Published online Sep 15, 2023. doi: 10.4239/wjd.v14.i9.1393
Peer-review started: May 6, 2023
First decision: May 19, 2023
Revised: May 24, 2023
Accepted: August 4, 2023
Article in press: August 4, 2023
Published online: September 15, 2023
Processing time: 130 Days and 8.6 Hours
Gestational diabetes mellitus (GDM) has become increasingly prevalent globally. Glycemic control in pregnant women with GDM has a critical role in neonatal complications.
To analyze the early neonatal complications in GDM, and examine the effect of blood glucose control level on neonatal infection.
The clinical data of 236 pregnant women with GDM and 240 healthy pregnant women and newborns during from March 2020 to December 2021 the same period were retrospectively analyzed, and the early complications in newborns in the two groups were compared. The patients were divided into the conforming glycemic control group (CGC group) and the non-conforming glycemic control group (NCGC group) based on whether glycemic control in the pregnant women with GDM conformed to standards. Baseline data, immune function, infection-related markers, and infection rates in neonates were compared between the two groups.
The incidence of neonatal complications in the 236 neonates in the GDM group was significantly higher than that in the control group (P < 0.05). Pregnant women with GDM in the NCGC group (n = 178) had significantly higher fasting plasma glucose, 2 h postprandial blood glucose and glycated hemoglobin A1C levels than those in the CGC group (n = 58) (P < 0.05). There were no differences in baseline data between the two groups (P > 0.05). Additionally, the NCGC group had significantly decreased peripheral blood CD3+, CD4+, CD8+ T cell ratios, CD4/CD8 ratios and immunoglobulin G in neonates compared with the CGC group (P < 0.05), while white blood cells, serum procalcitonin and C-reactive protein levels increased significantly. The neonatal infection rate was also significantly increased in the NCGC group (P < 0.05).
The risk of neonatal complications increased in pregnant women with GDM. Poor glycemic control decreased neonatal immune function, and increased the incidence of neonatal infections.
Core Tip: Gestational diabetes mellitus (GDM) is an important complication that affects pregnancy outcome. Pregnant women with GDM and long-term abnormal glucose metabolism are closely associated with the risk of adverse maternal and neonatal outcomes. Some studies suggest that the immune function of newborns may be significantly affected by GDM, and that the effect of glycemic control is related to pregnancy outcomes and neonatal prognosis. In this study, we confirmed that the risk of neonatal complications increased in pregnant women with GDM, and poor glycemic control leads to impairment of fetal immune system and ultimately increases the risk of neonatal infections.
- Citation: Wang BB, Xue M. Early neonatal complications in pregnant women with gestational diabetes mellitus and the effects of glycemic control on neonatal infection. World J Diabetes 2023; 14(9): 1393-1402
- URL: https://www.wjgnet.com/1948-9358/full/v14/i9/1393.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i9.1393
Gestational diabetes mellitus (GDM) is a glucose metabolism disorder during pregnancy. Studies have indicated that GDM tends to cause diabetes mellitus and cardiovascular disease in neonates after delivery by pregnant women with GDM[1]. Recently, studies reported that GDM incidence has shown a significant increasing trend worldwide[2] and has become a global health concern. Previous studies[3,4] have shown that GDM is considered to be one of the major risk factors for maternal and child complications in the perinatal phase and the incidence of cesarean section, polyhydramnios, premature birth, fetal deformities, neonatal infection, hyperbilirubinemia, fetal macrosomia, and neonatal hypoglycemia is higher in pregnant women with GDM who have abnormal glucose metabolism for a long period. Rational diet control, exercise, and glucose-lowering treatments have achieved good results in terms of glycemic control in pregnant women with GDM. However, 30% of pregnant women with GDM were reported to be affected by multiple factors, including irregular diet, lack of exercise, hormone secretion disorder, etc[5]. In addition, glycemic control was poor. This ultimately affected maternal and child health, and increased the risk of neonatal complications. A study found that the immune function of neonates may be significantly affected by GDM, and the effectiveness of glycemic control is strongly associated with adverse pregnancy outcomes and neonatal complications[6]. Early detection of abnormal glucose metabolism and achieving good glycemic control during pregnancy can effectively prevent adverse maternal and child outcomes in the perinatal stage of GDM patients[7]. However, there is still unclear whether glycemic control that does not conform to the standards in pregnant women with GDM decreases immune function in neonates and increases the incidence of neonatal infections.
In this study, we investigated the differences in neonatal complications between pregnant women with GDM and healthy controls to examine the effects of GDM on neonatal prognosis. We then divided the pregnant women with GDM into two groups based on whether glycemic control conformed to standards. Subsequently, we compared the blood glucose levels in pregnant women with GDM, immune function, infection-related marker levels, and the incidence of infection in neonates of the pregnant women with GDM in these two groups. The purpose of this study was to analyze the effects of glycemic control in GDM pregnant women on neonatal immune function and infection.
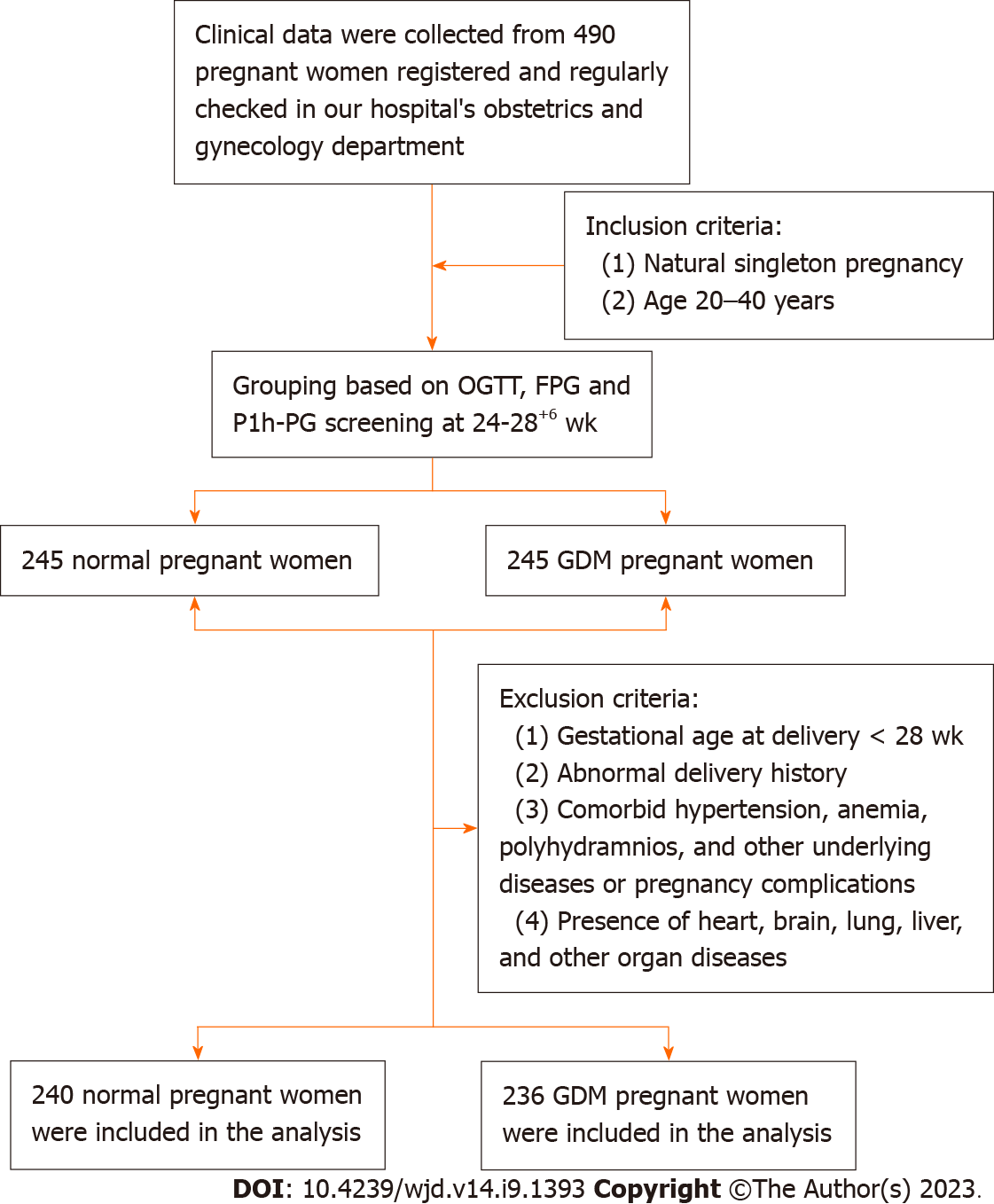
The newborns delivered by 236 pregnant women with GDM in Taizhou People’s Hospital of Jiangsu Province from March 2020 to December 2021 were retrospectively included in the GDM group. The neonates of 240 healthy pregnant women during the same period were selected as the control group. The patients were divided into the conforming glycemic control group (CGC group) and the non-conforming glycemic control group (NCGC group) based on whether glycemic control in pregnant women with GDM conformed to standards.
Inclusion criteria: (1) Natural singleton pregnancy; (2) age 20–40 years; and (3) for the GDM group, blood glucose measurement at week 24–28 of pregnancy conformed to the diagnostic criteria for GDM formulated by the American Diabetes Association in 2013[8]: 75 g oral glucose tolerance test result showed fasting plasma glucose (FPG) ≥ 5.1 mmol/L, blood glucose 1 h after test ≥ 10.0 mmol/L or blood glucose 2 h after test ≥ 8.5 mmol/L; the GDM group received diet and/or glucose-lowering treatment.
Exclusion criteria: (1) Comorbid hypertension, anemia, polyhydramnios, and other underlying diseases or pregnancy complications; (2) past history of adverse pregnancy outcomes; (3) gestational age at delivery < 28 wk; and (4) presence of heart, brain, lung, liver, and other organ diseases.
The screening process is shown in Figure 1. The study was reviewed and approved by the Taizhou People’s Hospital of Jiangsu Province Institutional Review Board.
Dietary control and/or insulin treatment was carried out in all pregnant women with GDM, which was specified as follows: total daily caloric intake was calculated based on 130 J/kg145 J/kg, and the proportions of carbohydrates, proteins, and fats were 55%–65%, 20%–25%, and 15%–25%, respectively. A routine diet complied with the principle of eating less in more meals and 4–6 meals were consumed each day. Insulin treatment of 0.6 U/kg–0.8 U/kg was administered every day, blood glucose level was closely monitored, and insulin dose was promptly adjusted in pregnant women with GDM with abnormal blood glucose after comprehensive dietary intervention. Follow-up was carried out every 2 wk in the form of hospital visits. Glycemic control criteria[9] were FPG ≤ 5.6 mmol/L, 2 h postprandial blood glucose (P2h-PG) ≤ 6.7 mmol/L, and glycated hemoglobin (HbA1C) < 6%. All three criteria must be met to conform to glycemic control standards. Otherwise, the patient was considered not to conform to glycemic control standards.
Baseline data and blood glucose in pregnant women and immune function, infectionrelated markers, and infection rate in neonates were observed and compared. (1) Blood glucose: This was measured 24 h before delivery. Venous whole blood was collected from pregnant women, and a low-speed centrifuge was used to extract serum samples at 3000 r/min for 10 min. A Mindray glucose assay kit (glucose oxidase assay) was used to measure FPG and P2h-PG in serum samples. Heparin anticoagulant tubes were used to collect venous whole blood from pregnant women. Ion exchange chromatography and gradient elution were used to measure HbA1C after hemolysis of blood samples using hemolysin; (2) immune function: 5 mL of umbilical vein blood was collected from neonates after delivery. Blood samples were mixed with allophycocyanin (APC)/cyanine dye 7 (Cy7) fluorescently labeled mouse anti-human CD3 antibody, phycoerythrin/Cy7 mouse anti-human CD4 antibody, and APC/Cy7 mouse anti-human CD8, and incubated at room temperature for 15 min. BD FACSTM lysis solution (BD Inc., USA) was added and incubated in the dark for 15 min. A BD FACSCanto II flow cytometer (BD Inc., USA) was used to measure the proportions of CD3+ T cells, CD4+T cells, and CD8+ T cells with different fluorescent labels in peripheral blood. The CD4/CD8 ratio was then calculated; (3) the levels of immunoglobulin G (IgG), IgA, and IgM in the peripheral blood of newborns in both groups were measured by immunoturbidimetry; (4) a Roche Cobas 8000 fully automatic biochemical analyzer was used to measure the white blood cell (WBC) count in the umbilical vein blood in neonates; (5) enzyme-linked immunosorbent assay (ELISA) was used to measure procalcitonin (PCT) and C-reactive protein (CRP) levels. ELISA kits were purchased from Beyotime Biotechnology Co., Ltd; and (6) neonatal infections were observed and recorded, including upper respiratory tract infection, lower respiratory tract infection, skin infection, intestinal infection, and sepsis.
Statistical data were processed using SPSS 20.0 software, and quantitative data were tested for normal distribution. Quantitative data with normal distribution were expressed as mean ± SD. An independent sample t-test was used for inter-group comparisons. Quantitative data with abnormal distribution are represented by median (quartile), and a non-parametric test was used for inter-group comparisons. Qualitative data were expressed as % and the χ2 test was used. A P value < 0.05 was considered statistically significant.
In the GDM group, the age of pregnant women ranged from 21–39 years and the mean age was 29.98 ± 4.65 years; body mass index (BMI) was 19.7–38.1 kg/m2 and mean BMI was (29.75 ± 2.68) kg/m2. There were 143 primipara and 93 multipara women. In the control group, the age ranged from 22–40 years and the mean age was 30.26 ± 4.74 years; BMI was 20.3–37.9 kg/m2 and mean BMI was 30.01 ± 3.12 kg/m2; there were 159 primipara and 181 multipara women. There were no differences in age, BMI, parity, and gravidity between the two groups and the groups were comparable (P > 0.05). as shown in Table 1.
| Groups | Cases | Age (yr) | BMI (kg/m2) | Type of pregnant woman, n (%) | |
| Primipara | Multipara | ||||
| CGC group | 178 | 30.05 ± 4.46 | 29.55 ± 2.82 | 109 (61.24) | 69 (38.76) |
| NCGC group | 58 | 29.47 ± 3.75 | 30.08 ± 2.57 | 34 (56.90) | 25 (43.10) |
| t/χ2 value | 0.893 | 1.270 | 0.344 | ||
| P value | 0.373 | 0.206 | 0.558 | ||
The incidence of premature births, fetal macrosomia, hypoglycemia, hypocalcemia, and hyperbilirubinemia in neonates delivered by pregnant women with GDM was significantly higher than that in neonates delivered by women in the control group. These differences were statistically significant (P < 0.05) as shown in Table 2.
| Complications | GDM group(n=236) | Control group(n=240) | χ2 value | P value |
| Premature birth | 45 (19.07) | 12 (5.00) | 22.341 | < 0.001 |
| Fetal macrosomia | 57 (24.15) | 23 (9.58) | 18.064 | < 0.001 |
| Hypoglycemia | 42 (17.80) | 7 (2.92) | 28.53 | < 0.001 |
| Hypocalcemia | 22 (9.32) | 5 (2.08) | 11.653 | < 0.001 |
| Hyperbilirubinemia | 29 (12.29) | 13 (5.42) | 6.984 | 0.008 |
| Polycythemia | 38 (16.10) | 22 (9.17) | 5.195 | 0.023 |
| Hyaline membrane disease | 13 (5.51) | 2 (0.83) | 8.522 | 0.004 |
| Fetal distress | 34 (14.41) | 6 (2.50) | 21.917 | < 0.001 |
| Congenital malformation | 11 (4.66) | 1 (0.42) | 8.723 | 0.003 |
| Neonatal asphyxia | 21 (8.90) | 6 (2.50) | 9.104 | 0.003 |
| Neonatal infection | 35 (14.83) | 11 (4.58) | 14.312 | < 0.001 |
Pregnant women with GDM were divided into the CGC group and the NCGC group based on whether their glycemic control conformed to standards. Blood glucose markers in the NCGC group, such as FPG, P2h-PG, and HbA1C levels, were significantly higher (P < 0.05) compared with pregnant women with GDM in the CGC group. These results are shown in Table 3.
| Groups | Cases | FPG (mmol/L) | P2h-PG (mmol/L) | HbA1C (%) |
| CGC group | 178 | 4.68 ± 0.60 | 5.51 ± 0.85 | 5.11 ± 0.45 |
| NCGC group | 58 | 5.96 ± 0.68 | 7.14 ± 1.04 | 6.38 ± 0.74 |
| t/χ2 value | 13.645 | 11.979 | 15.691 | |
| P value | < 0.001 | < 0.001 | < 0.001 |
Results of the flow cytometry analysis of peripheral blood T cell subsets were compared between the two groups of neonates. The ratio of peripheral blood CD3+T cells, CD4+T cells, and CD8+T cells, and the CD4/CD8 ratio in neonates in the NCGC group were all significantly lower than those in the CGC group (P < 0.05). These results showed that immune function was significantly decreased in neonates from the NCGC group. These results are shown in Table 4.
| Groups | Cases | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4/CD8 |
| CGC group | 178 | 52.01 ± 10.78 | 39.21 ± 7.80 | 25.69 ± 5.47 | 1.61 ± 0.54 |
| NCGC group | 58 | 45.25 ± 7.33 | 22.46 ± 5.48 | 19.42 ± 2.95 | 1.17 ± 0.33 |
| t value | 4.449 | 15.170 | 8.335 | 5.854 | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
The levels of IgG, IgM, and IgA, were compared between neonates in the two groups. The results showed that the level of IgG in the CGC group was significantly higher than that in the NCGC group, with a statistically significant difference (P < 0.05). There were no significant differences in the levels of IgM and IgA in peripheral blood between the two groups of newborns (P > 0.05), as shown in Table 5.
| Groups | Cases | IgG (g/L) | IgM (g/L) | IgA (g/L) |
| CGC group | 178 | 9.78 ± 1.38 | 0.181 ± 0.043 | 0.31 ± 0.08 |
| NCGC group | 58 | 7.21 ± 1.32 | 0.173 ± 0.040 | 0.29 ± 0.07 |
| t value | 12.447 | 1.251 | 1.688 | |
| P value | < 0.001 | 0.212 | 0.093 |
Infection-related inflammatory markers, including WBC, serum PCT and CRP levels, were compared between neonates in the two groups. WBC, PCT, and CRP levels in neonates in the NCGC group were significantly higher than those in the CGC group, and these differences were statistically significant (P < 0.05), as shown in Table 6.
| Groups | Cases | WBC (×109/L) | PCT (μg/L) | CRP (mg/L) |
| CGC group | 178 | 15.56 ± 5.47 | 0.43 ± 0.12 | 7.22 ± 2.07 |
| NCGC group | 58 | 25.80 ± 8.61 | 0.81 ± 0.24 | 12.38 ± 3.22 |
| t value | 10.618 | 15.920 | 14.212 | |
| P value | < 0.001 | < 0.001 | < 0.001 |
The incidence of upper respiratory tract infection, lower respiratory tract infection, and skin infection in neonates in the NCGC group was 31.03%, which was significantly higher than neonates in the CGC group (9.35%). These differences were statistically significant (P < 0.05) and are shown in Table 7.
| Groups | Cases | Upper respiratory tract infection | Lower respiratory tract infection | Skin infection | Intestinal infection | Sepsis | Total |
| CGC group | 178 | 10 (5.62) | 4 (2.25) | 1 (0.56) | 2 (1.12) | 0 (0.00) | 17 (9.55) |
| NCGC group | 58 | 7 (12.07) | 3 (5.17) | 2 (3.45) | 5 (8.62) | 1 (1.72) | 18 (31.03) |
| χ2 value | 15.985 | ||||||
| P value | < 0.001 |
Glucose metabolism disorders and long-term blood glucose abnormalities in pregnant women with GDM are associated with decreased pancreatic islet function and insulin resistance[10], and GDM has a major impact on adverse maternal and child outcomes in the perinatal phase compared to the normal diabetic population. Currently, there are 210 million neonates affected by GDM globally, as shown in the 2017 International Diabetes Federation report[11]. Furthermore, GDM has become a major global public health concern. The results of this study show that the incidence of premature births, fetal macrosomia, hypoglycemia, hypocalcemia, hyperbilirubinemia, and infection in neonates delivered by pregnant women with GDM was significantly higher than in neonates delivered by healthy pregnant women. These results demonstrate that the health of neonates is severely affected by blood glucose abnormalities in the mother during pregnancy. The main harm caused by GDM is an increase in maternal and child adverse outcomes and mortality rate during the perinatal phase, resulting in fetal distress, developmental abnormalities, and increases the risk of hypoglycemia, deformities, and infection in neonates[12]. Therefore, it can be seen from these results that stringent glycemic control in pregnant women with GDM is an essential measure to prevent neonatal complications in the perinatal stage. Capobianco et al[13] also reported the GDM is an important complication that affects maternal and pregnancy outcomes, and good glycemic control can decrease the risk of pregnancy complications and the cesarean section rate, increase the rate of natural vaginal delivery, and has positive effects in decreasing premature births, fetal macrosomia, hypoglycemia, asphyxiation, and infection.
A recent study[14] found that interleukin (IL)-6 and tumor necrosis factor (TNF)-α levels were significantly increased in the umbilical vein blood from neonates delivered by women with GDM. The neonates also showed varying degrees of immune dysfunction, suggesting that the risk of infection is higher in neonates of GDM patients. Our study results showed that compared with healthy pregnant women, the incidence of neonatal infection was significantly higher in pregnant women with GDM. Blood glucose markers and neonatal infection rates were significantly increased in pregnant women with GDM in the NCGC group compared to those in the CGC group. This suggested that glycemic control has significant effects in decreasing neonatal infection caused by blood glucose abnormalities in GDM patients. Zarrin et al[15] found that the condition of GDM patients worsened as gestational age increased, and glucose metabolism abnormalities during pregnancy may directly affect maternal and fetal immune function. Maternal immune defects and fetal T lymphocyte developmental abnormalities will affect neonatal immune function. Neonatal immune dysfunction is an independent risk factor for infection[16]. T lymphocytes are the most important cell population in the immune system, of which the CD3+ subset represents mature T lymphocytes and immune function[17]. CD4+ T cells mainly regulate humoral immunity while CD8+ T cells are inhibitory/cytotoxic T lymphocytes that mainly regulate cellular immunity and play a critical role in regulating CD3+ and CD4+ functions[18,19]. The CD4+/CD8+ ratio can normally be used to reflect the equilibrium between humoral/cellular immunity. This cell ratio is an important marker for evaluating immune function and a low CD4+/CD8+ ratio usually means that the body is in an immunosuppressed state[20]. Kugler et al[21] confirmed that neonatal immune function defects were related to the inheritance of abnormal T lymphocyte development and maternal immune function defects, and were independent risk factors for neonatal infection. We compared the differences in peripheral blood T cell subsets in this study between neonates in the two groups. The results showed that peripheral blood CD3+T cells, CD4+T cells, and CD8+T cells, and the CD4/CD8 ratio of neonates in the NCGC group were all significantly lower than those in the CGC group. These results demonstrated that blood glucose abnormalities in pregnant women with GDM may affect peripheral blood T cell subsets in neonates, resulting in decreased immune function and immune regulation disorders, thus reducing infection resistance.
Immunoglobulins are an important class of immune effector molecules, including IgG, IgA, IgM, and so on. IgG in the peripheral blood of newborns is mainly from the mother, accounting for about 75% of the total serum immunoglobulin content, and plays an important role in preventing infection[22]. This study found that IgG in neonates from women with GDM in the NCGC group significantly decreased compared to that in the CGC group, suggesting that poor blood glycemic control in GDM pregnant women can lead to a decline in neonatal immune function. It is speculated that for patients with GDM, abnormal glucose metabolism itself is an inflammatory reaction, which hinders the production of IgG, thereby reducing the amount of IgG entering the fetus via the placenta[23]. However, IgA and IgM cannot pass through the placental barrier, resulting in extremely low levels in the peripheral blood of newborns, leading to insignificant changes in levels.
WBC are the most commonly used marker for early diagnosis and treatment of neonatal infections, and PCT and CRP are important serum markers for diagnosing neonatal infection[24]. This study compared these markers in pregnant women with GDM and neonates from the two groups. The results revealed that WBC, serum PCT and CRP levels in neonates in the NCGC group were significantly greater than those in the CGC group, suggesting that GDM patients who did not meet glycemic control standards have neonates with elevated inflammatory markers and an increased risk of infection. This may be because blood glucose abnormalities during pregnancy can promote the transcription of placental CRP, IL-6, and PCT, which are important mechanisms that directly affect the fetal immune system[25]. Li et al[26] found that a hyperglycemic environment activated placental HIF-1a and TLR4/MyD88/NF-kB pathways in pregnant women with GDM, induced IL-6 and IL-8 secretions, promoted placental inflammation and autophagy to disrupt placental homeostasis and cell renewal, and increased the risk of infection in neonates delivered by pregnant women with GDM. From these findings combined with the results from the present study, we believe that poor glycemic control in pregnant women with GDM can result in long-term blood glucose abnormalities, which may stimulate inflammatory responses in the fetal placenta, thereby affecting the fetal immune system and ultimately increase the risk of neonatal infections.
The innovation of this study is that the relationship between the blood glucose control level in pregnant women with GDM and neonatal immune function was analyzed, which opens up a new direction for predicting neonatal infectious pathology. However, a larger multicenter clinical study is needed in the future to validate the results of this study as this was a single center study with a limited sample size due to the strict screening conditions. In addition, immunoglobulin, as an antibody related to the immune response in vivo, can be used as an early diagnostic indicator of infection, and IgG antibodies can also enhance the anti-infection ability of newborns and prevent related infectious diseases. However, this study lacks the assessment of neonatal peripheral blood immunoglobulin level to verify neonatal immune function. Finally, the neonates included in this study were only followed up for a short time. The influence of blood glucose control level in GDM patients on the long-term immune function of neonates still requires further research.
In summary, we speculate that glucose metabolism disorders and long-term blood glucose abnormalities in GDM patients with poor glycemic control may be considered a type of inflammatory response which affects the T lymphocyte subsets in neonates, resulting in immune dysfunction, and ultimately decreasing immune function and increasing the risk of infection.
Gestational diabetes mellitus (GDM) is related to obesity in pregnant women, older age in pregnant women, excessive nutrition during pregnancy, lack of exercise, genetic history of familial type 2 diabetes, excessive sugar consumption and other factors. GDM often causes obstetric complications, which seriously threaten the life and health of pregnant women and newborns. Blood sugar control measures have a considerable impact on pregnancy outcome and newborn status in patients with GDM.
The long-term abnormal glucose metabolism in GDM pregnant women affects the immune function of newborns, and it is unclear whether poor glucose control in GDM pregnant women increases the risk of neonatal infectious diseases.
The purpose of this study was to determine the correlation between GDM pregnant women and neonatal complications, and to analyze the impact of blood glucose control on the risk of neonatal infectious diseases.
The clinical data of 236 pregnant women with GDM and 240 healthy pregnant women and newborns were retrospectively analyzed to compare early neonatal complications in the two groups of pregnant women. The 236 pregnant women with GDM were divided into two groups based on whether their blood sugar control reached the standard. The baseline data, neonatal immune function, infection related indicators, and neonatal infection rate in the two groups of pregnant women with GDM were compared.
The incidence of neonatal complications in GDM pregnant women was significantly higher than that in normal pregnant women. Compared with GDM pregnant women who achieved glycemic control, the proportion of CD3+, CD4+, and CD8+T cells in peripheral blood and the ratio of CD4/CD8 cells in newborns from mothers who did not achieve glycemic control significantly decreased, while the white blood cell count, serum procalcitonin, and C-reactive protein levels significantly increased, and the neonatal infection rate significantly increased.
The risk of neonatal complications is increased in pregnant women with GDM, and poor glycemic control leads to impairment of the fetal immune system and ultimately increases the risk of neonatal infections.
The effect of blood glucose control is related to pregnancy outcome and neonatal prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ibenthal A, Germany; Pagotto U, Italy; Horowitz M, Australia S-Editor: Wang JL L-Editor: A P-Editor: Yu HG
| 1. | Schaefer-Graf UM, Pawliczak J, Passow D, Hartmann R, Rossi R, Bührer C, Harder T, Plagemann A, Vetter K, Kordonouri O. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005;28:1745-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep. 2016;16:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 865] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 3. | Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2150] [Cited by in RCA: 2121] [Article Influence: 106.1] [Reference Citation Analysis (0)] |
| 4. | Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, Duncan BB, Schmidt MI. Gestational diabetes and pregnancy outcomes--a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Miremberg H, Ben-Ari T, Betzer T, Raphaeli H, Gasnier R, Barda G, Bar J, Weiner E. The impact of a daily smartphone-based feedback system among women with gestational diabetes on compliance, glycemic control, satisfaction, and pregnancy outcome: a randomized controlled trial. Am J Obstet Gynecol. 2018;218:453.e1-453.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Buhary BM, Almohareb O, Aljohani N, Alzahrani SH, Elkaissi S, Sherbeeni S, Almaghamsi A, Almalki M. Glycemic control and pregnancy outcomes in patients with diabetes in pregnancy: A retrospective study. Indian J Endocrinol Metab. 2016;20:481-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Hashimoto K, Koga M. Indicators of glycemic control in patients with gestational diabetes mellitus and pregnant women with diabetes mellitus. World J Diabetes. 2015;6:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (2)] |
