Published online Jan 15, 2024. doi: 10.4239/wjd.v15.i1.53
Peer-review started: September 13, 2023
First decision: October 24, 2023
Revised: November 3, 2023
Accepted: December 13, 2023
Article in press: December 13, 2023
Published online: January 15, 2024
Processing time: 120 Days and 17.9 Hours
The lack of specific predictors for type-2 diabetes mellitus (T2DM) severely impacts early intervention/prevention efforts. Elevated branched-chain amino acids (BCAAs: Isoleucine, leucine, valine) and aromatic amino acids (AAAs: Tyrosine, tryptophan, phenylalanine)) show high sensitivity and specificity in predicting diabetes in animals and predict T2DM 10-19 years before T2DM onset in clinical studies. However, improvement is needed to support its clinical utility.
To evaluate the effects of body mass index (BMI) and sex on BCAAs/AAAs in new-onset T2DM individuals with varying body weight.
Ninety-seven new-onset T2DM patients (< 12 mo) differing in BMI [normal weight (NW), n = 33, BMI = 22.23 ± 1.60; overweight, n = 42, BMI = 25.9 ± 1.07; obesity (OB), n = 22, BMI = 31.23 ± 2.31] from the First People’s Hospital of Yunnan Province, Kunming, China, were studied. One-way and 2-way ANOVAs were conducted to determine the effects of BMI and sex on BCAAs/AAAs.
Fasting serum AAAs, BCAAs, glutamate, and alanine were greater and high-density lipoprotein (HDL) was lower
Heterogeneously elevated amino acids, especially BCAAs/AAAs, across new-onset T2DM patients in differing BMI categories revealed a potentially skewed prediction of T2DM development. The higher BCAA/AAA levels in obese T2DM patients would support T2DM prediction in obese individuals, whereas the lower levels of BCAAs/AAAs in NW-T2DM individuals may underestimate T2DM risk in NW individuals. This potentially skewed T2DM prediction should be considered when BCAAs/AAAs are to be used as the T2DM predictor.
Core Tip: Elevated branched-chain amino acids (BCAAs) and aromatic amino acids (AAAs) predict diabetes in animals with high sensitivity and specificity (both > 97%) and predict type-2 diabetes mellitus (T2DM) 10-20 years before T2DM onset. However, our results indicate that heterogeneously elevated BCAAs/AAAs among new-onset T2DM patients in differing BMI categories and sex may skew BCAA/AAA prediction of T2DM development among the general population: the greater BCAA/AAA elevation in obese individuals, especially males, would support T2DM prediction in these individuals, whereas the lack of or reduced BCAA/AAA elevation in NW and reproductive-aged females may compromise BCAA/AAA prediction of T2DM in these individuals. Potential nutritional, metabolic and molecular mechanisms are discussed.
- Citation: Wang M, Ou Y, Yuan XL, Zhu XF, Niu B, Kang Z, Zhang B, Ahmed A, Xing GQ, Su H. Heterogeneously elevated branched-chain/aromatic amino acids among new-onset type-2 diabetes mellitus patients are potentially skewed diabetes predictors. World J Diabetes 2024; 15(1): 53-71
- URL: https://www.wjgnet.com/1948-9358/full/v15/i1/53.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i1.53
According to the World Health Organization (WHO) (https://www.who.int/news-room/fact-sheets/detail/diabetes) and other global surveys[1], more than 465 million people worldwide will have type-2 diabetes mellitus (T2DM) in 2023, which is projected to double by 2050. T2DM was responsible for more than 4.2 million annual deaths in 2019. The global economic burden of T2DM in 2015 was 1.3 trillion United States dollars, which will double by 2030. Obesity (OB) is a major risk factor for T2DM and cardiovascular diseases (CVDs)[2]. More than 2.1 billion adults worldwide (39% of the adult population) are overweight (OW) or obese (700 million, 13% of the adult population) and are responsible for approximately 2.8 million deaths each year. China’s prevalence of OW and OB in 2019 was 34.3% and 16.4% for adults (≥ 18 years), respectively[3]. The prevalence of diabetes among Chinese adults increased from 10.9% in 2013 to 12.4% in 2018[3,4]. Moreover, 45.2% of the obese individuals had metabolically unhealthy OB (MUO) with comorbid diabetes (18.5%) or prediabetes (26.7%), whereas the other 55% of the obese individuals were metabolically healthy OW (MHO). Similarly, 32.7% of OW people have diabetes (12.8%) or prediabetes (19.9%), compared to 20.7% of normal weight (NW) people who have diabetes (7.6%) or prediabetes (13.1%)[3]. A 4-year follow-up study of 6748 nondiabetic middle-aged subjects (average 43 years old) showed that 55% of the population is metabolically healthy with a low risk of T2DM development, whereas 45% of the population is metabolically unhealthy and has a higher risk of diabetes development[5].
Early T2DM prediction could prevent or minimize the global impact of T2DM. T2DM onset can be delayed or prevented if intervened preclinically[6,7]. However, more than two-thirds of T2DM patients are not aware of having T2DM until their diagnosis. Elevated blood branched-chain amino acids (BCAAs: Leucine, isoleucine, valine) and aromatic amino acids (AAAs: Tyrosine, tryptophan and phenylalanine) are promising T2DM predictors, as their elevations have successfully predicted prediabetes[7-9], homeostasis model assessment-insulin resistance[10-12], and T2DM 10-20 years ahead of their onset[13-16]. However, these findings have not been replicated in heterogeneous populations or with an established cutoff for standard diagnosis. In fact, most of the BCAAs/AAAs findings were tentative and reported as odds ratios, hazard ratios (HRs) or relative risks (RRs), or the results of comparing the highest quartile vs lowest quartile individuals were reported without mentioning the sensitivity, specificity, accuracy, and positive and negative predictive values of standardized diagnosis criteria.
Furthermore, most of the T2DM BCAAs/AAAs findings are based on obese individuals. Significant confounding effects of OB on BCAA/AAA elevation in T2DM were reported[17]. Greater baseline body mass index (BMI) and metabolic syndromes (MetS) often coexist in individuals who subsequently develop T2DM than in those without T2DM, suggesting a confounding effect of baseline BMI/OB and MetS on BCAA/AAA elevation, CVD and T2DM development[18-20]. In an 8.5-year follow-up study of 6134 nondiabetic individuals resulting in 306 new T2DM cases, the HR of the highest vs lowest quartile dropped from 12.07 to 3.20 (nearly 4-fold) after adjustment for BMI, family history of T2DM, alcohol consumption, and MetS[10]. Furthermore, T2DM prevalence/incident rates in these BCAAs/AAAs studies (3%-5%) were often much lower than the population-based epidemiological data (approximately 10% for diabetes and approximately 20% for prediabetes) (WHO: https://www.who.int/news-room/fact-sheets/detail/diabetes, CDC: https://www.cdc.gov/diabetes/prevention/about-prediabetes.html)[21].
Although the exact mechanisms remain unknown, publication bias, systematic exclusions of T2DM comorbid conditions such cardiovascular disorders, cancer or other diseases, or selective inclusion of healthier control individuals or male subjects may have caused the discrepancies. One 19-year follow-up study of 1279 nondiabetic European and 1007 nondiabetic South Asian male individuals reported a 35% prevalence of T2DM in South Asian men and a 14% prevalence of T2DM in European men without reporting women’s data[14], which may compromise the results due to the sex-dependent elevation in BCAAs/AAAs[17,22,23].
BCAA/AAA elevation is further complicated by interactions of body composition, age, sex, genetics and dietary protein, fat, and energy intake. Higher dietary BCAA intake and elevated blood BCAAs are associated with increased risk of OB and IR in men but reduced risk in reproductive-aged women[24-26]. Higher animal protein but not plant protein intake is associated with higher longitudinal insulin resistance and risk of T2DM[27]. However, five years of consumption of a low-fat Mediterranean diet normalized BCAA levels and promoted T2DM remission[28].
The metabolic impact of BCAA supplementation is affected by BMI and/or adiposity status. A higher percentage intake of BCAAs in terms of total protein was associated with a significantly decreased risk of diabetes in lean/NW middle-aged Japanese men and women (BMI = 22)[29]. A higher dietary BCAA intake/ratio and elevated blood BCAAs were inversely associated with the risk/prevalence of OB in lean individuals (BMI < 24)[30,31]. Replacing animal protein with plant protein is associated with decreased T2DM risk in adult males[32]. Because a higher intake of animal protein is often associated with increased consumption of saturated fats and increased body fat/weight gain, a body fat/weight-dependent effect of BCAAs may be associated with T2DM risk/onset. In support of that, BCAA supplementation significantly increased hepatic gluconeogenesis, plasma lipid and muscular and renal lipid accumulation and reduced hepatic lipid accumulation in high-fat-diet-induced obese mice[33], whereas BCAA supplementation attenuated the severity of streptozotocin-induced diabetes in lean rats[34].
This study aimed to evaluate the effects of BMI and sex on BCAAs/AAAs in new-onset T2DM individuals in differing BMI categories.
New-onset T2DM patients were diagnosed at the Department of Endocrinology, the First People's Hospital of Yunnan Province, Kunming, China, from December 2016 to June 2018. Ninety-seven T2DM patients diagnosed with T2DM within 1 year were included in the analysis (53 male/44 female, 43.3 ± 11.2 years of age). The diagnosis and classification of T2DM were based on 1999 WHO standards[35]: (1) Fasting plasma glucose concentration ≥ 7.0 mmol (or ≥ 126 mg/dL); (2) ≥ 11.1 mmol (or ≥ 200 mg/dL) 2 h after a 75 g oral glucose load; and (3) HbA1c ≥ 6.5%. The exclusion criteria were as follows: (1) A history of diabetes that was diagnosed more than 12 mo prior; and (2) acute complications of diabetes and severe liver and/or kidney dysfunction or other serious health conditions. The study procedures were conducted in accordance with the Helsinki Declaration of 1975 and were approved by the Medical Ethics Review Committee of the First People's Hospital of Yunnan Province [No. 2016(001)].
Anthropometric measures of height, body weight and waist circumference (WC) were used to determine BMI and body weight status. The 2004 WHO classifications for the Asian/Chinese population were used for this study: (1) NW (BMI < 24); (2) OW (BMI ≥ 24 and < 28); and (3) OB (BMI ≥ 28), which differ from the standards of United States and European populations (NW: BMI < 25 kg/m2, OW: BMI ≥ 25, OB: ≥ 30). Visceral adipose tissue (VAT) at the level of the umbilicus was measured via an abdominal dual BIA machine following the manufacturer’s protocol (DUALSCAN HDS-2000, Omron Health care Co., Kyoto, Japan).
Fasting blood samples of the T2DM patients were collected and analyzed following the standard operation protocol. Briefly, overnight fasting (> 8 h) venous blood samples were collected and centrifuged immediately to separate the serum.
