Published online Sep 15, 2023. doi: 10.4239/wjd.v14.i9.1349
Peer-review started: March 3, 2023
First decision: April 26, 2023
Revised: May 4, 2023
Accepted: August 7, 2023
Article in press: August 7, 2023
Published online: September 15, 2023
Processing time: 194 Days and 0.5 Hours
Glycation is an important step in aging and oxidative stress, which can lead to endothelial dysfunction and cause severe damage to the eyes or kidneys of diabetics. Inhibition of the formation of advanced glycation end products (AGEs) and their cell toxicity can be a useful therapeutic strategy in the prevention of diabetic retinopathy (DR). Gardenia jasminoides Ellis (GJE) fruit is a selective inhibitor of AGEs. Genipin is an active compound of GJE fruit, which can be employed to treat diabetes.
To confirm the effect of genipin, a vital component of GJE fruit, in preventing human retinal microvascular endothelial cells (hRMECs) from AGEs damage in DR, to investigate the effect of genipin in the down-regulation of AGEs expression, and to explore the role of the CHGA/UCP2/glucose transporter 1 (GLUT1) signal pathway in this process.
In vitro, cell viability was tested to determine the effects of different doses of glucose and genipin in hRMECs. Cell Counting Kit-8 (CCK-8), colony formation assay, flow cytometry, immunofluorescence, wound healing assay, transwell assay, and tube-forming assay were used to detect the effect of genipin on hRMECs cultured in high glucose conditions. In vivo, streptozotocin (STZ) induced mice were used, and genipin was administered by intraocular injection (IOI). To explore the effect and mechanism of genipin in diabetic-induced retinal dysfunction, reactive oxygen species (ROS), mitochondrial membrane potential (MMP), and 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose (2-NBDG) assays were performed to explore energy metabolism and oxidative stress damage in high glucose-induced hRMECs and STZ mouse retinas. Immunofluorescence and Western blot were used to investigate the expression of inflammatory cytokines [vascular endothelial growth factor (VEGF), SCG3, tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-18, and nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing 3 (NLRP3)]. The protein expression of the receptor of AGEs (RAGE) and the mitochondria-related signal molecules CHGA, GLUT1, and UCP2 in high glucose-induced hRMECs and STZ mouse retinas were measured and compared with the genipin-treated group.
The results of CCK-8 and colony formation assay showed that genipin promoted cell viability in high glucose (30 mmol/L D-Glucose)-induced hRMECs, especially at a 0.4 μmol/L dose for 7 d. Flow cytometry results showed that high glucose can increase apoptosis rate by 30%, and genipin alleviated cell apoptosis in AGEs-induced hRMECs. A high glucose environment promoted ATP, ROS, MMP, and 2-NBDG levels, while genipin inhibited these phenotypic abnormalities in AGEs-induced hRMECs. Furthermore, genipin remarkably reduced the levels of the pro-inflammatory cytokines TNF-α, IL-1β, IL-18, and NLRP3 and impeded the expression of VEGF and SCG3 in AGEs-damaged hRMECs. These results showed that genipin can reverse high glucose induced damage with regard to cell proliferation and apoptosis in vitro, while reducing energy metabolism, oxidative stress, and inflammatory injury caused by high glucose. In addition, ROS levels and glucose uptake levels were higher in the retina from the untreated eye than in the genipin-treated eye of STZ mice. The expression of inflammatory cytokines and pathway protein in the untreated eye compared with the genipin-treated eye was significantly increased, as measured by Western blot. These results showed that IOI of genipin reduced the expression of CHGA, UCP2, and GLUT1, maintained the retinal structure, and decreased ROS, glucose uptake, and inflammation levels in vivo. In addition, we found that SCG3 expression might have a higher sensitivity in DR than VEGF as a diagnostic marker at the protein level.
Our study suggested that genipin ameliorates AGEs-induced hRMECs proliferation, apoptosis, energy metabolism, oxidative stress, and inflammatory injury, partially via the CHGA/UCP2/GLUT1 pathway. Control of advanced glycation by IOI of genipin may represent a strategy to prevent severe retinopathy and vision loss.
Core Tip: The formation of advanced glycation end products (AGEs) has been widely validated in pathological changes of diabetic retinopathy (DR). A new vital compound in Gardenia jasminoides Ellis fruit, genipin, can be used to treat DR and decrease AGEs. Genipin ameliorated AGEs-induced human retinal microvascular endothelial cell proliferation, apoptosis, energy metabolism, oxidative stress, and inflammatory injury, partially via the CHGA/UCP2/glucose transporter 1 pathway. Control of AGEs by intraocular injection of genipin may represent a strategy to prevent severe retinopathy and vision loss. Here, we confirmed the effectiveness of genipin to treat DR both in vivo and in vitro, and explored its related molecular mechanism.
- Citation: Sun KX, Chen YY, Li Z, Zheng SJ, Wan WJ, Ji Y, Hu K. Genipin relieves diabetic retinopathy by down-regulation of advanced glycation end products via the mitochondrial metabolism related signaling pathway. World J Diabetes 2023; 14(9): 1349-1368
- URL: https://www.wjgnet.com/1948-9358/full/v14/i9/1349.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i9.1349
The number of patients with diabetes mellitus (DM) has increased from less than 110 million in 1980 to approximately 420 million in 2015 worldwide and is expected to reach 642 million in 2040[1]. The estimated overall prevalence of DM is 10.9% among Chinese citizens based on national-wide surveillance[2]. Diabetic retinopathy (DR) is the most common microvascular complication of DM and is a vital reason for blindness in citizens aged over 55 years[3]. Some new treatments, such as intravitreal vascular endothelial growth factor (VEGF) inhibitors or steroid hormones, have been introduced for DR[4]. However, up to 50% of the patients failed to respond to such agents. Despite being an inherently destructive procedure, laser photocoagulation remains the mainstay therapy for people with proliferative DR (PDR). This necessitates other effective methods for DR treatment. In the pathogenesis of DR, the retinal circulation is damaged by microvascular lesions. If this change is not controlled in the early stages, continual progression leads to retinal detachment and vision loss[5]. The primary pathogenesis of DR involves angiogenesis, chronic inflammation, and oxidative stress. The retinal capillary endothelium is composed of endothelial cells, pericytes, and the basement membrane. Neovascularization of the retinal surface is the primary stage of PDR[6]. It is also the most important factor in retinal detachment. Endothelial dysfunction leads to angiogenesis, which involves the proliferation, migration, and formation of tubes and is central to the development of vascular complications[7]. Angiogenesis requires energy support such as adenosine triphosphate (ATP) or glucose. Damage to human retinal microvascular endothelial cells (hRMECs) in DR arises from metabolic abnormalities in glucose metabolism, which are principally caused by advanced glycation end products (AGEs).
Glycation is an important step in aging and oxidative stress[8]. Persistently elevated glucose concentrations lead to rapid and intensive glycation reactions. Both acute and chronic hyperglycemia can enhance AGEs production[9], which results in endothelial dysfunction and causes severe damage to the eyes or kidneys of diabetics. Inhibition of AGEs and its cell toxicity can be a useful strategy for the prevention of DR[10]. According to previous studies, reducing AGEs receptor/ligand interaction or breaking established AGEs crosslinks prevent AGEs formation[11].
Mitochondrial dysfunction and oxidative stress are largely involved in aging, cancer, age-related neurodegenerative disorder, and metabolic syndrome[12]. Mitochondrial dysfunction may predispose to the development of DM with the accompanying risk of developing DR or may contribute directly to diabetic metabolic dysregulation and thereby increase the risk of late diabetic complications including retinopathy[13,14]. The relation between mitochondrial dysfunction and diabetic eye complications has two elements: (1) Mitochondrial diseases may predispose to the development of DM and such type of diabetes may be accompanied with an increased risk of developing DR; and (2) metabolic dysregulation in DM may increase the risk of development of DR through a disturbance in mitochondrial function[13,14]. A hypothesis has recently been proposed that the metabolic dysfunction in diabetic patients induces the synthesis of a number of reactive oxygen species (ROS) that are normally eliminated in the mitochondria[13]. In addition, enhanced ROS may contribute to the induction of autophagy in the retina. Another mechanism of mitochondrial dysfunction leading to DR is the formation of free radicals[14-17]. These reactive compounds may contribute to a switch in the metabolism, e.g., by changing the activity of glyceraldehyde-3-phosphate dehydrogenase and the polyol pathway and activating protein kinase C, and consequent development of DR[13].
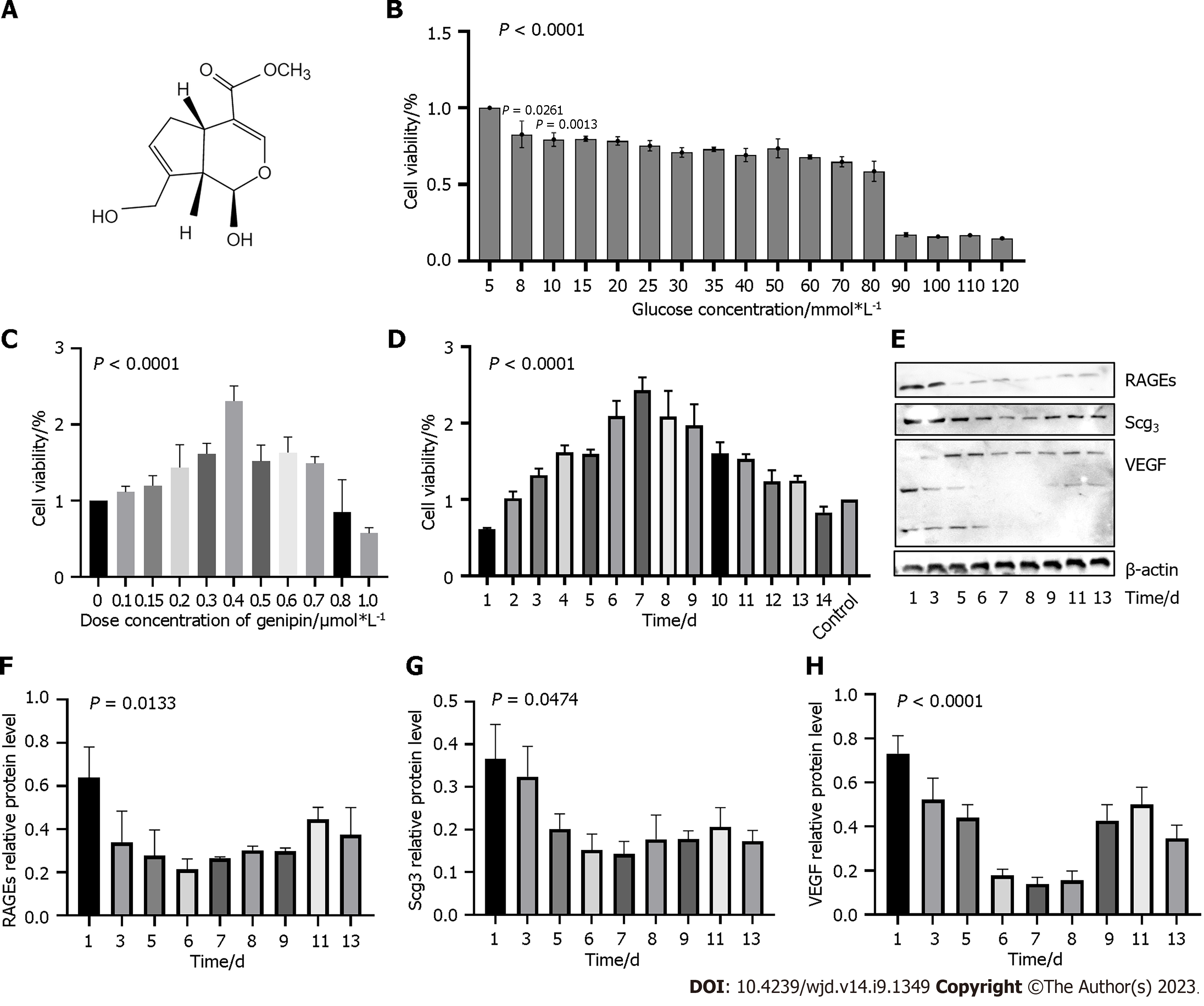
Genipin (Figure 1A), one of the principal bioactive components extracted from the Gardenia jasminoides Ellis (GJE, Chinese herbal name “Zhizi”), has multiple bioactivities, such as anti-inflammation, antitumor, antidepression, and the protection of hippocampal neurons from the toxicity of Alzheimer’s amyloid-β[18]. GJE, as a selective inhibitor of AGEs, prevents the development of diabetic vascular complications in experimental animal models; however, its possible role and molecular mechanism in the relief of DM symptoms are unknown[19]. To explore these, we searched the traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP) (https://old.tcmsp-e.com/tcmsp.php) using the keyword “Zhizi”. Genipin was the third most active constituent of Zhizi (Table 1). Other active constituents of Zhizi include quercetin and lutein. Recent studies demonstrated that quercetin inhibited the overexpression of TLR4 and NF-κB p65, and reduced the expression of VEGF and soluble intercellular adhesion molecule-1, thus exerting therapeutic effects in DR[20,21]. Many basic and clinical studies have reported that lutein has anti-oxidative and anti-inflammatory properties in the eye, suggesting its beneficial effects in protection and alleviation of ocular diseases such as age-related macular degeneration (AMD), DR, retinopathy of prematurity, myopia, and cataract[22,23]. However, some publications report that genipin, but not quercetin or lutein, can influence AGEs. In the present study, we administered streptozotocin (STZ)-treated mice by intraocular injection (IOI) of genipin to investigate whether genipin could attenuate the development of DR in experimental models through AGEs inhibition[24].
| MolID | Active component | OB (%) | DL | BBB |
| MOL009038 | GBGB | 45.58 | 0.83 | -5.43 |
| MOL001652 | 1H-2,6-dioxacyclopent(cd)inden-1-one, 4-((acetyloxy)methyl)-5-(beta-D-glucopyranosyloxy)-2a,4a,5,7b-tetrahydro-, (2aS-(2aalpha,5alpha,7balpha))- | 26.43 | 0.71 | -2.00 |
| MOL001648 | Genipin | 26.06 | 0.10 | -0.98 |
| MOL000098 | Quercetin | 46.43 | 0.28 | -0.77 |
| MOL000422 | Kaempferol | 41.88 | 0.24 | -0.55 |
| MOL007245 | 3-Methylkempferol | 60.16 | 0.26 | -0.49 |
| MOL001406 | Crocetin | 35.30 | 0.26 | -0.83 |
| MOL000551 | Hederagenol | 22.42 | 0.74 | -0.51 |
| MOL003515 | (3S,4S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-3-hydroxy-4,6a,6b,11,11,14b-hexamethyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicene-4,8a-dicarboxylic acid | 27.21 | 0.72 | -0.68 |
| MOL013377 | Lutein | 22.59 | 0.55 | -0.99 |
| MOL009548 | Desacetyl asperulosidic acid_qt | 32.49 | 0.10 | -1.85 |
| MOL004560 | SHANZHISIDE_qt | 117.77 | 0.10 | -1.44 |
| MOL001667 | Deacetyl asperuloside acid_qt | 62.46 | 0.11 | -1.62 |
| MOL007148 | Shanzhiside methyl ester_qt | 109.77 | 0.12 | -3.18 |
| MOL004555 | GARDENOSIDE_qt | 52.77 | 0.12 | -0.91 |
| MOL007994 | Ilexoside A_qt | 22.43 | 0.74 | -0.33 |
Genipin (99%, Figure 1) was purchased from Selleck (Shanghai, China). D-glucose (PB180418) and the complete endothelial cell medium of hRMECs (CM-H130) were purchased from Procell (Wuhan, Hunan Province, China). Trypsin-ethylenediamine tetraacetic acid (EDTA) solution (S310JV), Dulbecco’s modified Eagle’s medium (L170KJ), minimum essential medium (L510KJ), penicillin-streptomycin solution (S110JV), and phosphate buffer solution (B320KJ) were purchased from BasalMedia (Shanghai, China). Cell Counting Kit-8 (BS350A) and 4% paraformaldehyde fix solution (BL539A) were purchased from Biosharp (Hefei, Anhui Province, China). Endothelial cell medium (1001), endothelial cell growth supplement (1052), fetal bovine serum (0025), and penicillin and streptomycin solution (0503) were purchased from ScienCell (Shanghai, China).
All animal procedures were conducted in accordance with the China Animal Welfare Legislation. We purchased 4-wk-old C57/BL6 male or female mice (3-8 g, n = 60) from the Animal Ethics Committee of Chongqing Medical University (Yuzhong, Chongqing, China). They were housed in a light-temperature automatically controlled room with free access to food and water. One female mouse and four male mice were housed in a single cage. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Chongqing Medical University (2022-K45). All experiments were performed in accordance with the Guidelines and Regulations for the Care and Use of Laboratory Animals by the Chongqing Medical University (Yuzhong, Chongqing, China) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
STZ-induced hyperglycemia is a widely used DM model. At the beginning of the experiment, the mice were fed a high-sugar and high-fat diet (rodent diet with 60% calories from fat; XTHF60, XieTong Scientific Diets, Wuhan, China) for 70 d (20-35 g, n = 60). Using 1 mL syringes and 25 G needles, intraperitoneal injection (IPI) of freshly prepared STZ solution at 40 mg/kg (1.0 mL/100 g of citrate buffer, pH 4.5) was performed. This step was repeated on days 2 and 3 after fasting[25]. We used similar forage and feeding management practices. Fasting blood glucose (FBG) levels were measured with a Precision Plus blood glucose meter 14 d after STZ IPI. Mice with FBG levels > 11.1 mmol/L were considered diabetic.
In the genipin-treated group, IOI of genipin [genipin dissolved in penicillin-streptomycin solution (PBS)] at a dose of 10 mmol/L was administered to the right eye (OD) (0.4 μL/eye) of mice after 3 mo of STZ administration. The same volume of PBS was administered to the left eye (OS) of the same mouse. Mice were maintained on a high-sugar and high-fat diet with free access to water under standard conditions and were assessed once every 2 wk and 1 mo for their body weight and FBG, respectively. All animals were killed at the end of the third time of IOI administration. Both eyes were collected and frozen at -80 °C or fixed in 4% paraformaldehyde fix solution for further experiments.
The eyes of the mice were fixed overnight with 4% paraformaldehyde fix solution and embedded in paraffin. Coronal sections (5 mm thick) were dewaxed and stained with hematoxylin and eosin stain (H&E). The wound areas of each sample were observed under a Leica DM2000 microscope (Leica, Wetzlar, Germany).
hRMECs (BNCC 358978) were purchased from BeNa Culture Collection (Beijing, China). The cells were plated in T25 flasks and incubated in a humidified incubator with 5% CO2 at 37 °C.
hRMEC pellet was resuspended in 100 μL of endothelial cell medium (ECM) without FBS, incubated in 96-well plates for 1 d, and treated with various concentrations of D-glucose. Cell viability was assessed using the Cell Counting Kit-8 (CCK-8). We added CCK-8 (10%) with a culture medium of 110 μL was added to each well and incubated for 2.5 h (37 °C, 5% CO2). Absorbance at 450 nm and 600 nm was measured using a microplate reader (Thermo Fisher). The cell inhibition rate (I%) was calculated as (Acontrol-Atreated)/Acontrol × 100%.
hRMEC pellet was resuspended in 100 mL of ECM supplemented with 30 mmol/L of D-glucose (HG), incubated in 96-well plates for 1 d, and then treated with various concentrations of genipin. After 7 d of treatment, cell viability was assessed using CCK-8 assay.
Apoptosis was detected using an Annexin V-FITC Apoptosis Detection Kit (Beyotime) according to the manufacturer’s instructions (CytoFLEX, United States). Cells (2 × 106) were seeded in 6-well plates and incubated with a high concentration of glucose and genipin for 1 wk. They were collected after digestion with trypsin-EDTA (Canada). After washing with PBS twice, the supernatant was discarded. The pellet was resuspended using ice-cold 70% ethanol (30 min at 4 °C) and incubated at 37 °C for 30 min. Next, 500 μL of 1 × binding buffer was added, and the cells were stained with 5 μL of annexin V-FITC and 5 μL of propidine iodide in the dark at 4 °C for 20 min, followed by flow cytometry analysis.
Cells were lysed with ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime) and centrifuged at 12000 r/min for 15 min at 4 °C, and the supernatant was collected. Protein concentrations in the supernatant were measured using the BCA protein assay kit (ThermoFisher, Shanghai, China). Proteins (40 μg) were resolved by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% BSA in TBST (1 × Tris buffered saline, 0.1% TWEEN 20) for 2 h at room temperature. The blocked membranes were immunoblotted with rabbit anti-UCP2 (A4178, ABclonal, Wuhan, Hubei Province, China), rabbit anti-glucose transporter 1 (GLUT1)/SLC2A1 (A11208, ABclonal), rabbit anti-CHGA (A1668, ABclonal), rabbit anti-AGER (A13264, ABclonal), rabbit anti-SCG3 (A7799, ABclonal), and rabbit anti-VEGF (A12303, ABclonal) antibodies overnight at 4 °C. The membranes were then washed and probed with secondary antibody (horseradish peroxidase-labeled goat anti-rabbit IgG; A0208, Beyotime) for 2 h at room temperature. Chemiluminescence detection was performed using a SuperSignal West Atto chemiluminescence detection kit (Thermo Fisher, Shanghai, China) according to the manufacturer’s instructions.
hRMECs were seeded in 24-well plates with a cover glass (REF.10212424C, Shitai, Jiangsu, China) at a final cell density of 5.0 × 104 cells/mL. In the genipin group, 4 mmol/L genipin was added. When the cell confluence reached approximately 50%, the cells were transferred to the FBS-free and antibiotic-free medium and cultured for 1 d. They were washed with PBS three times. The cells were fixed in 4% paraformaldehyde fix solution (P0099, Beyotime) for 30 min and blocked for 0.5 h using goat serum (C0265, Beyotime). Then, the membranes were washed and immunoblotted with primary antibodies overnight at 4 °C, followed by probing with Alexa Fluor 488-labeled Goat Anti-Rabbit IgG (H + L) (A0423, Beyotime) as secondary antibody for 2 h at room temperature. 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride staining solution (C1005, Beyotime) was used for counterstaining. Antifade mounting medium (P0126, Beyotime) was used to reduce the luminescence delay. All pictures were obtained using a Leica fluorescence microscope (DMR, Deerfield, IL, United States).
hRMECs were seeded into 6-well plates, and the number of cells was adjusted to approximately 2000 cells/well. After allowing cells to attach the plates, ECM with 20% FBS was added. After 2 wk, the cells were fixed in 4% paraformaldehyde fix solution for 0.5 h and stained with crystal violet (Beyotime Institute of Biotechnology, Shanghai, China) for 15 min. Each well with > 50 cells was photographed and observed under a phase-contrast microscope.
hRMECs were seeded in 6-well plates at a final cell density of 2.0 × 105 cells/mL. After treatment for 5 d, the confluent cell monolayer was scraped with a sterile 200 µL tip to create a scratch across the center of the circle when the hRMECs confluence reached > 95%. The wound areas of each sample were observed under a microscope and evaluated using ImageJ software (Media Cybernetics, Silver Springs, MD, United States) at 0 h, 12 h, 24 h, 48 h, and 72 h.
We used 8 μm sized Transwell BD Matrigel chambers (Costar 3470, Corning, NY, United States) to measure cell migration. hRMECs were seeded into the upper chamber with 100 μL of serum-free ECM, and the number of cells was adjusted to approximately 2000 cells/well. The lower chamber contained 600 μL ECM with 20% of FBS. After 30 h of culturing, the chambers were removed and fixed with 4% paraformaldehyde fix solution for 0.5 h. The cells were stained with crystal violet for 15 min. Medical Cotton Stickers (Sanhe, Sichuan Province, China) were used to remove non-migratory cells from the upper chamber. Photographs were captured by using a microscope. Five fields with evenly distributed cells were examined.
To analyze the influence of high glucose and genipin on the tube-forming activity of hRMECs, we plated 50 μL Matrigel (BioCoat Matrigel 356234, Corning, NY, United States) onto a cold 96-well plate. hRMECs were added into each well at a cell density of 4.0 × 105. After 6 h, images were captured under a microscope.
hRMECs were plated onto 6-well plates and grown until they reached confluence. The cells were starved in an endothelial basal medium (EBM) without serum for 1 d. They were collected after transfection and washed twice with PBS. The cells were collected into 1.5 mL Eppendorf tubes, and the ATP concentration was measured using an ATP fluorometric assay kit (S0026, Beyotime).
hRMECs were plated onto 6-well plates and grown until they reached approximately 60% confluence. The cells were starved in an EBM medium without serum for 1 d. The ROS Assay Kit (S0033, Beyotime) and Apoptosis Detection Kit with Mito-Tracker Red CMXRos and annexin V-FITC (C1071S, Beyotime) were used to measure the ROS and mitochondrial membrane potential (MMP) changes.
hRMECs were plated onto 6-well plates and grown until they reached confluence. The cells were starved in an EBM medium without serum for 1 d. D-glucose analog (100 mmol/L; 2-NBDG) assay (HY-116215, MCE, Shanghai, China) was performed to measure the D-glucose transported into the hRMECs. Photographs were captured under a fluorescence microscope.
Pro-inflammatory cytokines [tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-18, and NLRP3] and other hallmark proteins of DR (VEGF and SCG3) were assayed by Western blot and immunofluorescence as described previously.
Statistical analyses were performed using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, United States) and GraphPad Prism 8 (GraphPad Software, LLC). All experiments were repeated at least three times independently. All data are presented as the mean ± SEM. Independent sample t-test was used for comparing variables with a normal distribution. One-way analysis of variance and multiple comparisons for trends were used for comparing continuous variables. The chi-square test (or Fisher’s exact test, when appropriate) was used to analyze dichotomous variables. Pearson’s correlation coefficient was used to determine the relationship between variables. A P value less than 0.05 was considered statistically significant.
First, we investigated the effects of high glucose and genipin on hRMEC viability and cytotoxicity using CCK-8 assay. Figure 1B depicts that 30 mmol/L D-glucose could decrease approximately 30% of the cell viability by approximately 30%, and cell viability decreased by < 30% until high glucose reached 90 mmol/L. Thus, 30 mmol/L of D-glucose was used for subsequent experiments. After treatment with different doses of genipin (0.1 μmol/L to 1.0 μmol/L) in a 30 mmol/L glucose medium, we detected cell proliferation by CCK-8 assay. The administration of 0.4 μmol/L genipin exerted a notable effect on hRMECs proliferation (Figure 1C). However, genipin at 0.8 μmol/L remarkably inhibited cell proliferation (P < 0.001). The optimal effective dose of high glucose was 30 mmol/L to 50 mmol/L and that of genipin was approximately 0.3-0.8 μmol/L; these doses did not exert any cytotoxic effects on the cells.
Next, we explored the optimal action time of genipin by CCK-8 assay (Figure 1D). Compared with normal glucose medium without genipin, treatment with 0.4 μM genipin for 4 d exerted a notable effect on high glucose-induced HRMECs (P < 0.001) (Figure 1D). In hRMECs cultured in ECM containing 30 mmol/L glucose, we observed the highest cell viability after 7 d of treatment with 0.4 μmol/L genipin (Figure 1D). We verified these results using Western blot (Figure 1E-H). Based on these results, treatment with 30 mmol/L high glucose and 0.4 μmol/L genipin for 7 d was used for the subsequent experiments.
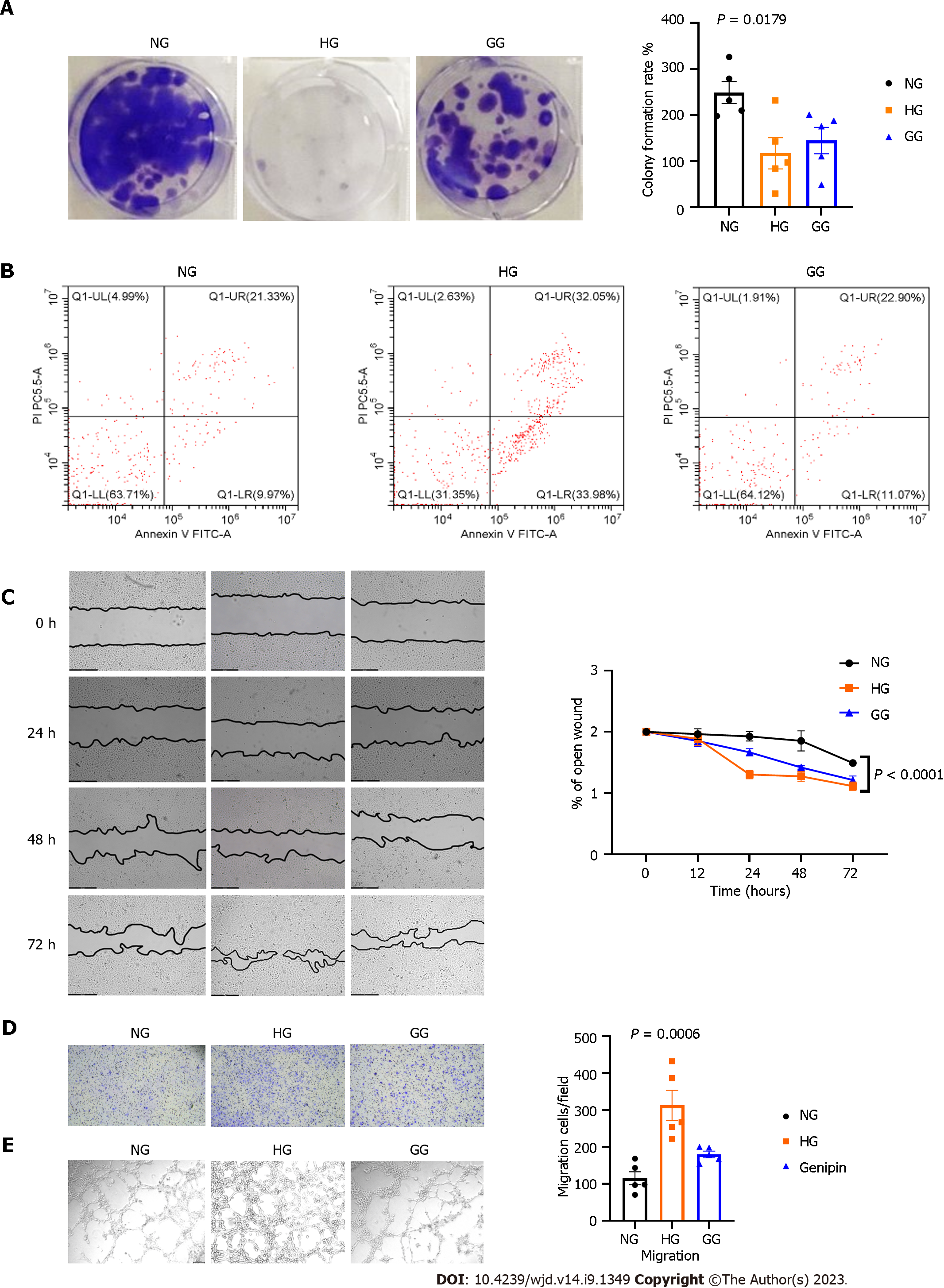
Action of glucose and genipin on hRMECs proliferation, apoptosis, and angiogenesis: CCK-8 assay demonstrated that genipin, particularly 0.4 μM genipin for 7 d, increased the viability of hRMECs in high-glucose ECM. The colony formation results supported this finding (Figure 2A). Results of flow cytometry (Figure 2B) suggested that the number of apoptotic hRMECs increased after the stimulation with 30 mmol/L glucose (61.05%), compared with normal medium stimulation (30.30%). However, the cell apoptosis ratio decreased after genipin treatment, and particularly, early cell apoptosis decreased from 33.98% to 11.07%.
To explore the effects of genipin on cell migration, we performed scratch wound healing and transwell migration assays. High glucose decelerated the closure of hRMECs, and genipin could restore this change (Figure 2C). Furthermore, high glucose levels increased the number of migrated cells, and genipin restored this change (Figure 2D).
Then, we analyzed the tube-forming capacity of hRMECs in high glucose, with or without genipin. High glucose significantly increased the number of newly formed tubes, while genipin reversed this change (Figure 2E).
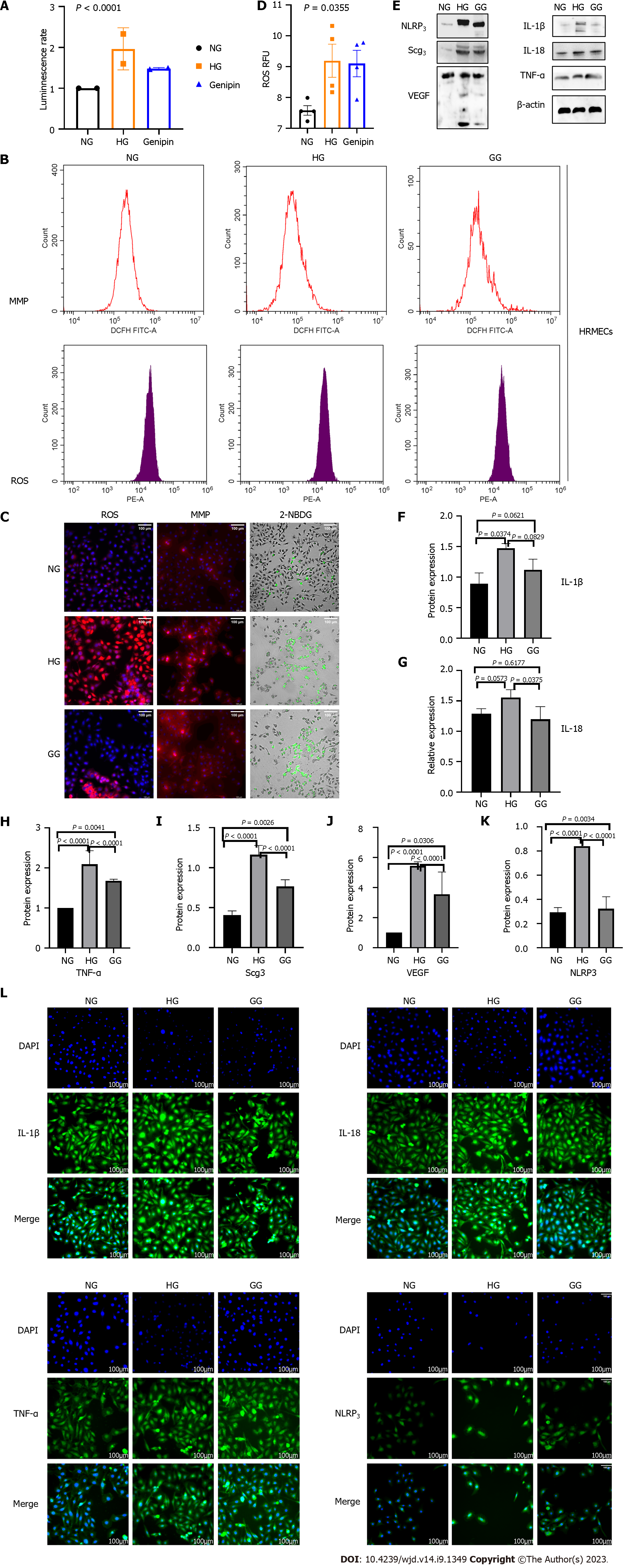
Action of glucose and genipin on intracellular ATP levels and MMP: Hyperglycemia in patients with DM is associated with abnormally elevated cellular glucose levels. ATP, as the primary energy storage molecule, is central to cell survival, proliferation, and migration. Increased cellular glucose levels alter glucose metabolism and influence intracellular ATP levels by inhibiting complexes I and II[26]. Subsequently, we measured the intracellular ATP levels and observed that high glucose increased the ATP level by 60%, and genipin could reduce this level by approximately 20% (Figure 3A).
Next, we explored the mitochondrial changes in DR. MMP increased after high glucose stimulation (Figure 3B and C), indicating that DR produced more ATP, which promotes the formation of mitochondria[27]. Simultaneously, these mitochondrial changes may lead to increased ROS accumulation.
Action of glucose and genipin on intracellular oxidative stress levels: To confirm the oxidative stress damage caused by high glucose levels, we measured intracellular ROS production. High glucose increased the fluorescence significantly; however, this increase was alleviated by genipin (Figure 3B-D).
Action of glucose and genipin on intracellular glucose metabolism levels: NBDG is a fluorescence-labeled 2-deoxy-glucose analog that works as a tracer for evaluating cellular glucose metabolism[28]. High glucose increased the fluorescence, and genipin might reverse the effects.
Action of glucose and genipin on inflammation: To evaluate the effects of genipin on inflammation following HG stimulation, we performed Western blot and Immunofluorescence to detect the protein levels of inflammatory cytokines (TNF-α, IL-1β, IL-18, and NLRP3) and DR biomarkers (VEGF and SCG3) in hRMECs inclubated with high glucose (Figures 3E-L and 5D). High glucose significantly increased the protein expression of TNF-α, IL-1β, IL-18, and NLRP3, and DR biomarkers. Interestingly, genipin down-regulated the expression of these proteins. Therefore, genipin may alleviate the damage caused by inflammation. Next, we will investigate the mechanisms underlying this effect.
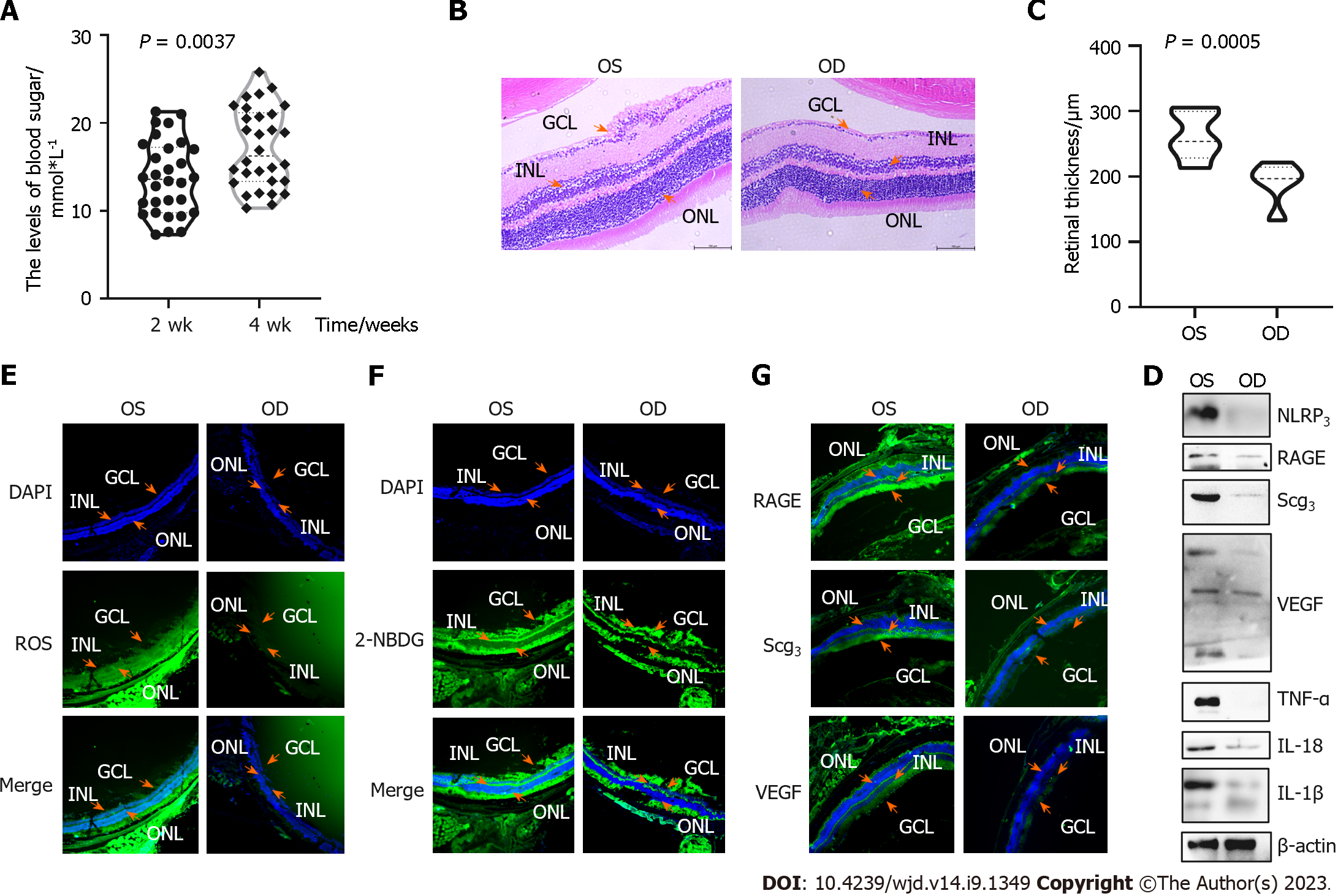
After continuous STZ IPI for 3 d, the blood glucose levels were > 7.5 mmol/L in 2 wk and > 11.1 mmol/L in 1 mo in C57 mice.
DM-induced neuronal loss was assessed by measuring the retinal thickness at 3 mo after DM induction using retinal sections from untreated eyes (OS) and genipin-injected eyes (Figure 4B and C). At 3 mo after DR induction, H&E staining was performed to assess the structural morphology of the retinal cells. We measured four spots of the retina; the thickness of genipin-treated retina (190.70 μm ± 58.32 μm) was lesser than that of DR-untreated retina (262.10 μm ± 55.52 μm, P = 0.045). Representative microphotographs demonstrate a disorder of the entire retina, vacuolization, and dissolution of the ganglion cell layer in the left eye.
We performed ROS and 2-NBDG assays to measure oxidative stress and glucose metabolism in the frozen sections. Genipin-treated cells had significantly increased oxidant stress and glucose metabolism. SCG3 and VEGF expression increased in the untreated group.
In addition, the protein levels of inflammatory cytokines (TNF-α, IL-1β, IL-18, and NLRP3) and DR biomarkers (VEGF and SCG3) were measured by Western blot. Genipin protected the cells from inflammation.
UCP2/GLUT1 pathway is involved in the protective effect of genipin on hRMECs (Figure 5): The decrease in glucose uptake in genipin-treated cells was mediated by the reduction of GLUT1 expression, as demonstrated by Western blot (Figure 5C and D). To investigate the glycation process that occurred after glucose, we explored the AGE-receptor interactions in both cells and tissues, which showed that genipin treatment decreased AGEs.
We used STRING (Version: 11.5, https://cn.string-db.org/cgi/input) to analyze the relationships among “RAGE”, “SCG3”, “UCP2”, “GLUT1”, and “VEGF” (Figure 5A). GLUT1 may be influenced by UCP2 expression in oxygen-induced retinopathy (OIR) at both the mRNA and protein levels, whereas elevated circulating CHGA can lead to UCP2 overexpression in the hypertensive phenotype[9]. Next, we performed Western blot and immunofluorescence to verify the expression of these proteins. The protein expression of these three proteins was increased in high-glucose conditions, and genipin could restore such change.
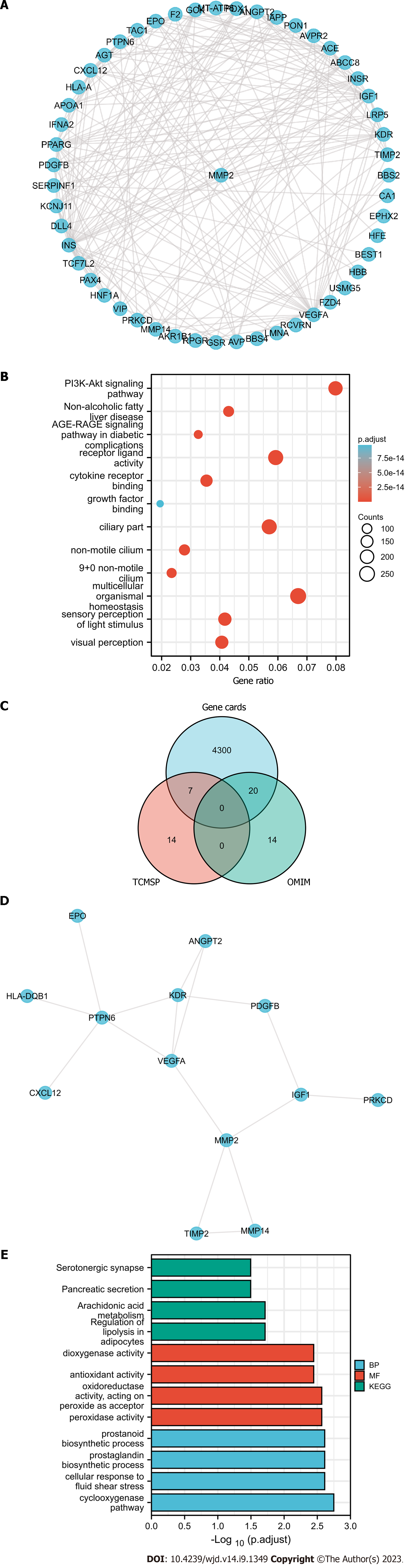
Genpin relives retinal endothelial damage in high-glucose conditions by influencing energy metabolism, oxidative stress, and inflammatory injury: We identified 33 and 4328 DR-related genes using GeneCards (https://www.genecards.org/) and OMIM (https://omim.org/), respectively, with the keywords “DR” or “diabetic retinopathy”. Next, we used STRING to analyze the relationships among these genes (Figure 6A); Gene Ontology (GO)/Kyoto Encyclopedia of Genes and Genomes function enrichment analyses revealed that AGE-RAGE plays a vital role in DR (Figure 6B). Restraining AGEs and alleviating toxicity may be a novel way to control DR.
We used TCMSP to identify 21 genes possibly targeted by genipin. UniProt (https://www.uniprot.org/) was used to assess and convert these gene names. A Wayne diagram was plotted to display the intersection of the genipin target and DR-related genes (Figure 6C and D). To determine the available phenotypic abnormalities, we used GO function enrichment and pathway enrichment analyses to explore the relationships among genes that were mixed by DR-related and genipin targets. Finally, oxidoreductase activity and arachidonic acid metabolism demonstrated distinct correlations (Figure 6E), indicating that genipin may influence ROS and inflammation as two vital phenotypic abnormalities in DR.
Vision loss in DR principally occurs in either diabetic macular edema (DME) or PDR[29]. DR is classified based on visible ophthalmologic changes and manifestations of retinal neovascularization[30]. Therefore, the early diagnosis and treatment of DR cover the complete range of retinopathies and are complex. Fortunately, regardless of the type of DR, abnormal AGE accumulation has the same basic reason.
DR has a mixed pathogenesis. It involves cell cycle regulation, apoptosis signal transduction, oxidative stress response, protein biosynthesis, carbohydrate metabolism, and other important proteins and proteases. The ROS assay demonstrated a high level of oxidative stress response in both cells in the high glucose group and STZ mice without treatment. Carbohydrate metabolism and mitochondrial injury in the high glucose group led to increased ATP levels and cell apoptosis. Glucose uptake was higher in the high glucose group than in the control group, which can be validated by GLUT1 expression. Genipin, an aglycone derived from the iridoid glycoside, is also used as an anti-tumor drug in oral squamous cell carcinoma, hepatocellular carcinoma, and gastric cancer via the signal transducer and activator of the transcription-3 pathway and upregulated MMP genes[31,32]; the relationships between genipin and glucose was rarely reported. We explained whether genipin could change the GLUT family in different cell types. This finding may help explain how genipin influences tumors and apoptosis in various diseases. VEGF is a classical biomarker of DR, and it is widely used to treat inflammation[32-35]. NLRP3 inflammasomes are involved in the production and persistence of inflammation in diabetic nephropathy[36-38]. We observed that high glucose stimulation could up-regulate IL-1β, IL-18, TNF-α and NLRP3, but not IL-6.
Upon exposure to sugar, proteins, lipids, and nucleic acids are oxidized and rearrange to form stable products, which can further undergo crosslinking, thus yielding AGEs[39]. AGEs are associated with a series of diseases such as Alzheimer’s disease, skin senescence, face splash capillary ectasia, and pathologies such as AMD, diabetic keratopathy, and DR. AGEs are reactive intermediates of chronic hyperglycemia with proteins, lipids, and nucleic acids in vivo, leading to the accumulation of pro-inflammatory cytokines and ROS. Furthermore, they damage the vasculature, eye, heart, and kidneys in patients with DM.
Consequently, we focused on glucose metabolism and AGEs production in hRMECs and addressed the hypothesis that high-glucose medium affects glucose uptake and mitochondrial metabolism, leading to intravitreal neovascularization in DR. UCP2, a mitochondrial carrier protein, can increase the cellular sensitivity to glucose and promote cellular glucose uptake by mediating proton leak across the inner mitochondrial membrane[40]. Genipin is a standard UCP2-inhibiting agent in various experimental models of ROS generation and mitochondrial activity[41]. Most tumor growth and proliferation processes involve excessive glucose uptake mediated by GLUTs[42]. The retina takes up glucose, principally through GLUT1[43]. Genipin promotes physiological retinal vascular development and improves endothelial function by inhibiting the UCP2/GLUT1 pathway in premature retinopathy.
Thus, we focused on the effects and molecular mechanisms of genipin on the proliferation, apoptosis, angiogenesis, energy metabolism, oxidative stress, and inflammation of AGEs-induced hRMECs.
Genipin have been used to treat several diseases due to its choleretic, anti-inflammatory, antioxidant, anti-apoptotic, autophagy-inducing, anti-necroptotic, and anti-pyroptotic properties[41,44-47]. Simultaneously, genipin is an aglycone of an iridoid glycoside termed geniposide. Moreover, it is a commonly used biological agent, e.g., as a cross-linker, because it can be better tolerated by cells. Despite its benefits and good biocompatibility, genipin is still unpopular in classical medicine because of its unclear mechanism of action. We analyzed the role of genipin as an important element of Zhizi against AGEs accumulation in DR. First, we evaluated the intracellular concentration of AGEs by assessing the expression level of RAGE. RAGE expression increased significantly with high glucose stimulation, and genipin decreased this increase. High glucose stimulation increased apoptosis, ROS, ATP, mitochondrial formation, glucose metabolism, and inflammation. High glucose could increase the accumulation of AGEs via pathways that can be affected by genipin and finally decrease GLUT1 expression. UCP2, as a binding site for genipin, decreases GLUT1 expression at both the mRNA and protein levels[40]. Then, we tried to connect RAGE and DR biomarkers with these pathways. We identified RAGE and DR biomarkers in these pathways. We analyzed the relationships between these five proteins and identified the “CHGA” protein. Elevated CHGA levels result in UCP2 overexpression in hypertension[48]. We evaluated these changes in hRMECs treated with high glucose and STZ mouse retinas and observed increased protein expression of CHGA, UCP2, and GLUT1. Hence, high glucose leads to the addition of AGEs, increases CHGA expression, and promotes UCP2 and GLUT1 expression, which influences cell proliferation, apoptosis, oxides, cellular energy metabolism, and inflammation. Genipin can inhibit this CHGA/UCP2/GLUT1 pathway not only by controlling UCP2 but also through other mechanisms.
Genipin is the third most active constituent of Zhizi (Table 1). The oral bioavailability of genipin is 26.06% and its topological polar surface area is 75.99, which suggests that genipin is preferentially administered by injection than orally. However, genipin is principally used as an oral formulation in previous research, whereas injection formulations have been less investigated. In addition, genipin is an extremely good natural cross-linking agent that contributes to sustained-release or delayed-release joint use with other drugs[49]. AGEs are a type of cross-linker that combines sugars and proteins and can also reduce this step by linking with proteins by physical methods. Furthermore, genipin is widely used in the clinic to treat corneal or scleral diseases[46,49].
Ocular drug delivery is difficult because of the lacrimal fluid-eye barrier and the retina-blood barrier[50]. Retinal diseases, such as retinal detachment, AMD, and DR, require an intravitreal injection to inhibit the formation of ocular neovascularization. Such common drugs include anti-VEGF medications and hormones. Our previous report describes the use of an AGE inhibitor that can penetrate the vitreous humor and treat retinal disease in STZ mice. We used the most effective dose of genipin in hRMECs for injection (C = m × V). The vitreous volume of mice was approximately 10 μL, and the maximum injection volume was 1 μL. We used 4 μmol/eye (5 mmol/L × 0.8 μL/eye, 10 mmol/L × 0.4 μL/eye) and 8 μmol/eye (10 mmol/L × 0.8 μL/eye), three different doses for IOI, and the results did not have a measurable difference. Finally, we selected the minimal volume for subsequent experiments.
Eventually, we identified the biomarkers of DR. Previous studies have reported on VEGF, monocyte chemoattractant protein-1, TNF-α, transforming growth factor-β, and nicotinamide adenine dinucleotide phosphate oxidase as the markers of inflammation. L-citrulline, indoleacetic acid, chenodeoxycholic acid, and eicosapentaenoic acid have been reported as markers of metabolism[51]. SCG3 is an angiogenic factor restricted to pathological conditions in DR[52]. Jiao et al[53] demonstrated that the concentration of SCG3 in the vitreous increased in 77 patients with DR. LeBlanc et al[54] reported that SCG3 antibodies alleviated retinal vascular leakage in diabetic mice with high efficacy. Furthermore, we assessed SCG3 expression in cells and tissues. Our results implied that SCG3 may be another biomarker of DR, which displayed a similar growing tendency in high glycogen to VEGF expression but with a higher sensitivity.
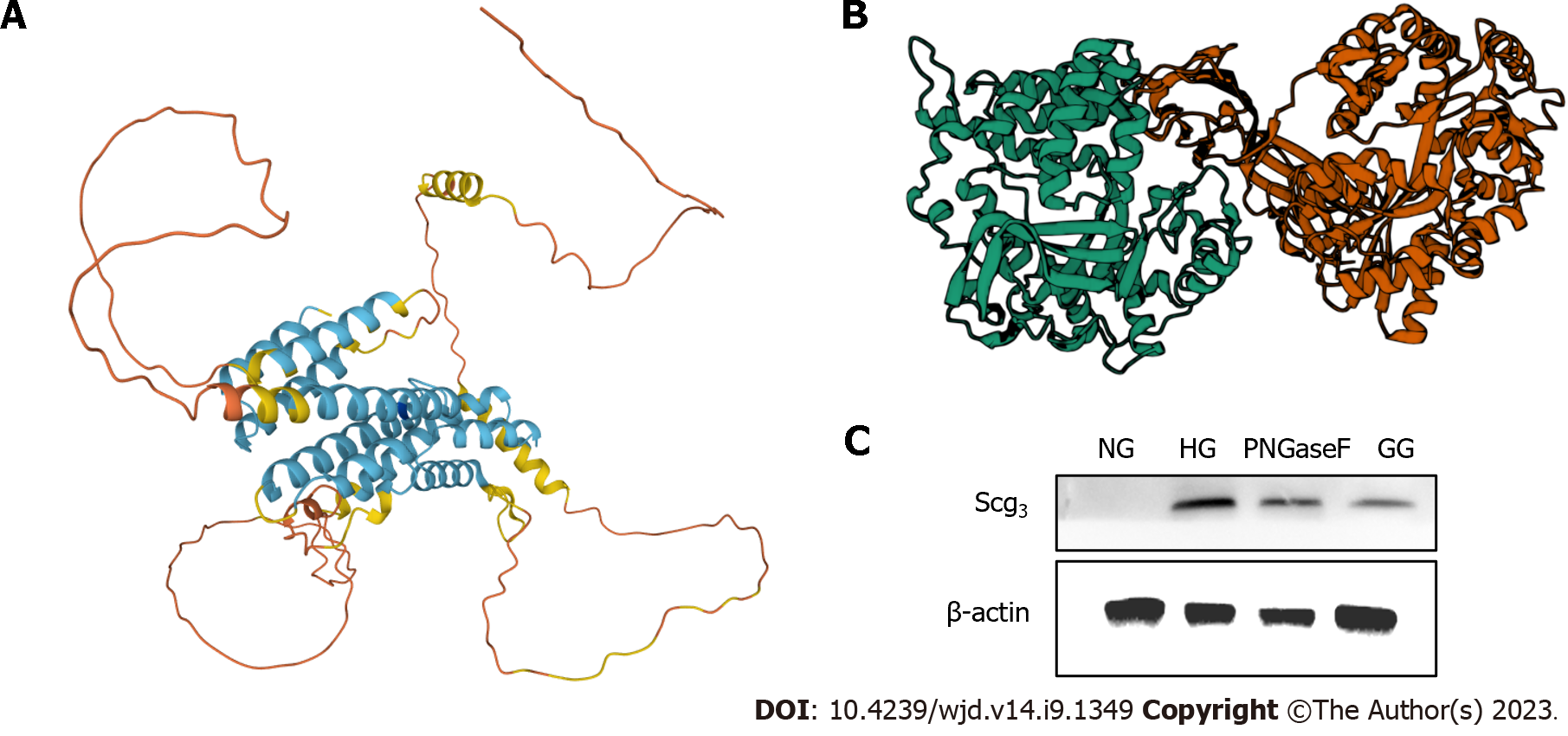
However, this study had several limitations. First, how the CHGA/UCP2/GLUT1 pathway is regulated by AGEs-RAGE binding requires further research. Some studies have demonstrated that they may form a GLUT1-UCP2 complex attached to the mitochondrial membrane; nonetheless, some details are still unknown. Second, we did not explain whether different doses of AGEs influence the severity of DR. Consequently, we added quantitative exogenous AGEs and observed changes in the cells and tissues. Third, the IOI group comprised limited doses in vivo. Next, we will add more dose groups injected with perfect genipin drug transduction in vivo and evaluate clinical medicine. Finally, we determined that SCG3 may replace VEGF in evaluating DR; however, the function of SCG3 in angiogenesis is unclear. Our future work will focus on the changes in SCG3 during abnormal physiological processes. Some researchers have mentioned that SCG3 is central to tumors, apoptosis, and other vital processes[55]. Togayachi et al[56] demonstrated that SCG3 is activated by adding N-glycosylation to change short-form SgIII into a long form in small-cell lung carcinoma. This biological process may be related with the GLUT family. To further explore the relevant mechanisms, we used UniProt (https://www.uniprot.org/) to determine the structure of SCG3 and explained the potential target site of glycosylation (Figure 7A, Table 2). The ZDOCK score for RAGE and SCG3 was 1277.337, which means that RAGE can be linked with SCG3 easily. There are totally three positions of RAGE associated with SCG3 (at sites 216, 231, and 359; Figure 7B). PNGase F, used to split SCG3, decreased SCG3 protein expression (Figure 7C).
| Type | Position | Description |
| Signal | 1-19 | - |
| Chain | 20-468 | Secretogranin-3 |
| Modified residue | 37 | Phosphoserine |
| Glycosylation1 | 216 | O-linked (GalNAcan) threonine |
| Glycosylation 2 | 231 | O-linked (GalNAcan) threonine |
| Glycosylation 3 | 359 | O-linked (GalNAcan) threonine |
| Modified residue | 362 | Phosphoserine |
Overall, apoptosis, angiogenesis, proliferation, ROS production, carbohydrate metabolism, and inflammation can reflect DR damage both in vivo and in vitro. Genipin is useful against AGEs, and its protective role in DR has been confirmed. This action may occur via AGEs-RAGE binding to control the CHGA/UCP2/GLUT1 pathway. SCG3 is a novel and sensitive biomarker for DR.
Diabetic retinopathy (DR) is a serious and common complication of diabetes. Advanced glycation end products (AGEs) are a group of reversible and poisonous products formed by nonenzymatic glycation of glucose with protein and lipids under hyperglycemia conditions. Both acute and chronic hyperglycemia can enhance AGEs production, which results in endothelial dysfunction and causes severe damage to diabetic retina. Some traditional Chinese herb like Gardenia jasminoides Ellis (GJE) fruit is a selective inhibitor of AGEs.
To confirm the effect of genipin, a vital component of GJE fruit, in preventing human retinal microvascular endothelial cells (hRMECs) from AGEs damage in DR and explored its mechanism.
To demonstrate whether genipin could lessen the development of DR in experimental models (4-wk-old C57/BL6 mice) through AGEs inhibition.
Cell Counting Kit-8 (CCK-8) assay, colony formation assay, flow cytometry, immunofluorescence, wound healing assay, transwell assay, and tube-forming assay were used to detect the effect of genipin on hRMECs in vivo. Streptozotocin induced mice were used to explore retinal dysfunction with DM in vitro. Reactive oxygen species (ROS), mitochondrial membrane potential (MMP), and 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose assays were used to evaluate energy metabolism and oxidative stress damage in high glucose-induced hRMECs and STZ mouse retinas. Immunofluorescence and Western blot were used to investigate the expression of inflammatory cytokines (VEGF, SCG3, TNF-α, IL-1β, IL-18, and NLRP3).
Our study confirmed that genipin ameliorated AGEs-induced hRMECs proliferation, apoptosis, energy metabolism, oxidative stress, and inflammatory injury, partially via the CHGA/UCP2/glucose transporter 1 pathway. Control of AGEs by IOI of genipin may represent a strategy to prevent severed retinopathy and vision loss.
Our study suggested that intraocular injection of genipin can ameliorate AGEs to control DR. Control of AGEs and using principal bioactive components extracted from herb by intraocular injection may represent strategies to prevent DR.
Our future work will focus on the changes in SCG3 during abnormal physiological processes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salceda R, Mexico; Wagner KD, France; Cai L, United States S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YX
| 1. | Simó-Servat O, Hernández C, Simó R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019;62:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Leley SP, Ciulla TA, Bhatwadekar AD. Diabetic Retinopathy in the Aging Population: A Perspective of Pathogenesis and Treatment. Clin Interv Aging. 2021;16:1367-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Pelikánová T. [Diabetic retinopathy in the Czech National Diabetes Programme 2012-2022]. Vnitr Lek. 2013;59:214-217. [PubMed] |
| 4. | Chua J, Lim CXY, Wong TY, Sabanayagam C. Diabetic Retinopathy in the Asia-Pacific. Asia Pac J Ophthalmol (Phila). 2018;7:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Pan CW, Wang S, Wang P, Xu CL, Song E. Diabetic retinopathy and health-related quality of life among Chinese with known type 2 diabetes mellitus. Qual Life Res. 2018;27:2087-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Lin Z, Wang Y, Li D, Wen L, Zhai G, Ding XX, Zang DX, Wang FH, Liang YB. Higher prevalence of diabetic retinopathy among female Chinese diabetic patients with metabolic syndrome. Jpn J Ophthalmol. 2022;66:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Hattori Y, Hashizume K, Nakajima K, Nishimura Y, Naka M, Miyanaga K. The effect of long-term treatment with sulindac on the progression of diabetic retinopathy. Curr Med Res Opin. 2007;23:1913-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Ellis MP, Lent-Schochet D, Lo T, Yiu G. Emerging Concepts in the Treatment of Diabetic Retinopathy. Curr Diab Rep. 2019;19:137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Angermann R, Rauchegger T, Nowosielski Y, Casazza M, Bilgeri A, Ulmer H, Zehetner C. Treatment compliance and adherence among patients with diabetic retinopathy and age-related macular degeneration treated by anti-vascular endothelial growth factor under universal health coverage. Graefes Arch Clin Exp Ophthalmol. 2019;257:2119-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Wang L, Wu Q, Wang RQ, Wang RZ, Wang J. Protection of leukemia inhibitory factor against high-glucose-induced human retinal endothelial cell dysfunction. Arch Physiol Biochem. 2023;129:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Arcadu F, Benmansour F, Maunz A, Willis JR, Prunotto M, Haskova Z. Deep learning algorithm for patient-level prediction of diabetic retinopathy (DR) response to vascular endothelial growth factor (VEGF) inhibition. Invest Ophthalmol & Vis Sci. 2019;60:2806. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 12. | Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1066-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1057] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 13. | Bek T. Mitochondrial dysfunction and diabetic retinopathy. Mitochondrion. 2017;36:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta. 2015;1852:2474-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 240] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 15. | Kersten HM, Roxburgh RH, Danesh-Meyer HV. Ophthalmic manifestations of inherited neurodegenerative disorders. Nat Rev Neurol. 2014;10:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Te BC, Goh BS. Case series of tuberculous otitis media: Spectrum of clinical presentation and outcome. Acta Otorrinolaringol Esp (Engl Ed). 2022;73:123-129. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Conlon TA, Hawkes CP, Brady JJ, Murphy NP. The presentation of congenital adrenal hyperplasia in an unscreened population. J Pediatr Endocrinol Metab. 2021;34:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Shi ZH, Han XY, Yao MD, Liu C, Jiang Q, Yan B. Differential MicroRNA Expression Pattern in Endothelial Progenitor Cells During Diabetic Retinopathy. Front Cell Dev Biol. 2021;9:773050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Malhotra NA, Greenlee TE, Iyer AI, Conti TF, Chen AX, Singh RP. Racial, Ethnic, and Insurance-Based Disparities Upon Initiation of Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema in the US. Ophthalmology. 2021;128:1438-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Yan L, Vaghari-Tabari M, Malakoti F, Moein S, Qujeq D, Yousefi B, Asemi Z. Quercetin: an effective polyphenol in alleviating diabetes and diabetic complications. Crit Rev Food Sci Nutr. 2022;1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Chai GR, Liu S, Yang HW, Chen XL. Quercetin protects against diabetic retinopathy in rats by inducing heme oxygenase-1 expression. Neural Regen Res. 2021;16:1344-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Bungau S, Abdel-Daim MM, Tit DM, Ghanem E, Sato S, Maruyama-Inoue M, Yamane S, Kadonosono K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid Med Cell Longev. 2019;2019:9783429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 23. | Li LH, Lee JC, Leung HH, Lam WC, Fu Z, Lo ACY. Lutein Supplementation for Eye Diseases. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Noma H, Funatsu H, Yamashita H, Kitano S, Mishima HK, Hori S. Regulation of angiogenesis in diabetic retinopathy: possible balance between vascular endothelial growth factor and endostatin. Arch Ophthalmol. 2002;120:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc. 2021;1:e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 432] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 26. | Wu Q, Liu H, Zhou M. Fangchinoline Ameliorates Diabetic Retinopathy by Inhibiting Receptor for Advanced Glycation End-Products (RAGE)-Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB) Pathway in Streptozotocin (STZ)-Induced Diabetic Rats. Med Sci Monit. 2019;25:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Ahmad Fadzil MH, Izhar LI, Nugroho H, Nugroho HA. Analysis of retinal fundus images for grading of diabetic retinopathy severity. Med Biol Eng Comput. 2011;49:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Ahmad Fadzil M, Ngah NF, George TM, Izhar LI, Nugroho H, Adi Nugroho H. Analysis of foveal avascular zone in colour fundus images for grading of diabetic retinopathy severity. Annu Int Conf IEEE Eng Med Biol Soc. 2010;2010:5632-5635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Lee JH, Cho YS, Jung KH, Park JW, Lee KH. Genipin enhances the antitumor effect of elesclomol in A549 lung cancer cells by blocking uncoupling protein-2 and stimulating reactive oxygen species production. Oncol Lett. 2020;20:374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Wang GF, Wu SY, Rao JJ, Lü L, Xu W, Pang JX, Liu ZQ, Wu SG, Zhang JJ. Genipin inhibits endothelial exocytosis via nitric oxide in cultured human umbilical vein endothelial cells. Acta Pharmacol Sin. 2009;30:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Cao H, Feng Q, Xu W, Li X, Kang Z, Ren Y, Du L. Genipin induced apoptosis associated with activation of the c-Jun NH2-terminal kinase and p53 protein in HeLa cells. Biol Pharm Bull. 2010;33:1343-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Shindo S, Hosokawa Y, Hosokawa I, Ozaki K, Matsuo T. Genipin inhibits MMP-1 and MMP-3 release from TNF-a-stimulated human periodontal ligament cells. Biochimie. 2014;107 Pt B:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med (Zagreb). 2020;30:030502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 34. | Semeraro F, Morescalchi F, Cancarini A, Russo A, Rezzola S, Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019;45:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 35. | Sheemar A, Soni D, Takkar B, Basu S, Venkatesh P. Inflammatory mediators in diabetic retinopathy: Deriving clinicopathological correlations for potential targeted therapy. Indian J Ophthalmol. 2021;69:3035-3049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Zou W, Luo S, Zhang Z, Cheng L, Huang X, Ding N, Pan Y, Wu Z. ASK1/p38mediated NLRP3 inflammasome signaling pathway contributes to aberrant retinal angiogenesis in diabetic retinopathy. Int J Mol Med. 2021;47:732-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Ge K, Wang Y, Li P, Li M, Zhang W, Dan H, Hu X, Zhou J, Yang Q, Wang J, Song Z. Down-expression of the NLRP3 inflammasome delays the progression of diabetic retinopathy. Microvasc Res. 2022;139:104265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Hao J, Zhang H, Yu J, Chen X, Yang L. Methylene Blue Attenuates Diabetic Retinopathy by Inhibiting NLRP3 Inflammasome Activation in STZ-Induced Diabetic Rats. Ocul Immunol Inflamm. 2019;27:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Li Z, Han Y, Ji Y, Sun K, Chen Y, Hu K. The effect of a-Lipoic acid (ALA) on oxidative stress, inflammation, and apoptosis in high glucose-induced human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2023;261:735-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 40. | Han X, Kong J, Hartnett ME, Wang H. Enhancing Retinal Endothelial Glycolysis by Inhibiting UCP2 Promotes Physiologic Retinal Vascular Development in a Model of Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2019;60:1604-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Cho YS. Genipin, an Inhibitor of UCP2 as a Promising New Anticancer Agent: A Review of the Literature. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Bernardo AF, Cortez E, Neves FA, Vieira AK, Soares Vde M, Rodrigues-Cunha AC, Andrade DC, Thole AA, Gabriel-Costa D, Brum PC, Moura AS, Garcia-Souza ÉP. Overnutrition during lactation leads to impairment in insulin signaling, up-regulation of GLUT1 and increased mitochondrial carbohydrate oxidation in heart of weaned mice. J Nutr Biochem. 2016;29:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Brandi J, Cecconi D, Cordani M, Torrens-Mas M, Pacchiana R, Dalla Pozza E, Butera G, Manfredi M, Marengo E, Oliver J, Roca P, Dando I, Donadelli M. The antioxidant uncoupling protein 2 stimulates hnRNPA2/B1, GLUT1 and PKM2 expression and sensitizes pancreas cancer cells to glycolysis inhibition. Free Radic Biol Med. 2016;101:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 44. | Shanmugam MK, Shen H, Tang FR, Arfuso F, Rajesh M, Wang L, Kumar AP, Bian J, Goh BC, Bishayee A, Sethi G. Potential role of genipin in cancer therapy. Pharmacol Res. 2018;133:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 45. | Koudouna E, Huertas-Bello M, Rodriguez CN, Consuelo Henao S, Navarrete ML, Avila MY. Genipin in an Ex Vivo Corneal Model of Bacterial and Fungal Keratitis. Transl Vis Sci Technol. 2021;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Donovan C, Koudouna E, Margo CE, Avila MY, Espana EM. Genipin Delays Corneal Stromal Enzymatic Digestion. Transl Vis Sci Technol. 2021;10:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Fan X, Lin L, Cui B, Zhao T, Mao L, Song Y, Wang X, Feng H, Qingxiang Y, Zhang J, Jiang K, Cao X, Wang B, Sun C. Therapeutic potential of genipin in various acute liver injury, fulminant hepatitis, NAFLD and other non-cancer liver diseases: More friend than foe. Pharmacol Res. 2020;159:104945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 48. | Mir SA, Li Y, Story JD, Bal S, Awdishu L, Street AA, Mehta RL, Singh P, Vaingankar SM. Mice overexpressing chromogranin A display hypergranulogenic adrenal glands with attenuated ATP levels contributing to the hypertensive phenotype. J Hypertens. 2018;36:1115-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Teimouri S, Kasapis S. Morphology of genipin-crosslinked BSA networks yields a measurable effect on the controlled release of vitamin B6. Food Chem. 2020;314:126204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Sun K, Hu K. Preparation and Characterization of Tacrolimus-Loaded SLNs in situ Gel for Ocular Drug Delivery for the Treatment of Immune Conjunctivitis. Drug Des Devel Ther. 2021;15:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Anton N, Dragoi EN, Tarcoveanu F, Ciuntu RE, Lisa C, Curteanu S, Doroftei B, Ciuntu BM, Chiselita D, Bogdanici CM. Assessing Changes in Diabetic Retinopathy Caused by Diabetes Mellitus and Glaucoma Using Support Vector Machines in Combination with Differential Evolution Algorithm. Appl Sci. 2021;11:3944. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Li W, Webster KA, LeBlanc ME, Tian H. Secretogranin III: a diabetic retinopathy-selective angiogenic factor. Cell Mol Life Sci. 2018;75:635-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Jiao MF, Sun S, Wang MM, Dong LJ, Hu BJ, Liu JP, Li W, Li XR. [The observation of secretogranin Ⅲ in the peripheral blood and vitreous of patients with diabetic retinopathy]. Zhonghua Yan Ke Za Zhi. 2020;56:933-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | LeBlanc ME, Wang W, Chen X, Caberoy NB, Guo F, Shen C, Ji Y, Tian H, Wang H, Chen R, Li W. Secretogranin III as a disease-associated ligand for antiangiogenic therapy of diabetic retinopathy. J Exp Med. 2017;214:1029-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 55. | LeBlanc ME, Wang W, Ji Y, Tian H, Liu D, Zhang X, Li W. Secretogranin III as a novel target for the therapy of choroidal neovascularization. Exp Eye Res. 2019;181:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Togayachi A, Iwaki J, Kaji H, Matsuzaki H, Kuno A, Hirao Y, Nomura M, Noguchi M, Ikehara Y, Narimatsu H. Glycobiomarker, Fucosylated Short-Form Secretogranin III Levels Are Increased in Serum of Patients with Small Cell Lung Carcinoma. J Proteome Res. 2017;16:4495-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |