TO THE EDITOR
The obesity tsunami has devastated the global healthcare budget in the recent years with a wide range of adiposity-related adverse health consequences including type 2 diabetes mellitus (T2DM), hypertension, metabolic dysfunction-associated fatty liver disease, obstructive sleep apnoea (OSA), polycystic ovary syndrome and a variety of obesity associated cancers[1,2]. Obesity and excess adiposity are associated with marked reduction of the quality-adjusted life years and the average life expectancy in the victims unless timely therapeutic interventions are enforced including lifestyle changes, pharmacotherapy and/or bariatric procedures.
Although bariatric interventions are the best management strategy associated with best therapeutic benefits in individuals with obesity, these surgical procedures are not simple and easy for everyone with the disease. Lack of wide availability in many clinical settings, high costs associated with the procedures, immediate and long-term complications associated with these interventions and poor acceptability by some of the patients are some of the constraints of bariatric interventions in day-to-day clinical practice[3,4]. It is important to assess the benefits and potential barriers to various bariatric interventions to appraise the current evidence for making informed clinical practice decisions between healthcare providers and patients, the theme of this editorial on a clinical review by He et al[5] published in a recent issue of World Journal of Diabetes.
Types of bariatric interventions and the metabolic implications
Bariatric procedures can be broadly classified into surgical procedures (bariatric or metabolic surgery) and endoscopic interventions (bariatric endoscopy). The decision regarding the appropriateness of these procedures depends on multiple factors such as the degree of intended weight loss, patients’ choice, the comorbidities to be addressed, and the surgical fitness of the patient for the individual procedure[3].
Surgical interventions
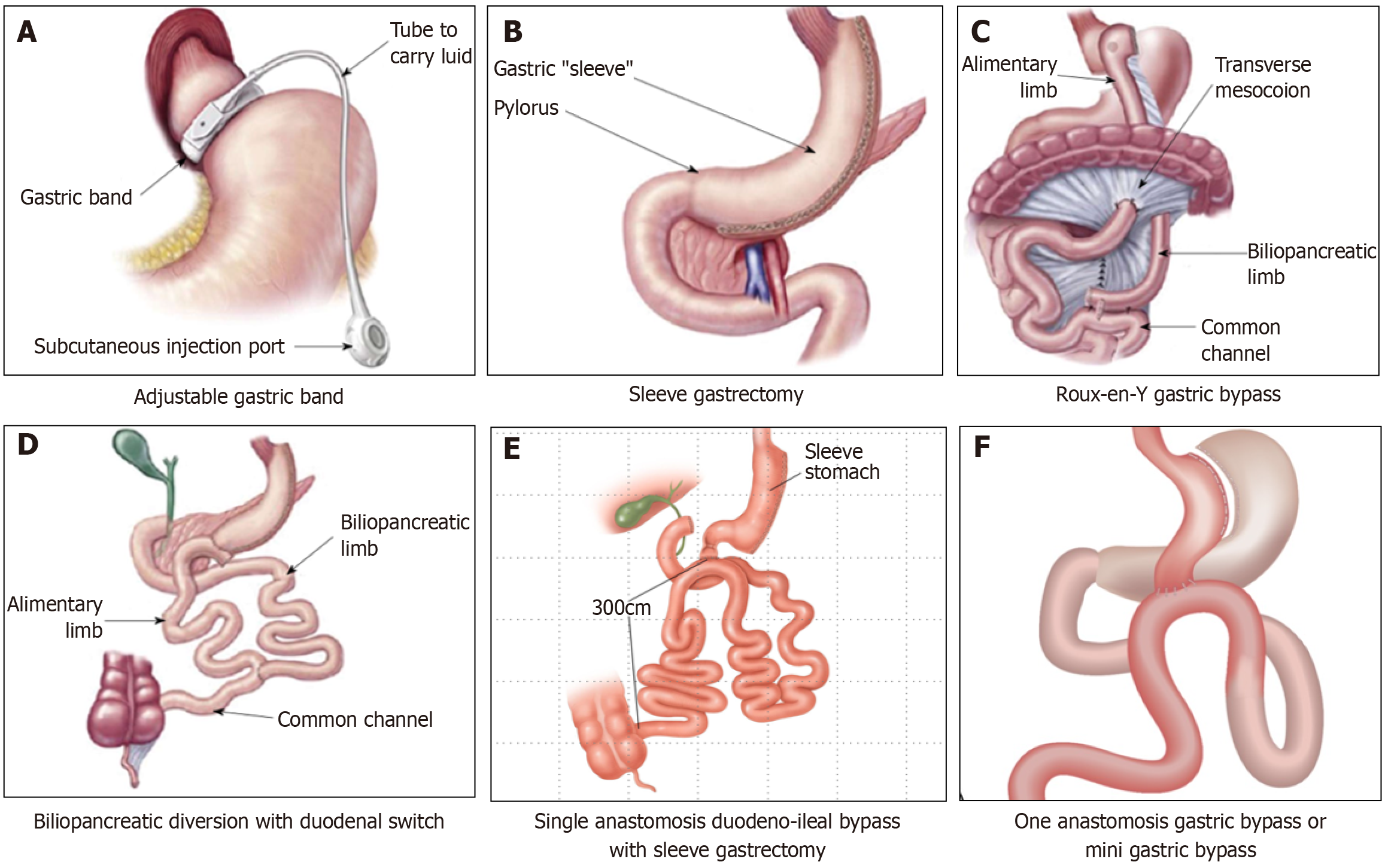
The surgical procedures can be subdivided into restrictive, malabsorptive and combined procedures[3,4]. Again the choice of the procedures depends on the factors mentioned above. One of the earliest surgical procedures among these was the placement of a laparoscopic adjustable gastric band (LAGB). Because of the relatively poor weight loss outcomes and complications with this surgery, LAGB is not favored by most center currently and not discussed here.
Sleeve gastrectomy: In this restrictive procedure, about 80% of the stomach is removed usually on the side of greater curvature to convert the stomach into a sleeve-like tube to reduce the storage capacity of the gastric reservoir[3-7]. The weight loss is not only from the reduced food portion size consumed by the patient, but also because of various hormonal changes including ghrelin, the hunger hormone mainly produced from the greater curvature of stomach. Both laparoscopic and robotic sleeve gastrectomy (SG) are associated with comparable efficacy and safety outcomes as shown by a recent systematic review[7]. However, longer procedural time, duration of hospitalization, and expenditure were the disadvantages of robotic SG which are expected to come down with further refinement of these procedures and more experience in future. Bleeding, leak from the anastomotic site, and gastro-oesophageal reflex disease (GORD) are the major adverse complications associated with SG. Mean percentage long term weight loss observed with SG was −18.67% (95%CI: −27.53%−9.81%) and T2DM remission was 42% (95%CI: 29%–56%) in a recent meta-analysis[6].
Roux-en-Y gastric bypass: This malabsorptive procedure results in nutrient deficit resulting in higher weight loss compared to SG as the proximal small gut from which most of the nutrient absorption occurs is bypassed. The mean percentage long-term weight loss and diabetes remission of −25.37% (95%CI: −28.88%−21.87%) and 47% (95%CI: 36%–59%) were observed in patients receiving this procedure[6]. However, higher short-term complications (15%) compared to SG (9%) were encountered in the Roux-en-Y gastric bypass (RYGB) group.
Biliopancreatic diversion with duodenal switch: This surgical technique is somewhat similar to RYGB but bypasses approximately 75% of the proximal small intestine resulting in much higher weight loss and multi-nutrient deficit[3,4]. Mean loss of excess body weight of 75%–80% on long term follow up[8], and mean T2DM remission in 54.12% at 3 years[9], were observed in patients. Because of the profound multi-nutrient deficits and extremely profound weight loss, this method is not often preferred by most patients and bariatric experts[10]. Therefore, the procedure may now be considered only for those patients with extreme obesity.
Stomach intestinal pylorus sparing surgery: This is a relatively new technique with modifications in the original duodenal switch procedure to reduce the complication rate. In a retrospective analysis of 123 patients undergoing stomach intestinal pylorus sparing surgery (SIPS) at two center, by the end of one year, there was a mean reduction of body mass index (BMI) by 19 units kg/m2, equaling almost 38% of total weight loss and 72% of excess weight loss (EWL)[11]. In a more recent prospective study on 185 patients undergoing SIPS, the mean weight loss at the end of 12 months was 35.6%, amounting to a weight reduction of 51.3 kg and BMI reduction of 17.8 kg/m2 which were maintained for all the patients who completed 24-months follow-up as well. Resolution of OSA, hypertension, T2D, and dyslipidemia were seen in 59.2%, 32.7%, 93.1%, and 87.6% respectively, at 12 months and maintained at 24 months. Complications were rare and not serious and nutritional deficiencies were not seen[12]. Also, a comparative retrospective matched cohort analysis of SIPS vs BPDDS showed similar weight loss with both procedures with lesser complication rates with SIPS vs the latter[13] (Figure 1).
Figure 1 Schematic representation of different surgical bariatric surgical procedures.
A: Adjustable gastric band; B: Sleeve gastrectomy; C: Roux-en-Y gastric bypass; D: Bilio-pancreatic diversion with duodenal switch; E: One-anastomosis gastric bypass; F: Single anastomosis duodeno-ileal bypass with sleeve gastrectomy.
Bariatric endoscopy
These procedures can be restrictive, malabsorptive or endoscopic revision procedures after previous bariatric surgery–for various reasons like weight regain, correction of complications of previous surgery or additional interventions to boost the benefits of previous surgery.
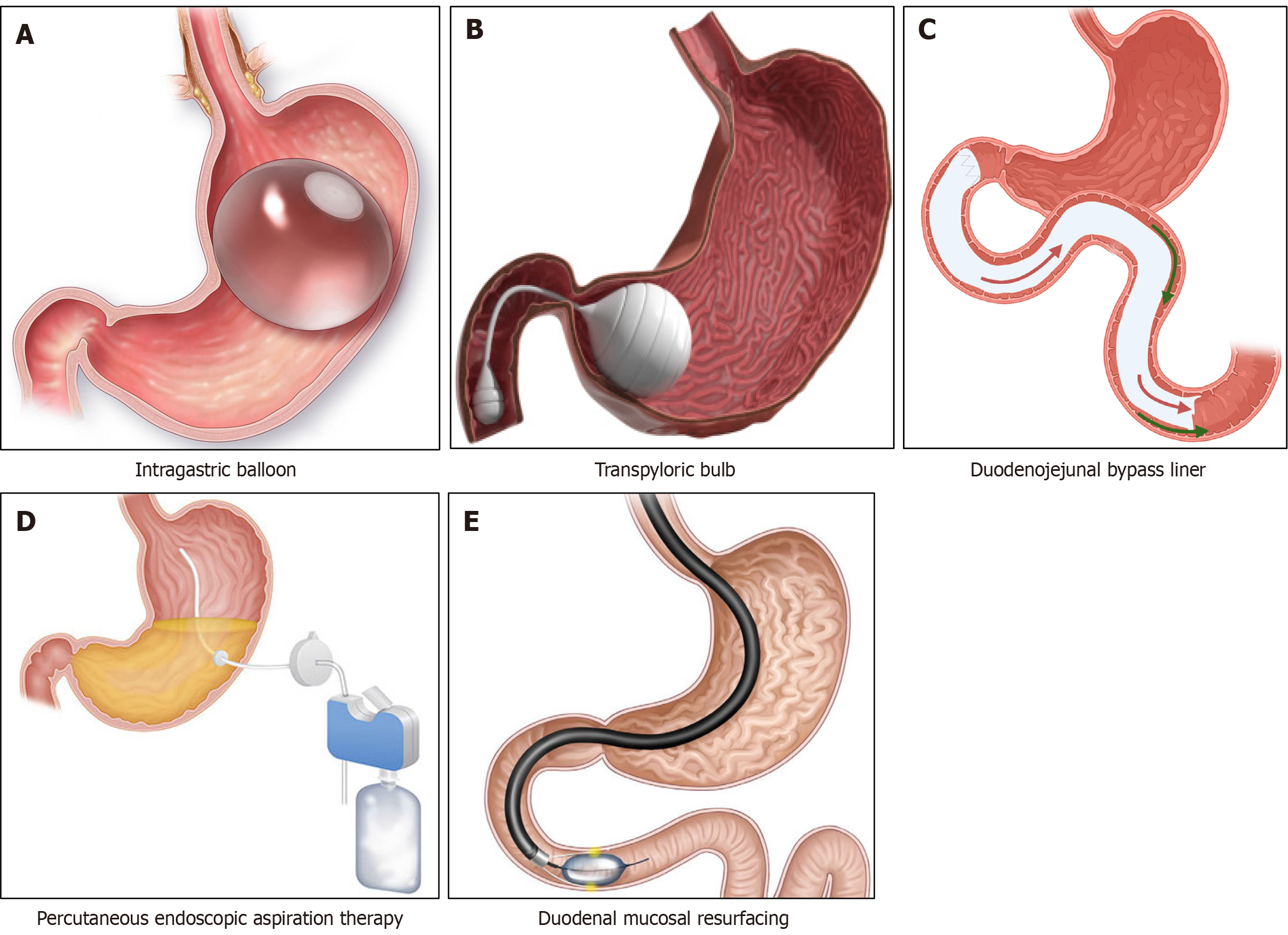
Intragastric balloon: By inserting saline/gas-filled silicon balloon(s) into the stomach, the capacity of the organ can be restricted, and the patient feel fullness after eating minimal amount of food. Intragastric balloon (IGB) also increases the gastric emptying time and results in significant weight loss depending on the gastric volume occupied by the IGB device[14]. Contraindications for use of IGB include gastric/oesophageal ulcers/varices, large hiatus hernia (> 5 cm), previous gastric surgery and anticoagulation therapy. The balloon should be removed at 6-12 months after insertion and patients often regain weight after IGB removal[14]. A recent meta-analysis of randomized controlled trials (RCTs) showed excess body weight loss of 17.11% (95%CI: 9.5%–24.8%) compared to lifestyle interventions and total body weight loss (TBWL) of 6.37% (3.4%–9.4%) at 6 months and TBWL of 4.13% (3.4%–4.9%) at 12 months[15]. Although there is not enough data on diabetes remission based on RCTs with the IGB use, an improvement of T2DM and other metabolic parameters are expected with significant weight loss. IGB is often used as a bridging procedure in morbidly obese individuals with very high BMI prior to a major bariatric procedure to improve the surgical outcomes[16].
Transpyloric shuttle: This device contains one large and a small bulb, made of silicon, connected by a tether placed endoscopically in the stomach for 12 months[17]. The gastric peristalsis after food intake propels the small bulb toward the pylorus which causes temporary gastric outlet obstruction, and delays food transit to duodenum, thereby causes calorie restriction. Patients treated with transpyloric shuttle (TPS) had a 9.3% TBWL, and 40% of them achieved ≥ 10% of TBWL (vs 14% in controls) at 12 months, making this an attractive option for people with obesity not willing for bariatric surgery or as an interim procedure in those with morbid obesity prior to definitive surgery[14,17]. Although there is not enough data on diabetes remission or improvement, improvements in metabolic parameters including T2DM is expected in patients with TPS. Abdominal discomfort was the main adverse event requiring early device removal in approximately 10% of patients[14].
Endoscopic gastroplasties: The stomach capacity is reduced in these procedures as in surgical SG through various methods such as suturing/stapling of the stomach endoscopically[14,17]. Significant improvements in short term and long-term weight loss and metabolic co-morbidities were seen in patients undergoing these procedures. A mean TBWL of 15.9%, with 90% of participants maintaining 5% TBWL and 61% maintaining 10% TBWL, was observed at 5 years in a recent long-term prospective study involving 216 patients[18]. A recent systematic review and meta-analysis showed that resolution of metabolic comorbidities such as T2DM, hypertension, dyslipidemia and OSA occurred in 55.4%, 62.8%, 56.3% and 51.7% respectively (in four studies involving 480 participants)[19].
Aspiration therapy: In this technique, a percutaneous endoscopic gastrostomy tube placed in the stomach that connected to an external device for aspiration of approximately 30% of the gastric contents after every meal resulting in caloric restriction[14,17]. The device (Aspire Assist® System; Aspire Bariatrics, Inc. King of Prussia, PA, United States) has Food and Drug Administration approval for use in patients with class II and III obesity. The mean weight loss associated with this technique was found to be 10.4% (95%CI: 7.0%-13.7%) in a meta-analysis[20]. Although data on remission/improvement of metabolic co-morbidities is not available based on RCTs, long term observational studies, significant improvement of these are expected with weight loss of > 10%.
Duodenal mucosal resurfacing: This is a catheter-based hydrothermal ablation technique for the duodenal mucosa between the ampulla of Vater and ligament of Treitz to reduce secretion of incretin hormone, gastric inhibitory polypeptide (GIP) which increases insulin resistance and is primarily designed for treatment of T2DM[14]. Duodenal mucosal resurfacing (DMR) was found to be associated with improvements in HbA1c, fasting plasma glucose and hepatic steatosis in a recent systematic review[21]. The study also concluded that 86% patients could discontinue insulin use when treated with newer antidiabetic agents after DMR. Abdominal discomfort and hypoglycemia were reported in 18% and 7.7% respectively in the first 30 days after DMR[14].
Duodenal-jejunal bypass liner: Placement of this device causes malabsorption of dietary contents from the proximal gut where maximum absorption of both micro- and macro-nutrients occurs in human beings. Popularized for clinical use in the brand name Endo Barrier® (GI Dynamics, Boston, MA, United States), is a 60 cm long flexible Teflon-coated tube, placed endoscopically and anchored to the duodenal bulb. The duodenal-jejunal bypass liner (DJBL) is usually removed after 12 months of placement[14]. The device works like RYGB as it prevents food items contacting the duodenal mucosal absorptive area to deliver nutrients to the proximal jejunum. A recent systematic review of 10 RCTs including 681 patients showed that DJBL use was associated with an EWL of 11.4% (95%CI: 7.75%–15.03%) with higher percentage reduction of HbA1c compared to the control group (-2.73 ± 0.5 vs -1.73 ± 0.4)[22]. Severe adverse events occurred in 19.7% of participants.
Gastroduodenojejunal bypass: This is a longer tube (120 cm) directly delivering the food items to jejunum mimicking RYGB without nutrient contact in the stomach or duodenum. This fluoropolymer sleeve (Endo Bypass System, ValenTx, Maple Grove, MN, United States) is anchored in the gastro-oesophageal junction endoscopically and removed after 12 months[14]. Although there are not many studies on the metabolic and weight loss benefits associated with this intervention, one small study showed promising results with 54% reduction in EWL at 12 months with maintenance of 30% EWL at 14 months after explanation of the device[14,23]. Improvement of metabolic comorbidities such as T2DM, hypertension, dyslipidemia with reduction of medications was observed in 70% of participants in this study.
The incisionless magnetic anastomosis system: This novel technique involves creation of an anastomosis using two octagonal magnets simultaneously by causing local tissue necrosis without any surgical incision in the intestine. These magnets are delivered into the proximal jejunum by enteroscopy and terminal ileum with colonoscopy simultaneously[14]. These magnets are usually expelled in the stool within 2 weeks once the anastomosis is completed. Incisionless magnetic anastomosis system (IMAS) results in nutrient and bile acid diversion into the ileum and thereby malnutrition and weight loss. IMAS was shown to be associated with a TBWL of 14.6% and an EWL of 40.2% in a pilot study with average participant BMI of 41 kg/m2[14,24]. An HbA1c reduction of 1.9% in T2DM and 1.0% in prediabetic patients were the metabolic benefits seen in this study. Diarrhoea and steatorrhea were the major adverse effects of IMAS (Figure 2).
Figure 2 Schematic representation of different endoscopic bariatric surgical procedures.
A: Intragastric balloon; B: Transplycoric bulb; C: Duodenojejunal bypass liner; D: Percutaneous endoscopic aspiration therapy; E: Duodenal mucosal resurfacing.
Revisional procedures after bariatric surgery
Revisional metabolic and metabolic surgery (RMBS) is often done in patients who regained weight after the initial bariatric surgical intervention or when the initial procedure gets complicated. Bariatric endoscopy is also an option for some of these revisional procedures in selected patients. Because of anatomical changes and adhesions from previous surgery, revisional procedures are more difficult with higher risk of morbidity, complications and length of hospital stay from complications[25]. The usual indications for RMBS are recurrent weight gain > 30%, suboptimal initial TBWL of < 20% or inadequate improvement of obesity-related complications and complications of surgery such as persistent GORD, abdominal pain, nausea and vomiting, strictures, fistulation, refractory marginal ulcers, internal herniation, and device-related complications[25,26].
Appropriate preoperative evaluation and correction of nutritional deficiencies should be done for optimizing RMBS outcomes[25]. After this, thorough endoscopic and imaging evaluation are done to plan the right revision surgical/ endoscopic revisional procedure. A multidisciplinary team approach involving surgeons, physicians, and nutritionists with prompt discussion with the patient regarding the targeted outcomes and potential complications should help best results for RMBS.
Bariatric interventions for specific population subgroups
Ethnic considerations: The pathobiological characteristics of adiposity-related chronic disorders are different in various ethnic subgroups therefore, the BMI cut-offs for these subgroups for consideration of bariatric procedures should be different. e.g., the BMI cut-off for defining obesity in South Asians[26] is 27.5 kg/m2 while that for Indians[27] is 25 kg/m2 because of the predominant abdominal adiposity in these populations compared to Caucasians. Although several studies and consensus statements have suggested lower cut-offs for diagnosing obesity in Asians[28], there is no clear international consensus on the BMI cut-offs for various bariatric interventions for different ethnic minorities across the globe.
Children and adolescents: Although a recent RCT[29] showed remarkable benefits of bariatric surgery among adolescents with obesity, there is not enough RCT-based evidence on the BMI cut offs for making routine recommendations for bariatric interventions among children and adolescents especially in presence of T2DM. The criteria for bariatric surgery in the pediatric age group patient are stricter than those used for adults. Usually, bariatric surgery is proposed in adolescents with BMI ≥ 35 kg/m2 with major comorbidities or in those with a BMI ≥ 40 kg/m2 (severe obesity) with minor comorbidities[30]. However, the European Society for Pediatric Gastroenterology Hepatology and Nutrition Position Statement suggested the role of bariatric surgery in adolescents with BMI ≥ 40 kg/m2 having severe comorbidities like T2DM; moderate-to-severe sleep apnea, pseudotumor cerebri or NASH with advanced fibrosis or in those with BMI ≥ 50 kg/m2 with mild comorbidities like hypertension, dyslipidemia, mild obstructive sleep apnea, gastroesophageal reflux disease, severe psychological distress, chronic venous insufficiency, urinary incontinence or obesity related arthropathies[31].
The most commonly used and recommended surgery procedure in adolescents is vertical SG which can lead to near-equivalent weight loss and resolution of co-morbidities as RYGB, with lesser adverse effects and lesser need for revision surgeries[32]. Unique challenges in adolescents undergoing bariatric surgery include a high risk for nutrient deficiency including effects on vitamin B12, thiamine, ferritin and also, chances of weight regain due to an increased risk for Loss of control with snacking and binge eating and psychologic distress[33].
Elderly individuals: The surgical risks due to various comorbidities including CVD in older individuals are the major concerns in any surgical interventions in such populations. However, advanced age alone should never dissuade offering bariatric procedures in the elderly. In fact, the clinical and metabolic outcomes, and the quality of life can be great in older individuals as noticed in the BASE Trial[34]. Therefore, bariatric intervention should be considered as an individualized care plan in the elderly with obesity and T2DM.
Bariatric surgery and pregnancy: Major elective surgeries are usually avoided during pregnancy and the immediate peripartum period. For this reason, bariatric interventions are avoided during, immediately prior and after pregnancy. Bariatric procedures among females in the fertility age group were found to be associated with beneficial effects in reducing the incidence of gestational diabetes mellitus, hypertension, large for gestational age (LGA), fetal macrosomia, and post-term birth in a recent systematic review[35]. However, there were also detrimental effects such as higher incidence of maternal anemia, perinatal mortality, preterm birth, neonatal intensive care unit admission, intrauterine growth restriction, congenital anomalies, and small-for gestational age babies. Therefore, females in the childbearing age-group should be appropriately counselled and properly monitored after surgery.
Type 1 diabetes mellitus: Management of obesity can be a real challenge in patients with type 1 diabetes mellitus (T1DM) with the development insulin resistance with a proportionately higher insulin requirement which can worsen their diabesity in a vicious circle[36]. Bariatric procedures in appropriately selected T1DM patient can be associated with significantly better metabolic outcomes and quality of life[37,38]. Patients must be carefully monitored for hypoglycemia, and microvascular complications which can get worse with rapid improvement of HbA1c levels in some of these patients.
Newer medications in managing obesity and T2DM
Recent studies using glucagon-like insulinotropic peptide-1 (GLP) receptor agonists such as semaglutide and GLP-1/GIP co-agonist like Tirzepatide showed remarkable weight loss benefits, sometimes at par with bariatric interventions at least in a subgroup of patients[36,39]. Identifying these individuals and prompt use of these medications might help in avoiding the invasive bariatric procedures in near future in this cohort.
Although bariatric procedures offer marked metabolic benefits with improvements in comorbidities such as hypertension, OSA, osteoarthritis, and quality of life, there are a lot of challenges in executing these in our day-to-day clinical practice. A clinical review by He et al[5] in the recent issue of World Journal of Diabetes address most of these challenges to enable clinicians to make better decisions for their practice. The authors concisely discussed the issues related to bariatric surgery in the elderly and pediatric age groups, special populations such as T1DM, people with T2DM and normal weight, those non-diabetics, immunodeficient and cancer patients, problems of weight regain and diabetes after surgery, optimal timing for surgery, selection of the appropriate candidates likely to get maximum benefits, and the reactive hypoglycemia and its management in this reasonably comprehensive review.
However, the authors did not visit several important issues such as revision surgery after initial bariatric procedures, advantages and disadvantages of bariatric endoscopy, ethnic-specific BMI cut offs for surgery, pregnancy planning and outcomes, cost implications of procedures in various economic settings including developing and developed countries, and emerging role of new medical management options for weight loss such as GLP-1/GIP co-agonists like Tirzepatide in avoiding bariatric procedures in at least some of the candidates with obesity and diabesity. Authors did not elaborate on the challenges and benefits in combining bariatric endoscopy prior to definitive surgery in some patients with morbid obesity. These limitations are understandable as the topic He et al[5] discussed is such a vast area of scientific research with a lot of uncertainties.