Published online Feb 15, 2024. doi: 10.4239/wjd.v15.i2.220
Peer-review started: November 11, 2023
First decision: November 30, 2023
Revised: December 13, 2023
Accepted: January 17, 2024
Article in press: January 17, 2024
Published online: February 15, 2024
Processing time: 84 Days and 22.8 Hours
The effects of viral hepatitis (VH) on type 2 diabetes (T2D) remain controversial.
To analyze the causal correlation between different types of VH and T2D using Mendelian randomization (MR).
Single nucleotide polymorphisms of VH, chronic hepatitis B (CHB), chronic hepatitis C (CHC) and T2D were obtained from the BioBank Japan Project, European Bioinformatics Institute, and FinnGen. Inverse variance weighted, MR-Egger, and weighted median were used to test exposure-outcome associations. The MR-Egger intercept analysis and Cochran’s Q test were used to assess horizontal pleiotropy and heterogeneity, respectively. Leave-one-out sensitivity analysis was used to evaluate the robustness of the MR analysis results.
The MR analysis showed no significant causal relationship between VH and T2D in Europeans [odds ratio (OR) = 1.028; 95% confidence interval (CI): 0.995-1.062, P = 0.101]. There was a negative causal association between CHB and T2D among East Asians (OR = 0.949; 95%CI: 0.931-0.968, P < 0.001), while there was no significant causal association between CHC and T2D among East Asians (OR = 1.018; 95%CI: 0.959-1.081, P = 0.551). Intercept analysis and Cochran’s Q test showed no horizontal pleiotropy or heterogeneity (P > 0.05). Sensitivity analysis showed that the results were robust.
Among East Asians, CHB is associated with a reduced T2D risk, but this association is limited by HBV load and cirrhosis. Although VH among Europeans and CHC among East Asians are not associated with the risk of T2D, focusing on blood glucose in patients with CHC is still relevant for the early detection of T2D induced by CHC-mediated pathways of hepatic steatosis, liver fibrosis, and cirrhosis.
Core Tip: The effects of hepatitis B and C on type 2 diabetes (T2D) remain controversial. The study aims to analyze the causal relationship of T2D with chronic hepatitis B (CHB) and chronic hepatitis C (CHC) by Mendelian randomization (MR). This MR analysis showed that in East Asians, CHC was not associated with T2D risk, whereas CHB was associated with a reduced risk of T2D. Although this MR analysis did not find a causal relationship between CHC and T2D, focusing on blood glucose in patients with CHC is still relevant, which helps early detect T2D induced by CHC-mediated other hepatic lesions.
- Citation: Yu YF, Hu G, Tong KK, Yang XY, Wu JY, Yu R. Effect of viral hepatitis on type 2 diabetes: A Mendelian randomization study. World J Diabetes 2024; 15(2): 220-231
- URL: https://www.wjgnet.com/1948-9358/full/v15/i2/220.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i2.220
Type 2 diabetes (T2D) is a chronic metabolic disease characterized by relative insulin deficiency and abnormally elevated blood glucose[1]. An epidemiological study has shown that as the prevalence of diabetes increases each year, approximately 1 in 10 adults globally now have diabetes, and it is projected that by 2045, the world will have 693 million individuals with diabetes[2,3]. As an incurable disease, the hyperglycemic state in T2D increases the risk of macro
VH, an inflammatory disease of the liver caused by infection with the hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), or hepatitis E virus (HEV), is a major global health problem[12]. These viruses cause acute hepatitis, and HBV, HCV, and HDV infections progress to chronic hepatitis[13,14]. Globally, approximately 257 million individuals have been reported to be infected with HBV, and 71 million are infected with HCV[15]. Chronic hepatitis B (CHB) from HBV infection and chronic hepatitis C (CHC) from HCV infection cause persistent damage to the liver, resulting in liver fibrosis, cirrhosis, liver cancer, and even death[13,16]. Relevant studies have shown that hepatitis B cirrhosis and HCV infection increased the risk of T2D by 74% and 1058%, respectively[11,17], suggesting that CHB and CHC are potential risk factors for T2D. This effect may be related to the signaling pathway by which hepatitis viruses alter hepatic glucose homeostasis by mediating the overexpression of protein phosphatase 2A to inhibit Akt and FoxO1 dephosphorylation[18]. However, some studies have reported that HBV and HCV infections do not increase the incidence of T2D[19,20], which indirectly negates the relationships of them. Whether different categories of VH, especially CHB and CHC, are associated with the risk of T2D remains controversial, and the causal relationship between them needs to be further explored.
Mendelian randomization (MR) is a method for assessing the causal relationship between exposure and outcome variables using genetic variants[21]. Due to the randomized nature of allele classification, MR has properties similar to those of randomized controlled trials[22]. Although MR cannot be used as a substitute for randomized controlled trials, it provides additional evidence for causality analysis[23]. This MR analysis explored the causal relationship between T2D and VH, CHB, and CHC from a gene prediction perspective, with the aim of providing additional evidence for risk factor studies in diabetes.
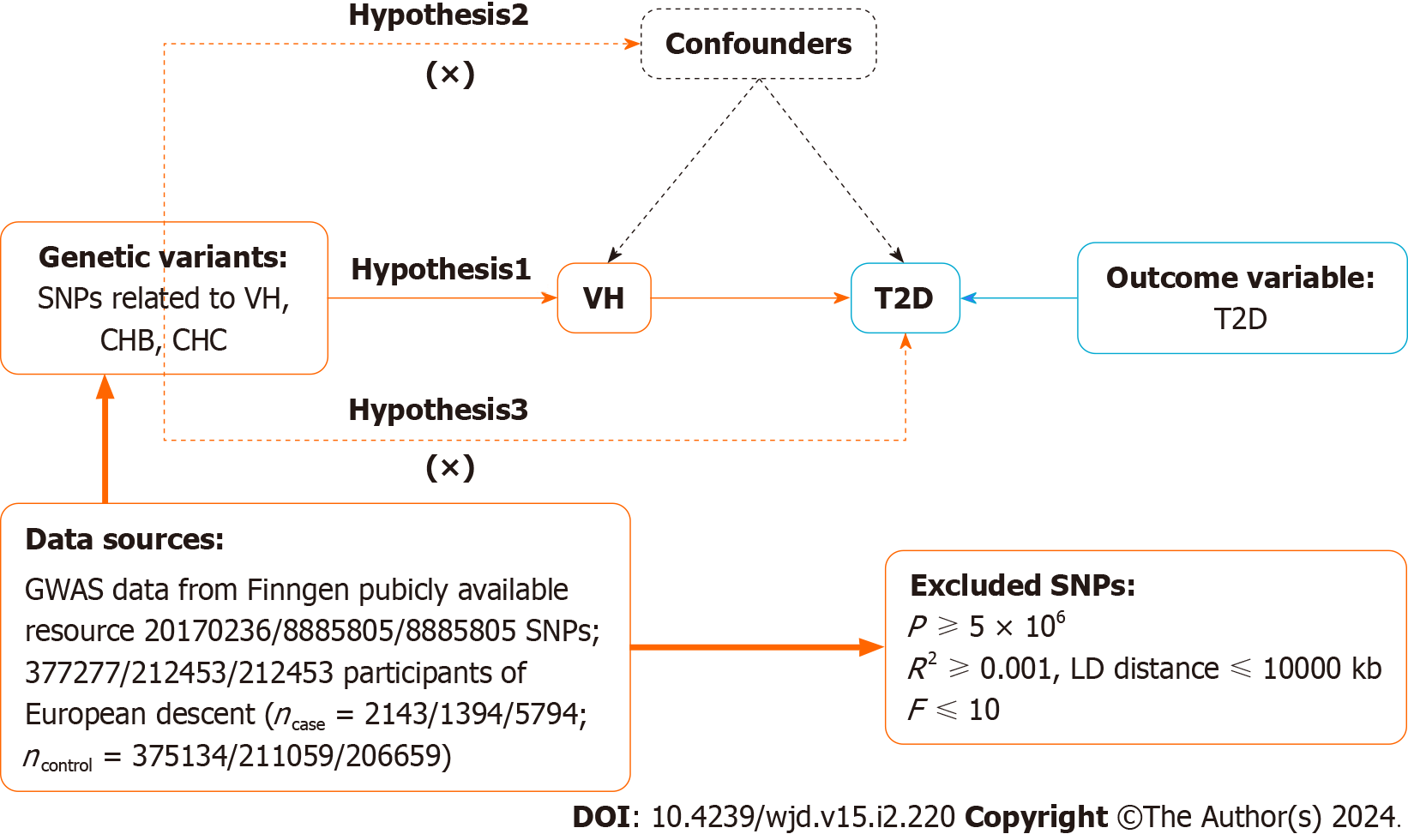
MR relies on three primary assumptions[24]: (1) Association assumption: Single nucleotide polymorphisms (SNPs) are strongly associated with exposure factors; (2) Independence assumption: SNPs are independent of confounding variables; and (3) Exclusivity assumption: SNPs do not act on outcome variables through pathways other than through exposure factors. The design is illustrated in Figure 1.
Data on VH, CHB, CHC, and T2D were obtained from the BioBank Japan Project (https://biobankjp.org/en/), European Bioinformatics Institute (https://www.ebi.ac.uk), and FinnGen (www.finngen.fi/fi). All the data were sourced from publicly available databases; therefore, no additional ethical approval was required.
First, SNPs strongly associated with exposure factors were screened in the genome-wide association studies (GWAS), according to a threshold of P < 5 × 10-6 to fulfill assumption 1. Second, independent SNPs were screened, according to R2 < 0.001 and kb = 10000, to avoid potential bias due to linkage disequilibrium. Third, the F-value of each SNP was calculated, and SNPs with F ≤ 10 were excluded. F-value was calculated publicly as F = [R2/(1 - R2)] × [(N - K - 1)/K], where R2 = 2 × (1 - MAF) × MAF × β2. R2: The cumulative explained variance of the selected instrument variables on exposure; MAF: The effect of minor allele frequency; β: The estimated effect of SNP; and N: Sample size of the GWAS. Finally, we referred to PhenoScanner (www.phenoscanner.medschl.cam.ac.uk) and related literature to remove SNPs potentially associated with T2D to fulfill assumption 2.
This study followed the STROBE-MR guidelines[25]. The “TwoSampleMR (0.5.7)” program package for R 4.3.1 was used to perform the two-sample MR analysis. Inverse variance weighting (IVW), MR-Egger, and weighted median were used as basic causality assessment methods. Among these methods, IVW was the primary analysis method[26] that achieved unbiased causal estimation without horizontal pleiotropy. MR-Egger and the weighted median are complementary methods to MR analysis, with the former providing valid causal estimation in some cases where pleiotropy exists, and the latter being less sensitive to outliers and measurement errors.
The MR results were corrected and analyzed using the MR-Pleiotropy Residual Sum and Outlier method (MR-PRESSO), and the MR analysis was re-executed after removing outlier SNPs (P < 1). Horizontal pleiotropy was assessed using MR-Egger’s intercept analysis, and P ≥ 0.05 suggested the absence of horizontal pleiotropy to fulfill assumption 3. Heterogeneity was assessed using Cochran’s Q test, and P ≥ 0.05 suggested the absence of heterogeneity. Leave-one-out sensitivity analysis was used to assess the robustness of the results and clarify individual SNP that significantly affected the pooled results.
The VH data were obtained from FinnGen, which included 377277 European participants (dataset
The T2D data for Europe were obtained from FinnGen, including 365950 European participants (dataset number: finngen_R9_T2D). Data on T2D for East Asia were obtained from the European Bioinformatics Institute, and it included 433540 East Asian individuals (dataset number: ebi-a- GCST010118) (Table 1).
| Year | Trait | Population | Sample size | Web source |
| 2023 | VH | European | 377277 | www.finngen.fi/en |
| 2023 | T2D | European | 365950 | www.finngen.fi/en |
| 2019 | CHB | East Asian | 212453 | https://biobankjp.org/en/ |
| 2019 | CHC | East Asian | 212453 | https://biobankjp.org/en/ |
| 2020 | T2D | East Asian | 433540 | https://www.ebi.ac.uk |
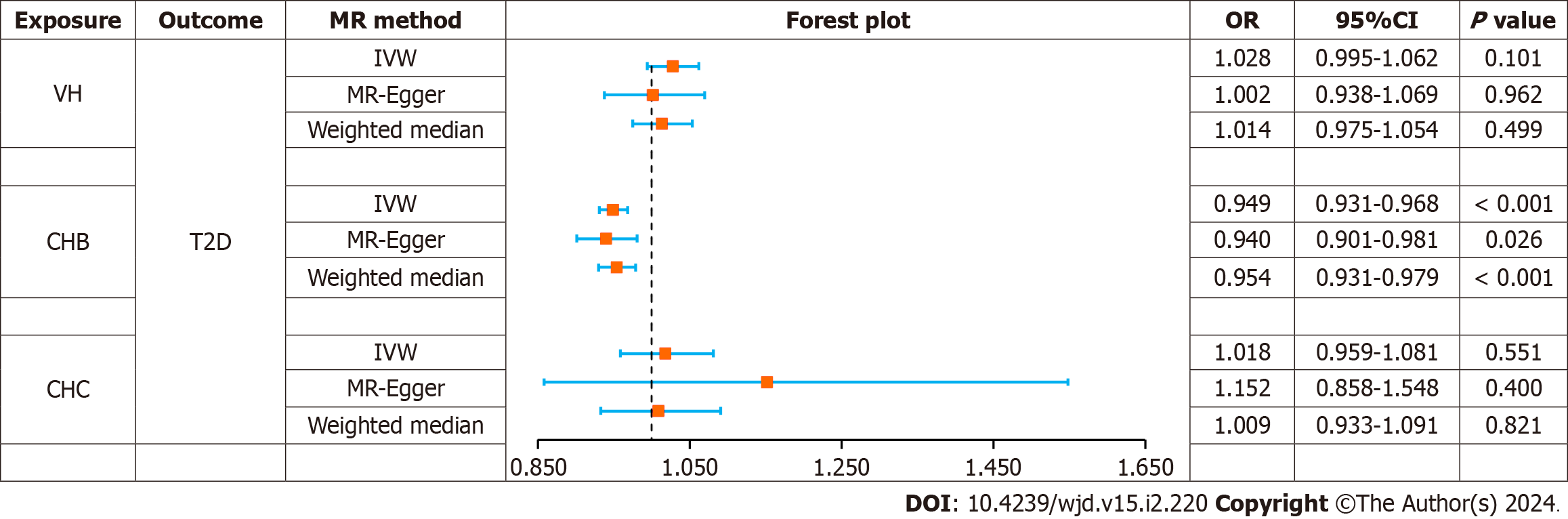
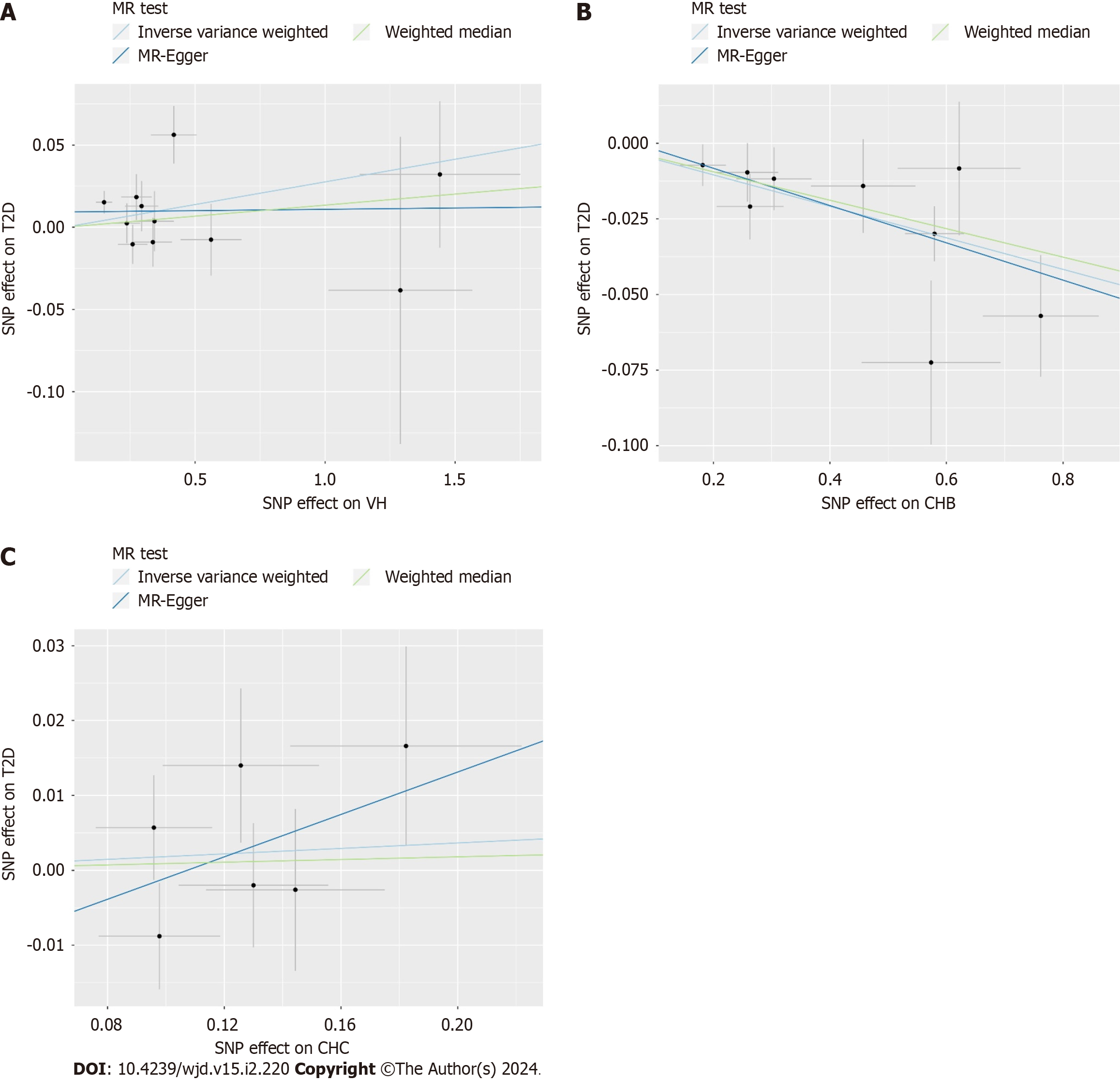
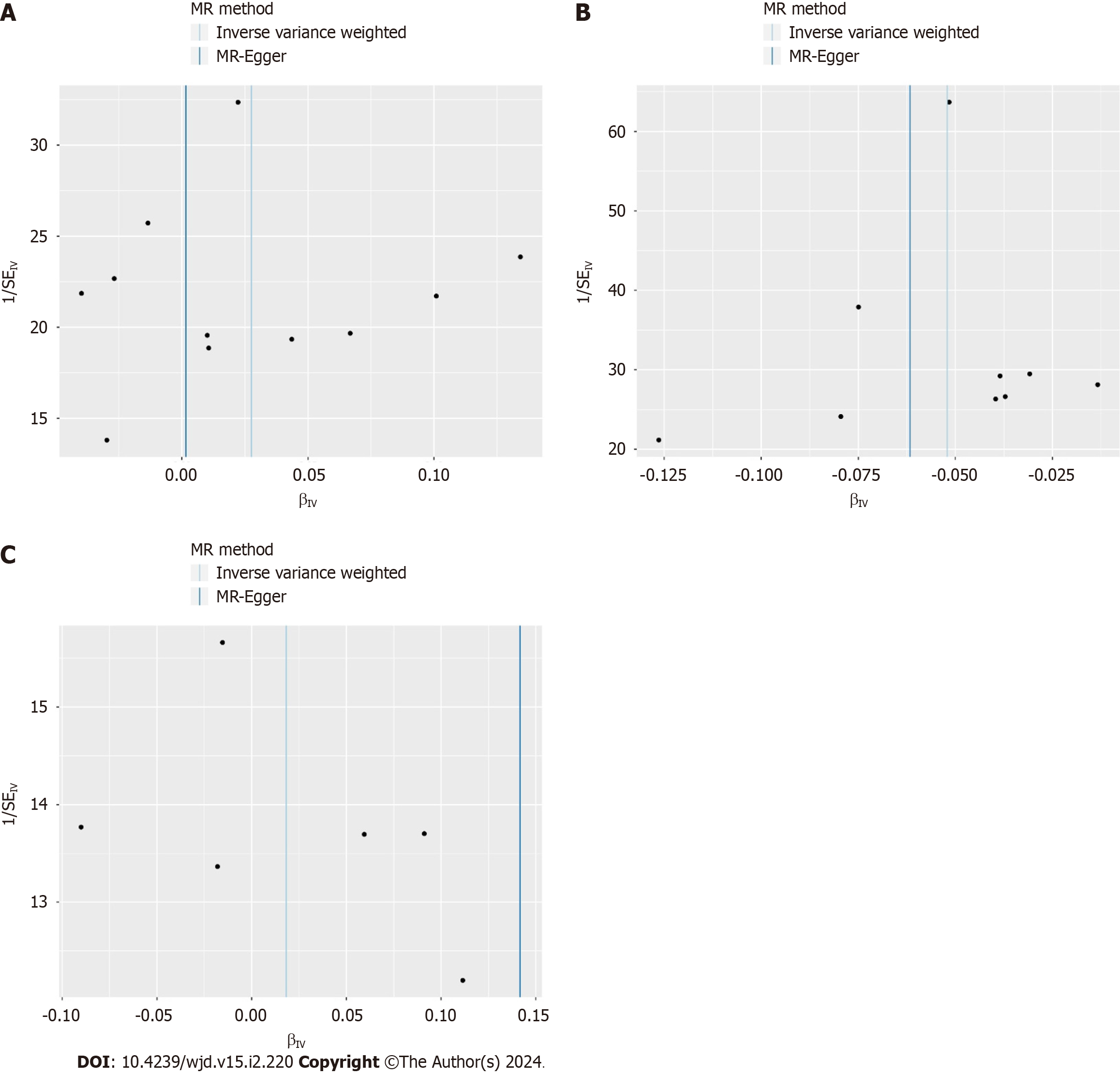
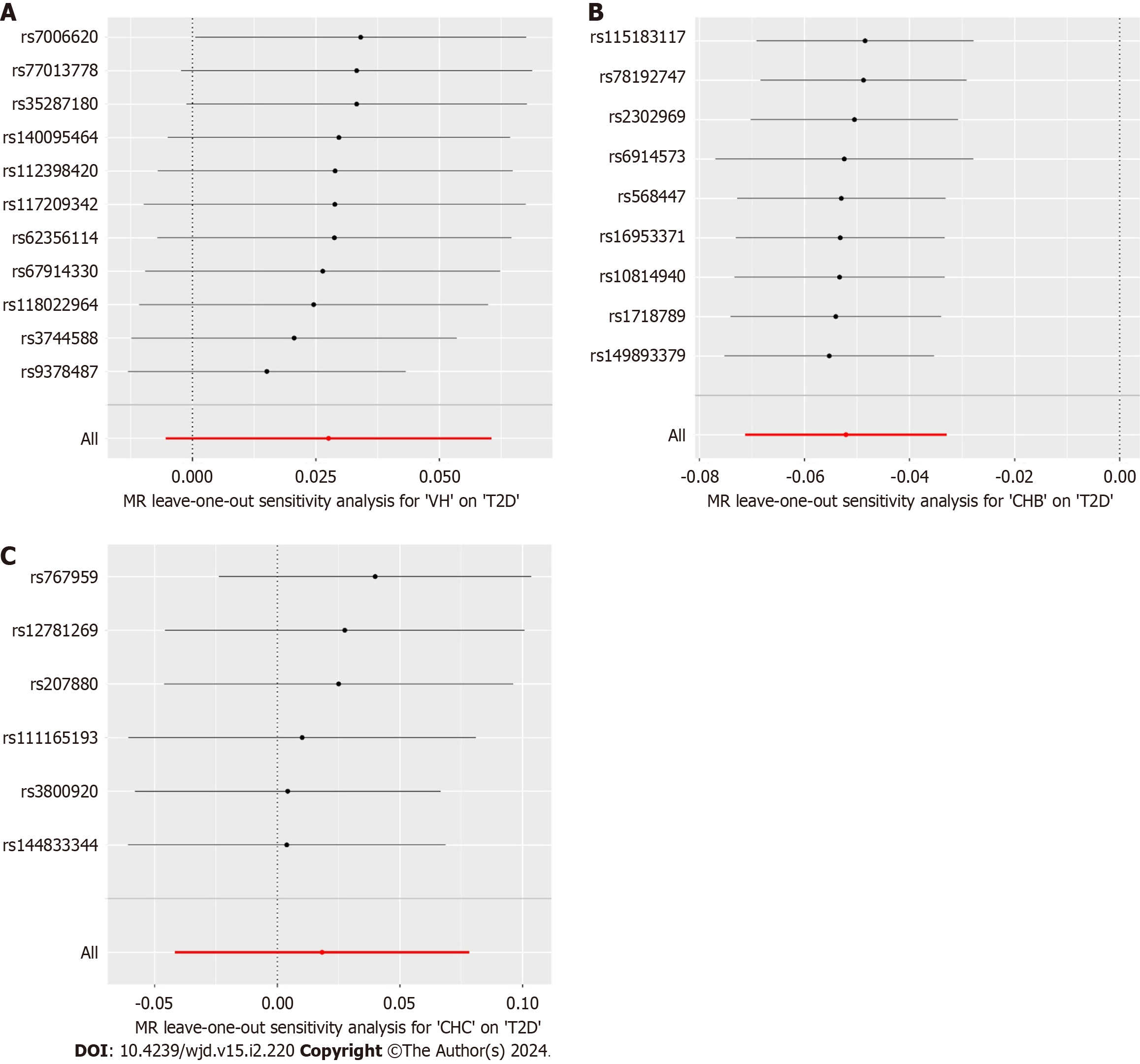
The causal effects between the exposure factors (VH, CHB, and CHC) and outcome variable (T2D) were analyzed using two-sample MR. A forest plot of the MR analysis is shown in Figure 2, and a scatter plot of the effect estimates for each SNP is shown in Figure 3. The results of the intercept analysis are shown in Supplementary Table 3. The results of the heterogeneity test are shown in Figure 4 and Supplementary Table 4. The sensitivity analysis is shown in Figure 5.
VH: None of the three methods of analysis showed significant causal relationships between VH and T2D in Europeans: IVW [odds ratio (OR) = 1.028; 95% confidence interval (CI): 0.995-1.062; P = 0.101], MR-Egger (OR = 1.002; 95%CI: 0.938-1.069; P = 0.962), or weighted median (OR = 1.014; 95%CI: 0.975-1.054; P = 0.499). Intercept analysis showed no horizontal pleiotropy (P = 0.391). Cochran’s Q test showed no heterogeneity (P = 0.119). Sensitivity analysis suggested that the results were robust.
CHB: All the three methods of analysis showed a negative causal association between CHB and T2D among East Asian individuals: IVW (OR = 0.949; 95%CI: 0.931-0.968; P < 0.001), MR-Egger (OR = 0.940; 95%CI: 0.901-0.981; P = 0.026), and weighted median (OR = 0.954; 95%CI: 0.931-0.979; P < 0.001). Intercept analysis showed no horizontal pleiotropy (P = 0.640). The Cochran’s Q test revealed no heterogeneity (P = 0.685). Sensitivity analysis suggested that the results were robust.
CHC: None of the three analysis methods showed a significant causal relationship between CHC and T2D in East Asians: IVW (OR = 1.018; 95%CI: 0.959-1.081, P = 0.551), MR-Egger (OR = 1.152; 95%CI: 0.858-1.548, P = 0.400), and weighted median (OR = 1.009; 95%CI: 0.933-1.091, P = 0.821). Intercept analysis showed no horizontal pleiotropy (P = 0.640). The Cochran’s Q test showed no heterogeneity (P = 0.376), and the sensitivity analysis suggested that the results were robust.
VH is an inflammatory liver disease caused by viruses, such as HAV, HBV, HCV, HDV, and HEV, and is one of the most common liver diseases[27]. Some evidence suggests that HBV and HCV infection are associated with impaired glucose tolerance and increased incidence of diabetes mellitus[28,29], which are potential risk factors for T2D. However, other studies have found that neither HBV nor HCV infections are associated with T2D risk[20,30]. The effects of HBV and HCV infections on T2D remain controversial, and their causal relationship remains unclear. To further understand the potential impact of different types of VH on T2D, MR was used to analyze the causal relationships of VH, CHB, and CHC with T2D.
This study found no significant causal association between VH and T2D risk in the European population. Among East Asians, CHB was associated with a lower risk of T2D, whereas CHC was not associated with a risk of T2D. The Cochrane’s Q-test and intercept analysis showed no heterogeneity or horizontal pleiotropy in these results, and the sensitivity analysis showed that the MR results were robust. As the GWAS did not include data on HAV, HDV, and HEV infections, this study did not assess the effect of these three types of VH on the risk of T2D. Additionally, the impact of CHB and CHC on T2D among Europeans is still being determined because GWAS do not contain available data on CHB and CHC in Europeans.
Our results showed that CHC was not associated with T2D risk, which is consistent with previous reports. A United States clinical trial, involving 15125 individuals found that neither the prevalence of prediabetes nor diabetes was associated with HCV infection, and the level of insulin resistance was not associated with HCV markers[20]. Additionally, an Iranian case-control study noted that CHC was not a risk factor for insulin resistance or diabetes in the Iranian population[31].
However, studies supporting the notion that CHC is not associated with T2D are few, and to date, most clinical studies have pointed to a correlation between CHC and T2D. Mehta et al[17] suggested that CHC is a potential risk factor for T2D, and they found a higher risk of T2D in patients with CHC than that in healthy individuals. An Italian single-arm trial noted that a significant effect of HCV on glucose load developed through increased insulin resistance in the liver and muscles[32]. The impact of HCV on blood glucose levels and risk of diabetes was more pronounced in patients aged 35-49 years and in those with severe liver disease[33]. The risk for T2D in patients with CHC has been reported to increase with increasing levels of liver fibrosis[34,35]. Additionally, there is evidence that patients with CHC infected with HCV1b and HCV3 have a higher incidence of T2D[36,37], implying that the HCV genotype is an essential factor influencing the risk of T2D. These results suggest that HCV increases the risk of developing T2D by affecting insulin sensitivity, and that this association is related to the degree of liver fibrosis and HCV genotype. Therefore, HCV eradication may help reduce blood glucose levels and T2D risk. Gilad et al[38] and Hussein et al[39] found that diabetic patients coinfected with HCV who were treated with direct-acting antiviral agents (DAAs) had significant improvements in glycosylated hemoglobin levels and insulin resistance, as well as a substantial reduction in diabetes-related microvascular complications[40]. Two meta-analyses have shown that DAAs restore HCV-induced alterations in glucose homeostasis by inducing a sustained virological response, thereby reducing insulin resistance and T2D risk[41,42]. These findings indicate that anti-HCV therapy benefits patients with T2D, and provide indirect evidence that CHC is a risk factor for T2D.
Although considerable evidence points to CHC as a potential risk factor for T2D, this MR analysis, based on genetic prediction, did not reveal a causal relationship between them. The MR analysis and clinical trial results differed, possibly because of intermediate factors between CHC and T2D. Ruhl et al[20] stated that diabetes risk is associated with elevated alanine aminotransferase and gamma-glutamyltransferase activities rather than HCV infection status. The authors suggested that the association between HCV infection and T2D reported in previous studies was related to elevated liver enzyme[20]. Related studies have shown that insulin resistance in individuals infected with HCV is associated with alterations in alanine transaminase, aspartate aminotransferase, and bilirubin levels[43]. Papatheodoridis et al[44] found that the risk of T2D in HCV-infected individuals was associated with hepatic fibrosis, cirrhosis, hepatic steatosis, and increased serum triglyceride levels.
Additionally, researchers have observed that HCV infection increases the risk of obesity and metabolic syndrome (MS) by affecting the liver. In a clinical study in Virginia, Younossi et al[45] found that HCV genotype three was associated with an increased risk of steatosis and fatty liver disease. A Taiwanese study has shown that HCV regulates host lipid metabolism and distribution to some extent[46]. Another Taiwanese study showed that the prevalence of MS was higher in individuals infected with HCV than that in non-infected patients (24.7% vs 13.2%)[47]. The effects of HCV infection on obesity and MS may be mediated through the promotion of hepatic steatosis and fibrosis. Hepatitis C core viral proteins in patients with HCV, especially in genotype 3a-infected patients, induced sterol regulatory element-binding protein 1 and peroxisome proliferator-activated receptor γ gene expression and activity, thereby increasing the transcription of genes involved in hepatic fatty acid synthesis, and ultimately promoting steatosis[48]. Hepatitis C core viral proteins, in turn, mediate oxidative stress, promote the expression of inflammatory factors, such as tumor necrosis factor-α, interleukin (IL)-6, and IL-8, and aggravate the degree of hepatic fibrosis, which exacerbates insulin resistance[49,50]. Insulin resistance plays a vital role in MS[47]. This evidence suggests that HCV may affect glucose metabolic homeostasis and increase the risk of T2D through intermediate pathways, such as hepatic steatosis, hepatic fibrosis, and cirrhosis. However, due to the assumption of the exclusivity of MR, SNPs associated with known risk factors for T2D, such as fatty liver, liver fibrosis, and cirrhosis, were excluded as confounding factors, which may be the main reason for the negative MR results.
This study suggests that CHB infection is associated with a reduced risk of T2D, which differs from the results of most clinical studies. Current studies support the notion that HBV infection is not an independent risk factor of T2D[19]. A Taiwanese study involving 1233 individuals found no significant differences in the prevalence of diabetes and glucose intolerance between asymptomatic chronic HBV-infected individuals and a non-HBV control group[30]. This is similar to the findings of another Taiwanese study that concluded that HBV itself does not confer a predisposition to diabetes[51]. Liu et al[52] supported this view from a serological perspective as they found that the serological status of HBV antigen (HBsAg) and hepatitis B surface antibody (HBsAb) was not associated with diabetes. Moreover, HBV infection did not increase the risk of macrovascular complications in diabetes mellitus[53].
Although most of the current literature suggests that CHB is not associated with T2D risk, some studies support CHB as a potential protective factor against T2D. A study of retired Chinese women showed that a HBsAb-positive status was associated with better metabolic status and a lower incidence of diabetes mellitus[54]. Another study found that a high HBV load is associated with a reduced risk of hepatic steatosis, a mechanism by which HBV reduces the risk of T2D[55]. This implies that a high HBV load may be an element of the reduced risk of T2D in patients with CHB rather than HBsAb positivity alone. However, most patients with CHB are treated with antiviral drugs, including tenofovir or entecavir, which reduce the HBV load in the body. As the viral load decreases, the role of HBV in regulating fat metabolism and reducing the risk of diabetes is significantly diminished, which may be the main reason why the results of this MR analysis differ from those of clinical studies.
The potential protective effects of HBV infection against obesity and MS provide indirect evidence that supports our findings. A cross-sectional study in China showed that the prevalence of MS was significantly lower in patients infected with HBV than that in non-infected patients (11.64% vs 12.66%)[56]. A Taiwanese clinical study included 3587 patients with HBV infection without cirrhosis and found that high HBV viral load was associated with a reduced risk of extreme obesity (OR = 0.30; 95%CI: 0.13-0.68) and centripetal obesity (OR = 0.53; 95%CI: 0.34-0.82)[57]. HBV infection may reduce the risk of hepatic steatosis by modulating lipid metabolism, which in turn reduces the risk of obesity and MS. A meta-analysis showed that the prevalence of steatosis was lower in CHB than that in the general population (OR = 0.81; 95%CI: 0.71-0.920)[58]. Another study reported that the prevalence of non-alcoholic fatty liver disease was lower in patients with HBV infection than that in non-infected patients[59]. A clinical study in Taiwan further showed that patients with positive HBsAg possessed lower hypertriglyceridemia (OR = 0.59; 95%CI: 0.52-0.66) and low-density lipoprotein-cholesterol levels (OR = 0.86; 95%CI: 0.79-0.93) than those with negative HBsAg[60]. Considering that steatosis is an essential factor that leads to the progressive impairment of glucose metabolism[61], the role of HBV in regulating hepatic lipid metabolism also contributes to the regulation of glucose metabolic homeostasis. This evidence suggests that HBV infection is associated with a lower risk of obesity and MS, and that the primary mechanism may be the modulation of hepatic fat metabolism, which corroborates our view.
Notably, the risk of T2D increases when CHB progresses to cirrhosis. A meta-analysis of 15 clinical studies showed that the incidence of diabetes was comparable between patients with non-cirrhotic CHB and those with asymptomatic HBV carriers and non-HBV[11]. In contrast, patients with hepatitis B cirrhosis had a significantly increased risk of T2D (OR = 1.99; 95%CI: 1.08-3.65)[11]. Epidemiological studies showed that only 2%-4% of patients infected with HBV each year worldwide will develop compensated cirrhosis, and only 1.5%-4% of compensated cirrhosis will further develop into decompensated cirrhosis[62]. Therefore, most patients with CHB do not have compensated or decompensated cirrhosis, which may explain why most clinical studies have not found an association between CHB and T2D. In summary, CHB is associated with a reduced risk of T2D; however, this association is limited by HBV load and cirrhosis. It weakens or disappears when patients with CHB receive antiviral therapy, and reverses when CHB progresses to cirrhosis.
Few studies have investigated the relationship between T2D and other VH, such as hepatitis A, D, and E. Lin et al[63] found that HAV infection was associated with an increased risk of diabetes (OR = 1.13; 95%CI: 1.08-1.18). However, HAV vaccination and successful HAV immunization were not associated with the risk of diabetes; therefore, they concluded that HAV infection was unlikely to cause diabetes[63]. Zitelli et al[64] found that among patients with chronic HCV infection receiving antiviral therapy, the incidence of diabetes was 3.65 times higher in HEV-positive patients than that in HEV-negative patients, suggesting that HEV is a potential risk factor for diabetes mellitus in chronic HCV-infected individuals. In summary, there are insufficient studies elucidating the effects of hepatitis caused by HAV, HDV, and HEV infections on T2D, and this issue needs to be further explored in subsequent studies.
Our study has some limitations. First, the data on CHB and CHC were derived from East Asians; therefore, the results mainly illustrate the effect of CHB and CHC on T2D among East Asians, and it is not yet clear how they affect other races. Second, the GWAS only provided an overall dataset on VH among Europeans with availability; it did not include a dataset of different types of VH. Therefore, the results of this study can only infer that VH is not associated with T2D risk in Europeans, and cannot explain the effects of different types of VH on T2D risk among Europeans. Third, data on HAV, HDV, and HEV were unavailable in the GWAS; therefore, their effects on T2D risk were not assessed. Fourth, our data were derived from the GWAS; therefore, it was impossible to stratify the analysis for populations with different viral loads. Given these limitations, we expect future studies to improve. First, we recommend continuing to promote human genome studies worldwide, and provide more comprehensive data for MR analysis of different races. Second, we recommend conducting stratified randomized controlled trials to explore the specific effects of the different types, stages, and viral loads of VH on T2D.
This MR analysis showed that neither VH among Europeans nor CHC among East Asians were associated with T2D risk, whereas CHB was associated with reduced T2D risk among East Asians. Although VH among Europeans and CHC among East Asians are not associated with T2D risk, focusing on blood glucose in patients with CHC is still relevant for the early detection of T2D induced by CHC-mediated pathways of hepatic steatosis, liver fibrosis, and cirrhosis. Further studies are needed to explore the causal relationships and mechanisms between different types of VH and T2D.
The causality between viral hepatitis (VH) and type 2 diabetes (T2D) remains unclear.
In this study, a Mendelian randomization (MR) analysis was applied to determine the causality between VH and T2D from genome-wide association study data.
We used a MR to identify the causality between VH, chronic hepatitis B (CHB), chronic hepatitis C (CHC) and T2D from genome-wide association study data.
Two-sample MR was performed to obtain the causality between VH, CHB, CHC and T2D. Summary statistics from the FinnGen were used for VH, BioBank Japan Project was used for CHB and CHC, and the European Bioinformatics Institute and FinnGen were utilized for T2D.
The MR analysis showed no significant causal relationship between VH and T2D in Europeans [odds ratio (OR) = 1.028; 95% confidence interval (CI): 0.995-1.062, P = 0.101] as well as between CHC and T2D in East Asians (OR = 0.949; 95%CI: 0.931-0.968, P < 0.001), while there was a negative causal association between CHB and T2D among East Asians (OR = 0.949; 95%CI: 0.931-0.968, P < 0.001). These MR analysis results showed no horizontal pleiotropy or heterogeneity (P > 0.05), and they were robust.
Among East Asians, CHB is associated with a reduced T2D risk, but this association is limited by hepatitis B virus (HBV) load and cirrhosis. Although CHC among East Asians are not associated with the risk of T2D, focusing on blood glucose in patients with CHC is still relevant for the early detection of T2D induced by CHC-mediated pathways of hepatic steatosis, liver fibrosis, and cirrhosis.
Whether different categories of VH, especially CHB and CHC, are associated with the risk of T2D remains controversial. CHB is associated with a reduced T2D risk among East Asians, but this association is limited by HBV load and cirrhosis. Although VH among Europeans and CHC among East Asians are not associated with T2D risk, focusing on blood glucose in patients with CHC is still relevant for the early detection of T2D induced by CHC-mediated pathways of hepatic steatosis, liver fibrosis, and cirrhosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Buechler C, Germany; Horowitz M, Australia; Yang SS, Taiwan S-Editor: Wang JJ L-Editor: A P-Editor: Zheng XM
| 1. | Sheng CY, Son YH, Jang J, Park SJ. In vitro skeletal muscle models for type 2 diabetes. Biophys Rev (Melville). 2022;3:031306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Majety P, Lozada Orquera FA, Edem D, Hamdy O. Pharmacological approaches to the prevention of type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1118848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4377] [Article Influence: 625.3] [Reference Citation Analysis (0)] |
| 4. | Zhang Y, Zhou H. Hyper-reactive platelets and type 2 diabetes. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:374-383. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Afsar B, Elsurer R. Increased renal resistive index in type 2 diabetes: Clinical relevance, mechanisms and future directions. Diabetes Metab Syndr. 2017;11:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Calles-Escandon J, Garcia-Rubi E, Mirza S, Mortensen A. Type 2 diabetes: one disease, multiple cardiovascular risk factors. Coron Artery Dis. 1999;10:23-30. [PubMed] |
| 7. | Park KS. Prevention of type 2 diabetes mellitus from the viewpoint of genetics. Diabetes Res Clin Pract. 2004;66 Suppl 1:S33-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Pfohl M, Schatz H. Strategies for the prevention of type 2 diabetes. Exp Clin Endocrinol Diabetes. 2001;109 Suppl 2:S240-S249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Alzahrani N. Hepatitis C virus, insulin resistance, and diabetes: A review. Microbiol Immunol. 2022;66:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 10. | Ciardullo S, Mantovani A, Ciaccio A, Carbone M, Invernizzi P, Perseghin G. Hepatitis C virus infection and diabetes: A complex bidirectional relationship. Diabetes Res Clin Pract. 2022;187:109870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Shen Y, Cai H, Liu YM, Qin G. Hepatitis B virus infection status and risk of type 2 diabetes mellitus: A meta-analysis. Hepatol Res. 2015;45:1100-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Nagra N, Kozarek RA, Burman BE. Therapeutic Advances in Viral Hepatitis A-E. Adv Ther. 2022;39:1524-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 13. | Liou JW, Mani H, Yen JH. Viral Hepatitis, Cholesterol Metabolism, and Cholesterol-Lowering Natural Compounds. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 14. | Farci P, Niro GA, Zamboni F, Diaz G. Hepatitis D Virus and Hepatocellular Carcinoma. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Lanini S, Ustianowski A, Pisapia R, Zumla A, Ippolito G. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect Dis Clin North Am. 2019;33:1045-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Leoni S, Casabianca A, Biagioni B, Serio I. Viral hepatitis: Innovations and expectations. World J Gastroenterol. 2022;28:517-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Bernsmeier C, Calabrese D, Heim MH, Duong HT. Hepatitis C virus dysregulates glucose homeostasis by a dual mechanism involving induction of PGC1α and dephosphorylation of FoxO1. J Viral Hepat. 2014;21:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Shen Y, Zhang S, Wang X, Wang Y, Zhang J, Qin G, Li W, Ding K, Zhang L, Liang F. Comparison of type 2 diabetes mellitus incidence in different phases of hepatitis B virus infection: A meta-analysis. Liver Int. 2017;37:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ruhl CE, Menke A, Cowie CC, Everhart JE. Relationship of hepatitis C virus infection with diabetes in the U.S. population. Hepatology. 2014;60:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Gao RC, Sang N, Jia CZ, Zhang MY, Li BH, Wei M, Wu GC. Association Between Sleep Traits and Rheumatoid Arthritis: A Mendelian Randomization Study. Front Public Health. 2022;10:940161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 22. | Julian TH, Boddy S, Islam M, Kurz J, Whittaker KJ, Moll T, Harvey C, Zhang S, Snyder MP, McDermott C, Cooper-Knock J, Shaw PJ. A review of Mendelian randomization in amyotrophic lateral sclerosis. Brain. 2022;145:832-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Ference BA, Holmes MV, Smith GD. Using Mendelian Randomization to Improve the Design of Randomized Trials. Cold Spring Harb Perspect Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 24. | Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1354] [Cited by in RCA: 2588] [Article Influence: 369.7] [Reference Citation Analysis (0)] |
| 25. | Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 3795] [Article Influence: 316.3] [Reference Citation Analysis (1)] |
| 26. | 1000 Genomes Project Consortium; Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526:68-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10217] [Cited by in RCA: 11988] [Article Influence: 1198.8] [Reference Citation Analysis (0)] |
| 27. | Bender D, Glitscher M, Hildt E. [Viral hepatitis A to E: prevalence, pathogen characteristics, and pathogenesis]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022;65:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Kukla M, Piotrowski D, Waluga M, Hartleb M. Insulin resistance and its consequences in chronic hepatitis C. Clin Exp Hepatol. 2015;1:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Wu D. Correlation of viral load of Hepatitis B with the gestation period and the development of diabetes mellitus. Saudi J Biol Sci. 2019;26:2022-2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Huang ZS, Huang TS, Wu TH, Chen MF, Hsu CS, Kao JH. Asymptomatic chronic hepatitis B virus infection does not increase the risk of diabetes mellitus: a ten-year observation. J Gastroenterol Hepatol. 2010;25:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Eshraghian K, Lankarani KB, Fattahi MR, Esmailnejad A, Peymani P. Low Prevalence of Insulin Resistance Among Iranian Patients with Chronic Hepatitis C Virus Infection: A Case-Control Study. Curr Diabetes Rev. 2018;14:446-450. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Gualerzi A, Bellan M, Smirne C, Tran Minh M, Rigamonti C, Burlone ME, Bonometti R, Bianco S, Re A, Favretto S, Bellomo G, Minisini R, Carnevale Schianca GP, Pirisi M. Improvement of insulin sensitivity in diabetic and non diabetic patients with chronic hepatitis C treated with direct antiviral agents. PLoS One. 2018;13:e0209216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Community-based study of hepatitis C virus infection and type 2 diabetes: an association affected by age and hepatitis severity status. Am J Epidemiol. 2003;158:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Fabiani S, Fallahi P, Ferrari SM, Miccoli M, Antonelli A. Hepatitis C virus infection and development of type 2 diabetes mellitus: Systematic review and meta-analysis of the literature. Rev Endocr Metab Disord. 2018;19:405-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Drazilova S, Janicko M, Skladany L, Kristian P, Oltman M, Szantova M, Krkoska D, Mazuchova E, Piesecka L, Vahalova V, Rac M, Schreter I, Virag L, Koller T, Liptakova A, Ondrasova M, Jarcuska P. Glucose Metabolism Changes in Patients with Chronic Hepatitis C Treated with Direct Acting Antivirals. Can J Gastroenterol Hepatol. 2018;2018:6095097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Memon MS, Arain ZI, Naz F, Zaki M, Kumar S, Burney AA. Prevalence of type 2 diabetes mellitus in hepatitis C virus infected population: a Southeast Asian study. J Diabetes Res. 2013;2013:539361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Zhao P, Wang JB, Jiao J. [Investigation on the incidence of diabetes in chronic hepatitis C patients and their HCV genotypes]. Zhonghua Gan Zang Bing Za Zhi. 2006;14:86-88. [PubMed] |
| 38. | Gilad A, Fricker ZP, Hsieh A, Thomas DD, Zahorian T, Nunes DP. Sustained Improvement in Type 2 Diabetes Mellitus is Common After Treatment of Hepatitis C Virus With Direct-acting Antiviral Therapy. J Clin Gastroenterol. 2019;53:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Hussein HA, Allam AS, Moaty ASA. Evaluation of Glycated Haemoglobin (HbA1c) Level in Type 2 Diabetic Chronic HCV Non-cirrhotic Treatment-Naïve Egyptian Patients Eradicated with Sofosbuvir Plus Daclatasvir. Curr Diabetes Rev. 2020;16:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Shiffman ML, Gunn NT. Impact of hepatitis C virus therapy on metabolism and public health. Liver Int. 2017;37 Suppl 1:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Sacco M, Saracco GM. The impact of direct-acting antiviral treatment on glycemic homeostasis in patients with chronic hepatitis C. Minerva Gastroenterol (Torino). 2021;67:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Ribaldone DG, Sacco M, Saracco GM. The Effect of Viral Clearance Achieved by Direct-Acting Antiviral Agents on Hepatitis C Virus Positive Patients with Type 2 Diabetes Mellitus: A Word of Caution after the Initial Enthusiasm. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Mishra PR, Bharti A, Arora R, Mir IA, Punia VPS. Increased Insulin Resistance in Hepatitis-C Infection-Association with Altered Hepatic Function Testing. Pathophysiology. 2022;29:326-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 44. | Papatheodoridis GV, Chrysanthos N, Savvas S, Sevastianos V, Kafiri G, Petraki K, Manesis EK. Diabetes mellitus in chronic hepatitis B and C: prevalence and potential association with the extent of liver fibrosis. J Viral Hepat. 2006;13:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Younossi ZM, McCullough AJ, Ong JP, Barnes DS, Post A, Tavill A, Bringman D, Martin LM, Assmann J, Gramlich T, Mullen KD, O'Shea R, Carey WD, Ferguson R. Obesity and non-alcoholic fatty liver disease in chronic hepatitis C. J Clin Gastroenterol. 2004;38:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Tsao YC, Chen JY, Yeh WC, Peng YS, Li WC. Association between visceral obesity and hepatitis C infection stratified by gender: a cross-sectional study in Taiwan. BMJ Open. 2017;7:e017117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Huang JF, Chuang WL, Yu ML, Yu SH, Huang CF, Huang CI, Yeh ML, Hsieh MH, Yang JF, Lin ZY, Chen SC, Dai CY, Chang WY. Hepatitis C virus infection and metabolic syndrome---a community-based study in an endemic area of Taiwan. Kaohsiung J Med Sci. 2009;25:299-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Del Campo JA, Romero-Gómez M. Steatosis and insulin resistance in hepatitis C: a way out for the virus? World J Gastroenterol. 2009;15:5014-5019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 50. | Lecube A, Hernández C, Genescà J, Simó R. Proinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: A case-control study. Diabetes Care. 2006;29:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Huang JF, Dai CY, Hwang SJ, Ho CK, Hsiao PJ, Hsieh MY, Lee LP, Lin ZY, Chen SC, Wang LY, Shin SJ, Chang WY, Chuang WL, Yu ML. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol. 2007;102:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Liu Y, Jiang C, Hao Y, Xu L, Zhang W, Jin YL, Zhu T, Lam TH. Association of hepatitis B surface antigen seropositivity and hepatitis B surface antibody seropositivity with diabetes: a cross-sectional study based on two Chinese populations in Guangdong, China. BMJ Open. 2020;10:e028968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Liu XY, Zhou Y. Influence of hepatitis B virus on the prevalence of diabetes complications in patients with type 2 diabetes. J Diabetes Investig. 2023;14:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 54. | Li M, Zhou H, Guan Y, Peng H, Wang S, Zhang P, Su B. Positive hepatitis B surface antibody is associated with reduced risk of diabetes mellitus in retired female Chinese workers. J Diabetes. 2016;8:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Yu MW, Lin CL, Liu CJ, Huang YW, Hu JT, Wu WJ, Wu CF. Hepatic steatosis and development of type 2 diabetes: Impact of chronic hepatitis B and viral specific factors. J Formos Med Assoc. 2022;121:1478-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Yan LB, Liao J, Han N, Zhou LY, Wang XE, Wang YJ, Tang H. Association between Hepatitis B Virus Infection and Metabolic Syndrome in Southwest China: A Cross-sectional Study. Sci Rep. 2020;10:6738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Chiang CH, Yang HI, Jen CL, Lu SN, Wang LY, You SL, Su J, Iloeje UH, Chen CJ; REVEAL-HBV Study Group. Association between obesity, hypertriglyceridemia and low hepatitis B viral load. Int J Obes (Lond). 2013;37:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Shi YW, Yang RX, Fan JG. Chronic hepatitis B infection with concomitant hepatic steatosis: Current evidence and opinion. World J Gastroenterol. 2021;27:3971-3983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 59. | Huang J, Jing M, Wang C, Wang M, You S, Lin S, Zhu Y. The impact of hepatitis B virus infection status on the prevalence of nonalcoholic fatty liver disease: A population-based study. J Med Virol. 2020;92:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Huang CY, Lu CW, Liu YL, Chiang CH, Lee LT, Huang KC. Relationship between chronic hepatitis B and metabolic syndrome: A structural equation modeling approach. Obesity (Silver Spring). 2016;24:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Kang SK, Chung TW, Lee JY, Lee YC, Morton RE, Kim CH. The hepatitis B virus X protein inhibits secretion of apolipoprotein B by enhancing the expression of N-acetylglucosaminyltransferase III. J Biol Chem. 2004;279:28106-28112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Zhao H, Wang Q, Luo C, Liu L, Xie W. Recompensation of Decompensated Hepatitis B Cirrhosis: Current Status and Challenges. Biomed Res Int. 2020;2020:9609731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Lin J, Ou HY, Karnchanasorn R, Samoa R, Chuang LM, Chiu KC. Role of hepatitis A virus in diabetes mellitus. World J Diabetes. 2021;12:1928-1941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Zitelli PMY, Gomes-Gouvêa M, Mazo DF, Singer JDM, Oliveira CPMS, Farias AQ, Pinho JR, Tanigawa RY, Alves VAF, Carrilho FJ, Pessoa MG. Hepatitis E virus infection increases the risk of diabetes and severity of liver disease in patients with chronic hepatitis C virus infection. Clinics (Sao Paulo). 2021;76:e3270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |