Published online Nov 15, 2024. doi: 10.4239/wjd.v15.i11.2189
Revised: June 10, 2024
Accepted: September 10, 2024
Published online: November 15, 2024
Processing time: 294 Days and 21.1 Hours
Diabetic retinopathy (DR), as one of the most common and significant micro
Core Tip: This article initially investigates the early pathological alterations of diabetic retinopathy (DR), with a particular emphasis on the retinal neurovascular unit (NVU) as a pivotal focal point in the initial stage of DR. Recent studies have revealed that ferroptosis may exert a significant role in the early phase of DR. Autophagy-dependent ferroptosis-related factors, including BECN1 and FABP4, could potentially serve as biomarkers for the onset and progression of DR, thereby representing promising targets for effective treatment strategies in the future. These findings provide novel insights into understanding the mechanisms underlying early pathological changes in DR and offer supplementary avenues for innovative research on treatment approaches.
- Citation: Sun WJ, An XD, Zhang YH, Tang SS, Sun YT, Kang XM, Jiang LL, Zhao XF, Gao Q, Ji HY, Lian FM. Autophagy-dependent ferroptosis may play a critical role in early stages of diabetic retinopathy. World J Diabetes 2024; 15(11): 2189-2202
- URL: https://www.wjgnet.com/1948-9358/full/v15/i11/2189.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i11.2189
The severe health threat and enormous healthcare costs associated with diabetes mellitus (DM) have become a major global public health concern. According to the International Diabetes Federation's 2021 conference, there are 537 million adults (aged 20 to 79) worldwide with DM, and it is projected that this number will rise to 784 million by 2045. Preventing and managing vascular complications is an essential aspect and ultimate goal of DM treatment. Diabetic retinopathy (DR), as one of the most common and significant microvascular complications of DM, was listed as a major cause of moderate to severe visual impairment and blindness in individuals over 50 years old in 2020[1]. In China, approximately 19.5 million DM patients have concurrent DR, with 3.8 million of them having vision-threatening DR[2]. Strict control of blood glucose, lipids, and blood pressure can partially slow down the development of DR[3]. For non-proliferative DR (NPDR), drugs such as calcium dobesilate, Qiming granules, and fenofibrate can be options[4,5]. Anti-vascular endothelial growth factor (VEGF) therapy or laser surgery are first-line treatment options for proliferative DR (PDR). While these treatments can reduce the risk of vision loss to some extent, they do not completely eliminate it, and only a few patients experience improved vision[6,7]. Additionally, the management of severe DR requires substantial medical resources, including ophthalmologists trained in laser and surgical procedures[8-10]. Despite these efforts, it is expected that the global burden of DR will remain high by 2045, with PDR continuing to be a leading cause of moderate to severe vision loss in most countries[11]. Therefore, gaining a deeper understanding of the physiology and pathology of DR in the early stages is of utmost importance for identifying potential effective drugs to halt or delay its progression, improve patients' quality of life, and reduce healthcare costs.
Although the medical community has been continuously enriching its understanding of the pathogenic mechanisms of DR, including inflammation and oxidative stress[12,13], targeted treatments for DR still fall short of effectively addressing its vision loss-related issues. Given the complexity of its associated mechanisms, it is essential to explore DR from new perspectives. Recent research indicates that characteristic neuroglial degeneration caused by DM, including reactive gliosis, decreased retinal neuron function, and neuronal apoptosis, occurs before significant microvascular lesions[14-16]. To comprehensively understand the early-stage pathological changes in DR, the retinal neurovascular unit (NVU) will become a crucial focal point for future research into the occurrence and progression of DR. Considering the disruption of the blood-retinal barrier (BRB) and the loss of NVU components, focusing on cell death will be a breakthrough in elucidating the pathogenesis of DR. Cell death encompasses various forms, such as apoptosis, autophagic cell death, and necrosis[17,18]. It was not until 2012 that Dixon et al[19] confirmed ferroptosis as a form of programmed cell death, characterized by abnormal iron metabolism resulting in lipid peroxidation and the reduced activity of the core enzyme of the antioxidant system, glutathione peroxidase 4 (GPX4)[19]. Although autophagy and ferroptosis are distinct cell death pathways in terms of mechanisms and morphology, increasing research suggests significant crosstalk between them[20,21], defining ferroptosis as an autophagy-dependent cell death mode[22,23]. Relevant studies indicate that autophagy-dependent ferroptosis mediates apoptosis in retinal NVU components, including pericytes and ganglion cells, with the primary pathological changes associated with abnormal iron metabolism and elevated levels of lipid peroxidation. By summarizing the current relationship between NVU and autophagy-dependent ferroptosis, our objective is to enhance the early-stage pathological information of DR and offer insights for future drug development.
DR is categorized into two critical stages based on severity[24,25]. NPDR represents the initial stage of DR, characterized primarily by the disruption of the BRB and features such as retinal hemorrhages, exudates, and microaneurysms. PDR, or late-stage DR, is characterized by neovascularization, which can promote tractional retinal detachment[26,27]. In this context, we will focus on the non-proliferative stage of DR to outline its pathological changes. Selective loss of pericytes is one of the earliest pathological changes in the retina. The reduction in pericytes is attributed to high glucose (HG) levels affecting their proliferation and division, and it is associated with abnormal expression of forkhead box protein 1 and transforming growth factor-beta[28,29]. Studies examining high-resolution imaging data have identified thickening of the basement membrane as one of the most critical abnormal structures in retinal capillaries. Hyperglycemia excessively induces the synthesis and thickening of the basement membrane, promoting structural and functional alterations, including cell death and vascular leakage in DR[30]. For many DR patients, the retina exhibits overproduction of VEGF, leading to excessive proliferation of endothelial cells, neovascularization, resulting in microaneurysms, fluid leakage, and tissue damage[31]. Additionally, neuroretinal degeneration also occurs in the pathogenic process of DR, primarily involving cellular apoptosis and alterations in neuroglial cells[32-34].
Although DR has long been regarded as a microvascular disorder, the latest position statement from the American Diabetes Association defines DR as a highly tissue-specific neurovascular complication[35]. Within the retina, neurons [including retinal ganglion cells (RGCs), bipolar cells, amacrine cells, and horizontal cells], neuroglia (Müller cells, astrocytes, and microglial cells), and vascular cells (endothelial cells and pericytes) are interconnected to form a critical structure known as the retinal NVU[36]. These cells communicate with each other through physical interactions, soluble ligands, and/or exosomes, and their interdependence helps maintain retinal homeostasis and function in a healthy state. The vascular system provides essential nutrient support to neural tissue, and neural cells, glial cells, and pericytes signal to the endothelial cells of the BRB, thus providing strict control over the neural environment[37]. Current research indicates that characteristic neuroglial changes induced by DM, including reactive neuroglial proliferation, reduced retinal neuronal function, and neural cell apoptosis, occur before significant microvascular changes[14-16]. NVU may emerge as a pivotal area of interest for early and efficacious treatment of DR, facilitating a comprehensive comprehension of the initial pathological alterations in this condition. In this regard, we will focus on introducing the physiological functions and pathological changes of both neural units (RGCs and neuroglial cells) and vascular units (pericytes, endothelial cells, and retinal pigment epithelial [RPE] cells).
Neural units: Neuronal units consist of RGCs, as well as glial cells such as microglia, Müller cells, and astrocytes. Ganglion cells play a primary role in transforming external stimuli projected onto the retina into electrical signals, which are then transmitted through the optic nerve to the visual center in the occipital lobe of the brain, forming the basis for our visual perception. Glial cells, including astrocytes and microglia, are crucial for maintaining retinal homeostasis. Their physiological functions encompass providing structural support, participating in immune regulation, modulating metabolism, and phagocytosing neuronal debris[38,39].
RGCs are neurons in the central nervous system responsible for processing and transmitting visual information from the retina to the brain[40,41]. In the early stages of DM, RGCs exhibit heightened sensitivity and vulnerability, and their limited regenerative capacity makes them difficult to repair after injury[42]. RGC apoptosis occurs in the early stages of DR, primarily associated with oxidative stress, extracellular glutamate accumulation, and aberrant expression of cytokines and neurotrophic factors[43]. Pathological damage to RGCs is mainly characterized by axonal degeneration, which affects the physiological processes of transmitting excitatory impulses generated by neuronal cell bodies to other neurons or effectors. High-fat diets can lead to Tau hyperphosphorylation, undermining the stability of microtubule tracks, disrupting microtubule-dependent synaptic targeting of mRNAs and mitochondria, and activating glycogen synthase kinase 3 to disrupt synaptic energy production in mitochondria, leading to visual defects and synaptic loss in RGCs[44,45]. Moreover, hyperglycemia also leads to increased Ca2+ release, subsequently activating Calpain-1 and Calpain-2, which in turn trigger caspase-3 activity, leading to synaptic plasticity impairment, neurodegeneration, and RGC apoptosis[46,47].
Microglia, as monocyte-derived phagocytes, transition from a resting state to an activated state in response to stimuli such as infection and abnormal glucose and lipid metabolism[48]. They exhibit a branched morphology and extend their distribution across various layers of the retina[49,50]. Furthermore, once they lose compensation, microglia often convert from M2 type (characterized by increased expression of anti-inflammatory factors) to M1 type (characterized by increased expression of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and chemotactic factors)[51-54]. Overactivated microglia can penetrate the inner BRB (iBRB) basement membrane, phagocytosing endothelial cells, resulting in acellular capillaries and increased albumin leakage[55,56]. Moreover, IL-6 released by M1 microglia promotes interactions between microglia and RPE cells, increasing VEGF expression in RPE cells, and disrupting the integrity of the outer BRB (oBRB), while released TNF-α can directly disrupt oBRB integrity[57]. Müller cells make up 90% of retinal glial cells and provide structural support, nutritional support, and waste transport to the retina[58,59]. Several factors contribute to inflammatory responses in Müller cells, including HG levels and endoplasmic reticulum stress[60,61]. CD40, as an immune co-stimulatory factor, induces ATP release in Müller cells, leading to increased P2X7 expression in microglial cells, promoting the release of inflammatory factors[62,63]. Additionally, Müller cell-derived VEGF leads to decreased tight junction-associated proteins, including ZO-1, which directly or indirectly results in BRB structural damage[64]. Astrocytes, located in the innermost layer of the retina, are crucial for maintaining the physiological and functional integrity of the BRB[59,65]. Abnormal microenvironments in DM, e.g., HG, inflammation, and hypoxia, activate astrocytes, leading to the release of inflammatory cytokines or chemotactic factors[39]. Astrocytes can also interact with T cells and microglia, promoting and amplifying inflammation[66].
Vascular units: The tight connections between the retinal pigment epithelium, endothelial cells, and pericytes serve as barriers that to some extent prevent the entry of large molecular substances from the choroidal vasculature into the retina, thus maintaining normal physiological barriers in the retina, including iBRB and oBRB. iBRB mainly consists of retinal endothelial cells, which are covered by the foot processes of astrocytes, pericytes, and Müller cells, and are crucial for maintaining the microenvironment of the inner retina. oBRB is primarily formed by the tight junctions between adjacent RPE cells and acts as a filter regulating solutes and nutrient filtration from the bloodstream[67-70].
Endothelial cells primarily reside within the interface between the bloodstream and vascular tissue, serving as a semi-permeable barrier responsible for facilitating metabolic exchanges and regulating vessel contraction and dilation. They play a crucial role in maintaining vascular homeostasis, including vessel generation, tension modulation, and other essential functions[71,72]. HG levels lead to reduced expression of CD31 and vascular endothelial cadherin in endothelial cells, resulting in the loss of cell-cell contact[62,73,74]. Alternatively, endothelial cells transition to a mesenchymal phenotype, known as endothelial-mesenchymal transition (EMT), characterized by increased expression of mesenchymal markers such as α-smooth muscle actin, smooth muscle 22, and fibroblast-specific protein 1[73]. This results in impaired capillary blood flow, leading to retinal ischemia and hypoxia. The excessive production of VEGF in the retina leads to endothelial cell proliferation, resulting in microaneurysms and fluid leakage[31,75]. Pericytes are located on retinal microvessels, surrounded by the basement membrane, and attached to endothelial cells, playing a role in regulating vascular development and blood flow[76,77]. Endothelial cells are connected to pericytes through N-cadherin, which is crucial for maintaining the integrity of the BRB[78,79]. Elevated blood glucose levels and other factors can induce endoplasmic reticulum stress and excessive autophagy in pericytes, leading to their apoptosis, which results in increased BRB permeability and vascular leakage[80-82]. This can also be accompanied by the degeneration of endothelial cell function and thickening of the basement membrane, promoting the formation of new non-capillary vessels[83,84]. Furthermore, the loss of pericytes itself can reduce the expression of VEGF, which, in turn, decreases the stability of the endothelial cell-pericyte interaction, further contributing to BRB dysfunction[85]. RPE cells are located between the neural retina and the choroid and are a critical component of the oBRB, essential for maintaining photoreceptor function[86]. RPE cells can also directly interact with neural tissue and participate in the phagocytosis of photoreceptor outer segments[67]. The retinal pigment epithelium is a major source of pigment epithelium-derived factor, which is one of the key anti-angiogenic factors[87]. HG levels can stimulate the migration and proliferation of RPE cells, reduce the expression of epithelial markers such as E-cadherin and ZO-1, and increase the levels of mesenchymal markers such as vimentin and α-SMA, indicating EMT[88]. Additionally, under DM conditions, RPE cells induce lysosomal membrane permeabilization, leading to the release of significant amounts of cathepsin B from lysosomes into the cytoplasm, followed by dysfunction of the autophagolysosomal pathway[89-92].
Given the increasing importance of the BRB disruption and the loss of components of the NVU in the development of DR, a focus on cell death has become a breakthrough point in elucidating the pathological mechanisms of DR. Among these, autophagy-dependent ferroptosis plays a crucial role in the development of DR. Ferroptosis is primarily characterized by iron metabolism abnormalities leading to lipid peroxidation and the reduction of the core enzyme in the antioxidant system, GPX4. While autophagy and ferroptosis are distinct cell death pathways in terms of mechanisms and morphology, increasing evidence suggests significant crosstalk between them[20,21], and ferroptosis has been defined as an autophagy-dependent cell death mode[22,23].
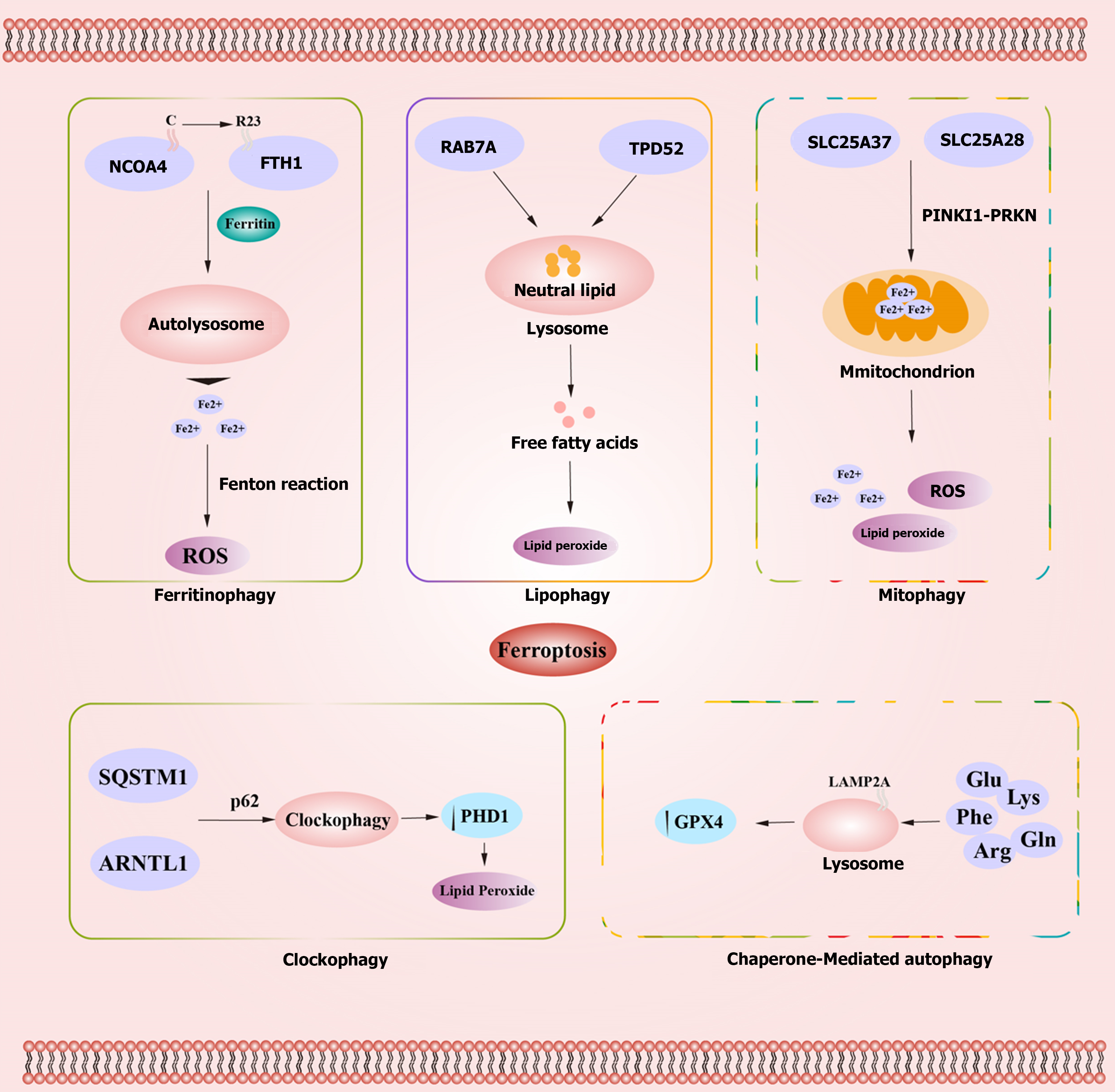
Studies have shown that autophagy can impact the occurrence and development of ferroptosis by regulating iron metabolism and ROS metabolism[93,94]. The autophagic processes influencing ferroptosis include ferritinophagy, lipophagy, mitophagy, clockophagy, and chaperone-mediated autophagy (CMA)[95,96]. Autophagy can modulate intracellular iron balance and lipid peroxidation as upstream mechanisms of ferroptosis[97]. Specific mechanisms include ferritinophagy induced by nuclear receptor coactivator 4 (NCOA4)-mediated ferritin degradation[93,98], the inhibition of the system Xc−-GPX4 pathway induced by recombinant Beclin 1 (BECN1)-soluble carrier family 7, member 11 (SLC7A11) complex[99], lipophagy mediated by the human RAS oncogene family member RAB7A[100], and aryl hydrocarbon receptor nuclear translocator-like protein (ARNTL)-mediated circadian clock protein-specific autophagy[97] (Figure 1).
NCOA4, acting as a selective receptor responsible for the autophagic degradation of ferritin, binds to the arginine residue R23 on the C-terminus of ferritin heavy chain (FTH1). This binding facilitates the transport of ferritin to the autophagic lysosome, a process known as ferritinophagy[101]. Studies have shown that silencing NCOA4 or autophagy-related genes 5/7 (ATG5/ATG7) in HT1080 cells can inhibit ferritinophagy, leading to reduced intracellular free iron levels and the suppression of ferroptosis. Conversely, overexpression of NCOA4 promotes ferroptosis[93]. Ferritinophagy, as a specialized form of selective autophagy for ferritin, reduces iron storage and promotes cellular iron accumulation by releasing free iron. Under normal physiological conditions, excess Fe2+ within cells is oxidized to Fe3+ and stored within ferritin or exported out of cells via the iron transporter protein 1 on the cell membrane. Under pathological conditions, excess Fe2+ can lead to the generation of reactive oxygen species (ROS) through the Fenton reaction, resulting in cellular toxicity and inducing cell death. Cell surface transferrin receptor is a key regulator of iron ion levels[102]. Nontransferrin bound iron, affects liver cells through solute carrier family 39 member A14 and increases Fe2+, promoting ferroptosis[103]. Additionally, iron-responsive element-binding protein 2 is involved in regulating intracellular iron homeostasis[104]. Therefore, iron overload and dysregulation of proteins involved in cellular iron homeostasis are pivotal factors contributing to the occurrence of ferroptosis.
Lipophagy is a selective form of autophagy involving the lysosomal degradation of lipid droplets, which are intracellular stores of neutral lipids, including triglycerides and cholesterol. The process of lipid degradation within lysosomes is termed lipophagy. This process generates free fatty acids (FFAs) that can, in turn, promote ATP production within mitochondria[105-107]. Additionally, lipid droplets protect cells from oxidative stress by sequestering FFAs away from the cell core[108]. Accumulation of lipid droplets may serve as a negative feedback mechanism to limit lipid peroxidation. A study has shown that upregulation of lipid storage mediated by tumor protein D52 inhibits ferroptosis induced by RSL3 in vitro[100]. However, excessive lipid degradation can increase lipid toxicity and lipid peroxidation levels, promoting ferroptosis. Members of the RAS oncogene family, such as RAB7A, are central regulators of hepatic lipophagy[107]. Research indicates that silencing RAB7A through shRNA can inhibit lipophagy, increase lipid storage, and subsequently inhibit ferroptosis induced by RSL3 in HEPG2 cells. Similarly, silencing tumor protein D52 to suppress lipid storage and upregulate lipophagy can promote ferroptosis[100]. Thus, abnormalities in lipid storage and lipophagy function are also crucial factors in the occurrence of ferroptosis.
Under physiological conditions, mitophagy selectively degrades mitochondria to maintain their quantity and quality. During the early stages of iron overload, some free iron can act as a buffer by being transported into mitochondria. Mitophagy can then surround free iron within autophagosomes, reducing the source of ROS related to ferroptosis. However, excessive iron overload can lead to mitochondrial damage, causing extensive abnormal mitophagy. This results in the release of free iron, ROS, and lipid peroxides, ultimately leading to ferroptosis. PTEN-induced kinase 1 (PINK1) and Parkin RBR E3 ubiquitin protein ligase (PRKN) are major regulators of mitophagy[109]. Solute carrier family 25 member 37 and solute carrier family 25 member 28 induce mitochondrial iron accumulation through the PINK1-PRKN pathway[110]. Research suggests that BNIP3 or PINK1-Parkin-mediated mitophagy can alleviate ferroptosis-induced damage in renal tubular epithelial cells by regulating the ROS/HO-1/GPX4 signaling axis[111].
Clockophagy, a selective autophagic process discovered in 2019, involves the autophagic cargo receptor SQSTM1 and circadian clock transcription factor ARNTL1. Through p62-mediated clockophagy, there is an upregulation of hypoxia-inducible factor prolyl hydroxylase 1 under hypoxic conditions. This, in turn, promotes lipid peroxidation within cells, further facilitating ferroptosis[97,112,113]. Nuclear factor erythroid 2-related factor 2/heme oxygenase-1 is a protein present in all human cells and can partially inhibit clockophagy by regulating calcium levels[114].
CMA is a selective form of autophagy that targets specific protein sequences, such as Lys-Phe-Glu-Arg-Gln (KFERQ), for protein degradation. In this process, molecular chaperones recognize the KFERQ motif within substrate proteins. Subsequently, these substrates bind to lysosome-associated membrane protein type 2A (LAMP2A) and enter lysosomes. Overexpression of LAMP2A can promote CMA degradation of GPX4, leading to ferroptosis[115-117]. GPX4, a key enzyme that converts toxic lipid peroxides into non-toxic lipid alcohols, is considered a major regulator of ferroptosis[118,119]. In an in vivo animal acute kidney injury (AKI) model, Legumain can cause AKI and ferroptosis, regulated by GPX4-mediated CMA[120]. Antimony (Sb), a neurotoxic pollutant, can induce neuronal damage. Sb can activate CMA and increase the expression of the chaperone heat shock cognate 70 (HSC70), heat shock protein 90, and lysosome receptor LAMP2A, accelerating lysosomal transport and subsequent GPX4 degradation, triggering neurotoxicity and leading to ferroptosis in neurons[121-123].
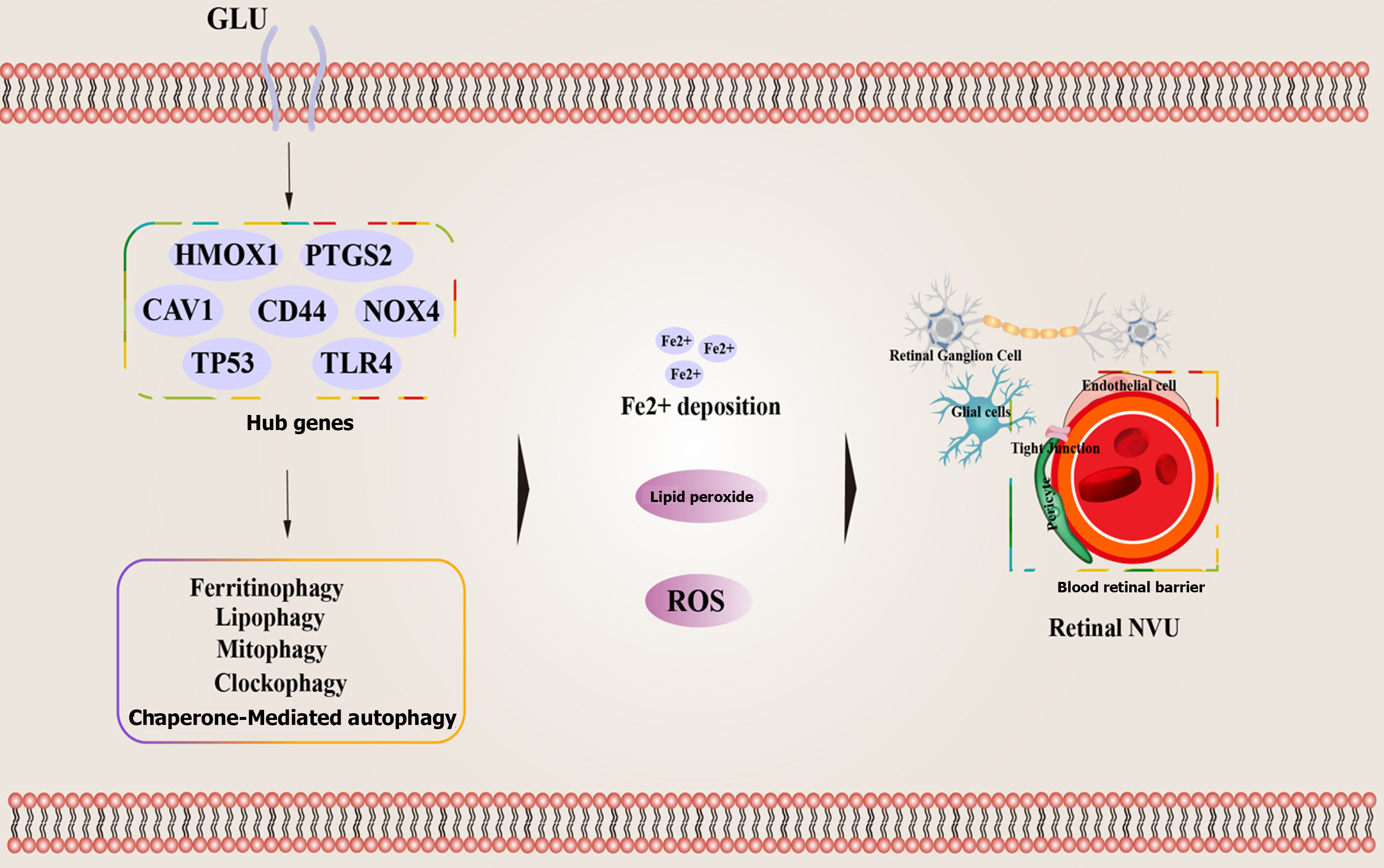
Currently, clinical examination of DR patient samples indicates an increase in the expression of markers associated with ferroptosis in their bodies, including iron ion accumulation and elevated ROS levels. Studies have shown that in DR patients, especially those with NPDR, there are higher concentrations of iron ions and ROS associated with ferroptosis[124]. A study based on 5321 patients revealed a correlation between disrupted serum iron metabolism and the occurrence of DR[125]. In both human and animal retinal tissues, increased iron accumulation is evident when compared to non-DR counterparts[126]. Based on bioinformatics techniques, cross-validated datasets, and previous research support, five hub genes related to ferroptosis (CAV1, CD44, NOX4, TLR4, and TP53) associated with the onset and progression of DR have been identified. Glutathione (GSH) has been shown to have an effective therapeutic effect on DR by targeting ferroptosis manifestations[127]. A study on a DR rat model showed increased oxidative stress, significant ferroptosis, and cell damage. Treatment with Ferrostatin-1 (an ferroptosis inhibitor) improved antioxidant capacity, reduced ferroptosis levels, and alleviated cell damage. Additionally, in HG-induced ARPE-19, treatment with Erastin (an ferroptosis activator) and Ferr-1 showed that Ferr-1 reversed the oxidative stress, ferroptosis, and cell damage induced by Erastin in HG-treated ARPE-19 cells[128]. In HG-treated ARPE-19 cells, circ-PSNE1 expression increased, and knocking out circ-PSEN1 regulated GSH and malondialdehyde (MDA) concentrations, increased cell viability, inhibited iron accumulation, and subsequently reduced ferroptosis[129]. Another study indicated that HG promoted the expression of miR-138-5p in RPE cells, reducing Sirt1/Nrf2 activity and antioxidant expression. Astragaloside IV, a major active ingredient in Astragalus[129], alleviated apoptosis caused by HG in RPE cells by regulating the miR-138-5p/Sirt1/Nrf2 pathway[130].
The current evidence also suggests a correlation between the onset and progression of DR and the buildup of lipid peroxides[131,132]. MDA generated during ferroptosis can interact with proteins and nucleic acids, disrupting cell membrane physiology and functional integrity[133]. Arachidonic acid and phosphatidylethanolamine of adrenaline can undergo further oxidation under the catalysis of lipoxygenase, inducing ferroptosis[134]. Within this context, the imbalance in iron homeostasis-induced oxidative reactions[135], activation of lipid ROS rather than cytoplasmic ROS, plays a critical role in initiating ferroptosis[102,136-139]. Accumulation of ROS-induced oxidative stress, or the induction of retinal vascular endothelial tissue damage and loss of endothelial cells due to retinaldehyde oxidation, further promotes DR development[140]. Besides pigment epithelial cells and endothelial cells, other cells within the NVU are also susceptible to iron accumulation, affecting normal physiological functions and exhibiting pathological changes. A study co-cultured neurons, astrocytes, and microglia, and treated the co-culture system with iron ions and RSL3. The results showed that all neurons, especially microglia, exhibited significant abnormal transcriptional states, released pro-inflammatory cytokines, and experienced marked ferroptosis. Among these, ferroptosis and GSH metabolism were the most affected pathways[141]. Accumulation of free iron in the retina disrupts the cell's redox system, leading to ferroptosis in RGCs. In this context, the NCOA4-mediated FTH1 signaling pathway may play a crucial role. Application of deferiprone, which can chelate excess free iron effectively, prevents RGCs' ferroptosis[142].
Certainly, there is a considerable amount of research suggesting that the occurrence and development of DR are also associated with abnormalities in autophagy and ferroptosis. A study, based on a comparison of RNAseq data from 15 DR patients and 3 healthy control subjects' retinas using the Gene Expression Omnibus database, identified a total of 52 genes related to ferroptosis when compared with ferroptosis-related genes in the FerrDb database. Among these genes, 43 were upregulated, and 9 were downregulated. These genes were significantly enriched in apoptosis signaling pathways, autophagy, iron ion binding, and the p53 signaling pathway. Key genes such as HMOX1 and PTGS2, along with their associated transcription factors and miRNAs, may be related to ferroptosis in the development of DR[143]. GSH is the most prominent antioxidant in RPE cells, with high concentrations in the retina and retinal pigment epithelium[144,145]. The efficiency of the GSH redox system decreases with age, leading to increased ROS generation and the induction of autophagy and ferroptosis in RPE cells[146,147]. Treatment of RPE cells with buthionine sulphoximine and erastin promotes GSH depletion, and increases lipid ROS production, resulting in iron accumulation, autophagy, and stress-induced premature senescence (SIPS). The supplementation of ferroptosis inhibitors can prevent cell death dependent on GSH depletion. Additionally, inducing autophagy using rapamycin can reduce SIPS, highlighting the crucial role of autophagy in ferroptosis and SIPS[147].
There is compelling direct evidence indicating that the pathogenesis and progression of DR are intricately linked to ferroptosis, which is mediated by ferritinophagy, CMA, mitochondrial autophagy, and other mechanisms. A study used differential expression analysis of the GSE146615 dataset to identify differentially expressed genes (DEGs) associated with ferroptosis in DR. A total of 8 DEGs were identified, among which BECN1, HERC2, ATG7, and BCAT2 may serve as potential biomarkers for DR. These genes may impact the occurrence and progression of DR by regulating ferritinophagy[148]. HSC70 acts as a receptor for CMA and can recognize ACSL4 protein, leading to its digestion in lysosomes. Abnormal autophagic lysosomal degradation due to HG-induced autophagic lysosomal degradation leads to the accumulation of ACSL4 in the retinal pigment epithelium, promoting the generation of harmful lipid compounds and inducing ferroptosis in RPE cells. The application of glia maturation factor-beta protein and the iron inhibitor liproxstatin-1 can mitigate RPE damage[149]. Eukaryotic fatty acid binding protein 4 (FABP4), as a companion protein for FFAs, is abnormally expressed in retinal lesions and positively correlates with the severity of DR. Therefore, FABP4 can serve as an independent prognostic marker for DR patients[150,151]. Animal studies have shown that inhibiting FABP4 expression can alleviate lipid peroxidation and oxidative stress in DR by regulating peroxisome proliferator-activated receptor γ-mediated ferroptosis[152] (Figure 2).
GPX4, as the primary regulator of ferroptosis, also plays a crucial role in ferroptosis mediated by MCA[118,119]. Currently, numerous studies suggest that GPX4, as a central core molecule, is closely associated with the occurrence and progression of DR. Under physiological conditions, lipid peroxidation is regulated by the GSH antioxidant system, which consists of GSH, GPX, and glutamine toxin, effectively preventing excessive ROS production[153,154]. Research indicates that GPX4 inactivation, which is necessary for ROS elimination, can induce ferroptosis even when cellular cysteine and GSH levels are normal[118]. The cysteine/glutamate reverse transporter (System Xc-)/GSH/GPX4 pathway is a critical pathway in regulating ferroptosis and plays an important role in inhibiting lipid peroxidation. System Xc- is located on the cell membrane and is composed of SLC7A11 and the heavy chain subunit solute carrier family 3 member 2. Its main function is to pump cysteine into cells and export glutamate out of cells[155]. Of 25(OH)D3, as a fat-soluble vitamin, can downregulate the expression of miR-93, reducing Fe2+ levels, GPX4, and SLC7A11 protein levels in human retinal microvascular endothelial cells induced by HG, effectively alleviating cell death, oxidative stress, and ferroptosis[156]. Human retinal endothelial cells (HRECs) treated with HG showed increased expression of TRIM46. Overexpression of TRIM46 reduced resistance to HG-induced ferroptosis. TRIM46 interacts with GPX4 to promote its ubiquitination. Overexpression of GPX4 improved the upregulation caused by TRIM46 and protected rabbit corneal endothelial cells (RCECs) from ferroptosis. Therefore, TRIM46 promotes HG-induced ferroptosis in human RCECs by promoting GPX4 ubiquitination[157]. A study explored the effects of amygdalin on ferroptosis and oxidative stress in HRECs stimulated by HG through the NRF2/ARE pathway. The results showed that HG stimulation reduced the levels of GSH, GPX4, SOD, and CAT but increased the levels of MDA, ROS, GSSG, and Fe2+ in HRECs. Amygdalin treatment upregulated the levels of NQO1 and HO-1 in HG-stimulated HRECs, and NRF2 inhibitors reversed the effects of amygdalin, suggesting that amygdalin treatment inhibits ferroptosis and oxidative stress in HRECs stimulated by HG by activating the NRF2/ARE signaling pathway[158].
Given that the management of severe DR requires more clinical resources, it is essential to focus on the pathological changes that occur in the early stages of DR, and explore and discover the pathological mechanisms affecting the occurrence and development of DR, as well as corresponding intervention measures. This is crucial for improving patients' quality of life and reducing healthcare expenditures. Based on current research and the understanding of scientists and clinicians, the retinal NVU, including ganglion cells, glial cells, endothelial cells, and surrounding cells, may become the focus of our future research. Based on existing evidence, ferroptosis, a form of cell death regulated by ferritinophagy and MCA, may serve as a potential core target and biomarker for the occurrence and development of DR. These findings provide new perspectives for understanding the pathological changes in the early stages of DR and exploring new intervention measures. However, it should be noted that the retinal NVU is composed of different types of cells and tissues. Most current studies have shown that the abnormal microenvironment caused by DM leads to iron accumulation, abnormal levels of lipid peroxidation, and the induction of ferroptosis in RPE cells, glial cells, and ganglion cells. Yet, these studies do not provide a comprehensive understanding of the negative impact of ferroptosis on the physiological and functional integrity of the entire NVU from a holistic and cellular interaction perspective. Therefore, future research may focus on discovering the most significant genetic loci affecting the impact of autophagy-dependent ferroptosis on the NVU using in vitro co-culture and organoid culture techniques, which are crucial for maintaining the physiological function of the retinal NVU. Certainly, our exploration of the pathological effects of autophagy-dependent ferroptosis on the retinal NVU has not yet led to the discovery of intervention measures with significant clinical value. Currently, several in vitro cell studies have shown that compounds such as astragaloside IV and amygdalin can inhibit ferroptosis by regulating iron ions and lipid peroxide levels. However, their efficacy for DR patients remains unclear. We may consider two different approaches to discovering effective treatments for DR. First, based on the currently available drugs that can effectively inhibit ferroptosis in retinal cells, exploratory clinical studies and new drug development research can be conducted to gradually clarify the effects of these potential drugs, providing treatment options for a wider range of DR patients. Second, we can select drugs that are currently effective in inhibiting ferroptosis but are used to treat other diseases and expand their clinical indications to DR. While expanding the range of drug treatments, this approach can provide more treatment options for DR. In conclusion, current research indicates a clear association between autophagy-dependent ferroptosis and the functional abnormalities, as well as cellular demise, in the retinal NVU. The aforementioned statement provides novel perspectives for comprehending the pathological alterations in the initial stages of DR and presents innovative ideas for further exploring additional therapeutic measures.
| 1. | GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144-e160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1680] [Cited by in RCA: 1496] [Article Influence: 374.0] [Reference Citation Analysis (0)] |
| 2. | Hou X, Wang L, Zhu D, Guo L, Weng J, Zhang M, Zhou Z, Zou D, Ji Q, Guo X, Wu Q, Chen S, Yu R, Chen H, Huang Z, Zhang X, Wu J, Wu J, Jia W; China National Diabetic Chronic Complications (DiaChronic) Study Group. Prevalence of diabetic retinopathy and vision-threatening diabetic retinopathy in adults with diabetes in China. Nat Commun. 2023;14:4296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 3. | Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, Genuth S, Goff DC, Leiter LA, Ismail-Beigi F, Ambrosius WT; Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 4. | An X, Jin D, Duan L, Zhao S, Zhou R, Lian F, Tong X. Direct and indirect therapeutic effect of traditional Chinese medicine as an add-on for non-proliferative diabetic retinopathy: a systematic review and meta-analysis. Chin Med. 2020;15:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Liu W, Wu S, Jin J, Li W, Wang N. Calcium dobesilate for diabetic retinopathy: a systematic review and meta-analysis. Sci China Life Sci. 2015;58:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Sacconi R, Giuffrè C, Corbelli E, Borrelli E, Querques G, Bandello F. Emerging therapies in the management of macular edema: a review. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Rodríguez ML, Pérez S, Mena-Mollá S, Desco MC, Ortega ÁL. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid Med Cell Longev. 2019;2019:4940825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Teo ZL, Tham YC, Yu M, Cheng CY, Wong TY, Sabanayagam C. Do we have enough ophthalmologists to manage vision-threatening diabetic retinopathy? A global perspective. Eye (Lond). 2020;34:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, Maia M, Mathenge W, Moreker S, Muqit MMK, Resnikoff S, Verdaguer J, Zhao P, Ferris F, Aiello LP, Taylor HR. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology. 2018;125:1608-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 481] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 10. | Li X, Tan TE, Wong TY, Sun X. Diabetic retinopathy in China: Epidemiology, screening and treatment trends-A review. Clin Exp Ophthalmol. 2023;51:607-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 11. | Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, Wong IY, Ting DSW, Tan GSW, Jonas JB, Sabanayagam C, Wong TY, Cheng CY. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 1018] [Article Influence: 254.5] [Reference Citation Analysis (1)] |
| 12. | Fanaro GB, Marques MR, Calaza KDC, Brito R, Pessoni AM, Mendonça HR, Lemos DEA, de Brito Alves JL, de Souza EL, Cavalcanti Neto MP. New Insights on Dietary Polyphenols for the Management of Oxidative Stress and Neuroinflammation in Diabetic Retinopathy. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Bandello F, Lattanzio R, Zucchiatti I, Del Turco C. Pathophysiology and treatment of diabetic retinopathy. Acta Diabetol. 2013;50:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 928] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 15. | Garcia-Ramírez M, Hernández C, Villarroel M, Canals F, Alonso MA, Fortuny R, Masmiquel L, Navarro A, García-Arumí J, Simó R. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia. 2009;52:2633-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Carrasco E, Hernández C, Miralles A, Huguet P, Farrés J, Simó R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care. 2007;30:2902-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G; Nomenclature Committee on Cell Death. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12 Suppl 2:1463-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 535] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 18. | Fearnhead HO, Vandenabeele P, Vanden Berghe T. How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 2017;24:1991-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11650] [Article Influence: 896.2] [Reference Citation Analysis (1)] |
| 20. | Kang R, Tang D. Autophagy and Ferroptosis - What's the Connection? Curr Pathobiol Rep. 2017;5:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 21. | Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem Biol. 2020;27:420-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 573] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 22. | Liu J, Guo ZN, Yan XL, Huang S, Ren JX, Luo Y, Yang Y. Crosstalk Between Autophagy and Ferroptosis and Its Putative Role in Ischemic Stroke. Front Cell Neurosci. 2020;14:577403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 23. | Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1314] [Cited by in RCA: 1197] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 24. | Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 25. | Fong DS, Aiello LP, Ferris FL 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27:2540-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 470] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Omori K, Nagata N, Kurata K, Fukushima Y, Sekihachi E, Fujii N, Namba-Hamano T, Takabatake Y, Fruttiger M, Nagasawa T, Uemura A, Murata T. Inhibition of stromal cell-derived factor-1α/CXCR4 signaling restores the blood-retina barrier in pericyte-deficient mouse retinas. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Kollias AN, Ulbig MW. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107:75-83; quiz 84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Arboleda-Velasquez JF, Valdez CN, Marko CK, D'Amore PA. From pathobiology to the targeting of pericytes for the treatment of diabetic retinopathy. Curr Diab Rep. 2015;15:573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R, Lois N. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 723] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 30. | Roy S, Kim D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2021;82:100903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Caprnda M, Kubatka P, Saxena S, Valaskova J, Stefanickova J, Kobyliak N, Zulli A, Kruzliak P. The Impact of Hyperglycemia on VEGF Secretion in Retinal Endothelial Cells. Folia Med (Plovdiv). 2017;59:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Simó R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 390] [Article Influence: 55.7] [Reference Citation Analysis (1)] |
| 33. | Villarroel M, Ciudin A, Hernández C, Simó R. Neurodegeneration: An early event of diabetic retinopathy. World J Diabetes. 2010;1:57-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Ramos H, Hernández C, Simó R, Simó-Servat O. Inflammation: The Link between Neural and Vascular Impairment in the Diabetic Retina and Therapeutic Implications. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 35. | Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:412-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 596] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 36. | Spaide RF. Measurable Aspects of the Retinal Neurovascular Unit in Diabetes, Glaucoma, and Controls. Am J Ophthalmol. 2019;207:395-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Ji L, Tian H, Webster KA, Li W. Neurovascular regulation in diabetic retinopathy and emerging therapies. Cell Mol Life Sci. 2021;78:5977-5985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 38. | Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51:1-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 567] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 39. | Rübsam A, Parikh S, Fort PE. Role of Inflammation in Diabetic Retinopathy. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 510] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 40. | Thomas CN, Berry M, Logan A, Blanch RJ, Ahmed Z. Caspases in retinal ganglion cell death and axon regeneration. Cell Death Discov. 2017;3:17032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Nguyen-Ba-Charvet KT, Rebsam A. Neurogenesis and Specification of Retinal Ganglion Cells. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Fu Y, Wang Y, Gao X, Li H, Yuan Y. Dynamic Expression of HDAC3 in db/db Mouse RGCs and Its Relationship with Apoptosis and Autophagy. J Diabetes Res. 2020;2020:6086780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Oshitari T. The Pathogenesis and Therapeutic Approaches of Diabetic Neuropathy in the Retina. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 44. | Zhu H, Zhang W, Zhao Y, Shu X, Wang W, Wang D, Yang Y, He Z, Wang X, Ying Y. GSK3β-mediated tau hyperphosphorylation triggers diabetic retinal neurodegeneration by disrupting synaptic and mitochondrial functions. Mol Neurodegener. 2018;13:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 45. | Shu XS, Zhu H, Huang X, Yang Y, Wang D, Zhang Y, Zhang W, Ying Y. Loss of β-catenin via activated GSK3β causes diabetic retinal neurodegeneration by instigating a vicious cycle of oxidative stress-driven mitochondrial impairment. Aging (Albany NY). 2020;12:13437-13462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Baudry M, Bi X. Calpain-1 and Calpain-2: The Yin and Yang of Synaptic Plasticity and Neurodegeneration. Trends Neurosci. 2016;39:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 47. | Liu XF, Zhou DD, Xie T, Hao JL, Malik TH, Lu CB, Qi J, Pant OP, Lu CW. The Nrf2 Signaling in Retinal Ganglion Cells under Oxidative Stress in Ocular Neurodegenerative Diseases. Int J Biol Sci. 2018;14:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Colton C, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 49. | Uddin MI, Kilburn TC, Duvall CL, Penn JS. Visualizing HIF-1α mRNA in a Subpopulation of Bone Marrow-Derived Cells to Predict Retinal Neovascularization. ACS Chem Biol. 2020;15:3004-3012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 417] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 51. | Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 1451] [Article Influence: 161.2] [Reference Citation Analysis (0)] |
| 52. | Li X, Yu ZW, Li HY, Yuan Y, Gao XY, Kuang HY. Retinal microglia polarization in diabetic retinopathy. Vis Neurosci. 2021;38:E006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Szabó K, Énzsöly A, Dékány B, Szabó A, Hajdú RI, Radovits T, Mátyás C, Oláh A, Laurik LK, Somfai GM, Merkely B, Szél Á, Lukáts Á. Histological Evaluation of Diabetic Neurodegeneration in the Retina of Zucker Diabetic Fatty (ZDF) Rats. Sci Rep. 2017;7:8891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Xiao Y, Hu X, Fan S, Zhong J, Mo X, Liu X, Hu Y. Single-Cell Transcriptome Profiling Reveals the Suppressive Role of Retinal Neurons in Microglia Activation Under Diabetes Mellitus. Front Cell Dev Biol. 2021;9:680947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Xie H, Zhang C, Liu D, Yang Q, Tang L, Wang T, Tian H, Lu L, Xu JY, Gao F, Wang J, Jin C, Li W, Xu G, Xu GT, Zhang J. Erythropoietin protects the inner blood-retinal barrier by inhibiting microglia phagocytosis via Src/Akt/cofilin signalling in experimental diabetic retinopathy. Diabetologia. 2021;64:211-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 56. | Yun JH, Park SW, Kim KJ, Bae JS, Lee EH, Paek SH, Kim SU, Ye S, Kim JH, Cho CH. Endothelial STAT3 Activation Increases Vascular Leakage Through Downregulating Tight Junction Proteins: Implications for Diabetic Retinopathy. J Cell Physiol. 2017;232:1123-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 57. | Jo DH, Yun JH, Cho CS, Kim JH, Kim JH, Cho CH. Interaction between microglia and retinal pigment epithelial cells determines the integrity of outer blood-retinal barrier in diabetic retinopathy. Glia. 2019;67:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 58. | Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 500] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 59. | Sorrentino FS, Allkabes M, Salsini G, Bonifazzi C, Perri P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. 2016;162:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 60. | Yang J, Chen C, McLaughlin T, Wang Y, Le YZ, Wang JJ, Zhang SX. Loss of X-box binding protein 1 in Müller cells augments retinal inflammation in a mouse model of diabetes. Diabetologia. 2019;62:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Gaonkar B, Prabhu K, Rao P, Kamat A, Rao Addoor K, Varma M. Plasma angiogenesis and oxidative stress markers in patients with diabetic retinopathy. Biomarkers. 2020;25:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Portillo JC, Lopez Corcino Y, Miao Y, Tang J, Sheibani N, Kern TS, Dubyak GR, Subauste CS. CD40 in Retinal Müller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes. 2017;66:483-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 63. | Abcouwer SF. Müller Cell-Microglia Cross Talk Drives Neuroinflammation in Diabetic Retinopathy. Diabetes. 2017;66:261-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297-2305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 65. | Fresta CG, Fidilio A, Caruso G, Caraci F, Giblin FJ, Leggio GM, Salomone S, Drago F, Bucolo C. A New Human Blood-Retinal Barrier Model Based on Endothelial Cells, Pericytes, and Astrocytes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 66. | Rothhammer V, Quintana FJ. Control of autoimmune CNS inflammation by astrocytes. Semin Immunopathol. 2015;37:625-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 67. | Naylor A, Hopkins A, Hudson N, Campbell M. Tight Junctions of the Outer Blood Retina Barrier. Int J Mol Sci. 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 68. | Das A, McGuire PG, Rangasamy S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology. 2015;122:1375-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 69. | Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild PR, Omri S, Gélizé E, Jonet L, Delaunay K, De Kozak Y, Berdugo M, Zhao M, Crisanti P, Behar-Cohen F. Mechanisms of macular edema: Beyond the surface. Prog Retin Eye Res. 2018;63:20-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 424] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 70. | Díaz-Coránguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017;139:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 71. | Yu PK, Balaratnasingam C, Morgan WH, Cringle SJ, McAllister IL, Yu DY. The structural relationship between the microvasculature, neurons, and glia in the human retina. Invest Ophthalmol Vis Sci. 2010;51:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Csortos C, Kolosova I, Verin AD. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the PPP family. Am J Physiol Lung Cell Mol Physiol. 2007;293:L843-L854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Thomas AA, Biswas S, Feng B, Chen S, Gonder J, Chakrabarti S. lncRNA H19 prevents endothelial-mesenchymal transition in diabetic retinopathy. Diabetologia. 2019;62:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 74. | Wu JH, Li YN, Chen AQ, Hong CD, Zhang CL, Wang HL, Zhou YF, Li PC, Wang Y, Mao L, Xia YP, He QW, Jin HJ, Yue ZY, Hu B. Inhibition of Sema4D/PlexinB1 signaling alleviates vascular dysfunction in diabetic retinopathy. EMBO Mol Med. 2020;12:e10154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 75. | Rattner A, Williams J, Nathans J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J Clin Invest. 2019;129:3807-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 76. | Caporarello N, D'Angeli F, Cambria MT, Candido S, Giallongo C, Salmeri M, Lombardo C, Longo A, Giurdanella G, Anfuso CD, Lupo G. Pericytes in Microvessels: From "Mural" Function to Brain and Retina Regeneration. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 77. | Attwell D, Mishra A, Hall CN, O'Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 466] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 78. | Monickaraj F, McGuire P, Das A. Cathepsin D plays a role in endothelial-pericyte interactions during alteration of the blood-retinal barrier in diabetic retinopathy. FASEB J. 2018;32:2539-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Eilken HM, Diéguez-Hurtado R, Schmidt I, Nakayama M, Jeong HW, Arf H, Adams S, Ferrara N, Adams RH. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun. 2017;8:1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 80. | Park DY, Lee J, Kim J, Kim K, Hong S, Han S, Kubota Y, Augustin HG, Ding L, Kim JW, Kim H, He Y, Adams RH, Koh GY. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun. 2017;8:15296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 81. | Lai DW, Lin KH, Sheu WH, Lee MR, Chen CY, Lee WJ, Hung YW, Shen CC, Chung TJ, Liu SH, Sheu ML. TPL2 (Therapeutic Targeting Tumor Progression Locus-2)/ATF4 (Activating Transcription Factor-4)/SDF1α (Chemokine Stromal Cell-Derived Factor-α) Axis Suppresses Diabetic Retinopathy. Circ Res. 2017;121:e37-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Mao XB, You ZP, Wu C, Huang J. Potential suppression of the high glucose and insulin-induced retinal neovascularization by Sirtuin 3 in the human retinal endothelial cells. Biochem Biophys Res Commun. 2017;482:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther. 2017;171:30-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 84. | Spencer BG, Estevez JJ, Liu E, Craig JE, Finnie JW. Pericytes, inflammation, and diabetic retinopathy. Inflammopharmacology. 2020;28:697-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 85. | Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1524] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 86. | Pavan B, Dalpiaz A. Retinal pigment epithelial cells as a therapeutic tool and target against retinopathies. Drug Discov Today. 2018;23:1672-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 87. | He X, Cheng R, Benyajati S, Ma JX. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond). 2015;128:805-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 88. | Yang J, Yang K, Meng X, Liu P, Fu Y, Wang Y. Silenced SNHG1 Inhibited Epithelial-Mesenchymal Transition and Inflammatory Response of ARPE-19 Cells Induced by High Glucose. J Inflamm Res. 2021;14:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 89. | Patel S, Homaei A, El-Seedi HR, Akhtar N. Cathepsins: Proteases that are vital for survival but can also be fatal. Biomed Pharmacother. 2018;105:526-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 90. | Feng L, Liang L, Zhang S, Yang J, Yue Y, Zhang X. HMGB1 downregulation in retinal pigment epithelial cells protects against diabetic retinopathy through the autophagy-lysosome pathway. Autophagy. 2022;18:320-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 91. | Xia T, Rizzolo LJ. Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vision Res. 2017;139:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 92. | Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52:2160-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 93. | Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1647] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 94. | Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1319] [Article Influence: 146.6] [Reference Citation Analysis (2)] |
| 95. | Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 715] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 96. | Chen P, Li FM, Zhou YF, Qian C, Li J, Jiang LR, Qian ZM. Effects of alpha-lipoic acid on expression of iron transport and storage proteins in BV-2 microglia cells. Pharmacol Rep. 2017;69:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ, Lotze MT, Zeh HJ, Kang R, Tang D. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5:eaaw2238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 98. | Latunde-Dada GO. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861:1893-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 614] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 99. | Kang R, Zhu S, Zeh HJ, Klionsky DJ, Tang D. BECN1 is a new driver of ferroptosis. Autophagy. 2018;14:2173-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 100. | Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, Kang R, Wang X, Tang D, Dai E. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. 2019;508:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 338] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 101. | Mancias JD, Pontano Vaites L, Nissim S, Biancur DE, Kim AJ, Wang X, Liu Y, Goessling W, Kimmelman AC, Harper JW. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 102. | Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell. 2015;59:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 1497] [Article Influence: 149.7] [Reference Citation Analysis (0)] |
| 103. | Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29:467-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 396] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 104. | Xia H, Wu Y, Zhao J, Cheng C, Lin J, Yang Y, Lu L, Xiang Q, Bian T, Liu Q. N6-Methyladenosine-modified circSAV1 triggers ferroptosis in COPD through recruiting YTHDF1 to facilitate the translation of IREB2. Cell Death Differ. 2023;30:1293-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 105. | Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3239] [Cited by in RCA: 3103] [Article Influence: 193.9] [Reference Citation Analysis (0)] |
| 106. | Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 398] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 107. | Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey CA, McNiven MA. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 2015;61:1896-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 108. | Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4608] [Cited by in RCA: 4944] [Article Influence: 618.0] [Reference Citation Analysis (0)] |
| 109. | Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1912] [Cited by in RCA: 2254] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
| 110. | Li C, Zhang Y, Cheng X, Yuan H, Zhu S, Liu J, Wen Q, Xie Y, Liu J, Kroemer G, Klionsky DJ, Lotze MT, Zeh HJ, Kang R, Tang D. PINK1 and PARK2 Suppress Pancreatic Tumorigenesis through Control of Mitochondrial Iron-Mediated Immunometabolism. Dev Cell. 2018;46:441-455.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 111. | Lin Q, Li S, Jin H, Cai H, Zhu X, Yang Y, Wu J, Qi C, Shao X, Li J, Zhang K, Zhou W, Zhang M, Cheng J, Gu L, Mou S, Ni Z. Mitophagy alleviates cisplatin-induced renal tubular epithelial cell ferroptosis through ROS/HO-1/GPX4 axis. Int J Biol Sci. 2023;19:1192-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 112] [Reference Citation Analysis (0)] |
| 112. | Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1043] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 113. | Liu J, Yang M, Kang R, Klionsky DJ, Tang D. Autophagic degradation of the circadian clock regulator promotes ferroptosis. Autophagy. 2019;15:2033-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 114. | Zhang X, Yu Y, Lei H, Cai Y, Shen J, Zhu P, He Q, Zhao M. The Nrf-2/HO-1 Signaling Axis: A Ray of Hope in Cardiovascular Diseases. Cardiol Res Pract. 2020;2020:5695723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 115. | Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, Shan B, Pan H, Yuan J. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci U S A. 2019;116:2996-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 116. | Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 635] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 117. | Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 118. | Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 5163] [Article Influence: 469.4] [Reference Citation Analysis (0)] |
| 119. | Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr Top Microbiol Immunol. 2017;403:143-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 120. | Chen C, Wang D, Yu Y, Zhao T, Min N, Wu Y, Kang L, Zhao Y, Du L, Zhang M, Gong J, Zhang Z, Zhang Y, Mi X, Yue S, Tan X. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021;12:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 121. | Yu S, Li Z, Zhang Q, Wang R, Zhao Z, Ding W, Wang F, Sun C, Tang J, Wang X, Zhang H, Huang R, Wu Q, Jiang J, Zhao X. GPX4 degradation via chaperone-mediated autophagy contributes to antimony-triggered neuronal ferroptosis. Ecotoxicol Environ Saf. 2022;234:113413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 122. | Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19:365-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 943] [Article Influence: 157.2] [Reference Citation Analysis (0)] |
| 123. | Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol. 2008;28:5747-5763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 124. | Mu L, Wang D, Dong Z, Wu J, Wu X, Su J, Zhang Y. Abnormal Levels of Serum Ferroptosis-Related Biomarkers in Diabetic Retinopathy. J Ophthalmol. 2022;2022:3353740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 125. | Chen YJ, Chen JT, Tai MC, Liang CM, Chen YY, Chen WL. Serum Iron and Risk of Diabetic Retinopathy. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 126. | Chaudhary K, Promsote W, Ananth S, Veeranan-Karmegam R, Tawfik A, Arjunan P, Martin P, Smith SB, Thangaraju M, Kisselev O, Ganapathy V, Gnana-Prakasam JP. Iron Overload Accelerates the Progression of Diabetic Retinopathy in Association with Increased Retinal Renin Expression. Sci Rep. 2018;8:3025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 127. | Liu J, Li X, Cheng Y, Liu K, Zou H, You Z. Identification of potential ferroptosis-related biomarkers and a pharmacological compound in diabetic retinopathy based on machine learning and molecular docking. Front Endocrinol (Lausanne). 2022;13:988506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 128. | Shao J, Bai Z, Zhang L, Zhang F. Ferrostatin-1 alleviates tissue and cell damage in diabetic retinopathy by improving the antioxidant capacity of the Xc(-)-GPX4 system. Cell Death Discov. 2022;8:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 129. | Zhu Z, Duan P, Song H, Zhou R, Chen T. Downregulation of Circular RNA PSEN1 ameliorates ferroptosis of the high glucose treated retinal pigment epithelial cells via miR-200b-3p/cofilin-2 axis. Bioengineered. 2021;12:12555-12567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 130. | Tang X, Li X, Zhang D, Han W. Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered. 2022;13:8240-8254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 131. | Zhou T, Zhou KK, Lee K, Gao G, Lyons TJ, Kowluru R, Ma JX. The role of lipid peroxidation products and oxidative stress in activation of the canonical wingless-type MMTV integration site (WNT) pathway in a rat model of diabetic retinopathy. Diabetologia. 2011;54:459-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 132. | Liu Y, Zhang D, Wu Y, Ji B. Docosahexaenoic acid aggravates photooxidative damage in retinal pigment epithelial cells via lipid peroxidation. J Photochem Photobiol B. 2014;140:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 133. | Nakamura T, Naguro I, Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta Gen Subj. 2019;1863:1398-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 134. | Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1637] [Cited by in RCA: 1994] [Article Influence: 249.3] [Reference Citation Analysis (0)] |
| 135. | Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim Biophys Acta Mol Cell Res. 2019;1866:118535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 536] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 136. | Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1703] [Article Influence: 154.8] [Reference Citation Analysis (1)] |
| 137. | Gao M, Monian P, Jiang X. Metabolism and iron signaling in ferroptotic cell death. Oncotarget. 2015;6:35145-35146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 138. | Zheng D, Liu J, Piao H, Zhu Z, Wei R, Liu K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol. 2022;13:1039241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 285] [Reference Citation Analysis (0)] |
| 139. | Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 2096] [Article Influence: 209.6] [Reference Citation Analysis (0)] |
| 140. | Khumaedi AI, Purnamasari D, Wijaya IP, Soeroso Y. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes Metab Syndr. 2019;13:1675-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 141. | Ryan SK, Zelic M, Han Y, Teeple E, Chen L, Sadeghi M, Shankara S, Guo L, Li C, Pontarelli F, Jensen EH, Comer AL, Kumar D, Zhang M, Gans J, Zhang B, Proto JD, Saleh J, Dodge JC, Savova V, Rajpal D, Ofengeim D, Hammond TR. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat Neurosci. 2023;26:12-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 206] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 142. | Yao F, Peng J, Zhang E, Ji D, Gao Z, Tang Y, Yao X, Xia X. Pathologically high intraocular pressure disturbs normal iron homeostasis and leads to retinal ganglion cell ferroptosis in glaucoma. Cell Death Differ. 2023;30:69-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 99] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 143. | Huang Y, Peng J, Liang Q. Identification of key ferroptosis genes in diabetic retinopathy based on bioinformatics analysis. PLoS One. 2023;18:e0280548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 144. | Saxena M, Singhal SS, Awasthi YC. A specific, sensitive, and rapid method for the determination of glutathione and its application in ocular tissues. Exp Eye Res. 1992;55:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 145. | Huster D, Hjelle OP, Haug FM, Nagelhus EA, Reichelt W, Ottersen OP. Subcellular compartmentation of glutathione and glutathione precursors. A high resolution immunogold analysis of the outer retina of guinea pig. Anat Embryol (Berl). 1998;198:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 146. | Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195-2209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1001] [Cited by in RCA: 1117] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 147. | Sun Y, Zheng Y, Wang C, Liu Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018;9:753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 404] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 148. | Yu F, Wang C, Su Y, Chen T, Zhu W, Dong X, Ke W, Cai L, Yang S, Wan P. Comprehensive analysis of ferritinophagy-related genes and immune infiltration landscape in diabetic retinopathy. Front Endocrinol (Lausanne). 2023;14:1177488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 149. | Liu C, Sun W, Zhu T, Shi S, Zhang J, Wang J, Gao F, Ou Q, Jin C, Li J, Xu JY, Zhang J, Tian H, Xu GT, Lu L. Glia maturation factor-β induces ferroptosis by impairing chaperone-mediated autophagic degradation of ACSL4 in early diabetic retinopathy. Redox Biol. 2022;52:102292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 150. | Itoh K, Furuhashi M, Ida Y, Ohguro H, Watanabe M, Suzuki S, Hikage F. Detection of significantly high vitreous concentrations of fatty acid-binding protein 4 in patients with proliferative diabetic retinopathy. Sci Rep. 2021;11:12382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 151. | Zhang XZ, Tu WJ, Wang H, Zhao Q, Liu Q, Sun L, Yu L. Circulating Serum Fatty Acid-Binding Protein 4 Levels Predict the Development of Diabetic Retinopathy in Type 2 Diabetic Patients. Am J Ophthalmol. 2018;187:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 152. | Fan X, Xu M, Ren Q, Fan Y, Liu B, Chen J, Wang Z, Sun X. Downregulation of fatty acid binding protein 4 alleviates lipid peroxidation and oxidative stress in diabetic retinopathy by regulating peroxisome proliferator-activated receptor γ-mediated ferroptosis. Bioengineered. 2022;13:10540-10551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 153. | Lv H, Zhen C, Liu J, Yang P, Hu L, Shang P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid Med Cell Longev. 2019;2019:3150145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 154. | Bajic VP, Van Neste C, Obradovic M, Zafirovic S, Radak D, Bajic VB, Essack M, Isenovic ER. Glutathione "Redox Homeostasis" and Its Relation to Cardiovascular Disease. Oxid Med Cell Longev. 2019;2019:5028181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 155. | Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond). 2018;38:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 565] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 156. | Zhan D, Zhao J, Shi Q, Lou J, Wang W. 25-hydroxyvitamin D3 inhibits oxidative stress and ferroptosis in retinal microvascular endothelial cells induced by high glucose through down-regulation of miR-93. BMC Ophthalmol. 2023;23:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 157. | Zhang J, Qiu Q, Wang H, Chen C, Luo D. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp Cell Res. 2021;407:112800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 158. | Li S, Lu S, Wang L, Liu S, Zhang L, Du J, Wu Z, Huang X. Effects of amygdalin on ferroptosis and oxidative stress in diabetic retinopathy progression via the NRF2/ARE signaling pathway. Exp Eye Res. 2023;234:109569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (1)] |