Published online Nov 15, 2024. doi: 10.4239/wjd.v15.i11.2245
Revised: September 22, 2024
Accepted: September 25, 2024
Published online: November 15, 2024
Processing time: 89 Days and 4.6 Hours
The rising prevalence of diabetes and prediabetes globally necessitates a deeper understanding of associated complications, including glymphatic system dysfunction. The glymphatic system, crucial for brain waste clearance, is imp-licated in cognitive decline and neurodegenerative diseases like Alzheimer’s disease. This letter explores recent research on glymphatic function across different glucose metabolism states. Tian et al’s study reveals significant glym-phatic dysfunction in type 2 diabetes mellitus patients, evidenced by lower diffusion tensor imaging analysis along perivascular space indices compared to those with normal glucose metabolism and prediabetes. The research also reveals a link between glymphatic dysfunction and cognitive impairment. Additional research underscores the role of glymphatic impairment in neurodegenerative diseases. These findings highlight the importance of integrating glymphatic health into diabetes management and suggest potential biomarkers for early diagnosis and targeted therapeutic interventions.
Core Tip: Integrating glymphatic system health into diabetes management can improve patient outcomes by addressing both cognitive and physiological complications. Recent studies show significant glymphatic dysfunction in type 2 diabetes mellitus patients and its correlation with cognitive decline. Identifying clinical variables influencing glymphatic function may offer biomarkers for early diagnosis and targeted inter-ventions, enhancing the management of diabetes-related cognitive impairments.
- Citation: Byeon H. Glymphatic system function in diverse glucose metabolism states. World J Diabetes 2024; 15(11): 2245-2250
- URL: https://www.wjgnet.com/1948-9358/full/v15/i11/2245.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i11.2245
The burgeoning prevalence of diabetes and its precursor, prediabetes, poses substantial public health challenges globally. Currently, about 410 million people have been diagnosed with diabetes, and an additional 32 million are considered prediabetic, therefore, there is an urgent need to understand the multifaceted complications associated with these conditions[1]. Diabetes complications manifest in various forms, affecting numerous bodily systems and functions. Among these complications, the dysfunction of the glymphatic system-a pivotal brain waste clearance mechanism-emerges as a critical area of concern, especially considering its implications for cognitive decline and the development of neurodegenerative diseases such as Alzheimer’s disease. The glymphatic system’s role in facilitating the clearance of metabolic waste from the central nervous system underscores its importance in maintaining neurological health and function. Dysfunction within this system, as observed in individuals with diabetes, poses significant implications for the accumulation of neurotoxic substances, including amyloid-beta protein, which is closely associated with the pathogenesis of Alzheimer’s disease[2].
The risk of cognitive impairment, including mild cognitive impairment and dementia, notably increases in individuals with diabetes, corroborating the connection between metabolic disorders and neurodegenerative conditions. Research findings suggest that patients with diabetes are up to 80% more likely to develop Alzheimer’s disease or exhibit glucose metabolism impairment[3]. Moreover, diabetes mellitus (DM) has been identified as a significant risk factor for dementia, including Alzheimer’s disease, due to its relationship with dysregulated energy metabolism, inflammation, and hypoperfusion, all of which detrimentally impact brain health[4].
Notably, insulin resistance, a hallmark feature of diabetes, has been implicated in contributing to the development of dementia. Insulin-signaling abnormalities not only affect cognitive function but also inhibit amyloid degradation, establishing a potential therapeutic avenue in tackling insulin resistance and enhancing insulysin activity in Alzheimer’s patients[5]. Moreover, diabetes compromise’s cerebrovascular function and alters glucose metabolism, affecting cognitive function and interacting with Alzheimer’s disease pathology[6].
Considering the glymphatic system’s role in neuroprotection, exploring therapeutic interventions aimed at enhancing its function could serve as a promising strategy for managing diabetes-induced cognitive complications. Maintaining optimal glycemic control and mitigating the vascular and metabolic derangements associated with diabetes are paramount in preserving cognitive health and curtailing the progression of neurodegenerative diseases such as Alzheimer’s disease. Therefore, this editorial aimed to investigate the potential therapeutic benefits of targeting the glymphatic system to alleviate cognitive decline in diabetic patients, highlighting the importance of integrating glymphatic health into standard diabetes care.
The glymphatic system, an intricate perivascular network, facilitates the exchange of cerebrospinal fluid (CSF) and interstitial fluid (ISF) within the brain. This network plays a crucial role in removing metabolic waste products, including amyloid-β and tau proteins, which are implicated in the pathogenesis of Alzheimer’s disease. The glymphatic pathway comprises three main components: The periarterial CSF inflow channel, the ISF outflow channel, and the astrocyte exchange channel, where aquaporin-4 on astrocyte end-feet is pivotal in facilitating this exchange.
DM, type 2 DM (T2DM), has been associated with a spectrum of cerebrovascular and cognitive complications. Hyperglycemia, a hallmark of diabetes, can induce microvascular damage, leading to neuropathy, retinopathy, and nephropathy, as well as macrovascular complications such as cardiovascular and cerebrovascular diseases. Notably, prolonged hyperglycemia can exacerbate oxidative stress, promote the formation of advanced glycation end products, and accelerate atherosclerosis, all of which can contribute to neuronal and vascular damage.
Advancements in magnetic resonance imaging, particularly diffusion tensor imaging (DTI), have facilitated the functional assessment of the glymphatic system. However, structural alterations may influence the DTI-derived parameters within the lymphatic system. To address these limitations, a novel noninvasive technique known as DTI analysis along the perivascular space (DTI-ALPS) has been developed to functionally assess the brain’s glymphatic system[7,8]. This method employs diffusion sequences to obtain water diffusivity measurements along the x, y, and z axes of the periventricular space white matter. These measurements are subsequently utilized to compute the ALPS index, which represents the DTI-ALPS.
The study conducted by Tian et al[1] employs (DTI-ALPS) to assess glymphatic function in individuals with different glucose metabolism states-normal glucose metabolism (NGM), prediabetes, and T2DM. The DTI-ALPS index, a new non-invasive marker, provides valuable insights into water movement along the perivascular space, thereby indicating the efficiency of the glymphatic system. The study by Tian et al[1] reveals that chronic hyperglycemia in T2DM significantly alters water diffusion characteristics in brain tissue, which may be associated with the accumulation of advanced glycation end products and atherosclerotic plaques. The decline in the DTI-ALPS index in T2DM patients suggests glymphatic dysfunction, is further exacerbated by the aggregation of amyloid β and tau proteins. The study also highlights the positive correlation between the DTI-ALPS index and 2-hour postprandial blood glucose (2hPG) levels in prediabetic individuals, indicating that glymphatic function remains relatively intact in managing increased blood glucose levels at this stage. Conversely, a negative correlation with glycated hemoglobin, total cholesterol, and low-density lipoprotein levels in prediabetic patients suggests that early metabolic changes may begin to impair glymphatic function. The study underscores the importance of managing physical symptoms and metabolic factors to improve glymphatic function, providing a more concrete understanding of the relationships between glymphatic function and glucose metabolism states.
Additional research by Kim et al[2] highlights that decreased glymphatic flow in Alzheimer’s disease serves as a marker of neurodegeneration associated with structural atrophy and cognitive decline, independent of cerebral amyloid deposition. Furthermore, Zhang et al[9] identify glymphatic system impairment in Alzheimer’s disease, emphasizing its associations with perivascular space volume and cognitive function. Zhong et al[10] presented a study unlocking the enigma of multiple cognitive dysfunctions linked to glymphatic impairment in early Alzheimer’s disease. Similarly, Tang et al[11] discussed the relationship between glymphatic system dysfunction and cognitive impairment in cerebral small vessel disease. This body of evidence reinforces the need for further investigation into the glymphatic system’s role in diabetes and prediabetes, particularly regarding cognitive functions and risks for Alzheimer’s disease.
Glymphatic dysfunction in T2DM: The study conducted by Tian et al[1] provides several key findings pivotal to understanding the relationship between glymphatic function and glucose metabolism states. Firstly, it was observed that glymphatic dysfunction is most pronounced in individuals with T2DM, as evidenced by significantly lower DTI-ALPS indices compared to those with NGM and prediabetes. This finding is critical as it underscores the heightened vulnerability of the glymphatic system to chronic hyperglycemia, suggesting that prolonged exposure to elevated blood glucose levels can severely impair the brain’s waste clearance mechanisms.
Secondly, the study highlighted the association between glymphatic dysfunction and cognitive impairment. Mini-mental state examination (MMSE) scores indicated that individuals with T2DM exhibit more pronounced cognitive decline compared to those with NGM and prediabetes. However, after adjusting for sex, no significant correlation was found between MMSE scores and DTI-ALPS indices, implying that the relationship between glymphatic dysfunction and cognitive impairment may be mediated through complex, multifactorial pathways that warrant further investigation.
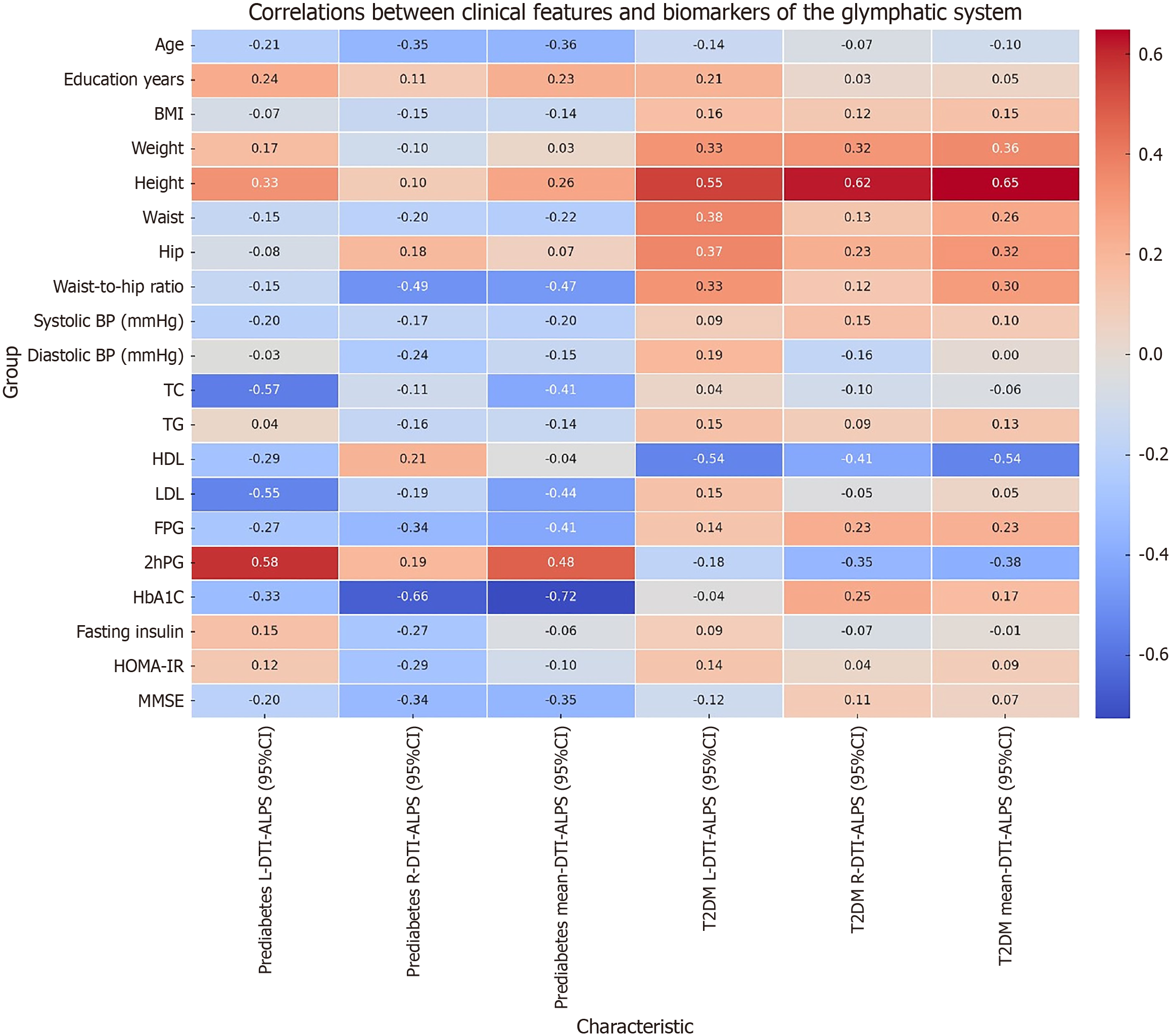
Additionally, the study pinpointed specific clinical variables that affect glymphatic function across various glucose metabolism states. Figure 1 presents the associations between clinical characteristics and glymphatic system biomarkers in prediabetes and Type 2 diabetes. In the prediabetes group, the left-side DTI-ALPS index exhibited a positive correlation with 2hPG levels and a negative correlation with total cholesterol and low-density lipoprotein levels. This suggests that early-stage glymphatic dysfunction may be influenced by fluctuating glucose levels and lipid profiles, indicating a potential window for early intervention.
In contrast, in the T2DM group, the DTI-ALPS index was positively correlated with height and negatively correlated with high-density lipoprotein levels, indicating that both anthropometric and metabolic factors play significant roles in glymphatic impairment in advanced glucose metabolism disorders.
These correlations suggest that glymphatic dysfunction in T2DM is multifactorial and may be influenced by a combination of systemic metabolic conditions and physical health metrics. Collectively, these findings not only enhance our understanding of the pathophysiological mechanisms underlying glymphatic dysfunction in diabetes but also highlight the potential for using specific clinical variables as biomarkers for early diagnosis and targeted therapeutic interventions aimed at preserving glymphatic function and mitigating cognitive decline in diabetic populations.
The findings from Tian et al’s[1] study contribute to a growing body of evidence suggesting that glymphatic dysfunction is a critical component of diabetic brain injury. By identifying specific clinical variables that influence the DTI-ALPS index, this research provides potential biomarkers for early diagnosis and monitoring of glymphatic health in individuals with impaired glucose metabolism.
Based on the findings of this study, it is imperative to develop specific recommendations for monitoring glymphatic function in diabetic patients and to propose potential therapeutic interventions. The investigation into the glymphatic system’s dysfunction within diabetic patients has illuminated significant areas of concern, particularly regarding the system’s role in neurodegenerative conditions and cognitive decline. The glymphatic system, a critical waste clearance network within the brain, is instrumental in the removal of soluble proteins and metabolites from the central nervous system, thereby maintaining cerebral homeostasis. Dysfunction within this system among diabetic patients can exacerbate the progression of neurodegenerative diseases and cognitive impairments by facilitating the accumulation of neurotoxic substances, such as amyloid-beta, implicated in Alzheimer’s disease pathology[12]. Given these associations, there arises a crucial need to establish specific recommendations for the regular monitoring of glymphatic function among diabetic populations.
The development of these recommendations demands a rigorous multidisciplinary approach, integrating insights from neurology, endocrinology, and radiology. Advanced imaging techniques, such as magnetic resonance imaging and positron emission tomography, offer potential pathways to visualize and quantify glymphatic flow and activity, enabling the early detection of glymphatic dysfunction[13]. Beyond diagnostic endeavors, the identification of glymphatic dysfunction in diabetic patients necessitates the exploration and implementation of targeted therapeutic interventions aimed at mitigating this impairment. Potential strategies may encompass the modification of lifestyle factors, such as diet and exercise, which have been shown to influence glymphatic activity positively.
Furthermore, pharmacological approaches targeting the underlying mechanisms of glymphatic dysfunction, such as inflammation and endothelial dysfunction, present promising avenues for intervention. The therapeutic modulation of sleep, an essential regulator of glymphatic clearance, through pharmacological or non-pharmacological means, could also serve as a critical component of these interventions, given the known enhancement of glymphatic flow during sleep[14].
To this end, future research must focus on elucidating the precise mechanisms underlying glymphatic dysfunction in diabetic patients and identifying modifiable risk factors conducive to therapeutic targeting. Through such efforts, it is anticipated that the development and implementation of tailored monitoring protocols and therapeutic interventions will markedly improve the neurocognitive outcomes of diabetic patients, ultimately enhancing their quality of life.
While the study[1] offers valuable insights, it also highlights the need for further research to elucidate the precise mechanisms underlying glymphatic dysfunction in diabetes. Larger, multicenter studies are essential to validate these preliminary findings and to explore the therapeutic potential of targeting glymphatic pathways in the prevention and treatment of diabetic cognitive decline and other neurological complications. This single study serves as a cornerstone for a complex, burgeoning field that is gradually unveiling the multifactorial nature of diabetes-induced cognitive decline. Understanding the role of glymphatic dysfunction in the broader context of diabetic neuropathology offers a promising avenue for developing targeted interventions. As such, harnessing DTI-ALPS and other innovative imaging techniques to visualize and quantify glymphatic function could revolutionize the approach to diagnosing and treating diabetes-associated brain disorders, making early intervention and personalized medicine a tangible reality.
The research by Tian et al[1] underscores the significance of glymphatic system health in the context of glucose metabolism disorders. By employing advanced imaging techniques, this study paves the way for a deeper understanding of the interplay between diabetes, glymphatic dysfunction, and cognitive decline. As we continue to confront the global diabetes epidemic, such insights are invaluable in guiding clinical practice and informing future therapeutic strategies.
| 1. | Tian B, Zhao C, Liang JL, Zhang HT, Xu YF, Zheng HL, Zhou J, Gong JN, Lu ST, Zeng ZS. Glymphatic function and its influencing factors in different glucose metabolism states. World J Diabetes. 2024;15:1537-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Kim YK, Nam KI, Song J. The Glymphatic System in Diabetes-Induced Dementia. Front Neurol. 2018;9:867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Lopes CM, Júnior JCdMV, Pessoa IA, Wan-Meyl FdS, Burbano R. Diabetes mellitus E A Doença De Alzheimer. Arq Catarin Med. 2018;47:159-168. |
| 5. | Sun MK, Alkon DL. Links between Alzheimer's disease and diabetes. Drugs Today (Barc). 2006;42:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Sato N, Morishita R. Brain alterations and clinical symptoms of dementia in diabetes: aβ/tau-dependent and independent mechanisms. Front Endocrinol (Lausanne). 2014;5:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, Kishimoto T, Naganawa S. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 537] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 8. | Kim M, Song YS, Han K, Bae YJ, Han JW, Kim KW. Impaired Glymphatic Flow on Diffusion Tensor MRI as a Marker of Neurodegeneration in Alzheimer's Disease: Correlation with Gray Matter Volume Loss and Cognitive Decline Independent of Cerebral Amyloid Deposition. J Alzheimers Dis. 2024;99:279-290. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Zhang X, Wang Y, Jiao B, Wang Z, Shi J, Zhang Y, Bai X, Li Z, Li S, Bai R, Sui B. Glymphatic system impairment in Alzheimer's disease: associations with perivascular space volume and cognitive function. Eur Radiol. 2024;34:1314-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 10. | Zhong J, Zhang X, Xu H, Zheng X, Wang L, Jiang J, Li Y. Unlocking the enigma: unraveling multiple cognitive dysfunction linked to glymphatic impairment in early Alzheimer's disease. Front Neurosci. 2023;17:1222857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 11. | Tang J, Zhang M, Liu N, Xue Y, Ren X, Huang Q, Shi L, Fu J. The Association Between Glymphatic System Dysfunction and Cognitive Impairment in Cerebral Small Vessel Disease. Front Aging Neurosci. 2022;14:916633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 12. | Pashkovska N. Cognitive impairment in type 2 diabetes mellitus: prospects for the use of metformin. Mìžnarodnij endokrinologìčnij žurnal. 2023;19:215-224. [DOI] [Full Text] |
| 13. | Telagamsetty DS. Type 2 diabetes mellitus and risk of Alzheimer's disease. Int J Homoeopathic Sci. 2021;5:241-245. [DOI] [Full Text] |