health Dis. 2018;17:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Yuan J, Zhang D, Wang Y, Zhu Z, Lin Q, Li M, Zhong W, Han J, Xu F, Dong J. Angiopoietin-Like 8 in Gestational Diabetes Mellitus: Reduced Levels in Third Trimester Maternal Serum and Placenta, Increased Levels in Cord Blood Serum. Int J Endocrinol. 2022;2022:1113811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Zhao Z, Deng X, Jia J, Zhao L, Wang C, Cai Z, Guo C, Yang L, Wang D, Ma S, Deng J, Li H, Zhou L, Tu Z, Yuan G. Angiopoietin-like protein 8 (betatrophin) inhibits hepatic gluconeogenesis through PI3K/Akt signaling pathway in diabetic mice. Metabolism. 2022;126:154921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Ke Y, Liu S, Zhang Z, Hu J. Circulating angiopoietin-like proteins in metabolic-associated fatty liver disease: a systematic review and meta-analysis. Lipids Health Dis. 2021;20:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Hong BS, Liu J, Zheng J, Ke W, Huang Z, Wan X, He X, Xiao H, Li Y. Angiopoietin-like protein 8/betatrophin correlates with hepatocellular lipid content independent of insulin resistance in non-alcoholic fatty liver disease patients. J Diabetes Investig. 2018;9:952-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Lee YH, Lee SG, Lee CJ, Kim SH, Song YM, Yoon MR, Jeon BH, Lee JH, Lee BW, Kang ES, Lee HC, Cha BS. Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Sci Rep. 2016;6:24013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Liao Z, Wu X, Song Y, Luo R, Yin H, Zhan S, Li S, Wang K, Zhang Y, Yang C. Angiopoietin-like protein 8 expression and association with extracellular matrix metabolism and inflammation during intervertebral disc degeneration. J Cell Mol Med. 2019;23:5737-5750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, Zafarmand MH. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021;41:261-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 234] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 27. | Luo M, Peng D. ANGPTL8: An Important Regulator in Metabolic Disorders. Front Endocrinol (Lausanne). 2018;9:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Cengiz M, Ozenirler S, Kocabiyik M. Serum β-trophin level as a new marker for noninvasive assessment of nonalcoholic fatty liver disease and liver fibrosis. Eur J Gastroenterol Hepatol. 2016;28:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Zhang Z, Yuan Y, Hu L, Tang J, Meng Z, Dai L, Gao Y, Ma S, Wang X, Zhang Q, Cai W, Ruan X, Guo X. ANGPTL8 accelerates liver fibrosis mediated by HFD-induced inflammatory activity via LILRB2/ERK signaling pathways. J Adv Res. 2023;47:41-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 30. | Guo W, Lu J, Qin P, Li X, Zhu W, Wu J, Xu N, Zhang Q. The triglyceride-glucose index is associated with the severity of hepatic steatosis and the presence of liver fibrosis in non-alcoholic fatty liver disease: a cross-sectional study in Chinese adults. Lipids Health Dis. 2020;19:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | Tutunchi H, Naeini F, Mobasseri M, Ostadrahimi A. Triglyceride glucose (TyG) index and the progression of liver fibrosis: A cross-sectional study. Clin Nutr ESPEN. 2021;44:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Amzolini AM, Forţofoiu MC, Barău Abu-Alhija A, Vladu IM, Clenciu D, Mitrea A, Forţofoiu M, Matei D, Enăchescu V, Predescu OI, Micu ES. Triglyceride and glucose index: a useful tool for non-alcoholic liver disease assessed by liver biopsy in patients with metabolic syndrome? Rom J Morphol Embryol. 2021;62:475-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |