Published online Oct 15, 2024. doi: 10.4239/wjd.v15.i10.2070
Revised: August 19, 2024
Accepted: September 2, 2024
Published online: October 15, 2024
Processing time: 64 Days and 20.5 Hours
Diabetic foot (DF) is a serious complication of type 2 diabetes. This study aimed to investigate the factors associated with DF occurrence and the role of delayed medical care in a cohort of patients with type 2 diabetes.
To reveal the impact of delayed medical treatment on the development of DF in patients with type 2 diabetes and to establish a predictive model for DF.
In this retrospective cohort study, 292 patients with type 2 diabetes who under
The DF group had significantly higher body mass index (BMI) (P < 0.001), disease duration (P = 0.012), plasma glucose levels (P < 0.001), and HbA1c (P < 0.001) than the NDF group. The NDF group had significantly higher Acute Thrombosis and Myocardial Infarction Health Service System (ATMHSS) scores (P < 0.001) and a significantly lower delayed medical treatment rate (72.38% vs 13.41%, P < 0.001). BMI, duration of diabetes, plasma glucose levels, HbA1c, diabetic peripheral neu
Delayed medical treatment significantly affects the probability of DF occurrence in patients with diabetes. Plasma glucose levels, HbA1c levels, and the combined predictive model of delayed medical treatment demonstrate good predictive value.
Core Tip: This retrospective cohort study investigates factors influencing diabetic foot (DF) in type 2 diabetes patients. Key findings highlight that increased body mass index, longer diabetes duration, elevated plasma glucose and HbA1c levels, as well as complications like diabetic neuropathy, are positively associated with DF occurrence. Additionally, a low Attitudes Toward Medical Help Seeking Scale score and delayed medical care over 3 months correlate with DF. These insights un
- Citation: Chen H, Xi Y. Delayed treatment of diabetic foot ulcer in patients with type 2 diabetes and its prediction model. World J Diabetes 2024; 15(10): 2070-2080
- URL: https://www.wjgnet.com/1948-9358/full/v15/i10/2070.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i10.2070
Type 2 diabetes is a long-term condition marked by the body's inability to properly use insulin or a reduced efficiency in insulin function, leading to persistently elevated blood sugar levels. Its epidemiological characteristics vary by region and population and are associated with genetic, lifestyle, and environmental factors[1,2]. Its typical manifestations include polyuria, polydipsia, polyphagia, and weight loss. Prolonged hyperglycemia can cause the occurrence and development of various complications[3,4].
Diabetic foot (DF) is a serious diabetes-related complication involving nerve damage in the lower extremities and various levels of vascular disease. This condition can cause infections, ulcers, and serious damage to deep tissues in patients. Severe symptoms can result in difficulty walking and even amputation, significantly influencing the quality of life of patients with diabetes[5,6]. At present, the cure rate for DF is improving and the amputation rate is gradually decreasing; however, its incidence is increasing year by year[7,8].
Delay in seeking medical care is defined as the behavior of individuals who do not seek timely medical care after discovering abnormal bodily symptoms due to objective or subjective reasons. This behavior occurs because the early symptoms of DF are not evident, and patients with diabetes do not actively seek foot examinations at hospitals to assess their risk of developing DF[9,10]. As a consequence, delayed medical care often leads to DF.
Although previous studies have analyzed the risk factors for DF[11-13], no research has focused on the impact of delayed medical care on the probability of DF occurrence in patients with diabetes. Therefore, investigating the impact of delayed medical care on DF occurrence in patients with diabetes is of utmost importance.
This retrospective cohort study included 292 individuals diagnosed with type 2 diabetes who received examinations at our hospital over the period from January 2023 to December 2023. They were divided into the DF group (n = 82) and nondiabetic foot group (n = 210, NDF group). This study was approved by the institutional review board and ethics committee of Shaanxi Provincial People’s Hospital. Given its retrospective design, this study only used data from unidentified patients and informed consent was waived.
The inclusion criteria were as follows: (1) Diagnosis of type 2 diabetes as per World Health Organization guidelines[14]; (2) Aged between 18 and 80 years; and (3) Ineligibility for surgical revascularization.
The exclusion criteria were as follows: (1) Prior occurrence of acute coronary syndrome, myocardial infarction, or transient ischemic stroke within the last 6 months; (2) Presence of uncontrolled immune disorders or active severe systemic infections; (3) Severe hematologic disorders or coagulation abnormalities; (4) History of malignant tumors; (5) Participation in other clinical trials within the preceding 3 months; or (6) Other concerns identified by the investigators that may impede compliance or safety.
The patients were grouped based on the presence or absence of DF: DF group (n = 82) and NDF group (n = 210). The diagnostic criteria were based on the 2012 Infectious Diseases Society of America clinical practice guidelines for DF infections[14].
Patient demographic data were acquired from the medical records system. Upon admission, 5 mL of fasting blood sample was obtained from the antecubital vein in the morning for blood testing. Hematological parameters such as hemoglobin (g/dL), hematocrit (%), white blood cell count (× 109/L), and platelet count (× 109/L) were measured using a fully automated coagulation analyzer (HC00608166, STA Compact, China).
Biochemical parameters and genomic and proteomic data were analyzed using venous blood samples of 5-7 mL (including whole blood, plasma, and serum). The measured biochemical markers included HbA1c, plasma glucose, total cholesterol (measured by the CHOD-PAP method), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, urea, and creatinine. All quantitative measurements were performed using a BS 400 auto-analyzer in accordance with the procedures provided by Dia Sys Diagnostic Systems GmbH, Germany. The glo
Delay in seeking medical care (patient delay) refers to the behavior of individuals who fail to seek timely medical at
Attitudes Toward Medical Help Seeking Scale (ATMHSS): The ATMHSS is a self-assessment scale consisting of 35 items divided into four dimensions: Behavioral intention (12 items), nonfatalism (11 items), medical trust (7 items), and nonavoidant attitudes (5 items). Each item is rated on a Likert 4-point scale, with “disagree” to “agree” corresponding to scores of 0-3. The total score ranges from 0 to 105, where a high score indicates a positive attitude toward seeking medical care. The scale has a Cronbach's α coefficient of 0.82.
The sample size was mainly depended on the number of patients included in the inclusion time frame complied with the events per variable > 10 principle. Using G*Power 3.1.9.7, we conducted a post hoc analysis based on the "Means: Dif
Patient characteristics were compared between the two groups using independent t-tests for continuous variables and χ2 tests for categorical variables. The normality of continuous variables was checked using the Shapiro-Wilk test. Normally distributed data were expressed as mean ± SD, and categorical variables were reported as counts and percentages. A P value of less than 0.05 was deemed statistically significant. All analyses were conducted with SPSS software, version 29.0 (SPSS Inc., Chicago, IL, United States).
Spearman correlation analysis was conducted. Indicators showing significant differences in the differential and correlation analyses were included as covariates in logistic regression analysis. The diagnostic efficiency of delayed medical care for DF was assessed using the area under the receiver operating characteristic (ROC) curve, and a combined predictive model was established by incorporating blood glucose levels, glycated protein levels, and delayed medical care.
Between-group comparison showed no statistically significant differences in general demographic data such as age and gender in the presence of DF (P > 0.05; Table 1). However, the body mass index (BMI) of the DF group was significantly higher than that of the NDF group (23.51 ± 3.57 vs 25.17 ± 3.57, P < 0.001). This finding suggests that an increase in BMI may elevate the incidence of DF.
| Characteristic | NDF group (n = 210) | DF group (n = 82) | t/χ2 | P value |
| Age (years) | 51.15 ± 9.32 | 52.69 ± 9.15 | 1.283 | 0.201 |
| BMI (kg/m2) | 23.51 ± 3.57 | 25.17 ± 3.57 | 3.588 | < 0.001 |
| Sex | 107 (50.95) | 47 (57.32) | 0.720 | 0.396 |
| Smoking history | 51 (24.29) | 19 (23.17) | 0.295 | 0.587 |
| Alcohol history | 65 (30.95) | 24 (29.27) | 0.060 | 0.807 |
| Family history of diabetes | 44 (20.95) | 21 (25.61) | 0.150 | 0.699 |
| Hypertension | 39 (18.57) | 18 (21.95)) | 1.229 | 0.268 |
| Hyperlipidemia | 37 (17.62) | 16 (19.51) |
Differential analysis was conducted on the occurrence probabilities of diabetes duration, classification, staging, and complications between the two groups (Table 2). No significant differences were observed in classification, staging, diabetic retinopathy, and diabetic vascular disease (P > 0.05). The duration of diabetes in the DF group was significantly longer than that in the NDF group (10.12 ± 4.95 vs 11.74 ± 4.84, P = 0.012). In addition, the occurrence rates of diabetic neuropathy (22.86% vs 40.2%, P = 0.005) and diabetic nephropathy (0.48% vs 4.88%, P = 0.035) were markedly lower in the NDF group compared with those in the DF group. These findings suggest that an increase in diabetes duration and the occurrence of some complications may contribute to the increased incidence of DF.
| Characteristic | NDF group (n = 210) | DF group (n = 82) | t/χ2 | P value | |
| Duration of diabetes (years) | 10.12 ± 4.95 | 11.74 ± 4.84 | 2.554 | 0.012 | |
| Types | Insulin resistance | 179 (85.24) | 70 (85.37) | 0.000 | 1.000 |
| Insufficient insulin secretion | 31 (14.76) | 12 (14.63) | |||
| Stages | Impaired glucose tolerance | 29 (13.81) | 11 (13.41) | 0.008 | 0.996 |
| Type 2 diabetes stage | 120 (57.14) | 47 (57.32) | |||
| Late stage of type 2 diabetes | 61 (29.05) | 24 (29.27) | |||
| Diabetic retinopathy | 43 (20.48) | 24 (29.27) | 2.105 | 0.147 | |
| Diabetic vascular disease | 69 (32.86) | 30 (36.59) | 0.218 | 0.640 | |
| Diabetic peripheral neuropathy | 48 (22.86) | 33 (40.24) | 8.048 | 0.005 | |
| Diabetic nephropathy | 1 (0.48) | 4 (4.88) | 4.426 | 0.035 | |
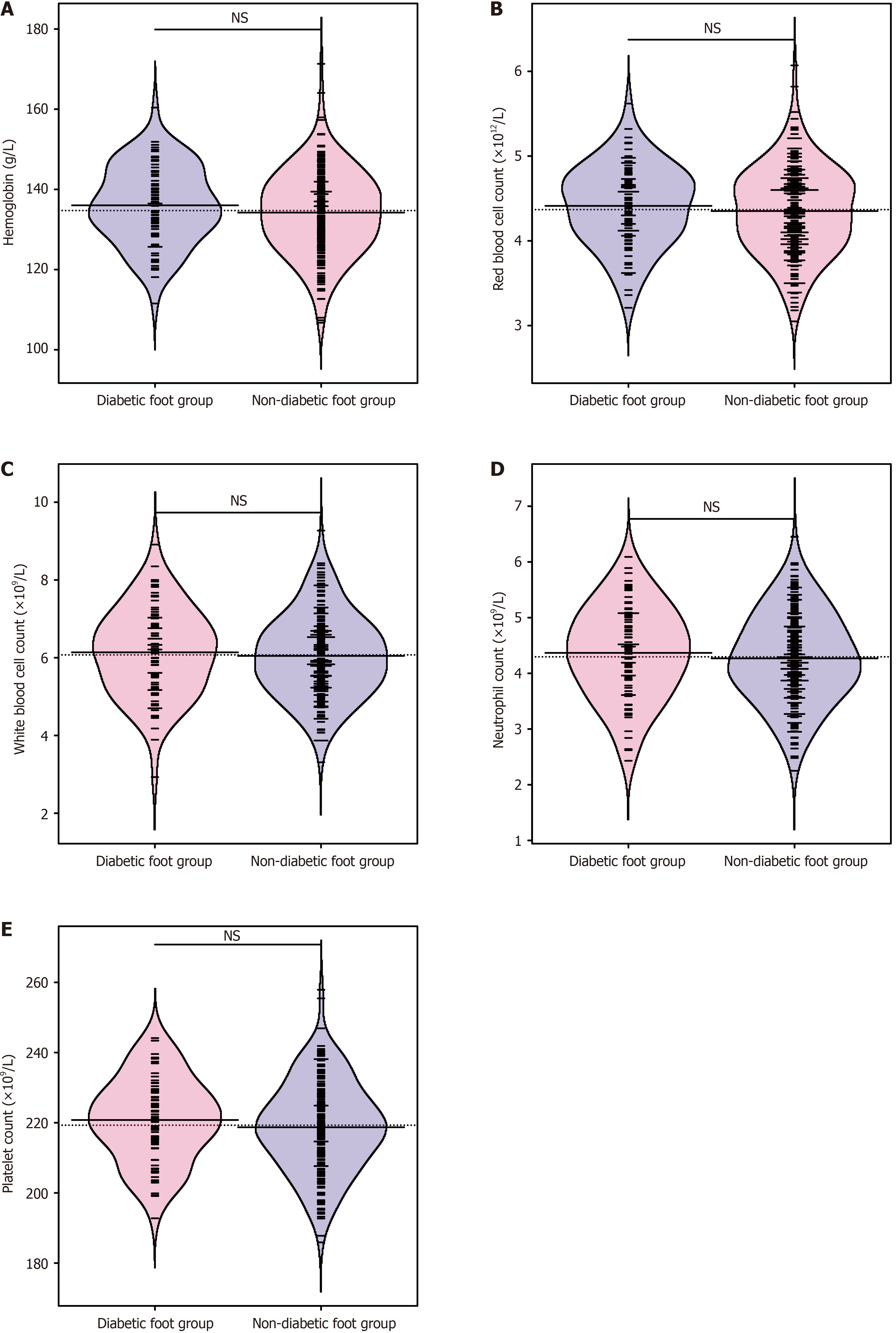
Routine blood examination indicated no statistical differences in hemoglobin concentration, white blood cell count, red blood cell count, neutrophil count, and platelet count between the two groups (P > 0.05; Figure 1). This finding suggests that preoperative blood routine indicators have no impact on the research results.
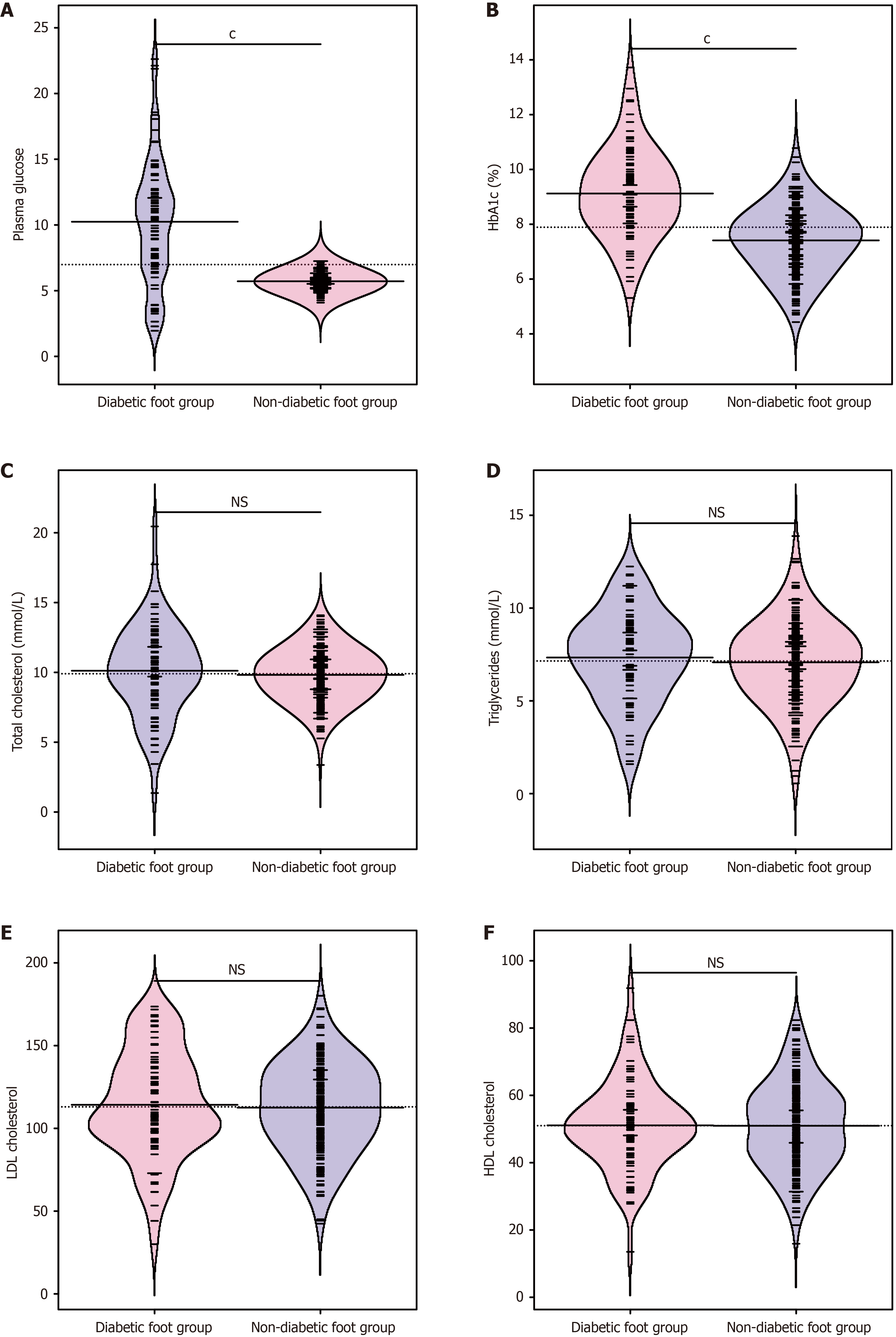
Examination of the patients' blood glucose and lipid levels indicated no statistical differences in total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol between the two groups (P > 0.05; Figure 2). This finding suggests that preoperative blood routine indicators have no impact on the research results. However, the DF group exhibited sig
Differential analysis was conducted on the renal function indicators of the two groups, including plasma creatinine, plasma urea, and estimated GFR (Table 3). No significant differences in renal function indicators were found between the two groups (P > 0.05). This finding suggests that intraoperative indicators have no impact on the research results.
| Characteristic | NDF group (n = 210) | DF group (n = 82) | t/χ2 | P value |
| Plasma creatinine (μmol/L) | 49.7 ± 4.12 | 50.27 ± 4.34 | 1.024 | 0.307 |
| Plasma urea (mmol/L) | 1.35 ± 0.59 | 1.41 ± 0.67 | 0.687 | 0.493 |
| eGFR, mL/min/1.73 m2 | 0.048 | 0.827 | ||
| ≥ 60 | 153 (72.86) | 58 (70.73) | ||
| < 60 | 57 (27.14) | 24 (29.27) |
The patients’ delayed medical care of more than 3 months was recorded, and their willingness to seek medical care was assessed using the ATMHSS (Table 4). The NDF group had significantly higher ATMHSS scores (68.71 ± 10.41 vs 59.84 ± 9.78, P < 0.001) and lower rate of delayed medical care (13.41% vs 72.38%) compared with the DF group. This finding indicates that low willingness to seek medical care and delayed medical care may lead to DF.
| Characteristic | NDF group (n = 210) | DF group (n = 82) | t/χ2 | P value | |
| ATMHSS score | 68.71 ± 10.41 | 59.84 ± 9.78 | 6.84 | < 0.001 | |
| Medical delay time | Less than 3 months | 152 (72.38) | 11 (13.41) | 80.773 | < 0.001 |
| More than 3 months | 58 (27.62) | 71 (86.59) | |||
A significant correlation was observed between various indicators and DF occurrence (Table 5). On the one hand, BMI, duration of diabetes, plasma glucose, HbA1c, and presence of diabetic peripheral neuropathy and diabetic nephropathy were positively correlated with DF occurrence. On the other hand, the ATMHSS score and duration of medical delay were negatively correlated with DF occurrence. These results underscore the predictive potential of these indicators for DF occurrence.
| Characteristic | Rho | P value |
| BMI (kg/m2) | 0.206 | < 0.001 |
| Duration of diabetes (years) | 0.147 | 0.012 |
| Plasma glucose | 0.626 | < 0.001 |
| HbA1c (%) | 0.498 | < 0.001 |
| ATMHSS score | -0.364 | < 0.001 |
| Diabetic peripheral neuropathy | 0.175 | 0.003 |
| Diabetic nephropathy | 0.152 | 0.009 |
| Medical delay time | -0.534 | < 0.001 |
Logistic regression analysis revealed a significant finding (Table 6). The multivariate regression model demonstrated that BMI, duration of diabetes, plasma glucose, HbA1c, presence of diabetic peripheral neuropathy and diabetic nephropathy, ATMHSS score, and medical delay time all had a strong diagnostic value for DF.
| Coef | Odds ratio | B | Beta | P value | |
| BMI (kg/m2) | 0.130 | 1.139 | 3.440 | 0.130 | < 0.001 |
| Duration of diabetes (years) | 0.067 | 1.069 | 2.481 | 0.067 | 0.013 |
| Plasma glucose | 0.775 | 2.171 | 6.459 | 0.775 | < 0.001 |
| HbA1c (%) | 0.902 | 2.463 | 7.363 | 0.902 | < 0.001 |
| ATMHSS score | 0.083 | 0.921 | 5.770 | -0.083 | < 0.001 |
| Diabetic peripheral neuropathy | 0.821 | 2.273 | 2.945 | 0.821 | 0.003 |
| Diabetic nephropathy | 2.372 | 10.718 | 2.107 | 2.372 | 0.035 |
| Medical delay time | 2.828 | 0.059 | 7.88 | -2.828 | < 0.001 |
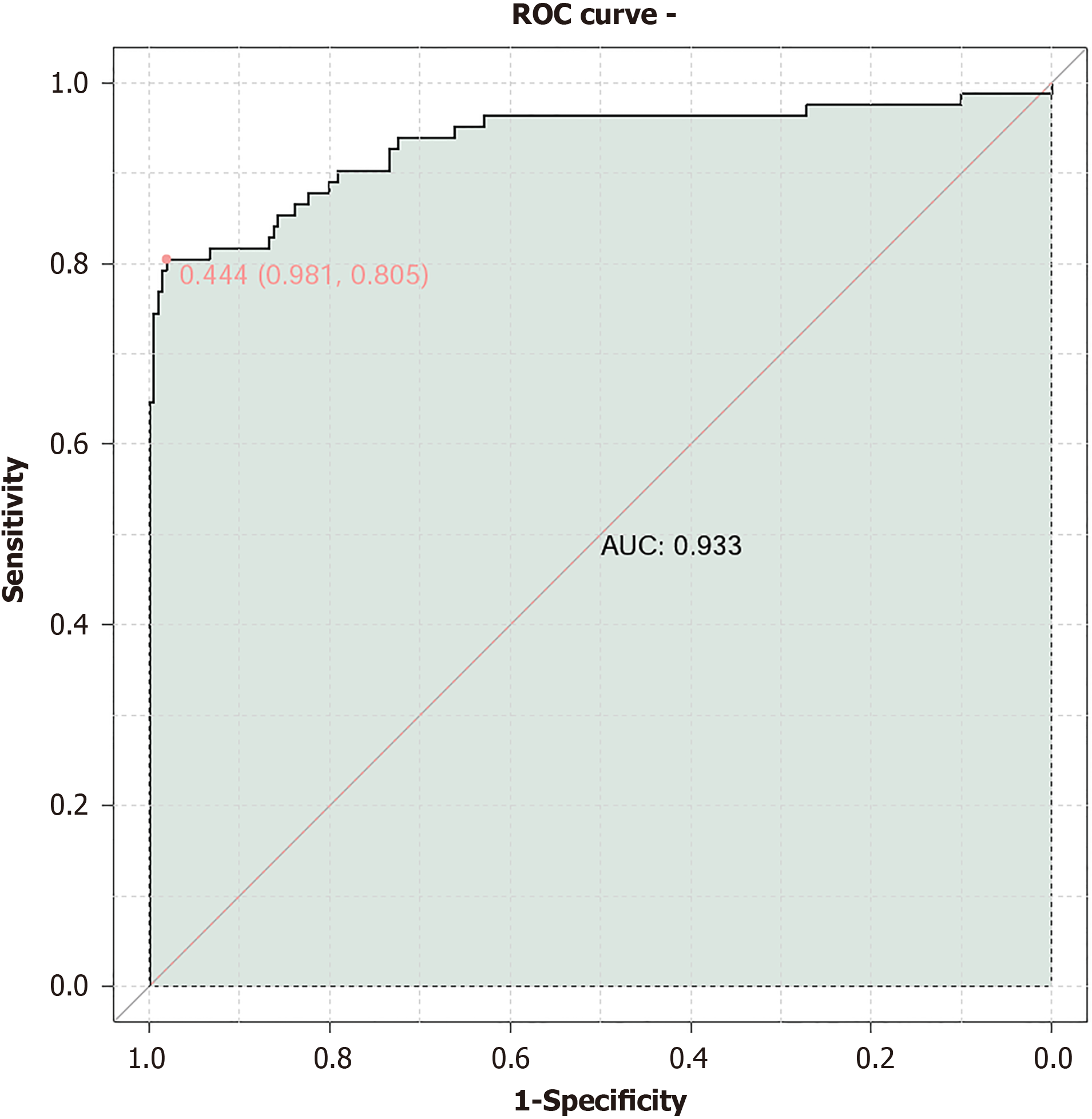
A validation cohort of patients was used to establish a predictive model for DF occurrence based on factors such as BMI, duration of diabetes, blood glucose levels, complications, and medical delay (Table 7). The results demonstrated that plasma glucose [area under the curve (AUC) = 0.819], HbA1c (AUC = 0.804), and medical delay (AUC = 0.795) exhibited good predictive value. The combined predictive model yielded an AUC of 0.933 (Figure 3).
| Sensitivities | Specificities | AUC | Youden index | |
| BMI (kg/m2) | 0.573 | 0.633 | 0.631 | 0.206 |
| Duration of diabetes (years) | 0.463 | 0.714 | 0.597 | 0.177 |
| Plasma glucose | 0.720 | 1.000 | 0.819 | 0.720 |
| HbA1c (%) | 0.646 | 0.867 | 0.804 | 0.513 |
| ATMHSS score | 0.646 | 0.700 | 0.731 | 0.346 |
| Diabetic peripheral neuropathy | 0.402 | 0.771 | 0.587 | 0.173 |
| Diabetic nephropathy | 0.049 | 0.995 | 0.522 | 0.044 |
| Medical delay time | 0.866 | 0.724 | 0.795 | 0.590 |
Diabetes represents an increasingly growing public health concern, with its associated complications drawing increasing attention[15,16]. Owing to the global prevalence of diabetes and the increased life expectancy of patients with diabetes, the incidence of DF has risen. Diabetes-related foot complications are one of the most common complications among patients with diabetes, constituting a significant healthcare burden[17-19].
Patient delay in seeking medical attention may result in the disease being in an advanced stage at the time of diagnosis. Medical delay can significantly reduce the clinical effectiveness of treatment, increase the treatment burden on patients, and even impact their short- and long-term prognoses.
The primary finding of this study is the impact of medical delay on the incidence of DF. Between-group comparison of the probability of medical delay and patients' willingness to seek medical care revealed that patients with medical delay and low willingness to seek medical care may have an increased likelihood of developing DF. The possible underlying mechanism is that patient delay in seeking medical attention may lead to the disease being in an advanced stage at the time of diagnosis, significantly reducing the clinical effectiveness of treatment, increasing the treatment burden on patients, and possibly affecting their short- and long-term prognoses. The low willingness of patients to seek medical care may be due to inadequate education, insufficient awareness of DF prevention, and lack of habit of timely check-ups and medical care[20,21]. In addition, the early symptoms of DF, such as coolness in the soles of the feet and delayed sensation, are often subtle and easily overlooked by patients, leading to medical delay.
Furthermore, the results of this study indicate that BMI, plasma glucose, and HbA1c levels are associated with DF. This relationship may be related to poor control of blood glucose levels in patients, leading to vascular changes in the lower extremities, insufficient blood supply to the lower limbs, and ultimately the occurrence of DF[22-24]. Chen et al[25] also illustrated that uncontrolled blood glucose levels in patients with diabetes can lead to diabetic complications. Consistent with the present findings, high blood glucose levels are associated with severe DF ulcers[25].
Diabetic neuropathy can affect the central and peripheral nervous systems, with the latter being particularly common. In particular, distal sensory neuropathy is the most prevalent and accounts for over 50% of all diabetic neuropathies[26,27]. Diabetic nephropathy is one of the most significant complications in patients with diabetes and often concurrently involves microvascular disease in other organs or systems[28,29]. Although the correlation of certain complications such as diabetic retinopathy and DF with diabetes is relatively low, the occurrence of diabetic neuropathy and diabetic neph
BMI, duration of diabetes, plasma glucose, HbA1c, and presence of diabetic peripheral neuropathy and diabetic nephropathy are all positively correlated with DF occurrence. Meanwhile, the ATMHSS score and delayed medical care are negatively correlated with DF occurrence. Logistic regression analysis indicates that these factors are risk factors for DF. ROC analysis demonstrates that the combined predictive model of blood glucose levels and delayed medical care has good predictive value.
This study investigates the predictive role of delayed medical care and other factors in patients with DF. However, it has certain limitations. First, the retrospective design imposes inherent constraints on causal inferences, as the observed correlation between personalized care interventions and postoperative outcomes does not establish a clear causal relationship. The reliance on retrospective data collection also introduces the possibility of information bias and con
Delayed medical care significantly influences the likelihood of DF occurrence in patients with diabetes. The combined predictive model of plasma glucose, HbA1c levels, and delayed medical care demonstrates good predictive value.
| 1. | Dugani SB, Mielke MM, Vella A. Burden and management of type 2 diabetes in rural United States. Diabetes Metab Res Rev. 2021;37:e3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 2. | Yan Y, Wu T, Zhang M, Li C, Liu Q, Li F. Prevalence, awareness and control of type 2 diabetes mellitus and risk factors in Chinese elderly population. BMC Public Health. 2022;22:1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 3. | Damanik J, Yunir E. Type 2 Diabetes Mellitus and Cognitive Impairment. Acta Med Indones. 2021;53:213-220. [PubMed] |
| 4. | Bellomo TR, Lee S, McCarthy M, Tong KPS, Ferreira SS, Cheung TP, Rose-Sauld S. Management of the diabetic foot. Semin Vasc Surg. 2022;35:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Reardon R, Simring D, Kim B, Mortensen J, Williams D, Leslie A. The diabetic foot ulcer. Aust J Gen Pract. 2020;49:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Rehman ZU, Khan J, Noordin S. Diabetic Foot Ulcers: Contemporary Assessment And Management. J Pak Med Assoc. 2023;73:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 7. | Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. 2023;330:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 490] [Article Influence: 245.0] [Reference Citation Analysis (0)] |
| 8. | Carro GV, Saurral R, Witman EL, Braver JD, David R, Alterini PA, Illuminati G, Carrió LM, Torres JC. [Diabetic foot attack. Pathophysiological description, clinical presentation, treatment and outcomes]. Medicina (B Aires). 2020;80:523-530. [PubMed] |
| 9. | Costa IG, Tregunno D, Camargo-Plazas P. Patients' Perceptions of Reasons Contributing to Delay in Seeking Help at the Onset of a Diabetic Foot Ulcer: A Grounded Theory Study. J Wound Ostomy Continence Nurs. 2022;49:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Crocker RM, Tan TW, Palmer KNB, Marrero DG. The patient's perspective of diabetic foot ulceration: A phenomenological exploration of causes, detection and care seeking. J Adv Nurs. 2022;78:2482-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Shao T, Wang J, Huang X, Deng X, Cao Y, Zhou M, Zhao C. An update on potential biomarkers for diagnosing diabetic foot ulcer at early stage. Biomed Pharmacother. 2021;133:110991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Sen P, Demirdal T, Emir B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab Res Rev. 2019;35:e3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 13. | Zhu Y, Xu H, Wang Y, Feng X, Liang X, Xu L, Liang Z, Xu Z, Li Y, Le Y, Zhao M, Yang J, Li J, Cao Y. Risk factor analysis for diabetic foot ulcer-related amputation including Controlling Nutritional Status score and neutrophil-to-lymphocyte ratio. Int Wound J. 2023;20:4050-4060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1152] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 15. | Laakso M. Biomarkers for type 2 diabetes. Mol Metab. 2019;27S:S139-S146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 16. | Tinajero MG, Malik VS. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol Metab Clin North Am. 2021;50:337-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 17. | Morbach S, Eckhard M, Lobmann R, Müller E, Reike H, Risse A, Rümenapf G, Spraul M. Diabetic Foot Syndrome. Exp Clin Endocrinol Diabetes. 2023;131:84-93. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Pérez-Panero AJ, Ruiz-Muñoz M, Cuesta-Vargas AI, Gónzalez-Sánchez M. Prevention, assessment, diagnosis and management of diabetic foot based on clinical practice guidelines: A systematic review. Medicine (Baltimore). 2019;98:e16877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Troisi N, Bertagna G, Juszczak M, Canovaro F, Torri L, Adami D, Berchiolli R. Emergent management of diabetic foot problems in the modern era: Improving outcomes. Semin Vasc Surg. 2023;36:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Lingyan L, Liwei X, Han Z, Xin T, Bingyang H, Yuanyuan M, Peiwei Q, Peifen M. Identification, influencing factors and outcomes of time delays in the management pathway of diabetic foot: A systematic review. J Tissue Viability. 2024;33:345-354. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Ogunlana MO, Govender P, Oyewole OO, Odole AC, Falola JL, Adesina OF, Akindipe JA. Qualitative exploration into reasons for delay in seeking medical help with diabetic foot problems. Int J Qual Stud Health Well-being. 2021;16:1945206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Guo Q, Ying G, Jing O, Zhang Y, Liu Y, Deng M, Long S. Influencing factors for the recurrence of diabetic foot ulcers: A meta-analysis. Int Wound J. 2023;20:1762-1775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 23. | Mariadoss AVA, Sivakumar AS, Lee CH, Kim SJ. Diabetes mellitus and diabetic foot ulcer: Etiology, biochemical and molecular based treatment strategies via gene and nanotherapy. Biomed Pharmacother. 2022;151:113134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 24. | Simoneau A, Rojubally S, Mohammedi K, Monlun M, Foussard N, Rigalleau V, Blanco L. Glucose control and infection of diabetic foot ulcer. J Diabetes Complications. 2021;35:107772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 25. | Chen W, Wang X, Jiang Q, Wu J, Shi W, Wang X, Yin Y, Zheng J, Hu X, Lin C, Zhang X. Association between triglyceride glucose index and severity of diabetic foot ulcers in type 2 diabetes mellitus. J Foot Ankle Res. 2023;16:68. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Røikjer J, Ejskjaer N. Diabetic Peripheral Neuropathy. Handb Exp Pharmacol. 2022;274:309-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Zakin E, Abrams R, Simpson DM. Diabetic Neuropathy. Semin Neurol. 2019;39:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Sagoo MK, Gnudi L. Diabetic Nephropathy: An Overview. Methods Mol Biol. 2020;2067:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 29. | Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021;2021:1497449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 489] [Article Influence: 122.3] [Reference Citation Analysis (0)] |