The frozen serum samples were thawed on ice and at 4°C followed by deproteinization by the addition of acetonitrile (1:3 ratio of serum to acetonitrile). The sample was then vortexed for 2 min followed by centrifugation at 12000 rpm for 10 min at 4°C to remove any precipitate from the supernatant before ultra-performance liquid chromatography (UPLC)/triple stage quadrupole mass spectrometer (TSQ/MS) analysis. A quality control sample consisting of 6 reference standards (isoleucine, leucine, valine, tyrosine, tryptophan, phenylalanine) was prepared and run after each of the 15 serum samples.
A 5 μL aliquot of extracted serum sample was injected into the UPLC column (DionexUltiMate3000-UPLC, United States) TSQ/MS (Thermo TSQ Endura, United States). Separation was achieved on a Luna Omega Polar C18 column (2.1 mm × 100 mm, 1.6 μm, Phenomenex) held at 40°C. The samples were eluted with A (water with 0.1% formic acid) and B (acetonitrile), and the gradient program was 1% B over 0-0.5 min, 1%-20% B over 0.5-9 min, 20%-75% B over 9-11 min, and 75%-99% B over 11-16 min. The composition was held at 99% B for 0.5 min and finally returned to 1% B at 20 min. The flow rate was 0.3 mL/min. Mass spectrometry was performed in positive ion electrospray (ESI +) mode. The temperature for the ion transfer tube and vaporizer was set at 350°C and 300°C, respectively. The pressures for the sheath gas, aux gas and sweep gas were set at 40, 15 and 1 Arb, respectively. The positive ion voltage was set to 3.5 kV. All the compounds were detected in selective reaction monitoring mode.
The data are presented as the mean ± standard deviation or median [25% quartile range (QR), 75% QR]. The effects of BMI and sex were analyzed using ANOVA for quantitative variables with a normal distribution or using the Wilcoxon rank sum test for nonnormally distributed parameters (SPSS 24, IBM). Post hoc multiple comparisons were assessed using Bonferroni correction. Pearson’s correlation coefficients were calculated among the variables. A 2-sided P≤0.05 was considered indicative of statistical significance.
Ninety-seven (97) T2DM patients, including 57 males (59%) and 40 females (41%), all within 1 year of T2DM diagnosis, were identified and included in this study. Of them, 33 (34%) were NW-T2DM (BMI = 22.23 ± 1.60), 42 (43.2%) were OW-T2DM (BMI = 25.90 ± 1.08), and 22 (22.8%) were OB-T2DM (BMI = 30.96 ± 2.59), based on the WHO body weight classification (Table 1).
| NW (33) | OW (42) | OB (22) | F value | P value | |
| Sex: M/F [n (%)] | 20/13 (33) | 24/18 (42) | 13/9 (22) | 0.095 | 0.954 |
| Age (yr, mean ± SD) | 43.64 ± 6.95 | 42.21 ± 9.76 | 36.77 ± 10.32a | 4.054 | 0.020a |
| T2DM onset (d) | 46.1 ± 96.8 | 41.8 ± 90.3 | 8.5 ± 20.2 | 1.569 | 0.214 |
| Medication (Y/N) | 13/20 | 13/29 | 6/16 | 1.016 | 0.602 |
| Metformin equiv dose (g, mean ± SD) | 176 ± 221 | 214 ± 225 | 546 ± 34 | 1.384 | 0.256 |
| Weight (kg) | 60.53 ± 6.87 | 73.49 ± 9.71b,c | 85.64 ± 11.35b,c | 49.593 | 0.000b |
| Height (cm) | 165.3 ± 8.26 | 168.5 ± 9.05 | 164.4 ± 6.91 | 1.407 | 0.250 |
| BMI index | 22.23 ± 1.60 | 25.88 ± 1.07b,c | 31.23 ± 2.31b,c | 208.086 | 0.000b |
| Waist circumference (cm) | 84.85 ± 6.21 | 89.76 ± 6.07b,c | 99.61 ± 7.05b,c | 35.920 | 0.000b |
| Waist: Height ratio | 0.514 ± 0.042 | 0.535 ± 0.034a | 0.604 ± 0.041b,c | 37.279 | 0.000b |
| Systolic blood pressure (mmHg) | 122.1 ± 17.6 | 125.6 ± 13.2 | 130.7 ± 18.2 | 12.68 | 0.07 |
| Diastolic blood pressure (mmHg) | 81.9 ± 12.8 | 83.8 ± 8.7 | 82.5 ± 15.7 | 0.509 | 0.602 |
No group differences were found in T2DM diagnosis, sex distribution, height, medication, or systolic and diastolic blood pressures (Table 1). The OB-T2DM group was significantly younger (36.77 ± 10.32 years) than the NW-T2DM and OW-T2DM groups (43.64 ± 6.95 and 42.21 ± 9.76 years, respectively, P < 0.05, each). The OB-T2DM group also showed greater values than the NW-T2DM and OW-T2DM groups in body weight, WC, WC-to-height ratio (WHR), and BMI (P < 0.01, each, Table 1).
The male and female patients showed similarities in T2DM onset age (30.4 ± 77.0 vs 43.3 ± 91.7 d), BMI (25.9 ± 4.2 vs 25.8 ± 2.9), WHR (0.535 ± 0.05 vs 0.555 ± 0.05), systolic blood pressure (125.46 ± 16.4 vs 125.88 ± 16.1 mmHg), diastolic blood pressure (84.0 ± 14.3 vs 81.2 ± 7.3 mmHg), and antidiabetic medication. The males were, however, significantly younger (39.7 ± 8.8 vs 44.0 ± 9.6 year), heavier (76.7 ± 13.7 vs 64.9 ± 8.2 kg), and taller (171.6 ± 6.3 vs 159.2 ± 4.9 cm) with greater WC (91.7 ± 8.7 vs 88.3 ± 7.7 cm, P < 0.05, all) than the females.
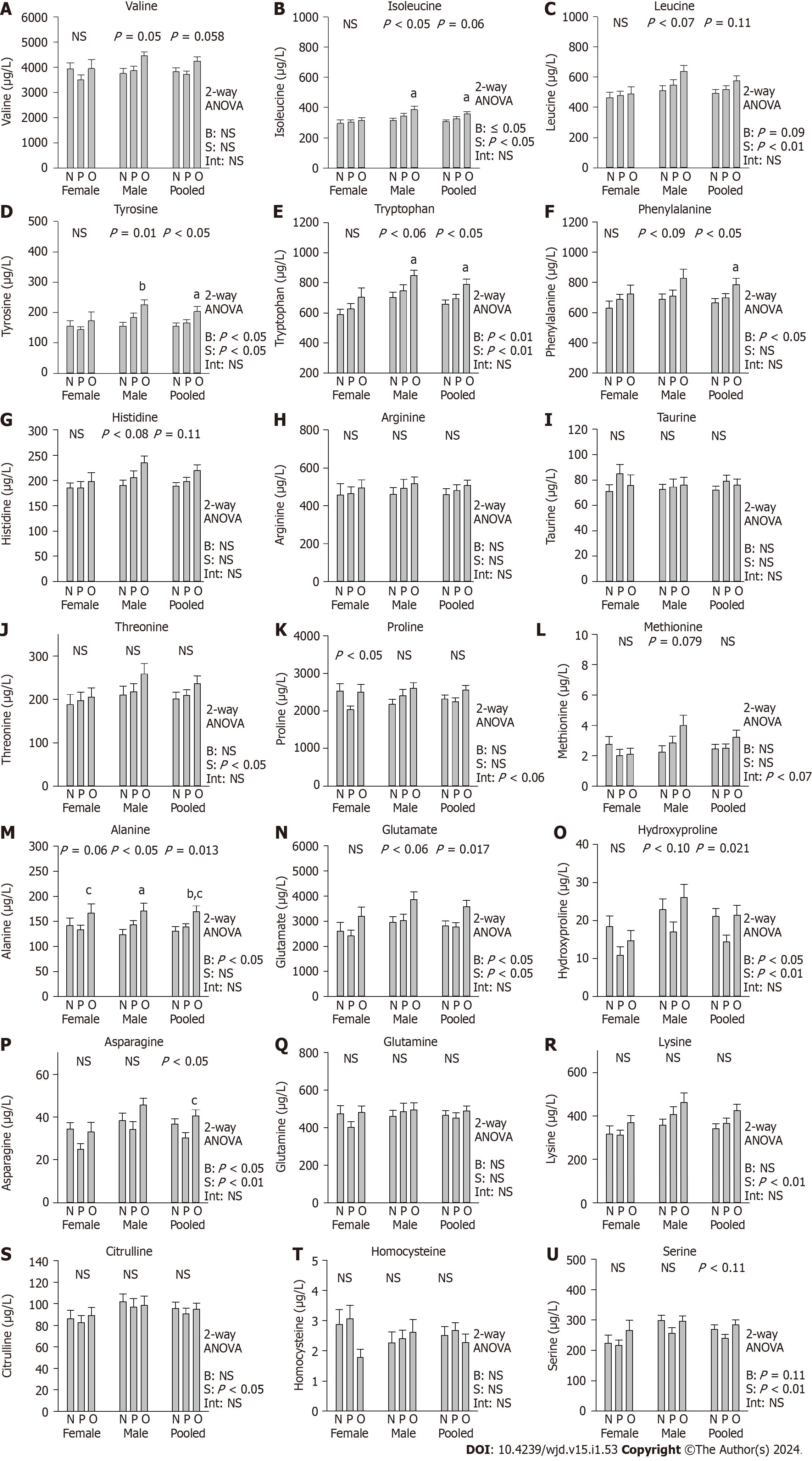
Two-way ANOVA of the effects of BMI and sex showed that among the 23 serum amino acids measured by using UPLC/TSQ-MS, 8 amino acids were significantly greater in obese than in NW and/or OW subjects (alanine, asparagine, carnosine, glutamate, hydroxyproline, proline, tyrosine, and tryptophan, P < 0.05, each) (Figure 1, Table 2). Valine, isoleucine, and phenylalanine were marginally greater in obese than in NW and/or OW subjects (P ≤ 0.1, each). Ten amino acids were significantly greater in male subjects than in female subjects (serine, asparagine, glutamate, lysine, hydroxyproline, citrulline, isoleucine, leucine, tyrosine, and tryptophan, P < 0.05, each). Histidine, methionine, phenylalanine, and threonine were marginally greater in male subjects than in female subjects (P < 0.1, each). Significant BMI and sex interactions were found for proline only.
| NW | OW | OB | Obesity, F, P (1-way ANOVA) | Obesity, F, P (2-way ANOVA) | Sex, F value, P value | Obesity × sex interaction, F value, P value | Age-adjusted F value, P value | Onset-adjusted, F value, P value | Metf. dose-adjusted, F value, P value | ||
| HbA1c (%) | Pooled | 10.96 ± 2.73 | 9.91 ± 2.69 | 9.09 ± 2.40a | 3.471, 0.035a | 2.888, 0.061d | 1.603, 0.209 | 0.376, 0.688 | NS | NS | NS |
| Male | 11.51 ± 2.93 | 10.07 ± 2.78 | 9.25 ± 2.16 | 3.012, 0.058d | |||||||
| Female | 10.12 ± 2.24 | 9.70 ± 2.64 | 8.85 ± 2.82 | 0.661, 0.522 | |||||||
| TC (mmol/L) | Pooled | 4.82 ± 1.36 | 4.68 ± 1.65 | 4.55 ± 1.10 | 0.229, 0.796 | 0.239, 0.788 | 1.020, 0.315 | 0.035, 0.965 | NS | NS | NS |
| Male | 4.91 ± 1.24 | 4.80 ± 1.96 | 4.73 ± 1.14 | 0.053, 0.948 | |||||||
| Female | 4.68 ± 1.58 | 4.52 ± 1.15 | 4.29 ± 1.04 | 0.243, 0.786 | |||||||
| TG (mmol/L) | Pooled | 3.12 ± 6.77 | 2.73 ± 3.18 | 4.66 ± 4.20 | 1.161, 0.318 | 0.992, 0.375 | 1.758, 0.188 | 0.253, 0.777 | NS | NS | NS |
| Male | 3.85 ± 8.61 | 2.91 ± 3.94 | 5.45 ± 4.99 | 0.711, 0.496 | |||||||
| Female | 2.00 ± 1.64 | 2.49 ± 1.80 | 3.53 ± 2.54 | 1.683, 0.200 | |||||||
| HDL (mmol/L) | Pooled | 1.07 ± 0.27 | 1.03 ± 0.27 | 0.86 ± 0.22 | 4.744, 0.011a | 4.478, 0.014a | 0.998, 0.320 | 0.337, 0.715 | NS | NS | NS |
| Male | 1.07 ± 0.26 | 0.98 ± 0.23 | 0.84 ± 0.24 | 3.602, 0.034a | |||||||
| Female | 1.08 ± 0.31 | 1.09 ± 0.32 | 0.88 ± 0.19 | 1.635, 0.209 | |||||||
| LDL (mmol/L) | Pooled | 2.82 ± 1.10 | 2.81 ± 0.87 | 2.34 ± 1.02 | 1.922, 0.152 | 1.670, 0.194 | 0.009, 0.925 | 0.121, 0.887 | NS | NS | NS |
| Male | 2.83 ± 0.96 | 2.87 ± 0.84 | 2.29 ± 0.99 | 1.870, 0.164 | |||||||
| Histidine (μg/L) | Pooled | 188.42 ± 40.62 | 197.28 ± 57.63 | 219.62 ± 53.34 | 2.488, 0.089 | 2.010, 0.140 | 3.614, 0.060d | 0.651, 0.524 | NS | NS | NS |
| Male | 190.30 ± 45.33 | 205.95 ± 62.41 | 234.87 ± 49.68 | 2.680, 0.078d | |||||||
| Female | 185.53 ± 33.68 | 185.72 ± 49.92 | 197.59 ± 53.32 | 0.234, 0.792 | |||||||
| Arginine (μg/L) | Pooled | 458.53 ± 180.93 | 480.08 ± 198.77 | 507.06 ± 127.36 | 0.487, 0.616 | 0.423, 0.656 | 0.219, 0.641 | 0.044, 0.957 | NS | NS | NS |
| Male | 459.85 ± 163.56 | 492.30 ± 231.02 | 516.31 ± 131.56 | 0.369, 0.693 | |||||||
| Female | 456.51 ± 211.98 | 463.80 ± 150.31 | 493.70 ± 127.59 | 0.140, 0.870 | |||||||
| β-Alanine (μg/L) | Pooled | 436.45 ± 188.57 | 445.91, 188.70 | 394.88, 202.89 | 0.530, 0.591 | 0.402, 0.670 | 0.569, 0.452 | 0.942, 0.394 | NS | NS | NS |
| Male | 454.86 ± 200.68 | 425.40 ± 177.60 | 357.16 ± 178.59 | 1.100, 0.340 | |||||||
| Female | 408.11 ± 172.11 | 473.25 ± 204.50 | 449.37 ± 233.59 | 0.395, 0.676 | |||||||
| Carnosine (μg/L) | Pooled | 5.99 ± 1.37 | 5.98 ± 2.06 | 6.95 ± 1.50 | 2.671, 0.074 | 3.122, 0.049 | 0.013, 0.908 | 1.193, 0.308 | NS | NS | NS |
| Male | 6.27 ± 1.41 | 5.93 ± 2.36 | 6.65 ± 1.34 | 0.632, 0.535 | |||||||
| Female | 5.55 ± 1.22 | 6.04 ± 1.63 | 7.39 ± 1.69a | 4.035, 0.026a | |||||||
| Serine (μg/L) | Pooled | 268.73 ± 91.46 | 239.17 ± 83.33 | 283.56 ± 79.43 | 2.258, 0.110 | 2.182, 0.119 | 7.451, 0.008b | 0.567, 0.569 | NS | NS | NS |
| Male | 297.99 ± 77.49 | 256.57 ± 87.30 | 295.74 ± 64.80 | 1.817, 0.172 | |||||||
| Female | 223.72 ± 95.80 | 215.97 ± 73.74 | 265.98 ± 98.35 | 1.038, 0.364 | |||||||
| Taurine (μg/L) | Pooled | 71.97 ± 18.22 | 78.96 ± 30.38 | 75.98 ± 22.23 | 0.721, 0.489 | 1.054, 0.353 | 1.714, 0.194 | 0.685, 0.507 | 9.335, 0.003b | NS | NS |
| Male | 72.61 ± 17.85 | 74.52 ± 30.24 | 76.10 ± 21.65 | 0.083, 0.921 | |||||||
| Female | 71.00 ± 19.47 | 84.88 ± 30.39 | 75.81 ± 24.38 | 1.131, 0.334 | |||||||
| Alanine (μg/L) | Pooled | 130.77 ± 49.32 | 139.06 ± 40.66 | 168.94 ± 56.03a | 4.531, 0.013a | 3.967, 0.022a | 0.007, 0.934 | 0.812, 0.447 | NS | NS | NS |
| Male | 123.80 ± 46.98 | 143.52 ± 40.18 | 170.88 ± 56.72a | 4.013, 0.024a | |||||||
| Female | 141.51 ± 52.77 | 133.10 ± 41.67 | 166.14 ± 58.32 | 1.357, 0.270 | |||||||
| Asparagine (μg/L) | Pooled | 36.73 ± 13.82 | 30.17 ± 16.07 | 40.40 ± 13.67c | 3.900, 0.024a | 3.817, 0.026a | 7.684, 0.007b | 0.586, 0.559 | NS | NS | NS |
| Male | 38.32 ± 15.37 | 34.05 ± 18.30 | 45.52 ± 11.83 | 2.161, 0.125 | |||||||
| Female | 34.28 ± 11.16 | 25.01 ± 10.95 | 33.01 ± 13.30 | 2.865, 0.070d | |||||||
| Glutamine (μg/L) | Pooled | 464.62 ± 156.05 | 450.21 ± 188.81 | 489.96 ± 120.18 | 0.422, 0.657 | 0.530, 0.590 | 0.652, 0.421 | 0.857, 0.428 | NS | NS | NS |
| Male | 459.31 ± 156.09 | 486.60 ± 219.88 | 495.68 ± 135.66 | 0.192, 0.826 | |||||||
| Female | 472.80 ± 162.00 | 401.70 ± 127.29 | 481.70 ± 100.88 | 1.534, 0.229 | |||||||
| Glutamate (μg/L) | Pooled | 2812.36 ± 1104.11 | 2768.39 ± 1153.78 | 3588.65 ± 1138.22 | 4.259, 0.017a | 4.028, 0.021a | 5.188, 0.025a | 0.176, 0.839 | NS | NS | NS |
| Male | 2949.39 ± 1011.65 | 3028.47 ± 1234.29 | 3866.48 ± 1088.44 | 3.039, 0.056d | |||||||
| Female | 2601.55 ± 1245.38 | 2421.62 ± 962.57 | 3187.33 ± 1147.67 | 1.470, 0.243 | |||||||
| Lysine (μg/L) | Pooled | 341.06 ± 128.62 | 365.48 ± 152.71 | 423.72 ± 142.42 | 2.262, 0.110 | 2.087, 0.130 | 6.688, 0.011a | 0.376, 0.687 | NS | NS | NS |
| Male | 357.60 ± 124.07 | 406.28 ± 172.85 | 462.00 ± 157.70 | 1.831, 0.170 | |||||||
| Female | 315.62 ± 136.32 | 311.08 ± 101.65 | 368.42 ± 100.58 | 0.833, 0.443 | |||||||
| Hydroxyproline (μg/L) | Pooled | 21.09 ± 11.77 | 14.33 ± 11.68a | 21.41 ± 12.09 | 4.050, 0.021a | 3.901, 0.024a | 9.074, 0.003b | 0.614, 0.543 | NS | NS | NS |
| Male | 22.84 ± 12.69 | 17.00 ± 12.59 | 26.07 ± 12.48 | 2.472, 0.094 | |||||||
| Female | 18.39 ± 10.06 | 10.78 ± 9.55 | 14.67 ± 7.98 | 2.490, 0.097 | |||||||
| Threonine (μg/L) | Pooled | 201.17 ± 88.38 | 208.93 ± 86.51 | 236.53 ± 82.51 | 1.173, 0.314 | 0.950, 0.390 | 3.035, 0.085d | 0.293, 0.746 | NS | NS | NS |
| Male | 209.66 ± 92.11 | 217.95 ± 89.86 | 258.54 ± 87.84 | 1.259, 0.292 | |||||||
| Female | 188.12 ± 84.22 | 196.90 ± 82.80 | 204.73 ± 66.12 | 0.118, 0.889 | |||||||
| Citrulline (μg/L) | Pooled | 95.53 ± 32.87 | 90.68 ± 34.25 | 94.71 ± 27.50 | 0.237, 0.790 | 0.527, 0.592 | 6.414, 0.013a | 0.001, 0.999 | 6.232, 0.014a | NS | NS |
| Male | 101.68 ± 34.73 | 96.78 ± 38.17 | 98.59 ± 30.95 | 0.105, 0.901 | |||||||
| Female | 86.06 ± 28.48 | 82.54 ± 27.12 | 89.11 ± 22.12 | 0.195, 0.824 | |||||||
| Proline (μg/L) | Pooled | 2313.70 ± 644.63 | 2244.77 ± 716.36 | 2561.43 ± 555.47 | 1.707, 0.187 | 3.138, 0.048a | 0.941, 0.335 | 3.279, 0.042a | 6.754, 0.011a | NS | NS |
| Male | 2176.16 ± 572.03 | 2404.58 ± 839.03 | 2604.08 ± 534.61 | 1.560, 0.220 | |||||||
| Female | 2525.30 ± 714.13 | 2031.68 ± 448.42a | 2499.81 ± 611.56 | 3.418, 0.043a | |||||||
| Homocysteine (μg/L) | Pooled | 2.50 ± 1.71 | 2.68 ± 1.64 | 2.27 ± 1.33 | 0.481, 0.620 | 0.800, 0.453 | 0.194, 0.661 | 1.812, 0.169 | NS | NS | NS |
| Male | 2.26 ± 1.66 | 2.39 ± 1.43 | 2.61 ± 1.52 | 0.205, 0.815 | |||||||
| Female | 2.88 ± 1.78 | 3.06 ± 1.85 | 1.77 ± 0.82 | 1.916, 0.161 | |||||||
| Valine (μg/L) | Pooled | 3839.99 ± 883.13 | 3718.96 ± 851.75 | 4259.02 ± 820.20 | 2.938, 0.058d | 2.627, 0.078d | 1.547, 0.217 | 1.385, 0.256 | NS | NS | NS |
| Male | 3763.81 ± 926.69 | 3876.21 ± 837.88 | 4465.80 ± 583.75 | 3.144, 0.051d | |||||||
| Female | 3957.18 ± 833.97 | 3509.28 ± 847.57 | 3960.35 ± 1041.61 | 1.264, 0.294 | |||||||
| Methionine (μg/L) | Pooled | 2.44 ± 1.86 | 2.48 ± 1.97 | 3.21 ± 2.24 | 1.201, 0.306 | 0.754, 0.473 | 3.091, 0.082d | 2.568, 0.082d | NS | NS | NS |
| Male | 2.23 ± 1.92 | 2.84 ± 2.11 | 3.99 ± 2.50 | 2.658, 0.079d | |||||||
| Female | 2.76 ± 1.78 | 2.01 ± 1.70 | 2.09 ± 1.15 | 0.879, 0.424 | |||||||
| Isoleucine (μg/L) | Pooled | 306.68 ± 75.83 | 326.23 ± 81.56 | 357.51 ± 75.72 | 2.778, 0.067 | 2.325, 0.104 | 6.977, 0.010b | 0.786, 0.459 | NS | NS | NS |
| Male | 313.67 ± 73.98 | 343.49 ± 87.07 | 386.75 ± 75.14a | 3.283, 0.045a | |||||||
| Female | 295.93 ± 80.37 | 303.21 ± 69.32 | 315.27 ± 56.43 | 0.200, 0.820 | |||||||
| Leucine(μg/L) | Pooled | 492.26 ± 136.62 | 518.94 ± 147.01 | 577.40 ± 153.91 | 2.301, 0.106 | 1.909, 0.154 | 8.751, 0.004b | 0.856, 0.428 | NS | NS | NS |
| Male | 511.46 ± 137.96 | 548.74 ± 163.13 | 638.14 ± 137.20 | 2.896, 0.064d | |||||||
| Female | 462.73 ± 134.45 | 479.21 ± 114.87 | 489.66 ± 138.87 | 0.129, 0.879 | |||||||
| Tyrosine (μg/L) | Pooled | 155.13 ± 57.40 | 166.00 ± 63.14 | 203.68 ± 74.79a | 3.998, 0.022a | 3.370, 0.039a | 5.255, 0.024a | 1.448, 0.240 | NS | NS | NS |
| Male | 154.79 ± 57.19 | 182.95 ± 70.70 | 225.47 ± 58.95b | 4.857, 0.011a | |||||||
| Female | 155.65 ± 60.07 | 143.39 ± 43.65 | 172.21 ± 87.10 | 0.685, 0.511 | |||||||
| Phenylalanine (μg/L) | Pooled | 664.47 ± 163.17 | 699.89 ± 172.50 | 785.74 ± 197.37a | 3.223, 0.044a | 2.909, 0.060d | 2.806, 0.097d | 0.384, 0.682 | NS | NS | NS |
| Male | 688.44 ± 154.62 | 709.87 ± 191.09 | 828.45 ± 208.14 | 2.534, 0.089d | |||||||
| Female | 627.58 ± 175.24 | 686.60 ± 148.44 | 724.03 ± 173.28 | 1.002, 0.377 | |||||||
| Tryptophan(μg/L) | Pooled | 658.00 ± 150.01 | 694.36 ± 188.43 | 790.15 ± 162.27a | 4.082, 0.020a | 4.277, 0.017a | 13.121, 0.000b | 0.051, 0.951 | NS | NS | NS |
| Male | 702.38 ± 151.16 | 746.40 ± 193.72 | 847.94 ± 127.90 | 3.062, 0.055d | |||||||
| Female | 589.72 ± 124.48 | 624.97 ± 161.06 | 706.66 ± 176.95 | 1.567, 0.222 |
Further 1-way ANOVA of BMI effects largely confirmed the 2-way ANOVA results. Within-sex 1-way ANOVA showed that most of the BMI differences were present among the male subjects only (alanine, asparagine, glutamate, valine, isoleucine, leucine, tyrosine, and tryptophan) (P ≤ 0.05, each) (Figure 1, Table 2), except that carnosine was significantly greater in the female obese group and proline was significantly lower in the female OW group than in the other two groups. Covariance analysis showed no difference in serum amino acids after controlling for day of T2DM diagnosis or controlling for metformin equivalent dose. of T2DM medicine. However, covariance analysis showed significant effects of BMI on taurine, citrulline and proline after controlling for age (P ≤ 0.05, each, Table 2).
Two-way ANOVA showed no significant effects of BMI and sex on triglycerides, total cholesterol, and low-density lipoprotein. However, high-density lipoprotein (HDL) was significantly affected by BMI, with significantly lower HDL levels found in obese T2DM patients than in their NW and OW counterparts (P < 0.01, P < 0.05, respectively), especially among male patients (Table 2).
Although multiple amino acids were significantly correlated with VAT, body weight and BMI and negatively correlated with HDL, in pooled samples (Table 3), the correlations with VAT were the strongest (tyrosine, r = 0.524, P < 0.0001; phenylalanine, r = 0.508, P < 0.0001; tryptophan r = 0.373, P < 0.01; isoleucine, r = 0.443, P = 0.002; leucine, r = 0.396, P = 0.006, valine, r = 0.375, P < 0.01; methionine, r = 0.379, P < 0.01; alanine, r = 0.429, P = 0.003; glutamate, r = 0.398, P < 0.01; lysine, r = 0.39, P < 0.01; hydroxyproline, r = 0293; P < 0.05, threonine, r = 0.388, P < 0.01; proline, r = 0.314, P < 0.05; and histidine, r = 0.278, P < 0.06), followed by body weight, BMI and HDL (Table 3). Taurine was negatively correlated with VAT (r = -0.367, P = 0.01), and HDL was negatively correlated with isoleucine (r = -0.309, P < 0.01), leucine (r = -0.276, P < 0.01) and phenylalanine (r = -0.307 P < 0.01). Within-BMI category correlation analysis showed no significant correlations in the NW group (Table 3). For the OW group, phenylalanine was highly correlated with VAT (r = 0.607, P = 0.002), and BCAAs and AAAs were significantly or marginally correlated with body weight (tyrosine, r = 0.343, P < 0.05; tryptophan r = 0.339, P < 0.05; isoleucine, r = 0.289, P = 0.063; leucine, r = 0.32, P = 0.039, valine, r = 0.286, P = 0.067, respectively), and HDL was negatively correlated with isoleucine (r = -0.417, P < 0.01), leucine (r = -0.345, P < 0.05) and phenylalanine (r =
| Amino acids | Pooled (n = 97) | Obese (n = 22) | Overweight (n = 42) | Normal weight (n = 33) | ||||||||||||
| VAT | Body weight | BMI | HDL | VAT | Body weight | BMI | HDL | VAT | Body weight | BMI | HDL | VAT | Body weight | BMI | HDL | |
| Valine | 0.375b | 0.236a | 0.212a | NS | 0.311 | 0.217 | 0.009 | NS | 0.140 | 0.286c | 0.223 | NS | 0.150 | 0.008 | 0.167 | NS |
| Isoleucine | 0.443b | 0.287b | 0.255a | -0.309b | 0.109 | 0.106 | -0.095 | NS | 0.347c | 0.289c | 0.301d | -0.417b | 0.351 | 0.047 | 0.114 | NS |
| Leucine | 0.396b | 0.315b | 0.249a | -.276b | 0.120 | 0.201 | -0.068 | NS | 0.312 | 0.320a | 0.340a | -0.345a | 0.133 | 0.159 | 0.173 | NS |
| Tyrosine | 0.524b | 0.341b | 0.301b | NS | 0.447 | 0.136 | 0.002 | NS | 0.251 | 0.343a | 0.094 | NS | -0.036 | 0.154 | 0.347a | NS |
| Phenylalanine | 0.508b | 0.264b | 0.262a | -0.307b | 0.177 | 0.176 | 0.027 | NS | 0.607b | 0.096 | 0.171 | -0.323a | 0.185 | 0.156 | 0.085 | NS |
| Tryptophan | 0.373a | 0.362b | 0.236a | NS | 0.382 | 0.076 | -0.230 | NS | 0.107 | 0.339a | 0.114 | NS | -0.042 | 0.280 | -0.012 | NS |
| Methionine | 0.379b | 0.251a | 0.228a | NS | 0.229 | 0.223 | 0.105 | NS | 0.377c | 0.327a | 0.357a | NS | -0.263 | 0.050 | 0.296c | NS |
| Taurine | -0.367a | -0.001 | 0.047 | NS | -0.479c | -0.043 | -0.164 | NS | -0.451a | -0.153 | 0.142 | NS | -0.345 | 0.083 | -0.082 | NS |
| Alanine | 0.429b | 0.194d | 0.268b | NS | 0.640a | 0.178 | 0.078 | NS | -0.027 | -0.041 | -0.095 | NS | 0.304 | -0.171 | 0.043 | NS |
| Asparagine | 0.278d | 0.218a | 0.079 | -0.222a | 0.200 | 0.333 | 0.049 | -0.478a | 0.129 | 0.287c | 0.008 | NS | -0.192 | 0.206 | -0.052 | NS |
| Glutamine | 0.253c | 0.157 | 0.052 | NS | 0.422 | 0.075 | 0.042 | NS | -0.021 | 0.282c | 0.043 | NS | 0.000 | 0.070 | -0.072 | NS |
| Glutamate | 0.398b | 0.263b | 0.234a | NS | 0.147 | 0.172 | 0.133 | NS | 0.291 | 0.181 | 0.021 | NS | -0.024 | 0.113 | -0.053 | NS |
| Lysine | 0.390b | 0.272b | 0.203a | NS | 0.372 | 0.124 | 0.053 | NS | 0.237 | 0.277c | 0.090 | NS | -0.246 | 0.059 | -0.054 | NS |
| Hydroxyproline | 0.293a | 0.025 | -0.008 | NS | -0.428 | 0.080 | 0.015 | -0.436a | 0.276 | 0.172 | 0.056 | NS | 0.192 | -0.105 | -0.171 | NS |
| Threonine | 0.388b | 0.237a | 0.190c | NS | 0.573a | 0.322 | 0.233 | NS | 0.108 | 0.190 | 0.177 | NS | 0.012 | 0.088 | 0.005 | NS |
| Citrulline | 0.118 | 0.035 | -0.046 | NS | -0.146 | -0.114 | -0.043 | NS | 0.068 | 0.231 | 0.015 | NS | -0.120 | -0.052 | -0.233 | NS |
| Proline | 0.314a | 0.099 | 0.104 | NS | 0.295 | -0.015 | -0.162 | NS | 0.059 | 0.205 | -0.061 | NS | 0.018 | -0.310c | 0.047 | NS |
| Homocysteine | -0.132 | 0.007 | -0.011 | NS | 0.232 | 0.475a | 0.431 | NS | -0.201 | -0.109 | -0.061 | NS | 0.251 | -0.015 | -0.020 | NS |
| Histidine | 0.278d | 0.331b | 0.250a | NS | 0.316 | 0.358 | 0.123 | NS | 0.085 | 0.275c | 0.231 | NS | -0.274 | 0.100 | 0.012 | NS |
| Arginine | 0.167 | 0.042 | 0.042 | NS | 0.097 | -0.189 | -0.226 | NS | 0.122 | 0.078 | -0.100 | NS | -0.215 | -0.182 | -0.088 | NS |
| β-Alanine | -0.134 | -0.073 | -0.108 | NS | 0.241 | -0.108 | 0.069 | NS | -0.360c | -0.175 | -0.329a | NS | 0.193 | 0.306c | -0.037 | NS |
| Carnosine | 0.278d | 0.162 | 0.157 | NS | 0.113 | -0.121 | -0.143 | NS | 0.056 | 0.062 | 0.054 | NS | -0.649a | 0.200 | -0.156 | NS |
| Serine | 0.149 | 0.151 | 0.039 | -0.240a | -0.157 | -0.031 | -0.154 | NS | 0.121 | 0.159 | -0.121 | NS | -0.220 | 0.465b | 0.111 | NS |
The global T2DM epidemic could be better managed or even prevented if potential T2DM candidates were identified and treated at the preclinical stage. BCAAs and AAAs are promising T2DM predictors because of their high diagnostic sensitivity and specificity (both > 97%) in predicting and discriminating diabetic rats from nondiabetic rats[37]. Such diagnostic sensitivity and accuracy, however, have not been demonstrated in T2DM prediction, possibly due to the confounding effects of unknown factors. Both OB and sex are implicated, although the exact mechanism remains unknown.
In this study, fasting serum levels of alanine, asparagine, carnosine, glutamate, hydroxyproline, proline, tyrosine, and tryptophan were significantly greater, whereas HDL was significantly lower in obese T2DM patients than in normal T2DM patients. Valine, isoleucine, leucine, and phenylalanine trended toward higher levels in obese than in NW-T2DM patients. The amino acid levels of the OW-T2DM patients were intermediate between those of the OB and NW groups. Ten amino acids (serine, asparagine, glutamate, lysine, hydroxyproline, citrulline, isoleucine, leucine, tyrosine, and tryptophan) were significantly greater in male patients than in female patients. Histidine, methionine, phenylalanine, and threonine also trended toward being greater in male patients. Serum AAAs, BCAAs and several other amino acids were significantly correlated with abdominal adiposity and less so with body weight or BMI, whereas BCAAs/AAAs and other amino acids were negatively correlated with HDL.
Our results are in agreement with previous reports that BMI-/abdominal adiposity-dependent BCAA/AAA elevations are associated with BMI, insulin resistance and T2DM development[38-40]. A 15-year metabolomics follow-up study of 11896 non-T2DM Finnish individuals [baseline age 24-45 years, 392 incident T2DM cases identified (3.2% T2DM of the study population vs 8% T2DM of the general Finnish population)] showed that BCAAs/AAAs are the strongest predictor of diabetes along with triacylglycerol, linoleic n-6 fatty acid and HDL-cholesterol[41]. Simultaneous hyperaminoacidemia and dyslipidemia precede prediabetes and T2DM onset in MUO individuals[42], whereas amino acid and lipid homeostasis in MHO individuals is intermediate between lean health and MUO individuals[43,44]. A meta-analysis shows significant confounding effects of OB and metabolic health on T2DM development in that MUO individuals pose 10 times higher risk, metabolically unhealthy OW (MUOW) pose 7 times higher risk, metabolically unhealthy NW (MUNW) pose 4 times higher risk, MHO group pose 3 times higher risk ratio and MHO individuals (MHOW) pose 2 times higher risk ratio for T2DM development than metabolically healthy NW (MHNW) individuals, respectively[45]. However, MUO poses a 3.5 times higher risk, MUOW poses a 4 times higher risk and MUNW poses a 4 times higher risk for T2DM development than its metabolically healthy counterparts of the same BMI categories, i.e., than MHO, MHOW, and MHNW, respectively[45]. Another 3-year follow-up study of 9623 non-T2DM Chinese adults showed decreased diabetes RR in the MHOW phenotype (0.65), no change in the MHO phenotype (0.99), and increased RR in the MUNW (1.81), MUOW (2.02) and MUO (2.48) phenotypes compared to MHNW[46].
The mechanism by which BCAAs/AAAs trigger T2DM development remains largely unknown and complex. Abnormal BCAA catabolism in muscle or loss of skeletal muscle mass may play a key role in the pathogenesis of elevated BCAAs in MetS, IR, liver cirrhosis, and T2DM, as skeletal muscle is the major site of BCAA catabolism due to the high activity of BCAA aminotransferase, which is absent in the liver[47-49]. Low skeletal muscle mass is associated with insulin resistance, diabetes, and MetS[50]. Furthermore, recent studies show that altered body composition, such as increased body fat percentage, abdominal fat mass and reduced lean muscle mass rather than BMI/body weight per se, could determine metabolic phenotypes in both obese and lean/NW children[51-54], adolescents and adults (including pre- and postmenopausal women)[55-59].
Both early-onset T2DM and typical T2DM show impaired expression of genes involved in branched-chain amino acid metabolism in muscle[60] but high circulating BCAAs and leucine treatment enhanced myotube lipid accumulation and oxidative stress in myotubes[61]. Increased BCAA catabolic flux may promote gluconeogenesis and glucose intolerance via glutamate transamination to alanine or trigger T2DM incidence by overstimulation of beta cell secretion and subsequent impairment of glucose-stimulated insulin secretion. Others show that 3-hydroxyisobutyrate, a catabolic intermediate of valine secreted from muscle cells, stimulates muscle fatty acid uptake and promotes lipid accumulation in muscle, causing insulin resistance in mice and in T2DM patients[62].
A direct association between tissue-specific alteration of BCAA-catabolizing enzymes and OB-related elevation in plasma BCAAs/branched-chain alpha-keto acids (BCKAs) has been demonstrated in rodent models of OB (OB/OB mice and Zucker rats) and in obese human subjects who underwent surgical weight loss intervention[63]. Plasma concentrations of BCAAs were significantly higher (56%-84%) in randomly fed obese mice and rats (OB/OB mice and Zucker rats) than in lean controls, and BCAA elevation diminished after overnight fasting due to reduced BCAA elevation (by 30%) in obese mice[63]. Therefore, fasting plasma BCAA levels did not differ between lean and obese animals. BCAA metabolism was altered in liver and adipose tissue but not in muscle in fed obese mice, which contributed to elevated plasma BCAA levels. In comparison with lean controls, obese rodents (OB/OB mice and Zucker rats) show decreased expression and activity of BCATm and BCKD E1α in liver and epididymal fat along with increased decreasing branched-chain α-keto acid dehydrogenase (BCKD) kinase (BCKDK) expression, whereas no such changes were found in skeletal muscle[63]. Because the postprandial elevation in BCAAs was more sensitive to fasting-induced BCAA reduction in obese rodents than in lean controls, this dynamic change in serum BCAAs suggests that postprandial samples rather than fasting samples could be better for analysis of potential T2DM predictors. The greater alteration in postprandial BCAAs/BCKAs in OB also indicates deficient BCAA/BCKA metabolic or disposal capabilities in the obese population. Furthermore, BCKAs are a more sensitive metabolic marker than BCAAs for OB[64]. Compared to wild-type animals, young male obese Zucker rats (with mutated leptin) show decreased BCKD activity in the kidney, heart, gastrocnemius and liver (-66% to -47%), increased plasma BCAAs (45%-69%) and BCKAs (100%) and hepatic BCKAs (193%-418%), leucine oxidation (23%), proteolysis (35%), urinary marker of proteolysis (183%-766%), increased dietary intake (23%), whole body protein synthesis (23%-29%), body weight (53%), liver weight (107%) and adiposity (300%)[64].
Among our findings, the male subjects exhibited higher levels of serum amino acids (including BCAAs and AAAs) and greater differences in amino acids between the obese and NW T2DM subjects than the female subjects. These sex differences are consistent with previous reports[65,66]. Although the underlying mechanism is unknown, animal studies have shown that the female steroid hormone 17β-estradiol stimulates BCAA catabolism by increasing the activity of the BCKD and by BCKDK, which is an inhibitor of BCKD activity[67]. As most of the female subjects in our study were of reproductive age, a potential confounding effect of estrogen may account for the sex difference in serum BCAA. Nevertheless, the moderate BMI-dependent BCAA elevation in the female subjects in our study may become significant if a large sample size or more postmenopausal women were included. Indeed, one cohort study of 2204 (most postmenopausal) women, including 115 T2DM patients, 192 individuals with impaired fasting glucose (IFG) and 1897 control individuals, who differed significantly in BMI (30.6 vs 27.9, vs 25.4, respectively) and age (63 vs 60 vs 50 years, respectively), showed significant BCAA elevation in subjects with T2DM and IFG compared with normal control individuals[68]. Moreover, among the elevated BCAA/BCKA metabolites, 3-methyl-2-oxovalerate was the strongest predictive biomarker for IFG with moderate heritability (h2 = 0.20), based on the single-nucleotide polymorphism rs1440581 of the gene encoding for protein phosphatase (PP2Cm), which is needed for maintaining the activity of BCKD, the rate-limiting enzyme for BCKA catabolism[68]. However, that study may be confounded by a potentially higher estrogen level in the younger peri-postmenopausal control women (< 50 years) than in the postmenopausal women of the T2DM and IFG groups (both ≥ 60 years). Another study showed that higher diet and plasma BCAA concentrations were associated with increased T2DM risk among women with gestational diabetes, independent of BMI and other risk factors[69].
In the present study, BCAAs and AAAs were more closely correlated with abdominal adiposity than with body weight or BMI, indicating an important role of adiposity in hyperaminoacidemia/BCAA/AAA elevation. Other studies showed that the association of dietary BCAAs with T2DM risk/remission was dependent on the type of dietary fat, baseline triglycerides and body weight, as a Mediterranean diet rich in extravirgin olive oil (Med-diet) significantly reduced blood BCAAs and attenuated the association between plasma BCAA levels and T2DM incidence after a 3.8-year follow-up of 945 people compared to a control low-fat diet (LF)[70], and the Med-diet was associated with T2DM remission and BCAA reduction measured after an oral glucose tolerance test[28]. In addition, baseline plasma BCAAs indicated whether the LF or Med diet was capable of inducing T2DM remission. In diet-induced obese mice, high dietary fat increased the circulating BCAA pool, BCAA catabolism and OB by altering the gut microbiota[71,72]. BCAA supplementation to a high-fat diet increased mTOR activity, whereas BCAA supplementation to a normal diet did not affect mTOR activity in animals[73].
While elevated BCAAs/BCKAs may induce and interact with FFA accumulation in obese T2DM candidates, T2DM development in lean/NW populations may arise from different mechanisms. Unlike the gold standard for predicting different subtypes of T2DM diagnosis (i.e., fasting glucose and HbA1c), so far, there is no established standard for T2DM prediction. Although BCAAs/AAAs is a promising predictor, its utility may be affected by a range of factors including race/ethnicity, age, sex, body weight/BMI, and subtypes of T2DM. The heterogeneous elevation of BCAAs/AAAs among new-onset T2DM patients indicates limitations and restricted utility of BCAAs/AAAs as the predictor for different subtypes of T2DM. While the greater BCAAs/AAAs elevation in obese T2DM patients would support its’ prediction in individuals with OB(-propensity) which account for a large portion of T2DM population, the lower level, or a lack of BCAAs/AAAs elevation in normal-weight and reproductive-aged females would diminish its’ predicting power in these individuals.
A longitudinal 12-year follow up study of old adults (56 ± 8 years) showed that when BMI-matched obese non-T2DM individuals (n = 189/group, BMI = 30) were compared, the highest quartile of individual with elevated baseline BCAAs/AAAs had a 2- to 3.5-fold higher odds of risk of developing diabetes per SD increment over a 12-year follow-up period based on individual BCAAs/AAAs, or a 5- to 7-fold higher odds of developing diabetes if all BCAAs/AAAs were combined, in comparison with those individuals whose plasma amino acid levels were in the lowest quartile[16]. However, such increments in odds of risk were reduced to 1.3 and 2.0, respectively if the obese T2D candidates were compared with NW controls (n = 400, BMI = 25) randomly selected form a larger pool. Thus, how the controls are selected can lead to very different outcomes.
Similarly, a meta-analysis show that MUO, MUOW and MUNW individuals would show similar 4-fold risk increase of developing T2DM if each of them is compared with healthy counterparts of their corresponding BMI category (vs MHO, MHOW, and MHNW, respectively), but MUO and MUOW would have 2-3-fold higher risks than the MU-NW group if each of them is compared with MH-NW[45]. Thus, BCAAs/AAAs elevation would be greater in obese T2DM candidates than in NW T2DM candidates if all were compared with NW controls, but similar BCAAs/AAAs elevations across different BMI categories if obese, OW and NW T2DM candidates were compared with same BMI categories controls, respectively.
T2DM is a highly heterogeneous disease that include latent autoimmune diabetes in adults (LADA, defined by the presence of glutamic acid decarboxylase antibodies (GADA), maturity onset diabetes of the young (MODY, defined by gene mutations that disrupt insulin production) and neonatal diabetes, in addition to insulin resistant and BMI-related subgroups.
A recent data-driven cluster analysis of 14755 European T2DM patients using six variables (GADA, age at diagnosis, BMI, HbA1c, β-cell function and insulin resistance) resulted in 5 well-separated novel subgroups of adult-onset diabetes with distinct outcomes: A cluster of more severe insulin resistant individuals associated with higher risk of diabetic kidney disease; insulin deficiency cluster associated with highest risk of retinopathy; relatively young insulin deficient individuals with poor glycemic control (high HbA1c) and; a larger group of elderly patients with benign disease course[74]. That finding has been confirmed and extended by another cluster analysis of 2316 Chinese T2D patients and 685 United States T2D patients using five variables (age at diagnosis, BMI, HbA1c/glucose, β-cell function and insulin resistance) that resulted in 4 clusters: Half of the patients were elders with milder metabolic derangements; 25% of the patients had the highest BMI values but average blood glucose, β-cell function and insulin resistance; 14% of the patients had severe insulin deficiency and highest blood glucose; 8% of the patients were elders with severe insulin resistance and β-cell dysfunction[75]. Similar results of cluster analysis were reported in 55777 individuals with prediabetes[76]. However, none of these studies included BCAAs/AAAs as the study variable. Thus, the contributions of BCAAs/AAAs to different clusters or subtypes of T2DM remain unknown.
Nevertheless, BCAAs/AAAs elevation, if standardized based on different age and BMI sub-groups, could be useful in screening future prediabetes and OB-related diabetes in infants and adolescents as blood BCAAs/AAAs were found significantly correlated with BMI standard deviation score, fasting glucose, HbA1c, triglycerides, cystatin C and creatinine in 2191 healthy participants aged 3 months to 18 years[77]. A 7.5-year longitudinal study of 396 nondiabetic Finnish girls showed that serum BCAA profile in childhood (11.2 ± 0.8 years at baseline) were associated with insulin resistance during pubertal development (significant both before and after menarche) independent of adiposity, and it predicted dysregulated glycemic and triglyceride levels in adulthood[78-80]. Blood BCAA/AAA were also found significantly elevated in OW and obese prepubertal children than in healthy controls[39-41,81].
The global prevalence of prediabetes in children and adults have reached alarming levels[81], with an annualized diabetes conversion rate of 5%–10%[82]. And 500 million adults in China (50% of China’s adult population) and 98 million adult Americans (38.0% of the United States adult population) have prediabetes (https://www.cdc.gov/diabetes/data/statistics-report/index.html#anchor_23827), the world T2DM population could double by 2050. In the United States, the total cost of diagnosed diabetes in 2022 was $413 billion, including $106 billion in indirect costs (https://www.cdc.gov/diabetes/health-equity/diabetes-by-the-numbers.html). Because preventive lifestyle modification can reduce the risk of diabetes by up to 70%[83], identification of “at-risk” individuals 10-20 years prior to T2DM onset based on BCAAs/AAAs elevation would offer plenty time for lifestyle modifications.
While BCAAs/AAAs elevation alone may not predict all subtypes of T2DM, its combined use with other anthropogenic, metabolic, and genetic biomarkers such as visceral adiposity index, muscle mass index[47-50], fasting glucose, GADA (associated with LADA), genetic polymorphisms (associated with MODY), and metabolic parameters associated with insulin deficiency, diabetic kidney disease and retinopathy should be evaluated in future studies.
This study has limitations. The lack of BMI-matched healthy control individuals makes it impossible to evaluate a potential low grade hyperaminoacidemia/BCAA/AAA elevation that may exist in NW-T2DM patients. Only fasting samples, not postprandial/oral glucose tolerance test samples or BCKAs, were studied, which may have missed the dynamic changes in BCAA catabolism. The moderate sample size of this cross-sectional study could not discern the causality of the findings. The lack of lifestyle data limits exploration of the influences of diet and social, psychological, and physical activities on BCAA/AAA. Anti-diabetic medication taken by some T2DM patients may have compromised the results[84,85]. In addition, no genetic, race/ ethnicity influences can be derived from this study, although sex differences and significant correlations between BCAAs/AAAs and anthropometric parameters were demonstrated.
Future studies should overcome the above limitations of this study. To date, most of the published BCAAs/AAAs findings were based on comparisons between obese T2DM patients and nonobese individuals, whereas the BCAAs/AAAs data of the lean/NW-T2DM groups were largely unreported. This publication bias should be addressed by stratified analyses for age, BMI, adiposity, sex, and genotype as discussed above and by comparisons between metabolically healthy vs metabolically unhealthy individuals of different BMI categories.
In summary, heterogeneous elevation of BCAAs/AAAs is found in new-onset T2DM patients. While the greater BCAA/AAA elevation in obese and male T2DM patients would support BCAA/AAA prediction of T2DM development in these individuals, the lower or lack of elevated BCAAs/AAAs in NW and reproductive-aged female T2DM patients could compromise its prediction in these people. This potentially skewed T2DM prediction should be considered when BCAAs/AAAs are to be used as the T2DM predictor. As BCAAs/AAAs can predict T2DM as early as from childhood to early adulthood and 1-2 decades prior to T2DM onset[86-89], and because BCAA/BCKA elevation can be effectively normalized through diet, exercise, weight control interventions and pharmacogenetic therapy[84-86], study and normalization of BCAA/AAA elevation could provide a novel opportunity for curbing global T2DM epidemic.
Type-2 diabetes mellitus (T2DM) is a major cause of comorbidity and mortality in society and was responsible for more than 4.2 million annual deaths in 2019 alone. The current world population of T2DM (approximately 450 million) is expected to double to 1 billion soon after 2050. This T2DM pandemic, however, can be curbed or prevented if the population at risk of T2DM can be identified and prophylactic actions be taken long before the onset of T2DM.
Research over the past decades has indicated that elevated branched-chain amino acids (BCAAs: Isoleucine, leucine, valine) and aromatic amino acids (AAAs: Tyrosine, tryptophan, phenylalanine) show high sensitivity and specificity (both > 97%) in predicting diabetes in animals and can successfully predict T2DM nearly 20 years before T2DM onset in select human populations. However, these findings have not been widely translated into clinical utilization due to unidentified factors.
We hypothesized that body weight and sex are potential confounding factors that could affect BCAAs/AAAs as general T2DM predictors. As the first step, the aim of our study was to determine the effects of body weight and sex on BCAAs/AAAs in new-onset T2DM individuals.
Fasting blood samples were collected from 97 new-onset T2DM patients (53 male/44 female, 43.3 ± 11.2 years of age, differing in body mass index (BMI): Normal weight (NW), n = 33, BMI = 22.23 ± 1.60; overweight, n = 42, BMI = 25.9 ± 1.07; and obesity, n = 22, BMI = 31.23 ± 2.31). All T2DM cases were diagnosed within 12 mo at the First People's Hospital of Yunnan Province, Kunming, China. Serum amino acids were analyzed using ultra-performance liquid chromatography/triple stage quadrupole mass spectrometry.
Fasting serum AAAs, BCAAs, glutamate, and alanine levels were significantly greater and high-density lipoprotein levels were significantly lower in obese T2DM patients than in NW-T2DM patients, especially among male patients. Arginine, histidine, leucine, methionine, and lysine were greater in male patients than in female patients. Moreover, histidine, alanine, glutamate, lysine, valine, methionine, leucine, isoleucine, tyrosine, phenylalanine, and tryptophan were significantly correlated with abdominal adiposity, body weight and BMI, respectively.
Heterogeneously elevated amino acids, especially BCAAs/AAAs, are found in new-onset T2DM patients in differing BMI categories, which may indicate a potentially skewed prediction of T2DM development by BCAA/AAAs, i.e., more accurate and reliable prediction in obese and male individuals than in NW individuals and females. This skewness may limit the universal application of this T2DM predictor.
This study has limitations. The lack of BMI-matched healthy control individuals makes it impossible to determine whether a potential low grade hyperaminoacidemia/BCAA/AAA elevation exists in normal-weight T2DM patients. The moderate sample size and lack of lifestyle and genetic data of this cross-sectional study could not discern the causality and/or mechanisms of the heterogeneity. Further studies should include both metabolically healthy and metabolically unhealthy individuals in differing BMI categories to overcome the abovementioned limitations.
The authors thank the staff of the Department of Endocrinology of the First People’s Hospital of Yunnan Province P. R. China for patient selection and sample collection. Evan Xing proofread the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gutiérrez-Cuevas J, Mexico; Wani I, India; Sanyal D, India S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1683] [Cited by in RCA: 1746] [Article Influence: 873.0] [Reference Citation Analysis (18)] |
| 2. | Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 3. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 885] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 4. | Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1356] [Article Influence: 169.5] [Reference Citation Analysis (0)] |
| 5. | Rhee EJ, Lee MK, Kim JD, Jeon WS, Bae JC, Park SE, Park CY, Oh KW, Park SW, Lee WY. Metabolic health is a more important determinant for diabetes development than simple obesity: a 4-year retrospective longitudinal study. PLoS One. 2014;9:e98369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Bansal N. Prediabetes diagnosis and treatment: A review. World J Diabetes. 2015;6:296-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 276] [Cited by in RCA: 323] [Article Influence: 32.3] [Reference Citation Analysis (9)] |
| 7. | Kivelä J, Meinilä J, Uusitupa M, Tuomilehto J, Lindström J. Longitudinal Branched-Chain Amino Acids, Lifestyle Intervention, and Type 2 Diabetes in the Finnish Diabetes Prevention Study. J Clin Endocrinol Metab. 2022;107:2844-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes. 2018;10:350-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. | de Almeida-Pititto B, Dualib PM, Jordão MC, Izar Helfenstein Fonseca M, Jones SR, Blaha MJ, Toth PP, Santos RD, Bensenor IM, Ferreira SRG, Lotufo PA; ELSA-Brasil Research Group. Branched-chain amino acids predict incident diabetes in the Brazilian Longitudinal Study of Adult Health - ELSA-Brasil. Diabetes Res Clin Pract. 2021;174:108747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Flores-Guerrero JL, Gruppen EG, Connelly MA, Shalaurova I, Otvos JD, Garcia E, Bakker SJL, Dullaart RPF. A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Flores-Guerrero JL, Osté MCJ, Kieneker LM, Gruppen EG, Wolak-Dinsmore J, Otvos JD, Connelly MA, Bakker SJL, Dullaart RPF. Plasma Branched-Chain Amino Acids and Risk of Incident Type 2 Diabetes: Results from the PREVEND Prospective Cohort Study. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Ramzan I, Ardavani A, Vanweert F, Mellett A, Atherton PJ, Idris I. The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Shi L, Brunius C, Lehtonen M, Auriola S, Bergdahl IA, Rolandsson O, Hanhineva K, Landberg R. Plasma metabolites associated with type 2 diabetes in a Swedish population: a case-control study nested in a prospective cohort. Diabetologia. 2018;61:849-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Tillin T, Hughes AD, Wang Q, Würtz P, Ala-Korpela M, Sattar N, Forouhi NG, Godsland IF, Eastwood SV, McKeigue PM, Chaturvedi N. Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia. 2015;58:968-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Walford GA, Davis J, Warner AS, Ackerman RJ, Billings LK, Chamarthi B, Fanelli RR, Hernandez AM, Huang C, Khan SQ, Littleton KR, Lo J, McCarthy RM, Rhee EP, Deik A, Stolerman E, Taylor A, Hudson MS, Wang TJ, Altshuler D, Grant RW, Clish CB, Gerszten RE, Florez JC. Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism. 2013;62:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2618] [Cited by in RCA: 2436] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 17. | Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2638] [Cited by in RCA: 2439] [Article Influence: 152.4] [Reference Citation Analysis (0)] |
| 18. | Lu Y, Wang Y, Ong CN, Subramaniam T, Choi HW, Yuan JM, Koh WP, Pan A. Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia. 2016;59:2349-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Ottosson F, Smith E, Melander O, Fernandez C. Altered Asparagine and Glutamate Homeostasis Precede Coronary Artery Disease and Type 2 Diabetes. J Clin Endocrinol Metab. 2018;103:3060-3069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B, Grallert H, Xu T, Bader E, Huth C, Mittelstrass K, Döring A, Meisinger C, Gieger C, Prehn C, Roemisch-Margl W, Carstensen M, Xie L, Yamanaka-Okumura H, Xing G, Ceglarek U, Thiery J, Giani G, Lickert H, Lin X, Li Y, Boeing H, Joost HG, de Angelis MH, Rathmann W, Suhre K, Prokisch H, Peters A, Meitinger T, Roden M, Wichmann HE, Pischon T, Adamski J, Illig T. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 547] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 21. | Rooney MR, Fang M, Ogurtsova K, Ozkan B, Echouffo-Tcheugui JB, Boyko EJ, Magliano DJ, Selvin E. Global Prevalence of Prediabetes. Diabetes Care. 2023;46:1388-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 226] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 22. | Xie G, Ma X, Zhao A, Wang C, Zhang Y, Nieman D, Nicholson JK, Jia W, Bao Y. The metabolite profiles of the obese population are gender-dependent. J Proteome Res. 2014;13:4062-4073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Lu J, Xie G, Jia W. Metabolomics in human type 2 diabetes research. Front Med. 2013;7:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Asoudeh F, Salari-Moghaddam A, Keshteli AH, Esmaillzadeh A, Adibi P. Dietary intake of branched-chain amino acids in relation to general and abdominal obesity. Eat Weight Disord. 2022;27:1303-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Liu M, Huang Y, Zhang H, Aitken D, Nevitt MC, Rockel JS, Pelletier JP, Lewis CE, Torner J, Rampersaud YR, Perruccio AV, Mahomed NN, Furey A, Randell EW, Rahman P, Sun G, Martel-Pelletier J, Kapoor M, Jones G, Felson D, Qi D, Zhai G. Restricting Branched-Chain Amino Acids within a High-Fat Diet Prevents Obesity. Metabolites. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Okekunle AP, Lee H, Provido SMP, Chung GH, Hong S, Yu SH, Lee CB, Lee JE. Dietary branched-chain amino acids and odds of obesity among immigrant Filipino women: the Filipino women's diet and health study (FiLWHEL). BMC Public Health. 2022;22:654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Chen Z, Franco OH, Lamballais S, Ikram MA, Schoufour JD, Muka T, Voortman T. Associations of specific dietary protein with longitudinal insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Clin Nutr. 2020;39:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Cardelo MP, Alcala-Diaz JF, Gutierrez-Mariscal FM, Lopez-Moreno J, Villasanta-Gonzalez A, Arenas-de Larriva AP, Cruz-Ares S, Delgado-Lista J, Rodriguez-Cantalejo F, Luque RM, Ordovas JM, Perez-Martinez P, Camargo A, Lopez-Miranda J. Diabetes Remission Is Modulated by Branched Chain Amino Acids According to the Diet Consumed: From the CORDIOPREV Study. Mol Nutr Food Res. 2022;66:e2100652. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Nagata C, Nakamura K, Wada K, Tsuji M, Tamai Y, Kawachi T. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: the Takayama study. Am J Epidemiol. 2013;178:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Okekunle AP, Zhang M, Wang Z, Onwuka JU, Wu X, Feng R, Li C. Dietary branched-chain amino acids intake exhibited a different relationship with type 2 diabetes and obesity risk: a meta-analysis. Acta Diabetol. 2019;56:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Li YC, Li Y, Liu LY, Chen Y, Zi TQ, Du SS, Jiang YS, Feng RN, Sun CH. The Ratio of Dietary Branched-Chain Amino Acids is Associated with a Lower Prevalence of Obesity in Young Northern Chinese Adults: An Internet-Based Cross-Sectional Study. Nutrients. 2015;7:9573-9589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Virtanen HEK, Koskinen TT, Voutilainen S, Mursu J, Tuomainen TP, Kokko P, Virtanen JK. Intake of different dietary proteins and risk of type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2017;117:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Zhao H, Zhang F, Sun D, Wang X, Zhang X, Zhang J, Yan F, Huang C, Xie H, Lin C, Liu Y, Fan M, Yan W, Chen Y, Lian K, Li Y, Zhang L, Wang S, Tao L. Branched-Chain Amino Acids Exacerbate Obesity-Related Hepatic Glucose and Lipid Metabolic Disorders via Attenuating Akt2 Signaling. Diabetes. 2020;69:1164-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 34. | Eizirik DL, Germano CM, Migliorini RH. Dietetic supplementation with branched chain amino acids attenuates the severity of streptozotocin-induced diabetes in rats. Acta Diabetol Lat. 1988;25:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 36. | She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 294] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 37. | Noguchi Y, Zhang QW, Sugimoto T, Furuhata Y, Sakai R, Mori M, Takahashi M, Kimura T. Network analysis of plasma and tissue amino acids and the generation of an amino index for potential diagnostic use. Am J Clin Nutr. 2006;83:513S-519S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Dong Q, Sidra S, Gieger C, Wang-Sattler R, Rathmann W, Prehn C, Adamski J, Koenig W, Peters A, Grallert H, Sharma S. Metabolic Signatures Elucidate the Effect of Body Mass Index on Type 2 Diabetes. Metabolites. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 39. | Gumus Balikcioglu P, Jachthuber Trub C, Balikcioglu M, Ilkayeva O, White PJ, Muehlbauer M, Bain JR, Armstrong S, Freemark M. Branched-chain α-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity. Endocrinol Diabetes Metab. 2023;6:e388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 40. | Polidori N, Grasso EA, Chiarelli F, Giannini C. Amino Acid-Related Metabolic Signature in Obese Children and Adolescents. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Ahola-Olli AV, Mustelin L, Kalimeri M, Kettunen J, Jokelainen J, Auvinen J, Puukka K, Havulinna AS, Lehtimäki T, Kähönen M, Juonala M, Keinänen-Kiukaanniemi S, Salomaa V, Perola M, Järvelin MR, Ala-Korpela M, Raitakari O, Würtz P. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia. 2019;62:2298-2309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 42. | Cheng D, Zhao X, Yang S, Cui H, Wang G. Metabolomic Signature Between Metabolically Healthy Overweight/Obese and Metabolically Unhealthy Overweight/Obese: A Systematic Review. Diabetes Metab Syndr Obes. 2021;14:991-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 43. | Badoud F, Lam KP, DiBattista A, Perreault M, Zulyniak MA, Cattrysse B, Stephenson S, Britz-McKibbin P, Mutch DM. Serum and adipose tissue amino acid homeostasis in the metabolically healthy obese. J Proteome Res. 2014;13:3455-3466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 44. | Telle-Hansen VH, Christensen JJ, Formo GA, Holven KB, Ulven SM. A comprehensive metabolic profiling of the metabolically healthy obesity phenotype. Lipids Health Dis. 2020;19:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Tajik S, Mirzababaei A, Ghaedi E, Kord-Varkaneh H, Mirzaei K. Risk of type 2 diabetes in metabolically healthy people in different categories of body mass index: an updated network meta-analysis of prospective cohort studies. J Cardiovasc Thorac Res. 2019;11:254-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Zhu X, Hu J, Guo H, Ji D, Yuan D, Li M, Yan T, Xue C, Ma H, Zhou X, Liu Y, Li Y, Sun K, Sun Z, Wang B. Effect of Metabolic Health and Obesity Phenotype on Risk of Diabetes Mellitus: A Population-Based Longitudinal Study. Diabetes Metab Syndr Obes. 2021;14:3485-3498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Kim G, Kim JH. Impact of Skeletal Muscle Mass on Metabolic Health. Endocrinol Metab (Seoul). 2020;35:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 48. | Gutierrez-Monreal MA, Harmsen JF, Schrauwen P, Esser KA. Ticking for Metabolic Health: The Skeletal-Muscle Clocks. Obesity (Silver Spring). 2020;28 Suppl 1:S46-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Holeček M. The role of skeletal muscle in the pathogenesis of altered concentrations of branched-chain amino acids (valine, leucine, and isoleucine) in liver cirrhosis, diabetes, and other diseases. Physiol Res. 2021;70:293-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Endocr J. 2014;61:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 51. | Chen F, Liu J, Yan Y, Mi J; China Child and Adolescent Cardiovascular Health (CCACH) Study Group. Abnormal Metabolic Phenotypes Among Urban Chinese Children: Epidemiology and the Impact of DXA-Measured Body Composition. Obesity (Silver Spring). 2019;27:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Ding WQ, Liu JT, Shang YX, Gao B, Zhao XY, Zhao HP, Wu WJ. DXA-measured visceral fat mass and lean body mass reflect abnormal metabolic phenotypes among some obese and nonobese Chinese children and adolescents. Nutr Metab Cardiovasc Dis. 2018;28:618-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Kang EY, Yim JE. Differences in dietary intakes, body compositions, and biochemical indices between metabolically healthy and metabolically abnormal obese Korean women. Nutr Res Pract. 2019;13:488-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Peppa M, Koliaki C, Papaefstathiou A, Garoflos E, Katsilambros N, Raptis SA, Hadjidakis DI, Dimitriadis GD. Body composition determinants of metabolic phenotypes of obesity in nonobese and obese postmenopausal women. Obesity (Silver Spring). 2013;21:1807-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Loos RJF, Kilpeläinen TO. Genes that make you fat, but keep you healthy. J Intern Med. 2018;284:450-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Salmón-Gómez L, Catalán V, Frühbeck G, Gómez-Ambrosi J. Relevance of body composition in phenotyping the obesities. Rev Endocr Metab Disord. 2023;24:809-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 57. | Blundell JE, Dulloo AG, Salvador J, Frühbeck G; EASO SAB Working Group on BMI. Beyond BMI--phenotyping the obesities. Obes Facts. 2014;7:322-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 58. | Xia L, Dong F, Gong H, Xu G, Wang K, Liu F, Pan L, Zhang L, Yan Y, Gaisano H, He Y, Shan G. Association between Indices of Body Composition and Abnormal Metabolic Phenotype in Normal-Weight Chinese Adults. Int J Environ Res Public Health. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Orsso CE, Tibaes JRB, Oliveira CLP, Rubin DA, Field CJ, Heymsfield SB, Prado CM, Haqq AM. Low muscle mass and strength in pediatrics patients: Why should we care? Clin Nutr. 2019;38:2002-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 60. | Hernández-Alvarez MI, Díaz-Ramos A, Berdasco M, Cobb J, Planet E, Cooper D, Pazderska A, Wanic K, O'Hanlon D, Gomez A, de la Ballina LR, Esteller M, Palacin M, O'Gorman DJ, Nolan JJ, Zorzano A. Early-onset and classical forms of type 2 diabetes show impaired expression of genes involved in muscle branched-chain amino acids metabolism. Sci Rep. 2017;7:13850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Johnson MA, Gannon NP, Schnuck JK, Lyon ES, Sunderland KL, Vaughan RA. Leucine, Palmitate, or Leucine/Palmitate Cotreatment Enhances Myotube Lipid Content and Oxidative Preference. Lipids. 2018;53:1043-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL, Arany Z. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 63. | She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552-E1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 64. | She P, Olson KC, Kadota Y, Inukai A, Shimomura Y, Hoppel CL, Adams SH, Kawamata Y, Matsumoto H, Sakai R, Lang CH, Lynch CJ. Leucine and protein metabolism in obese Zucker rats. PLoS One. 2013;8:e59443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Schauder P, Zavelberg D, Langer K, Herbertz L. Sex-specific differences in plasma branched-chain keto acid levels in obesity. Am J Clin Nutr. 1987;46:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Kobayashi R, Shimomura Y, Murakami T, Nakai N, Fujitsuka N, Otsuka M, Arakawa N, Popov KM, Harris RA. Gender difference in regulation of branched-chain amino acid catabolism. Biochem J. 1997;327:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Obayashi M, Shimomura Y, Nakai N, Jeoung NH, Nagasaki M, Murakami T, Sato Y, Harris RA. Estrogen controls branched-chain amino acid catabolism in female rats. J Nutr. 2004;134:2628-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Menni C, Fauman E, Erte I, Perry JR, Kastenmüller G, Shin SY, Petersen AK, Hyde C, Psatha M, Ward KJ, Yuan W, Milburn M, Palmer CN, Frayling TM, Trimmer J, Bell JT, Gieger C, Mohney RP, Brosnan MJ, Suhre K, Soranzo N, Spector TD. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62:4270-4276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 69. | Tobias DK, Clish C, Mora S, Li J, Liang L, Hu FB, Manson JE, Zhang C. Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk Among High-Risk Women with a History of Gestational Diabetes Mellitus. Clin Chem. 2018;64:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Ruiz-Canela M, Guasch-Ferré M, Toledo E, Clish CB, Razquin C, Liang L, Wang DD, Corella D, Estruch R, Hernáez Á, Yu E, Gómez-Gracia E, Zheng Y, Arós F, Romaguera D, Dennis C, Ros E, Lapetra J, Serra-Majem L, Papandreou C, Portoles O, Fitó M, Salas-Salvadó J, Hu FB, Martínez-González MA. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: case-cohort study within the PREDIMED Trial. Diabetologia. 2018;61:1560-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 71. | Gojda J, Cahova M. Gut Microbiota as the Link between Elevated BCAA Serum Levels and Insulin Resistance. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 72. | Zhang L, Yue Y, Shi M, Tian M, Ji J, Liao X, Hu X, Chen F. Dietary Luffa cylindrica (L.) Roem promotes branched-chain amino acid catabolism in the circulation system via gut microbiota in diet-induced obese mice. Food Chem. 2020;320:126648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 73. | Morio A, Tsutsumi R, Satomi S, Kondo T, Miyoshi H, Kato T, Kuroda M, Kitamura T, Hara K, Saeki N, Sakaue H, Tsutsumi YM. Leucine imparts cardioprotective effects by enhancing mTOR activity and mitochondrial fusion in a myocardial ischemia/reperfusion injury murine model. Diabetol Metab Syndr. 2021;13:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, Vikman P, Prasad RB, Aly DM, Almgren P, Wessman Y, Shaat N, Spégel P, Mulder H, Lindholm E, Melander O, Hansson O, Malmqvist U, Lernmark Å, Lahti K, Forsén T, Tuomi T, Rosengren AH, Groop L. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1662] [Cited by in RCA: 1377] [Article Influence: 196.7] [Reference Citation Analysis (1)] |
| 75. | Zou X, Zhou X, Zhu Z, Ji L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. 2019;7:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 76. | Zheng R, Xu Y, Li M, Gao Z, Wang G, Hou X, Chen L, Huo Y, Qin G, Yan L, Wan Q, Zeng T, Shi L, Hu R, Tang X, Su Q, Yu X, Qin Y, Chen G, Gu X, Shen F, Luo Z, Chen Y, Zhang Y, Liu C, Wang Y, Wu S, Yang T, Li Q, Mu Y, Zhao J, Hu C, Jia X, Xu M, Wang T, Zhao Z, Wang S, Lin H, Ning G, Wang W, Lu J, Bi Y; China Cardiometabolic Disease and Cancer Cohort (4C) Study Group. Data-driven subgroups of prediabetes and the associations with outcomes in Chinese adults. Cell Rep Med. 2023;4:100958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 77. | Hirschel J, Vogel M, Baber R, Garten A, Beuchel C, Dietz Y, Dittrich J, Körner A, Kiess W, Ceglarek U. Relation of Whole Blood Amino Acid and Acylcarnitine Metabolome to Age, Sex, BMI, Puberty, and Metabolic Markers in Children and Adolescents. Metabolites. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Wiklund P, Zhang X, Tan X, Keinänen-Kiukaanniemi S, Alen M, Cheng S. Serum Amino Acid Profiles in Childhood Predict Triglyceride Level in Adulthood: A 7-Year Longitudinal Study in Girls. J Clin Endocrinol Metab. 2016;101:2047-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Tricò D, Prinsen H, Giannini C, de Graaf R, Juchem C, Li F, Caprio S, Santoro N, Herzog RI. Elevated α-Hydroxybutyrate and Branched-Chain Amino Acid Levels Predict Deterioration of Glycemic Control in Adolescents. J Clin Endocrinol Metab. 2017;102:2473-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 80. | Zhang X, Ojanen X, Zhuang H, Wu N, Cheng S, Wiklund P. Branched-Chain and Aromatic Amino Acids Are Associated With Insulin Resistance During Pubertal Development in Girls. J Adolesc Health. 2019;65:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Bugajska J, Berska J, Wójcik M, Sztefko K. Amino acid profile in overweight and obese prepubertal children - can simple biochemical tests help in the early prevention of associated comorbidities? Front Endocrinol (Lausanne). 2023;14:1274011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Han C, Song Q, Ren Y, Chen X, Jiang X, Hu D. Global prevalence of prediabetes in children and adolescents: A systematic review and meta-analysis. J Diabetes. 2022;14:434-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1933] [Article Influence: 148.7] [Reference Citation Analysis (0)] |
| 84. | Preiss D, Rankin N, Welsh P, Holman RR, Kangas AJ, Soininen P, Würtz P, Ala-Korpela M, Sattar N. Effect of metformin therapy on circulating amino acids in a randomized trial: the CAMERA study. Diabet Med. 2016;33:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Zemdegs J, Martin H, Pintana H, Bullich S, Manta S, Marqués MA, Moro C, Layé S, Ducrocq F, Chattipakorn N, Chattipakorn SC, Rampon C, Pénicaud L, Fioramonti X, Guiard BP. Metformin Promotes Anxiolytic and Antidepressant-Like Responses in Insulin-Resistant Mice by Decreasing Circulating Branched-Chain Amino Acids. J Neurosci. 2019;39:5935-5948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 86. | Noh JW, Jung JH, Park JE, Lee JH, Sim KH, Park J, Kim MH, Yoo KB. The relationship between age of onset and risk factors including family history and life style in Korean population with type 2 diabetes mellitus. J Phys Ther Sci. 2018;30:201-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Uusitupa M, Khan TA, Viguiliouk E, Kahleova H, Rivellese AA, Hermansen K, Pfeiffer A, Thanopoulou A, Salas-Salvadó J, Schwab U, Sievenpiper JL. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 88. | Papakonstantinou E, Oikonomou C, Nychas G, Dimitriadis GD. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 89. | Khan TA, Field D, Chen V, Ahmad S, Mejia SB, Kahleová H, Rahelić D, Salas-Salvadó J, Leiter LA, Uusitupa M, Kendall CWC, Sievenpiper JL. Combination of Multiple Low-Risk Lifestyle Behaviors and Incident Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Diabetes Care. 2023;46:643-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |