Published online Feb 15, 2024. doi: 10.4239/wjd.v15.i2.170
Peer-review started: September 21, 2023
First decision: November 9, 2023
Revised: November 21, 2023
Accepted: December 27, 2023
Article in press: December 27, 2023
Published online: February 15, 2024
Processing time: 135 Days and 20 Hours
Helicobacter pylori (H. pylori) infection is related to various extragastric diseases including type 2 diabetes mellitus (T2DM). However, the possible mechanisms connecting H. pylori infection and T2DM remain unknown.
To explore potential molecular connections between H. pylori infection and T2DM.
We extracted gene expression arrays from three online datasets (GSE60427, GSE27411 and GSE115601). Differentially expressed genes (DEGs) commonly present in patients with H. pylori infection and T2DM were identified. Hub genes were validated using human gastric biopsy samples. Correlations between hub genes and immune cell infiltration, miRNAs, and transcription factors (TFs) were further analyzed.
A total of 67 DEGs were commonly presented in patients with H. pylori infection and T2DM. Five significantly upregulated hub genes, including TLR4, ITGAM, C5AR1, FCER1G, and FCGR2A, were finally identified, all of which are closely related to immune cell infiltration. The gene-miRNA analysis detected 13 miRNAs with at least two gene cross-links. TF-gene interaction networks showed that TLR4 was coregulated by 26 TFs, the largest number of TFs among the 5 hub genes.
We identified five hub genes that may have molecular connections between H. pylori infection and T2DM. This study provides new insights into the pathogenesis of H. pylori-induced onset of T2DM.
Core Tip: This bioinformatic research is the one of the first studies to identify the key genes and pathways associated with both Helicobacter pylori (H. pylori) infection and type 2 diabetes mellitus (T2DM), using integrated bioinformatics analyses. Five hub genes were identified, including TLR4, C5AR1, ITGAM, FCGR2A, FCER1G, and all of which were closely related to immune cell infiltration. We also verified their expression in clinical specimens. Hopefully, this study will shed some light on the pathogenesis of H. pylori-induced T2DM in the future. This study is of great clinical importance.
- Citation: Chen H, Zhang GX, Zhou XY. Identification of hub genes associated with Helicobacter pylori infection and type 2 diabetes mellitus: A pilot bioinformatics study. World J Diabetes 2024; 15(2): 170-185
- URL: https://www.wjgnet.com/1948-9358/full/v15/i2/170.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i2.170
The infection rate of Helicobacter pylori (H. pylori) is still increasing recently and it infects almost 50% of the world’ population. The prevalence rate is even higher in less developed countries[1]. It not only affects gastric disease but also affects extragastric diseases such as non-alcoholic fatty liver disease[2], cardiovascular disease[3], autoimmune disease[4], and endocrine disorders, such as diabetes[5]. In recent years, the prevalence rate of type 2 diabetes mellitus (T2DM) and its complications have also increased significantly[6]. The consequences of poor glycemic control in the long and short term can be significant on social and economic levels[7,8]. Patients with T2DM are more susceptible to H. pylori infection, according to our previous meta-analysis[9,10]. There is a significant decrease in the eradication rate of H. pylori infection in T2DM patients with H. pylori infection compared to T2DM patients without infection[11]. Additionally, H. pylori-infected T2DM patients have worse glycemic control capability[12]. All these clinical studies strongly suggest that there is an association between H. pylori infection and T2DM.
However, the detailed mechanisms underlying H. pylori infection and T2DM remain unclear. According to previous studies, both innate and adaptive immune reactions may be activated in the mucosa of the stomach as a result of H. pylori infection[13]. This local inflammation in the stomach may spread systematically as a result of proinflammatory cytokines released by the stomach[14]. Chronic low-grade inflammation, which is a feature of H. pylori-associated T2DM, would be more likely to develop as a result[15]. Our previous mechanistic study suggested that H. pylori infection induces hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway[16]. The gut microbiota may also play a role in the immune and metabolic homeostasis of the host, and the infection of H. pylori not only disrupts the balance of commensal bacterial species in the gastric mucosa but also causes alterations in the microbial composition of the human gut[17]. However, these hypotheses have not been formally confirmed and validated.
This study aimed to investigate the potential molecular connections between H. pylori infection and T2DM. We identified differentially expressed genes (DEGs) by analyzing gene expression datasets through comprehensive bioinformatics analysis. DEGs were screened by combining the results from GEO datasets. Protein-protein interaction (PPI) construction, Gene Ontology (GO) term analysis, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed to identify the hub genes linked to the two diseases. A miRNA-hub gene network and transcription factor (TF)-gene mRNA interaction network were also constructed. We sought to provide new insights into the pathogenesis of H. pylori-induced onset of T2DM.
The NCBI-GEO database is a publicly available database containing gene expression datasets[18,19]. Three datasets were retrieved from the GEO database (https://www.ncbi.nlm.nih.gov/geo/), including two gene expression profiles related to H. pylori (GSE60427 and GSE27411) and one dataset related to T2DM (GSE115601). Detailed information on the microarray datasets is provided in Supplementary Table 1. Gene expression profiles were set accordingly, including: (1) Tissue samples collected from diseased and normal gastric tissues; and (2) datasets with more than three samples.
The NCBI-GEO2R interactive tool was utilized to analyze and compare data under similar experimental conditions from two or more sample groups to identify genes significantly differentially expressed for both diseases (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?)[20]. Genes that satisfied the criteria of log fold change > 0.4 with adjusted P value less than 0.05 were identified as DEGs. Genes presenting upregulation or downregulation in both H. pylori and T2DM were selected using the Venn diagram web tool (http://bioinfogp.cnb.csic.es/tools/venny/).
DAVID (Database for Annotation, Visualization, and Integrated Discovery), as an online tool, was used to predict the functions of hub genes based on GO enrichment analysis and KEGG pathway analysis (https://david.ncifcrf.gov/)[21] at three levels: Biological process (BP), molecular function (MF), and cellular component (CC). Bubble maps were used for representing BP, MF, CC, and KEGG pathways, using R package of ggPlot2. A statistically significant P value was defined as P value less than 0.05.
A public online database, named STRING (https://string-db.org/), can be used to search for and predict PPIs. This inclusive resource facilitates the investigation of direct physical associations between proteins, as well as the detection of indirect functional connections unveiled through correlation analyses[22]. When common DEGs between different groups were identified, they were uploaded to STRING’s official website (https://cn.string-db.org/) and the interactions between DEGs and STRING database proteins were then assigned (with a minimum needed interaction score of 0.40). We followed the method of Liu et al[23], in which PPI interaction networks were visualized using Cytoscape (Version 3.6.1). Cytoscape is from National Institute of General Medical Sciences, United States. We used CytoHubba (Version 0.1) to identify hub genes using a maximal clique centrality algorithm.
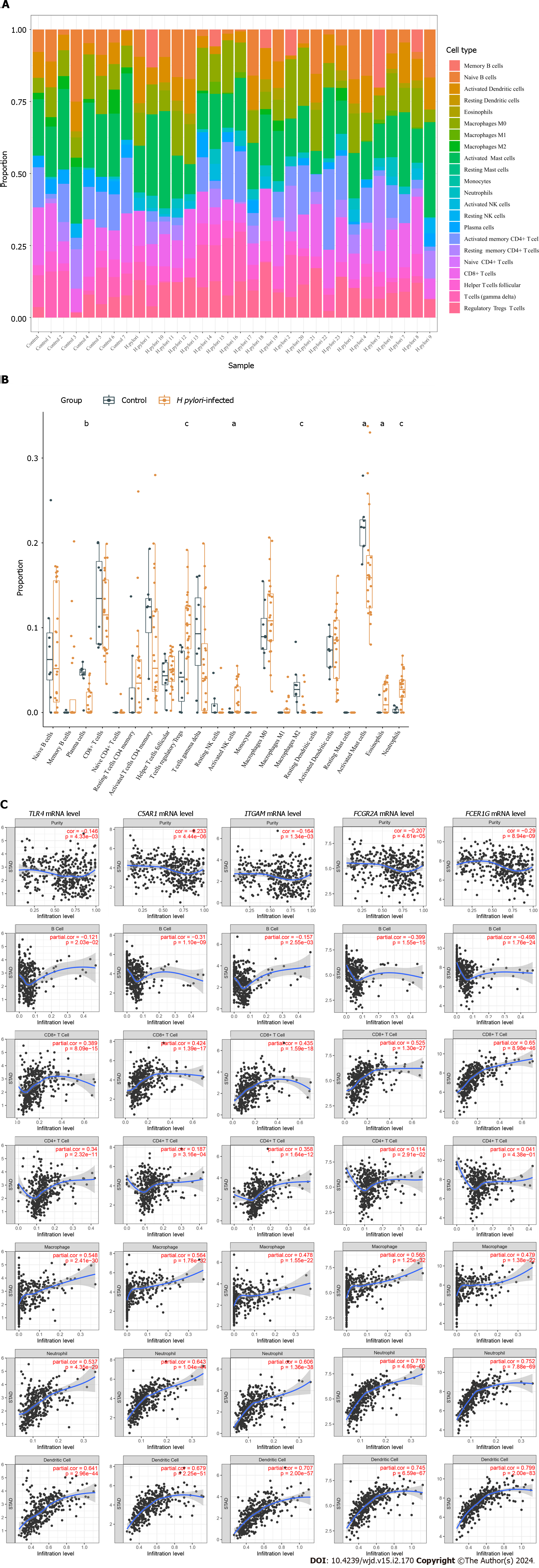
To explore the association between infiltrating immune cells and H. pylori infection, data on proportions of the 22 immune cell types were obtained using the “cell-type identification by estimating relative subsets of RNA transcripts” (CIBERSORT) algorithm (https://cibersort.stanford.edu/). As a result, only samples with a P value of < 0.05 were included in the immune cell infiltration matrix. Boxplots and violin plots were utilized to visualize the proportions of infiltrated immune cells in each sample and each group. The correlation between expression of the five hub genes and the abundance of six immune cell subsets [B cells, CD4+ T cells, CD8+ T cells, macrophages, dendritic cells (DCs), and neutrophils] was analyzed in the gene module of TIMER (http://timer.cistrome.org/)[24].
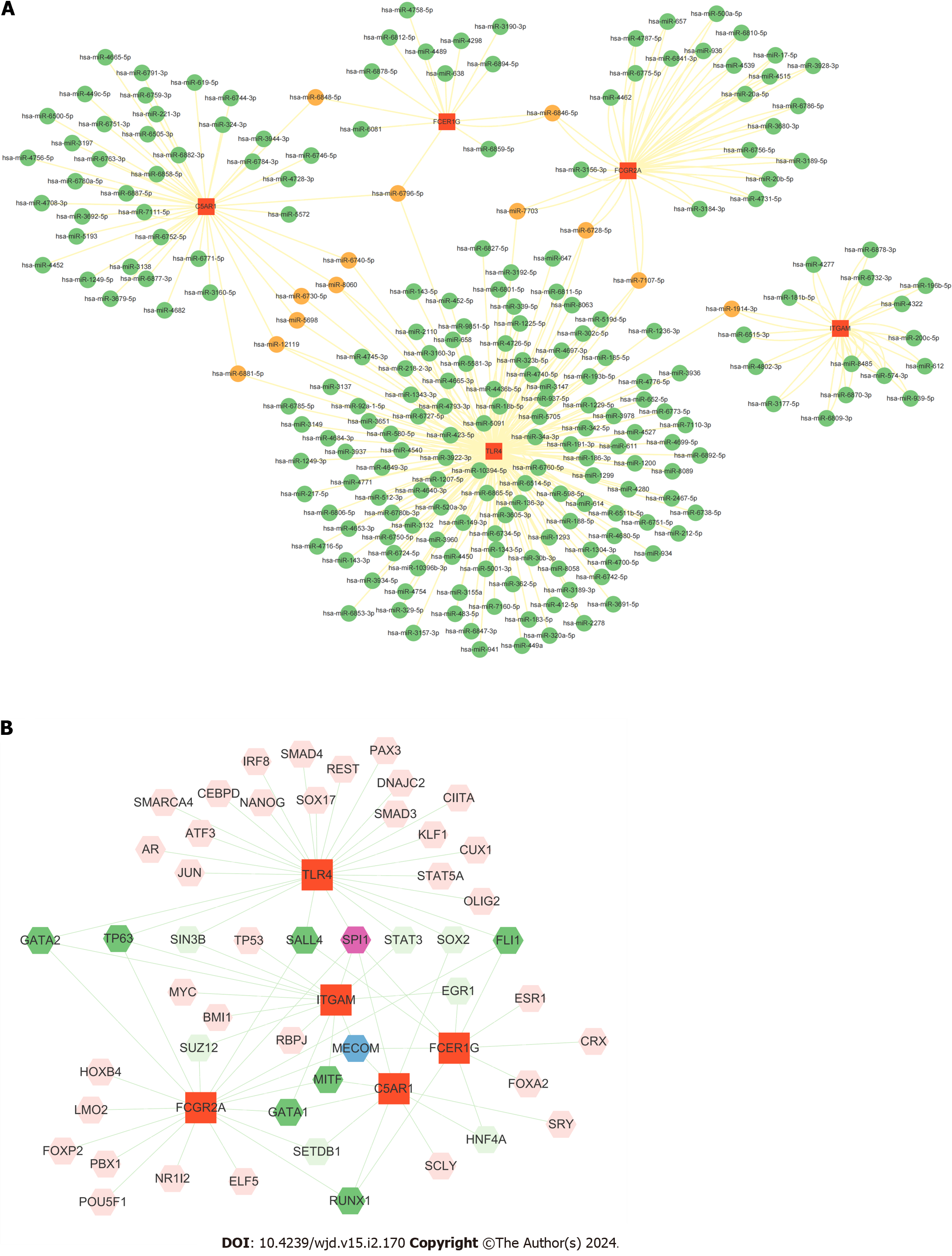
In order to predict their targeted miRNAs, hub genes were selected and analyzed using the miRWalk database (http://mirwalk.umm.uni-heidelberg.de/). The filter setting with a score of > 0.90 was implemented. The target gene binding region was the 3'-UTR, and the intersection with other databases was set to miRDB. Further data processing was carried out by Cytoscape.
The Network Analyst database (https://www.networkanalyst.ca/) was applied to identify human TFs of the related hub genes[25]. The database includes all three data sources named JASPAR, ENCODE and ChIP Enrichment Analysis. ChIP Enrichment Analysis was used to identify target TFs of hub genes in our current study. Moreover, the Cytoscape tool was used to visualize the TF-gene interaction network among TFs and hub genes.
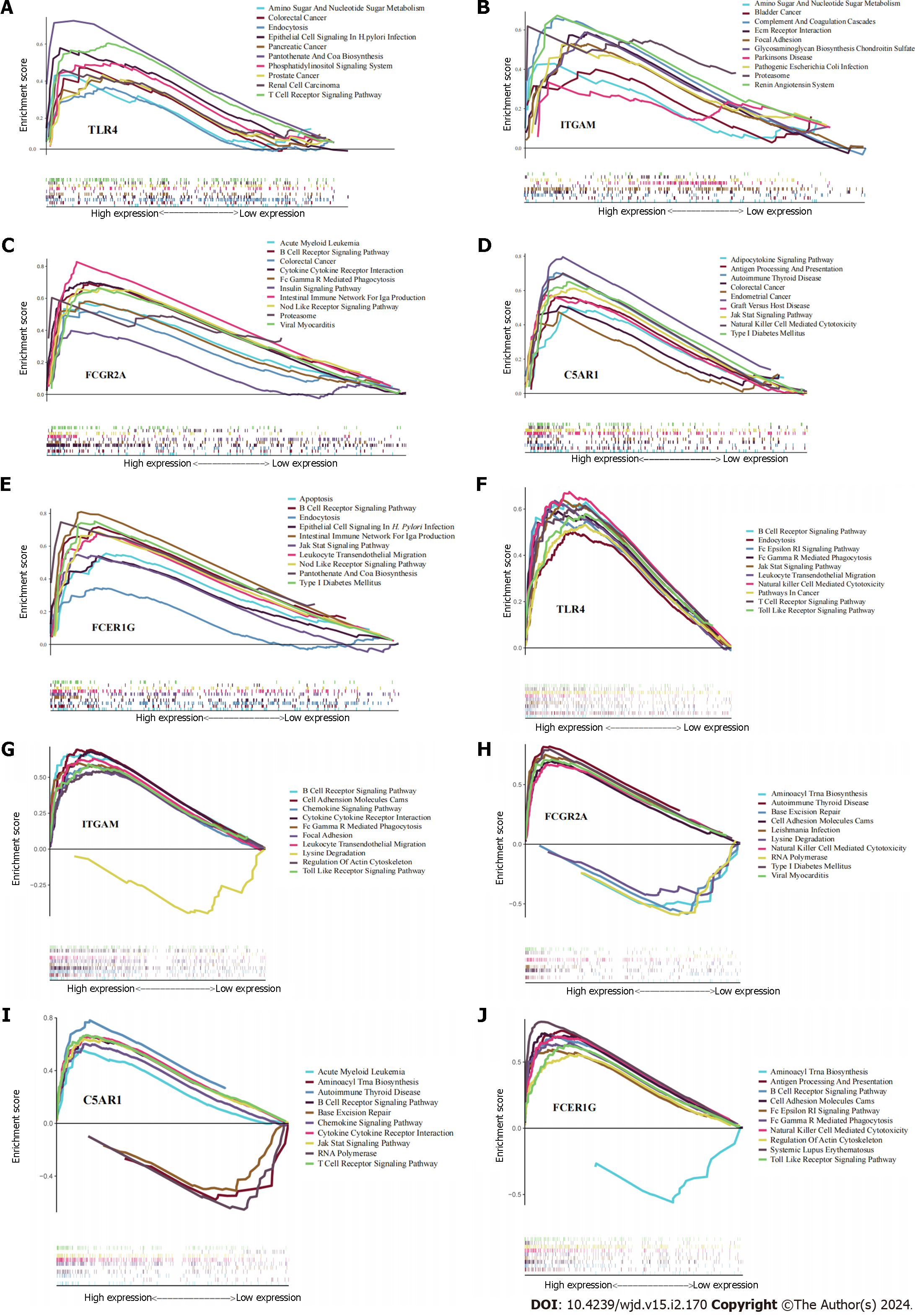
Gene set enrichment analysis (GSEA) of each hub gene was performed using the “clusterProfiler” R package to identify regulatory pathways and biological functions associated with each hub gene. An adjusted P < 0.05 was used to indicate significant thresholds for GSEA.
The results of our bioinformatics-based analysis were further verified by RT-qPCR assays. Gastric antrum tissues from patients and controls were collected (control: n = 30; T2DM: n = 30; H. pylori: n = 30; T2DM + H. pylori: n = 30).
H. pylori infection was diagnosed by the 13C-urea breath test (Headway Bio-Sci Co., Ltd, Shenzhen, China) according to the manufacturer’s instructions. A delta over baseline of > 4% indicates a positive H. pylori infection status. Patients with T2DM were diagnosed based on one of the following American Diabetes Association diagnostic criteria: fasting blood glucose level ≥ 7.0 mmol/L, 2-hour postload glucose level ≥ 11.1 mmol/L during an oral glucose tolerance test, glycated hemoglobin level ≥ 6.5%, or a random plasma glucose level ≥ 11.1 mmol/L in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis. This study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (2021-SRFA-034). Total RNA was extracted from each tissue sample using TRIzol (Invitrogen, F10488, Waltham, MA, United States), following the manufacturer’s instructions. The kit, EasyScript All-in-One First-Strand cDNA Synthesis SuperMix for RT-qPCR Kit (TransGen Biotech, Beijing, China), was utilized for reverse transcription, with incubations performed at a tempertature 42°C for 15 min and then at 85°C for 15 s. Subsequently, StarLighter SYBR Green RT-qPCR Mix (Universal) (Forever Star, Beijing, China) kit was utilized for RT-qPCR analysis, with an ABI 7500 system (Applied Biosystems, United States). The primers used are listed in Supplementary Table 2. The reaction conditions were as follows: Predenaturation (95°C for 5 min), 40 cycles of denaturation (94°C for 20 s), annealing and extension (60°C for 34 s). β-actin was served as an internal control for RT-qPCR. The 2-ΔΔCt method was utilized to determine relative the expression levels of genes. Statistical analysis was performed using GraphPad Prism (Version 9.0, Boston, MA, United States). Expression differences of hub genes were compared using one-way ANOVA in four groups (control, H. pylori infection, T2DM, and T2DM with H. pylori infection), and pairwise comparisons within the two groups were performed using Student’s t test. Statistically significant was defined as P < 0.05.
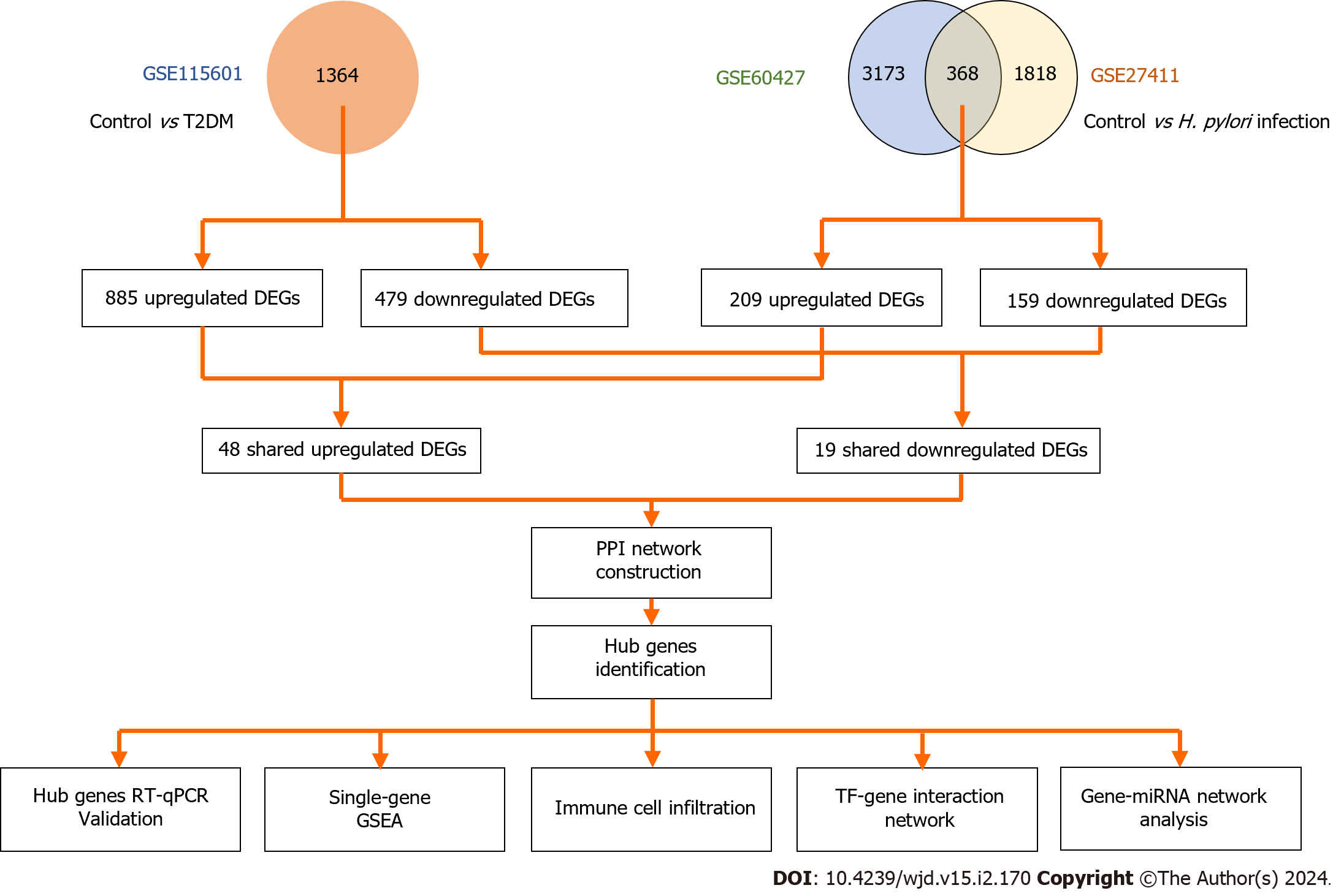
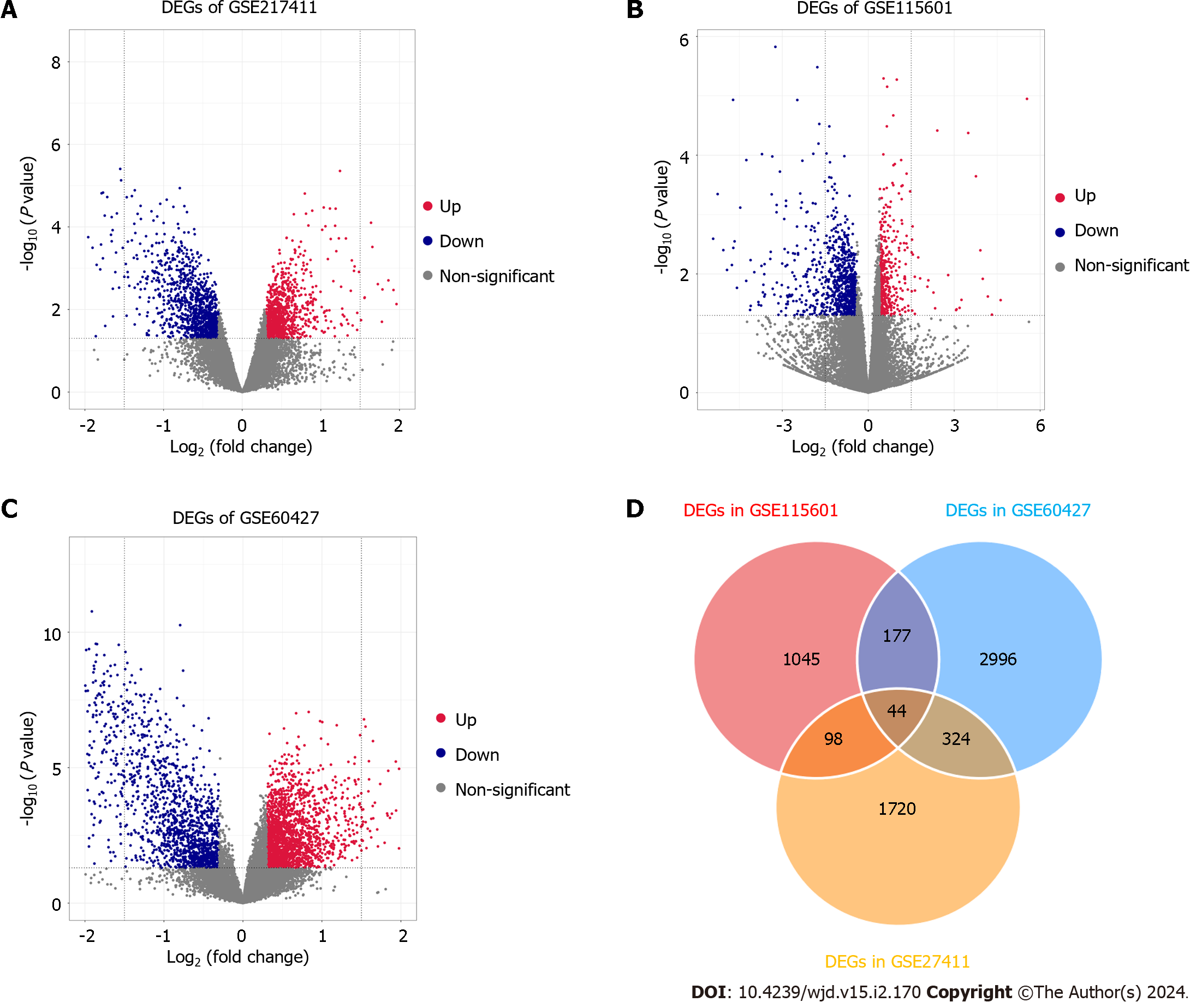
Figure 1 illustrated the overall study design. In brief, a total of 3541, 2186 and 1364 DEGs were identified from the GSE60427, GSE217411 and GSE115601 datasets, respectively. In the GEO datasets, volcano plots (Figure 2A-C) and heatmaps (Supplementary Figure 1) were used to illustrate the dysregulated genes (including upregulated and downregulated). Among these datasets, 67 common DEGs were extracted, including 48 upregulated and 19 downregulated genes
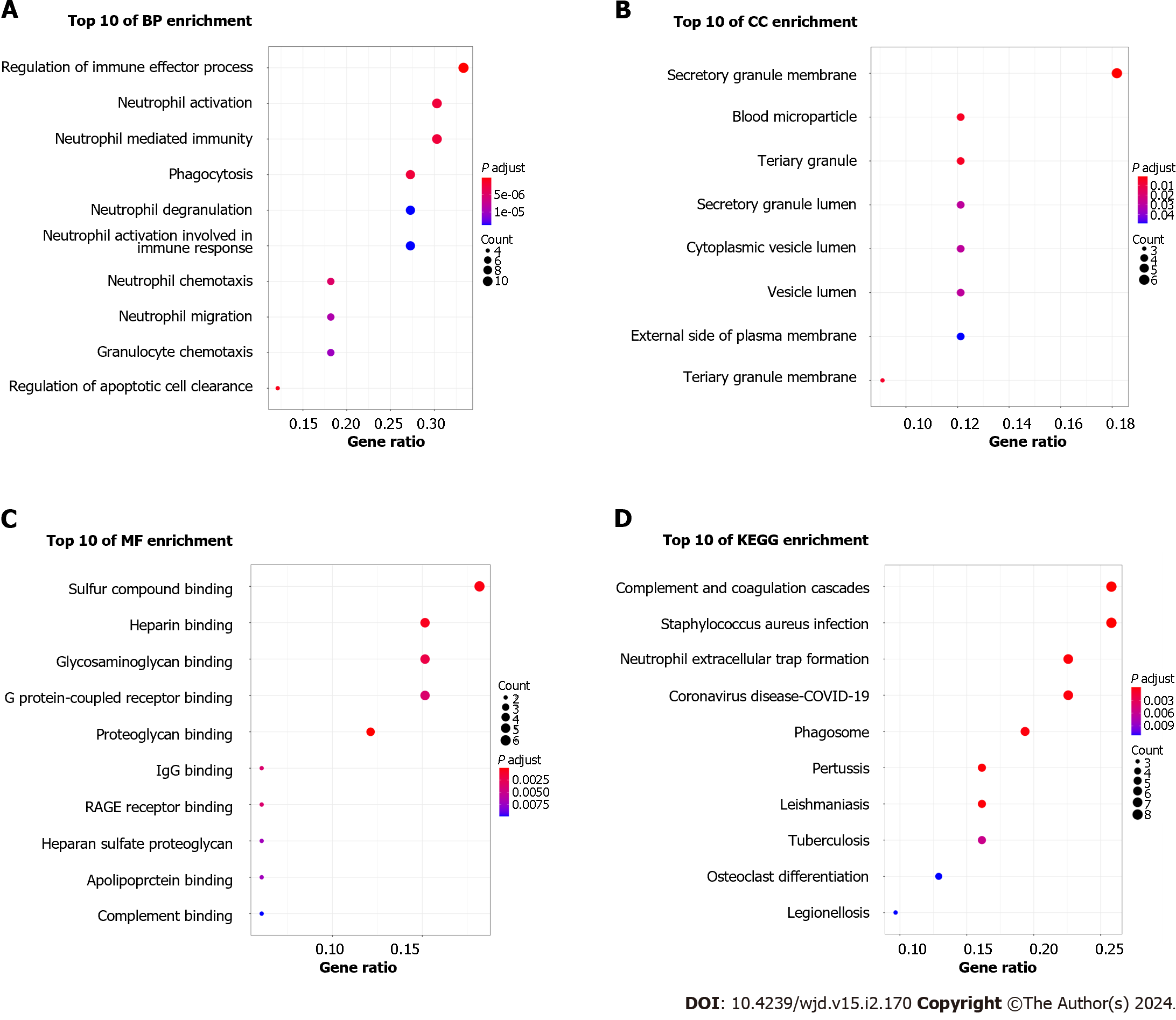
After DEGs were selected, GO and KEGG pathway enrichment analyses were performed to explore the biological functions of these genes involving three functional categories: BP, MF, and CC. Major BP terms associated with DEGs included regulation of the immune effector process, neutrophil activation and neutrophil mediated immunity (Figure 3A). Major CC terms associated with these DEGs included the secretory granule membrane, blood microparticle, and tertiary granule (Figure 3B). Finally, MF-associated GO terms were mainly associated with sulfur compound binding, heparin binding, glycosaminoglycan binding, etc. (Figure 3C). According to KEGG pathway analysis results, the DEGs were mainly enriched for pathways related to complement and coagulation cascades, Staphylococcus aureus infection, and neutrophil extracellular trap formation (Figure 3D).
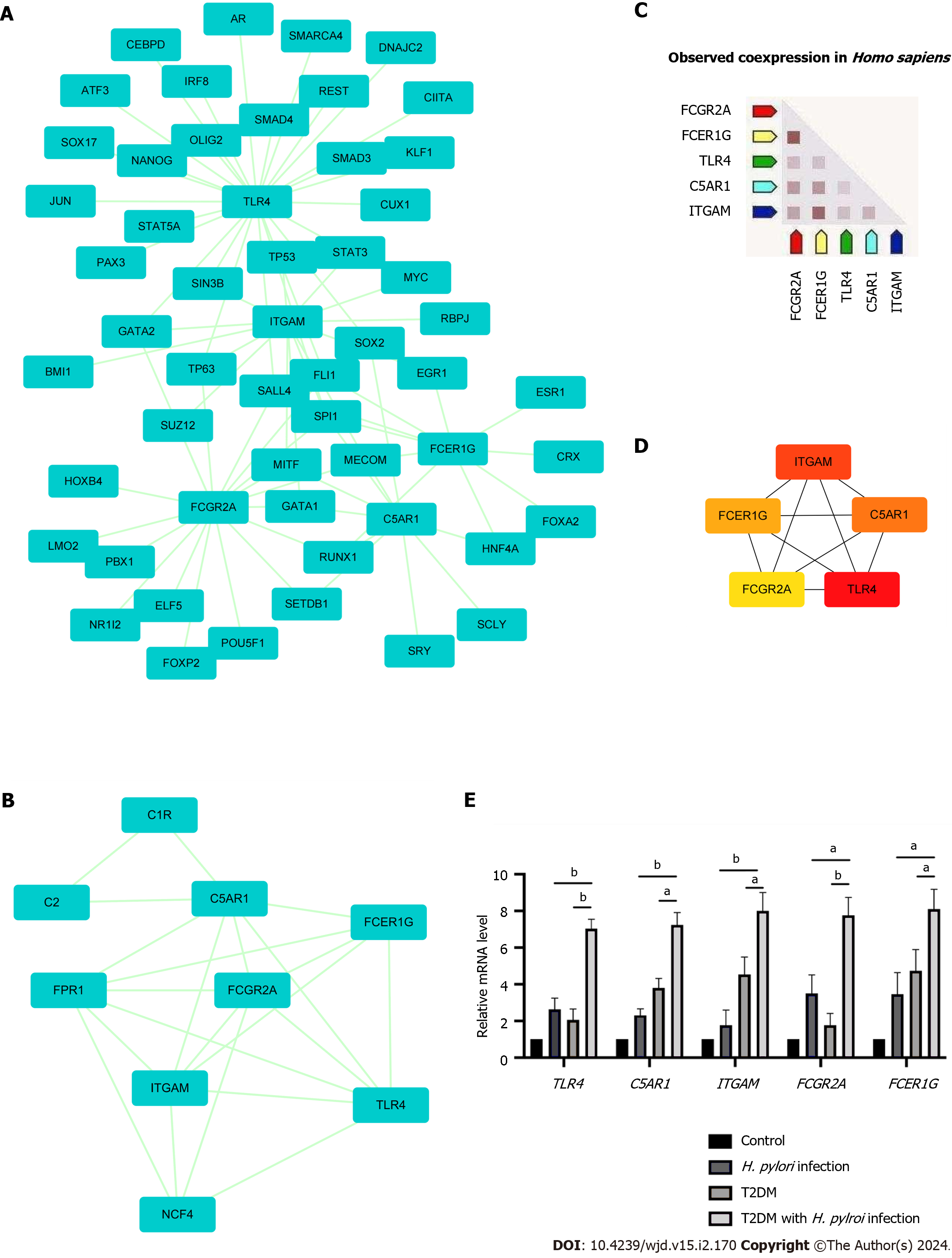
The PPI network of DEGs obtained from STRING was subjected to the MCODE plugin of Cytoscape to analyze significant modules. A total of 38 nodes and 84 edges were mapped in the PPI network (Figure 4A). From these modules, the top functional cluster of modules was selected based on the cutoff criteria of node > 3 and score > 3 (Figure 4B).
Then, the key genes with degree connectivity were ranked by the CytoHubba plugin of Cytoscape. Finally, five intersecting genes (TLR4, ITGAM, C5AR1, FCER1G and FCGR2A) with the highest degree were considered hub genes for further analyses (Figure 4C and D).
Expression levels of the five hub genes in the three datasets are shown in Supplementary Figure 2; and were significantly upregulated in patients with either H. pylori infection or T2DM alone compared to negative controls. Human gastric tissues from four groups were collected (control group, H. pylori infection alone group, T2DM alone group and T2DM with H. pylori infection group). All included patients underwent upper gastrointestinal endoscopy and were pathologically diagnosed with chronic superficial gastritis without acute inflammation or atrophy according to the Sydney System[26]. The baseline characteristics of the groups are shown in Supplementary Table 4. Through RT-qPCR analysis, we found that TLR4, ITGAM, C5AR1, FCER1G and FCGR2A were expressed at significantly higher levels in the T2DM with H. pylori infection group (P < 0.05) than in the T2DM group or the H. pylori infection group alone (Figure 4E).
Using the CIBERSORT algorithm, we explored differences in immune infiltration between H. pylori-infected versus normal gastric tissues. Compared with normal tissues, H. pylori-infected gastric tissues generally contained a higher proportion of regulatory T cells, activated NK cells, eosinophils and neutrophils, whereas the proportions of plasma cells, activated mast cells and M2 macrophages were lower in H. pylori-infected gastric tissues (Figure 5A and B).
The results obtained using TIMER showed that TLR4 and ITGAM expression correlated positively with CD8+ T cells, CD4+ T cells, macrophages, neutrophils, and DCs. C5AR1, FCER1G and FCGR2A expression was significantly associated with infiltration of B cells, CD8+ T cells, macrophages, neutrophils, and DCs, among which their mRNA expression levels all correlated negatively with B cells (Figure 5C).
A total of 225 miRNAs was predicted after we uploading the 5 identified hub genes to the miRWalk database. The gene–miRNA interaction network is shown in Figure 6A. We detected 13 miRNAs (miR-6848-5p, miR-6796-5p, miR-6740-5p, miR-8060, miR-6730-5p, miR-5698, miR-12119, miR-6881-5p, miR-6846-5p, miR-7703, miR-6728-5p, miR-7107-5p and miR-1914-3p) associated with at least two gene cross-links, as shown in Supplementary Table 5.
The top ranked TFs were SPI1, MECOM, GATA2, TP63, SALL4, GATA1, MITF, RUNX1 and FLI1 (Figure 6B). Based on the results, we found that TLR4 was coregulated by 26 TFs, the highest among the identified hub genes.
We performed GSEA on TLR4, ITGAM, C5AR1, FCER1G and FCGR2A to explore the role of these genes in the course of H. pylori infection and T2DM and found the top 10 significant items (Figure 7). According to GSEA results, it suggested that all these five genes play a direct or indirect role in the pathogenesis of H. pylori infection and T2DM. For example, FCG2A is involved in the signaling pathway of “type 1 diabetes mellitus” and the “insulin signaling pathway”, C5AR1 and FCER1G are involved in the signaling pathway of “type 1 diabetes mellitus”, and ITGAM is involved in the signaling pathway of “glycosaminoglycan biosynthesis chondroitin sulfate”.
Approximately 50% of the world’s population is infected with H. pylori, and the infection rate is even higher in patients with T2DM. Infected patients with T2DM have worse blood glucose control abilities, with great social and economic burdens[7,8]. However, the detailed mechanism of the interaction between T2DM and H. pylori infection remains unknown. Therefore, it is necessary to increase our understanding of the underlying mechanisms leading to the risk of H. pylori infection and T2DM to develop effective treatment approaches.
In this study, we investigated the biological functions, expression levels, and correlations with immune infiltrates of common genes with significantly altered expression in both H. pylori-infected individuals and T2DM patients through integrated bioinformatics analyses. Our results showed that expression of 67 overlapping genes was altered in gastric samples from both H. pylori-infected individuals and T2DM patients. Among these genes, 48 were upregulated and 19 downregulated. Five hub genes were further identified through PPI analysis. However, regardless of the statistical probability, the causality between a candidate genotype and the phenotype of the host remains uncertain[27]. To further identify the relationship between genotype (the 5 hub genes) and phenotype (H. pylori-associated T2DM), rigorous validation of mechanisms at the molecular, cellular, tissue, and whole-organism levels is needed.
Chronic low-grade inflammation has been definitively shown to correspond with obesity[28] and diabetes[29]. However, whether obesity and diabetes drive the inflammation or vice versa remains to be elucidated. Gut microbiota play a critical role in the development of the host immune system, making it an important immune organ[30]. Dis-turbance of the gut microbiota promotes inflammation within the lining of the intestines[31]. The dysbiosis of the gut results in bacterial infiltration, allowing microbes to contact the epithelium and causing inflammation[32]. Toll-like receptors (TLR) play a key role in host recognition of microbes[33]. TLR4 has been implicated in recognition of bacterial lipopolysaccharides, a key element of the cell walls of gram-negative bacteria. This triggers the expression of proinflammatory cytokines and chemokines, including tumor necrosis factor-alpha[34]. This inflammatory response is strongly linked to insulin resistance, and both TLR4 and its coreceptor CD14 are needed to induce insulin resistance in mice[35]. It is believed that TLR4, one of the TLR family members, possesses the potential to trigger nuclear factor-κB when confronted with short-chain fatty acids. Consequently, this leads to subsequent stimulation of the immune system[36]. Therefore, the inflammation caused by TLR4 serves a crucial function in the development of T2DM related to H. pylori. The study conducted by Devaraj et al[37] exhibited a notable rise in the level of TLR4 expression among individuals diagnosed with type 1 diabetes. This finding implies that TLR4 actively participates in the inflammatory state associated with diabetes. Moreover, knockout of TLR4 alleviated inflammation in rats with diabetes and TLR4 antagonists attenuated atherogenesis in mice with diabetes[38]. Based on our results, we speculated that TLR4 participates in the pathogenesis of H. pylori-associated T2DM via the TLR signaling pathway.
Other hub genes, ITGAM[39], C5AR1[40], FCER1G[41] and FCGR2A[42], are also reported to be associated with diabetes. ITGAM, a monocyte/macrophage marker, is upregulated in T2DM patients[39]. FCER1G was identified as a significant gene related to diabetic kidney disease. Gene Expression Omnibus validation using additional datasets showed that FCER1G is upregulated in diabetic glomerular lesions compared with normal tissues. This report also revealed that abnormal upregulation of FCER1G is related to diabetic glomerular lesions[41].
Clinical variability between individuals infected with any pathogen is enormous, ranging from silent to lethal. One of the main reasons is immunity differs among individuals[43]. Tumor-infltrating immune cells function together to defend the body against invading factors, such as bacterial infection. Therefore, they can be used as important predictors for diagnosis and treatment of diseases[44]. Based on KEGG pathway and immune cell infiltration analyses, we found that H. pylori infection is associated with multiple immune cell changes, especially NK cells and regulatory T cells. Through single-gene GSEA, we found that high expression of the hub genes TLR4, FCGR2A, and FCER1G was associated with NK cell-mediated cytotoxicity in diabetes, which suggests that H. pylori infection might change hub gene expression and downstream NK cells to induce T2DM. Further analysis suggested that these 5 hub genes all correlated with B cells, CD8+ T cells, macrophages, neutrophils, and DCs. It has been shown that isolated NK cells from T2DM subjects show defects in the NK cell-activating receptors NKG2D and NKp46, in association with functional defects in NK degranulation capacity[45]. Restrepo et al[46] demonstrated that chronic hyperglycaemia is significantly associated with defects in complement receptors and Fcγ receptors on isolated monocytes, resulting in phagocytosis impairment. An in vitro study using macrophages derived from mouse bone marrow and treated with high glucose showed reduced antibacterial activity and phagocytosis for the treated macrophages[47]. In the same study, reduced phagocytosis was shown in peritoneal macrophages from mice with T2DM. This might be related to the reduced glycolytic capacity and reserve of macrophages following long-term sensitization to high levels of glucose. Reactive oxygen species production was reportedly reduced in isolated neutrophils from T2DM tuberculosis patients following phorbol 12-myristate 13-acetate stimulation, and this defect in reactive oxygen species production was associated with increased levels of resistin in T2DM patient serum[48]. In a comparable study, Perner et al[49] documented the inhibition of superoxide production in neutrophils isolated from healthy individuals when subjected to a high-glucose environment. This hindrance was observed to be a consequence of the suppression of glucose-6-phosphate dehydrogenase, which disrupted the generation of nicotinamide adenine dinucleotide phosphate. Thus, we speculate that these 5 hub genes are involved in H. pylori-associated T2DM through immune infiltration. We will validate their relationship through experiments in the future.
This study provides some new insights into the pathogenesis of H. pylori-associated T2DM. However, several limitations should be mentioned. First of all, this study had a relatively small sample size and a larger sample size would be necessary for further investigations. Secondly, hub genes were identified using bioinformatics analysis and validated by a small clinical sample. Validation including RNA-seq from a larger clinical cohort is needed. It is necessary to investigate the potential underlying mechanisms involved in these findings in future large-scale prospective studies. Thirdly, despite statistical probability, the causality between a candidate genotype and the phenotype of the host is uncertain[27]. To identify the relationship between genotype (the 5 hub genes) and phenotype (H. pylori-associated T2DM), rigorous validation of mechanisms at the molecular, cellular, tissue, and whole-organism levels is needed.
We report 67 common DEGs and five hub genes (TLR4, ITGAM, C5AR1, FCER1G and FCGR2A) in H. pylori infection and T2DM. We validated expression of the five hub genes by RT-qPCR. All hub genes were significantly upregulated in T2DM patients with H. pylori infection compared with noninfected T2DM patients. Immune infiltration analysis showed that H. pylori-infected gastric tissues generally contained a higher proportion of regulatory T cells, activated NK cells, eosinophils and neutrophils. Our gene-miRNA analysis detected 13 miRNAs with at least two gene cross-links, and TF-gene interaction networks showed that TLR4 to be coregulated by 26 TFs, the largest number of TFs among the 5 hub genes. This study provides a new idea for elucidating the pathogenesis of H. pylori-associated T2DM at the genetic level.
This prevalence rate of Helicobacter pylori (H. pylori) is high, especially in less developed countries. Its infection related to not only gastric diseases but also extragastric diseases such as type 2 diabetes mellitus (T2DM). However, the underlying mechanisms connecting H. pylori infection and T2DM remains unclear.
The potential molecular connections between H. pylori infection and T2DM are needed to be identified, in order to further elucidate the pathogenesis and the new treatment strategy of H. pylori-infected T2DM.
We aimed to explore the potential molecular connections between H. pylori infection and T2DM using bioinformatics analysis. In the future research, we will investigating these identified genes and downstream signaling pathway to further understand their relationship.
Differentially expressed genes from three datasets commonly present in patients with H. pylori infection and T2DM were identified. Hub genes were validated by RT-qPCR using human gastric biopsy samples. Correlations between hub genes and immune cell infiltration, miRNAs, and transcription factors were further analyzed.
This is the first study to identify the key genes and pathways associated with H. pylori infection and T2DM using integrated bioinformatics analysis. We identified five hub genes, all of which were closely related to immune cell infiltration.
We were the first to find out that the 5 hub genes identified are playing important roles in the pathogenesis of H. pylori-infected T2DM.
It is necessary to investigate the potential underlying mechanisms involved in these findings in future large-scale prospective studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Horowitz M, Australia; Suzuki H, Japan S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Butt J, Epplein M. How do global trends in Helicobacter pylori prevalence inform prevention planning? Lancet Gastroenterol Hepatol. 2023;8:498-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Alvarez CS, Florio AA, Butt J, Rivera-Andrade A, Kroker-Lobos MF, Waterboer T, Camargo MC, Freedman ND, Graubard BI, Lazo M, Guallar E, Groopman JD, Ramírez-Zea M, McGlynn KA. Associations between Helicobacter pylori with nonalcoholic fatty liver disease and other metabolic conditions in Guatemala. Helicobacter. 2020;25:e12756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Zhang P, He Q, Song D, Wang Y, Liu X, Ding G, Xing W. Association of Helicobacter pylori Infection With Carotid Atherosclerosis in a Northern Chinese Population: A Cross-Sectional Study. Front Cardiovasc Med. 2021;8:795795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Wang L, Cao ZM, Zhang LL, Dai XC, Liu ZJ, Zeng YX, Li XY, Wu QJ, Lv WL. Helicobacter Pylori and Autoimmune Diseases: Involving Multiple Systems. Front Immunol. 2022;13:833424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 5. | Mansori K, Dehghanbanadaki H, Naderpour S, Rashti R, Moghaddam AB, Moradi Y. A systematic review and meta-analysis of the prevalence of Helicobacter pylori in patients with diabetes. Diabetes Metab Syndr. 2020;14:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Zhang K, Ma Y, Luo Y, Song Y, Xiong G, Sun X, Kan C. Metabolic diseases and healthy aging: identifying environmental and behavioral risk factors and promoting public health. Front Public Health. 2023;11:1253506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Pan Y, Zhong S, Zhou K, Tian Z, Chen F, Liu Z, Geng Z, Li S, Huang R, Wang H, Zou W, Hu J. Association between Diabetes Complications and the Triglyceride-Glucose Index in Hospitalized Patients with Type 2 Diabetes. J Diabetes Res. 2021;2021:8757996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Wang M, He Y, He Q, Di F, Zou K, Wang W, Sun X. Comparison of clinical characteristics and disease burden between early- and late-onset type 2 diabetes patients: a population-based cohort study. BMC Public Health. 2023;23:2411. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Zhou X, Zhang C, Wu J, Zhang G. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract. 2013;99:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Bener A, Ağan AF, Al-Hamaq AOAA, Barisik CC, Öztürk M, Ömer A. Prevalence of Helicobacter pylori Infection among Type 2 Diabetes Mellitus. Adv Biomed Res. 2020;9:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Yao CC, Kuo CM, Hsu CN, Yang SC, Wu CK, Tai WC, Liang CM, Wu KL, Huang CF, Bi KW, Lee CH, Chuah SK. First-line Helicobacter pylori eradication rates are significantly lower in patients with than those without type 2 diabetes mellitus. Infect Drug Resist. 2019;12:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Song X, Cai C, Jin Q, Chen X, Yu C. The efficacy of Helicobacter pylori eradication in diabetics and its effect on glycemic control: A systematic review and meta-analysis. Helicobacter. 2021;26:e12781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Thai TD, Chuenchom C, Donsa W, Faksri K, Sripa B, Edwards SW, Salao K. Helicobacter pylori extract induces purified neutrophils to produce reactive oxygen species only in the presence of plasma. Biomed Rep. 2023;19:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Han L, Shu X, Wang J. Helicobacter pylori-Mediated Oxidative Stress and Gastric Diseases: A Review. Front Microbiol. 2022;13:811258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Wu YY, Hsieh CT, Tsay GJ, Kao JT, Chiu YM, Shieh DC, Lee YJ. Recruitment of CCR6(+ ) Foxp3(+) regulatory gastric infiltrating lymphocytes in Helicobacter pylori gastritis. Helicobacter. 2019;24:e12550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Zhou X, Liu W, Gu M, Zhou H, Zhang G. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J Gastroenterol. 2015;50:1027-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Martin-Nuñez GM, Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Gut Microbiota: The Missing Link Between Helicobacter pylori Infection and Metabolic Disorders? Front Endocrinol (Lausanne). 2021;12:639856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Pfeifer SP. From next-generation resequencing reads to a high-quality variant data set. Heredity (Edinb). 2017;118:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1946] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 20. | Sufyan M, Ali Ashfaq U, Ahmad S, Noor F, Hamzah Saleem M, Farhan Aslam M, El-Serehy HA, Aslam S. Identifying key genes and screening therapeutic agents associated with diabetes mellitus and HCV-related hepatocellular carcinoma by bioinformatics analysis. Saudi J Biol Sci. 2021;28:5518-5525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC, Lempicki RA. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2023] [Cited by in RCA: 1800] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 22. | Rosandić M, Paar V, Gluncić M, Basar I, Pavin N. Key-string algorithm--novel approach to computational analysis of repetitive sequences in human centromeric DNA. Croat Med J. 2003;44:386-406. [PubMed] |
| 23. | Liu S, Ren W, Yu J, Li C, Tang S. Identification of Hub Genes Associated with Diabetes Mellitus and Tuberculosis Using Bioinformatic Analysis. Int J Gen Med. 2021;14:4061-4072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Zhao M, Zhang Y. Identification of fibronectin 1 (FN1) and complement component 3 (C3) as immune infiltration-related biomarkers for diabetic nephropathy using integrated bioinformatic analysis. Bioengineered. 2021;12:5386-5401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Cheng KP, Yang YJ, Hung HC, Lin CH, Wu CT, Hung MH, Sheu BS, Ou HY. Helicobacter pylori eradication improves glycemic control in type 2 diabetes patients with asymptomatic active Helicobacter pylori infection. J Diabetes Investig. 2019;10:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Casanova JL, Su HC; COVID Human Genetic Effort. A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell. 2020;181:1194-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 28. | She Y, Mangat R, Tsai S, Proctor SD, Richard C. The Interplay of Obesity, Dyslipidemia and Immune Dysfunction: A Brief Overview on Pathophysiology, Animal Models, and Nutritional Modulation. Front Nutr. 2022;9:840209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Koh GY, Rowling MJ, Pritchard SK. Possible role of type 1 and type 2 taste receptors on obesity-induced inflammation. Nutr Rev. 2022;80:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Bonde A, Daly S, Kirsten J, Kondapaneni S, Mellnick V, Menias CO, Katabathina VS. Human Gut Microbiota-associated Gastrointestinal Malignancies: A Comprehensive Review. Radiographics. 2021;41:1103-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Lock JY, Caboni M, Strandwitz P, Morrissette M, DiBona K, Joughin BA, Lewis K, Carrier RL. An in vitro intestinal model captures immunomodulatory properties of the microbiota in inflammation. Gut Microbes. 2022;14:2039002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Bui TI, Gill AL, Mooney RA, Gill SR. Modulation of Gut Microbiota Metabolism in Obesity-Related Type 2 Diabetes Reduces Osteomyelitis Severity. Microbiol Spectr. 2022;10:e0017022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Rong Z, Huang Y, Cai H, Chen M, Wang H, Liu G, Zhang Z, Wu J. Gut Microbiota Disorders Promote Inflammation and Aggravate Spinal Cord Injury Through the TLR4/MyD88 Signaling Pathway. Front Nutr. 2021;8:702659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Noori MS, Courreges MC, Bergmeier SC, McCall KD, Goetz DJ. Modulation of LPS-induced inflammatory cytokine production by a novel glycogen synthase kinase-3 inhibitor. Eur J Pharmacol. 2020;883:173340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Lu Z, Zhang X, Li Y, Lopes-Virella MF, Huang Y. TLR4 antagonist attenuates atherogenesis in LDL receptor-deficient mice with diet-induced type 2 diabetes. Immunobiology. 2015;220:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Yuan Y, Lu L, Bo N, Chaoyue Y, Haiyang Y. Allicin Ameliorates Intestinal Barrier Damage via Microbiota-Regulated Short-Chain Fatty Acids-TLR4/MyD88/NF-κB Cascade Response in Acrylamide-Induced Rats. J Agric Food Chem. 2021;69:12837-12852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 37. | Devaraj S, Tobias P, Jialal I. Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine. 2011;55:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 38. | Ekuni D, Yoneda T, Endo Y, Kasuyama K, Irie K, Mizutani S, Azuma T, Tomofuji T, Morita M. Occlusal disharmony accelerates the initiation of atherosclerosis in apoE knockout rats. Lipids Health Dis. 2014;13:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Westerbacka J, Cornér A, Kolak M, Makkonen J, Turpeinen U, Hamsten A, Fisher RM, Yki-Järvinen H. Insulin regulation of MCP-1 in human adipose tissue of obese and lean women. Am J Physiol Endocrinol Metab. 2008;294:E841-E845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Li L, Wei T, Liu S, Wang C, Zhao M, Feng Y, Ma L, Lu Y, Fu P, Liu J. Complement C5 activation promotes type 2 diabetic kidney disease via activating STAT3 pathway and disrupting the gut-kidney axis. J Cell Mol Med. 2021;25:960-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Liu S, Wang C, Yang H, Zhu T, Jiang H, Chen J. Weighted gene co-expression network analysis identifies FCER1G as a key gene associated with diabetic kidney disease. Ann Transl Med. 2020;8:1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Mehrbod P, Eybpoosh S, Farahmand B, Fotouhi F, Khanzadeh Alishahi M. Association of the host genetic factors, hypercholesterolemia and diabetes with mild influenza in an Iranian population. Virol J. 2021;18:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Casanova JL. Human genetic basis of interindividual variability in the course of infection. Proc Natl Acad Sci U S A. 2015;112:E7118-E7127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 44. | Qi Z, Yan F, Chen D, Xing W, Li Q, Zeng W, Bi B, Xie J. Identification of prognostic biomarkers and correlations with immune infiltrates among cGAS-STING in hepatocellular carcinoma. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 45. | Gajovic N, Jurisevic M, Pantic J, Radosavljevic G, Arsenijevic N, Lukic ML, Jovanovic I. Attenuation of NK cells facilitates mammary tumor growth in streptozotocin-induced diabetes in mice. Endocr Relat Cancer. 2018;25:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Restrepo BI, Twahirwa M, Rahbar MH, Schlesinger LS. Phagocytosis via complement or Fc-gamma receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. PLoS One. 2014;9:e92977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Srinontong P, Wandee J, Aengwanich W. Paraquat modulates immunological function in bone marrow-derived macrophages. Acta Vet Hung. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Chao WC, Yen CL, Wu YH, Chen SY, Hsieh CY, Chang TC, Ou HY, Shieh CC. Increased resistin may suppress reactive oxygen species production and inflammasome activation in type 2 diabetic patients with pulmonary tuberculosis infection. Microbes Infect. 2015;17:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Perner A, Nielsen SE, Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. 2003;29:642-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |