Published online Oct 15, 2024. doi: 10.4239/wjd.v15.i10.2135
Revised: August 10, 2024
Accepted: September 6, 2024
Published online: October 15, 2024
Processing time: 134 Days and 20.4 Hours
Glucagon-like peptide-1 receptor agonists (GLP-1RA) and sodium-glucose co-transporter-2 inhibitors (SGLT-2I) are associated with significant cardiovascular benefit in type 2 diabetes (T2D). However, GLP-1RA or SGLT-2I alone may not improve some cardiovascular outcomes in patients with prior cardiovascular co-morbidities.
To explore whether combining GLP-1RA and SGLT-2I can achieve additional benefit in preventing cardiovascular diseases in T2D.
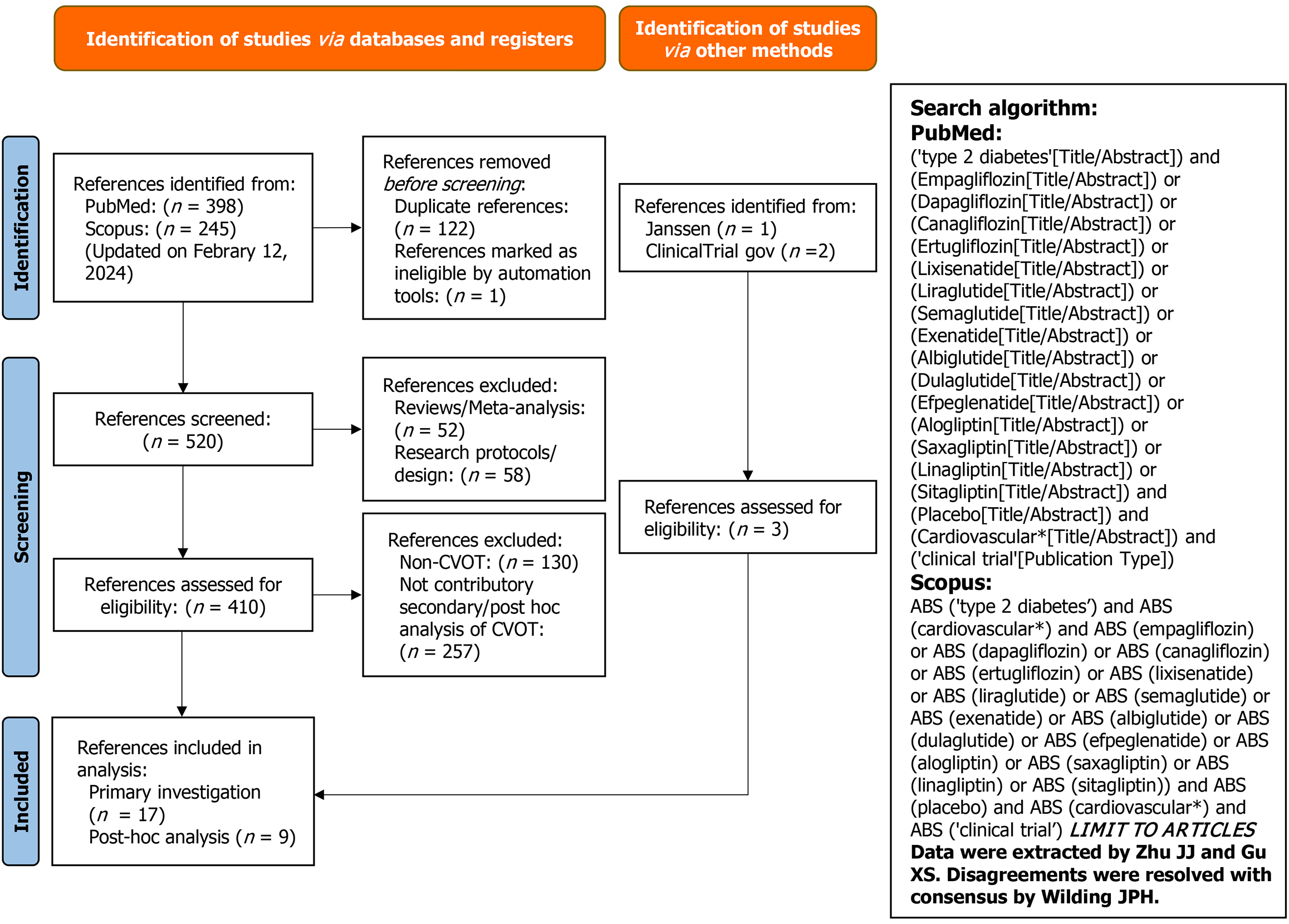
The systematic review was conducted according to PRISMA recommendations. The protocol was registered on PROSPERO (ID: 42022385007). A total of 107049 participants from eligible cardiovascular outcomes trials of GLP-1RA and SGLT-2I were included in network meta-regressions to estimate cardiovascular benefit of the combination treatment. Effect modification of prior myocardial infarction (MI) and heart failure (HF) was also explored to provide clinical insight as to when the combination treatment should be considered.
The estimated hazard ratios (HR)GLP-1RA/SGLT-2IvsPlacebo (0.75-0.98) and HRCombinationvsGLP-1RA/SGLT-2I (0.26-0.86) for primary and secondary cardiovascular outcomes suggested that the combination treatment may achieve additional cardiovascular benefit compared with GLP-1RA or SGLT-2I alone. In patients with prior MI or HF, the mono-therapies may not improve the overall cardiovascular outcomes, as the estimated HRMI+/HF+ (0.57-1.52) suggested that GLP-1RA or SGLT-2I alone may be associated with lower risks of hospitalization for HF but not cardiovascular death.
Considering its greater cardiovascular benefit in T2D, the combination treatment of GLP-1RA and SGLT-2I might be prioritized in patients with prior MI or HF, where the monotherapies may not provide sufficient cardiovascular protection.
Core Tip: Major cardiovascular outcome trials suggest that patients with prior cardiovascular co-morbidities may not gain sufficient cardiovascular protection from glucagon-like peptide-1 receptor agonists (GLP-1RA) or sodium-glucose co-transporter-2 inhibitors (SGLT-2I) alone. This systematic review with network meta-regression demonstrated that the combination treatment may provide greater cardiovascular benefit compared with GLP-1RA or SGLT-2I alone. In patients with prior myocardial infarction or heart failure, the monotherapies may not be associated with consistently improved cardiovascular outcomes, hence the combination treatment might be considered for cardiovascular disease prevention.
- Citation: Zhu JJ, Wilding JPH, Gu XS. Combining GLP-1 receptor agonists and SGLT-2 inhibitors for cardiovascular disease prevention in type 2 diabetes: A systematic review with multiple network meta-regressions. World J Diabetes 2024; 15(10): 2135-2146
- URL: https://www.wjgnet.com/1948-9358/full/v15/i10/2135.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i10.2135
The macro- and micro-vascular benefits of glucagon-like peptide-1 receptor agonists (GLP-1RA) and sodium-glucose co-transporter-2 inhibitors (SGLT-2I) are independent of their glucose-lowering effects[1]. In patients with type 2 diabetes (T2D), the major cardiovascular outcome trials (CVOT) showed that dipeptidyl peptidase-4 inhibitors (DPP-4I) did not improve cardiovascular outcomes[2], whereas cardiovascular benefit of GLP-1RA or SGLT-2I was significant[3,4]. Further subgroup analyses indicated that the background cardiovascular risk should be considered when examining the cardiovascular outcomes of these newer glucose-lowering medications. For instance, prevention of major adverse cardiovascular events (MACE) was only seen in those patients with baseline atherosclerotic cardiovascular disease[3,4]. Moreover, a series of CVOT conducted in patients with heart failure (HF) have demonstrated that (compared with placebo) SGLT-2I significantly reduced risk of hospitalization for HF or cardiovascular death, irrespective of their history of T2D[5-8]. However, similar cardiovascular benefits were not observed in those with myocardial infarction (MI)[9,10]. Cardiovascular co-morbidities are not only approximately twice as common but are also associated with disproportionately worse cardiovascular outcomes in patients with T2D, compared to the general population[11]. Therefore, it is of clinical importance to investigate whether the combination treatment of GLP-1RA and SGLT-2I could achieve greater cardiovascular benefit, particularly when considering patients with cardiovascular co-morbidities who may not gain sufficient cardiovascular protection from the monotherapies.
This systematic review with multiple network meta-regressions was mainly aimed to explore whether combining GLP-1RA and SGLT-2I can provide additional cardiovascular benefit in T2D. Cardiovascular outcomes of these newer antidiabetic medications were also estimated under effect modification of prior cardiovascular diseases. This was to provide clinical insight as to when the combination treatment might be prioritized.
We conducted a comprehensive systematic review with multiple network meta-regressions (and parallel network meta-analyses) according to PRISMA recommendations[12]. The protocol is registered in PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022385007). PubMed, Scopus, the ClinicalTrials.gov registry, and Center for Drug Evaluation and Research were searched for eligible CVOT and associated post-hoc analyses (Figure 1). The study search was initially performed on October 19, 2023 and further updated on February 12, 2024. Studies included in analysis were those only conducted in adult patients with T2D receiving DPP-4I, GLP-1RA, or SGLT-2I (Table 1 and Supplementary Figure 1). CVOT of these antidiabetic medications while recruiting patients without T2D (which were determined at baseline) were excluded. The Cochrane Collaboration’s Risk-of-Bias tool was applied for quality ass-essment (Supplementary Figure 2).
| Year | CVOT | Intervention | Median follow-up (year) | History of MI (yes, %) | History of HF (yes, %) | Post-baseline GLP-1RA/SGLT-2I (yes, %), intervention/placebo |
| 2013 | EXAMINE[25] | Alogliptin | 1.5 | N/A | N/A | N/A |
| 2013 | SAVOR-TIMI 53[26] | Saxagliptin | 2.1 | N/A | N/A | N/A |
| 2015 | TECOS[27] | Sitagliptin | 3.0 | N/A | N/A | N/A |
| 2019 2015 | CARMELINA[28] ELIXA[29] | Linagliptin Lixisenatide | 2.2 2.1 | N/A 22 | N/A 22 | N/A N/A |
| 2016 | SUSTAIN-6[30] | Semaglutide | 2.1 | 32 | 17[31] | 1.5/1.2 |
| 2016 | LEADER[32] | Liraglutide | 3.8 | 31 | 18[33] | 2.1/2.81 |
| 2017 | EXSCEL[34] | Exenatide | 3.2 | 32[35] | 16 | 4.4/5.8 |
| 2018 | HARMONY OUTCOMES[36] | Albiglutide | 1.5 | 47 | 20 | 9.7/10.8 |
| 2019 | REWIND[37] | Dulaglutide | 5.4 | 16[38] | 9 | 5.2/7.3 |
| 2019 | PIONEER-6[39] | Semaglutide | 1.3 | 36[31] | 12[31] | 13.5/15.8 |
| 2021 | AMPLITUDE-O[40] | Efpeglenatide | 1.8 | N/A | 18 | 17.5/21.2 |
| 2015 | EMPA-REG OUTCOME[41] | Empagliflozin | 3.1 | 47 | 10 | N/A |
| 2017 | CANVAS[42] | Canagliflozin | 2.4 | 29[43] | 14 | 6.2/7.72 |
| 2019 | DECLARE-TIMI 58[44] | Dapagliflozin | 4.2 | 21[45] | 10 | 9.5/11.5[19] |
| 2019 | CREDENCE[46] | Canagliflozin | 2.6 | 10[47] | 15 | 6.5/6.9 |
| 2020 | VERTIS CV[48] | Ertugliflozin | 3.5 | 48 | 24 | 4.9/5.6 |
Effect sizes [i.e., hazard ratios (HR)] indicating treatment effects of these newer antidiabetic medications on primary and secondary cardiovascular outcomes, were extracted from the eligible CVOT (Supplementary Figures 3-7), and converted to statistics including mean logHR and their standard errors [calculated using HR and 95% confidence intervals (95%CI)] for network meta-regressions and meta-analyses[13]. Covariates including percentages of patients receiving the co-treatment with GLP-1RA or SGLT-2I and having baseline prior MI or HF in the placebo and treatment groups, were retrieved from the CVOT of GLP-1RA and SGLT-2I for network meta-regressions (Table 1).
A set of Bayesian network meta-analyses were initially performed to compare the cardiovascular outcomes among these antidiabetic medications (including DPP-4I). The between-study heterogeneities were assessed using the I2 and τ2 statistics (Supplementary Figures 3-7). Surface under the cumulative ranking curve (SUCRA) was also calculated for efficacy comparisons.
Furthermore, we conducted multiple Bayesian network meta-regressions to explore the effect modification of GLP-1RA on treatment efficacies of SGLT-2I and vice versa, which is equivalent to answering the main research question of whether the combination treatment of GLP-1RA and SGLT-2I can provide additional cardiovascular benefit. The network meta-regression model was constructed to establish a correlation between the covariate and effect size (i.e., HR) observed in the CVOT. The correlation, namely, effect modification, can be numerically quantified as a coefficient (β)[14]. Given that this statistical model is linear[14], the percentages of patients ever receiving the co-treatment during the CVOT, namely, the postbaseline co-treatment, were incorporated as the covariate. This approach could yield results with more accuracy than those incorporating the baseline co-treatment. HR0/GLP-1RA/SGLT-2IvsPlacebo and HR1/CombinationvsGLP-1RA/SGLT-2I were thus estimated when assigning covariate = 0 or 1, assuming either 0% or 100% patients receiving the co-treatment in the CVOT, and compared with HRNA from the parallel network meta-analyses (indicating the effect size observed from the CVOT with the actual percentages of patients receiving the co-treatment).
Likewise, cardiovascular outcomes of GLP-1RA or SGLT-2I were explored under effect modification of prior cardiovascular diseases. Percentages of patients having baseline MI or HF were incorporated as the covariates. HR0/Disease- and HR1/Disease+, were estimated when assigning covariate = 0 or 1 with the assumption being that either 0% or 100% patients having MI or HF in the CVOT; and compared with HRNA from meta-analyses (indicating the effect size observed from the CVOT with the actual percentages of patients having the co-morbidities).
In addition, I2 or τ2 in the network meta-regressions and run-in-parallel network meta-analyses (without covariate incorporation) was compared to determine the covariate effects on between-study heterogeneity. All the analyses were conducted with R version 4.2.3 using the GEMTC packages. We used four Markov chains with 150000 iterations after an initial burn-in of 20000 and a thinning of 1 for all the analyses (Supplementary material). As all the eligible CVOT for analysis were double-blind and randomized placebo-controlled trials, inconsistency was not assessed (Supplementary Figure 1).
The credibility of all the proposed effect modifications was assessed using the instrument for the credibility of effect modification analyses (ICEMAN)[15]. For the credibility question 5, considering a Bayesian network meta-regression model applied in this study, 95%CI of the β (instead of P values) was included to indicate the results of the interaction test (Supplementary material).
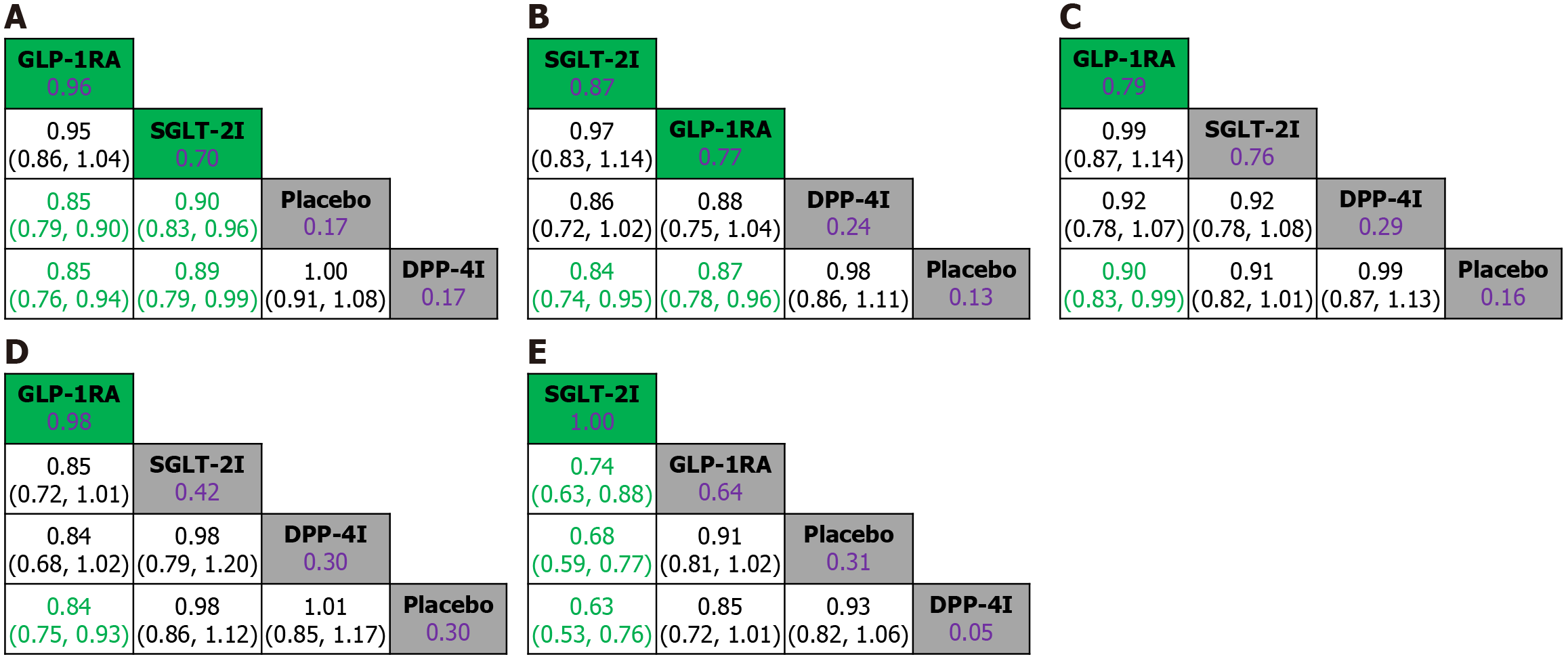
To determine the cardiovascular benefits of GLP-1RA and SGLT-2I, a total of 150423 participants in the CVOT were incorporated in the overall network meta-analysis to compare primary and secondary cardiovascular outcomes among DPP-4I, GLP-1RA, and SGLT-2I in T2D (Table 1, Supplementary Figure 1, and Figure 2). Based on our preliminary test results (not shown), network meta-analyses using relative risks would significantly underestimate the cardiovascular benefit of SGLT-2I, hence the results using survival (i.e., HR) rather than count statistics have greater robustness. The overall heterogeneities were low, with I2 of 0% and 19% and τ2 of 0.001 to 0.005 (Supplementary Figures 3-7). Compared with placebo, DPP-4I demonstrated no risk-reducing effects on any of the cardiovascular outcomes. Both GLP-1RA and SGLT-2I significantly reduced risks of MACE (HRGLP-1RAvsPlacebo = 0.85, 95%CI: 0.79-0.90; HRSGLT-2IvsPlacebo = 0.90, 95%CI: 0.83-0.96) and cardiovascular death (HRGLP-1RAvsPlacebo = 0.87, 95%CI: 0.74-0.95; HRSGLT-2IvsPlacebo = 0.84, 95%CI: 0.74-0.95). Moreover, GLP-1RA might have modest benefit over SGLT-2I in reducing risks of MACE (HRGLP-1RAvsSGLT-2I = 0.95, 95%CI: 0.86-1.04; SUCRAGLP-1RA = 0.95, SUCRASGLT-2I = 0.70). Whereas for cardiovascular death, SGLT-2I might be associated with lower risk compared with GLP-1RA (HRSGLT-2IvsGLP-1RA = 0.97, 95%CI: 0.83-1.14; SUCRASGLT-2I = 0.87, SUCRAGLP-1RA = 0.77). GLP-1RA also demonstrated significant risk-reducing effects on fatal and non-fatal MI (HRGLP-1RAvsPlacebo = 0.90, 95%CI: 0.83-0.99), and fatal and non-fatal stroke (HRGLP-1RAvsPlacebo = 0.84, 95%CI: 0.75-0.93). Compared with the other interventions, SGLT-2I achieved the most significant and superior benefit in reducing risk of hospitalization for HF (e.g., HRSGLT-2IvsGLP-1RA = 0.74, 95%CI: 0.63-0.88; SUCRASGLT-2I = 1; Figure 2).
Estimation of the combination treatment for cardiovascular disease prevention in T2D was further conducted in 107049 participants only in the CVOT of GLP-1RA and SGLT-2I. Potential effect modification of co-treatment with SGLT-2I on cardiovascular outcomes of GLP-1RA (and vice versa) was analyzed using network meta-regressions. The overall negative β (-0.13 to -0.01) indicated that there might be a positive effect modification of the co-treatment on improvement of the primary and secondary cardiovascular outcomes, i.e., the higher the percentages of patients receiving the combination treatment, the lower the HR (Supplementary Table 1), which is in consistent with comparisons among HR0, HR1, and HRNA (e.g., HR0 > HRNA > HR1/CombinationvsGLP-1RA/SGLT-2I; Table 2). In patients not receiving the co-treatment, GLP-1RA or SGLT-2I (alone) could improve cardiovascular outcomes (compared to placebo). The lack of statistical significance in some of the results could stem from EMPA-REG OUTCOME being excluded from the network meta-regressions, as this trial did not report percentages of patients receiving the post co-treatment of GLP-1RA in the placebo and SGLT-2I groups (Table 1). In patients receiving the co-treatment, the combination treatment was estimated to be associated with additional cardiovascular benefit in preventing MACE compared to either GLP-1RA (HR1 = 0.51, 95%CI: 0.16-1.65) or SGLT-2I (HR1 = 0.48, 95%CI: 0.15-1.54) alone. Similar effect sizes were also assessed for cardiovascular death and fatal and non-fatal MI. Although to a lesser extent, the combination treatment might further lower the risk of fatal and non-fatal stroke compared with GLP-1RA (HR1 = 0.86, 95%CI: 0.12-6.23) or SGLT-2I (HR1 = 0.74, 95%CI: 0.10-5.47) alone. Moreover, hospitalization for HF might be prevented to a greater extent in patients receiving the combination treatment rather than receiving GLP-1RA (HR1 = 0.26, 95%CI: 0.03-1.88) or SGLT-2I (HR1 = 0.33, 95%CI: 0.04-2.53; Table 2) alone. Taken together, the estimated effect sizes, i.e., HR1, were all numerically but not significantly favorable to the combination treatment, suggesting that the combination treatment may achieve greater benefit than the monotherapies in preventing cardiovascular diseases in patients with T2D.
| Cardiovascular outcome | Covariate | Intervention | HR with 95%CI |
| MACE | NA | GLP-1RA vs Placebo | 0.84 (0.77-0.90) |
| MACE | 0 | GLP-1RA vs Placebo | 0.89 (0.77-0.99) |
| MACE | NA | SGLT-2I vs Placebo | 0.90 (0.82-0.98) |
| MACE | 0 | SGLT-2I vs Placebo | 0.95 (0.82-1.08) |
| MACE | 1 | Combination vs GLP-1RA | 0.51 (0.16-1.65) |
| MACE | 1 | Combination vs SGLT-2I | 0.48 (0.15-1.54) |
| Cardiovascular death | NA | GLP-1RA vs Placebo | 0.85 (0.76-0.94) |
| Cardiovascular death | 0 | GLP-1RA vs Placebo | 0.88 (0.73-1.07) |
| Cardiovascular death | NA | SGLT-2I vs Placebo | 0.90 (0.79-1.02) |
| Cardiovascular death | 0 | SGLT-2I vs Placebo | 0.93 (0.76-1.16) |
| Cardiovascular death | 1 | Combination vs GLP-1RA | 0.58 (0.08-3.39) |
| Cardiovascular death | 1 | Combination vs SGLT-2I | 0.55 (0.07-3.25) |
| Fatal and non-fatal MI | NA | GLP-1RA vs Placebo | 0.89 (0.79-0.98) |
| Fatal and non-fatal MI | 0 | GLP-1RA vs Placebo | 0.94 (0.79-1.09) |
| Fatal and non-fatal MI | NA | SGLT-2I vs Placebo | 0.92 (0.81-1.05) |
| Fatal and non-fatal MI | 0 | SGLT-2I vs Placebo | 0.98 (0.81-1.19) |
| Fatal and non-fatal MI | 1 | Combination vs GLP-1RA | 0.45 (0.10-2.18) |
| Fatal and non-fatal MI | 1 | Combination vs SGLT-2I | 0.44 (0.09-2.10) |
| Fatal and non-fatal stroke | NA | GLP-1RA vs Placebo | 0.81 (0.72-0.91) |
| Fatal and non-fatal stroke | 0 | GLP-1RA vs Placebo | 0.82 (0.67-1.00) |
| Fatal and non-fatal stroke | NA | SGLT-2I vs Placebo | 0.94 (0.82-1.08) |
| Fatal and non-fatal stroke | 0 | SGLT-2I vs Placebo | 0.95 (0.75-1.20) |
| Fatal and non-fatal stroke | 1 | Combination vs GLP-1RA | 0.86 (0.12-6.23) |
| Fatal and non-fatal stroke | 1 | Combination vs SGLT-2I | 0.74 (0.10-5.47) |
| Hospitalization for HF | NA | GLP-1RA vs Placebo | 0.90 (0.79-1.02) |
| Hospitalization for HF | 0 | GLP-1RA vs Placebo | 0.97 (0.80-1.19) |
| Hospitalization for HF | NA | SGLT-2I vs Placebo | 0.68 (0.59-0.79) |
| Hospitalization for HF | 0 | SGLT-2I vs Placebo | 0.75 (0.59-0.96) |
| Hospitalization for HF | 1 | Combination vs GLP-1RA | 0.26 (0.03-1.88) |
| Hospitalization for HF | 1 | Combination vs SGLT-2I | 0.33 (0.04-2.53) |
Regarding the primary and secondary cardiovascular outcomes, low degrees of variations between I2 or τ2 in the meta-regressions and meta-analyses might eliminate the probability of the co-treatments being sources of between-study heterogeneity (Supplementary Table 1). However, for the effect modifications of the co-treatments, the overall credibility ratings ranged from low to moderate (Supplementary material).
Effect modification of prior MI or HF on cardiovascular outcomes in patients receiving GLP-1RA or SGLT-2I were likewise explored in network meta-regressions. The negative β (-0.07 to -0.01) indicated that GLP-1RA and SGLT-2I might be more effective in prevention of cardiovascular death and hospitalization for HF in trial populations with higher rates of MI and HF, respectively (Supplementary Table 2). In patients without prior MI, GLP-1RA were estimated to be associated with a significant risk reduction in cardiovascular death (HR0 = 0.88, 95%CI: 0.76-0.99), whereas the effect size might modestly increase in patients with prior MI (HR1 = 0.74, 95%CI: 0.26-2.01). Similarly, in patients without prior HF, SGLT-2I could significantly reduce the risk of hospitalization for HF (HR0 = 0.68, 95%CI: 0.60-0.76), and additional risk reduction was estimated in patients with prior HF (HR1 = 0.62, 95%CI: 0.14-2.80; Table 3). However, the estimated cardiovascular benefit of GLP-1RA and SGLT-2I was numerically but not statistically conclusive in patients with these preexisting cardiovascular co-morbidities.
| Cardiovascular outcome | Covariate | Intervention | HR with 95%CI | |
| Fatal and non-fatal MI | Prior history of MI | NA | GLP-1RA vs Placebo | 0.91 (0.84-1.01) |
| Fatal and non-fatal MI | Prior history of MI | 0 | GLP-1RA vs Placebo | 1.13 (0.85-1.51) |
| Fatal and non-fatal MI | Prior history of MI | 1 | GLP-1RA vs Placebo | 0.57 (0.30-1.05) |
| Fatal and non-fatal MI | Prior history of MI | NA | SGLT-2I vs Placebo | 0.91 (0.82-1.02) |
| Fatal and non-fatal MI | Prior history of MI | 0 | SGLT-2I vs Placebo | 0.84 (0.66-1.07) |
| Fatal and non-fatal MI | Prior history of MI | 1 | SGLT-2I vs Placebo | 1.09 (0.66-1.80) |
| Cardiovascular death | Prior history of MI | NA | GLP-1RA vs Placebo | 0.88 (0.76-0.99) |
| Cardiovascular death | Prior history of MI | 0 | GLP-1RA vs Placebo | 0.93 (0.59-1.48) |
| Cardiovascular death | Prior history of MI | 1 | GLP-1RA vs Placebo | 0.74 (0.26-2.01) |
| Cardiovascular death | Prior history of MI | NA | SGLT-2I vs Placebo | 0.84 (0.72-0.96) |
| Cardiovascular death | Prior history of MI | 0 | SGLT-2I vs Placebo | 0.92 (0.62-1.32) |
| Cardiovascular death | Prior history of MI | 1 | SGLT-2I vs Placebo | 0.68 (0.32-1.48) |
| Hospitalization for HF | Prior history of HF | NA | GLP-1RA vs Placebo | 0.91 (0.82-1.02) |
| Hospitalization for HF | Prior history of HF | 0 | GLP-1RA vs Placebo | 0.93 (0.61-1.42) |
| Hospitalization for HF | Prior history of HF | 1 | GLP-1RA vs Placebo | 0.84 (0.20-3.67) |
| Hospitalization for HF | Prior history of HF | NA | SGLT-2I vs Placebo | 0.68 (0.60-0.76) |
| Hospitalization for HF | Prior history of HF | 0 | SGLT-2I vs Placebo | 0.69 (0.52-0.90) |
| Hospitalization for HF | Prior history of HF | 1 | SGLT-2I vs Placebo | 0.62 (0.14-2.80) |
| Cardiovascular death | Prior history of HF | NA | GLP-1RA vs Placebo | 0.86 (0.76-0.97) |
| Cardiovascular death | Prior history of HF | 0 | GLP-1RA vs Placebo | 0.77 (0.51-1.08) |
| Cardiovascular death | Prior history of HF | 1 | GLP-1RA vs Placebo | 1.52 (0.30-10.07) |
| Cardiovascular death | Prior history of HF | NA | SGLT-2I vs Placebo | 0.84 (0.73-0.96) |
| Cardiovascular death | Prior history of HF | 0 | SGLT-2I vs Placebo | 0.76 (0.52-1.04) |
| Cardiovascular death | Prior history of HF | 1 | SGLT-2I vs Placebo | 1.51 (0.29-10.38) |
In contrast, the positive β (0.05-0.08) indicated that GLP-1RA and SGLT-2I might demonstrate reduced effectiveness in preventing cardiovascular death and recurrent MI as the prevalence of MI and HF within trial populations increased (Supplementary Table 2). In patients without prior HF, both GLP-1RA and SGLT-2I could significantly reduce the risk for cardiovascular death (HR0/GLP-1RA = 0.86, 95%CI: 0.76-0.97; HR0/SGLT-2I = 0.84, 95%CI: 0.73-0.96). However, these risk reduction effects were estimated to be neutral in patients with prior HF (HR1/GLP-1RA = 1.52, 95%CI: 0.30-10.07); HR1/SGLT-2I = 1.51, 95%CI: 0.29-10.38; Table 3).
Compared with fatal and non-fatal MI or hospitalization for HF, cardiovascular death demonstrated the greatest heterogeneities as I2 = 19% indicated (Supplementary Figures 4, 5, and 7). Notably, the I2 and τ2 were reduced when incorporating covariates of prior HF or MI, suggesting that these co-morbidities could be also considered sources of the between-study heterogeneities (Supplementary Table 2). With respect to the effect modifications of these cardiovascular diseases in patients receiving the mono-antidiabetic treatment with GLP-1RA or SGLT-2I, the overall credibility ratings ranged from low to moderate (Supplementary material).
The initial network meta-analyses confirmed the cardiovascular benefit of GLP-1RA and SGLT-2I in T2D. GLP-1RA demonstrated remarkable risk reductions in various adverse cardiovascular outcomes. SGLT-2I had superior benefit in preventing cardiovascular death and hospitalization for HF. Compared with previous analyses[16], our study exhibited lower heterogeneities and generated results with higher robustness. These advantages can be attributed to analysis using survival rather than count statistics and incorporation of CVOT exclusively conducted in patients with T2D.
To date, there has not been any systematic review examining whether the combination treatment of GLP-1RA and SGLT-2I can prevent cardiovascular diseases in T2D. It should be noted that running separate subgroup analyses is not a correct method to investigate effect modification in network meta-analysis as it cannot guarantee same estimates of between-trial variation nor produce test of interaction to reject the null hypothesis of equal effects[17]. Therefore, our study used a robust network meta-regression model to explore the cardiovascular benefit of the combination treatment via estimating the effect modification of GLP-1RA on treatment efficacies of SGLT-2I (and vice versa). Moreover, from a methodological standpoint, covariate incorporation in meta-regression can avoid unbalanced hazards between intervention groups (which can be introduced via covariate stratification in sub-group analysis[18-21]), thereby estimating effect sizes with greater precision. Consistent with previous post hoc subgroup and propensity score matching analyses[17-21], our results suggest that the combination treatment may achieve additional cardiovascular benefit in T2D[17-21]. A recent published real-world data based study further confirmed that the combination treatment was associated with both lower cardiovascular and risks compared with the monotherapies[22]. Mechanistically, their complementary actions on glucose, blood pressure, and lipid regulation might have contributed to the greater cardiovascular benefits[23].
Cardiovascular co-morbidities have been recognized as risk factors capable of potentially modifying cardiovascular benefit of GLP-1RA and SGLT-2I[3,4]. Our results indicated that SGLT-2I could significantly lower hospitalization for HF but not cardiovascular death in patients with HF, which are consistent with observations from the CVOT conducted in HF with preserved ejection fraction (e.g., EMPEROR-Preserved and DELIVER)[5,7]. In patients with prior MI, the EMPACT-MI trial showed that the SGLT-2I was not associated with improved cardiovascular outcomes[10], whereas our results indicated that the risk of cardiovascular death might be further reduced compared with those without prior MI, but the estimation remains statistically inconclusive as the 95%CI indicated. Similar effect modifications were also estimated in GLP-1RA. As GLP-1RA and SGLT-2I have become the most recommended second-line and, in some cases, first-line antidiabetic treatments, particularly for patients with “high risk” (e.g., atherosclerotic cardiovascular disease)[24], these specific cardiovascular conditions may be considered “above high risk” at which patients should receive the combination treatment of GLP-1RA and SGLT-2I to optimize the overall cardiovascular outcomes.
The overall credibility of these effect modifications was rated as low to moderate using ICEMAN. This is considered a major limitation of our study. Factors that underestimated the credibility include the over-specification of the network meta-regression model due to scarcity of the data points (e.g., only 13 available trials/baselines were included for analysis)[14]. Consequently, the β values were generated with less statistical power, which also contributes to the generally low to moderate credibility and may explain the very wide 95%CI of some estimated HR. Multiple interaction models using individual patient data should be undertaken in the future, to investigate cardiovascular and renal benefits of the combination treatment under effect modification of these cardiovascular co-morbidities. Nevertheless, further definitive trials are still in need to be able to support a strong recommendation to this effect.
The combination treatment of GLP-1RA and SGLT-2I may achieve additional cardiovascular benefit in T2D. In patients with prior cardiovascular co-morbidities including MI and HF, GLP-1RA or SGLT-2I alone may not significantly improve the overall cardiovascular outcomes, hence the combination treatment can be prioritized in such clinical scenarios.
The authors wish to thank Dr. Frank Vercruysse from Janssen Pharmaceuticals, Professor Rury Holman from University of Oxford, Dr. Susanna Stevens from Duke University, and Dr. Kajsa Kvist from Novo Nordisk for providing unpublished data (indicated in Table 1). The authors wish to thank Dr. Gert van Valkenhoef from the Cochrane Collabo
| 1. | Zhu J, Wilding JPH. Body Fat Depletion: the Yin Paradigm for Treating Type 2 Diabetes. Curr Atheroscler Rep. 2024;26:1-10. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Patoulias D, Boulmpou A, Teperikidis E, Katsimardou A, Siskos F, Tranidou A, Nikolaidis A, Mouselimis D, Doumas M, Papadopoulos CE, Vassilikos V. Meta-analysis of cardiovascular outcome trials assessing the cardiovascular efficacy and safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus. Eur Heart J. 2021;42:Suppl 1. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1914] [Article Influence: 319.0] [Reference Citation Analysis (0)] |
| 4. | Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 1058] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 5. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 2755] [Article Influence: 688.8] [Reference Citation Analysis (0)] |
| 6. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4429] [Article Influence: 738.2] [Reference Citation Analysis (0)] |
| 7. | Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022;387:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1426] [Article Influence: 475.3] [Reference Citation Analysis (0)] |
| 8. | Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 3163] [Article Influence: 632.6] [Reference Citation Analysis (0)] |
| 9. | James S, Erlinge D, Storey RF, McGuire DK, de Belder M, Eriksson N, Andersen K, Austin D, Arefalk G, Carrick D, Hofmann R, Hoole SP, Jones DA, Lee K, Tygesen H, Johansson PA, Langkilde AM, Ridderstråle W, Parvaresh Rizi E, Deanfield J, Oldgren J. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Evid. 2024;3:EVIDoa2300286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 141] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 10. | Butler J, Jones WS, Udell JA, Anker SD, Petrie MC, Harrington J, Mattheus M, Zwiener I, Amir O, Bahit MC, Bauersachs J, Bayes-Genis A, Chen Y, Chopra VK, Figtree G, Ge J, Goodman SG, Gotcheva N, Goto S, Gasior T, Jamal W, Januzzi JL, Jeong MH, Lopatin Y, Lopes RD, Merkely B, Parikh PB, Parkhomenko A, Ponikowski P, Rossello X, Schou M, Simic D, Steg PG, Szachniewicz J, van der Meer P, Vinereanu D, Zieroth S, Brueckmann M, Sumin M, Bhatt DL, Hernandez AF. Empagliflozin after Acute Myocardial Infarction. N Engl J Med. 2024;390:1455-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 134] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 11. | Chilton RJ, Dungan KM, Shubrook JH, Umpierrez GE. Cardiovascular risk and the implications for clinical practice of cardiovascular outcome trials in type 2 diabetes. Prim Care Diabetes. 2020;14:193-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4635] [Article Influence: 1158.8] [Reference Citation Analysis (0)] |
| 13. | Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol. 2010;10:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 14. | Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: Application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med. 2009;28:1861-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA, Gagnier J, Borenstein M, van der Heijden GJMG, Dahabreh IJ, Sun X, Sauerbrei W, Walsh M, Ioannidis JPA, Thabane L, Guyatt GH. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192:E901-E906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 16. | Lin DS, Lee JK, Hung CS, Chen WJ. The efficacy and safety of novel classes of glucose-lowering drugs for cardiovascular outcomes: a network meta-analysis of randomised clinical trials. Diabetologia. 2021;64:2676-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Sedgwick P. Meta-analyses: heterogeneity and subgroup analysis. BMJ. 2013;346:f4040. [RCA] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Clegg LE, Penland RC, Bachina S, Boulton DW, Thuresson M, Heerspink HJL, Gustavson S, Sjöström CD, Ruggles JA, Hernandez AF, Buse JB, Mentz RJ, Holman RR. Effects of exenatide and open-label SGLT2 inhibitor treatment, given in parallel or sequentially, on mortality and cardiovascular and renal outcomes in type 2 diabetes: insights from the EXSCEL trial. Cardiovasc Diabetol. 2019;18:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Cahn A, Wiviott SD, Mosenzon O, Murphy SA, Goodrich EL, Yanuv I, Rozenberg A, Wilding JPH, Leiter LA, Bhatt DL, McGuire DK, Litwak L, Kooy A, Gause-Nilsson IAM, Fredriksson M, Langkilde AM, Sabatine MS, Raz I. Cardiorenal outcomes with dapagliflozin by baseline glucose-lowering agents: Post hoc analyses from DECLARE-TIMI 58. Diabetes Obes Metab. 2021;23:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Lam CSP, Ramasundarahettige C, Branch KRH, Sattar N, Rosenstock J, Pratley R, Del Prato S, Lopes RD, Niemoeller E, Khurmi NS, Baek S, Gerstein HC. Efpeglenatide and Clinical Outcomes With and Without Concomitant Sodium-Glucose Cotransporter-2 Inhibition Use in Type 2 Diabetes: Exploratory Analysis of the AMPLITUDE-O Trial. Circulation. 2022;145:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Neves JS, Borges-Canha M, Vasques-Nóvoa F, Green JB, Leiter LA, Granger CB, Carvalho D, Leite-Moreira A, Hernandez AF, Del Prato S, McMurray JJV, Ferreira JP. GLP-1 Receptor Agonist Therapy With and Without SGLT2 Inhibitors in Patients With Type 2 Diabetes. J Am Coll Cardiol. 2023;82:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Simms-Williams N, Treves N, Yin H, Lu S, Yu O, Pradhan R, Renoux C, Suissa S, Azoulay L. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study. BMJ. 2024;385:e078242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 23. | Anderson JE. Combining Glucagon-Like Peptide 1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors to Target Multiple Organ Defects in Type 2 Diabetes. Diabetes Spectr. 2020;33:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S140-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 535] [Article Influence: 267.5] [Reference Citation Analysis (1)] |
| 25. | White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1897] [Article Influence: 158.1] [Reference Citation Analysis (0)] |
| 26. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2804] [Cited by in RCA: 2580] [Article Influence: 215.0] [Reference Citation Analysis (0)] |
| 27. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1831] [Cited by in RCA: 1876] [Article Influence: 187.6] [Reference Citation Analysis (0)] |
| 28. | Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK; CARMELINA Investigators. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019;321:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 787] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 29. | Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 1725] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 30. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4058] [Article Influence: 450.9] [Reference Citation Analysis (1)] |
| 31. | Husain M, Bain SC, Jeppesen OK, Lingvay I, Sørrig R, Treppendahl MB, Vilsbøll T. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22:442-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 32. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 4894] [Article Influence: 543.8] [Reference Citation Analysis (0)] |
| 33. | Marso SP, Baeres FMM, Bain SC, Goldman B, Husain M, Nauck MA, Poulter NR, Pratley RE, Thomsen AB, Buse JB; LEADER Trial Investigators. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Diabetes With or Without Heart Failure. J Am Coll Cardiol. 2020;75:1128-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 34. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1488] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 35. | Mentz RJ, Bethel MA, Merrill P, Lokhnygina Y, Buse JB, Chan JC, Felício JS, Goodman SG, Choi J, Gustavson SM, Iqbal N, Lopes RD, Maggioni AP, Öhman P, Pagidipati NJ, Poulter NR, Ramachandran A, Reicher B, Holman RR, Hernandez AF; EXSCEL Study Group. Effect of Once-Weekly Exenatide on Clinical Outcomes According to Baseline Risk in Patients With Type 2 Diabetes Mellitus: Insights From the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1213] [Article Influence: 173.3] [Reference Citation Analysis (0)] |
| 37. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1921] [Cited by in RCA: 1786] [Article Influence: 297.7] [Reference Citation Analysis (1)] |
| 38. | Branch KRH, Dagenais GR, Avezum A, Basile J, Conget I, Cushman WC, Jansky P, Lakshmanan M, Lanas F, Leiter LA, Pais P, Pogosova N, Raubenheimer PJ, Ryden L, Shaw JE, Sheu WHH, Temelkova-Kurktschiev T, Bethel MA, Gerstein HC, Chinthanie R, Probstfield JL. Dulaglutide and cardiovascular and heart failure outcomes in patients with and without heart failure: a post-hoc analysis from the REWIND randomized trial. Eur J Heart Fail. 2022;24:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC; PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1173] [Article Influence: 195.5] [Reference Citation Analysis (0)] |
| 40. | Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, Dyal L, Branch K; AMPLITUDE-O Trial Investigators. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N Engl J Med. 2021;385:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 472] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 41. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8280] [Article Influence: 828.0] [Reference Citation Analysis (1)] |
| 42. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5388] [Article Influence: 673.5] [Reference Citation Analysis (0)] |
| 43. | Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 392] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 44. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4242] [Article Influence: 707.0] [Reference Citation Analysis (0)] |
| 45. | Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Nicolau JC, Gause-Nilsson IAM, Fredriksson M, Langkilde AM, Sabatine MS, Wiviott SD. Dapagliflozin and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus and Previous Myocardial Infarction. Circulation. 2019;139:2516-2527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 46. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3967] [Article Influence: 661.2] [Reference Citation Analysis (0)] |
| 47. | Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Capuano G, de Zeeuw D, Greene T, Levin A, Pollock C, Sun T, Wheeler DC, Yavin Y, Zhang H, Zinman B, Rosenthal N, Brenner BM, Perkovic V. Canagliflozin and Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus and Chronic Kidney Disease in Primary and Secondary Cardiovascular Prevention Groups. Circulation. 2019;140:739-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 48. | Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK; VERTIS CV Investigators. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med. 2020;383:1425-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 1013] [Article Influence: 202.6] [Reference Citation Analysis (0)] |