| 8. | American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 Suppl 1:S11-S66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2371] [Cited by in RCA: 2481] [Article Influence: 206.8] [Reference Citation Analysis (0)] |
| 9. | Miazgowski T, Bikowska M, Ogonowski J, Taszarek A. The Impact of Health Locus of Control and Anxiety on Self-Monitored Blood Glucose Concentration in Women with Gestational Diabetes Mellitus. J Womens Health (Larchmt). 2018;27:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 985] [Article Influence: 164.2] [Reference Citation Analysis (1)] |
| 11. | Carracher AM, Marathe PH, Close KL. International Diabetes Federation 2017. J Diabetes. 2018;10:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (2)] |
| 12. | Egan AM, Bogdanet D, Griffin TP, Kgosidialwa O, Cervar-Zivkovic M, Dempsey E, Allotey J, Alvarado F, Clarson C, Cooray SD, de Valk HW, Galjaard S, Loeken MR, Maresh MJA, Napoli A, O'Shea PM, Wender-Ozegowska E, van Poppel MNM, Thangaratinam S, Crowther C, Biesty LM, Devane D, Dunne FP; INSPIRED research group. A core outcome set for studies of gestational diabetes mellitus prevention and treatment. Diabetologia. 2020;63:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Capobianco G, Gulotta A, Tupponi G, Dessole F, Pola M, Virdis G, Petrillo M, Mais V, Olzai G, Antonucci R, Saderi L, Cherchi PL, Dessole S, Sotgiu G. Materno-Fetal and Neonatal Complications of Diabetes in Pregnancy: A Retrospective Study. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 14. | Teng Y, Xuan S, Jiang M, Tian L, Tian J, Chang Q. Expression of H(2)S in Gestational Diabetes Mellitus and Correlation Analysis with Inflammatory Markers IL-6 and TNF-α. J Diabetes Res. 2020;2020:3085840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Zarrin M, Grossen-Rösti L, Bruckmaier RM, Gross JJ. Elevation of blood β-hydroxybutyrate concentration affects glucose metabolism in dairy cows before and after parturition. J Dairy Sci. 2017;100:2323-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Allam G, Alsulaimani AA, Alzaharani AK, Nasr A. Neonatal infections in Saudi Arabia: Association with cytokine gene polymorphisms. Cent Eur J Immunol. 2015;40:68-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Dong D, Zheng L, Lin J, Zhang B, Zhu Y, Li N, Xie S, Wang Y, Gao N, Huang Z. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature. 2019;573:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 18. | Ruterbusch M, Pruner KB, Shehata L, Pepper M. In Vivo CD4(+) T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu Rev Immunol. 2020;38:705-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 345] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 19. | Martin MD, Badovinac VP. Defining Memory CD8 T Cell. Front Immunol. 2018;9:2692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 324] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 20. | Behairy BE, Ehsan N, Anwer M, Allam A, El-Henawy I, Hameed NA, Zakaria HM. Expression of intrahepatic CD3, CD4, and CD8 T cells in biliary atresia. Clin Exp Hepatol. 2018;4:7-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Kugler DG, Flomerfelt FA, Costa DL, Laky K, Kamenyeva O, Mittelstadt PR, Gress RE, Rosshart SP, Rehermann B, Ashwell JD, Sher A, Jankovic D. Systemic toxoplasma infection triggers a long-term defect in the generation and function of naive T lymphocytes. J Exp Med. 2016;213:3041-3056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Gudelj I, Lauc G, Pezer M. Immunoglobulin G glycosylation in aging and diseases. Cell Immunol. 2018;333:65-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 23. | Dall'Olio F, Malagolini N. Immunoglobulin G Glycosylation Changes in Aging and Other Inflammatory Conditions. Exp Suppl. 2021;112:303-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Yang AP, Liu J, Yue LH, Wang HQ, Yang WJ, Yang GH. Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med. 2016;54:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Wojcik M, Zieleniak A, Mac-Marcjanek K, Wozniak LA, Cypryk K. The elevated gene expression level of the A(2B) adenosine receptor is associated with hyperglycemia in women with gestational diabetes mellitus. Diabetes Metab Res Rev. 2014;30:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Li YX, Long DL, Liu J, Qiu D, Wang J, Cheng X, Yang X, Li RM, Wang G. Gestational diabetes mellitus in women increased the risk of neonatal infection via inflammation and autophagy in the placenta. Medicine (Baltimore). 2020;99:e22152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |