Published online Sep 15, 2023. doi: 10.4239/wjd.v14.i9.1422
Peer-review started: April 17, 2023
First decision: June 1, 2023
Revised: June 14, 2023
Accepted: July 29, 2023
Article in press: July 29, 2023
Published online: September 15, 2023
Processing time: 148 Days and 21.7 Hours
Diabetic retinopathy (DR) is currently recognized as one of the most serious diabetic microangiopathies and a major cause of adult blindness. Commonly used clinical approaches include etiological control, microvascular improvement, and surgical intervention, but they are ineffective and have many side effects. Oral Chinese medicine (OCM) has been used for thousands of years to treat DR and is still widely used today, but it is unclear which OCM is more effective for DR.
To estimate relative effectiveness and safety profiles for different classes of OCMs for DR, and provide rankings of the available OCMs.
The search time frame was from the creation of the database to January 2023. RevMan 5.3 and Stata 14.0 software were used to perform the systematic review and Network meta-analyses (NMA).
A total of 107 studies and 9710 patients were included, including 4767 cases in the test group and 4973 cases in the control group. Based on previous studies and clinical reports, and combined with the recommendations of Chinese guidelines for the prevention and treatment of DR, 9 OCMs were finally included in this study, namely Compound Xueshuantong Capsules, Qiming Granules, Compound Danshen Dripping Pills, Hexue Mingmu Tablets (HXMM), Qiju Dihuang Pills (QJDH), Shuangdan Mingmu Capsules (SDMM), Danggui Buxue Decoction (DGBX), Xuefu Zhuyu Decoction and Buyang Huanwu Decoction. When these nine OCMs were analyzed in combination with conventional western medicine treatment (CT) compared with CT alone, the NMA results showed that HXMM + CT has better intervention effect on the overall efficacy of DR patients, HXMM + CT has better effect on improving patients' visual acuity, SDMM + CT has better effect on inhibiting vascular endothelial growth factor, DGBX + CT has better effect on reducing fundus hemorrhage area, HXMM + CT has better effect on reducing fasting blood glucose, and QJDH + CT has better effect on reducing glycated hemoglobin. When there are not enough clinical indicators for reference, SDMM + CT or HXMM + CT treatments can be chosen because they are effective for more indicators and demonstrate multidimensional efficacy.
This study provides evidence that combining OCMs with CT leads to better outcomes in all aspects of DR compared to using CT alone. Based on the findings, we highly recommend the use of SDMM or HXMM for the treatment of DR. These two OCMs have demonstrated outstanding efficacy across multiple indicators.
Core Tip: To our knowledge, this study represents the first network meta-analysis (NMA) examining the effectiveness of traditional Chinese medicine in treating diabetic retinopathy (DR). Notably, this NMA includes the largest number of original studies, subjects, and variety of Chinese medicines to date. While the efficacy of Chinese medicine for DR has been widely recognized in China, no previous studies have systematically evaluated which Chinese medicine treatment is the most effective. Therefore, this study fills an important gap in the field.
- Citation: Li HD, Li MX, Zhang WH, Zhang SW, Gong YB. Effectiveness and safety of traditional Chinese medicine for diabetic retinopathy: A systematic review and network meta-analysis of randomized clinical trials. World J Diabetes 2023; 14(9): 1422-1449
- URL: https://www.wjgnet.com/1948-9358/full/v14/i9/1422.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i9.1422
Diabetic retinopathy (DR) is currently recognized as one of the serious diabetic microangiopathies and is the leading cause of blindness in adults. According to the International Diabetes Federation, it is estimated that the number of people with diabetes will reach 642 million worldwide in 2040, and 34.6% of these patients will have DR[1]. DR causes irreversible visual impairment, including abnormal vision, blurred vision, and even blindness. In addition, the presence of DR implies an increased risk of life-threatening systemic vascular complications[2]. Currently, the main treatments for DR include retinal laser photocoagulation, pharmacotherapy, hormonal therapy, and surgery. However, these treatments may lead to adverse effects such as increased angiogenesis, increased intraocular pressure, and retinal hemorrhage[3,4]. Studies have shown that age is a key factor affecting DR, and the number of DR patients in the elderly population will reach new highs as the world ages[5]. In view of the current situation, the pathogenesis of DR is being actively explored around the world and effective therapeutic drugs are being explored.
In fact, traditional Chinese medicine (TCM) has long been considered a promising complementary therapy that dates back more than 1000 years. In China, many facts have proven that herbal medicine can effectively improve the fundus condition of patients, relieve the pain of the disease, and obtain a better quality of life for patients through multi-target and multi-path interventions in DR[6]. Many Oral Chinese medicine (OCM), including proprietary Chinese medicine preparations and herbal granules are widely used in the treatment of DR. For example, Qiming Granules (QM), the first OCM approved by the State Food and Drug Administration for the treatment of DR, whose main ingredients are Hedysarum Multijugum Maxim. (Huangqi in chinese), Radix Puerariae (Gegen in chinese), Lycii Fructus (Gouqizi in chinese), and Cassiae Semen (Juemingzi in chinese), etc., were shown to alleviate retinal hypoxia and ischemia by increasing retinal blood flow and improving blood circulation in a multicenter, randomized, parallel controlled clinical trial[7]. Then for example, Compound Xueshuantong Capsules (XST), an OCM commonly used for DR, was shown to effectively improve the disorder of retinal structure and edema in streptozotocin-induced type 2 diabetic rats by activating the PPAR signaling pathway, reversing the reduction in retinal thickness and retinal ganglion cell number, and reducing the apoptotic index of retinal cells[8]. Compound Danshen dripping pills, an oral proprietary Chinese medicine containing Danshen, was found to improve vision and clinical symptoms and reduce the incidence of macular edema compared to captopril in a retrospective study[9].
In addition to these well known OCMs, there are many lesser known OCMs that are widely used in clinical practice. However, the selection of these OCMs remains a challenge for patients with different disease states. Network meta-analyses (NMA) allow for the comparison of multiple treatments (i.e. three or more) using both direct comparisons of interventions within randomized controlled trials (RCT) and indirect comparisons across trials based on a common comparator[10]. To date, there are no studies comparing different types of OCMs used for the treatment of DR. Therefore, we searched all RCTs of OCMs used for the treatment of DR and initiated this NMA to compare the efficacy between them, hoping to provide some suggestions for clinical practice.
The study protocol was registered on PROSPERO (International Prospective Register of Systematic Reviews). Registration No. CRD42022352250 (https://www.crd.york.ac.uk/PROSPERO/#myprosperoID=CRD42022352250). This program was developed in accordance with the Preferred Reporting Items For Systematic Review And Meta-analysis Protocols (PRISMA-P)[11]. The PRISMA Extension Statement is used to ensure that all aspects of the methods and results are reported[12].
The Population-Intervention-Comparators-Outcomes-Studydesign framework was adopted as the eligibility criteria for the review as following.
The RCT is the original study that we agreed to include. We did not place any restrictions on the language, country, publication date, or phase of the RCT. Duplicate publications, summaries of personal experience, purely theoretical studies, reviews, animal or cellular experiments, and original studies with incorrect or incomplete data in the literature should also be excluded.
Patients with DR who meet the standard diagnosis rely on fundus fluorescence angiography and fundus signs to detect microangiomas, exudates, hemorrhages, neovascularization, and other fundus changes. No restriction of age, gender, occupation and region. No concomitant ocular diseases caused by non-hyperglycemic factors, such as primary glaucoma and senile cataract; no acute metabolic diseases, such as diabetic ketoacidosis, within a short period of time before enrollment.
Regarding the RCT of OCM for DR, the blinding and language are not limited. The control group received only oral western medicine, conventional western medicine treatment (CT) mainly included hypoglycemic drugs, lipid-lowering drugs and antihypertensive drugs, among which hypoglycemic drugs could include subcutaneous injection of insulin, in addition, other injectable drugs were not acceptable; antioxidant and microcirculation improvement drugs, such as calcium dobesilate, pancreatic kininogenase; drugs to promote retinal metabolism and nerve nutrition of the eye, such as lecithin complex iodine, etc. The test group was added to the control group with OCM. By combining previous studies and actual clinical observations, and also referring to the latest published Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition)[13], we decided to study the 9 most commonly used OCMs which are XST (composed of Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow/Figwort Root/Hedysarum Multijugum Maxim./
The main efficacy indicators in this study encompass total clinical effectiveness and visual acuity. Following the international DR efficacy determination standard, the efficacy after treatment was categorized into three groups: Significantly effective, effective, and ineffective. Significantly effective cases were identified based on two criteria: (1) Improvement in visual acuity by ≥ 2 lines; and (2) improvement in two or more of the three fundus indices (microvessel count, hemorrhage, and exudation), or significant improvement in one or more of the three indices without deterioration in the remaining indices. Effective cases were determined by improvement in visual acuity and improvement in at least one of the three fundus indices without deterioration in the remaining indices. Ineffective cases were those that did not meet the criteria for effectiveness based on the indices. For assessing visual acuity, the international standard visual acuity table was utilized, where the counting included 2 rows for no light perception to light perception, and 1 row for each interval including light perception, manual, index, 0.02, 0.04, 0.06, 0.08, and 0.1, while refractive error measurements accounted for corrected visual acuity. The efficacy index considered the total clinical efficiency (comprising significantly effective and effective populations), reflecting the actual determination of clinical effects. Secondary indicators include fundus hemorrhagic area (FHA), fasting blood glucose (FBG), glycated hemoglobin (HbA1c) and vascular endothelial growth factor (VEGF).
A total of seven databases were searched by computer: China national knowledge infrastructure, Wanfang Database, Weipu Journal Database, Chinese Biomedical Literature Database, PubMed, Cochrane Library, and Web of Science database, and the search time of each database was built until January 2023. In addition, references to the literature were incorporated retrospectively to supplement access to relevant literature. The search takes a combination of subject terms and free words. English search terms include: "Diabetic Retinopathy", "Diabetic Retinopathies", "Traditional Chinese medicine", "Compound Xueshuantong Capsules", "Compound Danshen Dripping Pills", "Qiju Dihuang Pills", "Qiming Granules", "Hexue Mingmu Tablets", "Randomized Controlled Trial", etc. The detailed search strategy is described in Supplementary Table 1.
Two investigators (LMX and LHD) performed literature screening and data extraction, excluding duplicates and then first read the title and abstract to exclude literature that clearly did not meet the requirements, and then read the remaining literature in full to clarify whether it met the inclusion criteria, and if there was disagreement, a third investigator (WZ) had to be consulted for a decision after discussion. Data extraction included: (1) Basic information: Article title, first author, publication time, country/region, etc.; (2) study characteristics: Interventions in the trial and control groups, number of cases, age, duration of intervention, and adverse effects in the study subjects; (3) key information required for risk of bias evaluation in the literature; and (4) outcome indicators included in the test and control groups.
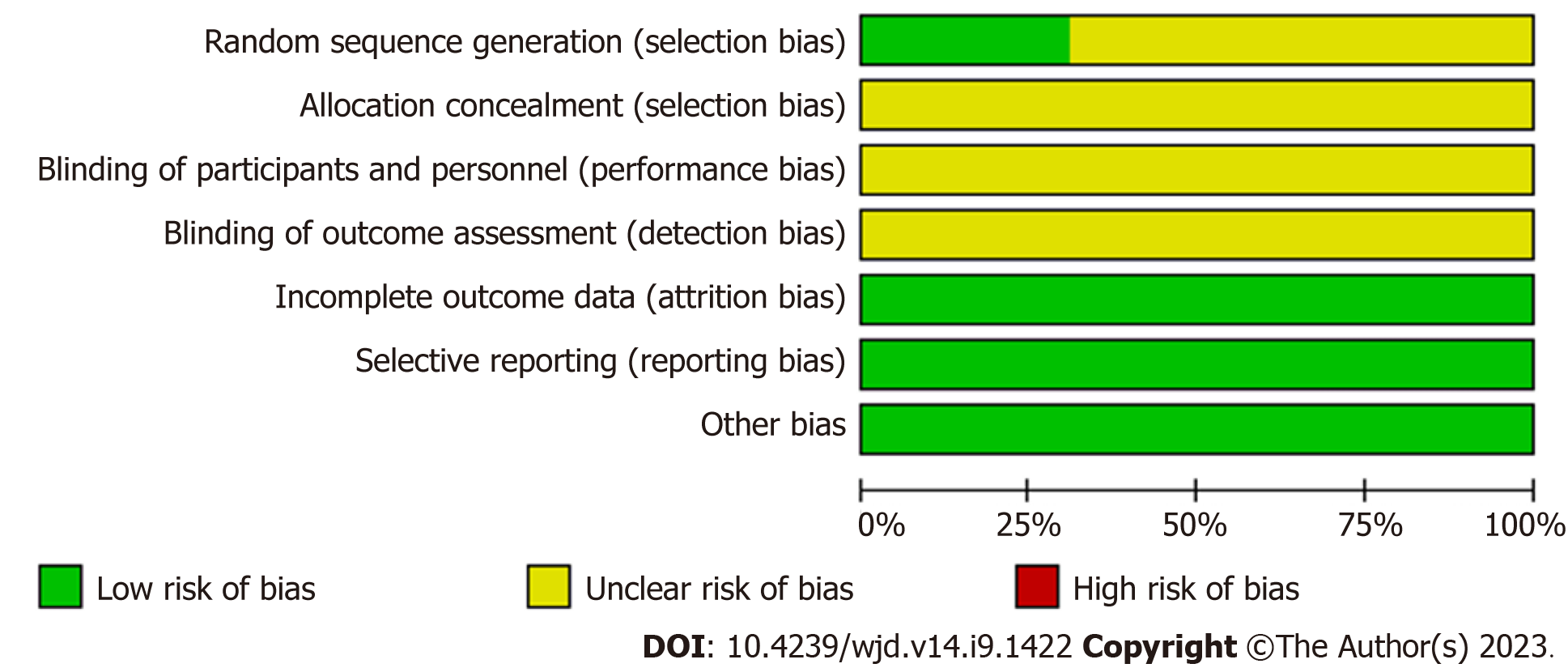
Quality evaluation of RCTs was performed usingRevMan 5.3 (Cochrane Collaboration, Copenhagen, The Nordic Cochrane Centre). Assessed by 2 investigators (HL and ML) using the tool for assessing risk of bias recommended in the Cochrane systematic reviewers' handbook 5.1[14], including the following 7 aspects: Random-sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); others bias. Each aspect can be further categorized as “low risk of bias”, “unclear risk of bias”, and “high risk of bias”. When there is a disagreement, it can be decided by mutual discussion or by consulting 3 investigators (SZ).
Stata 14.0 (MRC Biostatistics Unit, Cambridge, United Kingdom) software was used to implement the statistical process. The odds ratio (OR) and its 95% confidence interval (CI) were used for statistical data, and the mean difference and its 95%CI were used for measurement data. Data were preprocessed using the "Network" command, and two comparisons were made between different interventions, according to this case there is no closed loop, so the consistency model is used. The efficacy was ranked according to the surface under the cumulativeranking curve (SUCRA), and the results of the NMA analysis were finally presented in tabular form to obtain the relatively best interventions. Considering the inclusion of multiple observations, we combined the individual observations two by two and used a multivariate approach to determine the dependencies between the results. Use the "Clustering" command to obtain a clustering ranking chart[15]. Sensitivity analysis is performed for factors that may affect the stability of the results. Finally, "correction-comparison" funnel plots were drawn for publication bias assessment.
A total of 1667 relevant papers were identified and screened for inclusion in 107 randomized controlled trials, involving a total of 9710 patients (4767 in the trial group and 4973 in the control group). Baseline characteristics were carefully matched between the groups. The individual sample sizes of the trials ranged from 30 to 256 individuals, and the obser-vation periods varied from 4 wk to 24 wk. The trials encompassed 9 different OCMs, namely XST (26 studies[16-41]), QM (23 studies[42-64]), DS (21 studies, references[65-85]), HXMM (9 studies[86-94]), QJDH (6 studies[95-100]), SDMM (5 studies[101-105]), DGBX (6 studies[106-111]), XFZY (7 studies[112-118]), and BYHW (4 studies[119-122]). All studies evaluated at least one of the two primary efficacy measures, namely total clinical effectiveness and visual acuity. It should be noted that all included studies were conducted exclusively in China. The literature screening process is shown in Figure 1, the characteristics of the literature are shown in Table 1, and the OCMs involved in the study and their details are shown in Supplementary Tables 2 and 3, respectively.
| Ref. | Sample size | Random method | Interventions | Period of treatment | Age | Outcomes | Adverse reactions | |
| C/E | C | E | C/E | C/E | ||||
| Men[16], 2020 | 40/40 | TRD | CT + Calcium Dobesilate | C + XST | 8 wk | 67.46 ± 2.52/63.12 ± 2.21 | 1, 2, 3, 4 | - |
| Bai[17], 2016 | 38/38 | - | CT + Calcium Dobesilate | C + XST | 24 wk | 51.08 ± 4.73/50.63 ± 5.51 | 1, 2, 7 | 3/2 |
| An[18], 2021 | 30/30 | - | Calcium Dobesilate | C + XST | 20 wk | 69.51 ± 5.19/70.32 ± 5.39 | 1, 2 | - |
| Yan and Song[19], 2020 | 46/46 | TRD | Calcium Dobesilate | C + XST | 12 wk | 47.4 ± 4.6/48.5 ± 4.9 | 1, 7 | 22/8 |
| Li[20], 2021 | 20/20 | - | Calcium Dobesilate | C + XST | 20 wk | 59.67 ± 1.78/58.51 ± 1.31 | 1, 3 | - |
| Jin[21], 2020 | 40/40 | - | Calcium Dobesilate | C + XST | 20 wk | 66.12 ± 3.45/66.58 ± 3.16 | 2, 3 | - |
| Wu[22], 2015 | 50/50 | TRD | CT | C + XST | 12 wk | 54.1 ± 6.6/54.6 ± 6.2 | 1 | - |
| Qu[23], 2018 | 35/35 | - | CT + Calcium Dobesilate | C + XST | 20 wk | 59.7 ± 6.3/60.4 ± 7.2 | 1 | - |
| Sui[24], 2017 | 47/49 | - | CT + Calcium Dobesilate | C + XST | 4 wk | - | 1 | - |
| Jiang et al[25], 2019 | 46/46 | - | Calcium Dobesilate | C + XST | 20 wk | 58.72 ± 2.20/58.69 ± 2.15 | 1, 2, 4 | - |
| Zhu et al[26], 2016 | 48/48 | - | CT | C + XST | 12 wk | 56.38 ± 12.19/56.15 ± 12.21 | 2 | - |
| Sun[27], 2021 | 18/18 | - | CT | C + XST | 12 wk | 61.38 ± 3.69/62.47 ± 3.01 | 1 | - |
| Liu et al[28], 2018 | 45/45 | TRD | CT + Calcium Dobesilate | C + XST | 12 wk | 48.56 ± 7.64/48.34 ± 6.49 | 1 | - |
| Li[29], 2019 | 49/49 | TRD | CT + Calcium Dobesilate | C + XST | 12 wk | 66.41 ± 4.11/66.82 ± 4.03 | 1, 2, 4 | - |
| Xiao[30], 2016 | 110/110 | - | CT + Calcium Dobesilate | C + XST | 12 wk | 50.2 ± 6.4/49.5 ± 5.9 | 1, 7 | 0/0 |
| Chen[31], 2019 | 39/39 | - | Calcium Dobesilate | C + XST | 12 wk | 58.17 ± 3.82/59.34 ± 3.27 | 1, 3, 4 | - |
| Zhao[32], 2020 | 43/44 | - | Calcium Dobesilate | C + XST | 24 wk | 53.71 ± 5.52/53.66 ± 5.49 | 3 | - |
| An[33], 2020 | 35/35 | - | Calcium Dobesilate | C + XST | 12 wk | 52.12 ± 15.76/51.17 ± 17.83 | 1, 4, 7 | 1/2 |
| Zhang[34], 2019 | 19/19 | - | Calcium Dobesilate | C + XST | 8 wk | 51.86 ± 1.92/53.28 ± 2.64 | 1, 7 | 0/0 |
| Wei et al[35], 2017 | 34/34 | TRD | CT | C + XST | 35 wk | 62.94 ± 3.48/61.31 ± 3.54 | 1, 3, 4, 7 | 0/0 |
| Meng et al[36], 2012 | 38/40 | - | CT + Calcium Dobesilate | C + XST | 24 wk | 49.2 ± 7.8/48.8 ± 6.7 | 1 | - |
| Zhu and Sui[37], 2022 | 76/76 | TRD | CT + Calcium Dobesilate | C + XST | 12 wk | 55.89 ± 4.17/55.94 ± 4.13 | 1, 2, 3, 7 | 12/13 |
| Li[38], 2017 | 33/33 | - | Calcium Dobesilate | C + XST | 12 wk | - | 2 | - |
| Wang et al[39], 2020 | 42/44 | TRD | CT + Calcium Dobesilate | C + XST | 20 wk | 68.35 ± 6.82/69.52 ± 7.11 | 1, 3, 7 | 4/3 |
| Hu[40], 2017 | 30/30 | - | Calcium Dobesilate | C + XST | 20 wk | 55.30 ± 2.15/55.67 ± 2.08 | 1, 3, 7 | 0/0 |
| Xu and Ru[41], 2020 | 46/46 | - | Calcium Dobesilate | C + XST | 12 wk | 52.3 ± 3.2/52.1 ± 3.6 | 1, 3 | - |
| Wang[42], 2016 | 38/38 | - | Iodized Lecithin | C + QM | 8 wk | 43 ± 6/42 ± 5 | 1 | - |
| Wang[43], 2018 | 44/44 | TRD | Calcium Dobesilate | C + QM | 12 wk | 58.4 ± 7.5/57.8 ± 6.2 | 1, 7 | 12/5 |
| Wang et al[44], 2020 | 32/32 | - | Amlodipine besylate | C + QM | 12 wk | 38.94 ± 4.89/39.87 ± 5.13 | 1, 2 | - |
| Chen[45], 2016 | 45/45 | TRD | CT | C + QM | 12 wk | 62.05 ± 5.47/63.11 ± 5.64 | 1 | - |
| Wang et al[46], 2015 | 38/41 | - | Calcium Dobesilate | C + QM | 24 wk | 52.1 ± 5.6/52.5 ± 5.3 | 1, 2, 7 | 0/0 |
| Sui et al[47], 2014 | 43/43 | - | Calcium Dobesilate | C + QM | 12 wk | 50.53 ± 11.28/50.22 ± 14.82 | 1, 2, 5, 6, 7 | 0/0 |
| Huang[48], 2017 | 63/63 | - | CT + Calcium Dobesilate | C + QM | 12 wk | 55.9 ± 4.1/55.6 ± 4.2 | 1 | - |
| Feng et al[49], 2016 | 41/42 | Lottery - | Calcium Dobesilate | C + QM | 12 wk | 55.89 ± 6.13/55.26 ± 6.29 | 1, 2, 5, 6 | - |
| Ge[50], 2018 | 53/53 | - | CT + Calcium Dobesilate | C + QM | 24 wk | 50.87 ± 3.71/51.25 ± 3.64 | 1 | - |
| Yan[51], 2020 | 38/46 | Lottery | CT + Calcium Dobesilate | C + QM | 8 wk | 56.96 ± 4.59/56.65 ± 4.02 | 1, 2, 5, 6, 7 | 8/2 |
| Meng et al[52], 2016 | 21/21 | - | Calcium Dobesilate | C + QM | 24 wk | - | 1 | - |
| Zhang et al[53], 2016 | 46/45 | - | CT | C + QM | 24 wk | - | 1 | - |
| Zhang[54], 2013 | 34/34 | - | CT | C + QM | 36 wk | - | 1 | - |
| Wu et al[55], 2022 | 50/50 | Lottery | Pancreatic Kininogenase | C + QM | 24 wk | 53.82 ± 5.42/54.06 ± 4.93 | 1, 2, 4, 7 | 3/5 |
| Zhou and Femng[56], 2018 | 60/60 | - | CT + Calcium Dobesilate | C + QM | 12 wk | 58.5 ± 6.7/57.9 ± 6.2 | 1, 2 | - |
| Wang et al[57], 2019 | 48/52 | - | CT + Calcium Dobesilate | C + QM | 24 wk | 66.8 ± 6.3/66.7 ± 6.2 | 1, 7 | 0/0 |
| Wang[58], 2017 | 47/47 | - | CT + Calcium Dobesilate | C + QM | 12 wk | 54.3 ± 4.9/54.5 ± 4.8 | 1, 4, 7 | 0/0 |
| Yin[59], 2018 | 46/50 | - | Calcium Dobesilate | C + QM | 12 wk | 55.27 ± 5.42/54.63 ± 5.28 | 1, 2, 7 | 0/0 |
| Xin et al[60], 2019 | 38/38 | - | Epalrestat | C + QM | 12 wk | 55.1 ± 3.3/55.5 ± 3.2 | 1, 3, 7 | 0/0 |
| Zhang[61], 2017 | 39/39 | - | Calcium Dobesilate | C + QM | 12 wk | 56.8 ± 2.5/56.9 ± 2.1 | 1, 2, 5, 6, 7 | 0/0 |
| Yue[62], 2016 | 38/57 | - | CT | C + QM | 24 wk | 49.82 ± 6.17/50.67 ± 5.23 | 1 | - |
| Zheng et al[63], 2014 | 15/15 | - | CT | C + QM | 24 wk | 50.4 ± 3.1/55.2 ± 4.7 | 1 | - |
| Yang et al[64], 2013 | 36/35 | - | CT | C + QM | 24 wk | 50.94 ± 8.01/50.23 ± 7.15 | 1 | - |
| Huang[65], 2020 | 20/20 | - | Calcium Dobesilate | C + DS | 16 wk | 52.16 ± 2.45/53.16 ± 2.26 | 1, 2, 3, 5, 6 | - |
| Zheng and Ji[66], 2021 | 43/44 | - | CT | C + DS | 8 wk | 57.52 ± 6.41/58.21 ± 6.35 | 1, 4 | - |
| Meng et al[67], 2011 | 28/30 | - | CT + Calcium Dobesilate | C + DS | 24 wk | 51.20 ± 7.90/50.60 ± 8.70 | 1, 7 | 0/0 |
| Wang[68], 2004 | 16/28 | - | CT + Calcium Dobesilate | C + DS | 16 wk | 50.5 ± 9.36/50.4 ± 8.70 | 1 | - |
| Zhao[69], 2019 | 53/53 | TRD | CT | C + DS | 8 wk | 57.5 ± 14.8/56.8 ± 13.4 | 1, 3, 4, 7 | 0/0 |
| Ma et al[70], 2016 | 34/48 | - | CT | C + DS | 24 wk | 59.16 ± 9.73/59.01 ± 10.58 | 5, 6, 7 | 0/0 |
| Chen et al[71], 2007 | 25/25 | - | CT | C + DS | 8 wk | 60.56/62.42 | 2, 3 | - |
| Xu[72], 2019 | 43/43 | TRD | Calcium Dobesilate | C + DS | 16 wk | 53.06 ± 4.39/53.11 ± 4.41 | 1, 3, 7 | 0/0 |
| Huang et al[73], 2021 | 45/45 | TRD | CT + Calcium Dobesilate | C + DS | 24 wk | 67.3 ± 5.1/67.5 ± 5.3 | 2, 4, 7 | 3/4 |
| Jiao[74], 2018 | 75/75 | - | CT + Calcium Dobesilate | C + DS | 8 wk | 56.31 ± 2.19/56.24 ± 3.86 | 1 | - |
| Li[75], 2017 | 89/89 | - | CT + Calcium Dobesilate | C + DS | 8 wk | 55.8 ± 6.8/56.5 ± 7.2 | 1, 5, 6 | - |
| Yan and Yuan[76], 2014 | 20/60 | - | CT | C + DS | 24 wk | 68.8/65.6 | 2, 3 | - |
| Zhou[77], 2008 | 18/28 | - | Calcium Dobesilate | C + DS | 24 wk | 50.50 ± 9.36/50.40 ± 8.70 | 1 | - |
| Miao[78], 2020 | 24/34 | TRD | Calcium Dobesilate | C + DS | 16 wk | 57.46 ± 4.41/57.33 ± 4.26 | 2, 3 | - |
| Ruan et al[79], 2017 | 35/35 | - | Calcium Dobesilate | C + DS | 16 wk | 52.8 ± 1.7/52.5 ± 1.1 | 1, 3, 5, 6, 7 | 0/0 |
| Yin et al[80], 2013 | 50/50 | - | CT + Calcium Dobesilate | C + DS | 8 wk | 59.7/57.9 | 1, 3 | - |
| Zhu[81], 2018 | 57/57 | - | CT | C + DS | 12 wk | 64.12 ± 1.36/64.17 ± 1.38 | 1, 2, 3 | - |
| Yang et al[82], 2013 | 32/33 | - | CT | C + DS | 8 wk | 54.2 ± 10.8/55.4 ± 12.1 | 5, 6 | - |
| Bai[83], 2017 | 38/38 | TRD | CT + Calcium Dobesilate | C + DS | 16 wk | - | 1, 2, 3, 4, 7 | 0/0 |
| Guo[84], 2015 | 35/100 | - | CT | C + DS | 24 wk | 59.6 ± 9.7/59.0 ± 10.6 | 5, 6 | - |
| Liu[85], 2018 | 89/89 | TRD | CT | C + DS | 4 wk | 54.97 ± 4.88/55.02 ± 5.01 | 5, 6 | - |
| Liu[86], 2019 | 41/42 | - | Pancreatic Kininogenase | C + HXMM | 12 wk | - | 3, 7 | 2/2 |
| Zhao and Liu[87], 2021 | 30/30 | - | CT + Calcium Dobesilate | C + HXMM | 12 wk | 64.84 ± 4.26/65.09 ± 4.37 | 1, 7 | 4/2 |
| Gao et al[88], 2020 | 128/128 | TRD | CT + Calcium Dobesilate | C + HXMM | 12 wk | 57.65 ± 7.82/58.14 ± 7.63 | 1, 3, 7 | 4/3 |
| Li[89], 2021 | 34/34 | Lottery | CT + Calcium Dobesilate | C + HXMM | 12 wk | 68.49 ± 4.62/67.84 ± 4.57 | 1, 3 | - |
| Wang et al[90], 2018 | 100/100 | - | Pancreatic Kininogenase | C + HXMM | 12 wk | 50.62 ± 6.91/50.96 ± 6.71 | 1, 3, 7 | 4/2 |
| Zhang and Wang[91], 2018 | 39/39 | - | CT | C + HXMM | 12 wk | - | 1, 2 | - |
| Ye et al[92], 2019 | 88/88 | TRD | Calcium Dobesilate | C + HXMM | 12 wk | 60.9 ± 13.4/60.5 ± 13.4 | 1, 5, 6 | - |
| Yu[93], 2019 | 30/30 | - | Epalrestat | C + HXMM | 12 wk | - | 1, 7 | 2/3 |
| Du et al[94], 2015 | 26/25 | TRD | CT | C + HXMM | 12 wk | 53.39 ± 4.96/52.13 ± 5.01 | 1, 7 | 0/0 |
| Song[95], 2013 | 40/40 | - | CT | C + QJDH | 12 wk | - | 1, 7 | 0/0 |
| Li[96], 2019 | 40/40 | - | Mecobalamin | C + QJDH | 24 wk | 61.25 ± 6.75/60.85 ± 6.57 | 1, 3, 4, 7 | 0/0 |
| Li and Wei[97], 2019 | 54/54 | - | CT + Calcium Dobesilate | C + QJDH | 20 wk | 56.1 ± 3.7/55.4 ± 3.1 | 1, 3, 4, 5, 6, 7 | 0/0 |
| Wu[98], 2018 | 29/29 | TRD | Pancreatic Kininogenase | C + QJDH | 12 wk | 52.4 ± 10.8/52.3 ± 9.2 | 1, 5, 7 | 0/0 |
| Guan[99], 2017 | 40/40 | - | CT + Calcium Dobesilate | C + QJDH | 24 wk | 40.0 ± 3.1/41.1 ± 2.0 | 1, 5, 6, 7 | 6/5 |
| Ainu et al[100], 2019 | 50/50 | - | CT + Calcium Dobesilate | C + QJDH | 4 wk | 53.02 ± 5.39/52.61 ± 5.39 | 2, 3, 4 | - |
| Fu[101], 2019 | 40/40 | - | Calcium Dobesilate | C + SDMM | 24 wk | - | 5, 7 | 7/2 |
| Ji and Liu[102], 2022 | 52/52 | Lottery | Calcium Dobesilate | C + SDMM | 12 wk | 56.53 ± 4.09/56.63 ± 4.02 | 1, 3, 4, 7 | 7/5 |
| Jin and Zhang[103], 2019 | 71/71 | - | CT + Calcium Dobesilate | C + SDMM | 16 wk | 62.39 ± 8.34/63.07 ± 8.08 | 1, 7 | 9/2 |
| Liu et al[104], 2019 | 60/60 | TRD | CT + Calcium Dobesilate | C + SDMM | 16 wk | 57.10 ± 9.26/57.54 ± 8.11 | 1, 3, 4, 7 | 0/0 |
| Pang[105], 2015 | 40/40 | - | CT | C + SDMM | 16 wk | 49.6 ± 5.3/49.4 ± 5.7 | 1 | - |
| Deng et al[106], 2018 | 40/40 | TRD | Calcium Dobesilate | C + DGBX | 12 wk | 59.48 ± 8.22/59.62 ± 8.30 | 1, 3, 7 | 0/0 |
| Wang and Chen[107], 2020 | 75/75 | - | Calcium Dobesilate | C + DGBX | 12 wk | 62.38 ± 2.00/62.40 ± 2.02 | 1, 4 | - |
| Wu[108], 2013 | 33/34 | - | CT | C + DGBX | 12 wk | - | 1, 2, 5, 6, 7 | 0/0 |
| Sun et al[109], 2019 | 90/92 | - | CT | C + DGBX | 12 wk | 55.3 ± 3.7/55.9 ± 3.5 | 1, 3, 7 | 10/6 |
| Yu[110], 2020 | 28/28 | - | CT + Calcium Dobesilate | C + DGBX | 12 wk | 60.01 ± 8.26/60.48 ± 8.11 | 1, 2 | - |
| Xu[111], 2018 | 43/43 | TRD | CT | C + DGBX | 12 wk | 56.2 ± 7.3/56.6 ± 7.1 | 1, 7 | 4/0 |
| Huang[112], 2017 | 54/54 | TRD | Iodized Lecithin | C + XFZY | 8 wk | 62.15 ± 11.80/61.84 ± 12.11 | 2 | - |
| Zhu[113], 2010 | 36/36 | TRD | CT | C + XFZY | 8 wk | 55.42 ± 5.35/55.73 ± 5.10 | 1, 5, 6, 7 | 0/0 |
| Xiong and Chen[114], 2019 | 43/43 | TRD | Calcium Dobesilate | C + XFZY | 16 wk | 53.14 ± 7.25/54.28 ± 7.13 | 2, 5, 6 | - |
| Liu[115], 2020 | 57/57 | Lottery | CT | C + XFZY | 16 wk | 50.38 ± 3.67/50.47 ± 3.22 | 2 | - |
| Gong et al[116], 2014 | 40/40 | - | CT | C + XFZY | 12 wk | 57.24 ± 10.60/55.36 ± 9.28 | 1, 5 | - |
| Hao[117], 2012 | 84/66 | - | CT | C + XFZY | 4 wk | - | 1 | - |
| Huang[118], 2004 | 50/70 | - | CT | C + XFZY | 12 wk | 64.5/65 | 1 | - |
| Zhang[119], 2019 | 46/46 | - | CT | C + BYHW | 8 wk | 64.37 ± 9.13/66.46 ± 9.90 | 1 | - |
| Yang[120], 2019 | 40/40 | TRD | CT + Calcium Dobesilate | C + BYHW | 8 wk | 48.66 ± 8.82/50.35 ± 9.06 | 1, 2, 5 | - |
| Tian[121], 2019 | 27/27 | - | Metformin | C + BYHW | 12 wk | 53.05 ± 1.18/53.12 ± 1.12 | 1 | - |
| Qu and Yao[122], 2009 | 30/32 | - | CT + Iodized Lecithin | C + BYHW | 12 wk | - | 1 | - |
The quality of the included literature was evaluated using the "Risk Assessment Tool" recommended by the Cochrane Collaboration: 33 studies[16,19,22,28,29,35,37,39,43,45,49,51,55,69,72,73,78,83,85,88,89,92,94,98,102,104,106,111-115,120] mentioned the specific randomization method used and therefore assessed as "Low risk". The other 74 studies only mentioned the randomized grouping without mentioning the specific method used for allocation and were therefore evaluated as "Unclear risk". None of the included studies mentioned allocation concealment and blinding, and were evaluated as "Unclear risk". All studies had clear outcome indicators and were evaluated as "Low risk"; no duplicate publications or published biases were found in any of the studies and were evaluated as "Low risk"; other biases were unknown and were evaluated as "Unclear risk". All data were reported completely and were comparable between groups (Figure 2 and Supplementary Figure 1).
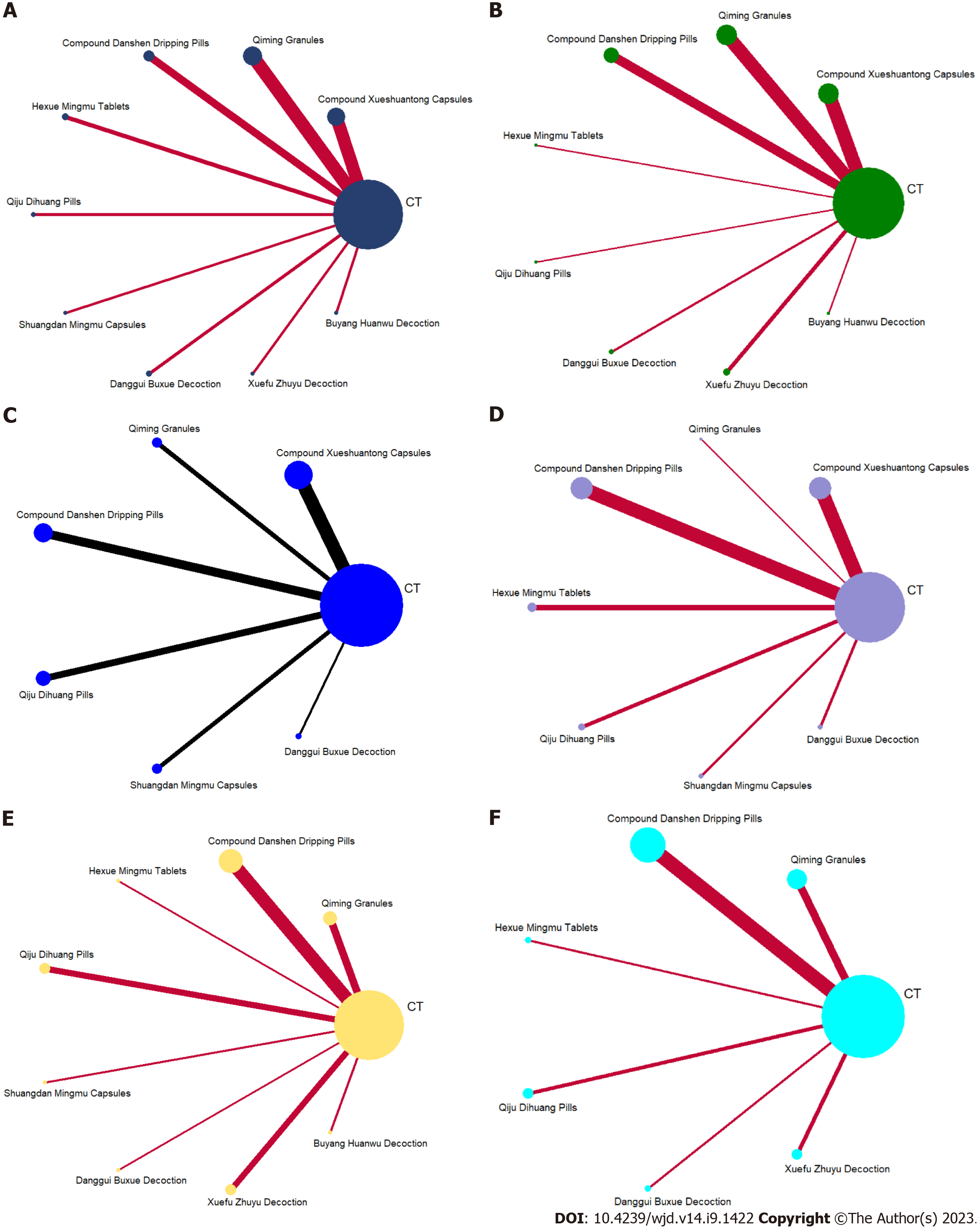
Mesh relationship diagram and consistency testing: The reticulation between the nine included OCM is shown in Figure 3. The total number of arms in the 107 papers totals 214. Lines between nodes indicate direct comparative evidence between the two interventions, no lines indicate no direct comparison, indirect comparisons can be made through reticulated Meta-analysis. The thickness of the line represents the number of included studies comparing each treatment, and the circular area represents the sample size of the population using the measure. All interventions involved in this study did not form a closed loop and did not require consistency testing.
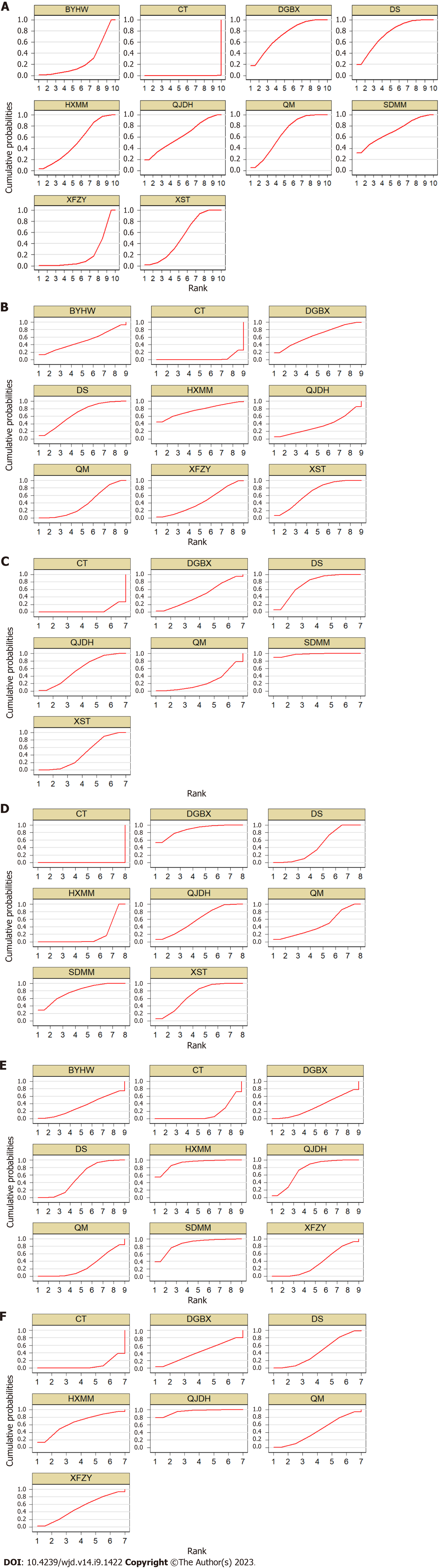
Total effective rate: A total of 89 studies[16-20,22-25,27-31,33-37,39-69,72,74,75,77,79-81,83,87-99,102-111,113,116-122] were included to compare the total effective rate after OCM + CT treatment. The evidence diagram is shown in Figure 4A. The difference was statistically significant (P < 0.05) for the total effective rate for these nine OCM + CT treatments compared with CT (Figure 5A). The nine OCMs + CT total effective rate in descending order are: DS (SUCRA = 75.0%) > DGBX (SUCRA = 72.8%) > SDMM (SUCRA = 71.4%) > QM (SUCRA = 66.4%) > QJDH (SUCRA = 62.4%) > XST = HXMM (SUCRA = 52.5%) > BYHW (SUCRA = 26.8%) > XFZY (SUCRA = 20.1%) > CT (SUCRA = 0.0%). The probability ranking is shown in Figure 6A.
Visual acuity: A total of 34 studies[16-18,21,23,25,26,29,37,38,44,46,47,49,51,55,56,59,60,65,71,73,76,78,81,83,91,100,108,110,112,114,115,120] with 8 OCMs were included to compare visual acuity after OCM + CT treatment. The evidence diagram is shown in Figure 4B. In terms of visual acuity, supplemental XST, QM, DS, HXMM, QJDH, DGBX and XFZY treatments were statistically significant compared with CT treatment alone (P < 0.05) (Figure 5B). The eight OCMs + CT in order of highest to lowest improvement in visual acuity are: HXMM (SUCRA = 76.3%) > XST (SUCRA = 67.4%) > DS (SUCRA = 67.2%) > DGBX (SUCRA = 64.8%) > BYHW (SUCRA = 50.3%) > XFZY (SUCRA = 44.4%) > QM (SUCRA = 40.1%) > QJDH (SUCRA = 36.2%) > CT (SUCRA = 3.4%). The probability ranking is shown in Figure 6B.
VEGF: A total of 18 studies[16,25,29,31,33,35,55,58,66,69,73,83,96,97,100,102,104,107] with 6 OCMs were included to compare VEGF after OCM + CT treatment. The evidence diagram is shown in Figure 4C. In terms of VEGF, supplemental XST, DS, QJDH and SDMM were statistically significant compared with CT treatment alone (P < 0.05) (Figure 5C). The seven OCMs + CT in order of VEGF reduction from highest to lowest are: SDMM (SUCRA = 97.7%) > DS (SUCRA = 74.4%) > QJDH (SUCRA = 57.9%) > DGBX (SUCRA = 45.9%) > XST (SUCRA = 44.8%) > QM (SUCRA = 24.7%) > CT (SUCRA = 4.7%). The probability ranking is shown in Figure 6C.
FHA: A total of 32 studies[16,20,21,31,32,35,37,39-41,60,65,69,71,72,76,78-81,83,86,88-90,96,97,100,102,104,106,109] with 7 OCMs were included to compare FHA after OCM + CT treatment. The evidence diagram is shown in Figure 4D. These seven OCM + CT were statistically significant (P < 0.05) compared with CT for FHA, respectively (Figure 5D). The seven OCMs + CT reduce FHA in the following order from highest to lowest: DGBX (SUCRA = 87.5%) > SDMM (SUCRA = 77.7%) > XST (SUCRA = 67.9%) > QJDH (SUCRA = 58.8%) > DS (SUCRA = 45.9%) > QM (SUCRA = 45.1%) > HXMM (SUCRA = 17.0%) > CT (SUCRA = 0.0%). The probability ranking is shown in Figure 6D.
FBG: A total of 21 studies[47,49,51,61,65,70,75,79,82,84,85,92,97-99,101,108,113,114,116,120] with 8 OCMs were included to compare FBG after OCM + CT treatment. The evidence diagram is shown in Figure 4E. In terms of FBG, supplemental DS, HXMM, QJDH and SDMM treatments were statistically significant compared with CT treatment alone (P < 0.05) (Figure 5E). The eight OCMs + CT lower FBG in the following order from highest to lowest: HXMM (SUCRA = 91.1%) > SDMM (SUCRA = 86.4%) > QJDH (SUCRA= 73.8%) > DS (SUCRA = 54.2%) > XFZY (SUCRA = 36.1%) > BYHW (SUCRA = 34.0%) > DGBX (SUCRA = 33.2%)> QM (SUCRA =27.8%)> CT (SUCRA =13.5%). The probability ranking is shown in Figure 6E.
HbA1c: A total of 17 studies[47,49,51,61,65,70,75,79,82,84,85,92,97,99,108,113,114] with 6 OCMs were included to compare HbA1c after OCM + CT treatment. The evidence diagram is shown in Figure 4F. In terms of HbA1c, supplemental DS and QJDH treatments were statistically significant compared with CT treatment alone (P < 0.05) (Figure 5F). The six OCMs + CT lower HbA1c in the following order from highest to lowest: QJDH (SUCRA = 95.5%) > HXMM (SUCRA = 65.3%) > XFZY (SUCRA = 50.8%) > QM (SUCRA = 45.0%) > DS (SUCRA = 43.6%) > DGBX (SUCRA = 42.2%) > CT (SUCRA = 7.6%). The probability ranking is shown in Figure 6F.
Cluster analysis and meta-analysis two-by-two comparison results: The key outcome indicators included in this study were cluster analyzed to derive the intervention of different OCMs + CT for two outcome indicators at the same time. In terms of total effective rate and visual acuity, HXMM, XST, DS and DGBX in the upper right corner of Figure 7A performed better; in terms of total effective rate and VEGF, SDMM and DS performed better (Figure 7B); in terms of total effective rate and FHA, DGBX and SDMM in the upper right corner of Figure 7C performed better; in terms of FBG and HbA1c, QJDH and HXMM in the upper right corner of Figure 7D performed better.
A two-by-two comparison of the nine OCMs + CT and CT was performed at each index. The total clinical efficiency of DR treatment with all 9 OCMs + CT was found to be higher than that of CT alone; CT in combination with XST, QM, DS, HXMM, DGBX and XFZY were superior to CT alone in improving visual acuity, respectively; in anti-VEGF, CT in combination with XST, DS, QJDH and SDMM were better than CT alone, respectively; CT in combination with XST, QM, DS, HXMM, QJDH, SDMM and DGBX were superior to CT alone in reducing FHA, respectively; CT in combination with DS, HXMM, QJDH and SDMM were superior to CT alone in lowering FBG, respectively; CT in combination with DS and QJDH were superior to CT alone in lowering HbA1c, respectively. All results are plotted as forest plots shown in Supp
To verify the stability of the above results, we performed NMA with sample size and duration of treatment as sensitivity factors that may affect the results. Of the total 107 studies, 54 studies[16-18,20,21,23,27,31-36,38,40,42,44,46,52,54,60,61,63-68,71,76-79,82,83,87,89,91,93-96,98,99,101,105,106,108,110,113,116,120-122] with a case load of no more than 80 were included in the sensitivity analysis. The results revealed that there was no significant difference in total effective rate for CT + SDMM compared with CT (OR: 0.94, 95%CI: 0.94-23.98, P > 0.05), unlike the original NMA; for HbA1c, DGBX + CT was effective in reducing HbA1c compared with CT (OR: 0.67, 95%CI: 0.48-0.94, P < 0.05), unlike the original NMA. There was no significant change in the remaining indicators, so the sample size was considered as a possible factor influencing the results (Supplementary Figure 3). When using duration of treatment as a sensitivity factor, we divided all studies according to 12 wk of treatment, and a total of 65 studies[16,19,22,24,26-31,33,34,37,38,41-45,47-49,51,56,58-61,66,69,71,72,75,80-82,85-95,98,100,102,106-113,116-122] with no more than 12 wk of treatment were included in the sensitivity analysis. Results found that DGBX + CT was more effective than CT in terms of VEGF (OR: 0.29, 95%CI: 0.16-0.53, P < 0.05), unlike the original NMA; for FBG, CT + QJDH was not significantly different from CT (OR: 0.67, 95%CI: 0.39-1.16, P > 0.05), unlike the original NMA; for HbA1c, the effect of DS + CT was not significantly different from CT (OR: 0.77, 95%CI: 0.38-1.57, P > 0.05), unlike the original NMA. Consideration of sample size may have influenced this result (Supple
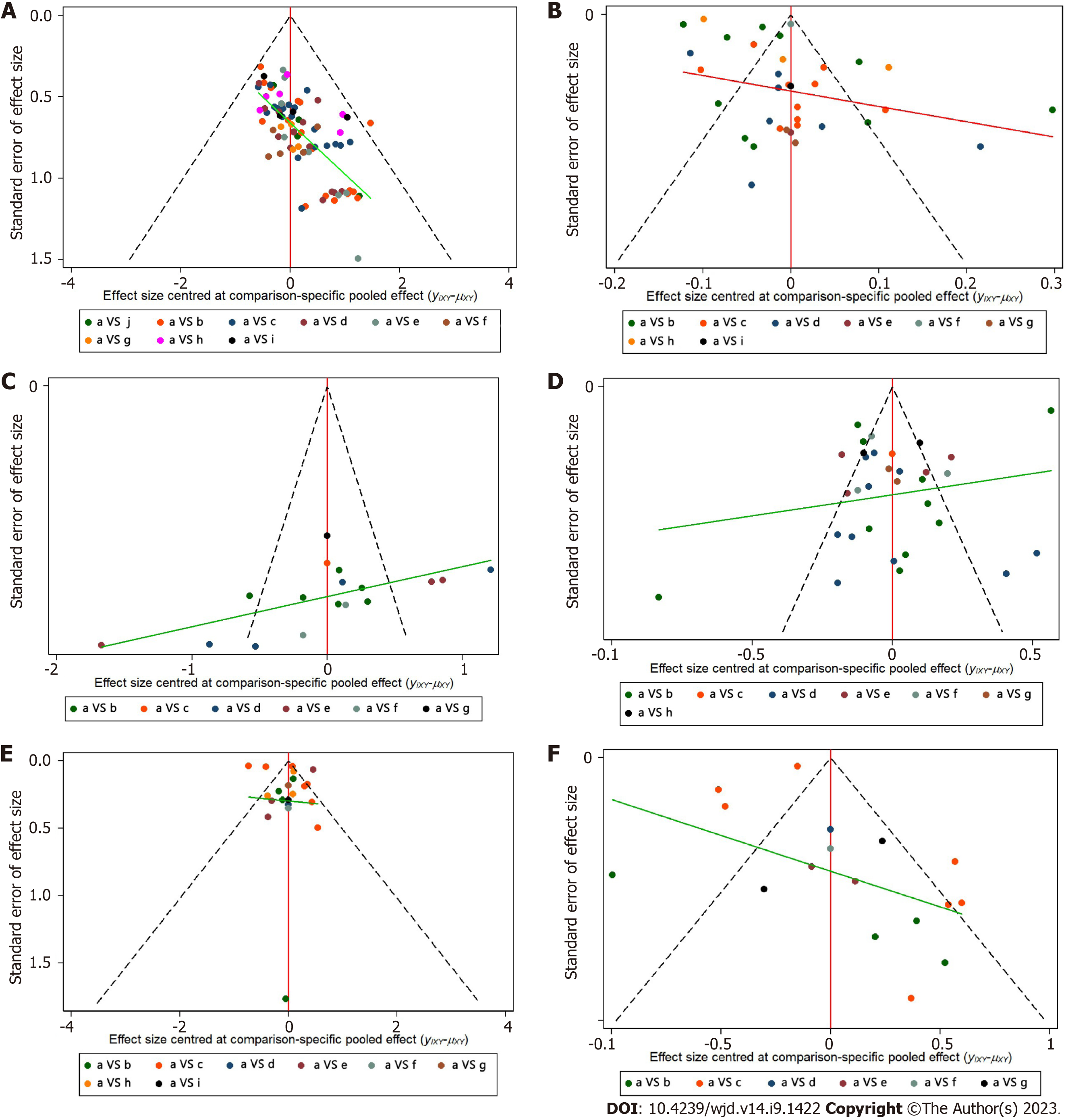
The total effective rate (significantly effective + effective), visual acuity, VEGF, FHA, FBG and HbA1c were used as evaluation indicators to produce a comparative corrected funnel plot of the study to assess the small sample effect, see Figure 8. The results showed that the total effective rate and FBG comparison corrected funnel plots showed basic symmetry, with studies roughly symmetrically distributed on both sides of the midline, suggesting that a small sample effect is less likely. The poor symmetry of the corrected funnel plot for visual acuity, VEGF, FHA, and HbA1c comparisons suggests the possibility of a small sample effect. The reasons may be related to the mixed quality of included studies, small sample size, inconsistent treatment regimens, and different pathological stages of study subjects. Further analysis of publication bias using Begg's and Egger's tests revealed the presence of publication bias for total clinical effectiveness (PBegg < 0.001, PEgger < 0.001). Additionally, there was a potential publication bias for FBG (PBegg = 0.01; PEgger = 0.151) and FHA (PBegg = 0.224, PEgger = 0.041). However, no publication bias was observed for visual acuity, VEGF, and HbA1c
Of the total 107 RCTs, 47 mentioned adverse reactions, but only 20 of these studies[17,19,33,37,39,43,51,55,73,86-88,90,93,99,101-103,109,111] had patients with adverse reactions, and the other 26 studies[30,34,35,40,46,47,57-61,67-70,72,79,83,94-98,104,106,108,113] in which all patients had no adverse reactions. A total of 76 patients in the experimental group had adverse reactions during the treatment, and 127 patients in the control group had adverse reactions. A total of 7 OCMs were involved, and the results are shown in Table 2.
| OCMs compound | Group | Sample | Hypoglycemia | Stomach upset (n) | Loss of appetite | Insomnia | Fever | Dizzy | Nausea | Liver damage | Kidney damage | Macular edema | Corneal damage | Itchy skin | Fatigue |
| Xueshuantong Capsules | C | 42 | 0 | 14 | 5 | 0 | 0 | 0 | 6 | 8 | 9 | 0 | 0 | 0 | 0 |
| E | 28 | 0 | 8 | 8 | 0 | 0 | 0 | 8 | 2 | 2 | 0 | 0 | 0 | 0 | |
| Qiming Granules | C | 23 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 8 | 7 | 1 | 1 |
| E | 12 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 3 | 1 | 2 | |
| Compound Danshen Dripping Pills | C | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hexue Mingmu Tablets | C | 16 | 2 | 4 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 | 1 |
| E | 12 | 0 | 6 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | |
| Qiju Dihuang Pills | C | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| E | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Shuangdan Mingmu Capsules | C | 23 | 0 | 5 | 0 | 0 | 5 | 2 | 8 | 0 | 0 | 0 | 0 | 0 | 3 |
| E | 9 | 0 | 2 | 0 | 0 | 2 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Danggui Buxue Decoction | C | 14 | 2 | 4 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| E | 6 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
To the best of our knowledge, this study is the first NMA focused on the combination of OCM with conventional Western medicine for the treatment of DR, and is the NMA with the largest number of original studies included, the largest number of subjects, and the largest variety of OCM included to date. Only five NMAs[123-127] have reported the therapeutic effect on DR, and only one[125] of them is about the efficacy of herbal injections for DR. This study comprehensively collected RCTs involving 9 commonly used OCMs in China and included 6 clinical indicators commonly used to evaluate the efficacy of DR, the largest number of included indicators we are aware of to date in a similar report.
Although surgical treatment and intravitreal drug injection for DR are becoming popular, there are still some problems that are difficult to solve in a short period of time. First, invasive therapies have a limited audience. Total retinal photocoagulation is the primary treatment for proliferative DR and is not advocated for the treatment of non-proliferative DR; intraocular injections of VEGF inhibitors are more effective primarily in the treatment of DR with macular edema. In some forms of non-clearing vitreous hemorrhage, vitrectomy has been shown to remain the only method for removing fibrous proliferation and relieving traction detachment, with mixed results[128]. Second, safety issues need to be kept in mind, for example, one study found that although intraocular steroid injections led to rapid regression of dimethyl ether, however, this improvement did not persist and was associated with a significant increase in the incidence of elevated intraocular pressure and cataracts[129]. Furthermore, we must take into account the additional financial burden incurred by this type of treatment. A study from Canada reported that Grid laser therapy adds an additional cost benefit per quality of life adjusted year[130]. In contrast, OCM is not only increasingly proving to have surprising clinical efficacy[131], is affordable[132], and is indicated for patients in almost all stages of DR with a broad universal indication. Therefore, a systematic and comprehensive evaluation of the therapeutic efficacy of OCM for DR is essential.
This study found that DS showed excellent efficacy in improving visual acuity levels and total clinical effectiveness. DS is composed of three herbs Radix Salviae (Danshen in Chinese), Panax notoginseng (Burkill) F. H. Chen ex C. H. (Sanqi in Chinese) and borneol (bingpian in Chinese). According to Chinese medicine, Danshen and Sanqi have the effect of activating blood circulation and dispelling blood stasis, and are commonly used herbs for treating diseases of blood stasis and obstruction; Bingpian is obtained from the stem of Blumea balsamifera (L.) DC. or the leaves of Cinnamomum camphora by water steam distillation and recrystallization, and is documented in the famous Chinese medical work Annotation of Materia Medica from the Tang Dynasty (about 659 AD) as a treatment for eye diseases. Each of the three herbs has its own characteristics and at the same time exerts the effect of activating blood circulation and removing blood stasis, which is in line with modern pharmacological research. It was found[133] that Tanshinone IIa (the main active component of Danshen) promoted phosphorylation of AMP-activated protein kinase AMPK at T172 in retinal pigment epithelial cells and inhibited monolayer permeability of human retinal epithelial cells under high glucose conditions, similar to that under normal glucose, while apparently preventing co-localization of NF-κB and p300 and inhibiting their binding, thereby reducing ARPE-19 cell monolayer permeability. A study by Fan et al[134] found that Tanshinone IIa significantly downregulated the expression levels of VEGF and intercellular adhesion molecule-1 (ICAM-1) in a dose-dependent manner under HG conditions, probably by mediating proliferation, migration and inhibition of angiogenesis in human retinal endothelial cells. Sanqi and its extracts have anti-inflammatory[135-138], antioxidant, inhibit platelet aggregation, regulate blood glucose[139,140], regulate blood pressure[141-143], improve insulin resistance[144,145], inhibit neuronal apoptosis[146-148] and neuronal protection[149-152]. In particular, ginsenoside Rb1 (the main active compound extracted from the rhizome of Sanqi) has been widely demonstrated to be promising as an antidiabetic and its complications, improving diabetes-related complications by regulating oxidative stress, apoptosis, inflammatory response, enhancing insulin sensitivity, improving leptin resistance, activating the activation of lipocalin signaling pathway, and inhibiting fibronectin expression[153-161]. In addition to showing good clinical treatment rates, DGBX has shown excellent performance in improving the fundus hemorrhage area. According to TCM theory, DGBX is mainly used for treating diseases caused by fatigue and internal injury, blood deficiency and qi weakness. Although there are only two herbs in the formula, Radix Astragali Mongolici (Huangqi in Chinese) and Angelicae Sinensis Radix (Danggui in Chinese), the formula is short but powerful and can promote the production of tangible blood from invisible qi, which is a classic OCM with the effect of tonifying qi and promoting blood production. It is a classic OCM with the effect of nourishing Qi and promoting blood circulation. DGBX was found to affect lipid metabolism in the early stages of atherosclerosis in diabetic Goto-Kakizaki rats, and the mechanism may be related to the regulation of intravascular lipid metabolism genes[162]. Astragalus polysaccharides are the main active components of Huangqi, can reduce the levels of tumor necrosis factor-α, ICAM-1, vascular VEGF and p-Akt in the retina of diabetic rats, and affect the Akt-VEGF signaling pathway by anti-inflammatory and reducing the adhesion of leukocytes to the diabetic retina[163], while inhibiting peripapillary cell apoptosis and basement membrane thickening. Dangui and its active ingredients were able to inhibit the VEGF-α pathway to improve the inflammatory response and apoptosis in the retina of diabetic rats[164], and inhibit α-glucosidase, protein tyrosine phosphatase 1B, rat lens aldose reductase, acetylcholinesterase, butyrylcholinesterase andβ-site amyloid precursor protein cleaving enzyme 1 to exert antidiabetic effects[165].
An interesting finding is that SDMM, QJDH and HXMM are all OCMs with the main effect of nourishing the Yin of the liver and kidney, and they all showed good results in lowering glucose. Chinese medicine theory believes that diabetes is a disease with Yin deficiency as the fundamental pathogenesis, and DR develops from diabetes, so the treatment should focus on replenishing Yin. Ligustrum lucidum (Nvzhenzi in Chinese) and Ecliptae Herba (Mohanlian in Chinese) are common components of HXMM and SDMM, which are widely used in China for the treatment of liver-kidney yin deficiency syndrome[166] (A TCM pathological diagnostic pattern caused by the imbalance of yin and yang[167,168], which is closely related to the development of diabetes). They were found to increase insulin sensitivity, enhance the function of islet β-cells INS-1 and β-tc-6[169], reduce retinal oxidative stress levels, repair diabetes-induced abnormal transcriptome[170], improve retinal cell apoptosis[171], inhibit NLRP3 inflammasome and autophagy signaling pathways[172], regulate homocysteine pathway, reduce lipid peroxidation and scavenge free radicals[173], thereby reducing fundus microangiopathy and protecting normal retinal barrier function in diabetic mice. Meanwhile, SDMM was confirmed to be the most effective complementary and alternative drug for inhibiting VEGF in this study, exerting anti-VEGF effects by inhibiting VEGF-induced RF/6A cell tube formation[174], inhibiting NF-κB activity to regulate advanced glycosylation end-product accumulation, oxidative stress and mitochondrial function, and thus improving retinal cell apoptosis by downregulating PKCδ, P47phox and ERK1/2[175,176]. QJDH is a very well-known Chinese OCM for the treatment of eye diseases, which is composed of Liuwei Dihuang Pills (an ancient remedy with very good treatment of diabetes and its complications) plus Chrysanthemi Flos and Lycii Fructus, and has been shown to possibly inhibit the development of DR by interfering with multiple biological pathways such as regulation of response to insulin, glucose homeostasis, and angiogenesis[177]. It was found that Lycii Fructus extract and the active ligand taurine dose-dependently enhanced cell viability, reduced apoptosis, downregulated caspase-3 protein expression and caspase-3 enzymatic activity, and downregulated mRNA encoding pro-inflammatory mediators of MMP-9 and fibronectin as well as COX-2 and iNOS protein expression in human retinal epithelial cell lines after HG treatment in order to achieve a protective effect on human retinal epithelial cell lines under HG exposure, thereby delaying the progression of DR[178,179]. Several network pharmacological and experimental validations found that chemical components such as luteolin, kaempferol, beta-sitosterol, and thymol were able to improve apoptosis-related protein expression by regulating the NLRP/NOX4 signaling pathway, downregulate network hub genes of tumor necrosis factor, and other multibiological pathways, inhibit VEGF-induced RF/6A cell tube formation, and slow down the DR process[171,174,180,181], and these chemicals were also found in Chrysanthemi Flos.
It is noteworthy that metabolic diseases, such as hyperglycemia, hyperlipidemia, and hypertension, serve as crucial risk factors contributing to the development of DR[2]. Effective management of these risk factors is imperative in the prevention and treatment of DR. In this regard, incorporating exercise training and neuromuscular electrical stimulation[182,183] can prove to be a valuable strategy. The guidelines established by the American College of Sports Medicine and the American Diabetes Association emphasize the significance of initial guidance from a qualified exercise trainer for individuals with type 2 diabetes[184]. These guidelines advocate for the implementation of appropriate exercise training to optimize outcomes related to glycemic control, blood pressure, lipid levels, and cardiovascular risk management.
Despite the clear strengths of this study, there are some limitations. First, the quality of the included studies in this study warrants improvement, as most of them were short-term, single-center, and had small sample sizes. Second, clinical effectiveness, being a commonly used measure in clinical practice, may vary slightly in its definition across different randomized controlled trials, leading to a certain degree of heterogeneity in the results. And then, due to the relatively strict inclusion criteria applied in this study, some high-quality individualized TCM treatment studies were excluded, which may limit the comprehensive representation of the characteristics of TCM. Finally, the included literature lacked direct comparisons between different TCMs, and a closed loop was not formed in the evidence network, thereby allowing only indirect comparisons to assess the efficacy advantages and disadvantages of different interventions. Despite these limitations, the present study remains one of the most comprehensive studies available and holds significant clinical reference value.
This study aims to optimize patient outcomes by tailoring treatment strategies to each patient's unique condition. We recommend the utilization of SDMM + CT or HXMM + CT for treatment due to their demonstrated efficacy across multiple indicators. Specifically, HXMM + CT has shown greater effectiveness in improving patients' visual acuity, while SDMM + CT exhibits stronger inhibitory effects on VEGF. Furthermore, DGBX + CT has proven to be more effective in reducing FHA, HXMM + CT excels in reducing FBG, and QJDH + CT demonstrates superior efficacy in reducing HbA1c. Additionally, we suggest combining OCMs with western drugs for the treatment of DR, as this combination has been shown to yield superior outcomes compared to interventions with western drugs alone. Hence, it is crucial to select appropriate treatment methods in clinical practice based on the individual circumstances of patients with DR to attain maximum benefits from combined Chinese and Western medicine interventions.
This study provides evidence that combining OCMs with western drugs leads to better outcomes in all aspects of DR compared to using western drugs alone. Based on the findings, we highly recommend the use of SDMM or HXMM for the treatment of DR. These two OCMs have demonstrated outstanding efficacy across multiple indicators.
Diabetic retinopathy (DR) is one of the most important factors in adult blindness, yet rationalized DR treatment protocols are currently not systematically updated.
Current traditional Chinese medicine treatment options for DR need to be re-evaluated.
To investigate which complementary alternative treatment with herbs is the most effective for the different clinical characteristics of DR patients.
Alternative treatment options to traditional Chinese medicine were incorporated and assessed by employing a mesh meta-analysis to prioritize the therapeutic effects of these options based on various clinical observations.
When these nine Oral Chinese medicines were analyzed in combination with conventional western medicine treatment (CT) compared with CT alone, the results showed that Hexue Mingmu Tablets has better intervention effect on the overall efficacy, visual acuity and reducing fasting blood glucose, Shuangdan Mingmu Capsules has better effect on inhibiting vascular endothelial growth factor, Danggui Buxue Decoction has better effect on reducing fundus hemorrhage area, and Qiju Dihuang Pills has better effect on reducing glycated hemoglobin.
Shuangdan Mingmu Capsules or Hexue Mingmu Tablets in combination with western drugs for DR may be the ideal treatment option.
Bringing guidance to the clinical use of DR, as well as providing direction to basic experiments.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rahmati M, Iran; Salceda R, Mexico; Horowitz M, Australia S-Editor: Fan JR L-Editor: A P-Editor: Zhang YL
| 1. | Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Resnikoff S, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR; Vision Loss Expert Group. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888-e897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1070] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 2. | Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 2283] [Article Influence: 152.2] [Reference Citation Analysis (0)] |
| 3. | Whitcup SM, Cidlowski JA, Csaky KG, Ambati J. Pharmacology of Corticosteroids for Diabetic Macular Edema. Invest Ophthalmol Vis Sci. 2018;59:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R, Lois N. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 717] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 5. | Youngblood H, Robinson R, Sharma A, Sharma S. Proteomic Biomarkers of Retinal Inflammation in Diabetic Retinopathy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Zhao ZH, Xu M, Fu C, Huang Y, Wang TH, Zuo ZF, Liu XZ. A Mechanistic Exploratory Study on the Therapeutic Efficacy of Astragaloside IV Against Diabetic Retinopathy Revealed by Network Pharmacology. Front Pharmacol. 2022;13:903485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Luo XX, Duan JG, Liao PZ, Wu L, Yu YG, Qiu B, Wang YL, Li YM, Yin ZQ, Liu XL, Yao K. Effect of qiming granule on retinal blood circulation of diabetic retinopathy: a multicenter clinical trial. Chin J Integr Med. 2009;15:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Sun HH, Chai XL, Li HL, Tian JY, Jiang KX, Song XZ, Wang XR, Fang YS, Ji Q, Liu H, Hao GM, Wang W, Han J. Fufang Xueshuantong alleviates diabetic retinopathy by activating the PPAR signalling pathway and complement and coagulation cascades. J Ethnopharmacol. 2021;265:113324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Wang F, Li L, Xiao H. Analysis of the efficacy and safety of Danshen Dripping pills on the eyes of diabetic retinopathy patients with Qi stagnation and blood stasis. Am J Transl Res. 2021;13:13059-13066. [PubMed] |
| 10. | Li T, Puhan MA, Vedula SS, Singh S, Dickersin K; Ad Hoc Network Meta-analysis Methods Meeting Working Group. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 2011;9:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 11. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8213] [Article Influence: 821.3] [Reference Citation Analysis (0)] |
| 12. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5230] [Article Influence: 523.0] [Reference Citation Analysis (1)] |
| 13. | Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society; Geriatric Professional Committee of Beijing Medical Award Foundation; National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua Nei Ke Za Zhi. 2022;61:12-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 14. | Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2871] [Article Influence: 478.5] [Reference Citation Analysis (0)] |
| 15. | Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1217] [Cited by in RCA: 1851] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 16. | Men LB. [Effect of Compound Xueshuantong Capsules combined with Calcium Dobesilate on visual acuity and inflammatory factor levels in patients with diabetic retinopathy]. Linchuang Yixue. 2020;40:124-125. [DOI] [Full Text] |
| 17. | Bai YX. [Observation on treating diabetic retinopathy in integrative medicine and the safety evaluation]. Zhongyi Linchang Yanjiu. 2016;8:17-19. [DOI] [Full Text] |
| 18. | An LN. [Efficacy of compound thromboxane capsules supplemented with calcium hydroxybenzenesulfonate tablets in the treatment of non-proliferative diabetic retinopathy and its effect on retinal hemodynamics]. Shiyong Tangniaobing Zazhi. 2021;17:127-128. |
| 19. | Yan H, Song JH. [Effectiveness of compound thromboxane capsules in the adjuvant treatment of non-proliferative diabetic retinopathy and the effect on OA hemodynamics in patients]. Haixia Yaoxue. 2020;32:141-142. [DOI] [Full Text] |
| 20. | Li D. [Effect of compound thromboxane capsule combined with calcium hydroxybenzenesulfonate in the treatment of diabetic retinopathy with macular edema]. Tangniaobing Tiandi. 2021;18:88. |
| 21. | Jin SJ. [Observation on the effect of treatment with compound thromboxane capsules combined with calcium hydroxybenzenesulfonate in patients with diabetic retinopathy]. Kang Yi. 2020;17:249. [DOI] [Full Text] |
| 22. | Wu ZX. [Clinical effects and blood rheology of thromboxane in the treatment of type 2 diabetic retinopathy]. Haixia Yaoxue. 2015;27:3. [DOI] [Full Text] |
| 23. | Qu ZX. [Therapeutic effect of compound Xueshuantong capsule combined with calcium dobesilate in the treatment of diabetic retinopathy]. ZhongguoXiandai Yisheng. 2018;56:30-32. |
| 24. | Sui YN. [Efficacy of compound Xueshuantong Capsules combined with Calcium Dobesilatein the treatment of diabetic retinopathy]. Neimenggu Zhongyiyao. 2017;36:93. [DOI] [Full Text] |
| 25. | Jiang YN, Jiang XD, Li S, Wang HM. [Effect of Compound Thrombosis on Early Diabetic Retinopathy and Its Influence on Serum Levels of Vascular Endothelial Growth Factor and NSE]. Xiandai Zhenduan Zhiliao. 2019;30:1782-1784. |
| 26. | Zhu YX, Li J, Ying J. [Efficacy of compound Xueshuantong capsules in the treatment of non-proliferative diabetic retinopathy]. Zhongguo Xiandai Yisheng. 2016;54:60-62+66. |
| 27. | Sun H. [Observation on the effect of compound thromboxane capsule in the treatment of diabetic retinopathy]. Tangniaobing Tiandi. 2021;18:57. |
| 28. | Liu X, Zhou Q, Zhang Y. [Effects of compound Xueshuantong combined with calcium dobesilate on the levels of HIF-1 and SDF-1 in patients with non-proliferative diabetic retinopathy]. Hebei Yiyao. 2018;40:1838-1841. [DOI] [Full Text] |
| 29. | Li Y. [Effect of compound Xueshuantong combined with calcium dobesilate on vascular growth factor levels and visual acuity in patients with diabetic retinopathy]. Tangniaobing Xintiandi. 2019;22:180-181. [DOI] [Full Text] |
| 30. | Xiao LP. [Clinical efficacy of compound Xueshuantong combined with calcium dobesilate in the treatment of non-proliferative diabetic retinopathy]. Jilin Yixue. 2016;37:2254-2255. [DOI] [Full Text] |
| 31. | Chen KF. [Clinical effect observation of compound Xueshuantong combined with calcium dobesilate in the treatment of early diabetic retinopathy]. Huaihai Yiyao. 2019;37:72-74. [DOI] [Full Text] |
| 32. | Zhao Y. [Clinical effects of compound Xueshuantong combined with calcium dobesilate in the treatment of early diabetic retinopathy]. Shoudu Shipin Yiyao. 2020;27:95. |
| 33. | An LN. [Clinical study of compound Xueshuantong combined with calcium dobesilate in the treatment of diabetic retinopathy]. Shiyong Zhongxiyi Jiehe Linchuang. 2020;20:69-71. [DOI] [Full Text] |
| 34. | Zhang L. [Clinical efficacy of compound Xueshuantong combined with calcium dobesilate in the treatment of early diabetic retinopathy]. Tangniaobing Tiandi. 2019;16:91. |
| 35. | Wei M, Chen C, Liao T, Peng J, Liu J. [Clinical Effect of Compound Xueshuantong on Early Diabetic Retinopathy and Serum Levels of VEGF, IGF-1 and NSE]. Xiandai Shengwu Yixue Jinzhan. 2017;17:3901-3904. [DOI] [Full Text] |
| 36. | Meng X, Zhang S, Duan Y. [Clinical observation on the treatment of diabetic retinopathy with Compound Xueshuantong]. Henan Daxue Xuebao. 2012;31:151-152. [DOI] [Full Text] |
| 37. | Zhu Y, Sui A. [Effects of two different treatment regimens on fundus microvasculature and blood rheology in patients with early diabetic retinopathy]. Zhongguo Yixue Chuangxin. 2022;19:35-38. [DOI] [Full Text] |
| 38. | Li BJ. Clinical efficacy of compound Xueshuantong combined with calcium dobesilate in the treatment of proliferative diabetic retinopathy. 2017 Fifth World Congress of Integrative Medicine Abstracts Collection (Previous); 2017 Dec 6; Guangzhou, GuangDong, China: Guoji Huiyi, 2017: 13. Available from: https://kns.cnki.net/kcms2/article/abstract?v=zrtWY6fLGG6aXSpR1SYn5cOFoTKcnoLRVUAXqH0FI1QCAceY09lAbBhMYYljvCp5i5xrdbocLTN1kW6aMDBZd6Xc0kqT6nyytP6hkxdUyiWvGTu0upJMqfkNJ8vE-khRHBn_yfbae5-aiQpCcLN7og==&uniplatform=NZKPT. |
| 39. | Wang J, Du W, Li Y. [Efficacy of compound Xueshuantong combined with calcium dobesilate capsules in the treatment of diabetic retinopathy in the elderly and its effect on blood rheology]. Zhongguo Laonianxue Zazhi. 2020;40:1603-1606. [DOI] [Full Text] |
| 40. | Hu D. [Clinical efficacy of Compound Xueshuantong combined with calcium dobesilate in patients with visual field defects due to diabetic retinopathy]. Yixue Lilun Shijian. 2017;30:670-671. [DOI] [Full Text] |
| 41. | Xu J, Ru J. [Effect of Compound Xueshuantong combined with calcium dobesilate on ocular blood flow in patients with early diabetic retinopathy]. Xiandai Zhongxiyi Jiehe Zazhi. 2020;29:4064-4067. [DOI] [Full Text] |
| 42. | Wang X. [Clinical observation of Iodized Lecithin combined with Qiming Granules in the treatment of macular cystoid edema in diabetic retinopathy]. Zhongyiyao Xinxi. 2016;33:111-113. |
| 43. | Wang Q. [Efficacy of Qiming Granules in non-proliferative diabetic retinopathy. Shenzhen Zhongxiyi Jiehe Zazhi. 2018;28:48-49. [DOI] [Full Text] |
| 44. | Wang M, Yan ZJ, Zhang LM, Zhang YH, Xu WH. [Clinical efficacy of Qiming Granules combined with Levamlodipine Besylate in the treatment of diabetic retinopathy combined with hypertension]. Hubei Zhongxiyi Jiehe Zazhi. 2020;22:58-60. [DOI] [Full Text] |
| 45. | Chen JQ. [Clinical observation of 45 cases of diabetic retinopathy treated with Qiming Granules combined with conventional drugs]. Xinzhongyi. 2016;48:50-51. [DOI] [Full Text] |
| 46. | Wang B, Li XY, Liu MC, Gao ZN. [Efficacy of Qiming Granules combined with calcium dobesilate in diabetic retinopathy]. Zhongwai Yiliao. 2015;34:114-115. [DOI] [Full Text] |
| 47. | Sui HL, Yu CY, Xue HM, Wang RN. [Clinical efficacy of Qiming Granules combined with calcium dobesilate Capsules in the treatment of patients with non-proliferative diabetic retinopathy]. Zhongwai Yixue Chuangxin. 2014;11:99-102. [DOI] [Full Text] |
| 48. | Huang Q. [Efficacy of Qiming Granules combined with calcium dobesilate in the treatment of type 2 diabetic retinopathy]. Zhongxiyi Jiehe Xinxueguan Dianzi Zazhi. 2017;5:34-35. [DOI] [Full Text] |
| 49. | Feng JL, Zhou P, Wu QL, Chen YN, Tian J. [Observation on the effect of Qiming Granules combined with calcium dobesilate in the treatment of diabetic retinopathy in phase III]. Hebei Yiyao. 2016;38:3430-3433. [DOI] [Full Text] |
| 50. | Ge AL. [Clinical observation on the treatment of diabetic retinopathy with Qiming Granules combined with western medicine]. Zhongguo Minjian Liaofa. 2018;26:76-77. [DOI] [Full Text] |
| 51. | Yan JH. [Effectiveness of Qiming Granules combined with calcium dobesilate in the treatment of non-proliferative diabetic retinopathy]. Henan Yixue Yanjiu. 2020;29:6477-6479. [DOI] [Full Text] |
| 52. | Meng FC, Yu GJ, Hou J. [Clinical efficacy of Qiming Granules for diabetic retinopathy]. Zhongguo Shequ Yishi. 2016;32:111,113. [DOI] [Full Text] |
| 53. | Zhang Y, Ma JY, Wang C. [Analysis of the effect of Qiming Granules in the treatment of non-proliferative diabetic retinopathy]. Zhongguo Shiyong Yiyao. 2016;11:151-152. [DOI] [Full Text] |
| 54. | Zhang M. [Analysis of the efficacy of Qiming Granules in the treatment of simple diabetic retinopathy]. Zhongguo Yaowu Jingjixue. 2013;S3:93-94. |
| 55. | Wu Z, Dong N, Shang LX. [Clinical study on Qiming Granules combined with pancreatic kininogenase in treatment of type 2 diabetic retinopathy]. Xiandai Yaowu Linchuang. 2022;37:795-799. [DOI] [Full Text] |
| 56. | Zhou P, Feng JL. [Analysis of the effect of Qiming Granules combined with calcium dobesilate in the treatment of non-proliferative diabetic retinopathy]. Dongfang Shiliao Baojian. 2018;1:108. [DOI] [Full Text] |
| 57. | Wang SQ, Lei Y, Zhang R. [Improvement of Qiming granule combined with calcium dobesilate on fundus microcirculation in the treatment of patients with non-proliferative diabetic retinopathy]. Linchuang Yixue Yanjiu Shijian. 2019;4:117-118. [DOI] [Full Text] |
| 58. | Wang Z. [Qiming Granules combined with calcium dobesilate in treatment of non-proliferative diabetic retinopathy]. Guoji Yanke Zazhi. 2017;17:4. [DOI] [Full Text] |
| 59. | Yin XD. [Efficacy and safety evaluation of the combination regimen of Qiming Granules and calcium dobesilate in the treatment of non-proliferative diabetic retinopathy]. Yixue Lilun Shijian. 2018;31:83-84. [DOI] [Full Text] |
| 60. | Xin W, Yang L, Cui S, Wang D, Guo YH, Yang ZX. [Effects of Qiming Granules combined with Epalrestat on fundus angiography, blood rheology and oxidative stress in patients with diabetic retinopathy]. Xiandai Zhongxiyi Jiehe Zazhi. 2019;28:2677-2680. [DOI] [Full Text] |
| 61. | Zhang JT. [Curative effect and safety analysis of calcium dobesilate combined with Qiming granules to treated with 78 cases diabetic retinopathy]. Dangdai Yixue. 2017;23:88-90. [DOI] [Full Text] |
| 62. | Yue YJ. [Clinical effect of Qiming Granules on treating non-proliferative diabetic retinopathy]. Zhongguo Yiyao Zhinan. 2016;14:8-9. [DOI] [Full Text] |
| 63. | Zheng JQ, Huang JS, Liu ZF. [Efficacy of Qiming Granules in the treatment of diabetic retinopathy]. Manxing Bingxue Zazhi. 2014;15:657-658. [DOI] [Full Text] |
| 64. | Yang L, Zhang GY, Quan XL, Jia N. [Efficacy of Qiming granules in 71 cases of non-proliferative diabetic retinopathy]. Hangkong Hangtian Zazhi. 2013;24:686-687. [DOI] [Full Text] |
| 65. | Huang H. [Analysis of the effect of Compound Danshen Dripping Pills in the adjuvant treatment of patients with diabetic retinopathy]. Beifang Yaoxue. 2020;17:100-101. [DOI] [Full Text] |
| 66. | Zheng XH, Ji YJ. [Effect of Compound Danshen Dripping Pills on the efficacy of diabetic retinopathy patients]. Beifang Yaoxue. 2021;18:41-42. [DOI] [Full Text] |
| 67. | Meng XM, Zhang SS, Duan YC. [Clinical observation on the treatment of diabetic retinopathy with Compound Danshen Dripping Pills combined with Calcium Dobesilate]. Yixue Luntan Zazhi. 2011;32:137-138. |
| 68. | Wang B. [Clinical efficacy of Compound Danshen Dripping Pills in the treatment of diabetic retinopathy]. Zhonghua Yixue Quanke Zazhi. 2004;3:58-59. |
| 69. | Zhao CQ. [Clinical study on the treatment of diabetic retinopathy with Compound Danshen Dripping Pills]. Yixue Linchuang Yanjiu. 2019;36:4-7. [DOI] [Full Text] |
| 70. | Ma MJ, Tian CG, Zhao ZG, Qin GJ, Zheng LL, Jiang HW. [The Curative Effect of Compound Danshen Dripping Pills Treatment Early Diabetic Retinopathy]. Shijie Zhongyiyao. 2016;11:450-453. [DOI] [Full Text] |
| 71. | Chen Y, Guo TS, Chen XW. [Clinical observation on the treatment of early diabetic retinopathy with Compound Danshen Dripping Pills]. Shizhen Guoyi Guoyao. 2007;18:2539-2540. [DOI] [Full Text] |
| 72. | Xu HT. [Observation on the effect of Compound Danshen Dripping Pills combined with Calcium Dobesilate in the treatment of diabetic retinopathy]. Zhongguo Liaoyang Yixue. 2019;28:884-886. [DOI] [Full Text] |
| 73. | Huang YX, Sun HP, Tang YQ, Chen X. [Effects of Compound Danshen Dripping Pills on inflammatory mediators, cytokines and visual function in patients with diabetic retinopathy]. Zhongxiyi Jiehe Xinnao Xueguanbing Zazhi. 2021;19:1364-1366, 1408. [DOI] [Full Text] |
| 74. | Jiao SF. [Application of Calcium Dobesilate combined with Compound Danshen Dripping Pills in the treatment of diabetic retinopathy]. Zhongwai Nvxing Jiankang Zazhi. 2018;24:82-83. [DOI] [Full Text] |
| 75. | Li Y. [Clinical efficacy evaluation of the combination regimen of Compound Danshen Dripping Pills and Calcium Dobesilate in the treatment of diabetic retinopathy]. Hangkong Hangtian Yixue Zazhi. 2017;28:1229-1231. [DOI] [Full Text] |
| 76. | Yan XQ, Yuan MR. [Observation on the efficacy of applying Compound Danshen Dripping Pills in the treatment of 60 cases of senile simple diabetic retinopathy]. Shizhen Guoyi Guoyao. 2014;25:2187-2188. [DOI] [Full Text] |
| 77. | Zhou LJ. [Clinical efficacy of Compound Danshen Dripping Pills in the treatment of diabetic retinopathy]. Zhongguo Yiyao Daobao. 2008;5:77. [DOI] [Full Text] |
| 78. | Miao CX. [Clinical efficacy of Compound Danshen Dripping Pills with Calcium Dobesilate in the treatment of diabetic retinopathy]. Dongfang Yaoshan. 2020;3:87. |
| 79. | Ruan YX, Chen M, Liu ZQ, Wang YL. , Sun N, Huang X, Gan H. [Clinical study on the treatment of retinopathy in diabetic patients with Compound Danshen Dripping Pills combined with Calcium Dobesilate]. Zhongnan Yixue Kexue Zazhi. 2017;45:18-20,23. [DOI] [Full Text] |
| 80. | Yin L, Zhang DL, Ren Q, Li L, Sun P, Sun RX, Yu H, Sun CH. [Observation on the effect of Compound Danshen Dripping Pills with Calcium Dobesilate in the treatment of diabetic retinopathy]. Xiandai Zhongxiyi Jiehe Zazhi. 2013;22:2449-2450. [DOI] [Full Text] |
| 81. | Zhu J. [Efficacy of Compound Danshen Dripping Pills in the treatment of early diabetic retinopathy]. Jiankang Shiye. 2018;10:99-100. [DOI] [Full Text] |
| 82. | Yang PJ, Li SM, Lv YP, Huang ZY, Huang H. [Effects of Compound Danshen Dripping Pills on vascular endothelial function in patients with early diabetic retinopathy]. Zhongguo Shiyan Fangjixue Zazhi. 2013;19:340-343. [DOI] [Full Text] |
| 83. | Bai YX. [Efficacy of Calcium Dobesilate combined with Compound Danshen Dripping Pills in the treatment of diabetic retinopathy and the effect on serum inflammatory factors]. Qiqihar Yixueyuan Xuebao. 2017;38:2641-2643. [DOI] [Full Text] |
| 84. | Guo BD. [Efficacy of Compound Danshen Dripping Pills in the treatment of early diabetic retinopathy]. Linchuang Yixue Wenxian Dianzi Zazhi. 2015;8:1418-1418, 1419. [DOI] [Full Text] |
| 85. | Liu J. [Effect of Compound Danshen Dripping Pills on vascular endothelial function in patients with early diabetic retinopathy]. Xinli Yisheng. 2018;24:118-119. |
| 86. | Liu YZ. [Clinical analysis of Hexue Mingmu Tablets + Pancreatic Kininogenase Enteric-coated Tablets in the treatment of diabetic retinopathy]. Yixue Lilun Shijian. 2019;32:3. [DOI] [Full Text] |
| 87. | Zhao ZH, Liu JX. [Clinical effects of Hexue Mingmu Tablets with Calcium Dobesilate in the treatment of early diabetic retinopathy]. Jiankang Nvxing. 2021;13:136. |
| 88. | Gao L, Qi T, Xu WB, Wan L. [Clinical study on the treatment of early diabetic retinopathy with Hexue Mingmu Tablets combined with Calcium Dobesilate]. Zhongguo Yaoye. 2020;29:133-135. [DOI] [Full Text] |
| 89. | Li M. [Clinical observation on the treatment of early diabetic retinopathy with Hexue Mingmu Tablets combined with Calcium Dobesilate. Jiankang Nvxing. 2021;35:99. |
| 90. | Wang YA, Wang WM, Kong FN. [Efficacy observation of Hexue Mingmu Tablets combined with Pancreatic Kininogenase Enteric-coated Tablets in the treatment of diabetic retinopathy]. Xiandai Yaowu Linchuang. 2018;33:2978-2981. [DOI] [Full Text] |
| 91. | Zhang YR, Wang WX. [Effect of Hexuemingmu Tablet on Diabetic Retinopathy]. Zhongguo Yiyao Zhinan. 2018;16:42-43. [DOI] [Full Text] |
| 92. | Ye XL, Guo XN, Xiong F. [Clinical observation of oral calcium dobesilate combined with HeXue MingMu tablet in the treatment of nonproliferative diabetic retinopathy]. Chuanbei Yixueyuan Xuebao. 2019;34:223-225. [DOI] [Full Text] |
| 93. | Yu MX. [Clinical study on the treatment of diabetic retinopathy with Epalrestat Tablets in combination with Hexue Mingmu Tablets]. Linchuang Yiyao Shijian. 2019;28:573-576. [DOI] [Full Text] |
| 94. | Du J, Li SG, Lv YY. [Clinical observation on the treatment of diabetic retinopathy with Hexue Mingmu Tablets]. Zhejiang Zhongxiyi Jiehe Zazhi. 2015;25:1140-1141. [DOI] [Full Text] |
| 95. | Song R. [Efficacy of Mingmu Dihuang Pills in diabetic retinopathy]. Zhongyi Linchuang Yanjiu. 2013;11:36-37. [DOI] [Full Text] |
| 96. | Li YP. [Clinical study on the treatment of diabetic retinopathy with Mingmu Dihuang Pills combined with Mecobalamin Tablets]. Xiandai Yaowu Linchuang. 2019;34:3044-3049. [DOI] [Full Text] |
| 97. | Li JB, Wei XY. [Clinical study on Mingmu Dihuang Pills combined with calcium dobesilate in treatment of early diabetic retinopathy]. Xiandai Yaowu Linchuang. 2019;34:1197-1201. [DOI] [Full Text] |
| 98. | Wu YF. [Observation on the effect of Qiju Dihuang Pills in the adjuvant treatment of diabetic retinopathy]. Zhongguo Xiangcun Yiyao. 2018;25:22-23. [DOI] [Full Text] |
| 99. | Guan W. [Analysis of the efficacy of Qiju Dihuang Pills combined with calcium hydroxybenzene sulfonate in the treatment of diabetic retinopathy]. Zhongxiyi Jiehe Yanjiu. 2017;9:90-92. [DOI] [Full Text] |
| 100. | Ainu N, Wang Y, Bu Q, Zhao Y. [Clinical efficacy of calcium dobesilate dispersible tablets combined with Mingmu Dihuang pills in treatment of NPDR]. Guoji Yanke Zazhi. 2019;19:992-996. [DOI] [Full Text] |
| 101. | Fu ZJ. [Safety analysis of Shuangdan Mingmu Capsules combined with Calcium Dobesilate in the treatment of diabetic retinopathy]. Shijie Zuixin Yixue Xinxi Wenzhai. 2019;19:205-206. [DOI] [Full Text] |
| 102. | Ji XD, Liu WT. [Safety and efficacy of Shuangdan Mingmu capsule combined with calcium dobesilate dispersible tablets in the treatment of diabetic retinopathy]. Linchuang Yixue Yanjiu Shijian. 2022;7:85-87. [DOI] [Full Text] |
| 103. | Jin L, Zhang LJ. [Clinical study on Shuangdan Mingmu Capsules combined with calcium dobesilate in treatment of diabetic retinopathy]. Xiandai Yaowu Linchuang. 2019;34:159-163. [DOI] [Full Text] |
| 104. | Liu JP, Kong YH, Wang L. [Clinical Efficacy of Shuangdan Mingmu Capsules Combined with Calcium Dobesilate in the Treatment of Diabetic Retinopathy and its Effects on Serum Levels of Vascular Endothelial Growth Factor, Platelet-Derived Growth Factor and Interleukin-1]. Zhongguo Yiyuan Yongyao Pingjia Fenxi. 2019;19:1332-1334,1338. [DOI] [Full Text] |
| 105. | Pang YH. [Clinical observation on the treatment of diabetic retinopathy with Shuangdan Mingmu Capsules]. Tangniaobing Xinshijie. 2015;3:39-39. [DOI] [Full Text] |
| 106. | Deng C, Li J, Tang XZ. [Effect of Danggui Buxue Decoction on the efficacy of diabetic retinopathy and the effect of serum ICAM-1 and ET-1 levels]. Zhongyiyao Xinxi. 2018;35:90-93. [DOI] [Full Text] |
| 107. | Wang D, Chen SF. [Clinical Effect of Danggui Buxue Decoction Combined with Calcium Hydroxybenzenesulfonate on Diabetic Retinopathy]. Zhongwai Yiliao. 2020;39:162-164. [DOI] [Full Text] |
| 108. | Wu YL. [Clinical Survey of Xi-shiyin in Treatment of Non-proliferative Diabetic Retinopathy]. Fujian Zhongyiyao Daxue. 2013;(401):12-18. [DOI] [Full Text] |
| 109. | Sun XH, Qian KW, Zhou Y, Gu HY. [Study on the efficacy and mechanism of Danggui Buxue Decoction in the treatment of diabetic retinopathy]. Xiandai Zhongxiyi Jiehe Zazhi. 2019;28:3152-3155,3170. [DOI] [Full Text] |
| 110. | Yu RC. [Effects of the Danggui Buxue decoction on non-proliferative diabetic retinopathy and ICAM-1 and ET-1]. Zhongwai Linchuang Yanjiu. 2020;12:62-64. [DOI] [Full Text] |
| 111. | Xu W. [Study on the therapeutic effect of Chinese traditional Chinese medicine on the treatment of diabetic retinopathy]. Yiyao Qianyan. 2018;8:362-363. |
| 112. | Huang SC. [Observation on the efficacy of Xuefu Zhuyu Decoction in the treatment of simple diabetic retinopathy]. Shenzhen Zhongxiyi Jiehe Zazhi. 2017;27:27-28. [DOI] [Full Text] |
| 113. | Zhu JX. [Observation of Xuefu Zhuyu Decoction on Clinical Efficacy of Qi and Yin Deficiency and Blood Stasis type Diabetic Retinopathy Bleeding Period]. Shandong Zhongyiyao Daxue. 2015;401:10-12. |
| 114. | Xiong P, Chen R. [Effects of Xuefu Zhuyu Decoction treatment on glucolipid metabolism, inflammatory factors and visual acuity levels in patients with non-proliferative diabetic retinopathy]. Sichuan Zhongyi. 2019;37:137-139. |
| 115. | Liu T. [The effect of Xuefu Zhuyu Decoction treatment on the visual acuity level of patients with non-proliferative diabetic retinopathy]. Jiankang Zhiyou. 2020;3:262-263. |
| 116. | Gong MF, Xiao QF, Huang XY. [Treatment of 40 cases of simple diabetic retinopathy with Xuefu Zhuyu Decoction]. Liaoning Zhongyi Zazhi. 2014;41:500-501. [DOI] [Full Text] |
| 117. | Hao XF. [Treatment of 66 cases of simple diabetic retinopathy with Xuefu Zhuyu Decoction]. Henan Zhongyi. 2012;32:916. [DOI] [Full Text] |
| 118. | Huang YF. [Combination of Chinese and Western medicine in the treatment of diabetic retinopathy in 70 cases. Sichuan Zhongyi. 2004;08:94. [DOI] [Full Text] |
| 119. | Zhang FF. [Synergistic Effect of Buyang Huanwu Decoction on Non-proliferative Diabetic Retinopathy]. Fujian Zhongyiyao Daxue. 2019;(401):12-16. |
| 120. | Yang L. [Clinical observation on the treatment of diabetic retinopathy by combining Buyang Huanwu Decoction with Calcium Dobesilate Tablets]. Zhongguo Minjian Liaofa. 2019;27:60-61. [DOI] [Full Text] |
| 121. | Tian L. [Efficacy of the Yiqi Huoxue Therapy in the Adjunctive Treatment of Diabetic Retinopathy]. Shenzhen Zhongxiyi Jiehe Zazhi. 2019;29:30-31. [DOI] [Full Text] |
| 122. | Qu L, Yao XY. [Efficacy of combined Chinese and Western medicine in the treatment of simple diabetic retinopathy]. Henan Zhongyi. 2009;29:1019-1020. [DOI] [Full Text] |
| 123. | Li S, Liu H, Zhu X. The effect of psychotherapy on anxiety, depression, and quality of life in patients with diabetic retinopathy: A protocol for systematic review and network meta-analysis. Medicine (Baltimore). 2021;100:e28386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | Zhang B, Zhou Z, Zhang B, Wang D. Efficacy and Safety of Various Treatments for Proliferative Diabetic Retinopathy: A Systematic Review and Network Meta-Analysis. Front Pharmacol. 2021;12:709501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 125. | Sun W, Li J, Yan X, Liao L, Li S, Wang X, Xiao C, Shang M, Chao G, Zhou J. Traditional Chinese Medicine Injections for Diabetic Retinopathy: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J Integr Complement Med. 2022;28:927-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 126. | Fallico M, Maugeri A, Lotery A, Longo A, Bonfiglio V, Russo A, Avitabile T, Pulvirenti A, Furino C, Cennamo G, Barchitta M, Agodi A, Reibaldi M. Intravitreal anti-vascular endothelial growth factors, panretinal photocoagulation and combined treatment for proliferative diabetic retinopathy: a systematic review and network meta-analysis. Acta Ophthalmol. 2021;99:e795-e805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 127. | Wang DY, Zhao XY, Zhang WF, Meng LH, Chen YX. Perioperative anti-vascular endothelial growth factor agents treatment in patients undergoing vitrectomy for complicated proliferative diabetic retinopathy: a network meta-analysis. Sci Rep. 2020;10:18880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Hooper P, Boucher MC, Cruess A, Dawson KG, Delpero W, Greve M, Kozousek V, Lam WC, Maberley DAL. Excerpt from the Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of diabetic retinopathy. Can J Ophthalmol. 2017;52 Suppl 1:S45-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 129. | Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447-1449, 1449.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 390] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 130. | Sharma S, Brown GC, Brown MM, Hollands H, Shah GK. The cost-effectiveness of grid laser photocoagulation for the treatment of diabetic macular edema: results of a patient-based cost-utility analysis. Curr Opin Ophthalmol. 2000;11:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | An X, Jin D, Duan L, Zhao S, Zhou R, Lian F, Tong X. Direct and indirect therapeutic effect of traditional Chinese medicine as an add-on for non-proliferative diabetic retinopathy: a systematic review and meta-analysis. Chin Med. 2020;15:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Wang H, Jin C, Jiang Q. Price adjustment for traditional Chinese medicine procedures: Based on a standardized value parity model. Biosci Trends. 2017;11:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 133. | Guo ZL, Li Y, Liu XW, Wu MY, Guo Q, Yao XC, Wang YD, Wu WY. Sodium Tanshinone IIA Silate Alleviates High Glucose Induced Barrier Impairment of Human Retinal Pigment Epithelium through the Reduction of NF-κB Activation via the AMPK/p300 Pathway. Curr Eye Res. 2020;45:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 134. | Fan K, Li S, Liu G, Yuan H, Ma L, Lu P. Tanshinone IIA inhibits high glucoseinduced proliferation, migration and vascularization of human retinal endothelial cells. Mol Med Rep. 2017;16:9023-9028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 135. | Allison DJ, Ditor DS. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation. 2014;11:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 136. | Jeon SW, Kim YK. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J Psychiatry. 2016;6:283-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 140] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (1)] |
| 137. | Nagata H, Sai B, Tada F, Tonomura T, Ise C. [Anemia and milk secretion - with special reference to anemia in the postpartum period]. Josanpu Zasshi. 1979;33:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 138. | Zheng X, Liang Y, Kang A, Ma SJ, Xing L, Zhou YY, Dai C, Xie H, Xie L, Wang GJ, Hao HP. Peripheral immunomodulation with ginsenoside Rg1 ameliorates neuroinflammation-induced behavioral deficits in rats. Neuroscience. 2014;256:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 139. | Xie T, Zhou XP, Lin LL, Xu JY, Shen CS, Feng Z, Zhou LL, Shan JJ. [Metabolomics analysis of Tripterygium wilfordii formulation based on theory of detoxicity compatibility]. Zhongguo Zhong Yao Za Zhi. 2016;41:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 140. | Zhang H, Li Z, Zhou Z, Yang H, Zhong Z, Lou C. Antidepressant-like effects of ginsenosides: A comparison of ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, compound K, and 20(S)-protopanaxadiol in mice models of despair. Pharmacol Biochem Behav. 2016;140:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 141. | Pang HH, Li MY, Wang Y, Tang MK, Ma CH, Huang JM. Effect of compatible herbs on the pharmacokinetics of effective components of Panax notoginseng in Fufang Xueshuantong Capsule. J Zhejiang Univ Sci B. 2017;18:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 142. | Tian Z, Pang H, Du S, Lu Y, Zhang L, Wu H, Guo S, Wang M, Zhang Q. Effect of Panax notoginseng saponins on the pharmacokinetics of aspirin in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1040:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 143. | Zhao S, Zheng MX, Chen HE, Wu CY, Wang WT. Effect of panax notoginseng saponins injection on the p38MAPK pathway in lung tissue in a rat model of hypoxic pulmonary hypertension. Chin J Integr Med. 2015;21:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 144. | Zhai Y, Meng X, Luo Y, Wu Y, Ye T, Zhou P, Ding S, Wang M, Lu S, Zhu L, Sun G, Sun X. Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Oncotarget. 2018;9:9344-9363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 145. | Zhang TT, Jiang JG. Active ingredients of traditional Chinese medicine in the treatment of diabetes and diabetic complications. Expert Opin Investig Drugs. 2012;21:1625-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 146. | Hou QL, Wang Y, Li YB, Hu XL, Wang SL. [Protective effect of notoginsenoside R1 on neuron injury induced by OGD/R through ATF6/Akt signaling pathway]. Zhongguo Zhong Yao Za Zhi. 2017;42:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 147. | Li W, Ling S, Yang Y, Hu Z, Davies H, Fang M. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett. 2014;35:104-109. [PubMed] |
| 148. | Yang X, Yang S, Hong C, Yu W, Guonian W. Panax Notoginseng Saponins attenuates sevofluraneinduced nerve cell injury by modulating AKT signaling pathway. Mol Med Rep. 2017;16:7829-7834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 149. | Wang T, Guo R, Zhou G, Zhou X, Kou Z, Sui F, Li C, Tang L, Wang Z. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: A review. J Ethnopharmacol. 2016;188:234-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 289] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 150. | Berge LI, Riise T. Comorbidity between Type 2 Diabetes and Depression in the Adult Population: Directions of the Association and Its Possible Pathophysiological Mechanisms. Int J Endocrinol. 2015;2015:164760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 151. | Xie W, Meng X, Zhai Y, Zhou P, Ye T, Wang Z, Sun G, Sun X. Panax Notoginseng Saponins: A Review of Its Mechanisms of Antidepressant or Anxiolytic Effects and Network Analysis on Phytochemistry and Pharmacology. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 152. | Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G, Sun X. Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 153. | Chen CF, Chiou WF, Zhang JT. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008;29:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 154. | Chen F, Wen Q, Jiang J, Li HL, Tan YF, Li YH, Zeng NK. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J Ethnopharmacol. 2016;179:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 155. | Li S, Tang Y, Liu C, Zhang Y. Development of a method to screen and isolate potential α-glucosidase inhibitors from Panax japonicus C.A. Meyer by ultrafiltration, liquid chromatography, and counter-current chromatography. J Sep Sci. 2015;38:2014-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 156. | Li X, Wu L, Fan X, Zhang B, Gao X, Wang Y, Cheng Y. [Network pharmacology study on major active compounds of Fufang Danshen formula]. Zhongguo Zhong Yao Za Zhi. 2011;36:2911-2915. [PubMed] |
| 157. | Park MJ, Bae CS, Lim SK, Kim DI, Lim JC, Kim JC, Han HJ, Moon JH, Kim KY, Yoon KC, Park SH. Effect of protopanaxadiol derivatives in high glucose-induced fibronectin expression in primary cultured rat mesangial cells: role of mitogen-activated protein kinases and Akt. Arch Pharm Res. 2010;33:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 158. | Tabandeh MR, Jafari H, Hosseini SA, Hashemitabar M. Ginsenoside Rb1 stimulates adiponectin signaling in C2C12 muscle cells through up-regulation of AdipoR1 and AdipoR2 proteins. Pharm Biol. 2015;53:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 159. | Wu Y, Yu Y, Szabo A, Han M, Huang XF. Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet. PLoS One. 2014;9:e92618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 160. | Xue B, Sun L, Li X, Wang X, Zhang Y, Mu Y, Liang L. Ginsenoside Rb1 relieves glucose fluctuation-induced oxidative stress and apoptosis in Schwann cells. Neural Regen Res. 2012;7:2340-2346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 161. | Yu X, Ye L, Zhang H, Zhao J, Wang G, Guo C, Shang W. Ginsenoside Rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J Ginseng Res. 2015;39:199-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 162. | Xue M, Bian Y, Liu Y, Zhou J, Xu J, Zhang L, Huang X. Danggui Buxue decoction ameliorates lipid metabolic defects involved in the initiation of diabetic atherosclerosis; identification of active compounds. J Tradit Chin Med. 2020;40:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 163. | Liu JH, Li CJ, Li YT. The protective effects of Astragalus polysaccharide on diabetic rats with retinopathy. Hebei Yike Daxue Xuebao. 2017;38:797-800. [DOI] [Full Text] |
| 164. | Yang B, Ma G, Liu Y. Z-Ligustilide Ameliorates Diabetic Rat Retinal Dysfunction Through Anti-Apoptosis and an Antioxidation Pathway. Med Sci Monit. 2020;26:e925087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 165. | Yousof Ali M, Jung HA, Choi JS. Anti-diabetic and anti-Alzheimer's disease activities of Angelica decursiva. Arch Pharm Res. 2015;38:2216-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 166. | Zhai Y, Xu J, Feng L, Liu Q, Yao W, Li H, Cao Y, Cheng F, Bao B, Zhang L. Broad range metabolomics coupled with network analysis for explaining possible mechanisms of Er-Zhi-Wan in treating liver-kidney Yin deficiency syndrome of Traditional Chinese medicine. J Ethnopharmacol. 2019;234:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 167. | Jiang M, Lu C, Zhang C, Yang J, Tan Y, Lu A, Chan K. Syndrome differentiation in modern research of traditional Chinese medicine. J Ethnopharmacol. 2012;140:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 168. | Lee S, Park J, Lee H, Kim K. Development and validation of Yin-Deficiency Questionnaire. Am J Chin Med. 2007;35:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 169. | Xu H, Su ZR, Huang W, Choi RC, Zheng YZ, Lau DT, Dong TT, Wang ZT, Tsim KW. Er Zhi Wan, an ancient herbal decoction for woman menopausal syndrome, activates the estrogenic response in cultured MCF-7 cells: an evaluation of compatibility in defining the optimized preparation method. J Ethnopharmacol. 2012;143:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 170. | Yao F, Jiang X, Qiu L, Peng Z, Zheng W, Ding L, Xia X. Long-Term Oral Administration of Salidroside Alleviates Diabetic Retinopathy in db/db Mice. Front Endocrinol (Lausanne). 2022;13:861452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 171. | Yang Y, Zhou M, Liu H. Luteolin, an aryl hydrocarbon receptor antagonist, alleviates diabetic retinopathy by regulating the NLRP/NOX4 signalling pathway: Experimental and molecular docking study. Physiol Int. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 172. | Zhou L, Zhang T, Lu B, Yu Z, Mei X, Abulizi P, Ji L. Lonicerae Japonicae Flos attenuates diabetic retinopathy by inhibiting retinal angiogenesis. J Ethnopharmacol. 2016;189:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 173. | Li R, Chen L, Yao GM, Yan HL, Wang L. Effects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy. Int J Ophthalmol. 2021;14:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 174. | Wang S, Du S, Wang W, Zhang F. Therapeutic investigation of quercetin nanomedicine in a zebrafish model of diabetic retinopathy. Biomed Pharmacother. 2020;130:110573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 175. | Kim CS, Kim J, Kim YS, Jo K, Lee YM, Jung DH, Lee IS, Kim JH, Kim JS. Improvement in Diabetic Retinopathy through Protection against Retinal Apoptosis in Spontaneously Diabetic Torii Rats Mediated by Ethanol Extract of Osteomeles schwerinae C.K. Schneid. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 176. | Chen B, He T, Xing Y, Cao T. Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Exp Ther Med. 2017;14:6022-6026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 177. | Yuan M, He Q, Long Z, Zhu X, Xiang W, Wu Y, Lin S. Exploring the Pharmacological Mechanism of Liuwei Dihuang Decoction for Diabetic Retinopathy: A Systematic Biological Strategy-Based Research. Evid Based Complement Alternat Med. 2021;2021:5544518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 178. | Song MK, Salam NK, Roufogalis BD, Huang TH. Lycium barbarum (Goji Berry) extracts and its taurine component inhibit PPAR-γ-dependent gene transcription in human retinal pigment epithelial cells: Possible implications for diabetic retinopathy treatment. Biochem Pharmacol. 2011;82:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 179. | Song MK, Roufogalis BD, Huang TH. Reversal of the Caspase-Dependent Apoptotic Cytotoxicity Pathway by Taurine from Lycium barbarum (Goji Berry) in Human Retinal Pigment Epithelial Cells: Potential Benefit in Diabetic Retinopathy. Evid Based Complement Alternat Med. 2012;2012:323784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 180. | Li H, Li B, Zheng Y. Exploring the Mechanism of Action Compound-Xueshuantong Capsule in Diabetic Retinopathy Treatment Based on Network Pharmacology. Evid Based Complement Alternat Med. 2020;2020:8467046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 181. | Li Y, Huang Y, Tu C. Systems-Pharmacology-Based Identification of Antitumor Necrosis Factor Effect in Mimeng Flower Decoction for the Treatment of Diabetic Retinopathy. Evid Based Complement Alternat Med. 2019;2019:5107103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 182. | Rahmati M, Gondin J, Malakoutinia F. Effects of Neuromuscular Electrical Stimulation on Quadriceps Muscle Strength and Mass in Healthy Young and Older Adults: A Scoping Review. Phys Ther. 2021;101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 183. | Rahmati M, Malakoutinia F. Aerobic, resistance and combined exercise training for patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis. Physiotherapy. 2021;113:12-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 184. | Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B; American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692-2696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 463] [Article Influence: 30.9] [Reference Citation Analysis (0)] |