Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.361
Peer-review started: November 30, 2023
First decision: December 29, 2023
Revised: January 4, 2024
Accepted: February 7, 2024
Article in press: February 7, 2024
Published online: March 15, 2024
Processing time: 106 Days and 0.4 Hours
Diabetes, one of the world's top ten diseases, is known for its high mortality and complication rates and low cure rate. Prediabetes precedes the onset of diabetes, during which effective treatment can reduce diabetes risk. Prediabetes risk factors include high-calorie and high-fat diets, sedentary lifestyles, and stress. Consequences may include considerable damage to vital organs, including the retina, liver, and kidneys. Interventions for treating prediabetes include a healthy lifestyle diet and pharmacological treatments. However, while these options are effective in the short term, they may fail due to the difficulty of long-term implementation. Medications may also be used to treat prediabetes. This review examines prediabetic treatments, particularly metformin, glucagon-like peptide-1 receptor agonists, sodium glucose cotransporter 2 inhibitors, vitamin D, and herbal medicines. Given the remarkable impact of prediabetes on the progression of diabetes mellitus, it is crucial to intervene promptly and effectively to regulate prediabetes. However, the current body of research on prediabetes is limited, and there is considerable confusion surrounding clinically relevant medications. This paper aims to provide a comprehensive summary of the pathogenesis of pre-diabetes mellitus and its associated therapeutic drugs. The ultimate goal is to facilitate the clinical utilization of medications and achieve efficient and timely control of diabetes mellitus.
Core Tip: Addressing the global impact of diabetes, this review underscores the pivotal role of pre-diabetes as a precursor and the window of opportunity it offers for reducing diabetes risk. While interventions like lifestyle changes and pharmacological treatments prove effective in the short term, sustained implementation remains challenging. The review delves into the potential of medications, including metformin and other agents, shedding light on the current limitations in research and clinical confusion. By providing a comprehensive overview, the paper aims to enhance understanding, enabling more efficient and timely control of diabetes mellitus.
- Citation: Ping WX, Hu S, Su JQ, Ouyang SY. Metabolic disorders in prediabetes: From mechanisms to therapeutic management. World J Diabetes 2024; 15(3): 361-377
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/361.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.361
Prediabetes, also known as impaired fasting glucose or impaired glucose tolerance (IGT), is a condition that has affected approximately 213000 young individuals in the United States as of 2017, with an estimated 239000 individuals projected to be affected by 2060, based on current growth trends[1]. Timely management of prediabetes can reduce the incidence of diabetes, particularly type 2 diabetes[2]. Prediabetes refers to blood glucose levels that are higher than normal but below the glucose levels detected in patients with diabetes[3,4]. Moreover, individuals with prediabetes are in a sub-healthy state, somewhere between being healthy and clinically diabetic. Effective treatment of prediabetes can prevent the development of diabetes and help individuals to return to a healthy state.
Interventional therapy for prediabetes primarily includes lifestyle interventions and pharmacological treatments. Commonly used clinical drugs include metformin, glucagon-like peptide (GLP-1) agonists, sodium–glucose cotransporter 2 (SGLT2) inhibitors, vitamin D supplements, and Chinese herbal medicines. In this review, we provide an overview of prediabetes and its therapeutic agents. The ultimate aim of this article is to offer insights into prediabetes and contribute to the development of effective treatment strategies.
Prediabetic contributory factors may include genetics and diets high in calories and fat[5]. Such diets contribute to excess fat accumulation and compensatory lipolysis within the body, resulting in an increased free fatty acid (FFA) content. The FFAs can disrupt cellular homeostasis, hinder cellular insulin response, reduce cellular uptake and utilization, increase the risk of insulin resistance in the liver, and damage muscles and the liver, ultimately leading to the development of diabetes[6].
The criteria for diagnosing prediabetes include a fasting plasma glucose level of 100-125 mg/dL (5.6-6.9 mmol/L), a 2-h oral glucose tolerance test (OGTT; 75 g 2 h) result of 140-199 mg/dL (7.8-11.0 mmol/L), and a glycated hemoglobin (HbA1c) level of 5.7%-6.4% (39-47 mmol/mol)[7]. It is important to note that, among these criteria, the HbA1c test is only applicable to adults. IGT is a key diagnostic criterion for prediabetes; however, HbA1c and fasting blood glucose (FBG) levels are also used in the diagnosis, as shown in Table 1[8-11].
A clinical survey in the United States reported that the prevalence of prediabetes is as high as 30%, indicating that approximately one in three adults has a fasting glycemic index or HbA1c level that meets the criteria for prediabetes[12]. Meanwhile, in India, the number of individuals with IGT reached 25.2 million in 2019 and is expected to reach 35.7 million by 2045[13]. The prevalence of prediabetes has notably increased from 15.5% in 2008 to 38.1% in 2018 in China (Table 2)[14-16].
Patients with prediabetes may exhibit characteristics associated with diabetes, including weight and blood glucose abnormalities and systemic insulin resistance. Systemic insulin resistance plays a key role in prediabetes, as it leads to decreased ability of the body to respond to insulin, resulting in an imbalance in glucose homeostasis which, in turn, leads to insulin resistance. The decreased ability of muscle cells to uptake and process glucose reduces the storage capacity for both glucose and triglycerides, resulting in abnormally elevated levels of free glucose and triglycerides in the blood, ultimately increasing the risk of developing diabetes[17,18].
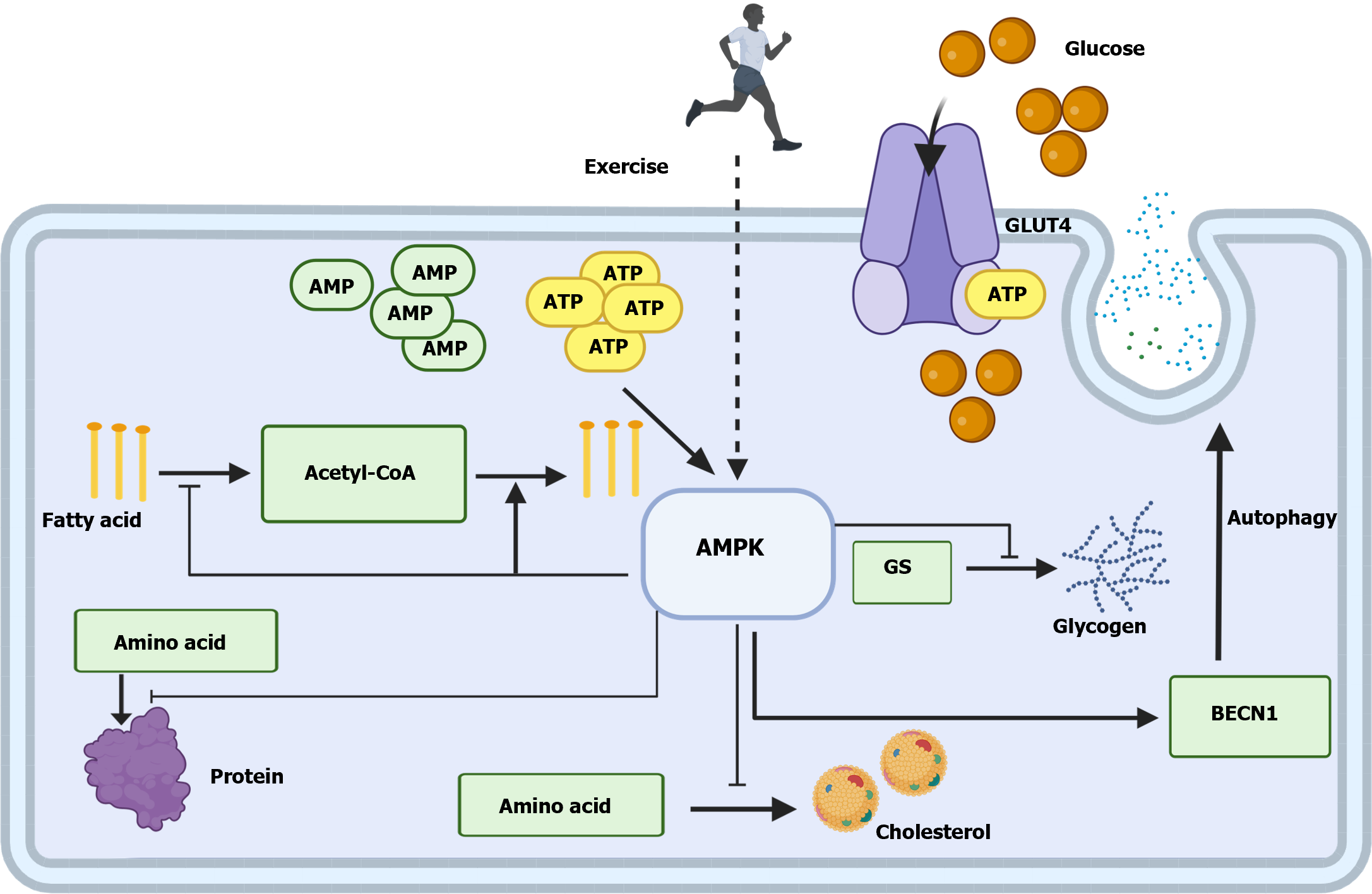
Most prediabetic states progress to diabetes mellitus, accompanied by complications including microvascular complications, retinopathy, and cardiovascular disease. Insulin resistance affects normal oxidative stress in nerves, leading to mitochondrial dysfunction, which causes retinopathy and drives neurological and vascular pathology. The incidence rate of retinopathy is approximately 8.2%-20.9% in prediabetic patients[19-22], while the risk of stroke increases by 0.74% compared to that in patients without diabetes[23]. Additionally, the prevalence of metabolic syndrome is approximately 37.6% higher than that of normoglycemic patients, and the vascular risk ratio score is increased by 0.43[24,25]. Prediabetes is characterized by hyperglycemia and insulin resistance, partly due to the disruption of glucose homeostasis, primarily caused by the compromised function of pancreatic islet β-cells. Adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK) is an insulin sensitizer that exists in the form of a heterotrimeric complex with major subunits comprising AMPKα, AMPKβ, and AMPKγ. As blood glucose levels transition from fasting to postprandial levels, the decline in phosphorylated AMPK levels within islets triggers the activation of AMPK phosphorylation, enhancing glucose-stimulated insulin secretion (GSIS). This promoted glucose uptake in muscle tissues while reducing glucose production in the liver to maintain constant blood glucose levels. The activity of AMPK activity is lowest when ATP occupies the subunit site of AMPKγ under high-energy conditions. Liver kinase B1 (LKB1) is required to regulate AMPK activity through AMP/ADP or AMPK phosphatase inhibition. LKB1 primarily phosphorylates AMPKα by binding to Thr172, while LKB1 deficiency in β-cells inhibits the phosphorylation of Thr172 and AMPK target proteins. In contrast, the variable binding of the subunit to AMPKγ, phosphorylation of the downstream kinase Thr172, and impaired downstream dephosphorylation determine the degree of AMPK activation. This kinase in pancreatic β-cells may be protein phosphatase 1, which prevents the sustained activation of AMPK in the presence of high glucose, leading to GSIS failure and insulin resistance[26]. In damaged pancreatic β-cells, a sustained high-glucose environment results in sustained AMPK phosphorylation in the pancreatic β-cells, inhibiting GSIS and promoting insulin resistance.
Apart from hyperglycemia and insulin resistance, prediabetes also presents elevated endoplasmic reticulum (ER) stress levels and abnormal apoptosis of pancreatic islet β-cells. ER stress induces senescence of pancreatic β-cells due to the over-activation of the mammalian target of rapamycin (mTOR), a serine-threonine kinase, encompassing mTOR1 and mTOR2. mTOR1 is responsible for protein synthesis and ribosome genesis, while mTOR2 activates AKT-serine 473. Protein synthesis occurs in the ER.
The unfolded protein response (UPR) is activated when misfolded proteins accumulate in the ER. Over-activation of mTOR1 promotes excessive protein synthesis, increasing the likelihood of misfolded protein synthesis. This, in turn, sustains UPR activation, impairs cellular autophagy mechanism, and leads to pancreatic β-cell death. In patients with prediabetes, prolonged over-activation of the mTOR complex 1 signaling pathway in the β islets results in increased pancreatic β-cell numbers and inhibition of the β-cell autophagy protection mechanism, increasing the likelihood of apoptosis[27].
Insulin resistance and pancreatic islet β-cell apoptosis due to insufficient insulin secretion impedes the normal glucose-lowering effect. Reduced insulin target cell receptor sensitivity leads to diminished insulin signaling, thereby decreasing glucose uptake and increasing extracellular free glucose. Furthermore, the body’s negative feedback leads to more insulin release, causing hyperinsulinemia and creating a vicious cycle. The resulting insulin resistance promotes the development of prediabetes. Additionally, excessive free extracellular glucose promotes glucose uptake by the cells, leading to an imbalance in blood glucose homeostasis[27].
The primary preventive measures for prediabetes include lifestyle interventions and pharmacotherapy (Table 3). These interventions primarily aim to reduce glycemic weight, improve insulin resistance, reduce pancreatic β-cell apoptosis, and reduce oxidative stress, thereby reducing islet resistance. Additionally, lifestyle interventions are intended to assist patients with prediabetes in improving unhealthy lifestyles and dietary habits, among others, while naturally reversing the imbalance in blood glucose homeostasis. An advantage of lifestyle interventions is their rapid effectiveness; however, lifestyle regulation is time-consuming[28-30]. Dietary and lifestyle changes can considerably improve the weight and blood glucose levels of individuals who have followed high-fat and-calorie diets over a long time. However, sustained improvement in blood glucose with weight loss may be minimal[30]. Additionally, maintaining a healthy diet over the long term may be challenging for individuals in the contemporary context. Pharmacological management is another form of prediabetes intervention that is remarkably more effective in controlling weight and blood glucose than dietary control. It is also adaptable to modern, high-stress, fast-paced lifestyles[31-36]. The main available drugs include metformin, GLP-1 receptor agonists, SGLT2 inhibitors, vitamin D supplements, and Chinese herbal medicine, among others.
| Intervention method | Cycle time, follow-up time | Effect | Ref. |
| Lifestyle intervention | 4 months, 1 yr follow up | Blood glucose and lipids can be effectively controlled | Gokulakrishnan et al[28] |
| Lifestyle intervention | 1 yr | Effective reduction of disease risk in patients with prediabetes | Hu et al[29] |
| Lifestyle intervention | 1 yr | Weight loss 34.1% higher than in the diatomic group | Apolzan et al[30] |
| Metformin | 10 yr | Enhanced glycemic control to improve health outcomes | Jonas et al[31] |
| Metformin | 1 yr | More effective in body weight reduction. Better results than life interventions | O’Brien et al[32] |
| Metformin | 5 yr | Weight loss 2.5% higher than life intervention group | Apolzan et al[30] |
| Metformin | 15 yr | Compared with the placebo group, 17% lower incidence of diabetes | Diabetes Prevention Program Research Group[33] |
| GLP-1 receptor agonist | 3 yr | Significant weight loss and improved blood sugar | le Roux et al[34] |
| GLP-1 receptor agonist | 17 months | Significant weight loss | Wilding et al[35] |
| GLP-1 receptor agonist | 14 wk | Significant reduction of body weight and improved relevant glucose tolerance indicators | Kim et al[36] |
Metformin is a primary hypoglycemic agent that can lower glucose levels by impeding glucose production and enhancing its uptake and utilization[37-39]. Metformin stimulates AMPK, considerably ameliorating abnormalities in glycolipid metabolism[40]. Metformin can promote AMPK phosphorylation, reduce oxidative stress in skeletal muscle, and reverse glucose intolerance, leading to a hypoglycemic effect on the body[41]. Ma et al[42] explored the relationship between metformin, presenilin enhancer 2 (PEN2), and AMPK by knocking down the PEN2 gene or reintroducing the PEN1 mutant gene into Cryptobacterium. They reported that metformin can bind PEN2, activate ATP6AP1 and AMPK, and initiate glucose metabolism-related signaling pathways, exerting its hypoglycemic effect[42].
AMPK acts as a cellular energy sensor[43,44] and is closely related to the body’s activity level. ATP decreases with strenuous exercise, and the ATP/ADP and ATP/AMP ratios subsequently decrease. The concomitant activation of the closely related AMPK positively regulates pathways that replenish the cellular ATP supply, including increasing glucose uptake, activating cellular autophagy, and promoting fatty acid oxidation, negatively regulating biosynthetic processes that consume ATP, including gluconeogenesis[45,46], cholesterol synthesis, protein synthesis, and fatty acid synthesis (Figure 1)[47].
Indeed, metformin effectively reduces the risk of developing diabetes during the prediabetic stage[2,48]. The American Diabetes Association states that metformin is the most effective drug for diabetes prevention and recommends its use for prediabetes intervention[2]. Long-term metformin administration results in marked weight loss in a few patients, with minimal gastrointestinal upset. Therefore, it is considered safe, effective, and well-tolerated for the treatment of patients with prediabetes and abnormally elevated fasting glucose and IGT levels[49].
Metformin is clinically prescribed at a starting dose of 500 mg, which can be increased to 1000 mg twice daily. The dosage varies according to the individualized requirements of the patient. Reported doses used during prediabetic interventions are listed in Table 4. In previous safety trials, patients exhibited symptoms of anemia after long-term use of metformin due to the diminished concentrations of vitamin B12[50]. Therefore, the Diabetes Prevention Program recommends that long-term metformin users should be tested for vitamin B12 levels, with B12 supplementation.
| Trial population | Prescribed dosage | Associated or not | Treatment cycle | Reversal rate | Ref. |
| Adolescents | 1000 mg/d | Rosiglitazone (2 mg) | 3.9 yr | 80% improvement in glucose tolerance | Zinman et al[137] |
| Adolescents | 1000 mg/d | No | 6 months | 45% increase in insulin sensitivity | Srinivasan et al[138] |
| Adults | 850 mg/d | No | 1 yr | 7% reduction in the incidence of diabetes | Andreadis et al[139] |
| Adults | 2000 mg/d | No | 1 yr | Increased insulin sensitivity (P < 0.01) | Malin et al[134] |
| Adults | 1500-2000 mg/d | Exenatide (10-20 μg/d) | 1 yr | 64% improvement in prediabetes remission rates | Tao et al[140] |
Metformin use has certain shortcomings, including gastrointestinal symptoms, such as abdominal pain and diarrhea, which occur in 30% of users. The incidence of such symptoms increases with the duration of use[49]. Metformin use may also cause lactic acidosis or even death in patients with severe renal impairment (estimated glomerular filtration rate < 30 mL/min/1.73 m). Additionally, metformin is a biologically active molecule with a low environmental decomposition capacity and may cause aquatic environmental contamination[51].
GLP-1, a large peptide hormone comprising 30 or 31 amino acids, is primarily secreted by distal enteroendocrine L cells, pancreatic α-cells, and the central nervous system. GLP-1 participates in regulating glucose homeostasis by acting on the GLP-1 receptor (GLP-1R). GLP-1R agonists (GLP-1RAS) approved for marketing in China primarily include the six types listed in Table 5.
| Name | Molecular formula | Number of amino acids | Recommended initial dosage | Recommended dosage for prediabetes | Ref. |
| Exenatide | C149H234N40O47S | 39 | 10 μg/day | 10-20 μg/day | Tavlo et al[51] |
| Liraglutide | C172H265N43O51 | 9 | 0.6-1.2 mg/day | 3 mg/day | le Roux et al[34] |
| Dulaglutide | C40H50N8O5 | 8 | 0.75 mg/week | – | – |
| Lixisenatide | C215H347N61O65S | 44 | 10 μg/day | – | – |
| Polyethylene glycol loxenatide | C210H325N55O69S(C2H4O)2n | 38 | 0.1 mg/week | – | – |
| Benarutide | C149H225N39O46 | 29 | 0.3 mg/day | – | – |
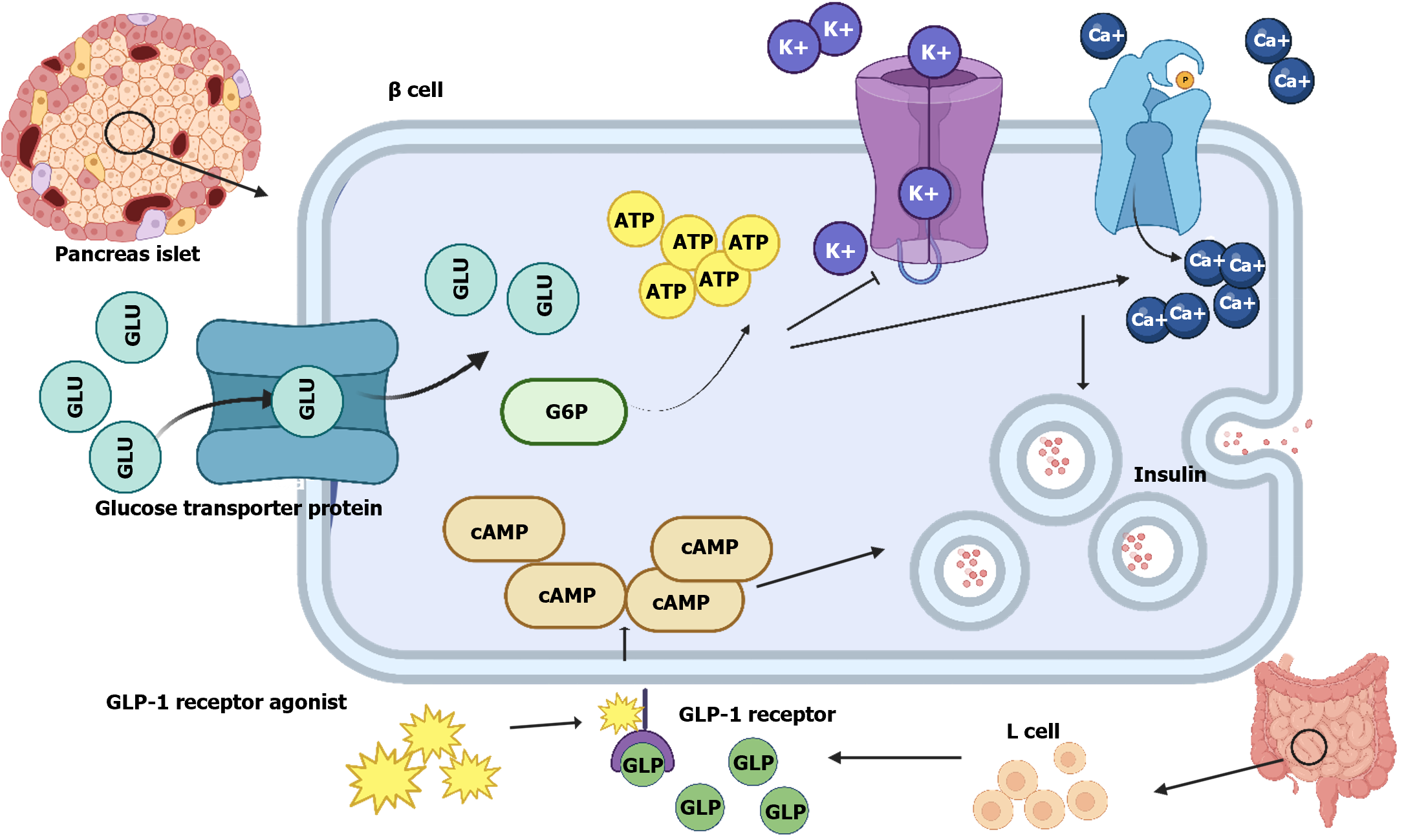
GLP-1 is a type of entero-insulin, a hormone-stimulated and secreted by intestinal food and endothelial cells, res-pectively, that acts via GLP-1R on pancreatic β-cells to generate more intracellular cyclic AMP and ATP, thereby promoting insulin release from pancreaticβ-cells. Additionally, GLP-1 can inhibit abnormal secretion of glucagon from pancreatic α-cells[52]. It regulates glucose abnormalities by lowering HbA1c concentration, promoting insulin secretion from pancreatic β-cells, reducing body weight, contributing to postprandial glucose regulation[53], and reducing glucagon secretion from pancreatic α-cells in a glucose concentration-dependent manner. This inhibitory function is achieved via the paracrine effect of the islets[54]. However, GLP-1 glucose regulation is limited due to its short half-life in plasma. Hence, GLP-1RAS was developed to achieve longer-lasting glucose regulation by extending the half-life.
GLP-1RAS promotes the uptake and utilization of glucose via several mechanisms[55]. GLP-1RAS can activate pancreatic β-cell GLP-1R by increasing the affinity of GLP-1 to GLP-1R or by binding directly to GLP-1R, promoting insulin secretion by facilitating the conversion of glucose to ATP, enhancing calcium ions inflow and inhibiting K+ outflow from cells (Figure 2)[56]. GLP-1RAS can inhibit glucagon secretion while promoting glucose-dependent insulin secretion owing to its high affinity and similarity to the natural GLP-1RAS and GLP-1, respectively, counteracting the increase in blood glucose caused by diet. The effect of maintaining blood glucose levels in a normal state is known as the entero-insulin effect[57].
Glucose metabolism is significantly improved after 68 wk of treatment with semaglutide[58]. Oral semaglutide therapy causes HbA1c levels and weight reduction[59]. Meanwhile, tirzepatide upregulates insulin sensitivity in the body and restores islet β-cell function[60]; tirzepatide and semaglutide reduce HbA1c levels[61]. Thus, GLP-1RAS notably aids the restoration of glucose homeostasis, improves islet function, enhances insulin sensitivity, and controls body weight.
Currently, the main adverse reactions associated with GLP-1RAS include nausea, vomiting, and gastrointestinal discomfort[62]. GLP-1RAS is a biomolecular formulation that can only be administered via dermal injection. Therefore, it lacks the portability and comfort of small-molecule drugs that can be orally administered.
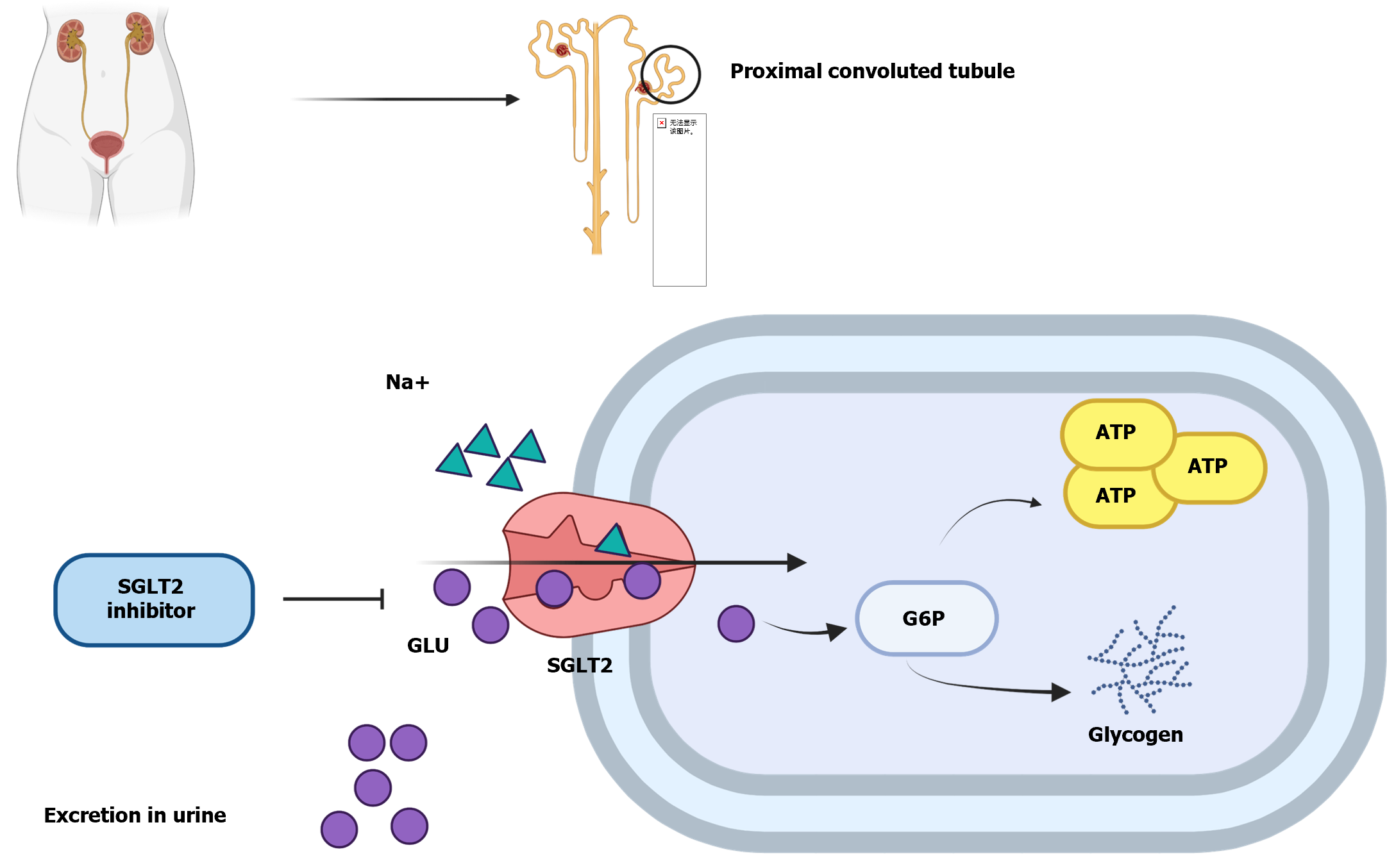
SGLT 1 and SGLT2 play a prominent role in the reabsorption of filtered glucose by the glomerulus. SGLT2 inhibitors reduce SGLT2 activity and the efficiency of glucose uptake in the proximal tubules of the kidney, which increases the urinary glucose concentration and reduces blood glucose. The main SGLT2 inhibitors currently on the market are listed in Table 6[63-65].
| Name | Molecular formula | Recommended initial dosage | In China, Listed or not | Recommended dosage for prediabetes | Ref. |
| Canagliflozin | C24H25FO5S | 100 mg/day | No | – | – |
| Dapagliflozin | C21H25CIO6•C3H8O2 | 5 mg/day | Listed | 10 mg/day | Lundkvist et al[63] |
| Empagliflozin | C23H27CIO7 | 10 mg/day | No | 10 mg/day | Lee et al[64] |
| Ipragliflozin | C21H21FO5S | 50 mg/day | No | – | – |
| Luseogliflozin | C23H30O6S | 2.5 mg/day | No | – | – |
| Tofogliflozin | C22H26O6 | 5 mg/day | No | 40 mg/day | Pafili et al[65] |
SGLT2 is an important member of the cotransport protein family. SGLT2 is mainly expressed in the proximal renal tubule, where it facilitates the reabsorption of glucose in the primary filtrate and converts it into ATP or glycogen (Figure 3)[66]. Under normal circumstances, the amount of glycosuria produced by the body after consuming a large quantity of carbohydrates is extremely small, mainly attributed to the filtering and reabsorption ability of SGLT2. SGLT2 inhibitors are a class of hypoglycemic drugs that inhibit the activity of sodium–glucose transport proteins on the luminal surface of the proximal tubule of the kidney, preventing glucose and Na+ from normally entering the cells in the proximal tubule. In addition to lowering blood glucose and body weight, SGLT2 inhibitors improve insulin sensitivity and enhance pancreatic β-cell function, among other effects[39,67-69].
Dapagliflozin and empagliflozin reduce HbA1c by an average of 0.66%[70]. SGLT2 inhibitors delayed the development of diabetes in four randomized trials involving 5655 patients with prediabetes[71]. Moreover, dapagliflozin administration to obese and overweight individuals resulted in weight loss and marked reductions in blood lipids and glucose, including associated OGTTs[72]. These findings show that SGLT2 inhibitors are highly effective in preventing diabetes.
However, the increased glycosuria level caused by SGLT2 inhibitors increases the risk of fungal infections. In eight clinical trials, 3.5% of SGLT2 inhibitor users experienced ketoacidosis. SGLT2 inhibitors may also accelerate the loss of minerals from bone, thereby increasing the risk of fracture. Additionally, SGLT2 inhibitors facilitate Na+ excretion and may cause adverse effects, such as acute kidney injury and renal function impairment[73,74].
Prediabetes is treated with medications similar to those used for treating diabetes. Table 7 summarizes the dosages, main results, and related conclusions of the use of metformin, GLP-1 agonists, and SGLT2 inhibitors in individuals with prediabetes, non-diabetic individuals, and individuals with obesity.
| Name | Ref. | Participants | Grouping and dosage | Result | Conclusion |
| Metformin | O’Brien et al[32] | 92 | Metformin group (850 mg/daily), standard diet group | Compared with the standard diet group, the metformin group lost an average of 1.1% body weight, and a normal blood glucose ratio of 28.7% was restored | Reduces weight and restores normal blood glucose levels in prediabetics |
| Metformin | Tavlo et al[51] | 183 | 1500-2000 mg/d over 12 wk | The impaired glucose tolerance remission rate was 32% | Improves postprandial insulin secretion |
| Metformin + exenatide | Tavlo et al[51] | 183 | Metformin: 1500-2000 mg/d; exenatide: 10-20 μg/d over 12 wk | The impaired glucose tolerance remission rate was 64% | Combined administration of drugs is more effective in alleviating glucose tolerance compared with monotherapy |
| Exenatide | Tavlo et al[51] | 183 | 10-20 μg/d over 12 wk | Impaired glucose tolerance remission rate of 56% | Improves postprandial insulin secretion |
| Liraglutide | le Roux et al[34] | 749 | Placebo group (n = 749), liraglutide group (n = 1505, 3 mg/d) over 160 wk | 4.2% weight loss and 2.7 times longer onset of diabetes in the liraglutide group than the placebo group | 3 mg liraglutide reduces weight gain and diabetes risk |
| Liraglutide | Pi-Sunyer et al[141] | 3731 | Placebo (n = 1244), liraglutide (n = 2487, 3 mg/d) over 56 wk | Body weight in the liraglutide group decreased by 36.1%; glycated hemoglobin, fasting blood glucose, and fasting insulin were reduced; and the prevalence of diabetes was reduced, compared with the placebo group | 3 mg liraglutide may reduce the incidence of urine disease |
| Dapagliflozin | Veelen et al[142] | 30 | Dapagliflozin (2 mg/d) vs placebo, over 10 wk | The plasma glucose level was reduced in the dapagliflozin group compared with the placebo group, and no extensive changes were observed in the glycogen and lipid content of the liver | Dapagliflozin improves fat oxidation and exhibits a marked hypoglycemic effect |
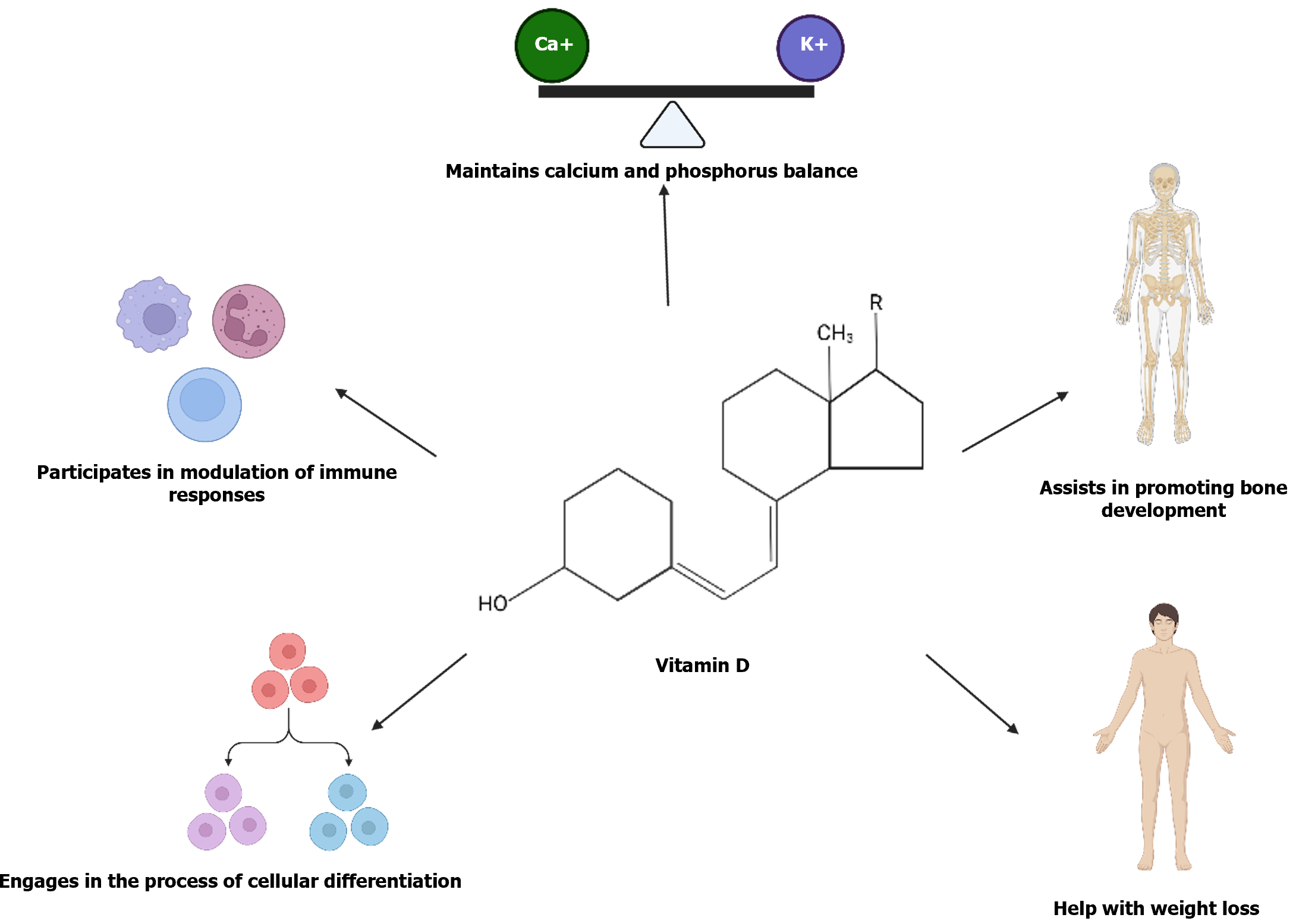
Vitamin D plays an important role in maintaining Ca2+ and phosphorus homeostasis, enhancing bone strength[75], increasing bone growth[76], reducing body weight[77], participating in cell differentiation[78], supporting immune function[79] (Figure 4), and delaying the progression of diabetes by lowering blood glucose and maintaining glucose metabolism homeostasis[80,81]. Randomized double-blind and placebo human trials have found that increasing and maintaining serum vitamin D levels reduce the risk of diabetes[82,83].
Recent research indicates that vitamin D reduces the risk of diabetes and its related conditions via various mechanisms[84,85]. First, vitamin D promotes insulin synthesis and secretion by improving the function of pancreatic β-cells[86]. Second, vitamin D reduces insulin resistance and improves sensitivity by modulating insulin’s target sites (liver, muscle, and adipose tissue)[87].
Research indicates a marked reduction in the insulin resistance index (HOMA-IR) in a vitamin intervention group compared with a placebo group[88]. Additionally, a considerable improvement was found in the glycemic index with dosages of vitamin D > 2000 IU/d.
A study of baseline serum vitamin D concentrations in more than 6000 patients with abnormal blood glucose levels found that individuals with high levels of serum vitamin D have a considerably reduced prevalence of elevated blood glucose and associated complications compared with those with serum vitamin < 25 nmol/L[89]. Another study in 2423 individuals with prediabetes identified the lowest risk of diabetes in individuals with serum vitamin D levels of ≥ 125 mmol/L; serum vitamin D levels of 100-124 mmol/L reduced the risk of developing diabetes in some individuals[90]. Furthermore, 43 randomized controlled trials have reported that high doses of vitamin D (≥ 1000 IU/d) markedly reduced the risk of developing diabetes in 55936 individuals with prediabetes[91]. Four trials, including 896 participants, have found that vitamin D supplementation effectively reduces the risk of prediabetes progressing to diabetes[83,92].
Vitamin D use for therapeutic interventions may cause hypercalcemia[93]. Vitamin D is present in the body mainly as vitamins D2 or D3, which are not biologically active. The inactive forms are catabolized and metabolized in the liver to 25-hydroxyvitamin D (ossified diol) and the kidney to 1,25-dihydroxy vitamin D (ossified triol), with both metabolites being biologically active[94]. Osteotriol is the main metabolite of vitamin D in the body and mediates Ca2+ and phosphorus uptake. Excessively elevated osteotriol levels can lead to hypercalcemia and hyperphosphatemia, which increases the risk of vascular calcification. Therefore, phosphate levels should be strictly monitored with vitamin D intervention.
Patients may also include vitamin D-enriched foods in their daily dietary regimen, such as those listed in Table 8[95].
| Food | Vitamin D content |
| Fresh shiitake mushrooms (0.0992 kg) | 600-1000 IU D2 |
| Sun-dried shiitake mushrooms (0.0992 kg) | 600-1000 IU D2 |
| Egg yolk | 20 IU D3, 0.2-0.8 μg 25-(OH)D |
| Cod liver oil (0.006 kg) | 400-1000 IU D3 |
| Beef liver (0.4536 kg) | 0-2500 IU D3, 0.3-3.5 μg 25-(OH)D |
| Beef muscle (0.4536 kg) | 0-180 IU D3, 0.1-2.6 μg 25-(OH)D |
| Pork muscle (0.4536 kg) | 10-250 IU D3, 0-31.4 μg 25-(OH)D |
The use of herbal medicine for treating diabetes and prediabetes dates back to the Qin Dynasty (approximately 221 BC). Currently, herbal medicines are considered useful for preventing and treating diabetes and its complications[96]. The herbs used for diabetes and prediabetes generally have multiple effects, including counteracting hypoglycemia, reducing insulin resistance, reducing oxidative stress, lowering lipids, and regulating intestinal flora. Numerous Chinese herbal medicines are currently used for treating prediabetes, including Huanglian, Qingqianliu, Mulberry [Morus alba (M. alba)] leaf, Astragalus, Guajia, Lady’s mantle, Dendrobium, Dry lotus, Ginseng, Wolfberry, Pentaphyllum, and Sanguisorba[97]. The functions and mechanisms of action of the herbs, including berberine, safranin, cyanotis, M. alba leaf, and Astragalus, in alleviating diabetes and prediabetic symptoms are briefly discussed here.
Berberine, a main active ingredient in Huanglian used in treating diabetes and prediabetes, plays crucial roles in treating hypoglycemia and other aspects. It positively affects mucin increase and promotes the improvement of intestinal mucosal morphology. Additionally, berberine can down-regulate the expression of Toll-like receptor 4, nuclear factor kappa B (NF-κB), and tumor necrosis factor-α, alleviating the chronic inflammation caused by diabetes. Furthermore, it counteracts the reduction of intestinal microbial diversity caused by IGT; reduces FBG and HOMA-IR; improves liver and kidney function; reduces cholesterol, blood lipid, and high-density fatty acid levels; and increases the number of cupped cells and villi length in IGT rats[98,99]. Berberine induces accelerated closure of KCNH6 K+ channels, decreases KCNH6 currents, prolongs glucose-dependent cell membrane depolarization, and promotes insulin secretion[100]. However, it exhibits an IC50 of 713.57 mg/kg in acute toxicity tests in rats[101]. Therefore, the use of berberine as an alkaloidal constituent of Coptis chinensis should be approached with due consideration of its toxicity.
Phellodendrin (PAL) is an active constituent of Phellodendron that improves blood glucose and insulin resistance levels in rats with IGT. Furthermore, PAL ameliorates the defective insulin secretion in insulinoma cells induced by chondroitin (PA) via the c-Jun N-terminal kinase signaling pathway. It also extensively inhibits PA-induced cell-induced β-cell apoptosis[102]. However, PAL elicits toxic effects with an IC50 of 1533.68 mg/kg in acute toxicity tests in rats[101]. Accordingly, attention to dosage is required in PAL use for prediabetes prophylaxis[103].
Cycads have various therapeutic properties, including hypoglycemic, hypolipidemic, hypotensive, anticancer, anti-fatigue, and antioxidant effects[104,105]. Cyanus was used in ancient times to treat diabetes[106-108]. Cyanidin improves insulin secretion by reducing apoptosis of pancreatic β-cells, reducing excessive oxidative stress in the pancreas, and maintaining the balance of glucose and lipid metabolism in the liver, thereby regulating blood glucose and lipid regulatory homeostasis[109]. Cryptococcus can also relieve hyperglycemic symptoms by modulating the intestinal microflora[110,111]. Cyclocarya paliurus is a traditional medicinal plant with various active effects; however, its safety issues should not be overlooked. Rats have shown good tolerance in acute toxicity studies; however, adverse changes in hematology, serum chemistry, urinalysis parameters, organ weights, and histopathology occur. Currently, C. paliurus is regarded as safe for use in the treatment of prediabetes[112].
M. alba is an Asian medicinal plant with roots, fruits, and seeds used to lower glucose levels, reduce liver damage, and improve oxidative stress[113,114]. Flavonoids, polysaccharides, and alkaloids are key active components in M. alba leaves that alleviate symptoms of hyperglycemia. M. alba leaf extract intervention in mice with IGT reduces insulin resistance and IGT while improving glucose uptake in a hepatocyte islet resistance model[115]. M. alba extract considerably improves glucose lipid levels, islet function, and insulin resistance index. It also substantially inhibits PA-induced apoptosis and markedly activates the AMPK/mTOR signaling pathway, inducing islet cell autophagy and improving the functional utilization of islet cells[115,116]. The aqueous extract of M. alba downregulates the expression levels of relevant inflammatory factors, ameliorating chronic inflammation. Additionally, M. alba eliminates oxidative stress caused by IGT by modulating the advanced glycation end-products (AGEs)/receptor of AGEs/NADPH oxidase 4/NF-κB signaling pathway[117]. Importantly, ensuring the safety of M. alba leaves is crucial due to the abundance of biologically active phytochemicals and their many beneficial components. Acute toxicity, subacute toxicity, and genotoxicity studies in rats have shown no mortality or abnormal behavioral changes; no parameter changes in blood, biochemistry, or histopathology; and no mutagenic activity in the Ames assay. These findings weaken the claim that the aqueous extract of M. alba leaves may induce chromosomal aberrations or sperm abnormalities[118]. Therefore, the medicinal use of the aqueous extract of M. alba leaves is considered safe.
Astragalus membranaceus (A. membranaceus) has a long history of medical applications in China, including tonifying qi, lowering lipid and blood pressure levels, nurturing the heart, and regulating blood glucose[119,120]. Moreover, flavonoids of A. membranaceus regulate intestinal microflora, reduce FBG, and improve brain damage caused by IGT[121]. Water-soluble A. membranaceus polysaccharides considerably reduce blood glucose levels and the insulin resistance index. They enhance glucose intolerance; improve insulin resistance in mice; and reduce oxidative stress, inflammation, and liver injury, while increasing the concentration of short-chain fatty acids in the intestinal flora. Notably, they augment the levels of Allobaculum, Lactobacillus, Akkermansia, Faecia, Akkermansia, and Faecalibaculum in the intestinal flora of mice with IGT[122,123]. These functions have positive implications for alleviating symptoms associated with diabetes[124]. Unfortunately, studies on the toxicity of A. membranaceus are limited, and toxicology tests are required before considering it as an intervention for prediabetes.
In addressing prediabetes, obesity is a key factor. To delay diabetes onset, increasing exercise intensity and duration is crucial. For prediabetic patients, a weekly increase of 150 min in exercise or 30 min daily can significantly lower the fasting glycemic index[125]. Six months of high-intensity exercise effectively improves oral glucose tolerance[126], and 20 wk of sustained exercise normalizes blood glucose levels[127]. A Meta-analysis confirms that both aerobic and resistance training, individually or combined, benefit insulin resistance and glycemic control in prediabetic patients[128].
Enhancing behavioral interventions requires a comprehensive, adaptable strategy that accounts for patient preferences, risks, and comorbidities, ensuring long-term adherence. This strategy should include building supportive relationships that encourage healthy behaviors, timely plan adjustments based on patient progress, and incorporating incentives for sustained motivation and adherence[129].
A high-calorie diet contributes to prediabetes development. Epidemiologic evidence supports increasing intake of non-starchy vegetables, fruits, and whole grains[130], while limiting added sugars to effectively reduce glycated HbA1c, fasting glycemic index, serum insulin, insulin resistance, cholesterol levels, body weight, and body mass index[131]. This approach also lowers type 2 diabetes risk[132]. Early time-restricted feeding, involving a 6-h eating window ending by 3 p.m., improves insulin sensitivity, β-cell responsiveness, blood pressure, oxidative stress, and appetite within 5 wk, aiding diabetes prevention[133]. This study aims to concisely highlight the importance of dietary protein and fiber in mitigating prediabetes.
Pharmacologic and lifestyle interventions can be combined for the prevention of diabetes. Metformin is the most commonly used drug in combination with lifestyle interventions. The administration of metformin (500-2000 mg/d) combined with exercise has improved insulin sensitivity in prediabetic patients[134]. However, this combination does not offer an advantage[135] and may even diminish the glucose-lowering effects of exercise[136] compared with metformin alone (1000 mg twice daily) and exercise training alone.
Diabetes has a high incidence with a low reversal rate[137]. Prediabetes, a common precursor often accompanied by microvascular complications like retinopathy, cardiovascular disease, and other issues, underscores the importance of effective intervention and management to slow or even prevent the development of diabetes.
Unhealthy lifestyles play a pivotal role in prediabetes development. Achieving complete remission of prediabetes necessitates the long-term maintenance of a healthy lifestyle. A combination of a nutritious diet and increased physical activity plays a crucial role in preventing or delaying the onset of diabetes and its complications. While lifestyle interventions are effective in the short term, their long-term efficacy is limited. Therefore, pharmacological interventions become necessary. These interventions can correct glucose homeostasis dysregulation, delay diabetes progression, and control glucose and lipid metabolism disorders. Common pharmacological interventions for prediabetes include metformin, GLP-1RAS, SGLT2 inhibitors, vitamin D, and Chinese herbal medicine[138-142]. These drugs have multifaceted effects, including blood sugar regulation, improved insulin sensitivity, and reduced insulin resistance.
Current treatments for prediabetic patients predominantly consist of lifestyle and pharmacological interventions. However, patient adherence to lifestyle interventions is often challenging to maintain in the long term, and a single lifestyle change is typically insufficient to extensively improve glycemic regulation. In contrast, a single pharmacological intervention can promptly lower blood glucose levels and enhance insulin sensitivity, yet prolonged use may lead to drug resistance over time. Combining lifestyle interventions with appropriate medication, as opposed to monotherapy, can yield a more favorable therapeutic outcome by promoting sustained weight loss, normal blood glucose control, pancreatic islet cell repair, and improved insulin sensitivity. Integrating lifestyle and pharmacological interventions is likely to be more acceptable to prediabetic patients and aligns with current treatment trends. Furthermore, the prevention and management of prediabetes take precedence over treating diabetes. Regular monitoring of daily blood glucose, weight, and medical parameters enables timely diabetes detection and disease progression control. Safe and effective interventions for prediabetes remain a necessity. Future efforts should focus on improving standardized prediabetes diagnosis to facilitate early detection, management, and treatment of prediabetic patients. The integration of pharmacological and lifestyle interventions is poised to become a new direction in prediabetes treatment.
This paper provides a comprehensive overview of the mechanisms behind prediabetes development and its associated therapeutic drugs. Prediabetes is significantly influenced by unhealthy lifestyles. To achieve complete remission, it is crucial to maintain a healthy lifestyle, including a balanced diet and regular physical activity. These practices are key in preventing or delaying the onset of diabetes and its complications. While lifestyle changes are effective short-term, their long-term efficacy is limited, making pharmacological treatments essential. Treatments such as metformin, GLP-1RAS, SGLT2 inhibitors, vitamin D, and Chinese herbal medicine play a pivotal role in regulating glucose homeostasis, decelerating diabetes progression, and controlling glucose and lipid metabolism disorders. They also help regulate blood sugar levels, improve insulin sensitivity, and reduce insulin resistance.
We would like to thank Prof. Qi Chen for providing suggestions regarding the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Chinese Society for Immunology, M200956803S.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shamseldeen AM, Egypt S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Tönnies T, Brinks R, Isom S, Dabelea D, Divers J, Mayer-Davis EJ, Lawrence JM, Pihoker C, Dolan L, Liese AD, Saydah SH, D'Agostino RB, Hoyer A, Imperatore G. Projections of Type 1 and Type 2 Diabetes Burden in the U.S. Population Aged <20 Years Through 2060: The SEARCH for Diabetes in Youth Study. Diabetes Care. 2023;46:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S29-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Rett K, Gottwald-Hostalek U. Understanding prediabetes: definition, prevalence, burden and treatment options for an emerging disease. Curr Med Res Opin. 2019;35:1529-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2251] [Article Influence: 321.6] [Reference Citation Analysis (0)] |
| 5. | Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1964] [Cited by in RCA: 2397] [Article Influence: 342.4] [Reference Citation Analysis (0)] |
| 6. | Softic S, Gupta MK, Wang GX, Fujisaka S, O'Neill BT, Rao TN, Willoughby J, Harbison C, Fitzgerald K, Ilkayeva O, Newgard CB, Cohen DE, Kahn CR. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017;127:4059-4074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 7. | Wallace AS, Wang D, Shin JI, Selvin E. Screening and Diagnosis of Prediabetes and Diabetes in US Children and Adolescents. Pediatrics. 2020;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2128] [Article Influence: 425.6] [Reference Citation Analysis (0)] |
| 9. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 1871] [Article Influence: 467.8] [Reference Citation Analysis (0)] |
| 10. | American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1409] [Article Influence: 469.7] [Reference Citation Analysis (1)] |
| 11. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1301] [Article Influence: 650.5] [Reference Citation Analysis (70)] |
| 12. | Khan T, Wozniak GD, Kirley K. An assessment of medical students' knowledge of prediabetes and diabetes prevention. BMC Med Educ. 2019;19:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69:2932-2938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 14. | Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1356] [Article Influence: 169.5] [Reference Citation Analysis (0)] |
| 15. | Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, Zhang X, Li C, Huang Z, Sun X, Wang L, Zhou M, Wu J, Wang Y. Prevalence and Treatment of Diabetes in China, 2013-2018. JAMA. 2021;326:2498-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 557] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 16. | Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, Shi B, Sun H, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Ning G, Mu Y, Zhao J, Teng W, Shan Z. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1030] [Cited by in RCA: 996] [Article Influence: 199.2] [Reference Citation Analysis (1)] |
| 17. | Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazari A, Gundmi S, Jadhav R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 18. | Merz KE, Thurmond DC. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr Physiol. 2020;10:785-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 19. | Cleveland KH, Schnellmann RG. Pharmacological Targeting of Mitochondria in Diabetic Kidney Disease. Pharmacol Rev. 2023;75:250-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 20. | White NH, Pan Q, Knowler WC, Schroeder EB, Dabelea D, Chew EY, Blodi B, Goldberg RB, Pi-Sunyer X, Darwin C, Schlögl M, Nathan DM; Diabetes Prevention Program Outcome Study (DPPOS) Research Group. Risk Factors for the Development of Retinopathy in Prediabetes and Type 2 Diabetes: The Diabetes Prevention Program Experience. Diabetes Care. 2022;45:2653-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Kirthi V, Nderitu P, Alam U, Evans J, Nevitt S, Malik RA, Jackson TL. Prevalence of retinopathy in prediabetes: protocol for a systematic review and meta-analysis. BMJ Open. 2021;11:e040997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Asare-Bediako B, Noothi SK, Li Calzi S, Athmanathan B, Vieira CP, Adu-Agyeiwaah Y, Dupont M, Jones BA, Wang XX, Chakraborty D, Levi M, Nagareddy PR, Grant MB. Characterizing the Retinal Phenotype in the High-Fat Diet and Western Diet Mouse Models of Prediabetes. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Ng CH, Chan KE, Chin YH, Zeng RW, Tsai PC, Lim WH, Tan DJH, Khoo CM, Goh LH, Ling ZJ, Kulkarni A, Mak LL, Huang DQ, Chan M, Chew NW, Siddiqui MS, Sanyal AJ, Muthiah M. The effect of diabetes and prediabetes on the prevalence, complications and mortality in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2022;28:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 24. | Welsh C, Welsh P, Celis-Morales CA, Mark PB, Mackay D, Ghouri N, Ho FK, Ferguson LD, Brown R, Lewsey J, Cleland JG, Gray SR, Lyall DM, Anderson JJ, Jhund PS, Pell JP, McGuire DK, Gill JMR, Sattar N. Glycated Hemoglobin, Prediabetes, and the Links to Cardiovascular Disease: Data From UK Biobank. Diabetes Care. 2020;43:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Manouchehri M, Cea-Soriano L, Franch-Nadal J, Ruiz A, Goday A, Villanueva R, Diez-Espino J, Mata-Cases M, Giraldez-García C, Regidor E; PREDAPS Study Group. Heterogeneity in the association between prediabetes categories and reduction on glomerular filtration rate in a 5-year follow-up. Sci Rep. 2022;12:7373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Rourke JL, Hu Q, Screaton RA. AMPK and Friends: Central Regulators of β Cell Biology. Trends Endocrinol Metab. 2018;29:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Guillén C, Benito M. mTORC1 Overactivation as a Key Aging Factor in the Progression to Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 2018;9:621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Gokulakrishnan K, Ranjani H, Weber MB, Pandey GK, Anjana RM, Balasubramanyam M, Prabhakaran D, Tandon N, Narayan KM, Mohan V. Effect of lifestyle improvement program on the biomarkers of adiposity, inflammation and gut hormones in overweight/obese Asian Indians with prediabetes. Acta Diabetol. 2017;54:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Hu Z, Qin L, Xu H. One-Year Results of a Synthetic Intervention Model for the Primary Prevention of T2D among Elderly Individuals with Prediabetes in Rural China. Int J Environ Res Public Health. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Apolzan JW, Venditti EM, Edelstein SL, Knowler WC, Dabelea D, Boyko EJ, Pi-Sunyer X, Kalyani RR, Franks PW, Srikanthan P, Gadde KM; Diabetes Prevention Program Research Group. Long-Term Weight Loss With Metformin or Lifestyle Intervention in the Diabetes Prevention Program Outcomes Study. Ann Intern Med. 2019;170:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 31. | Jonas DE, Crotty K, Yun JDY, Middleton JC, Feltner C, Taylor-Phillips S, Barclay C, Dotson A, Baker C, Balio CP, Voisin CE, Harris RP. Screening for Prediabetes and Type 2 Diabetes: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 32. | O'Brien MJ, Perez A, Scanlan AB, Alos VA, Whitaker RC, Foster GD, Ackermann RT, Ciolino JD, Homko C. PREVENT-DM Comparative Effectiveness Trial of Lifestyle Intervention and Metformin. Am J Prev Med. 2017;52:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Diabetes Prevention Program Research Group. Long-term Effects of Metformin on Diabetes Prevention: Identification of Subgroups That Benefited Most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2019;42:601-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 34. | le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, Ortiz RV, Wilding JPH, Skjøth TV, Manning LS, Pi-Sunyer X; SCALE Obesity Prediabetes NN8022-1839 Study Group. 3 years of liraglutide vs placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a random-ised, double-blind trial. Lancet. 2017;389:1399-1409. [RCA] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 499] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 35. | Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 2177] [Article Influence: 544.3] [Reference Citation Analysis (0)] |
| 36. | Kim SH, Abbasi F, Nachmanoff C, Stefanakis K, Kumar A, Kalra B, Savjani G, Mantzoros CS. Effect of the glucagon-like peptide-1 analogue liraglutide versus placebo treatment on circulating proglucagon-derived peptides that mediate improvements in body weight, insulin secretion and action: A randomized controlled trial. Diabetes Obes Metab. 2021;23:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Herman R, Kravos NA, Jensterle M, Janež A, Dolžan V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 38. | Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321:1926-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 322] [Article Influence: 53.7] [Reference Citation Analysis (1)] |
| 39. | Inzucchi SE, Docherty KF, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Verma S, Bělohlávek J, Böhm M, Chiang CE, de Boer RA, Diez M, Dukát A, Ljungman CEA, Bengtsson O, Langkilde AM, Sjöstrand M, Jhund PS, McMurray JJV; DAPA-HF Investigators and Committees. Dapagliflozin and the Incidence of Type 2 Diabetes in Patients With Heart Failure and Reduced Ejection Fraction: An Exploratory Analysis From DAPA-HF. Diabetes Care. 2021;44:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 40. | Kaneto H, Kimura T, Obata A, Shimoda M, Kaku K. Multifaceted Mechanisms of Action of Metformin Which Have Been Unraveled One after Another in the Long History. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 41. | Cheng J, Xu L, Yu Q, Lin G, Ma X, Li M, Guan F, Liu Y, Huang X, Xie J, Chen J, Su Z, Li Y. Metformin alleviates long-term high-fructose diet-induced skeletal muscle insulin resistance in rats by regulating purine nucleotide cycle. Eur J Pharmacol. 2022;933:175234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 42. | Ma T, Tian X, Zhang B, Li M, Wang Y, Yang C, Wu J, Wei X, Qu Q, Yu Y, Long S, Feng JW, Li C, Zhang C, Xie C, Wu Y, Xu Z, Chen J, Huang X, He Y, Yao L, Zhang L, Zhu M, Wang W, Wang ZC, Zhang M, Bao Y, Jia W, Lin SY, Ye Z, Piao HL, Deng X, Zhang CS, Lin SC. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603:159-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 337] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 43. | Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, O'Rourke B, He L. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019;29:1511-1523.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 44. | Jia J, Bissa B, Brecht L, Allers L, Choi SW, Gu Y, Zbinden M, Burge MR, Timmins G, Hallows K, Behrends C, Deretic V. AMPK, a Regulator of Metabolism and Autophagy, Is Activated by Lysosomal Damage via a Novel Galectin-Directed Ubiquitin Signal Transduction System. Mol Cell. 2020;77:951-969.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 45. | Li M, Li X, Zhang H, Lu Y. Molecular Mechanisms of Metformin for Diabetes and Cancer Treatment. Front Physiol. 2018;9:1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 46. | Nojima I, Wada J. Metformin and Its Immune-Mediated Effects in Various Diseases. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 47. | LaMoia TE, Shulman GI. Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev. 2021;42:77-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 512] [Cited by in RCA: 433] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 48. | Herman WH, Ratner RE. Metformin Should Be Used to Treat Prediabetes in Selected Individuals. Diabetes Care. 2020;43:1988-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Nishimura R, Taniguchi M, Takeshima T, Iwasaki K. Efficacy and Safety of Metformin Versus the Other Oral Antidiabetic Drugs in Japanese Type 2 Diabetes Patients: A Network Meta-analysis. Adv Ther. 2022;39:632-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, Bray GA, Schade DS, Temprosa MG, White NH, Crandall JP; Diabetes Prevention Program Research Group. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101:1754-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 303] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 51. | Tavlo M, Skakkebæk NE, Mathiesen ER, Kristensen DM, Kjær KH, Andersson AM, Lindahl-Jacobsen R. Hypothesis: Metformin is a potential reproductive toxicant. Front Endocrinol (Lausanne). 2022;13:1000872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 52. | Smith NK, Hackett TA, Galli A, Flynn CR. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem Int. 2019;128:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 53. | Goldenberg RM, Ahooja V, Clemens KK, Gilbert JD, Poddar M, Verma S. Practical Considerations and Rationale for Glucagon-Like Peptide-1 Receptor Agonist Plus Sodium-Dependent Glucose Cotransporter-2 Inhibitor Combination Therapy in Type 2 Diabetes. Can J Diabetes. 2021;45:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Tan Q, Akindehin SE, Orsso CE, Waldner RC, DiMarchi RD, Müller TD, Haqq AM. Recent Advances in Incretin-Based Pharmacotherapies for the Treatment of Obesity and Diabetes. Front Endocrinol (Lausanne). 2022;13:838410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 55. | Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 1096] [Article Influence: 156.6] [Reference Citation Analysis (1)] |
| 56. | Gilbert MP, Pratley RE. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front Endocrinol (Lausanne). 2020;11:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 57. | Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 850] [Article Influence: 212.5] [Reference Citation Analysis (0)] |
| 58. | Perreault L, Davies M, Frias JP, Laursen PN, Lingvay I, Machineni S, Varbo A, Wilding JPH, Wallenstein SOR, le Roux CW. Changes in Glucose Metabolism and Glycemic Status With Once-Weekly Subcutaneous Semaglutide 2.4 mg Among Participants With Prediabetes in the STEP Program. Diabetes Care. 2022;45:2396-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 59. | Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzík M; PIONEER 1 Investigators. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care. 2019;42:1724-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 295] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 60. | Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J Clin Endocrinol Metab. 2021;106:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 183] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 61. | Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 974] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 62. | Andersen A, Knop FK, Vilsbøll T. A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes. Drugs. 2021;81:1003-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 63. | Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: A 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab. 2017;19:49-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 64. | Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, Dreisbach JG, Labinjoh C, Lang NN, Lennie V, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Speirits IA, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark PB, McMurray JJV, Jhund PS, Petrie MC, Sattar N. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 2021;143:516-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 65. | Pafili K, Papanas N. Tofogliflozin: the road goes ever on. Expert Opin Pharmacother. 2014;15:1197-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Ferrannini E, Murthy AC, Lee YH, Muscelli E, Weiss S, Ostroff RM, Sattar N, Williams SA, Ganz P. Mechanisms of Sodium-Glucose Cotransporter 2 Inhibition: Insights From Large-Scale Proteomics. Diabetes Care. 2020;43:2183-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 67. | Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 68. | Op den Kamp YJM, de Ligt M, Dautzenberg B, Kornips E, Esterline R, Hesselink MKC, Hoeks J, Schrauwen-Hinderling VB, Havekes B, Oscarsson J, Phielix E, Schrauwen P. Effects of the SGLT2 Inhibitor Dapagliflozin on Energy Metabolism in Patients With Type 2 Diabetes: A Randomized, Double-Blind Crossover Trial. Diabetes Care. 2021;44:1334-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 69. | Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE, Testani JM. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation. 2020;142:1028-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 288] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 70. | Kramer CK, Zinman B. Sodium-Glucose Cotransporter-2 (SGLT-2) Inhibitors and the Treatment of Type 2 Diabetes. Annu Rev Med. 2019;70:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Mori Y, Duru OK, Tuttle KR, Fukuma S, Taura D, Harada N, Inagaki N, Inoue K. Sodium-Glucose Cotransporter 2 Inhibitors and New-onset Type 2 Diabetes in Adults With Prediabetes: Systematic Review and Meta-analysis of Randomized Controlled Trials. J Clin Endocrinol Metab. 2022;108:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Lundkvist P, Pereira MJ, Katsogiannos P, Sjöström CD, Johnsson E, Eriksson JW. Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: Sustained reductions in body weight, glycaemia and blood pressure over 1 year. Diabetes Obes Metab. 2017;19:1276-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 73. | Klen J, Dolžan V. Treatment Response to SGLT2 Inhibitors: From Clinical Characteristics to Genetic Variations. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Beitelshees AL, Leslie BR, Taylor SI. Sodium-Glucose Cotransporter 2 Inhibitors: A Case Study in Translational Research. Diabetes. 2019;68:1109-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | de la Puente Yagüe M, Collado Yurrita L, Ciudad Cabañas MJ, Cuadrado Cenzual MA. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 76. | Jagannath VA, Fedorowicz Z, Asokan GV, Robak EW, Whamond L. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev. 2010;CD008422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Rozmus D, Ciesielska A, Płomiński J, Grzybowski R, Fiedorowicz E, Kordulewska N, Savelkoul H, Kostyra E, Cieślińska A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms-The Risk of Malignant Tumors and Other Diseases. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 78. | Pop TL, Sîrbe C, Benţa G, Mititelu A, Grama A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 79. | Lemke D, Klement RJ, Schweiger F, Schweiger B, Spitz J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front Immunol. 2021;12:655739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 80. | Cojic M, Kocic R, Klisic A, Kocic G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients With Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front Endocrinol (Lausanne). 2021;12:610893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 81. | Pittas AG, Kawahara T, Jorde R, Dawson-Hughes B, Vickery EM, Angellotti E, Nelson J, Trikalinos TA, Balk EM. Vitamin D and Risk for Type 2 Diabetes in People With Prediabetes : A Systematic Review and Meta-analysis of Individual Participant Data From 3 Randomized Clinical Trials. Ann Intern Med. 2023;176:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 82. | Infante M, Ricordi C, Sanchez J, Clare-Salzler MJ, Padilla N, Fuenmayor V, Chavez C, Alvarez A, Baidal D, Alejandro R, Caprio M, Fabbri A. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 83. | Zhang Y, Tan H, Tang J, Li J, Chong W, Hai Y, Feng Y, Lunsford LD, Xu P, Jia D, Fang F. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients With Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2020;43:1650-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 84. | Lu L, Bennett DA, Millwood IY, Parish S, McCarthy MI, Mahajan A, Lin X, Bragg F, Guo Y, Holmes MV, Afzal S, Nordestgaard BG, Bian Z, Hill M, Walters RG, Li L, Chen Z, Clarke R. Association of vitamin D with risk of type 2 diabetes: A Mendelian randomisation study in European and Chinese adults. PLoS Med. 2018;15:e1002566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 85. | Kawahara T, Suzuki G, Mizuno S, Inazu T, Kasagi F, Kawahara C, Okada Y, Tanaka Y. Effect of active vitamin D treatment on development of type 2 diabetes: DPVD randomised controlled trial in Japanese population. BMJ. 2022;377:e066222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 86. | Rasouli N, Brodsky IG, Chatterjee R, Kim SH, Pratley RE, Staten MA, Pittas AG; D2d Research Group. Effects of Vitamin D Supplementation on Insulin Sensitivity and Secretion in Prediabetes. J Clin Endocrinol Metab. 2022;107:230-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 87. | Lemieux P, Weisnagel SJ, Caron AZ, Julien AS, Morisset AS, Carreau AM, Poirier J, Tchernof A, Robitaille J, Bergeron J, Marette A, Vohl MC, Gagnon C. Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: a randomised, placebo-controlled trial. Eur J Endocrinol. 2019;181:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 88. | Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 89. | Zhang P, Guo D, Xu B, Huang C, Yang S, Wang W, Liu W, Deng Y, Li K, Liu D, Lin J, Wei X, Huang Y, Zhang H. Association of Serum 25-Hydroxyvitamin D With Cardiovascular Outcomes and All-Cause Mortality in Individuals With Prediabetes and Diabetes: Results From the UK Biobank Prospective Cohort Study. Diabetes Care. 2022;45:1219-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 90. | Dawson-Hughes B, Staten MA, Knowler WC, Nelson J, Vickery EM, LeBlanc ES, Neff LM, Park J, Pittas AG; D2d Research Group. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults With Prediabetes: A Secondary Analysis From the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 91. | Barbarawi M, Zayed Y, Barbarawi O, Bala A, Alabdouh A, Gakhal I, Rizk F, Alkasasbeh M, Bachuwa G, Manson JE. Effect of Vitamin D Supplementation on the Incidence of Diabetes Mellitus. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 92. | Mohammadi S, Hajhashemy Z, Saneei P. Serum vitamin D levels in relation to type-2 diabetes and prediabetes in adults: a systematic review and dose-response meta-analysis of epidemiologic studies. Crit Rev Food Sci Nutr. 2022;62:8178-8198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 93. | Brandenburg V, Ketteler M. Vitamin D and Secondary Hyperparathyroidism in Chronic Kidney Disease: A Critical Appraisal of the Past, Present, and the Future. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 94. | Bjelakovic M, Nikolova D, Bjelakovic G, Gluud C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2021;8:CD011564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Charoenngam N, Shirvani A, Holick MF. Vitamin D for skeletal and non-skeletal health: What we should know. J Clin Orthop Trauma. 2019;10:1082-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 96. | Chen ZJ, Ma F, Sun XM, Zhao XS, Luo R. Renoprotective Effect of a Chinese Herbal Formula, Qidan Dihuang Decoction, on Streptozotocin-Induced Diabetes in Rat. Evid Based Complement Alternat Med. 2018;2018:7321086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Wang D, Ren Y, Sun W, Gong J, Zou X, Dong H, Xu L, Wang K, Lu F. Berberine Ameliorates Glucose Metabolism in Diabetic Rats through the alpha7 Nicotinic Acetylcholine Receptor-Related Cholinergic Anti-Inflammatory Pathway. Planta Med. 2022;88:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Xu X, Gao Z, Yang F, Yang Y, Chen L, Han L, Zhao N, Xu J, Wang X, Ma Y, Shu L, Hu X, Lyu N, Pan Y, Zhu B, Zhao L, Tong X, Wang J. Antidiabetic Effects of Gegen Qinlian Decoction via the Gut Microbiota Are Attributable to Its Key Ingredient Berberine. Genomics Proteomics Bioinformatics. 2020;18:721-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 99. | Wang Y, Liu H, Zheng M, Yang Y, Ren H, Kong Y, Wang S, Wang J, Jiang Y, Yang J, Shan C. Berberine Slows the Progression of Prediabetes to Diabetes in Zucker Diabetic Fatty Rats by Enhancing Intestinal Secretion of Glucagon-Like Peptide-2 and Improving the Gut Microbiota. Front Endocrinol (Lausanne). 2021;12:609134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 100. | Zhao MM, Lu J, Li S, Wang H, Cao X, Li Q, Shi TT, Matsunaga K, Chen C, Huang H, Izumi T, Yang JK. Author Correction: Berberine is an insulin secretagogue targeting the KCNH6 potassium channel. Nat Commun. 2021;12:6342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Yi J, Ye X, Wang D, He K, Yang Y, Liu X, Li X. Safety evaluation of main alkaloids from Rhizoma Coptidis. J Ethnopharmacol. 2013;145:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 102. | Tian X, Zhang Y, Li H, Li Y, Wang N, Zhang W, Ma B. Palmatine ameliorates high fat diet induced impaired glucose tolerance. Biol Res. 2020;53:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Long J, Song J, Zhong L, Liao Y, Liu L, Li X. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie. 2019;162:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 104. | Chen Z, Jian Y, Wu Q, Wu J, Sheng W, Jiang S, Shehla N, Aman S, Wang W. Cyclocarya paliurus (Batalin) Iljinskaja: Botany, Ethnopharmacology, phytochemistry and pharmacology. J Ethnopharmacol. 2022;285:114912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 105. | Kakar MU, Naveed M, Saeed M, Zhao S, Rasheed M, Firdoos S, Manzoor R, Deng Y, Dai R. A review on structure, extraction, and biological activities of polysaccharides isolated from Cyclocarya paliurus (Batalin) Iljinskaja. Int J Biol Macromol. 2020;156:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 106. | Li Q, Hu J, Xie J, Nie S, Xie MY. Isolation, structure, and bioactivities of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja. Ann N Y Acad Sci. 2017;1398:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Zheng XR, Zhang MJ, Shang XL, Fang SZ, Chen FM. Etiology of Cyclocarya paliurus Anthracnose in Jiangsu Province, China. Front Plant Sci. 2020;11:613499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Yang HM, Yin ZQ, Zhao MG, Jiang CH, Zhang J, Pan K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry. 2018;151:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 109. | Li J, Luo M, Hu M, Guo AY, Yang X, Zhang Q, Zhu Y. Investigating the Molecular Mechanism of Aqueous Extract of Cyclocarya paliurus on Ameliorating Diabetes by Transcriptome Profiling. Front Pharmacol. 2018;9:912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Yao Y, Yan L, Chen H, Wu N, Wang W, Wang D. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine. 2020;77:153268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 111. | Qiu M, Peng J, Deng H, Chang Y, Hu D, Pan W, Wu H, Xiao H. The Leaves of Cyclocarya paliurus: A Functional Tea with Preventive and Therapeutic Potential of Type 2 Diabetes. Am J Chin Med. 2022;50:1447-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Liu C, Zhao M, Wen L, Zhao H. The chemical composition and toxic effects of aqueous extracts of Cyclocarya paliurus leaves. Front Nutr. 2022;9:994055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 113. | Han JH, Lee HW, Jung SH, Cho CW, Kim TJ, Kang JS, Myung CS. The anti-obesity effect of mulberry leaf (Mori Folium) extracts was increased by bioconversion with Pectinex. Sci Rep. 2022;12:20375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 114. | Lim SH, Yu JS, Lee HS, Choi CI, Kim KH. Antidiabetic Flavonoids from Fruits of Morus alba Promoting Insulin-Stimulated Glucose Uptake via Akt and AMP-Activated Protein Kinase Activation in 3T3-L1 Adipocytes. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 115. | Lv Q, Lin J, Wu X, Pu H, Guan Y, Xiao P, He C, Jiang B. Novel active compounds and the anti-diabetic mechanism of mulberry leaves. Front Pharmacol. 2022;13:986931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 116. | Ji S, Zhu C, Gao S, Shao X, Chen X, Zhang H, Tang D. Morus alba leaves ethanol extract protects pancreatic islet cells against dysfunction and death by inducing autophagy in type 2 diabetes. Phytomedicine. 2021;83:153478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 117. | Liu C, Zhu R, Liu H, Li L, Chen B, Jia Q, Wang L, Ma R, Tian S, Wang M, Fu M, Niu J, Orekhov AN, Gao S, Zhang D, Zhao B. Aqueous Extract of Mori Folium Exerts Bone Protective Effect Through Regulation of Calcium and Redox Homeostasis via PTH/VDR/CaBP and AGEs/RAGE/Nox4/NF-κB Signaling in Diabetic Rats. Front Pharmacol. 2018;9:1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Li Y, Zhang X, Liang C, Hu J, Yu Z. Safety evaluation of mulberry leaf extract: Acute, subacute toxicity and genotoxicity studies. Regul Toxicol Pharmacol. 2018;95:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 119. | Zheng Y, Ren W, Zhang L, Zhang Y, Liu D, Liu Y. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front Pharmacol. 2020;11:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 120. | Wang L, Zheng W, Yang J, Ali A, Qin H. Mechanism of Astragalus membranaceus Alleviating Acquired Hyperlipidemia Induced by High-Fat Diet through Regulating Lipid Metabolism. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 121. | Li X, Zhao T, Gu J, Wang Z, Lin J, Wang R, Duan T, Li Z, Dong R, Wang W, Hong KF, Liu Z, Huang W, Gui D, Zhou H, Xu Y. Intake of flavonoids from Astragalus membranaceus ameliorated brain impairment in diabetic mice via modulating brain-gut axis. Chin Med. 2022;17:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 122. | Chen X, Chen C, Fu X. Hypoglycemic activity in vitro and vivo of a water-soluble polysaccharide from Astragalus membranaceus. Food Funct. 2022;13:11210-11222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 123. | Chen X, Chen C, Fu X. Hypoglycemic effect of the polysaccharides from Astragalus membranaceus on type 2 diabetic mice based on the "gut microbiota-mucosal barrier". Food Funct. 2022;13:10121-10133. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 124. | Song Q, Cheng SW, Li D, Cheng H, Lai YS, Han Q, Wu HY, Shaw PC, Zuo Z. Gut microbiota mediated hypoglycemic effect of Astragalus membranaceus polysaccharides in db/db mice. Front Pharmacol. 2022;13:1043527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 125. | Sénéchal M, Slaght J, Bharti N, Bouchard DR. Independent and combined effect of diet and exercise in adults with prediabetes. Diabetes Metab Syndr Obes. 2014;7:521-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 126. | Slentz CA, Bateman LA, Willis LH, Granville EO, Piner LW, Samsa GP, Setji TL, Muehlbauer MJ, Huffman KM, Bales CW, Kraus WE. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia. 2016;59:2088-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 127. | Huang L, Fang Y, Tang L. Comparisons of different exercise interventions on glycemic control and insulin resistance in prediabetes: a network meta-analysis. BMC Endocr Disord. 2021;21:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 128. | Koenigsberg MR, Corliss J. Diabetes Self-Management: Facilitating Lifestyle Change. Am Fam Physician. 2017;96:362-370. [PubMed] |
| 129. | McAtee JR, Tao MH, King C, Chai W. Association of Home Food Availability with Prediabetes and Diabetes among Adults in the United States. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 130. | Gardner CD, Landry MJ, Perelman D, Petlura C, Durand LR, Aronica L, Crimarco A, Cunanan KM, Chang A, Dant CC, Robinson JL, Kim SH. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am J Clin Nutr. 2022;116:640-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 131. | Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020;17:e1003053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 132. | Augustin LSA, Kendall CWC, Jenkins DJA, Willett WC, Astrup A, Barclay AW, Björck I, Brand-Miller JC, Brighenti F, Buyken AE, Ceriello A, La Vecchia C, Livesey G, Liu S, Riccardi G, Rizkalla SW, Sievenpiper JL, Trichopoulou A, Wolever TMS, Baer-Sinnott S, Poli A. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25:795-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 432] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 133. | Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27:1212-1221.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 952] [Article Influence: 136.0] [Reference Citation Analysis (0)] |
| 134. | Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35:131-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 135. | Madsen KS, Chi Y, Metzendorf MI, Richter B, Hemmingsen B. Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst Rev. 2019;12:CD008558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 136. | Viskochil R, Malin SK, Blankenship JM, Braun B. Exercise training and metformin, but not exercise training alone, decreases insulin production and increases insulin clearance in adults with prediabetes. J Appl Physiol (1985). 2017;123:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 137. | Zinman B, Harris SB, Neuman J, Gerstein HC, Retnakaran RR, Raboud J, Qi Y, Hanley AJ. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet. 2010;376:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 138. | Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, Yap F, Ward GM, Cowell CT. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 139. | Andreadis EA, Katsanou PM, Georgiopoulos DX, Tsourous GI, Yfanti GK, Gouveri ET, Diamantopoulos EJ. The effect of metformin on the incidence of type 2 diabetes mellitus and cardiovascular disease risk factors in overweight and obese subjects--the Carmos study. Exp Clin Endocrinol Diabetes. 2009;117:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 140. | Tao T, Zhang Y, Zhu YC, Fu JR, Wang YY, Cai J, Ma JY, Xu Y, Gao YN, Sun Y, Fan W, Liu W. Exenatide, Metformin, or Both for Prediabetes in PCOS: A Randomized, Open-label, Parallel-group Controlled Study. J Clin Endocrinol Metab. 2021;106:e1420-e1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 141. | Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1571] [Article Influence: 157.1] [Reference Citation Analysis (0)] |
| 142. | Veelen A, Andriessen C, Op den Kamp Y, Erazo-Tapia E, de Ligt M, Mevenkamp J, Jörgensen JA, Moonen-Kornips E, Schaart G, Esterline R, Havekes B, Oscarsson J, Schrauwen-Hinderling VB, Phielix E, Schrauwen P. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on substrate metabolism in prediabetic insulin resistant individuals: A randomized, double-blind crossover trial. Metabolism. 2023;140:155396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |