INTRODUCTION
Studies on the dependency of pancreatic enzyme synthesis on insulin release started 60 years ago and resulted in the discovery of the so-called “halo phenomenon”[1-5]. For several years we have explored the ‘reverse arm’ of regulation; namely the dependency of insulin release on pancreatic enzyme secretion and the role of the interaction between these parameters on glucose metabolism[6]. Our studies indicate the local and peripheral dependency of insulin secretion on pancreatic alpha-amylase. Alpha-amylase, beyond its digestive functions, actively participates in glucose metabolism, both via inhibition of glucose absorption from the gut[7,8] and by lowering insulin release[9].
It should be mentioned that other pancreatic-like enzymes, such as proteinases and lipases of microbial origin, were tested with respect to their effect on insulin secretion and glucose utilization. We have shown that lipase does not affect the afore mentioned parameters, while proteinase decreases the insulin sensitivity index and stimulates insulin release[6].
The above-described alpha-amylase-dependent inhibition of glucose absorption, through its storage or metabolism in the gut tissues could reduce insulin release, making the secretion adequate to glucose levels entering the circulation. Thus, there appears to be some sort of intra-pancreatic down regulation effect of alpha-amylase on insulin secretion and/or synthesis. Insulin stimulates the secretion of alpha-amylase and other pancreatic enzymes, while alpha-amylase inhibits insulin release[10]. In fact, chronic hyperinsulinemia could lead not only to insulin resistance, but also to the destruction of pancreatic acinar cells, with the further development of exocrine pancreatic insufficiency (EPI)[11].
The above observations were confirmed in several in vitro and in vivo studies in our lab[9,10,12-14]. Both the alpha-amylase-insulin reciprocal acini-islet-acinar (AIA) local interactions, as well as the peripheral interactions originating in the gut and blood, which attenuate and downregulate insulin secretion, belong to the postulated new type of regulations where alpha-amylase exhibits a hormonal-like action. In 1976, Arnesjö and Lundquist[15] demonstrated a decrease in insulin release and the improvement of glucose tolerance in rats with long-term CCK-PZ stimulated pancreatic enzymes secretion[15]. We noted the stronger suppression of insulin release after pancreatin administration in the meal glucose tolerance test (44%), when compared to the intravenous glucose tolerance test (31%)[13]. In the same year, Lindqvist et al[16] observed a decrease in levels of active glucagon-like peptide-1 (GLP-1) after Roux-and-Y gastric bypass (RYGB) surgery in pigs[16], and in early 2018 our group showed a consequential increase in plasma amylase activity in the EPI porcine model[14]. We recognized that the effects of amylase on the regulation of insulin secretion are para-hormonal and considering all our observations, we thought it would be suitable to locate the anti-incretin action of amylase in the same place where incretins e.g., GLP-1 usually act.
Reciprocal AIA regulations are likely species- and individual-dependent, thus they are in some way immanent. We recognize that dietary factors e.g., carbohydrates (glucose), acting from the duodenum, stimulate insulin and alpha-amylase secretion in a species-specific manner and in individuals’ adequate amount in islands and acini surrounding them. Previous studies have shown that an iv infusion of glucose does not stimulate the secretion of pancreatic alpha-amylase[17]. Considering the observed counteraction of alpha-amylase to glucose-dependent insulinotropic polypeptide (GIP)-1 we used the term anti-incretin to describe metabolic effects of alpha-amylase. This "anti-incretin" hypothesis needs to be confirmed by future studies, with specific focus on enteral-insular regulatory mechanisms. In fact, comparative studies on the exclusive effect of pancreatic alpha-amylase on insulin secretion, stimulated by oral or intravenous glucose administration, have not yet been carried out. The effect of alpha-amylase on the secretion of incretins (GLP-1 and GIP) has also not yet been assessed to date.
Incretin-dependent regulation of insulin secretion is coupled with the intake of “quick” energy in the form of carbohydrates and thus could be recognized as temporal. These regulatory mechanisms need to be abruptly inhibited after the digestion of simple, ‘easily-digested’ carbohydrates in order to prevent the overproduction of insulin by beta cells and thus beta cell exhaustion. The overlooked alpha-amylase dependent downregulation of insulin levels protects both pancreatic beta and acinar cells from metabolic failure.
BILIOPANCREATIC DIVERSION SURGERY REVEALS THE INHIBITORY EFFECT OF ALPHA-AMYLASE ON INSULIN SECRETION
The clinical efficacy of bariatric surgery has encouraged scientific investigation of the gut as a major endocrine organ. Manipulation of gastrointestinal anatomy, through bariatric surgery, has profound effects. Even though these procedures were designed to restrict food intake and increase nutrient malabsorption, evidence suggests that the contribution of the above-mentioned factors to weight loss is minimal. Instead, these interventions reduce body weight gain by decreasing hunger, increasing satiation during a meal, changing food preferences and energy expenditure. GLP-1 analogues, currently used as a treatment for diabetes type 2 (DT2) or slimming injections, are now recognized as alternatives to bariatric surgery in terms of their weight loss and anti-diabetic effects. However, not much is known about their safety with regards to beta cell function in the long run, since the primary target of GLP-1 analogues is the stimulation of insulin release[18].
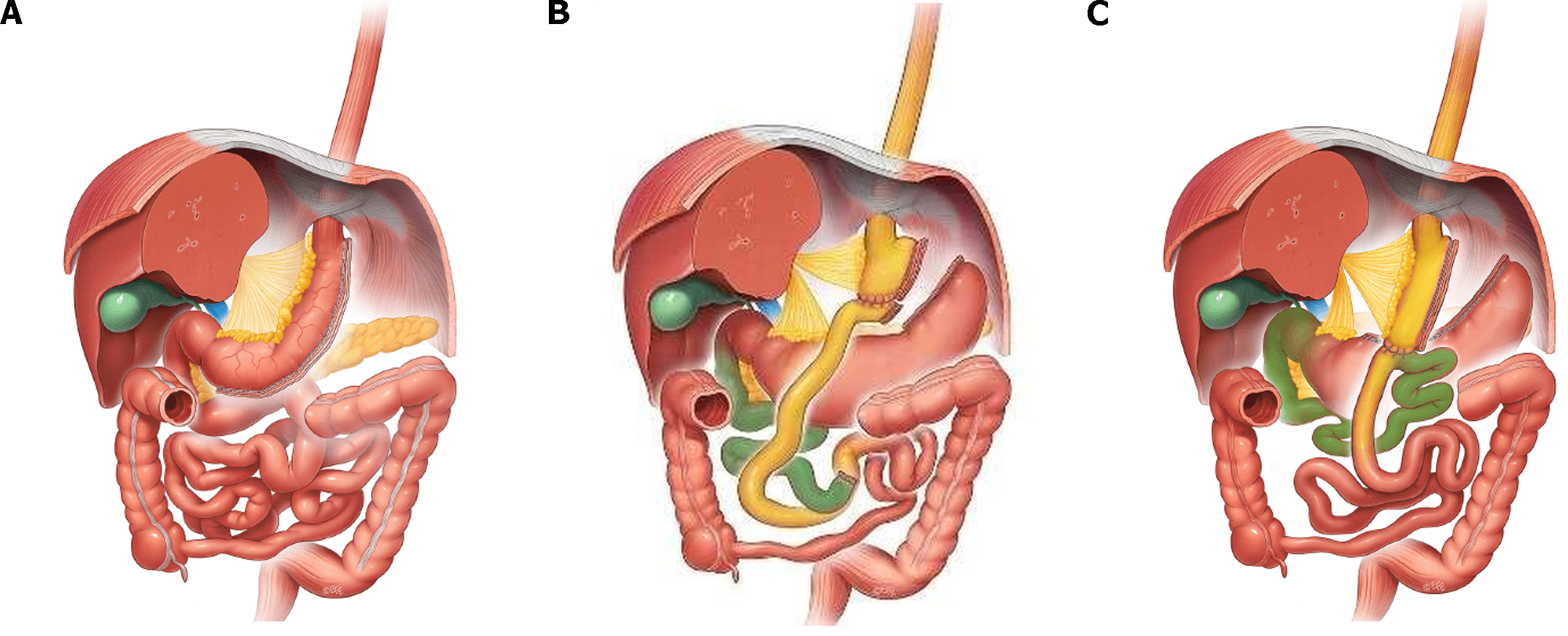
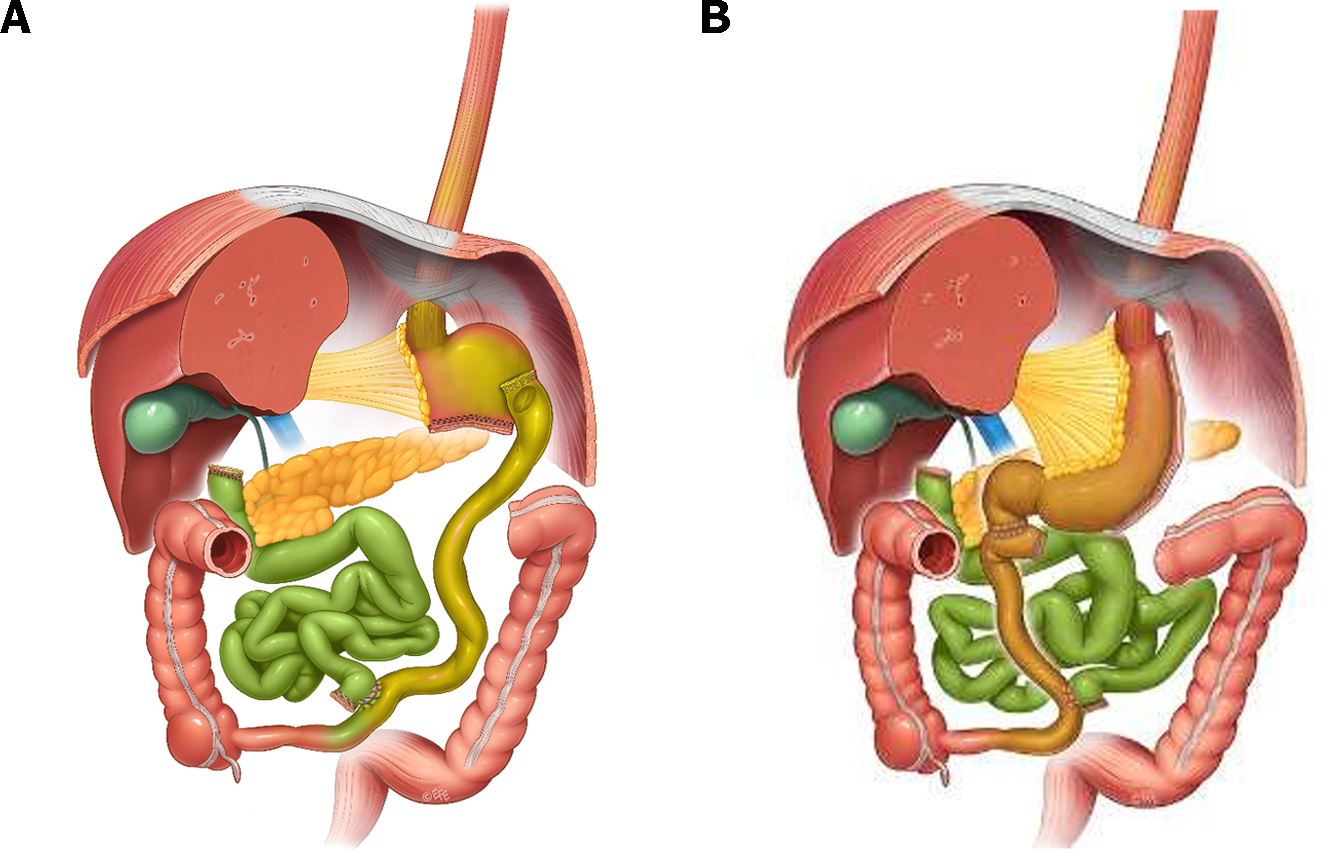
It is worth noticing that RYGB and sleeve gastrectomy (SG)[19-21] (Figure 1) have very similar outcomes both in short-term excessive weight loss and long term total weight loss, as well as in type II diabetes resolution and both procedures are superior when compared to the adjustable gastric banding[22-24]. At the same time, the greatest diabetes remission was observed for patients undergoing biliopancreatic diversion with duodenal switch (BPD-DS) (Figure 2[25,26]) (95.1% resolved), followed by gastric bypass (80.3%), gastroplasty (79.7%), and then laparoscopic adjustable gastric banding (56.7%) The same pattern was observed even for an excessive weight loss and total body weight loss in the long term perspective[27-29]. However, despite better outcomes in terms of obesity and obesity-related comorbidities, BPD-DS counts only for ca 2% of bariatric surgeries performed worldwide due to increased risk of complications and deve-lopment of malnutrition[30].
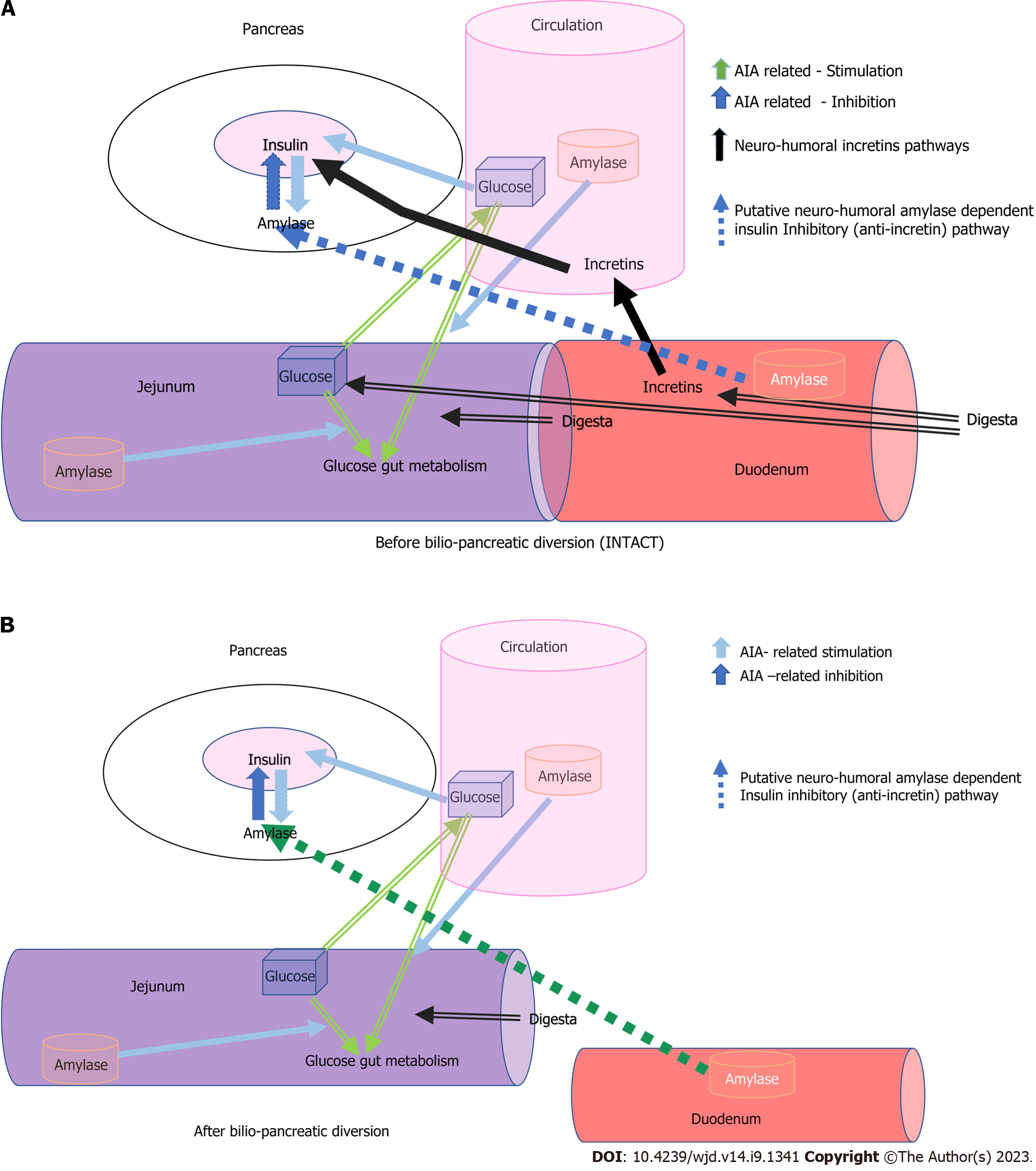
We describe the possible pathways of alpha-amylase-dependent AIA regulation of insulin secretion and their integration with food-related “dietary” incretin regulation of insulin secretion, which are usually eliminated or ameliorated by successful bariatric surgery, using the example of BPD or BPD-DS surgery (Figure 3). Breaking contact between the duodenal mucosa and the digesta coming from the stomach through BPD/BPD-DS surgery, in a broad sense interrupts the enteral-insular axis and thus the resulting decrease in insulin secretion could be due to other mechanisms-it is obvious. However, alpha-amylase still flows into the duodenum as a result of stimulation by gastro-pancreatic reflexes and by the attenuated (yet still existing) reflexes which originate in the alimentary limb (stimulated by digesta). Thus, the inhibitory effect of alpha-amylase on insulin secretion can result in the enhanced elimination of incretins.
Figure 3 A schematic view of acini-islet-acinar- and alpha-amylase-dependent inhibitory pathway involved in the regulation of glucose metabolism before and after Biliopancreatic diversion surgery.
A: Before biliopancreatic diversion surgery; B: After biliopancreatic diversion surgery. Incretin-dependent, quick stimulatory pathways (black) and acini-islet-acinar–dependent intrapancreatic inhibitory pathway and downregulating alpha-amylase dependent pathways, originating in the duodenum, of insulin secretion (orange and green respectively). AIA: Acini-islet-acinar.
It seems that BPD/BPD-DS bariatric surgery followed by immediate and long-term remission of DT2[22-24,27-30] has allowed us, for the first time, to observe and reveal these alpha-amylase-dependent inhibitory effects on insulin secretion and glucose metabolism. BPD/BPD-DS surgery prominently highlights the role of pancreatic alpha-amylase in the down regulation of insulin secretion, not only by means of elimination of the duodenal incretin effects on insulin release.
As shown in Figures 3, the remission of DT2 is related to the elimination of the action of incretins from the duodenum in BPD-SD patients. In these patients, insulin production is mainly dependent on blood glucose levels and its local (in pancreas) down regulation by alpha-amylase and in the diverted duodenum, as was proven in a pig model[13].
Attenuation of incretin release, due to the absence of contact between the chyme and the gastro-duodenal mucosa in the biliopancreatic limb after BPD-SD surgery, results in lower insulin release. At the same time, the passage of food to the alimentary limb is responsible for the slight stimulation of pancreatic enzyme secretion. Since alpha-amylase from the pancreatic juice which is being secreted into the biliopancreatic limb does not interact with its’ starch substrate, the other functions of alpha-amylase, over and above that of starch digestion, e.g., inhibitory effect on insulin secretion, can be highlighted. Whole or partially digested alpha-amylase attenuates glucose absorption and improves the metabolism and/or storage of glucose in the gut tissues[13]. One should bear in mind that alpha-amylase, as well as the other pancreatic enzymes being digested in the BP limb, could serve as a source of different types of biologically active peptides which could affect insulin secretion, as has been proven for alpha-amylase-derived peptides[13]. At the same time, alpha-amylase from the acini surrounding the pancreatic islets can additionally slow down insulin release, which can be recognized as the inhibitory action of alpha-amylase on insulin release. These events are most probably responsible for the elimination of DT2, following BPD/BPD-DS bariatric surgery.
To highlight the efficacy of classical RYGB bariatric surgery, in terms of DT2 remission, one should consider that the residual secretion of stomach/gastric juice is probably the most important factor which could influence the overall success of bariatric metabolic surgery. Lack of stomach juice entering the biliopancreatic limb-which is what happens in patients after BPD/BPD-DS surgery-eliminates the release of most incretins (GIP, GLP-1), thus insulin secretion is stimulated to a much lower extent. The above is not true for other types of bariatric surgery where small amounts of gastric juice still enter the duodenum, thus stimulating incretin release. The role of alpha-amylase in AIA regulations[10,13] has been experimentally proven both in the pig model of BPD[13], with complete isolation of the stomach from the biliopancreatic limb, and in in vitro experiments, where alpha-amylase directly inhibited insulin release from an insulinoma cell line, BRIN-11[9].
Importantly, the pharmacological duodenal inhibition of the actions of incretins, through the use of GLY-200 polymer[31], which mimics bariatric (metabolic) surgery, is being studied in a clinical trial. Mechanical duodenal exclusion, realized via endoscopy, is now being tested in clinics and has produced very good results to date with respect to the remission of DT2[32]. Both strategies assume the exclusion of incretin action and the mechanical prevention of contact between the duodenal mucosa and digesta from the stomach (duodenum isolation), thus allowing AIA pancreatic reflexes, including the anti-incretin action of alpha-amylase, to be realized to the full extent.
Pancreatic alpha-amylase, which is synthesized in the acinar cells and then appears in the pancreatic juice, also leaks into the periinsular interstitial fluid, downregulating insulin secretion. Additionally, digesta entering the alimentary arm (proximal jejunum) stimulates reflexes responsible for insulin and enzyme secretion to a lower extent, since in the proximal jejunum the number of incretins and other gastrointestinal hormone receptors is markedly decreased, when compared to the duodenum.
PANCREATIC ENZYME THERAPY AMELIORATES EPI-DEPENDENT HYPERINSULINEMIA
EPI is frequently diagnosed in both DT1 and DT2 patients[11]. Our own studies on streptozotocin (STZ)-induced diabetes[9] and on EPI[12] pig models have clearly shown that both iv administration of alpha-amylase of microbial origin (Amano, Japan) and the oral administration of pig pancreatic enzymes (enteric coated pancrelipase-Abbot Healthcare Products Ltd, Southampton, United Kingdom), lowers insulin and glucose levels during an iv-glucose tolerance test (IvGTT). However, responses to oral glucose loading are very weak compared to that observed following intravenous loading of a similar amount of glucose. Moreover, studies on healthy pigs[6] have shown that different pancreatic-like enzymes of microbial origin (Sigma-Aldrich), after oral administration, affect insulin release differently; alpha-amylase lowers insulin release, while protease enhances insulin release during an IvGTT and lipase has no effect on insulin release. The above-mentioned studies on EPI and STZ pigs have allowed us to explore the potency of the alpha-amylase-induced, long-lasting AIA regulations of glucose homeostasis, revealing at the same time the protective/downregulating effects of enteral and blood alpha-amylase on the survival of pancreatic beta cells.
Enteral or parenteral alpha-amylase, of microbial or porcine origin, lowers blood levels of insulin and glucose during IvGTT and oral glucose tolerance tests. Undoubtfully, in this scenario, high levels of alpha-amylase in the gut and blood should be treated as a factor which protects pancreatic beta cells from metabolic failure. Nowadays more and more reports about the negative correlation between high plasma amylase activity and the development of metabolic diseases have become available in the literature[33,34].
The “anti-incretin” theory of metabolic surgery mode of action was presented by Rubino et al[35-37] in the beginning of century. The authors considered the gut as a primary organ involved in the regulation of metabolism and describe the balance of “anti-incretins” as a crucial factor which regulates glucose metabolism and could be impaired in metabolic disorders. In their comments to the article by Lindqvist et al[38] describing an increase in b-cell mass after RYGB surgery, Rubino and Amiel[37] point out that proliferation is not always beneficial and in fact, could be due to impaired “incretin/anti-incretin” balance[37]. The same research group demonstrated an improvement in insulin sensitivity in diabetic rats after duodenal-jejunal bypass surgery, without changes in incretin and insulin secretion[39] and revealed the anti-incretin effect of orally administered glucose[40].
However, no endogenic “anti-incretin” factor have been recognized until now what is conditio sine qua non to complete anti-incretin hypotheses. At the same time, the number of reports confirming the alternative, anti-incretin-like role of alpha-amylase, has been increasing over the last few years. For example, the active role of alpha-amylase of duodenal origin in the regulation of enterocytes’ proliferation was shown by Date et al[41,42]; there are reports about antiproliferative effects of alpha-amylase in cancer cell lines[43,44]; alpha-amylase is currently even being considered as one of the main energy regulators in the brain[45]. Although almost all tissues express their own amylase and the extra-digestive roles of amylase have become more and more obvious, the possible role of amylase in the regulation of glucose metabolism is still ignored and overlooked.
The overarching question is how are alpha-amylase signals from the gut lumen and from the blood transferred to pancreatic beta and acinar cells? Due to the nature of the portal vascular system in the pancreas[46,47], local peri-islet insulin levels are very high compared to systemic levels. Our supraphysiological doses of iv infused alpha-amylase were much higher than physiological alpha-amylase levels and thus could possibly have increased the alpha-amylase concentration in the peri-islet circulation to a level which inhibited insulin release[12]. The inhibitory action of alpha-amylase on insulin from the gut lumen requires the existence of a gut-pancreas, alpha-amylase-dependent reflex, which ends in the peri-islet interstitial tissue. The more physiological mechanism, however, would be the direct action of alpha-amylase or alpha-amylase-derived peptides on gut glucose absorption[9,13] or on its storage and metabolism in the gut.
To confirm this intriguing postulation, it is necessary to perform in vivo studies on direct infusion of alpha-amylase alone and/or as a mixture with other pancreatic enzymes, to the artery suppling the pancreas, during iv glucose loading. The expected inhibition of insulin release during such an experiment will finally confirm the role of alpha-amylase in the protection of beta cells from the overproduction of insulin.
CONCLUSION
In summary, we suggest that bariatric BPD/BPD-DS surgery (which is actually seldom performed due to the development of severe EPI syndrome) highlights the never before described alpha-amylase-induced, anti-incretin-like regulation of glucose metabolism, which protects the pancreatic beta cells from exhaustion and subsequent failure. BPD/BPD-DS surgery eliminates the incretin-dependent vicious cycle driven from the duodenum in obese diabetic patients. Food stimulates the synthesis of insulin and alpha-amylase in parallel. Alpha-amylase synthesis is extraordinarily stimulated by insulin-halo phenomenon. In turn, alpha-amylase, through its ability to inhibit glucose absorption and direct downregulation of insulin release[9] limits hyperglycemia and hyperinsulinemia to a certain degree.
ACKNOWLEDGEMENTS
The authors would like to express their thanks to Dr. Janine Donaldson for her constructive feedback and valuable input with regards to the editing of the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Sweden
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gong N, China; Shuang W, China; Horowitz M, Australia S-Editor: Fan JR L-Editor: A P-Editor: Chen YX