Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.311
Peer-review started: November 11, 2023
First decision: January 12, 2024
Revised: January 14, 2024
Accepted: February 19, 2024
Article in press: February 19, 2024
Published online: March 15, 2024
Processing time: 124 Days and 23.9 Hours
While chronic hyperglycaemia resulting from poorly controlled diabetes mellitus (DM) is a well-known precursor to complications such as diabetic retinopathy, neuropathy (including autonomic neuropathy), and nephropathy, a paradoxical intensification of these complications can rarely occur with aggressive glycemic management resulting in a rapid reduction of glycated haemoglobin. Although, acute onset or worsening of retinopathy and treatment induced neuropathy of diabetes are more common among these complications, rarely other problems such as albuminuria, diabetic kidney disease, Charcot’s neuroarthropathy, gastroparesis, and urinary bladder dysfunction are also encountered. The World Journal of Diabetes recently published a rare case of all these complications, occurring in a young type 1 diabetic female intensely managed during pregnancy, as a case report by Huret et al. It is essential to have a comprehensive understanding of the pathobiology, prevalence, predisposing factors, and management strategies for acute onset, or worsening of microvascular complications when rapid glycemic control is achieved, which serves to alleviate patient morbidity, enhance disease management compliance, and possibly to avoid medico-legal issues around this rare clinical problem. This editorial delves into the dynamics surrounding the acute exacerbation of microvascular complications in poorly controlled DM during rapid glycaemic control.
Core Tip: New onset, or acute worsening of preexisting microvascular complications of diabetes mellitus (DM), is an uncommon complication of rapid improvement of chronic hyperglycaemia from intensive management of DM. Worsening of diabetic retinopathy and treatment induced neuropathy of diabetes are the two common microvascular diseases complicating intensive DM treatment with a rapid glycated haemoglobin reduction more than 2% points within a period of 3 months, though less commonly other complications such as Charcot’s neuroarthropathy, diabetic nephropathy, gastroparesis and urinary bladder dysfunction are also encountered. This editorial discusses the case of a young female type 1 diabetic, intensively managed during pregnancy, developing all these complications, published as a case report in the World Journal of Diabetes, with an appraisal of the current evidence on this uncommon phenomenon.
- Citation: Blaibel D, Fernandez CJ, Pappachan JM. Acute worsening of microvascular complications of diabetes mellitus during rapid glycemic control: The pathobiology and therapeutic implications. World J Diabetes 2024; 15(3): 311-317
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/311.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.311
Uncontrolled chronic hyperglycaemia is associated with both micro- and macrovascular complications. The microvascular complications including diabetic nephropathy (DN), retinopathy, and neuropathy could lead to dialysis, blindness, and amputation, respectively[1,2]. On the other hand, macrovascular complications including myocardial infarction, heart failure, and stroke could increase the mortality risk[3]. Studies in type 2 diabetes mellitus (T2DM) patients have shown that certain microvascular complications are associated with increased risk for macrovascular complications and cardiovascular mortality[4]. Though intensive control of chronic hyperglycaemia could reduce the incidence of microvascular (statistically significant) and macrovascular (statistically not significant) complications[5], the global burden of these complications is still alarmingly high due to the increased prevalence of diabetes mellitus and increased life expectancy[6].
However, rapid control of chronic hyperglycaemia in those with longstanding poorly controlled diabetes can be associated with worsening microvascular complications including diabetic retinopathy (DR)[7,8], painful diabetic neuropathy (PDN)[9,10], Charcot’s neuroarthropathy (CN)[11-14], and rarely DN[15]. This paradoxical worsening of diabetic microvascular disease can occur in patients with both type 1 diabetes mellitus (T1DM) and T2DM, with insulin and non-insulin-based therapies, during pregnancy, post-bariatric surgery, and post-pancreas transplant[16].
The diabetes control and complications trial (DCCT) trial observed an early worsening of DR among T1DM patients with high glycated haemoglobin (HbA1c) levels at baseline and a marked HbA1c reduction in the initial 6 months of treatment[17]. Despite this early worsening, the intensively treated T1DM group had an equivalent or superior long-term retinopathy outcome in comparison to the conventionally treated group without early worsening. Coexistent renal impairment, presence of advanced DR (severe non-proliferative DR or proliferative DR), younger age at T1DM diagnosis, raised serum triglyceride levels, and increased retinal venular diameters are the known predictors for excessive risk of DR progression in those patients achieving rapid glycaemic control[17,18].
There are several studies in the literature that describe early worsening of DR with tighter glycaemic control[7,8,16-18]. For instance, in a study conducted at Oslo, half of the T1DM patients managed with continuous subcutaneous insulin infusion (CSII) experienced early worsening of DR within three months of intense glycemic control by the treatment, as opposed to those on short or intermediate-acting insulin regime[7,19]. Furthermore, similar findings were described in the Kroc Collaboration study, in which 47% of T1DM patients receiving CSII developed worsening DR at 8 months, while only 27% in the conventional treatment group had DR worsening[20]. The mechanism underlying the early worsening of retinopathy is not well established, however, there are several proposed modes of pathogenesis. The clinical or morphological early worsening of DR is defined as the new development of dot-and-blot haemorrhages, microaneurysms, intraretinal microvascular abnormalities (IRMAs), cotton-wool spots (also known as soft exudates), and capillary-free areas[7]. For example, in the aforementioned Kroc Collaboration study, higher numbers of cotton wool spots and IRMAs were observed in the intensely treated CSII group compared to the conventionally treated group at the 8 months of treatment[7,20].
However, it is noteworthy that at the end of 2 years of treatment, both groups exhibited a similar number of cotton wool spots and IRMAs indicating probable arrest of progression of disease after an initial worsening of DR. There are also some discrepancies in the observed DR outcomes between different studies comparing intense vs conventional glycemic control. In the Oslo study for instance, the CSII group had a significantly higher number of microaneurysms compared to the control group at three months of treatment while this group had considerably fewer microaneurysms at baseline (prior to the study)[19]. Similar findings were reported in DCCT trial in which an early DR worsening was observed in the intensively treated group with a rapid reduction of HbA1c at 6 months of treatment[17].
It is suggested that two factors contribute to early worsening DR, namely a glycaemia-related mechanism as well as a potential role for blood pressure (BP) control. A rapid drop of HbA1c with intensive glycaemic treatment lowers intravascular osmotic pressure[21]. The resultant osmotic gradient between intra- and extracellular compartments causes water movement across blood vessels with fluid exudation from the vulnerable retinal microcirculation[7]. Another proposed mechanism is a possible synergistic effect of insulin and vascular endothelial growth factor (VEGF) on retinal vessels with proliferation of retinal vessels and worsening of DR[22]. This overexpression of VEGF coupled with the release of reactive oxygen species in the context of hyperglycaemia might contribute to worsening of DR. Tight BP control also might contribute to worsening DR though this mechanism remains controversial with contrasting evidence[7].
The commonest microvascular complication associated with intensive glycaemic control is Treatment Induced Neuropathy of Diabetes (TIND), previously also known as insulin neuritis, characterised by painful sensory neuropathy and at times, autonomic neuropathy. TIND is defined as the occurrence of an acute neuropathy within 8 wk of a rapid decrease in HbA1c often following rapid improvement of a chronic hyperglycaemic state[10]. The actual prevalence of TIND is unknown but may be encountered in up to 10% of tertiary referrals for evaluation of acute diabetic neuropathies[9]. Although TIND is more commonly seen in individuals with T1DM, it may also occur in T2DM managed with hypoglycaemic medications or even diet control[9].
Although the exact mechanisms are not fully understood, experiments in animal models suggested various concepts. One is the hypothesis of neural “energy crisis” wherein, chronic hyperglycaemia might lead to a reduction in nerve blood flow and endoneural oedema, creating a long-standing hypoxic endoneural microenvironment[10]. When an abrupt drop in glucose levels occurs in such as situation, it might lead to a relative endoneural hypoglycaemia, leading to an “energy crisis”, resulting in acute neuropathy.
Another factor is the presence of macrophage infiltration in peripheral nerves leading to chronic inflammation and the release of macrophage-derived cytokines and neuroinflammatory regulators which result in the activation of sensory neurons resulting in neuropathic pain[10]. Neural regeneration after improved glycemic control also can be an additional source of neuropathic pain[23]. However, this concept requires further studies for a comprehensive understanding of how the interplay between nerve degeneration and regeneration contributes to TIND.
CN is a chronic destructive disease in patients with peripheral neuropathy characterized by painful or painless bone and joint destruction in limbs that have lost sensory innervation[16]. The estimated prevalence of CN is 0.1% to 0.4% in individuals with diabetes, but the prevalence can be as high as 35% in patients with diabetic peripheral neuropathy[16]. Both types of diabetes can increase the risk, though the prevalence may be higher in those with T1DM and one or both feet may be involved.
Bone modelling factors such as RANKL (receptor activator of nuclear factor-κB ligand) and its antagonist osteoprotegerin (OPG) play an important role in the development of CN[16]. Acute CN may occur with rapid correction of hyperglycaemia as in patients who underwent a concurrent kidney–pancreas transplantation. This is possibly from an interplay of OPG and RANKL levels resulting from intensive glycemic control in such situations[16]. Inhibition of OPG by reduction of HbA1c might induce an increase in the RANKL level leading to rapid maturation of the osteoblasts, and hence bone lysis with the development of acute CN.
Like the observed development of DR and TIND with the rapid reduction of HbA1c, there is evidence, however, limited, in the literature that indicates a rapid correction of hyperglycaemia may contribute to the early worsening of DN. For instance, in an article by Cundy et al, the authors describe a rapid and sustainable decline of eGFR (estimated glomerular filtration rate) in four subjects with type 2 diabetes after their HbA1c had fallen from 118 mmol/mol (12.9%) to 48 mmol/mol (5.5%)[15]. The renal microcirculation is usually protected by hemodynamic autoregulation when compared to the retina and the vasa nervorum of peripheral nerves which would explain the rarity of this phenomenon.
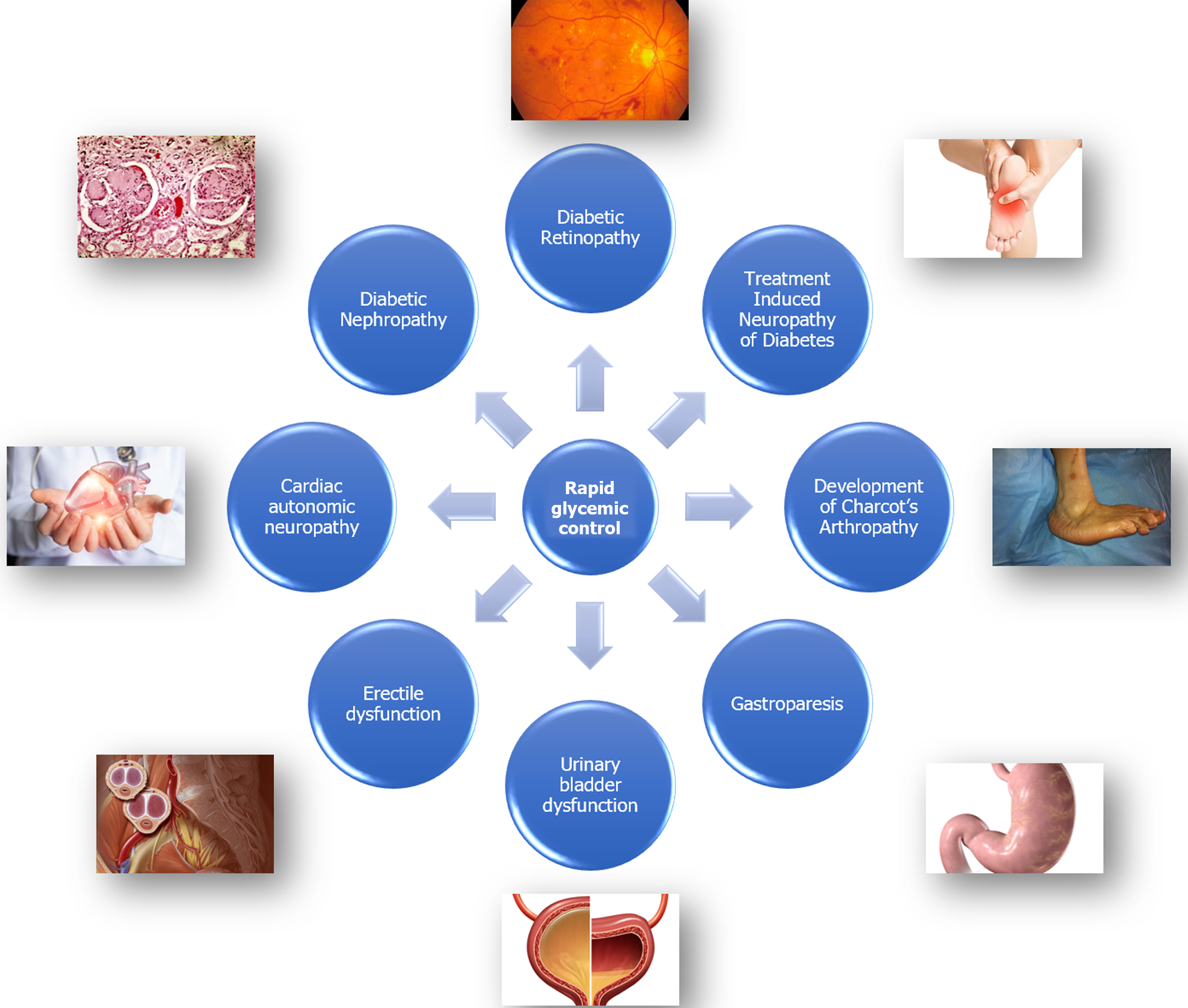
There is a strong correlation between the proportionate risk of development of TIND and the magnitude of HbA1c reduction as observed by a large series by Gibbon and Freeman[24]. For instance, a rapid HbA1c reduction of 2%-3% points in 3 months period was shown to be associated with an absolute risk of TIND in 20% of diabetic subjects while a reduction of > 4% was associated with an absolute risk of > 80% in this study. Complications of diabetic autonomic neuropathy such as gastroparesis, urinary bladder dysfunction, erectile dysfunction, and cardiac autonomic neuropathy are all consequences of TIND[24]. The Figure 1 shows the microvascular complications that can be associated with rapid improvement of chronic hyperglycaemia in patients with poorly controlled diabetes.
Although there are several cohort studies, case series and reports in the published literature, the simultaneous occurrence of a multitude of microvascular diabetic complications following rapid correction of severe chronic hyperglycaemia is rarely reported. Huret et al[25] in the recent issue of the World Journal Diabetes, reported a unique case of multifocal microvascular disease complicating the management of a 25-year-old woman with poorly controlled T1DM after intense reduction of chronic hyperglycaemia during pregnancy. The patient developed crippling CN (culminating in left knee replacement and foot amputation) followed by the development of severe sight-threatening DR, gastroparesis, urinary bladder dysfunction, and rapidly progressive DN terminating in dialysis dependence within three years of the index disease onset.
Although the development of microvascular complications because of rapid reduction of chronic hyperglycaemia occurs usually within a short period of time, the multifocal microvascular disease in the above case except CN occurred only after several months of marked improvement of her T1DM, which is slightly difficult to explain. We are unclear whether pregnancy as such impacted the delay in the development of some of these complications, in this case, owing to the marked immune alterations at the childbearing period in females. It is noteworthy that some of the microvascular complications are believed to develop through immune/cytokine-mediated mechanisms, and the immunological and cytokine milieu during pregnancy are very different from those in normal adults.
Pregnancy as such is a risk factor for the development and/or progression of microvascular complications in patients with preexisting diabetes possibly because of marked glycaemic fluctuations during this period[26], for which reason, women are regularly monitored during each trimester of their pregnancy for screening these complications. Although rigorous control of hyperglycaemia was very important during pregnancy to improve maternal and foetal outcomes, this patient, unfortunately, developed catastrophic complications of intense diabetes control, an unanticipated and possibly unavoidable iatrogenic complication.
A few potential medicolegal issues may arise in relation to an acute worsening of microvascular complications of diabetes from tight glycaemic control. For instance, there is an issue with informed decision making, and establishing a standard of care. Physicians and healthcare providers should ensure that patients are adequately informed about the potential risks associated with tight glycaemic control and the possibility of early worsening of microvascular complications. Informed consent should include discussions about the benefits and risks of treatment options, potential complications, and alternatives. Failing to obtain informed consent may lead to legal challenges if complications arise.
Moreover, healthcare providers are expected to adhere to the standard of care when managing diabetes and its complications. If a provider fails to follow established guidelines and recommendations, it may be considered medical malpractice. However, it is critical to note that there are no current large-scale studies that examined the cut-off points for HbA1c that is optimal for evading these complications simultaneously, while aiming to curb the long-term effects of poor glycaemic control.
Situations, where marked reduction of hyperglycaemia and HbA1c are anticipated, as in cases of bariatric surgery, pregnancy (as in the case described by Huret et al[25]), pancreas transplantation, and even in those with massive weight loss from lifestyle changes, incretin-based therapies, or bariatric surgery, patients may be counselled well in advance regarding likelihood of development of these microvascular complications. Patients also must be monitored rigorously and regularly in such situations.
Optimal management strategy of TIND is still not clear because of the lack of adequate data from global scientific literature. Some experts suggest relaxing the glycaemic targets transiently to slow down the intensity of rigorous control of hyperglycaemia, while others do not support this concept and advise supportive management[27-29]. There is some evidence favouring the concept of relaxing the tight glycaemic control (permissive hyperglycaemia) for short periods in patients developing acute DR/worsening DR complicating the abrupt diabetes improvement[27,30]. However, maintaining good glycaemic control over long-term periods with only symptomatic supportive management of TIND has resulted in improvement of the disease in a good proportion of patients, while marked fluctuations and poor glucose control have been associated with worsening of all the microvascular complications including DR, TIND, and nephropathy[30]. Therefore, it is better to target an HbA1c reduction of < 2% points within the first three months of glycaemic management, if possible, in patients with poor baseline glycaemic control.
As there is inadequate data on the appropriate treatment of acute onset/worsening of existing microvascular complications in patients with poor baseline diabetes control, a patient-cantered management strategy must be adopted depending on the individual clinical situation. Wherever an abrupt reduction of HbA1c of more than 2% points is expected (e.g., bariatric surgery, marked weight loss in obesity with T2DM, and in eating disorders), patients should be counselled and regularly monitored. Management options as mentioned above may be considered, though improvement may not occur in some patients as reported by Huret et al[25].
Acute worsening of microvascular complications is a challenging aspect of managing uncontrolled diabetes. Uncontrolled chronic hyperglycaemia is a well-established risk factor for both microvascular and macrovascular complications, leading to significant morbidity and mortality. However, the paradoxical exacerbation of microvascular complications, such as DR, PDN, CN, and DN, during rapid glycaemic control can be an important clinical concern with probable medicolegal implications. The pathobiology, actuarial prevalence, predisposing factors, and the management strategies for acute onset/ worsening of microvascular complications in patients achieving rapid glycaemic control of their poorly controlled diabetes are yet to be fully elucidated. More research with emerging new evidence on this enigmatic disease may help us to optimally manage patients like the one reported by Huret et al in their unique clinical case report.
We thank Dr. Marina George Kudiyirickal for providing the voice clip for the audio Core Tip.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiang FX, Australia; Mao RF, China; Tavan H, Iran S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | American Diabetes Association Professional Practice Committee. Addendum. 11. Chronic Kidney Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45(Suppl. 1): S175-S184. Diabetes Care. 2022;45:2182-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association Professional Practice Committee. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S185-S194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | American Diabetes Association Professional Practice Committee. Addendum. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45(Suppl. 1):S144-S174. Diabetes Care. 2022;45:2178-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Yen FS, Wei JC, Shih YH, Hsu CC, Hwu CM. Impact of individual microvascular disease on the risks of macrovascular complications in type 2 diabetes: a nationwide population-based cohort study. Cardiovasc Diabetol. 2023;22:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Hemmingsen C, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;CD008143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | International Diabetes Federation. Annual Report 2022. [cited 24 October 2023] Available from: https://idf.org/media/uploads/2023/07/IDF_Annual_Report_2022_Final.pdf. |
| 7. | Bain SC, Klufas MA, Ho A, Matthews DR. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes Metab. 2019;21:454-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (1)] |
| 8. | Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2014:CD009122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Gibbons CH. Treatment induced neuropathy of diabetes. Auton Neurosci. 2020;226:102668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Baum P, Koj S, Klöting N, Blüher M, Classen J, Paeschke S, Gericke M, Toyka KV, Nowicki M, Kosacka J. Treatment-Induced Neuropathy in Diabetes (TIND)-Developing a Disease Model in Type 1 Diabetic Rats. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Dardari D, Van GH, M'Bemba J, Laborne FX, Bourron O, Davaine JM, Phan F, Foufelle F, Jaisser F, Penfornis A, Hartemann A. Rapid glycemic regulation in poorly controlled patients living with diabetes, a new associated factor in the pathophysiology of Charcot's acute neuroarthropathy. PLoS One. 2020;15:e0233168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Dardari D, Schuldiner S, Julien CA, Ha Van G, M'Bemba J, Bourgeon M, Sultan A, Lepeut M, Grandperret-Vauthier S, Baudoux F, François M, Clavel S, Martini J, Vouillarmet J, Michon P, Moret M, Monnier A, Chingan-Martino V, Rigalleau V, Dumont I, Kessler L, Stifii I, Bouillet B, Bonnin P, Lemoine A, Da Costa Correia E, Faraill MMB, Muller M, Cazaubiel M, Zemmache MZ, Hartemann A. Trends in the relation between hyperglycemia correction and active Charcot neuroarthropathy: results from the EPICHAR study. BMJ Open Diabetes Res Care. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 13. | Dardari D, Dardari R. Why the Risk of Developing Neuroarthropathy Is Higher After Simultaneous Kidney and Pancreatic Transplantation Compared to Kidney Transplantation Only: The Role of Euglycemia. Ann Transplant. 2021;26:e928449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Xiang GD, Sun HL, Zhao LS, Hou J, Yue L, Xu L. [Changes of osteoprotegerin before and after insulin therapy in type 1 diabetic patients]. Zhonghua Yi Xue Za Zhi. 2007;87:1234-1237. [PubMed] |
| 15. | Cundy T, Holden A, Stallworthy E. Early Worsening of Diabetic Nephropathy in Type 2 Diabetes After Rapid Improvement in Chronic Severe Hyperglycemia. Diabetes Care. 2021;44:e55-e56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Poonoosamy J, Lopes P, Huret P, Dardari R, Penfornis A, Thomas C, Dardari D. Impact of Intensive Glycemic Treatment on Diabetes Complications-A Systematic Review. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 352] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Perais J, Agarwal R, Evans JR, Loveman E, Colquitt JL, Owens D, Hogg RE, Lawrenson JG, Takwoingi Y, Lois N. Prognostic factors for the development and progression of proliferative diabetic retinopathy in people with diabetic retinopathy. Cochrane Database Syst Rev. 2023;2:CD013775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 19. | Brinchmann-Hansen O, Dahl-Jørgensen K, Hanssen KF, Sandvik L. Effects of intensified insulin treatment on various lesions of diabetic retinopathy. Am J Ophthalmol. 1985;100:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Kroc Collaborative Study Group. Blood glucose control and the evolution of diabetic retinopathy and albuminuria. A preliminary multicenter trial. N Engl J Med. 1984;311:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 347] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Jingi AM, Tankeu AT, Ateba NA, Noubiap JJ. Mechanism of worsening diabetic retinopathy with rapid lowering of blood glucose: the synergistic hypothesis. BMC Endocr Disord. 2017;17:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Meng D, Mei A, Liu J, Kang X, Shi X, Qian R, Chen S. NADPH oxidase 4 mediates insulin-stimulated HIF-1α and VEGF expression, and angiogenesis in vitro. PLoS One. 2012;7:e48393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 24. | Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain. 2015;138:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (2)] |
| 25. | Huret P, Lopes P, Dardari R, Penfornis A, Thomas C, Dardari D. Rapid correction of hyperglycemia: A necessity but at what price? A brief report of a patient living with type 1 diabetes. World J Diabetes. 2023;14:1710-1716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 26. | Relph S, Patel T, Delaney L, Sobhy S, Thangaratinam S. Adverse pregnancy outcomes in women with diabetes-related microvascular disease and risks of disease progression in pregnancy: A systematic review and meta-analysis. PLoS Med. 2021;18:e1003856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Stainforth-Dubois M, McDonald EG. Treatment-induced neuropathy of diabetes related to abrupt glycemic control. CMAJ. 2021;193:E1085-E1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Siddique N, Durcan R, Smyth S, Tun TK, Sreenan S, McDermott JH. Acute diabetic neuropathy following improved glycaemic control: a case series and review. Endocrinol Diabetes Metab Case Rep. 2020;2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Chandler E, Brown M, Wintergerst K, Doll E. Treatment-Induced Neuropathy of Diabetes (TIND) in Pediatrics: A Case Report and Review of the Literature. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Gibbons CH. Treatment induced neuropathy of diabetes-Long term implications in type 1 diabetes. J Diabetes Complications. 2017;31:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |