TO THE EDITOR
Diabetic peripheral neuropathy (DPN) is the most frequent and early-onset chronic complication of diabetes mellitus. Patients often experience symmetrical numbness, formication, coldness, and other abnormal feelings at the distal ends of the limbs, as well as spontaneous burning pain in the limbs. Pain, which serves as a primary reason for 25% of patients diagnosed with DPN to seek medical care, intensifies after physical activity[1,2]. In severe cases, neurogenic arthropathy, ischemic gangrene, and foot ulcers are the primary causes of disability in diabetic patients. DPN can occur in the pre-diabetic stage[3], and approximately 50% of diabetes patients will eventually develop DPN[4,5]. Furthermore, previous studies have indicated that individuals with type 2 diabetes have a significantly greater risk of peripheral neuropathy, DPN, diabetic retinopathy, and hypertension than those with type 1 diabetes[6].
However, the precise mechanisms underlying DPN remain unclear. The pathogenesis of diabetes is multifactorial. Among the potential causative factors, high blood glucose levels play a central role in pathogenesis and neuronal hypoxic injury, oxidative stress, excessive activation of the polyol pathway, increased advanced glycation end products, elevated protein kinase C levels, deficiency in Y-linolenic acid, lack of growth factors, and abnormal inflammatory responses[7]. There may be undiscovered interactions between various pathogenic mechanisms, and most possible mechanisms may have complicated relationships that pose challenges in the treatment of DPN. Currently, only a few effective therapies are available for the treatment of DPN[8]. In the treatment algorithm developed based on current pain and headache reports for painful diabetic neuropathy, adequate glucose control and symptomatic pain relief remain the most important methods[9]. The strict control of blood glucose is fundamental in the management of diabetes and its complications. However, even patients with type 2 diabetes and well-controlled blood glucose levels can still experience secondary neuropathy, which demonstrates progressive deterioration over time. Pain sufferers can only be treated symptomatically, and duloxetine and pregabalin remain the first-line therapies for neuropathic pain in DPN. The effectiveness of drugs is limited, and only 1/3 of patients experience pain relief. Moreover, once neuropathic pain occurs, the reversal of neuropathic pain symptoms is very limited, even after controlling for blood sugar fluctuations[10-12]. The development of drugs specifically targeting DPN also faces obstacles, such as limited targets, high costs, and obvious side effects, which limit their application in clinical practice. For example, aldose reductase inhibitors have been found to exhibit potential adverse effects and limit the amelioration of clinical symptoms, leading to their discontinuation[13]. Therefore, there is an urgent need to explore novel therapeutic approaches for DPN.
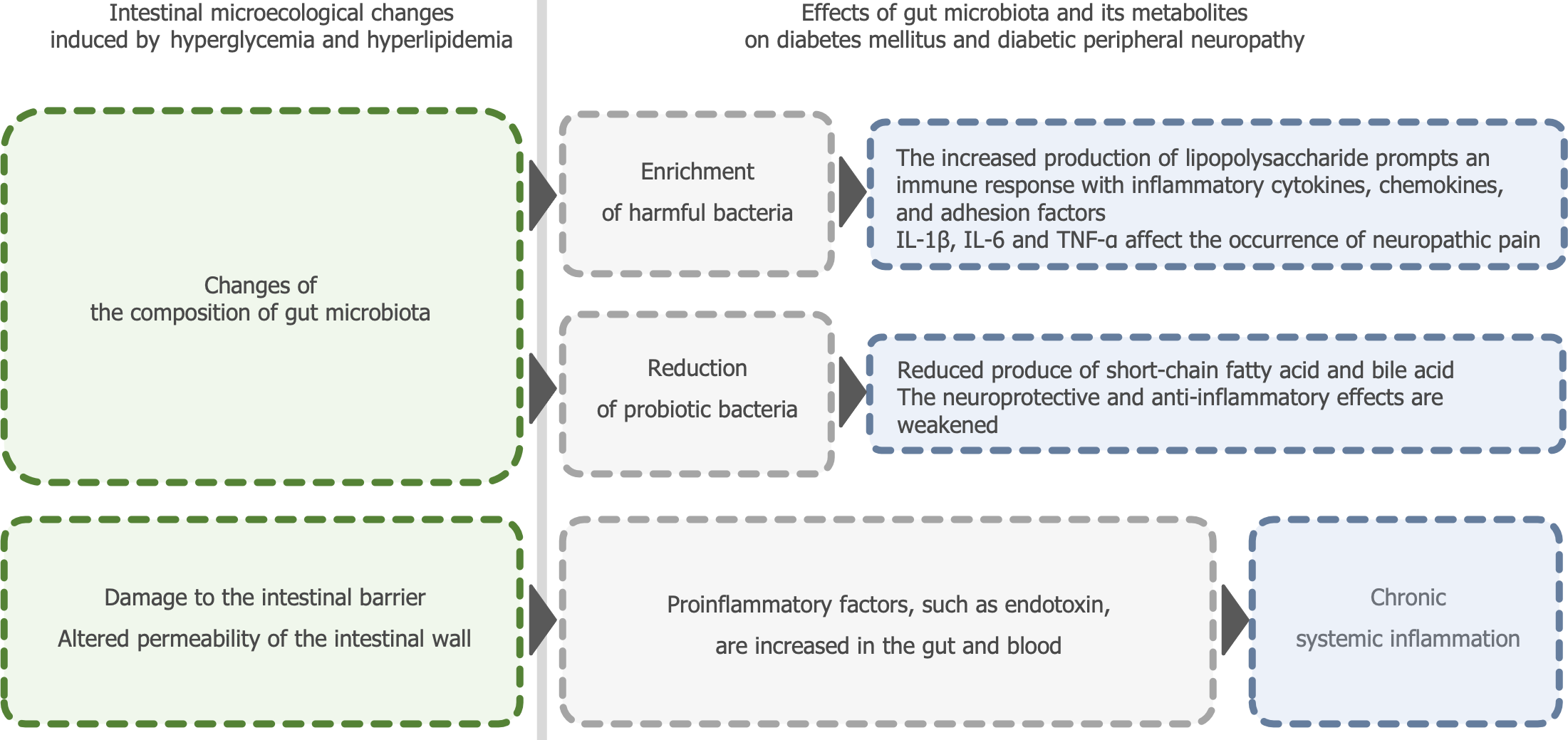
In recent years, the modulation of the gut microbiota has attracted extensive attention in the fields of inflammatory bowel disease, obesity, diabetes, and cancer[14-16]. Evidence from multiple animal models and populations has confirmed that differences in gut microbiota composition between diabetic and healthy hosts contribute to various diabetic complications[17,18]. The gut microbiota can affect patients with DPN in numerous ways (Figure 1). Studies aiming to treat DPN by modulating the gut microbiota through the use of probiotics, fecal microbiota transplantation (FMT), or adjusting diet have accumulated preclinical evidence[19]. Li et al[20] provided new insights into the effects of traditional Chinese medicine (TCM) on DPN by modulating the gut microbiota. They reviewed and summarized several articles on the treatment of diabetes and its complications using TCM compounds and found that several TCM compounds, such as Shenqi Dihuang Decoction, Huangkui Capsules, and Qidi Tangshen Granules, can modulate the gut microbiota, thereby reducing serum total bile acid levels, mediating immune responses, regulating and promoting the release of various signaling factors, and ultimately facilitating the recovery of DPN. Based on these studies, the authors propose that using TCM to regulate the types and quantities of gut microbiota to achieve balance and diversity in the gut microbiota may be a potential strategy to alleviate the clinical symptoms of DPN.
Figure 1 Effects of gut microbiota and its metabolites on diabetes mellitus and diabetic peripheral neuropathy.
IL: Interleukin; TNF-α: Tumor necrosis factor-alpha.
THE DIVERSE INTERACTIONS OF TCM AND GUT MICROBIOTA
Considering the complexity of the pathological mechanisms of DPN, multi-component, multi-target, and multi-pathway TCMs are promising drug candidates for the treatment of DPN. TCM, one of the oldest healing systems, can be used to treat diseases by regulating the overall internal environment of the human body through herbal medicine formula, acupuncture, moxibustion, and massage. TCM is characterized by its personalized and holistic nature, emphasizing the comprehensive assessment of the patient's overall health status and conducting tailored treatment strategies that align with the individual's unique characteristics. Therapies targeting gut microbiota have emerged as viable options for the treatment of diabetes and its associated complications. TCM is primarily administered orally, so its natural compounds can interact extensively with the gut microbiota[21]. Increasing evidence suggests that the gut microbiota plays a key role in TCM therapy via complex interactions with TCM components. The enrichment of beneficial gut microbes and the reduction of harmful gut microbes preceded the amelioration of disease symptoms, suggesting that the restoration of the gut microbiota balance may promote symptom improvement rather than being a mere consequence thereof[22]. The possible interactions can be summarized into the following three aspects[19,23,24]. Metabolites derived from TCM undergo biotransformation by the intestinal microbiota, resulting in altered bioavailability and varying levels of bioactivities/toxicities compared to their original forms. Some herbal ingredients, such as resveratrol, epigallocatechin gallate, and isoflavones (daidzin, genistin, glycitin) can be converted to more active forms by the metabolism of the gut microbiota[25]. TCM compounds contribute to the regulation of the gut microbiota composition, thereby mitigating gut microbiota dysfunction and associated pathological conditions. TCM shows an inhibitory effect on opportunistic or potential pathogenic bacteria in the gut of preclinical models of chronic kidney disease[26]. TCM can also exert prebiotic effects by selectively stimulating the growth of commensal probiotics. Blautia and Allobaculum, two strains that produce short-chain fatty acids (SCFAs), were specifically enhanced through the incorporation of Berberine (BBR)[27]. Moreover, the interactions, including both synergistic and antagonistic effects, among different chemical constituents in TCM are modulated by the intestinal microbiota. Substantial clinical evidence and research data have been accumulated in Chinese medicine for the treatment of DPN. Many TCMs have been extensively studied in this context, particularly their monomeric extracts, which are commonly investigated for their effects on neurological complications in diabetes[28]. Polyphenolic compounds such as curcumin and resveratrol have anti-inflammatory, antioxidant, and protective properties against cell apoptosis. Research has found that curcumin can inhibit the expression of IL-1β and caspase-3 induced by high glucose in Schwann cells. It also significantly reduces the expression of NF-κB and p-NF-κB proteins, indicating that it may protect Schwann cells from apoptosis by inhibiting the NF-κB pathway to alleviate inflammatory reactions[29]. Resveratrol has anti-inflammatory and antioxidant effects. In rat experiments, resveratrol can protect rats’ nerves by improving nerve conduction velocity, increasing cerebral blood flow, lowering serum malondialdehyde, tumor necrosis factor-alpha, interleukin (IL)-6, and cyclooxygenase-2 levels, and reducing lipid peroxidation[30]. Studies have demonstrated that TCM alleviates diabetes symptoms by maintaining homeostasis of the gut microbiota. TCM treatments enhance the abundance of SCFA-producing bacteria and anti-inflammatory bacteria in the guts of patients with diabetes[23]. BBR effectively inhibits the activity of Ruminococcus bromii (R. bromii), a crucial intestinal bacterium involved in converting primary bile acids (BAs) into secondary bile acids, particularly in deoxycholic acid (DCA) biosynthesis. By suppressing the metabolic activity of R. bromii, BBR reduced DCA production, which was associated with a significant decrease in glycated hemoglobin (HbA1c), a key indicator of improved blood glucose control[30]. Jinmaitong, a TCM compound specifically designed for DPN, has shown neuroprotective effects by regulating the gut microbiota and neuregulin 1 level in DPN rats[31]. However, it is imperative to acknowledge that while these TCM extracts and compounds can serve as effective adjunctive treatment regimens for anti-diabetic drugs in patients with DPN, they cannot entirely substitute hypoglycemic agents in clinical practices. The incidence and severity of diabetic neuropathy are positively correlated with the duration of hyperglycemia and the level of blood glucose. Diabetic microangiopathy induced by hyperglycemia persists and is irreversible, even if blood glucose levels are subsequently maintained within the normal range. Therefore, early and positive glycemic control remains of significant importance. The absence of relevant literature on the adverse effects also represents a significant limitation of TCM in the management of DPN[32]. This underscores the necessity of compiling and analyzing adverse reactions associated with TCM treatments, implementing appropriate control measures, and harnessing their pleiotropic effects to establish TCM as a promising candidate for the prevention and treatment of DPN.
In addition to herbal medicine, acupuncture, moxibustion, and acupuncture have also shown effective treatment outcomes in some cases of DPN[23]. Wang et al[34] observed that electrical acupuncture could improve neuropathic hypersensitivity and reduce pro-inflammatory cytokines in a rat model of type 2 diabetes mellitus (T2DM) induced by low-dose streptozotocin and a high-fat diet.
GUT MICROBIOTA RESEARCH IS EXPECTED TO PARTICIPATE IN THE ENTIRE PROCESS OF DISEASE PREVENTION, DIAGNOSIS, AND TREATMENT OF DPN
Although significant progress has been made in understanding the intricate interactions between the gut microbiota and the host, we are still in the early stages of elucidating the direct role of intestinal bacteria in the prevention, diagnosis, and management of DPN. Further studies, such as fecal transplantation of "pathogenic bacterias" or "probiotics" in germ-free animal models, are needed to identify and ascertain the gut microbiota closely associated with the occurrence and development of DPN, explore the interaction mechanism between the gut microbiota and DPN, to help doctors and scholars to assess the risk of DPN during the entire course of diabetes dynamically, form a more precise and personalized treatment plan for DPN. Furthermore, it will enable a more accurate investigation of TCM's therapeutic mechanisms and the evaluation of its efficacy in clinical experiments.
DPN presents without noticeable symptoms in its initial phases. However, once it begins to exhibit symptoms and significant impairment, the process is irreversible. It is crucial to promptly diagnose DPN and intervene to prevent its escalation and deterioration, creating the demand for new treatments and dependable biomarkers that can track the emergence and development of early neuropathic alterations characteristic of DPN[13]. Benn et al[35] studied the correlation between the risk of peripheral neuropathy and biomarkers traditionally regarded as markers of heart disease, diabetes, and kidney disease. High-sensitivity cardiac troponin T, NT-proBNP, fasting blood glucose, and HbA1c levels are correlated with peripheral neuropathy and are expected to become biomarkers for predicting peripheral neuropathy. Evidence from specific cases indicates that disruptions in the gut microbiota can be observed several years before disease onset. These disruptions provide biomarkers for early detection of disease risk and the possibility of preventive interventions[15]. This highlights the potential significance of gut microbiota in the initial diagnosis of DPN.
In the past, the monitoring endpoints of most TCM treatment studies on DPN were mainly improvement of clinical symptoms, lacking systematic studies on the pathogenesis and pathology. Many studies have demonstrated the interaction between gut microbiota and diabetes and its complications. In the future, the regulatory effect of TCM on the gut microbiota may become an observable indicator for evaluating the therapeutic effect of TCM in the treatment of DPN. In particular, when most chemical substances in certain TCM compounds or formulas have no biological activity or bioavailability, exploring the role of TCM in regulating the gut microbiota can better explain the mechanism of the effect of TCM[36]. Owing to individual biological variations, differences between animal and human experiments, and the potential impact of drugs taken by the tested individuals on the gut microbiota, there is still a long way to go to fully comprehend the gut microbiota. Animal and in vitro experiments have identified certain gut microbiota that may be associated with DPN. Parabacteroides can participate in the management of metabolic diseases, particularly those related to glycolipid metabolism. They can transform bile acids and improve metabolic health by producing succinic acid and affecting secondary bile acid levels[37]. Bifidobacteria, a well-known probiotic, produce SCFA such as acetic acid, propionic acid, and butyric acid. These SCFA are negatively correlated with low-grade inflammation and insulin resistance and help maintain intestinal health and regulate the host's immune response[6]. Akkermansia decomposes mucin in the intestinal mucus layer, helping to maintain the integrity of the intestinal barrier, and is also associated with reducing inflammation and improving metabolic health[33]. Bacteroides vulgatus and Bacteroides dorei maintain intestinal barrier integrity and reduce lipopolysaccharide production by upregulating tight junction expression[37]. The genus of Lactobacillus is positively associated with T2DM, but some species have anti-inflammatory properties. For example, lactic acid bacteria can induce the production of the anti-inflammatory cytokine IL-10, which helps improve insulin sensitivity in muscles[38]. Gut microbiota has been applied to disease treatment. For instance, gut microbiota detection is used for dynamic assessment of the efficacy of tumor immunotherapy. Preliminary clinical trials have shown that transferring fecal microbiota from patients who respond to anti-PD1 therapy to resistant patients can overcome primary and acquired resistance to immune checkpoint blockade treatment[39,40]. Similar attempts in the field of DPN are also worthy of research on the gut microbiota.
The oral microbiome, as the initial segment of the digestive system, is gaining recognition for its significance and its connection to the gut microbiome[41]. Because multiple dental pathogenic bacteria in the saliva of patients with periodontitis are significantly enriched, researchers hypothesize that these bacteria may enter the intestine through saliva and disrupt intestinal microbial homeosta[42]. Researchers found that when Porphyromonas gingivalis, a periodontal pathogen, was administered orally to mice, it caused changes in their intestinal microbiota, systemic inflammation, and insulin resistance[43]. Further investigation is needed to understand the interactions between the oral microbiome linked to periodontal disease and the gut microbiome, identify which oral bacteria are crucial in this interaction, and explore how alterations in these microbial communities influence diabetes development.
CONCLUSION
The applications of TCM in the prevention and treatment of DPN have emerged as a significant research focus for diabetes-related microvascular complications. Adjusting the intestinal microbiota using TCM may be an effective strategy to alleviate the clinical symptoms of DPN. As research on the intestinal microbiota continues to progress, it is potentially valuable to diagnose DPN through intestinal microbiota, analyze the mechanisms of action of Chinese medicine, and evaluate the efficacy of Chinese medicine through modulating intestinal microbiota in the study of DPN and TCM. By conducting 16S rRNA gene sequencing, a technique that can accurately identify the gut microbiota at the genus level, on the fecal microbiota of patients with diabetes or prediabetes may offer valuable insights to clinicians and researchers regarding the progression of diabetes and the efficacy of TCM treatment by showing the increase of probiotics and the reduction of harmful bacteria. To make accurate predictions, it is essential to perform FMT experiments in germ-free animal models and conduct large-scale cohort studies in humans to observe changes in both gut microbiota composition and host physiology. This will help determine the bacteria most closely associated with diabetes and its complications, such as Bacteroidetes, Firmicutes, and Bifidobacterium. Moreover, employing deep sequencing whole-genome metagenomics technology combined with strain-level analysis can facilitate the identification of disease-related strains and their functions[44]. These findings will further explore the causal relationship between gut microbiota and diabetes, leading to the development of targeted therapeutic strategies.
ACKNOWLEDGEMENTS
Anonymous reviewers provided helpful and constructive comments that greatly improved the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C, Grade D
Novelty: Grade B, Grade B, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade C
P-Reviewer: Altarawneh HB; Casu C; Cheng G S-Editor: Liu H L-Editor: A P-Editor: Chen YX