Published online Sep 15, 2023. doi: 10.4239/wjd.v14.i9.1385
Peer-review started: May 4, 2023
First decision: May 15, 2023
Revised: May 25, 2023
Accepted: August 7, 2023
Article in press: August 7, 2023
Published online: September 15, 2023
Processing time: 132 Days and 3.8 Hours
Diabetic nephropathy (DN) is frequently seen in the development of diabetes mellitus, and its pathogenic factors are complicated. Its current treatment is controversial, and there is a lack of a relevant efficacy prediction model.
To determine the effects of paricalcitol combined with hemodiafiltration on bone-metabolism-related indexes in patients with DN and chronic renal failure (CRF), and to construct an efficacy prediction model.
We retrospectively analyzed 422 patients with DN and CRF treated in Cangzhou Central Hospital between May 2020 and May 2022. We selected 94 patients who met the inclusion and exclusion criteria. Patients were assigned to a dialysis group (n = 45) and a joint group (n = 49) in relation to therapeutic regimen. The clinical efficacy of the two groups was compared after treatment. The changes in laboratory indexes after treatment were evaluated, and the two groups were compared for the incidence of adverse reactions. The predictive value of laboratory indexes on the clinical efficacy on patients was analyzed.
The dialysis group showed a notably worse improvement in clinical efficacy than the joint group (P = 0.017). After treatment, the joint group showed notably lower serum levels of serum creatinine, uric acid (UA) and blood urea nitrogen (BUN) than the dialysis group (P < 0.05). After treatment, the joint group had lower serum levels of phosphorus, procollagen type I amino-terminal propeptide (PINP) and intact parathyroid hormone than the dialysis group, but a higher calcium level (P < 0.001). Both groups had a similar incidence of adverse reactions (P > 0.05). According to least absolute shrinkage and selection operator regression analysis, UA, BUN, phosphorus and PINP were related to treatment efficacy. According to further comparison, the non-improvement group had higher risk scores than the improvement group (P < 0.0001), and the area under the curve of the risk score in efficacy prediction was 0.945.
For treatment of CRF and DN, combined paricalcitol and hemodiafiltration can deliver higher clinical efficacy and improve the bone metabolism of patients, with good safety.
Core Tip: This study confirmed that paricalcitol combined with hemodiafiltration can effectively improve the condition of patients with diabetic nephropathy (DN) and chronic renal failure (CRF) and alleviate calcium–phosphorus metabolism disorder. This study has also successfully constructed a predictive model. It provides a new reference for evaluating the efficacy of treatment of combined DN and CRF.
- Citation: Ma XY, Sheng YP, Yang XM, Zhang HR, Sun FY. Effects of paricalcitol combined with hemodiafiltration on bone-metabolism-related indexes in patients with diabetic nephropathy and chronic renal failure. World J Diabetes 2023; 14(9): 1385-1392
- URL: https://www.wjgnet.com/1948-9358/full/v14/i9/1385.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i9.1385
Improvement of socioeconomic and living standards has resulted in an increase in the global prevalence of diabetes mellitus (DM), bringing an increasing incidence of diabetic nephropathy (DN)[1]. DN is one of the most frequent causes of chronic renal failure (CRF) worldwide, and 30%–40% of DM patients develop DN[2]. The pathogenesis of DN is complex, and is bound up with abnormal balance in the body, including hemodynamics, metabolic disorder, inflammation and fibrosis[3]. The imbalance between renal injury and renal protection factors is the primary cause of disease progression[4]. DN has a heavy burden on the global economy and a high mortality risk in DM patients.
DN is a most common complication in DM patients and a common cause of CRF[5]. Approximately 30%-40% of the patients with DM will develop DN, leading to CRF[6,7]. When DN progresses to the advanced stage, the patients may need dialysis or kidney transplantation to help maintain renal function and quality of life[8]. Hemodiafiltration is an effective method of treatment for CRF, which can prolong the survival of patients[9]. However, patients receiving hemodialysis often have abnormal serum calcium and phosphorus, and adverse reactions such as joint calcification, bone pain, arrhythmia, pruritus and platelet insufficiency[10].
Paricalcitol is a vitamin D analog that is used to treat secondary hyperparicalcitolism triggered by renal insufficiency[11]. Paricalcitol can promote intestinal absorption of calcium and inhibit secretion and differentiation of Paricalcitol cells by binding to vitamin D receptor, thus reducing the synthesis and secretion of Paricalcitol hormone (PTH)[12]. Paricalcitol can also alleviate kidney damage triggered by inflammation and apoptosis, thus helping to protect kidney function[13].
However, there is controversy about the impact of paricalcitol combined with hemodiafiltration on serum calcium, phosphorus and intact PTH (iPTH) in patients with both DN and CRF. Accordingly, this study aimed to provide a basis for clinical treatment of comorbid DN and CRF.
We retrospectively analyzed 422 patients with both DN and CRF treated in Cangzhou Central Hospital between May 2020 and May 2022. The patients were screened according to the following criteria. Inclusion criteria: patients who met the diagnostic criteria of DN[14] and were not allergic to the drugs used in this study. Exclusion criteria: patients with primary nephropathy or secondary DM; patients comorbid severe dysfunction of the heart, liver or brain; patients with proteinuria due to other reasons; and patients who had received angiotensin-converting enzyme inhibitors or diuretics before admission. According to the above standards, 94 patients with DN and CRF were selected. The patients were assigned to a dialysis group (n = 45) and a joint group (n = 49) according to therapeutic regimen. This study was carried out with approval of the Medical Ethics Committee of Cangzhou Central Hospital.
Both groups received symptomatic treatment, including correction of fluid and electrolyte imbalance, anti-allergy, anti-hemolysis and correction of acid poisoning. The dialysis group was treated by hemodiafiltration through an Fx80 dialyzer (Fresenius, Germany) , with blood flow of 200–300 mL/min, and dialysis time of 4 h (3 times/wk). Twelve sessions were taken as a course of dialysis treatment, and two courses of treatment were conducted. The joint group was treated with paricalcitol while being treated with hemodiafiltration. Paricalcitol was injected at a dose of 0.04–0.1 μg/kg (2.8–7.0 μg) each time, and the dose was adjusted according to the serum iPTH level. The frequency of administration was no more than once every other day, and it was administered for eight continuous weeks. During treatment, serum calcium, phosphorus and iPTH were detected every 2 wk, and the dose was adjusted according to the test results.
The clinical data were collected through the medical record system of our hospital and included: age, gender, body mass index, course of renal decompensation, history of hypertension, history of hyperlipidemia, and history of smoking and alcoholism. Laboratory indexes included renal-function-related indexes [serum creatinine (SCr), uric acid (UA) and urea nitrogen (BUN)], and bone-metabolism-related indexes [serum phosphorus, serum calcium, procollagen type I N-terminal propeptide (PINP) and iiPTH]. Improvement of clinical efficacy in patients after treatment and the incidence of adverse reactions during treatment were evaluated.
Markedly effective: After treatment, the related symptoms of the patients subsided, and the levels of renal-function-related indexes such as SCr, UA and BUN decreased by over 30%. Effective: After treatment, the related symptoms were relieved, and the levels of SCr, UA and BUN decreased by 5%–30%. Ineffective: The related symptoms and condition of the patients were not notably alleviated, and there was even some aggravation of disease, with the indexes such as SCr, UA and BUN decreased by < 5%, or even increased. Total effective rate = (number of cases with markedly effectively treatment + number of cases with effective treatment]/total number of cases × 100%.
Primary outcome measures: The clinical efficacy on the two groups was compared. The changes in laboratory indexes in patients after treatment were evaluated. Secondary outcome measures: The clinical data of the two groups were compared. The adverse reactions of the two groups were also compared. The predictive value of laboratory indexes on the clinical efficacy on patients was analyzed.
This study used R language 4.1.1 software (R Foundation for Statistical Computing, Vienna, Austria) for data reduction and data analysis, and established a model. The predictors of non-zero coefficient were screened by least absolute shrinkage and selection operator (LASSO) regression, and the nomogram was drawn by R (R3.5.3) software package and rms package. The consistency index (C-index) was calculated by rms package, and its clinical value was verified by the receiver operating characteristic curve. We used Graph Pad Prism 8.0 (La Jolla, CA, USA) for visualization of data. P < 0.05 implies a notable difference.
According to inter-group comparison of clinical data, the dialysis and joint groups were similar (P > 0.05, Table 1).
| Factors | Dialysis group (n = 45) | Joint group (n = 49) | χ2 value | P value |
| Age (yr) | 2.042 | 0.153 | ||
| > 60 | 25 | 20 | ||
| ≤ 60 | 20 | 29 | ||
| Gender | 0.641 | 0.423 | ||
| Male | 22 | 28 | ||
| Female | 23 | 21 | ||
| BMI (kg/m2) | 0.300 | 0.583 | ||
| > 25 | 15 | 19 | ||
| ≤ 25 | 30 | 30 | ||
| Course of renal decompensation (yr) | 0.526 | 0.468 | ||
| > 2 | 29 | 35 | ||
| ≤ 2 | 16 | 14 | ||
| History of hypertension | 0.512 | 0.474 | ||
| Yes | 12 | 10 | ||
| No | 33 | 39 | ||
| History of hyperlipidemia | 0.566 | 0.451 | ||
| Yes | 8 | 6 | ||
| No | 37 | 43 | ||
| History of smoking | 0.641 | 0.423 | ||
| Yes | 22 | 28 | ||
| No | 23 | 21 | ||
| History of alcoholism | 0.222 | 0.637 | ||
| Yes | 6 | 5 | ||
| No | 39 | 44 |
The dialysis group showed a significantly lower improvement in clinical efficacy than the joint group (P = 0.017, Table 2).
| Group | Markedly effective | Effective | Ineffective | Total effective rate |
| Dialysis group (n = 45) | 25 (55.56) | 8 (17.78) | 12 (26.66) | 33 (73.34) |
| Joint group (n = 49) | 33 (67.35) | 12 (24.48) | 4 (8.16) | 45 (91.84) |
| χ2 value | 5.686 | |||
| P value | 0.017 |
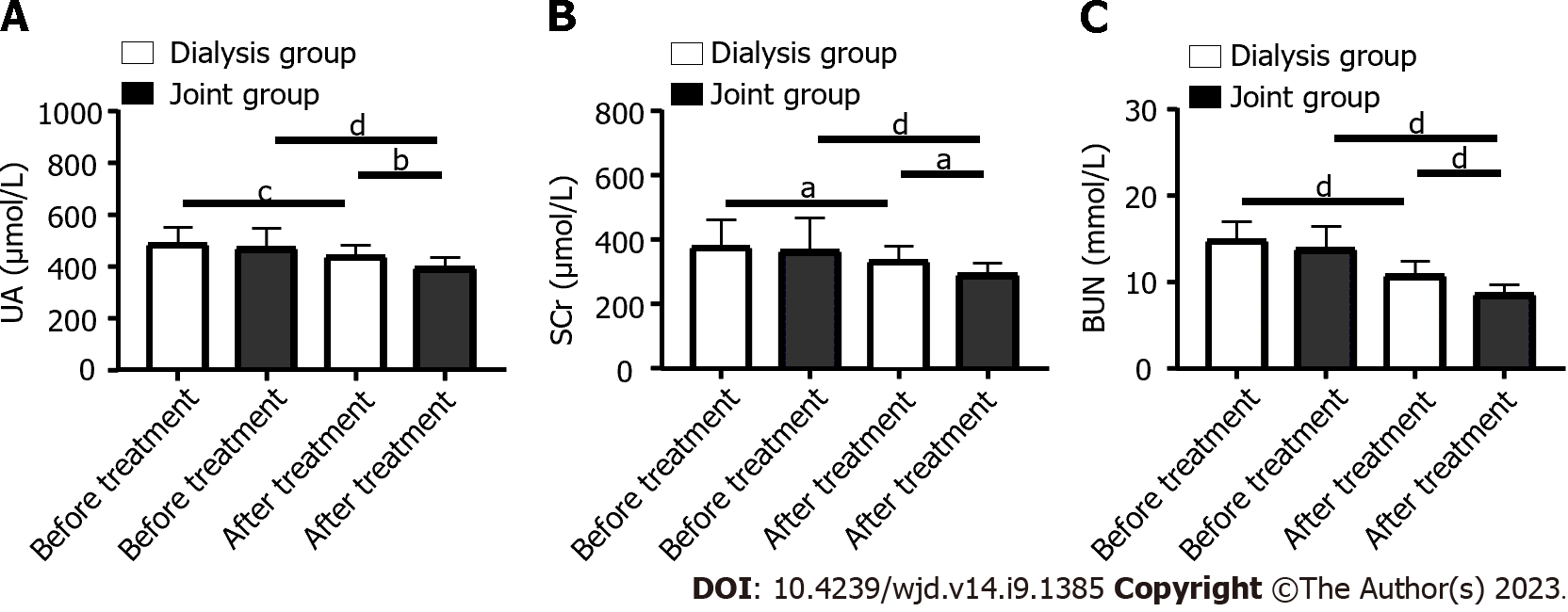
Before treatment, SCr, UA and BUN levels were similar in the dialysis and joint groups (P > 0.05). After treatment, SCr, UA and BUN levels in both groups decreased significantly (P < 0.05), with greater decreases in the joint group than in the dialysis group (P < 0.05, Figure 1).
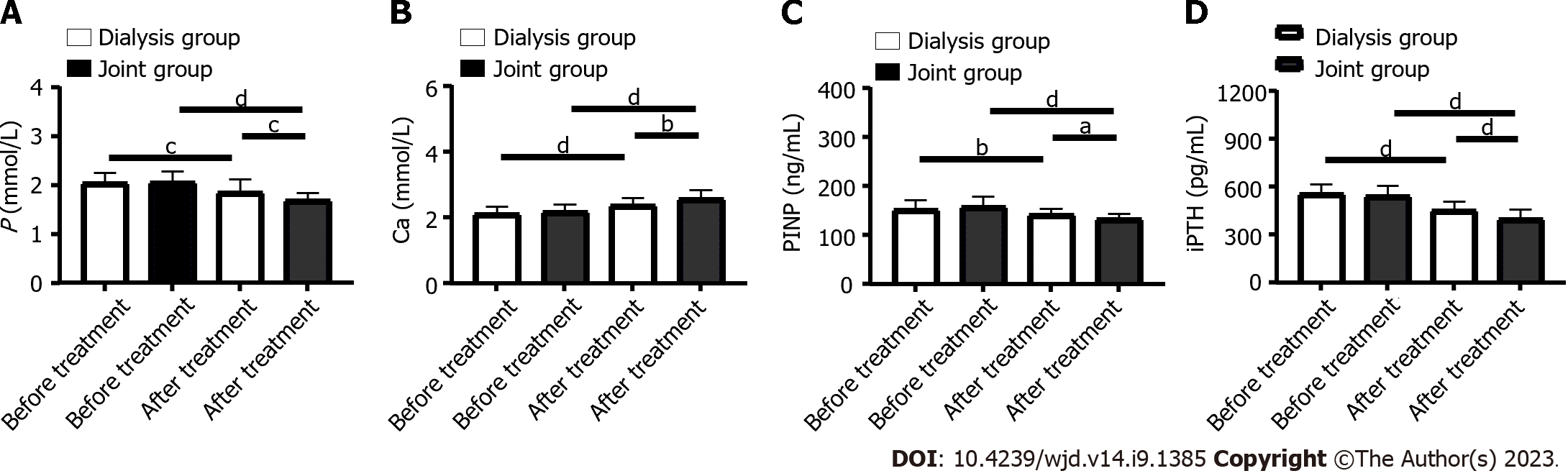
Before treatment, the levels of phosphorus, calcium, PINP and iPTH did not differ significantly between the dialysis and joint groups (P > 0.05). After treatment, phosphorus, PINP and iPTH in both groups decreased significantly (P < 0.0001), while calcium increased significantly. After treatment, the joint group had significantly lower serum levels of phosphorus, PINP and iPTH than the dialysis group, and a significantly higher Ca level (P < 0.001, Figure 2).
The incidence of adverse reactions was similar in the joint and dialysis groups (P > 0.05, Table 3).
| Group | Nausea and vomiting | Loss of appetite | Phlebitis | Gastrointestinal reaction | Rash | Total incidence rate |
| Dialysis group (n = 45) | 2 (4.44) | 2 (4.44) | 1 (2.22) | 2 (4.44) | 1 (2.22) | 8 (17.78) |
| Joint group (n = 49) | 1 (2.04) | 1 (2.04) | 2 (4.08) | 1 (2.04) | 1 (2.04) | 6 (12.24) |
| χ2 value | 0.566 | |||||
| P value | 0.451 |
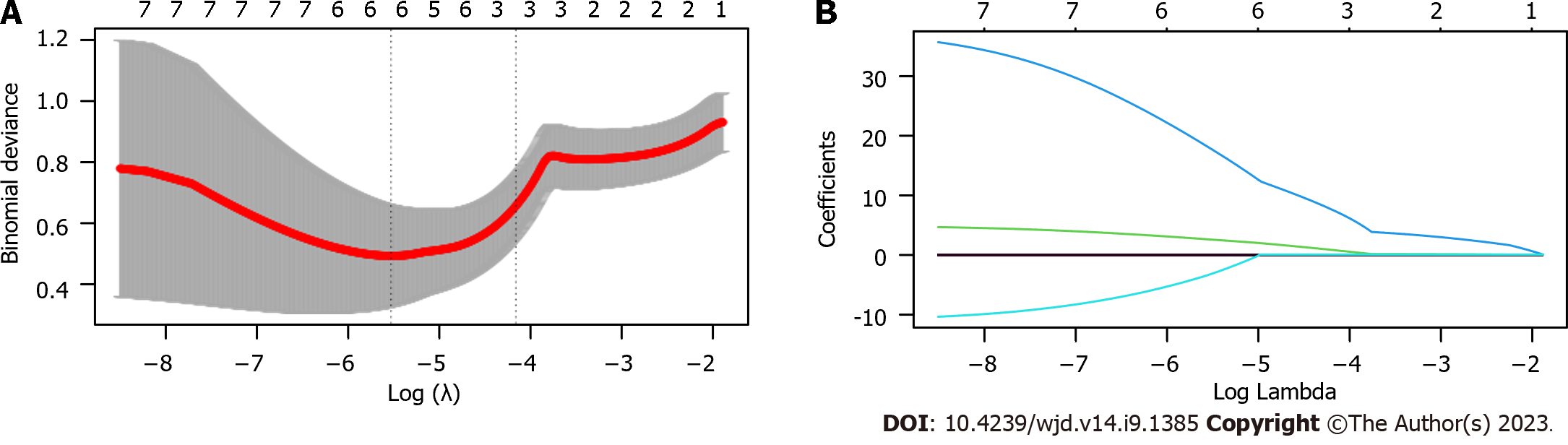
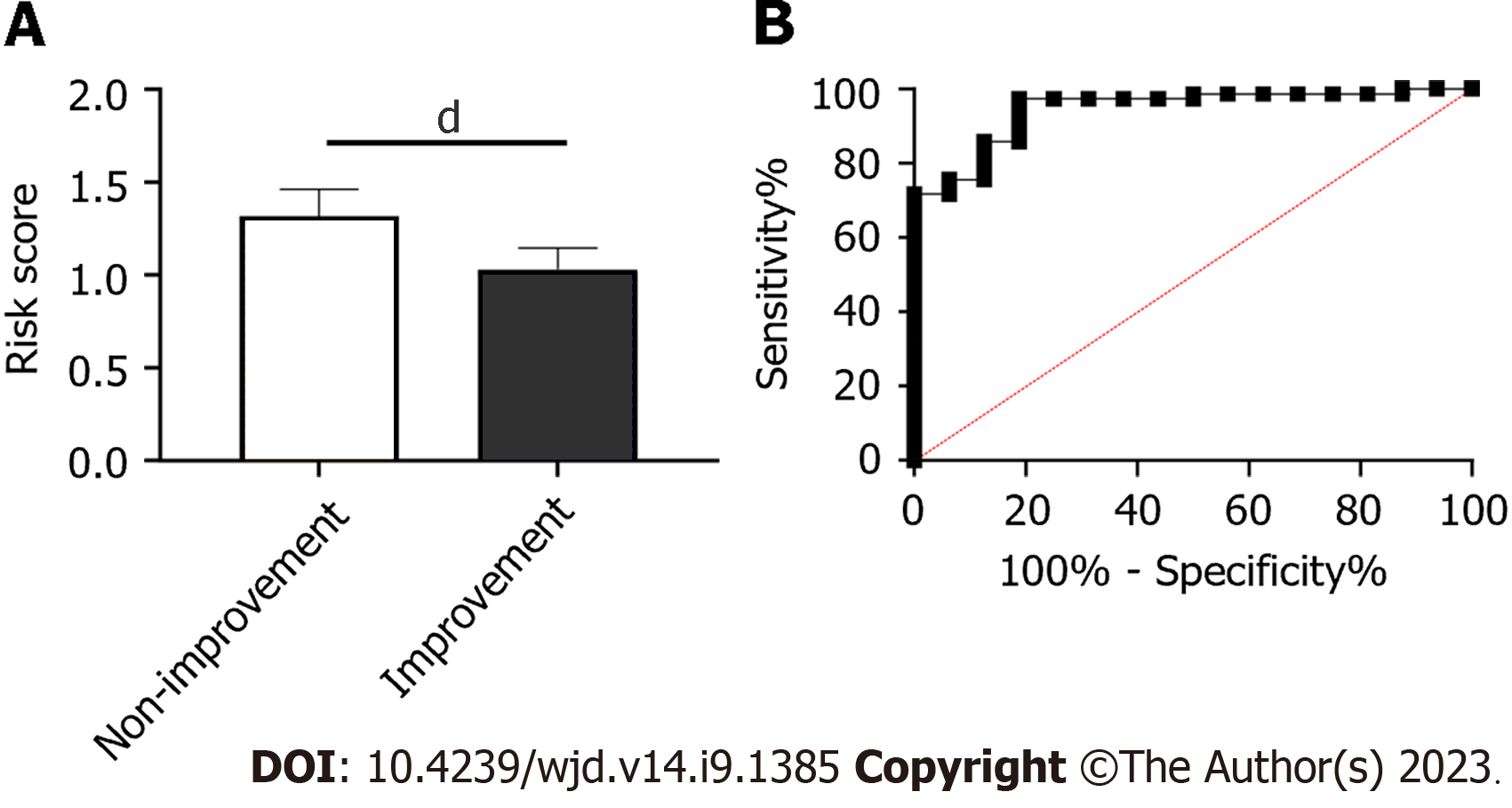
The value of laboratory indexes for predicting treatment efficacy was determined using LASSO regression. Patients with markedly effective treatment and those with effective treatment were assigned to an improvement group (n = 78), while patients with ineffective treatment were assigned to a non-improvement group (n = 16). Through LASSO regression analysis, UA, BUN, phosphorus and PINP were related to treatment efficacy (Figure 3). A predictive equation was constructed according to the risk coefficient: = -0.0056463379 × UA + 0.1334320595 × BUN + 0.9356101817 × P + -0.0002297355 × PINP. The non-improvement group had higher risk scores than the improvement group (Figure 4A, P < 0.0001), and the area under the curve of risk score in forecasting efficacy was 0.945 (Figure 4B).
DM is characterized by high incidence, low control rate and various complications worldwide[15]. DN is a common complication of DM, especially in patients with a disease course > 10 years[16]. The long-term presence of hyperglycemia triggers activation of the polyol pathway and protein kinase C pathway, resulting in a series of pathophysiological changes, such as oxidative stress, disorder of renal glucose metabolism, inflammatory reaction, abnormal metabolism, and abnormal hemodynamics, and finally inducing CRF[17]. The kidneys of patients with CRF shrink and lose the ability to maintain normal renal function, which results in serious consequences such as imbalance of acid and potassium, retention of metabolites, and fluid and electrolyte imbalance, which increases the risk of death[18].
Hemodiafiltration is a type of renal replacement therapy, which is primarily used for blood purification in patients with renal failure[19]. This method removes metabolites, toxins, and excess liquids and electrolytes from the blood through a series of filtration and dialysis processes. In hemodiafiltration treatment, the patient’s blood is filtered and dialyzed by special filters and dialyzers, and the removed waste and excess liquid are excreted through the urine[20]. Hemodiafiltration can ameliorate the symptoms and quality of life of patients with kidney disease, but it also needs close monitoring and adjustment of treatment regimens to avoid complications[21]. According to prior research[22], patients often have calcium–phosphorus metabolism disorder during dialysis, which compromises its efficacy. Continuous hemodialysis can reduce urinary toxins, but it cannot replace normal renal metabolism and endocrine function. In this study, the clinical efficacy and renal function improvement of the joint group were significantly higher than those in the dialysis group after treatment. The results suggest that paricalcitol combined with hemodiafiltration can deliver significantly greater efficacy in patients with comorbid DN and CRF, and improve renal function.
Due to the loss of renal function, dialysis patients often have abnormal bone metabolism, including hyperphosphatemia, hypocalcemia, and hyperparicalcitolism. These abnormalities increase the risk of osteoporosis and fracture[23]. In hemodialysis therapy, the use of auxiliary drugs can improve this situation and reduce the occurrence of adverse reactions[24]. Therefore, in the bone metabolism management of dialysis patients, it is necessary to comprehensively consider factors including the clinical situation of patients and serum bone-metabolism-related indexes. In this study, the joint group showed significantly lower levels of phosphorus, PINP and iPTH than the dialysis group, and a significantly higher calcium level. The two groups showed no significant difference in the incidence of adverse reactions. The results indicate that paricalcitol combined with hemodiafiltration can control serum phosphorus and calcium levels, lower iPTH level, and improve bone-metabolism-related indexes, without increasing safety risks.
Predicting the clinical efficacy on patients is important in the treatment process[25]. For example, predictive models can help healthcare providers make more informed decisions and recommend specific treatments for individual patients, which can bring better results and more personalized care[26]. Predictive models can also help providers identify high-risk patients in certain situations, so that they can carry out early intervention to prevent or mitigate the development of these situations. In this study, we established a risk model to predict the efficacy based on laboratory indexes. In this study, UA, BUN, phosphorus and PINP were related to the efficacy in patients. The risk score of each patient was calculated. The non-improvement group had significantly higher risk scores than the improvement group. According to receiver operating characteristic curve-based analysis, the area under the curve of risk score for predicting efficacy was 0.945.
Our study confirmed that paricalcitol combined with hemodiafiltration improved the condition of patients with comorbid DN and CRF and corrected calcium–phosphorus metabolism disorder. We also successfully constructed a predictive model. However, the study still had some limitations. First, we did not collect data about long-term prognosis, and we only evaluated short-term efficacy, so whether there is any influence on long-term efficacy after treatment needs further study. Second, we need to verify whether the predictive model has high generality or not needs verification by more data. Finally, in such a single-center study, the lack of research samples may have led to bias in the analysis. We hope to carry out more studies in the future to improve the research conclusions.
In the treatment of comorbid DN and CRF, the combined use of paricalcitol and hemodiafiltration delivered greater clinical efficacy and improved the bone metabolism of patients, with good safety.
Diabetic nephropathy (DN) is one of the common complications of diabetes, mainly manifested as glomerular damage. As it progresses, DN may lead to chronic renal failure (CRF), which seriously affects quality of life and life expectancy.
Hemodialysis filtration is an effective method for treating CRF, but patients receiving hemodialysis often experience abnormalities in blood calcium and phosphorus. Paroxycarbinol can promote intestinal calcium absorption and inhibit the secretion and differentiation of Paricalcitol cells by binding to vitamin D receptors. However, it is still unclear whether periostenol has an effect on calcium phosphate metabolism disorder in patients with CRF due to DN.
Supplement the blank of paracalcitol combined with hemodiafiltration in the treatment of CRF, and increases the clinical treatment plan for disorder of calcium and phosphate metabolism during hemodialysis and filtration.
We retrospectively analyzed and observed the effect of paricalcitol combined with hemodiafiltration on calcium phosphate metabolism disorder in patients with CRF due to DN. For the first time, a risk model for predicting efficacy was established using a least absolute shrinkage and selection operator (LASSO) regression model.
We found that the combination of paricalcitol and hemodialysis filtration significantly improved the metabolic disorder of calcium phosphate metabolism disorder in patients, and improved efficacy. Using the LASSO model, we established a risk score to predict efficacy, which provides a new reference for clinical treatment and efficacy prediction.
Paricalcitol can improve calcium phosphate metabolism disorder in hemodialysis patients, and the risk model established by LASSO regression model effectively predicts clinical efficacy.
As a retrospective study, we cannot collect more samples and observe the prognosis of patients. We hope to conduct randomized controlled trials in future studies to observe the long-term prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kramer JR, United States; Negera WG, Germany; Horowitz M, Australia S-Editor: Wang JL L-Editor: A P-Editor: Chen YX
| 1. | Qi C, Mao X, Zhang Z, Wu H. Classification and Differential Diagnosis of Diabetic Nephropathy. J Diabetes Res. 2017;2017:8637138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 2. | Li X, Lu L, Hou W, Huang T, Chen X, Qi J, Zhao Y, Zhu M. Epigenetics in the pathogenesis of diabetic nephropathy. Acta Biochim Biophys Sin (Shanghai). 2022;54:163-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021;2021:1497449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 489] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 4. | Khan NU, Lin J, Liu X, Li H, Lu W, Zhong Z, Zhang H, Waqas M, Shen L. Insights into predicting diabetic nephropathy using urinary biomarkers. Biochim Biophys Acta Proteins Proteom. 2020;1868:140475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Iyengar SK, Adler SG. The application of the HapMap to diabetic nephropathy and other causes of chronic renal failure. Semin Nephrol. 2007;27:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Pazmino PA. Current Concepts on Diabetic Nephropathy and 2014 Data on Diabetic Renal Failure in Texas. Tex Med. 2016;112:e1. [PubMed] |
| 7. | Takada T, Masaki T, Hoshiyama A, Toki T, Kamata Y, Shichiri M. Tolvaptan alleviates excessive fluid retention of nephrotic diabetic renal failure unresponsive to furosemide. Nephrology (Carlton). 2018;23:883-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Aniort J, Heng AÉ, Deteix P, Souweine B, Lautrette A. [Epidemiology of acute renal failure]. Nephrol Ther. 2019;15:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Iyengar R, Franzese J, Gianchandani R. Inpatient Glycemic Management in the Setting of Renal Insufficiency/Failure/Dialysis. Curr Diab Rep. 2018;18:75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Kelly DM, Ademi Z, Doehner W, Lip GYH, Mark P, Toyoda K, Wong CX, Sarnak M, Cheung M, Herzog CA, Johansen KL, Reinecke H, Sood MM. Chronic Kidney Disease and Cerebrovascular Disease: Consensus and Guidance From a KDIGO Controversies Conference. Stroke. 2021;52:e328-e346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 11. | Bae E, Kim JH, Jung MH, Jang SJ, Lee TW, Jung S, Lee S, Jang HN, Chang SH, Park DJ. Paricalcitol Attenuates Contrast-Induced Acute Kidney Injury by Regulating Mitophagy and Senescence. Oxid Med Cell Longev. 2020;2020:7627934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Martínez-Arias L, Panizo S, Alonso-Montes C, Martín-Vírgala J, Martín-Carro B, Fernández-Villabrille S, García Gil-Albert C, Palomo-Antequera C, Fernández-Martín JL, Ruiz-Torres MP, Dusso AS, Carrillo-López N, Cannata-Andía JB, Naves-Díaz M. Effects of calcitriol and paricalcitol on renal fibrosis in CKD. Nephrol Dial Transplant. 2021;36:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Lim JH, Yook JM, Oh SH, Jeon SJ, Noh HW, Jung HY, Choi JY, Cho JH, Kim CD, Kim YL, Park SH. Paricalcitol Improves Hypoxia-Induced and TGF-β1-Induced Injury in Kidney Pericytes. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45:3075-3090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 344] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 15. | Thipsawat S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature. Diab Vasc Dis Res. 2021;18:14791641211058856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 16. | Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14:361-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 504] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 17. | Selby NM, Taal MW. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22 Suppl 1:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 18. | Borisov VV, Shilov EM. [Chronic renal failure]. Urologiia. 2017;11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Bouchard J, Macedo E, Soroko S, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL; Program to Improve Care in Acute Renal Disease. Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2010;25:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Filler G, Clark WF, Huang SH. Tandem hemodialysis and plasma exchange. Pediatr Nephrol. 2014;29:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Navarrete JE, Rajabalan A, Cobb J, Lea JP. Proportion of Hemodialysis Treatments with High Ultrafiltration Rate and the Association with Mortality. Kidney360. 2022;3:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 23. | He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, Fu J, Lin X, Zheng G, Yu G, Chen J. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY). 2020;12:8583-8604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 24. | Greenfield EM. Anabolic effects of intermittent PTH on osteoblasts. Curr Mol Pharmacol. 2012;5:127-134. [PubMed] |
| 25. | Kate RJ, Pearce N, Mazumdar D, Nilakantan V. A continual prediction model for inpatient acute kidney injury. Comput Biol Med. 2020;116:103580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Archer L, Snell KIE, Ensor J, Hudda MT, Collins GS, Riley RD. Minimum sample size for external validation of a clinical prediction model with a continuous outcome. Stat Med. 2021;40:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |