Published online Feb 15, 2024. doi: 10.4239/wjd.v15.i2.240
Peer-review started: October 13, 2023
First decision: November 23, 2023
Revised: December 20, 2023
Accepted: January 22, 2024
Article in press: January 22, 2024
Published online: February 15, 2024
Processing time: 111 Days and 0.1 Hours
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide, the global burden of which is rising. It is still unclear the extent to which prediabetes contributes to the risk of CVD in various age brackets among adults. To develop a focused screening plan and treatment for Chinese adults with prediabetes, it is crucial to identify variations in the connection between prediabetes and the risk of CVD based on age.
To examine the clinical features of prediabetes and identify risk factors for CVD in different age groups in China.
The cross-sectional study involved a total of 46239 participants from June 2007 through May 2008. A thorough evaluation was conducted. Individuals with prediabetes were categorized into two groups based on age. Chinese atherosclerotic CVD risk prediction model was employed to evaluate the risk of deve
In total, 6948 people were diagnosed with prediabetes in this study. In pre-diabetes, prevalences of CVD were 5 (0.29%) in the younger group and 148 (2.85%) in the older group. Overall, 11.11% of the younger group and 29.59% of the older group were intermediate/high-risk of CVD for prediabetes without CVD based on the Prediction for ASCVD Risk in China equation in ten years. In the younger age group, the 10-year risk of CVD was found to be more closely linked to family history of CVD rather than lifestyle, whereas in the older age group, resident status was more closely linked.
The susceptibility to CVD is age-specific in newly diagnosed prediabetes. It is necessary to develop targeted approaches for the prevention and management of CVD in adults across various age brackets.
Core Tip: Cardiovascular disease (CVD) is a leading cause of illness and death on a global scale, with its worldwide impact steadily increasing. However, it is still unclear the extent to which prediabetes contributes to the risk of CVD in various age brackets among adults. In this study, we analyzed our prediabetes data from 17 centers between June 2007 and May 2008. We found the influential features of different age brackets for the 10-year risk of CVD based on Prediction for ASCVD Risk in China. Given our findings, specific prevention strategies are needed for different age groups.
- Citation: Xie S, Yu LP, Chen F, Wang Y, Deng RF, Zhang XL, Zhang B. Age-specific differences in the association between prediabetes and cardiovascular diseases in China: A national cross-sectional study. World J Diabetes 2024; 15(2): 240-250
- URL: https://www.wjgnet.com/1948-9358/full/v15/i2/240.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i2.240
Cardiovascular disease (CVD) is a prominent contributor to illness and death on a global scale, with its worldwide impact steadily increasing[1]. Addressing CVD is a paramount concern for public health worldwide. The prediabetic population constitutes a substantial pool of individuals who are at risk of developing diabetes, a contributing factor for CVD. Prediabetes refers to a rise in blood sugar levels that is higher than the normal range but lower than the levels seen in clinical diabetes. Impaired fasting glucose (IFG) level or impaired glucose tolerance (IGT) is the designated term for this condition. According to previous studies, the approximate occurrence of prediabetes in China was 35.7% [95% confidence interval (95%CI): 34.2%-37.3%] in 2013 and 38.1% (95%CI: 36.4%-39.7%) in 2018[2]. Moreover, prediabetes has been linked to a higher likelihood of combined cardiovascular incidents, coronary artery disease, cerebrovascular accidents, and overall mortality[3]. During an examination of the data collected from the National Health and Nutrition Examination Surveys 2011-2014, it was found that individuals with prediabetes, as determined by ADA-fasting plasma glucose (FPG) or hemoglobin A1c (HbA1c), exhibited a significant occurrence of hypertension (36.6%), dyslipidemia (51.2%), albuminuria (7.7%), and reduced estimated glomerular filtration rate (4.6%). In total, 24.3% of the individuals were presently smoking, exhibiting a heightened projected 10-year cardiovascular event risk of around 7%[4].
It is still uncertain the extent to which prediabetes contributes to the risk of CVD in various age brackets among adults. To develop a focused glycemic screening plan and treatment for Chinese adults with prediabetes, it is crucial to identify variations in the connection between prediabetes and the risk of CVD based on age. Given that the occurrence of CVD events gradually develops, a predictive model can be utilized to estimate the likelihood for individuals without CVD.
Currently, numerous CVD risk evaluation instruments exist worldwide, with the renowned Framingham Risk Score (FRS) being the creation of Framingham Heart Research Institute. Nevertheless, these models rely on the European and American sample populations, which predominantly consist of White and Black individuals, and have a comparatively limited representation of Asians[5]. The Prediction for ASCVD Risk in China (China-PAR) CVD risk assessment model was developed in 2016 to predict the risk of atherosclerotic CVD in China. This model was specifically designed for the Chinese population and allowed for the quantitative assessment of CVD incidence risk over 10 years. The China-PAR model’s development offered a significant and efficient evaluation tool for predicting CVD risk and promoting primary prevention in China[6]. The objective of this study was to forecast the likelihood of CVD in China’s prediabetic population by utilizing the FRS and China-PAR models. Additionally, it aimed to analyze the disparities in CVD risk prediction between these two models and identify distinct risk factors among younger and older age groups, ultimately establishing a targeted prevention strategy.
The study’s development set was obtained from a China National Diabetes and Metabolic Disorders Survey, which was a comprehensive cross-sectional study. From June 2007 to May 2008, a large epidemiological study was conducted across the nation. It involved 17 clinical centers located in 14 provinces and municipalities throughout the country. In the general population, individuals who were 20 years of age or older were chosen using a multistage stratified cluster sampling technique. The study design, eligibility criteria, and sampling have been previously published in great detail[7,8].
Individuals who had resided in their present locality for more than five years were qualified to take part in the research. A total of 54240 people were chosen and asked to take part in the research, yet only 46239 grown-ups finished the questionnaire.
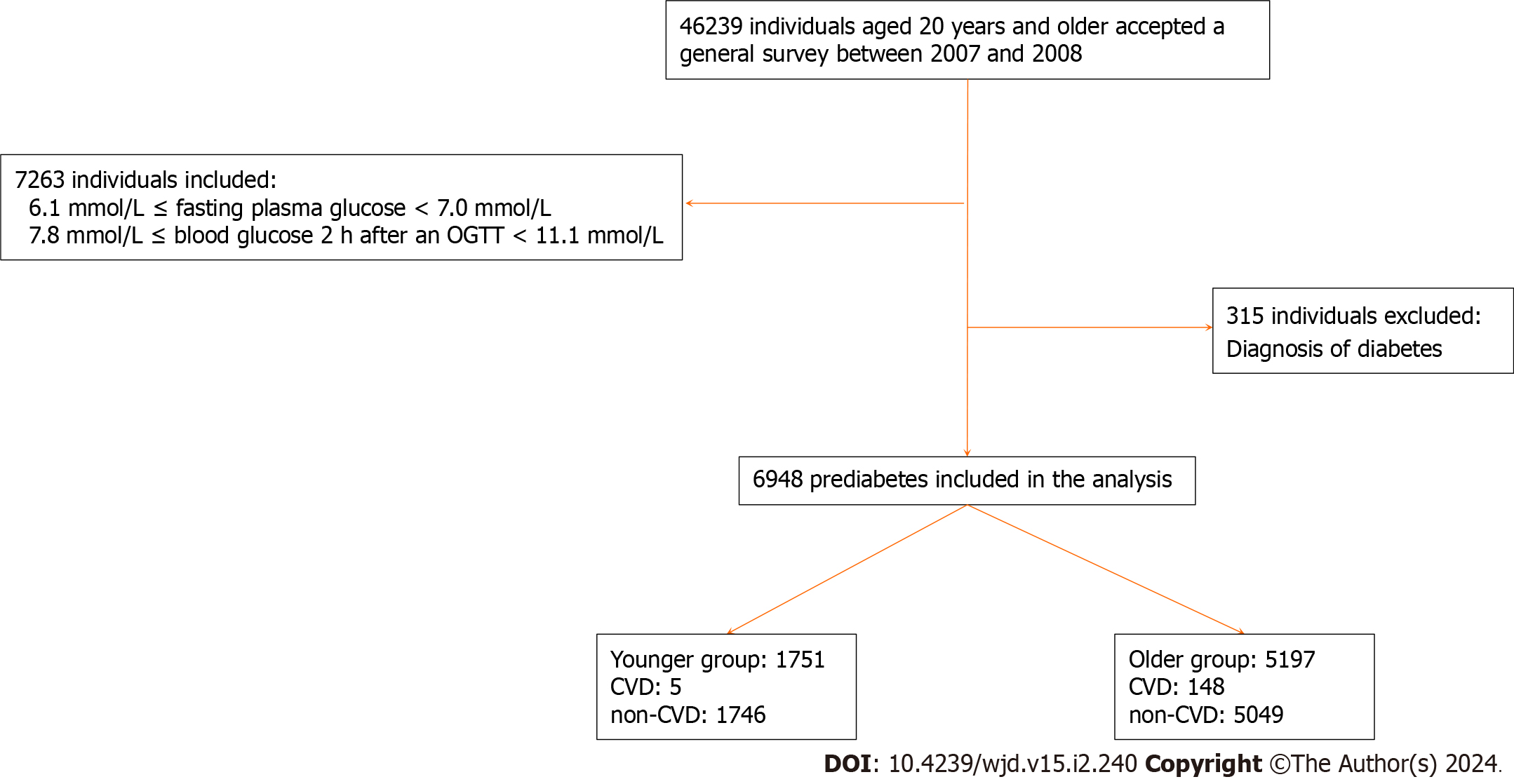
We included participants who were diagnosed with prediabetes using the oral glucose test (n = 7263) and excluded those who had been previously diagnosed with diabetes (n = 315). Consequently, our final analysis encompassed a total of 6948 adults, whom we subsequently categorized into two groups based on age range (as depicted in Figure 1).
Trained personnel administered a typical survey to gather data on demographic traits, individual and familial medical backgrounds, and factors that pose risks to one’s lifestyle[8].
Before the oral glucose tolerance test, participants were given instructions to continue with their regular physical activity and diet for a minimum of 3 d. Following a minimum of 10 hours of fasting overnight, a blood sample was obtained from a vein using a vacuum tube that contained sodium fluoride. This sample was collected to measure the glucose levels in the plasma. Individuals without any documented record of diabetes were administered a typical 75 g glucose solution, while individuals who self-reported having diabetes were provided with a steamed bun comprising roughly 80 g of intricate carbohydrates for precautionary purposes. Glucose concentrations were measured by drawing blood samples at 0, 30 min, and 120 min following the glucose or carbohydrate load[8].
Plasma glucose levels were assessed utilizing an enzymatic method involving hexokinase. Serum cholesterol and triglyceride levels were enzymatically assessed using commercially available reagents at the clinical biochemical laboratories in each province. Before starting this study, all research laboratories have successfully finished a program for standardization and certification.
Prediabetes was diagnosed using the diagnostic criteria from the World Health Organization in 1999[9]. The plasma glucose testing results were classified into three categories: isolated IFG (fasting glucose level of ≥ 6.1 mmol/L and < 7.0 mmol/L, and PG2h level of < 7.8 mmol/L); isolated IGT (fasting glucose level of < 7.0 mmol/L, and PG2h level of ≥ 7.8 mmol/L and < 11.1 mmol/L); and undiagnosed diabetes (fasting glucose level of ≥ 7.0 mmol/L, PG2h level of ≥ 11.1 mmol/L, or both). Diabetes that had been diagnosed before was determined when the participant answered positively to the inquiry, “Has a medical professional ever informed you that you have diabetes?” The overall count of diabetes encompassed both previously diagnosed cases and those that had not been identified[8]. Prediabetes was characterized by either IFG or IGT.
To assess the CVD risk, the China-PAR and FRS were employed. The China-PAR model is a tool for evaluating developed by the China-PAR Risk Assessment Research. A single expert researcher inputted the personal details and test outcomes of the volunteers via the online platform (http://www.cvdrisk.com.cn), which encompassed gender, age, present address (urban or rural), location (north or south), waist size, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), existing blood pressure level, usage of antihypertensive medication, presence of diabetes, smoking habits, and family history of CVD. On the China-PAR, the 10-year absolute risk percentage for CVD was categorized as low (< 5%), intermediate (5%-10%), and high risk (> 10%). The FRS scores were computed by considering six risk factors, which encompassed age, gender, TC, HDL-c, systolic blood pressure (SBP), and smoking patterns. To calculate FRS, the thresholds were set as TC < 160, 160-199, 200-239, 240-279, and ≥ 280 mg/dL; for SBP, the ranges were < 120, 120-129, 130-139, 140-159, and ≥ 160 mmHg; and for HDL-c, the values were < 40, 40-49, 50-59, and ≥ 60 mg/dL. The percentage of ten-year risk was determined by adding up the points (1 point, 6%; 2 points, 8%; 3 points, 10%; 4 points, 12%; 5 points, 16%; 6 points, 20%; 7 points, 25%; 10 points or more, greater than 30%). The percentage of absolute CVD risk over 10 years was categorized as low (less than 10%), moderate (10%-20%), and high (greater than 20%) according to classification[10].
The objective of our research was to obtain precise evaluations of the risk elements associated with CVD among various age categories in the Chinese population, specifically individuals who are 20 years old or above and have prediabetes. To ensure accuracy in a complex survey design, the estimated sample sizes were determined to align with the commonly advised criteria[11]. The calculations were adjusted to reflect the entire Chinese adult population (20 years or older) using the 2006 Chinese population data and the study’s sampling method. Corrections were made for various aspects of the survey, such as oversampling of women and urban dwellers, nonresponse, highly developed economic regions, and demographic or geographic disparities between the sample and the overall population[8].
The occurrence rates of CVD were computed for the subcategories based on age factors. To investigate the correlation between the 10-year risk of CVD and demographic, lifestyle, and metabolic factors, we employ random forest (RF) analysis. SHapley Additive exPlanation (SHAP) values to provide consistent and locally accurate attribution values for each feature. This is a unified approach to explain the outcome of RF. SHAP values evaluate the importance of the output resulting from the inclusion of feature A for all combinations of features other than A. All P values were not adjusted for multiple testing and were considered two-tailed. The R software, version 4.3.2, was utilized for all statistical analyses. Two-tailed P values < 0.05 were considered significant.
This study involved a total of 6948 individuals who were diagnosed with prediabetes. Among this total, 1751 individuals (25.2%) were between the ages of 20 and 40, while 5197 individuals (74.8%) were above the age of 40, as shown in Table 1. In comparison to the younger participants, the older group exhibited a higher proportion of males. The older individuals with prediabetes were more likely to engage in smoking, alcohol consumption, and exercise. Additionally, they exhibited higher measurements of waist circumference (WC), PG2h, TC, HDL-c, low-density lipoprotein-cholesterol, SBP, and diastolic blood pressure (DBP). Moreover, it was observed that 5 individuals (0.29%) in the younger group and 148 individuals (2.85%) in the older group were found to have CVD.
| Younger group | Older group | P value | |
| Total | 1751 (25.20) | 5197 (74.80) | |
| Men | 744 (42.49) | 3035 (58.40) | 0.015 |
| Smoking | 402 (22.96) | 2204 (42.41) | 0.001 |
| Alcohol drinking | 412 (23.53) | 2328 (44.80) | 0.009 |
| Regular physical activity | 424 (24.21) | 2385 (45.89) | < 0.001 |
| Family history of CVD | 277 (15.82) | 1133 (21.80) | 0.173 |
| Antihypertensive drugs | 81 (4.63) | 346 (6.66) | 0.155 |
| Dyslipidemia | 84 (4.80) | 249 (4.79) | < 0.001 |
| Lipid-lowering drugs | 13 (0.74) | 87 (1.67) | 0.007 |
| BMI (kg/m2, 95%CI) | 25.0 (24.6-25.3) | 24.9 (24.7-25.1) | 0.688 |
| WC (cm, 95%CI) | 83.2 (82.0-84.4) | 84.8 (84.2-85.3) | 0.021 |
| FPG (mmol/L, 95%CI) | 5.6 (5.5-5.6) | 5.5 (5.5-5.6) | 0.586 |
| PG2h (mmol/L, 95%CI) | 8.2 (8.1-8.3) | 8.6 (8.5-8.6) | < 0.001 |
| TC (mmol/L, 95%CI) | 4.8 (4.7-4.9) | 5.0 (5.0-5.1) | < 0.001 |
| TG (mmol/L, 95%CI) | 1.9 (1.7-2.0) | 1.9 (1.8-1.9) | 0.985 |
| HDL-c (mmol/L, 95%CI) | 1.3 (1.2-1.3) | 1.3 (1.3-1.3) | 0.029 |
| LDL-c (mmol/L, 95%CI) | 2.7 (2.6-2.8) | 2.9 (2.9-3.0) | < 0.001 |
| SBP (mmHg, 95%CI) | 120.0 (118.9-121.2) | 131.9 (130.7-133.1) | < 0.001 |
| DBP (mmHg, 95%CI) | 79.1 (78.2-79.9) | 81.2 (80.5-82.0) | < 0.001 |
| Abnormal ECG (%) | 366 (20.90) | 2358 (45.37) | < 0.001 |
| Prediabetes category | |||
| IFG (%) | 357 (20.39) | 1447 (27.84) | < 0.001 |
| IGT (%) | 1394 (79.61) | 3750 (72.16) | < 0.001 |
| CVD (%) | 5 (0.29) | 148 (2.85) | 0.003 |
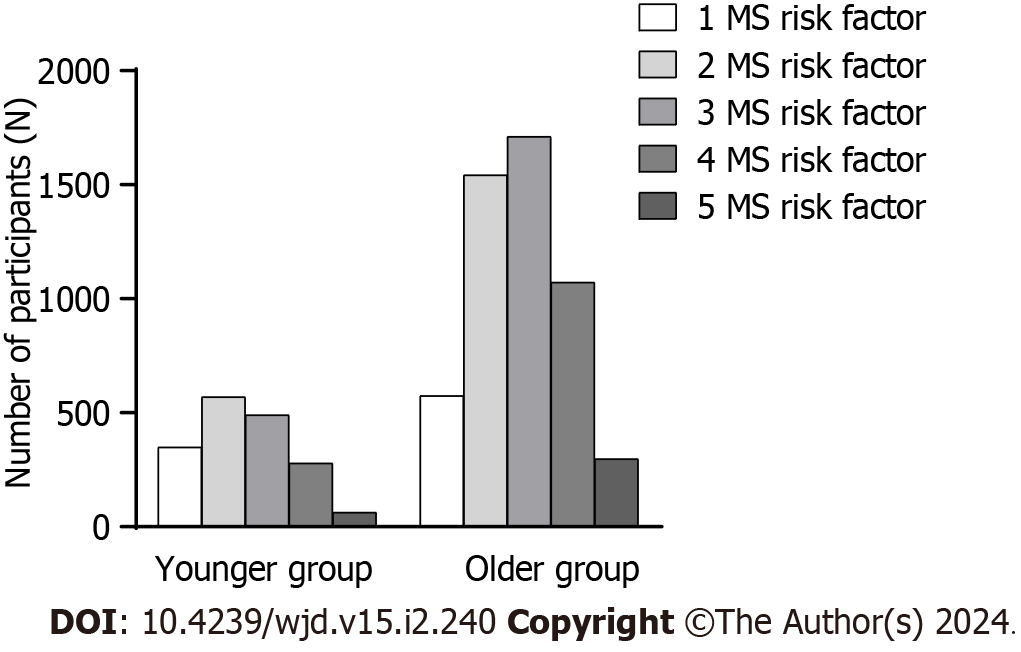
Figure 2 and Table 2 exhibit the occurrence of metabolic syndrome components according to age intervals. According to the data presented in Table 2, prediabetes in older age exhibited a higher tendency towards central obesity and elevated blood pressure.
| Younger group | Older group | P value | |
| Central obesity | 636 (36.32) | 4575 (88.03) | < 0.001 |
| High glucose | 1751 (100.00) | 5197 (100.00) | - |
| High blood pressure | 532 (30.38) | 2959 (56.94) | < 0.001 |
| High TG | 708 (40.43) | 2280 (43.87) | 0.334 |
| Low HDL-c | 367 (20.96) | 1161 (22.32) | 0.500 |
For prediabetes without CVD (n = 6795), the age stratification was used to compare the 10-year absolute risk grading of CVD. The findings indicated that there were statistically significant variations in the assessment outcomes of the low-, intermediate-, and high-risk categories in different age brackets on the FRS and China-PAR models (P < 0.001). However, the deductions made from the disease risk grading remained consistent. In other words, the higher the age, the higher the 10-year risk level of CVD (Table 3). Among the participants, a total of 5320 individuals (which accounts for 78.29% of the total) were simultaneously classified as low-, medium-, or high-risk based on both scores. The kappa test revealed a low level of agreement between the two methods (weighted κ coefficient of agreement = 0.395-0.400; P < 0.001) (Table 4).
| Younger group (n = 1746) | Older group (n = 5049) | Total (n = 6795) | P value | |
| China-PAR | ||||
| Low (< 5%) | 1552 (88.89) | 3540 (70.11) | 5092 | < 0.001 |
| Intermediate (5%-10%) | 127 (7.27) | 575 (11.39) | 702 | < 0.001 |
| High (> 10%) | 67 (3.84) | 934 (18.50) | 1001 | < 0.001 |
| FRS | ||||
| Low (< 10%) | 1717 (98.34) | 3928 (77.80) | 5645 | < 0.001 |
| Intermediate (10%-20%) | 27 (1.55) | 904 (17.90) | 931 | < 0.001 |
| High (> 20%) | 2 (0.11) | 217 (4.30) | 219 | < 0.001 |
| FRS | China-PAR | Total | ||
| Low risk | Intermediate risk | High risk | ||
| Low risk | 4923 | 492 | 230 | 5645 |
| Intermediate risk | 147 | 205 | 579 | 931 |
| High risk | 22 | 5 | 192 | 219 |
| Total | 5092 | 702 | 1001 | 6795 |
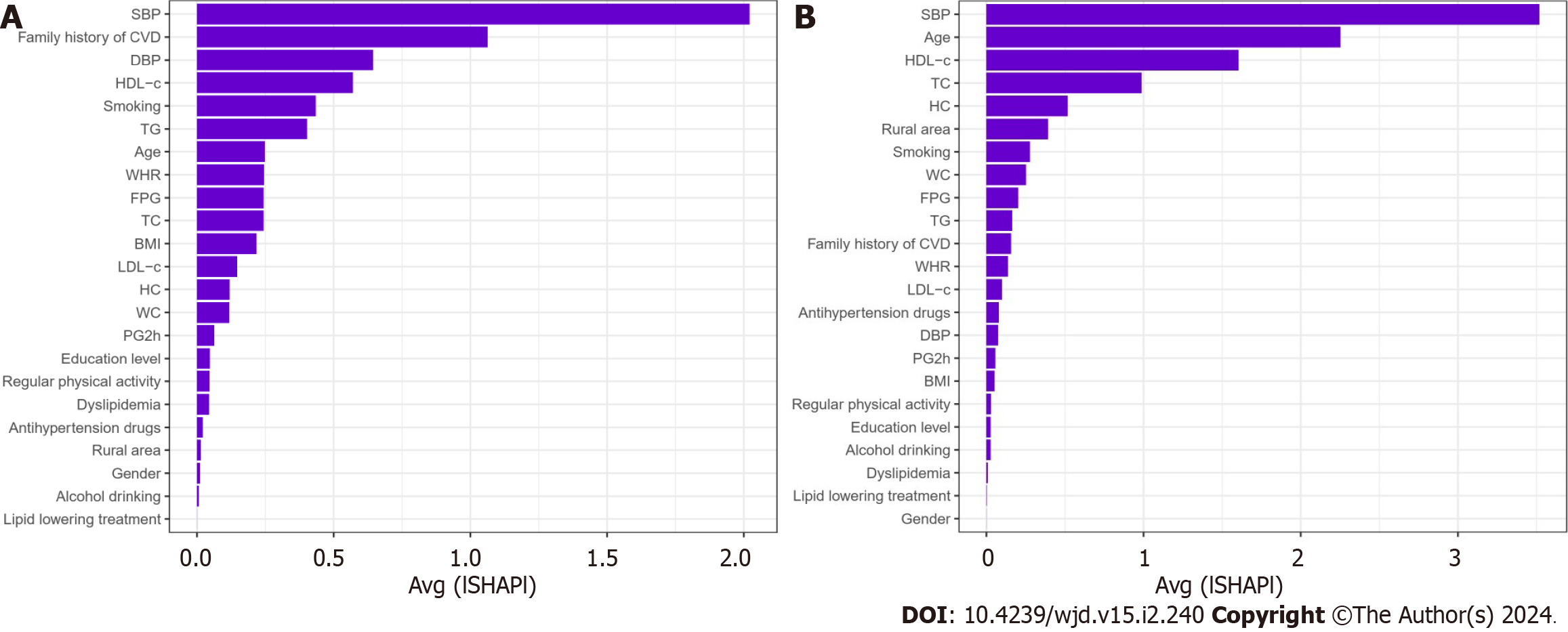
Therefore, we use China-PAR to predict the 10-year risk of CVD for the Chinese. For prediabetes, intermediate/high risk of CVD (n = 194 in the younger group and n = 1509 in the older group) is more noteworthy. We utilized the RF with all the variables as input variables. The importance matrix plot for the RF method is shown in Figure 3 and revealed that the top 10 most important variables contributing to the younger group model were SBP, age, HDL-c, TC, HC, rural area, smoking, WC FPG, and TG. For the older group, the top 10 most important variables were SBP, family history of CVD, DBP, HDL-c, smoking, TG, age, WHR, FPG, and TC.
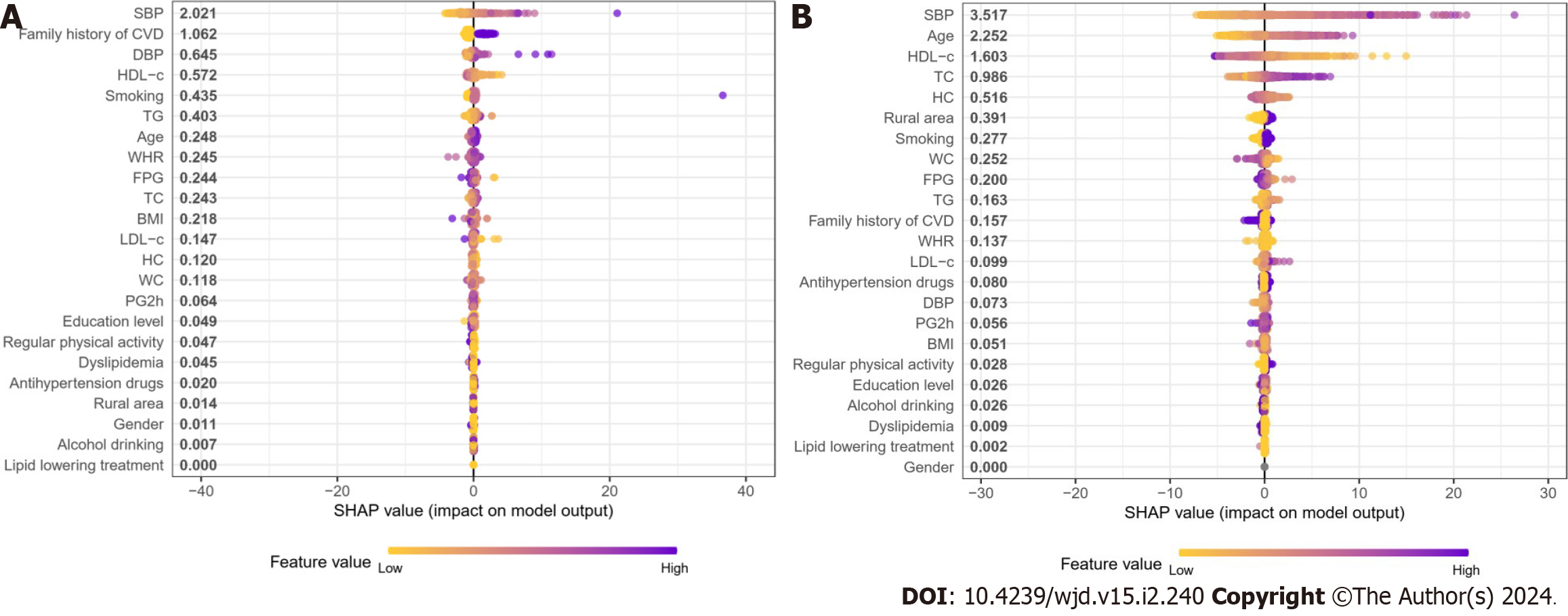
To identify the features that had the most influence, we depicted the SHAP summary plot of RF (Figure 4) for both age groups. This plot provided a visually concise figure by presenting the range and distribution of importance. It showed how high and low features’ values were with SHAP values. Each dot represented the SHAP value of the feature from the individual. It was plotted horizontally and was stacked vertically to show the density of the same SHAP value. Then, each dot was colored by the value of the feature, from low (yellow) to high (purple). The higher the SHAP value of a feature, the more likely occurrence of CVD in 10 years.
In the general population, fatal CVD is commonly associated with male sex, hypertension, dyslipidemia, diabetes, and smoking. Nevertheless, information is scarce concerning the presence of age-related disparities in the influence of these risk factors[12]. In China, we conducted a cross-sectional survey to examine how age and risk factors for 10-year risk of CVD interact and to determine variations in CVD risk factors among different age groups. Previous studies[13,14] support the results indicating that the younger group with hyperglycemia had a higher prevalence of CVD compared to the older group. Based on previous studies conducted locally and globally, age is a significant determinant that escalates the susceptibility to CVD[15]. Age was determined to have a significant impact on the risk of CVD after eliminating other variables that could distort the results.
Individuals with prediabetes have accompanying metabolic risk factors[16]. Metabolic syndrome is characterized by a group of metabolic risk factors, such as insulin resistance, central obesity, hyperglycemia, dyslipidemia, and high blood pressure[17]. Given that metabolic syndrome encompasses a comprehensive set of metabolic risk factors for cardiovascular events, it becomes imperative to anticipate the likelihood of CVD in these individuals. With the onset of the 21st century, CVD emerged as the primary reason for untimely death and illness globally, affecting 80% of individuals in underprivileged developing nations, following societal and economic progress. Extensive studies have been conducted since the mid-1900s to investigate the causes and risk elements, leading to the identification of various factors like tobacco use, high blood pressure, diabetes, and abnormal lipid levels as contributors to CVD[18].
Nonetheless, the correlation between prediabetes and CVD occurrences might compromise the precision of our results due to the limited number of CVD patients in our research. Hence, by utilizing the risk score, which includes the initial indications of CVD, as substitute measures, we can enhance the precision in identifying the connection between prediabetes and risk elements for CVD.
This study estimated the risk of CVD in prediabetes in the next 10 years, as shown by the China-PAR model. China-PAR incorporates the disease spectrum and prevalence of risk factors in China, including novel factors like WC and place of residence, by thoroughly considering the risk factors associated with the previous model. At the same time, the examination of the correlation between age and different risk factors was also conducted. A CVD risk prediction model suitable for the Chinese population was created, and the cut-off point of different risk stratification was proposed and verified; hence, its prediction results were more accurate. The study additionally discovered that both the FRS and China-PAR models demonstrated a positive correlation between age and the 10-year incidence risk of CVD in the prediabetes population. This suggests that the two methods consistently predict the risk level across various age groups.
In the general populace, diabetes raises the likelihood of both microvascular and macrovascular complications as well as premature death leading to a substantial financial burden on society. While there have been limited reports on the link between prediabetes diagnosed at a later stage and the risks of CVD and mortality, numerous studies have examined the connection between prediabetes diagnosed early and the risks of CVD or mortality. The identification of prediabetes at an early stage is considered a separate contributor to the risk of CVD and is linked to a mortality rate of 15%. According to the findings of the Emerging Risk Factors Collaboration, an elevated mortality risk was observed among 820900 participants from 97 prospective studies when fasting glucose levels exceeded 5.5 mmol/L, rather than falling within the range of 3.9-5.5 mmol/L[19]. We discovered that the occurrence of prediabetes in younger patients who were recently diagnosed was strongly linked to the incidence of CVD. This implies that the prevention and treatment of CVD in the future should prioritize prediabetes, especially among the younger prediabetes population.
Regardless of age group, the timely and precise prediction of CVD risk and the subsequent adoption of preventive measures significantly improve patients’ well-being and quality of life.
Implementing tactics to prevent both primary and secondary occurrences of CVD and/or its associated risk factors will alleviate the financial impact caused by this ailment. CVD risk factors can be categorized into modifiable or non-modifiable factors. Age, genetics, family history, gender, and race are among the factors involved. The risk factors that can be changed are categorized as: (1) Cardiometabolic factors, including high blood pressure, abnormal blood lipid levels, diabetes, and being overweight (which collectively make up the metabolic syndrome); and (2) lifestyle factors, such as tobacco use, lack of physical activity, poor diet, and low socio-economic status. Furthermore, there is growing evidence indicating that apart from genetic predisposition, early family-based environmental factors such as early nutrition, socioeconomic status, housing, and neighborhood play a significant role in the occurrence of CVD. Young individuals who have a familial background of CVD already possess an unfinished/unusual CVD risk profile. The authors Kataria and colleagues[20] examined the variation in plasma lipid levels and systemic blood pressure among healthy young college students who have a positive family history of CVD.
Nevertheless, in the case of elderly individuals, a family history of CVD does not pose a substantial threat to the ailment. Lifestyle and the environment in which one lives are the primary contributors to the most notable hazards. In the elderly population[21], health disparities persist among various regions and residential areas, playing a crucial role in determining overall health. Globally, it has been confirmed that there are differences in CVD mortality and levels of risk factors between urban and rural areas[22]. According to findings from a future urban-rural investigation, cardiovascular event rates were greater in rural regions compared to urban communities in middle- and low-income nations, despite urban settings having higher risk factors than rural areas[23]. Moreover, findings from a previous study conducted in Finland indicated that older individuals residing in rural regions had a higher occurrence of increased serum cholesterol levels and obesity compared to those residing in urban localities[24].
Notably, alcohol consumption has complex and sometimes paradoxical associations with CVD. In recent times, a considerable number of epidemiological studies[25] have been released concerning this subject. Experimental evidence strongly supports the advantageous impact of moderate alcohol intake, excluding instances of excessive drinking. Epidemiological data suggest that alcohol consumption protects some people against ischemic diseases to some degree. A J-shaped correlation was observed between the mean intake of alcohol and CVD, as reported in reference[26], which means for low to moderate alcohol consumption, a lower CVD risk is observed compared to abstaining and excessive drinking. Nevertheless, as most of the protective evidence of low to moderate alcohol consumption on CVD is from observational studies, it is uncertain whether this effect is a result of different forms of bias. According to a quantitative meta-analysis, individuals who consumed less than 30 g/d of alcohol and did not engage in heavy drinking episodes had the lowest risk of ischemic heart disease (relative risk = 0.64, 95%CI: 0.53, 0.71)[27]. Due to the lack of RCT, which is the gold standard, the focus in research has now shifted to new analytical methods, such as Mendelian randomization studies. However, none of these studies could truly resolve the pressing question of whether alcohol is the protective factor of CVD. Therefore, there is remaining controversy regarding the effects of moderate alcohol consumption on CVD.
In individuals with prediabetes, randomized clinical trials have demonstrated that interventions incorporating diet and physical activity can decrease the likelihood of developing diabetes. To alleviate the effects of newly diagnosed diabetes, it is imperative to enforce public health interventions. According to the latest ADA guidelines, it is recommended to annually screen individuals with prediabetes for diabetes and refer them to a lifestyle intervention aimed at promoting weight loss[28]. The authors Qiao et al[29] discovered that when analyzing combined data from Asian groups, 75% of individuals with prediabetes exhibited isolated IGT following glucose loading. The presence of insulin resistance increases the likelihood of developing CVD in both the general population and individuals with diabetes. Additionally, it serves as an indicator of the cardiovascular outlook for patients with CVD[30]. The findings of this research validated a correlation between age and other contributing elements, which could be significant in elucidating the variations in CVD risk factors among younger and older individuals. To prevent and manage CVD, community health centers can offer health advice to individuals across various age brackets.
This study has several strengths, including the incorporation of a vast, nationwide study sample; a thorough evaluation of their blood sugar levels, encompassing FPG and PG2h; and meticulous recognition of CVD by China-PAR.
This study has some limitations. To define prediabetes, ADA now suggests utilizing HbA1c within the range of 5.7%-6.4% (39-47 mmol/mol), according to their latest recommendation[31]. Nevertheless, in our research, we detected prediabetes by assessing FPG and PG2h. The HbA1c level was not measured, resulting in a decrease in the number of prediabetes diagnoses. Furthermore, we meticulously accounted for variables that could influence the results in the analyses, although there is a possibility of biases arising from unmeasured confounding and reverse causality. Furthermore, the present study’s cross-sectional design poses challenges in determining the causal relationship between variables. Further confirmation through prospective research is needed to establish the causal relationship between the research factors and conclusions, as the relationship is currently exploratory.
In summary, our findings suggest that prediabetes detected through FPG and PG2h might have a stronger association with CVD in younger individuals compared to older individuals. The findings of our study validated that the risk factors associated with CVD vary across age groups during the diagnosis of prediabetes. Therefore, age should be specifically considered in the care of adults with prediabetes for CVD prevention.
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide, the global burden of which is rising. It is still unclear the extent to which prediabetes contributes to the risk of CVD in various age brackets among adults.
To develop a focused screening plan and treatment for Chinese adults with prediabetes, it is crucial to identify variations in the connection between prediabetes and the risk of CVD based on age.
To examine the clinical features of prediabetes and identify risk factors for CVD in different age groups in China.
We analyzed age-specific differences in prediabetes to identify features for the 10-year risk of CVD in a large, representative population divided by age (younger < 40 and older > 40 years old). Chinese atherosclerotic CVD risk prediction model was employed to evaluate the risk of developing CVD over 10 years. Random forest was established in both age groups. SHapley Additive exPlanation method prioritized the importance of features from the perspective of assessment contribution.
In total, 6948 people were diagnosed with prediabetes in this study. In prediabetes, prevalences of CVD were 5 (0.29%) in the younger group and 148 (2.85%) in the older group. Overall, 11.11% of the younger group and 29.59% of the older group were intermediate/high-risk of CVD for prediabetes without CVD based on the China-PAR equation in ten years. In the younger age group, the 10-year risk of CVD was found to be more closely linked to family history of CVD rather than lifestyle, whereas in the older age group, resident status was more closely linked.
The susceptibility to CVD is age-specific in newly diagnosed prediabetes. It is necessary to develop targeted approaches for the prevention and management of CVD in adults across various age brackets.
Identification of prediabetes may help develop strategies to prevent and control CVD in China.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea; Zhou M, Japan S-Editor: Chen YL L-Editor: A P-Editor: Zhao S
| 1. | Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, Guerrero M, Kunadian V, Lam CSP, Maas AHEM, Mihailidou AS, Olszanecka A, Poole JE, Saldarriaga C, Saw J, Zühlke L, Mehran R. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. 2021;397:2385-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 792] [Article Influence: 198.0] [Reference Citation Analysis (0)] |
| 2. | Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, Zhang X, Li C, Huang Z, Sun X, Wang L, Zhou M, Wu J, Wang Y. Prevalence and Treatment of Diabetes in China, 2013-2018. JAMA. 2021;326:2498-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 561] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 3. | Echouffo-Tcheugui JB, Selvin E. Prediabetes and What It Means: The Epidemiological Evidence. Annu Rev Public Health. 2021;42:59-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 196] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 4. | Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol. 2018;6:392-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 5. | Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Yang X, Li J, Hu D, Chen J, Li Y, Huang J, Liu X, Liu F, Cao J, Shen C, Yu L, Lu F, Wu X, Zhao L, Gu D. Predicting the 10-Year Risks of Atherosclerotic Cardiovascular Disease in Chinese Population: The China-PAR Project (Prediction for ASCVD Risk in China). Circulation. 2016;134:1430-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 7. | Yang W, Xiao J, Yang Z, Ji L, Jia W, Weng J, Lu J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J; China National Diabetes and Metabolic Disorders Study Investigators. Serum lipids and lipoproteins in Chinese men and women. Circulation. 2012;125:2212-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J; China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 2309] [Article Influence: 153.9] [Reference Citation Analysis (2)] |
| 9. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 10. | Truett J, Cornfield J, Kannel W. A multivariate analysis of the risk of coronary heart disease in Framingham. J Chronic Dis. 1967;20:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 497] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Li L, Wu M, Yu Z, Niu T. Nutritional Status Indices and Monoclonal Gammopathy of Undetermined Significance Risk in the Elderly Population: Findings from the National Health and Nutrition Examination Survey. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Bergami M, Scarpone M, Bugiardini R, Cenko E, Manfrini O. Sex beyond cardiovascular risk factors and clinical biomarkers of cardiovascular disease. Rev Cardiovasc Med. 2022;23:19. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Huo X, Gao L, Guo L, Xu W, Wang W, Zhi X, Li L, Ren Y, Qi X, Sun Z, Li W, Ji Q, Ran X, Su B, Hao C, Lu J, Guo X, Zhuo H, Zhang D, Pan C, Weng J, Hu D, Yang X, Ji L. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Kim SM, Lee G, Choi S, Kim K, Jeong SM, Son JS, Yun JM, Kim SG, Hwang SS, Park SY, Kim YY, Park SM. Association of early-onset diabetes, prediabetes and early glycaemic recovery with the risk of all-cause and cardiovascular mortality. Diabetologia. 2020;63:2305-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 640] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 16. | Chan JC, Lau ES, Luk AO, Cheung KK, Kong AP, Yu LW, Choi KC, Chow FC, Ozaki R, Brown N, Yang X, Bennett PH, Ma RC, So WY. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med. 2014;127:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Lemieux I, Després JP. Metabolic Syndrome: Past, Present and Future. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 18. | Teo KK, Rafiq T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can J Cardiol. 2021;37:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 19. | Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2142] [Cited by in RCA: 2022] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 20. | Kataria N, Panda A, Singh S, Patrikar S, Sampath S. Risk factors for cardiovascular disease in a healthy young population: Family matters. Med J Armed Forces India. 2022;78:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 21. | Anderson TJ, Saman DM, Lipsky MS, Lutfiyya MN. A cross-sectional study on health differences between rural and non-rural U.S. counties using the County Health Rankings. BMC Health Serv Res. 2015;15:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Vaughan AS, Quick H, Pathak EB, Kramer MR, Casper M. Disparities in Temporal and Geographic Patterns of Declining Heart Disease Mortality by Race and Sex in the United States, 1973-2010. J Am Heart Assoc. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G; PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 641] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 24. | Fogelholm M, Valve R, Absetz P, Heinonen H, Uutela A, Patja K, Karisto A, Konttinen R, Mäkelä T, Nissinen A, Jallinoja P, Nummela O, Talja M. Rural-urban differences in health and health behaviour: a baseline description of a community health-promotion programme for the elderly. Scand J Public Health. 2006;34:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Zhao J, Stockwell T, Roemer A, Naimi T, Chikritzhs T. Alcohol Consumption and Mortality From Coronary Heart Disease: An Updated Meta-Analysis of Cohort Studies. J Stud Alcohol Drugs. 2017;78:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 26. | Roerecke M. Alcohol's Impact on the Cardiovascular System. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 27. | Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 28. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 1883] [Article Influence: 470.8] [Reference Citation Analysis (0)] |
| 29. | Qiao Q, Nakagami T, Tuomilehto J, Borch-Johnsen K, Balkau B, Iwamoto Y, Tajima N; International Diabetes Epidemiology Group; DECODA Study Group. Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia. 2000;43:1470-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 490] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 31. | Kester LM, Hey H, Hannon TS. Using hemoglobin A1c for prediabetes and diabetes diagnosis in adolescents: can adult recommendations be upheld for pediatric use? J Adolesc Health. 2012;50:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |