Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1301
Peer-review started: March 28, 2023
First decision: May 8, 2023
Revised: May 21, 2023
Accepted: June 5, 2023
Article in press: June 5, 2023
Published online: August 15, 2023
Processing time: 135 Days and 15.6 Hours
Diabetes mellitus (DM) is one of the largest global health emergencies of the 21st century. In recent years, its connection with environmental pollutants, such as bisphenol A (BPA), has been demonstrated; consequently, new structurally similar molecules are used to replace BPA in the plastics industry (BPS, BPF and BPAF).
To carry out a systematic review to allow coherent evaluation of the state of the art. Subsequently, a meta-analysis was performed to unify the existing quantitative data.
Firstly, a systematic review was carried out, using the terms “(bisphenol) AND (Diabetes OR Hyperglycemia)”, to maximize the number of results. Subsequently, three authors analyzed the set of articles. Finally, a meta-analysis was performed for each BP, using RevMan software. In addition, funnel plots were developed to study publication bias.
The systematic analysis of the literature revealed 13 recent articles (2017–2023) related to the study paradigm. The qualitative analysis showed interesting data linking diabetes to the three most widely used substitute BPs in the industry: BPS, BPF and BPAF. Finally, the meta-analysis determined a positive relationship with BPS, BPF and BPAF, which was only statistically significant with BPS.
There is a need to apply the precautionary principle, regulating the use of new BPs. Therefore, replacing BPA with BPS, BPF or BPAF is unlikely to protect the population from potential health risks, such as DM.
Core tip: The present study analyzed the potential dangers that society faces with the replacement of bisphenol A (BPA) by new BPs. Thus, using PRISMA methodologies, a systematic review and meta-analysis of the relationship between new BPs and diabetes mellitus (DM) in humans was carried out. The results showed a positive relationship between BPS, BPF and BPAF and DM, which was statistically significant only with BPS. Consequently, new BPs could represent a health risk equivalent to that of BPA.
- Citation: Moreno-Gómez-Toledano R, Delgado-Marín M, Cook-Calvete A, González-Cucharero C, Alcharani N, Jiménez-Guirado B, Hernandez I, Ramirez-Carracedo R, Tesoro L, Botana L, Sánchez-Esteban S, Diez-Mata J, Zamorano JL, Bosch RJ, Zaragoza C, Saura M. New environmental factors related to diabetes risk in humans: Emerging bisphenols used in synthesis of plastics. World J Diabetes 2023; 14(8): 1301-1313
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1301.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1301
Diabetes mellitus (DM) is one of the largest global health emergencies of the 21st century[1]. The prevalence of DM in recent decades has increased substantially. In 1980, the number of people affected was around 108 million adults aged 20–79 years; currently, the prevalence is 10.5% of the world population (536 million people affected), and it is estimated that could increase to 12.2% in 2045 (783.2 million)[2]. DM risk factors include numerous environmental and/or genetic factors, including covariates such as age, weight, diet, and smoking[3]. Therefore, the idea that environmental pollutants could play a role in the development or progression of the disease is coherent. In the literature, there is evidence that suggests a possible relationship between DM and environmental pollutants[4].
Plastics are one of the main environmental pollutants that modern society faces. Thanks to their multiplicity of uses and low cost, plastics have become one of the main axes of modern industry. The central element of the plastics industry is bisphenol A (BPA); a monomer of epoxy resins and polycarbonates used as an additive and improver of the physical properties of different polymers[5]. In the mid-1970s, BPA was part, directly or indirectly, of all major American industries[6]. Currently, the production volume of plastics has increased from 2 million tons (in 1950) to 368 million tons in 2019[7]. Economic studies estimate that plastic production will double in the next 20 years[8].
In recent years the scientific community has highlighted the potential health risks associated with BPA exposure, related with numerous pathologies, such as hormonal alterations[9,10], DM[11], obesity[12], hypertension[13], chronic kidney disease/diabetic nephropathy[14,15] or even with disorders of embryonic development[16,17]. For this reason, the new emerging regulations limit the use of BPA in various contexts, such as baby products[18,19], thermal paper (used in purchase receipts)[20], or containers[21]. Consequently, industries are replacing BPA with substitute molecules with similar structure and molecular weight. The three most important molecules in the plastic industry are BPS, BPF and BPAF[22-24]. In terms of European legislation, BPA is the only monomer that has a harmonized EU classification as toxic to reproduction 1B, H360F (may damage fertility). However, BPS is self-classified under REACH (EU chemicals legislation) as toxic to reproduction 2 (H361f), and BPAF is self-classified as toxic to reproduction 1B (H360F)[25]. BPF has not been classified, but it also has the potential to induce reproductive toxicity[25], showing a hormonal activity as active as BPA or BPS[22]. Despite the small number of publications exploring the possible effects of these new molecules on human health, their presence has already been detected in air, water, and food of many parts of the world[24,26-28].
The present study was a systematic review of the literature to allow a coherent evaluation of the state of the art. Subsequently, a meta-analysis was performed to unify the existing quantitative data. The primary outcome measures were serum/plasma or urinary BPs (except BPA) in relation to DM. The analysis was limited to humans and English language, but no restriction was applied in the academic search engines.
The study was conducted using the PRISMA guidelines[29,30] as a methodological basis. The main objective of the study was to identify and analyze the state of the art of the new bisphenols–diabetes paradigm. In recent years, the number of evidence related to BPA has increased; however, the new BPA substitute molecules continue to be relegated to the background in the literature, with a small amount of available evidence. For this reason, all those original studies that studied the possible implications of any BP (except for BPA), in the context of human populations, were selected. From the set of publications selected for the qualitative analysis, manuscripts with logistic regression analyzes were selected to quantitative analysis.
The search for articles of interest was performed in December 2022, using the reference academic search engines PubMed (PubMed.ncbi.nlm.nih.gov, accessed on 20 December 2022) and Web of Science (webofscience.com/wos/alldb/basic-search, accessed on 20 December 2022). To maximize the results and avoid losing potential articles of interest, a strategy focused on the generic terms was used. The terms “(Bisphenol) AND (Diabetes OR Hyperglycemia)” were used, without adding any restrictions in academic search engines. The search was carried out by three researchers independently (RMGT, MDM and ACC) and their decisions in each of the bibliographic search and evaluation steps were determined by consensus.
After removal of duplicate articles using the Mendeley bibliography manager (Mendeley Ltd., Elsevier, London, UK), the articles were evaluated by title/abstract. All the articles that were not original (such as reviews), in vitro or in vivo research models, exclusively BPA study models, or studies of compounds that were not BPs (such as phthalates), and all those articles that did not study DM, were excluded (Table 1). Subsequently, the full text of the manuscripts was analyzed and evaluated.
| Criteria | Description |
| Inclusion criteria | Studies published in peer-reviewed journals |
| Studies published as original article accepted and published | |
| Studies conducted in human populations, regardless of the population subgroup | |
| Studies focused on bisphenols, except BPA | |
| Exclusion criteria | Reviews, hypotheses, proyect reports, letters or comments |
| In vitro or in vivo study models | |
| Studies performed only on BPA, or on compounds other than bisphenols | |
| Studies not developed in diabetes |
After the full-text analysis, a descriptive analysis of the selected articles was performed. In addition, relevant data for the qualitative study and subsequent quantitative analysis were extracted. All the studies that provided odds ratio (OR) and 95% confidence interval (CI) were selected. Studies that performed correlations, linear regressions, or multivariate analyzes were only included in the descriptive analysis. Discrepancies between independent reviews were resolved by consensus.
RMGT, MDM, ACC, CGC, NA, BJG, IH, RRC, LT and LB extracted the data for Tables 2 and 3: first author, year of publication, country, population group, number of individuals included, age, study period, type of study (Table 2), BP, biological fluid analyzed, analysis method, detection frequency, and metabolite concentration determined (Table 3).
| Ref. | Country | Poblation group | N | Age | Study period | Type of study |
| Kataria et al[32], 2017 | USA | Healthy children | 41 (19 males; 22 females) | 10-13 | 2013-2014 | Cross-sectional |
| Li et al[33], 2018 | Saudi Arabia | Diabetic vs Control | 54 (28 males and 26 females) vs 47 (20 males and 27 females) | 28-68 | 2015-2016 | Cross-sectional (case-control) |
| Duan et al[34], 2018 | China | Diabetic vs Control | 251 vs 251 | D: 58 ± 10; C: 51 ± 10 | 2016-2017 | Cross-sectional (case-control) |
| Zhang et al[35], 2019 | China | Pregnant women | 1841 (167 GDM and 1674 Non-GDM) | GDM: 30.07 ± 4.11; non-GDM: 28.44 ± 3.14 | 2013-2015 | Prospective study |
| Lee et al[36], 2019 | Korea | Premenopausal adult women | 459 | 20-48 | 2015-2016 | Cross-sectional |
| Rancière et al[37], 2019 | France | Diabetic vs Control | 201 vs 584 | 30-65 | 1994-1996 + 3, 6 and 9 years | Longitudinal study |
| van der Meer et al[38], 2021 | Netherlands | Subjects with impaired fasting glucose (i.e., fasted glucose 6.1 mmol/L to 7.0 mmol/L) | 500 (299 males and 201 females) | 53.4 ± 10.3 | 2009-2013 and 2014-2015 | Longitudinal study |
| Tang et al[39], 2023 | China | GDM vs non-GDM pregnant women | 100 vs 400 | 30.62 ± 6.46 vs 30.60 ± 6.41 | From 2015 | Cross-sectional (case-control) |
| Lee et al[40], 2021 | USA | Diabetes-free women | 1299 | 45-56 | 1999-2000, 2002-2003 | Longitudinal study |
| Duan et al[41], 2021 | China | Diabetic vs Control | 60 vs 60 | 56 ± 7 vs 56 ± 7 | 2016-2017 | Cross-sectional (case-control) |
| Moreno-Gómez-Toledano et al[43], 2022 | USA | General population | 3658 (641 diabetic) | Non-D: 41.11, D: 58.33 | 2013-2016 | Cross-sectional |
| Zhu et al[44], 2022 | USA | GDM vs non-GDM pregnant women | 333 | 31.2 ± 4.6 | Cross-sectional (case-control) |
| Biological fluid | Bisphenol analyzed | Analysis method | Detection frequency (%) | GM (95%CI)/median (IQR) |
| Urine | BPS/BPF | HPLC-MS/MS | - | 2.06 (1.56-2.69)/0.141 (0.141-0.141) |
| Urine | BPF/BPS/BPAP | HPLC-MS/MS | D: 81.5/15.9/0.0; C: 48.9/0.0/17.0 | D: 3.6/0.10/0.05 |
| Urine | BPS/BPAF/BPF | HPLC-MS/MS | D: 68.1/57.4/26.3; C: 47.8/39.4/37.1 | D: 0.199 (ND-0.56)/0.093 (ND-0.84)/ND (ND-0.12); C: ND (ND-0.25)/ND (ND-0.05)/ND (ND-0.23) |
| Urine | BPS/BPAF/BPF | UHPLC-TQMS | 90.06/42.59/94.72 | 0.36 (0.33, 0.38)/0.030 (0.028, 0.031)/2.01 (1.75, 2.32) |
| Urine | BPS/BPF/BPB/BPAP | HPLC-MS/MS | 83.7/3.7/1.3/4.8 | 0.08 (0.03-0.24)/-/-/- |
| Urine | BPS-glucuronide | HPLC-MS/MS | Baseline: 14; year 3:9 | < LOD (< LOD-< LOD) |
| Urine | BPS/BPF | LC-MS/MS | Baseline: 13/55; follow-up: 18/53 | Baseline: < LOD (< LOD-< LOD)/0.29 (< LOD; 0.81); follow-up: < LOD (< LOD; < LOD)/0.25 (< LOD; 0.77) |
| Serum | BPS/BPF/BPB | UPLC-MS | 82.2/67.2/88.8 | 0.097 (0.050-0.107)/0.605 (> LOD-0.609)/0.236 (0.233-0.269) |
| Urine | BPF | HPLC-MS/MS | Baseline: 73.7; follow-up: 80.6 | Baseline: 0.99 (2.86); follow-up: 1.11 (2.64) |
| Urine | BPS/BPF/BPAF | HPLC-MS/MS | D: 66.7/31.7/45.0; C: 40.0/40.0/41.7 | D: 0.21 (ND-0.35)/ND (ND-0.23)/ND (ND-0.15); C: ND (ND-0.23)/ND (ND-0.31)/ND (ND-0.05) |
| Urine | BPS/BPF | HPLC-MS/MS | 88.4/57.1 | D: 0.59 (0.53-0.64)/0.43 (0.38-0.48); C: 0.50 (0.48-0.52)/0.41 (0.39-0.43) |
| Urine | BPS/BPF | HPLC-MS/MS | 75.1-90.0/- | 0.497 (0.436-0.559)/not calculated1 |
Review Manager software (RevMan 5.3, Cochrane, London, UK) was used to perform the inverse variance method. An analysis was performed for each type of BP present in the literature (BPS, BPF and BPAF). Heterogeneity between studies was calculated by applying the 2 and I2 tests. The I2 statistic was calculated as a percentage, and the results were interpreted as low, medium or high heterogeneity, reaching 25%, 50% and 75%, respectively[31]. The fixed-effect model was used when no heterogeneity was detected among studies, while the random-effect model was preferred when variance existed. P < 0.05 was considered statistically significant for all the analyses performed.
The individual quality of the articles was evaluated considering the use of urinary creatinine or urine gravity as a normalization factor for glomerular filtration rate, and the use of covariates related to diabetes in the development of binomial and multinomial logistic regression models. For the evaluation of publication bias in the meta-analysis, funnel plots were used to identify symmetry or asymmetry in the distribution of results.
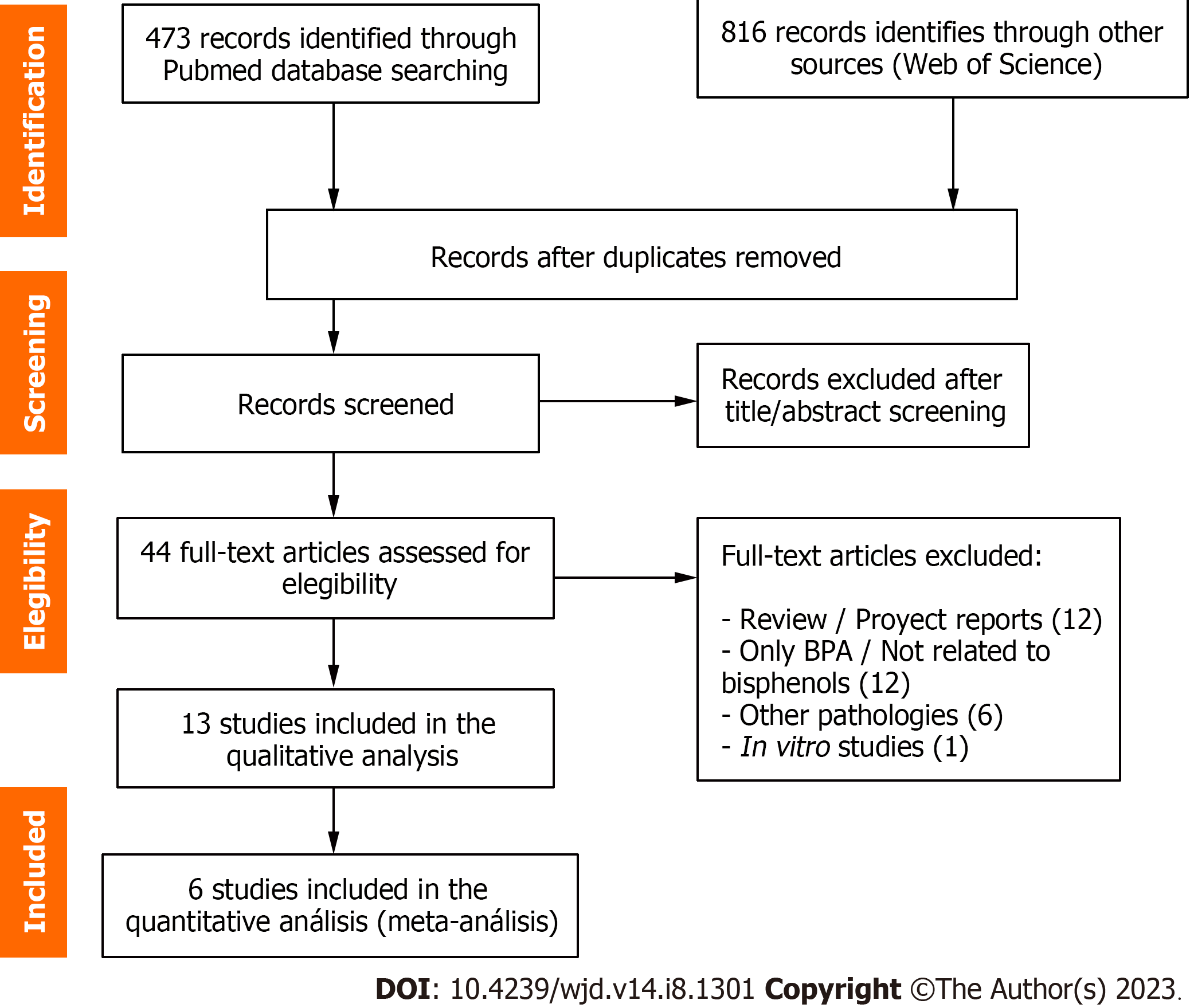
The initial search identified 472 articles in PubMed and 816 in Web of Science. After exporting the set of references to the Mendeley desktop application and removing duplicates, a total of 928 items were obtained. The first analysis carried out based on the title and abstract eliminated 884 articles that did not meet the selection criteria, yielding a total of 44 articles. From them, 13 manuscripts that met the search criteria were selected for qualitative analysis, and six were finally included in the quantitative analysis. As can be seen in Figure 1, the rest of the academic papers corresponded to reviews or project reports (n = 12), studies of BPA or compounds not related to BPs (n = 12), pathologies other than diabetes (n = 6) and an in vitro study. The degree of novelty of the topic is reflected in the temporality of the 13 selected articles, published from 2017 to 2022.
From a chronological point of view, the first work of interest was Kataria et al[32], published in 2017 (see Table 2 for qualitative manuscript details). The authors studied urinary BPs (BPA, BPS and BPF), and blood glucose and insulin levels in a small cohort of children aged 10–13 years. Subsequently, multivariate regression analysis of overweight, body mass index (BMI), insulin resistance, and albumin to creatinine ratio (ACR) showed only significant differences between BPS and ACR. In conclusion, the authors stated that BPS exposure was associated with renal function, but neither BPS nor BPF were related to DM.
In 2018, the first significant evidence between the new substitutes for BPA and DM in human populations appeared: Li et al[33] and Duan et al[34]. Li et al[33] observed a significant relationship between BPF and DM risk in an adult human cohort from Saudi Arabia. In the multinomial logistic regression model performed between quartile 4 (Q4) versus Q1 of BPF, corrected for creatinine, using age, gender, nationality, smoking status, and occupation as covariates, an OR (95%CI) of 8.02 (1.68–38.3) was observed. Due to the low presence of BPS and BPAF, they did not develop statistical association models with the BPA derivatives (Table 3). Duan et al[34] only observed statistically significant results for urinary BPS and BPAF. They performed binomial logistic regression analyzes for DM in a cohort of 251 DM patients versus 251 controls. After correcting for urinary creatinine and including the covariates sex, age, BMI, smoking and alcohol consumption, exercise status, education level, family history of DM, and blood pressure, an OR (95%CI) of 1.73 (1.37–2.18) for BPS and 4.95 (3.15–7.79) for BPAF was obtained.
In 2019, three articles relevant to the context of this manuscript were published: Zhang et al[35], Lee et al[36] and Rancière et al[37]. The studies were conducted in pregnant women, premenopausal adult women, and in the general population. Zhang et al[35] observed a significant association between BPAF and risk of gestational DM (GDM) in pregnant women with healthy BMI, determining an OR (95%CI) of 1.70 (1.03–2.72). The authors normalized the values of urinary metabolites correcting with specific gravity. Additionally, the logistic regression models performed in women with normal or high BMI, were corrected with the covariates maternal age, pre-pregnancy BMI, educational levels, parity, passive smoking and fetal sex. Lee et al[36] performed multipollutant models. The results showed a significant relationship between urinary BPS and the homeostasis model assessment for insulin resistance (HOMA-IR), reaffirming the possible relationship between BPS and DM. Finally, Rancière et al[37], in a 9-year longitudinal study carried out in the DESIR cohort, associated the glucuronidated form of BPS (BPS-G) with an increased risk of DM. Due to the small number of samples with the presence of BPS-G, they subdivided the population between the presence or absence of BPS-G, obtaining a hazard ratio value (95% CI) of 2.81 (1.74–4.53).
In 2021 five articles relevant to the analysis were published: van der Meer et al[38], Tang et al[39], Lee et al[40], Duan et al[41] and An et al[42]. van der Meer et al[38] analyzed the presence of endocrine-disrupting metabolites in the urine of subjects with impaired fasting glucose levels (6.1–7.0 mmol/L). The authors collected two samples per individual in two different times (first sample 2009–2013; second sample 2014–2015) and investigated the BP metabolite excretion over time both within and between individuals. Interestingly, while BPA median concentrations decreased (50% reduction), BPF levels remained stable within individuals and over time. BPS was detected only in 18% of the samples, so it was excluded from subsequent analysis. Tang et al[39] performed a case–control study in pregnant women with and without GDM. Multinomial logistic regression models performed with serum BPS and BPF, corrected for pregnancy BMI, area of residence, passive smoking during pregnancy, gravity, parity, and exercise regularly, showed positive but nonsignificant results, with OR (95%CI) of 1.68 (0.95–2.99) for highest levels of BPS, and 1.18 (0.68–2.05) for BPF. Lee et al[40] analyzed urinary BPF in a longitudinal study with 1299 nondiabetic women (45–56 years) and were followed 3 years later. Individual phenols were examined using Cox regression, and the overall joint effects using quantile-based g-computation. The results showed no significant associations between BPF and DM in middle-aged women. Duan et al[41] published a new case–control study in 60 type 2 DM patients and 60 controls, matched by age, sex and BMI. They analyzed 19 serum metabolic biomarkers using multiple linear regression models, and observed a significant association between BPS, BPAF (but not BPF) with several serum metabolites (Pyridoxal, L-histidine and L-citrulline) that could be related to DM (and other pathologies related to endothelial dysfunction). Finally, An et al[42] used a different methodological approach. Published datasets related to the genes, proteins and metabolites disturbed by BPS were investigated through omics methods. An interesting conclusion revealed by this analysis was that high concentrations of BPS tended to downregulate biomolecules, while low BPS concentrations tended to enhance metabolic reactions. Furthermore, the authors found evidence of DM-related metabolic disturbances influenced by BPS exposure, such as vitamin or glutathione metabolism.
Finally, Moreno-Gómez-Toledano et al[43] and Zhu et al[44] published two retrospective cohort studies, in the general population and pregnant women, respectively. In the Moreno-Gómez-Toledano et al[43] study, urinary BPS and BPF, corrected with creatinine, were analyzed using binomial and multinomial logistic regression models, corrected by age, sex, BMI, smoking, hypertension, and DM. For the urinary BPS, the results were 1.099 (1.016–1.188), OR (95%CI) in the binomial, and 1.28 (0.99–1.67) in the multinomial analysis. Urinary BPF showed OR of 0.991 (0.928–1.059) and 0.92 (0.70–1.20), respectively. Zhu et al[44] did not analyze BPF because the proportion of results below the limit of detection (LOD) was too high to provide a valid result. Urinary BPS was analyzed through multinomial logistic regression models, adjusted for urinary creatinine levels, age, pre-pregnancy BMI, and race/ethnicity (White, Black, Hispanic, and other). The results showed an OR (95% CI) in Asian/Pacific Islanders (A/PIs) of 2.12 (1.0–4.5) and 4.60 (1.55–13.7) in non-A/PIs.
As previously detailed, the BPA substitutes–diabetes paradigm comprises a small number of heterogeneous but potentially significant publications.
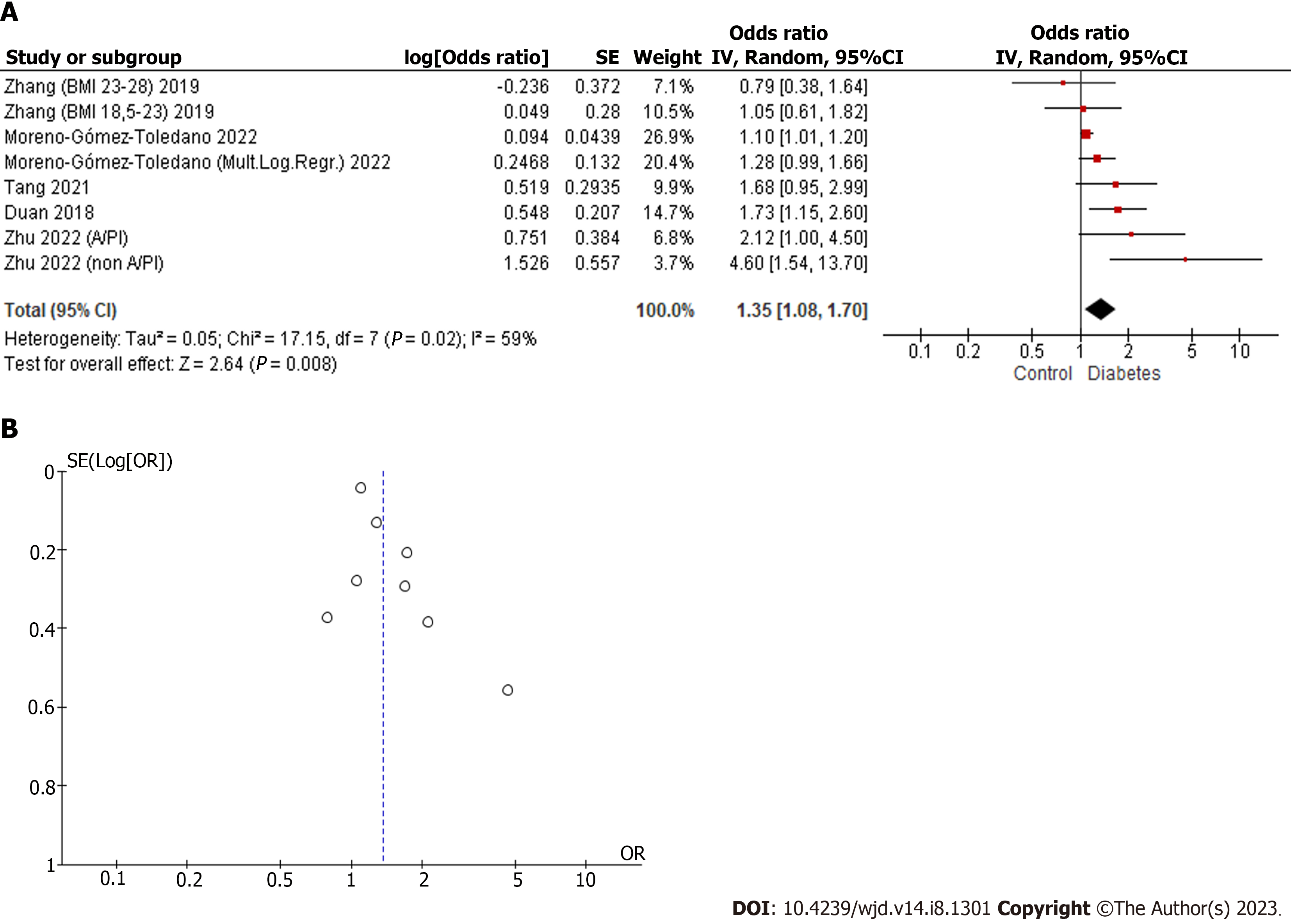
For the BPS meta-analysis, the works of Duan et al[34], Zhang et al[35], Tang et al[39], Zhu et al[44] and Moreno-Gómez-Toledano et al[43] were used. For the combined analysis, binomial and multinomial logistic regression analyses of the different population groups were selected, including a total of eight elements in the meta-analysis. In the work of Zhu et al[44], the population was subdivided into two differentiated groups: A/PIs and non-A/PIs. In Zhang et al[35], pregnant women with normal pre-pregnancy BMI (18.5–23.0) and high pre-pregnancy BMI (23–28) were included. Finally, in the work of Moreno-Gómez-Toledano et al[43], binomial and multinomial logistic regression model analyses were performed with a multiethnic American cohort of adult individuals.
The results of the combined analysis, as can be seen in Figure 2, showed a moderate heterogeneity, (I2 = 59%). The combined odds ratio was 1.35 (1.08–1.70), with a highly significant P = 0.008. The positive and significant results increased the strength of the evidence that BPS could be an environmental factor that could be related to DM.
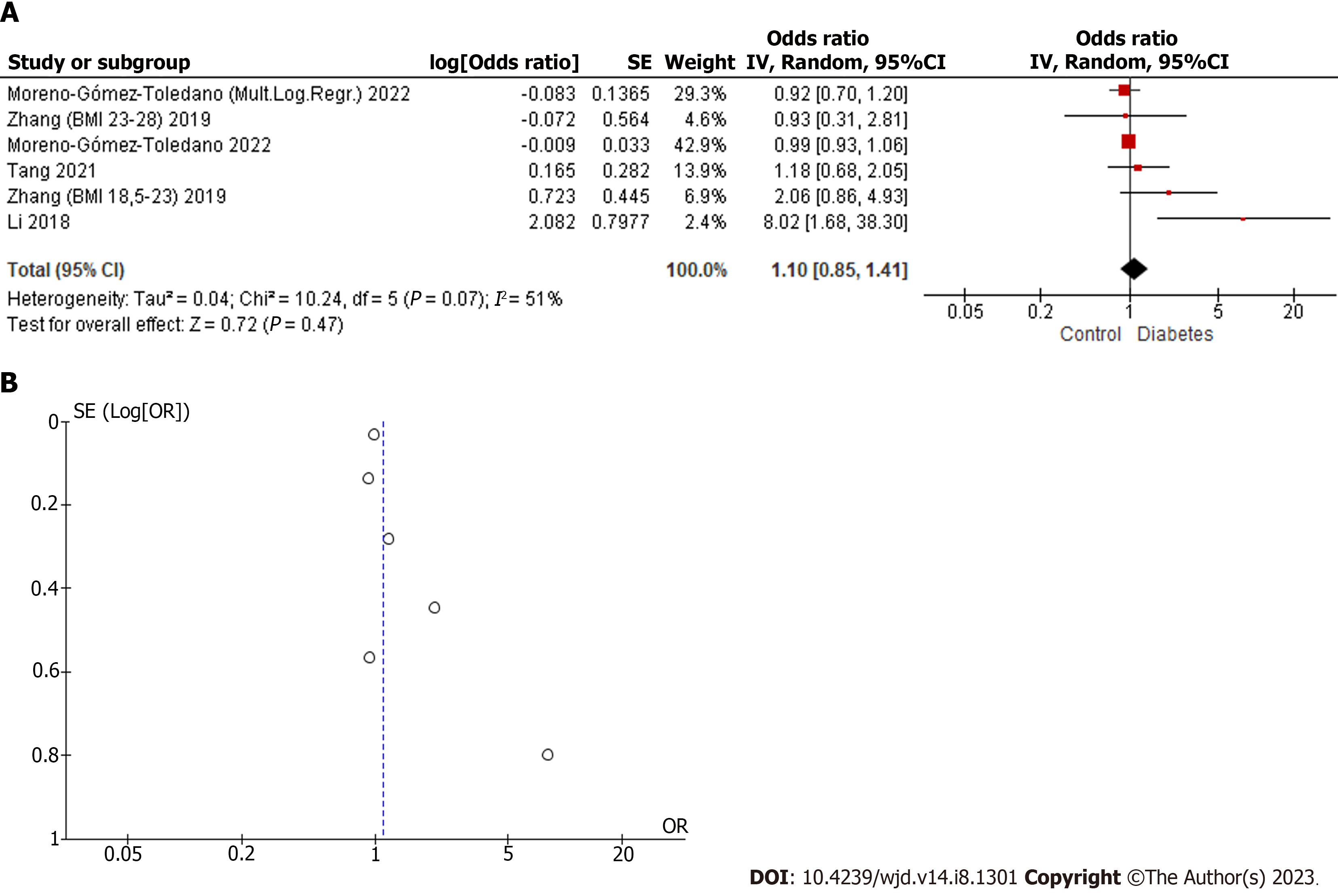
Articles with relevant data for qualitative analysis were Zhang et al[35], Tang et al[39], Moreno-Gómez-Toledano et al[43] and Li et al[33]. The same subgroups used in the quantitative analysis of the BPS were included, in addition to the multinomial logistic regression performed in the case–control study by Li et al[33]. The results did not show a significant combined result, although they showed a positive trend with DM (Figure 3). Except for the work of Li et al[33], none of the other study models showed a significant relationship between BPF and DM, which agrees with the result of the combined model. The I2 of 51% and combined odds ratio of 1.10 (0.85–1.41), with P = 0.47, showed the moderate heterogeneity of the studies and confirmed that there was no evidence to connect BPF with DM.
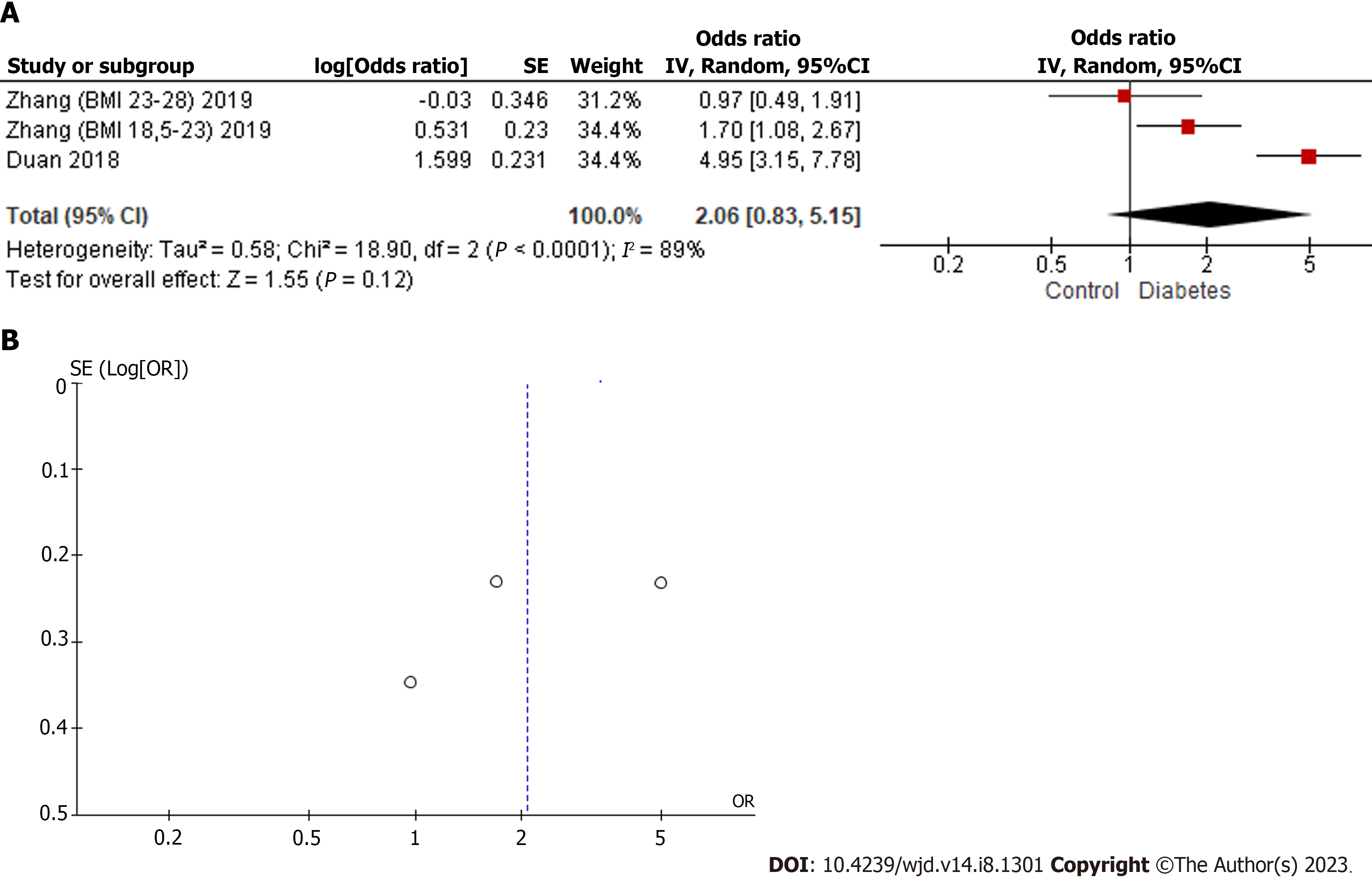
The third most widely used BP is the one with the least amount of evidence in the literature. As can be seen in Figure 4, three population groups from Duan et al[34] and Zhang et al[35] were included. I2 showed a high degree of heterogeneity (89%). The combined result (2.06; 0.83–5.15) showed a positive trend with DM, but due to the small amount of evidence and the absence of a significant result, it was concluded that it is necessary to increase the number of studies to explore the possible implications of BPAF for the risk of development or progression of DM.
Despite the moderate degree of heterogeneity of the studies included in the quantitative analyzes of BPS and BPF, the funnel plots showed symmetry, as can be seen in Figures 2 and 3. In the case of the BPAF, there was insufficient evidence. As can be seen in Figure 4, it is essential to increase the number of studies related to the BPAF–diabetes paradigm.
The present study was the first systematic review and meta-analysis of the new emerging bisphenols–diabetes paradigm. The systematic analysis of the literature has identified 13 studies with evidence for the context of the study in humans. The detailed analysis of the genealogy of the paradigm provided qualitative and quantitative data, which were used for the subsequent meta-analysis for each of the three most widely used BPA substitutes used in the plastic industry.
The new BPA substitute molecules retain a similar structure, with the presence of two phenolic rings. The monomers only differ in their interphenolic linker, characterized by the presence of sulfur in BPS, fluorine in BPAF, and the absence of methyl groups (CH3) in BPF[25,45]. Possibly due to their structural homology, there is evidence that suggests similarities in the hormonal activity of the new BPs[22]. In wild-type mice, Marroqui et al[46] observed that treatment with BPS and BPF rapidly increased insulin release and decreased ATP-sensitive K+ channel activity. In contrast, treatment in beta estrogen receptor knockout (BERKO) mice did not cause DM-related changes. For BPAF, Wei et al[47] demonstrated an important relationship with the development of DM in zebrafish (Danio rerio) exposed to environmentally relevant concentrations of the phenolic molecule. Animals exposed to µg/L doses suffered a significant increase in fasting blood glucose levels, hepatic glycogen content, and hepatosomatic indexes, and decreased muscular glycogen content. In addition, they observed alterations in insulin regulation, and quantitative PCR revealed alteration of genes involved in glycometabolic networks, which might promote hepatic gluconeogenesis and inhibit glycogenesis and glycolysis in the muscle and/or liver.
The quantitative results of the meta-analysis showed that the evidence analyzed in the literature related to BPS and DM showed a positive and significant relationship. There was a moderate degree of heterogeneity between studies and the symmetrical pattern observed in the funnel plot added robustness to the combined analysis. The OR (95% CI) of 1.35 (1.08–1.70), with a P value of 0.008 confirmed the qualitative evidence described in the qualitative analysis. However, BPF showed a positive trend, but did not show a significant result. Similarly, in the case of the BPAF, probably due to the small amount of evidence available, a significant result (although markedly positive) was not obtained either.
There were two examples in the literature that pointed to BPS as a potentially more dangerous monomer than BPA, because there was alarming evidence related to the pharmacokinetics and biodegradability of BPS. Gayrard et al[48] observed that the bioavailability of BPS was 250 times greater than BPA in a porcine study model, and Danzl et al[49] demonstrated that BPA and BPF were biodegradable in the marine environment; a phenomenon that does not occur with BPS.
Duan et al[41] (described in the qualitative analysis), observed metabolome alterations in a cohort of 60 patients with DM and 60 control subjects. Cohort analysis revealed a significant association (linear regression models) between BPS and pyridoxal 5’-phosphate (PLP). PLP deregulation has been linked to DM and blood glucose regulation. In addition, PLP may improve insulin sensitivity by controlling expression of the gene related to adipogenesis[41]. Metabolome analysis also revealed a significant association between BPAF and pyridoxal, L-histidine and L-citrulline. Histidine supplementation has been shown to be effective for insulin resistance, plasma lipid levels, and inflammatory markers, and delayed the development of atherosclerosis in several rodent models of diaDMbetes and metabolic syndrome[50]. Furthermore, citrulline is involved in the production of nitric oxide by nitric oxide synthase, and it plays a crucial role in DM[51], since it is a strong vasodilatory and anti-inflammatory signaling molecule that plays diverse roles in maintaining vascular homeostasis[52].
The body of evidence analyzed in this study revealed interesting relationships between the new BPA substitute molecules and DM. The quantitative results showed a positive relationship with BPS, BPF and BPAF, which was only significant with BPS. The present work revealed the small amount of scientific evidence related to the paradigm in the human context, as well as the need to deepen the study of the emerging BPA substitute molecules. Our results suggest the need to apply the precautionary principle, regulating the use of new BPs. In conclusion, replacing BPA with molecules such as BPS, BPF or BPAF is unlikely to protect the population from potential health risks, such as DM.
Diabetes mellitus (DM) is one of the largest global health emergencies of the 21st century. The prevalence of DM has increased substantially, from 108 million adults in 1980 to 536 million. In parallel, the consumption of plastic products has increased substantially in recent decades, which implies chronic exposure to monomers, such as bisphenol (BP)A, or its new substitute molecules, BPS, BPF and BPAF.
In recent years, the relationship between BPA and DM has been demonstrated. The new BPA substitute molecules have high structural homology with BPA, as well as similar hormonal activity. Therefore, the study of new BPs is potentially linked to population health.
The present systematic review of the literature allowed a coherent evaluation of the state of the art of the new bisphenols–diabetes paradigm. Subsequently, a meta-analysis was performed to unify the existing quantitative data in human cohorts.
Using the PRISMA guidelines as a reference, a systematic review of the literature was carried out. Using the qualitative data, a chronological review was performed, and all quantitative data of interest were identified. Subsequently, a meta-analysis was performed for each BP identified using the RevMan software, and a funnel plot was also performed for risk of bias.
Qualitative analysis identified 13 recently published articles (2017–2022) that contextualized the new evidence between emerging BPs and DM. The subsequent meta-analysis showed positive results with the three BPs, but only BPS was significant.
The present study was the first systematic review and meta-analysis of the new BPA substitute molecules and DM. The results support the possible positive relationship between the new BPs and the risk of DM, especially with BPS. Consequently, the substitution of BPA may not improve population health, and government institutions should consider applying the precautionary principle.
The results support the need to deepen the paradigm, increasing the evidence in basic and translational research, to determine the real risk to which the human population is exposed.
We want to thank Rosalía Gómez-Toledano (philologist and English teacher for 37 years) for proofreading the manu-script.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belosludtseva NV, Russia; Lei XH, China; Zhao W, China S-Editor: Chen YL L-Editor: Kerr C P-Editor: Cai YX
| 1. | Fan W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc Endocrinol. 2017;6:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4813] [Article Influence: 1604.3] [Reference Citation Analysis (36)] |
| 3. | Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep. 2019;21:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 402] [Article Influence: 67.0] [Reference Citation Analysis (1)] |
| 4. | Yang BY, Fan S, Thiering E, Seissler J, Nowak D, Dong GH, Heinrich J. Ambient air pollution and diabetes: A systematic review and meta-analysis. Environ Res. 2020;180:108817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 5. | Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P, Duflos G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere. 2017;182:781-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 622] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 6. | Vogel SA. The politics of plastics: the making and unmaking of bisphenol a "safety". Am J Public Health. 2009;99 Suppl 3:S559-S566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Lee T, Jung S, Baek K, Tsang YF, Lin KA, Jeon YJ, Kwon EE. Functional use of CO(2) to mitigate the formation of bisphenol A in catalytic pyrolysis of polycarbonate. J Hazard Mater. 2022;423:126992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Walker TR. (Micro)plastics and the UN Sustainable Development Goals. Curr Opin Green Sustain Chem. 2021;30:100497. [DOI] [Full Text] |
| 9. | Teng C, Goodwin B, Shockley K, Xia M, Huang R, Norris J, Merrick BA, Jetten AM, Austin CP, Tice RR. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact. 2013;203:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 919] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 12. | Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res. 2011;111:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 13. | Saura M, Marquez S, Reventun P, Olea-Herrero N, Arenas MI, Moreno-Gómez-Toledano R, Gómez-Parrizas M, Muñóz-Moreno C, González-Santander M, Zaragoza C, Bosch RJ. Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 2014;28:4719-4728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Moreno-Gómez-Toledano R, Arenas MI, Muñoz-Moreno C, Olea-Herrero N, Reventun P, Izquierdo-Lahuerta A, Antón-Cornejo A, González-Santander M, Zaragoza C, Saura M, Bosch RJ. Comparison of the renal effects of bisphenol A in mice with and without experimental diabetes. Role of sexual dimorphism. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Moreno-Gómez-Toledano R, Arenas MI, González-Martínez C, Olea-Herrero N, Reventún P, Di Nunzio M, Sánchez-Esteban S, Arilla-Ferreiro E, Saura M, Bosch RJ. Bisphenol A impaired cell adhesion by altering the expression of adhesion and cytoskeleton proteins on human podocytes. Sci Rep. 2020;10:16638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Bano U, Memon S, Shahani MY, Shaikh P, Gul S. Epigenetic effects of in utero bisphenol A administration: Diabetogenic and atherogenic changes in mice offspring. Iran J Basic Med Sci. 2019;22:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Nuñez P, Fernandez T, García-Arévalo M, Alonso-Magdalena P, Nadal A, Perillan C, Arguelles J. Effects of bisphenol A treatment during pregnancy on kidney development in mice: a stereological and histopathological study. J Dev Orig Health Dis. 2018;9:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Tyl RW. Abbreviated assessment of bisphenol A toxicology literature. Semin Fetal Neonatal Med. 2014;19:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Metz CM. Bisphenol A: Understanding the Controversy. Workplace Health Saf. 2016;64:28-36; quiz 37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Comisión Europea. Reglamento (Ue) 2016/2235 De La Comisión de 12 de diciembre de. Diario Oficial de la Unión Europea. April 5, 2016. [cited 15 May 2023]. Available from: https://www.boe.es/doue/2016/337/L00003-00005.pdf. |
| 21. | Jefatura de Estado. Ley 7/2022, de 8 de abril, de residuos y suelos contaminados para una economía circular. 2022. [cited 15 May 2023]. Available from: https://www.boe.es/buscar/act.php?id=BOE-A-2022-5809. |
| 22. | Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect. 2015;123:643-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 898] [Cited by in RCA: 1049] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 23. | Skledar DG, Schmidt J, Fic A, Klopčič I, Trontelj J, Dolenc MS, Finel M, Mašič LP. Influence of metabolism on endocrine activities of bisphenol S. Chemosphere. 2016;157:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Song S, Ruan T, Wang T, Liu R, Jiang G. Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China. Environ Sci Technol. 2012;46:13136-13143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 25. | den Braver-Sewradj SP, van Spronsen R, Hessel EVS. Substitution of bisphenol A: a review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit Rev Toxicol. 2020;50:128-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 26. | Dos Santos B, Ivantsova E, Guzman AP, Martyniuk CJ. Critical review of the toxicity mechanisms of bisphenol F in zebrafish (Danio rerio): Knowledge gaps and future directions. Chemosphere. 2022;297:134132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Wu LH, Zhang XM, Wang F, Gao CJ, Chen D, Palumbo JR, Guo Y, Zeng EY. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci Total Environ. 2018;615:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 28. | Catenza CJ, Farooq A, Shubear NS, Donkor KK. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues. Chemosphere. 2021;268:129273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 29. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47198] [Article Influence: 2949.9] [Reference Citation Analysis (0)] |
| 30. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13355] [Article Influence: 834.7] [Reference Citation Analysis (0)] |
| 31. | Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2053] [Cited by in RCA: 2637] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 32. | Kataria A, Levine D, Wertenteil S, Vento S, Xue J, Rajendiran K, Kannan K, Thurman JM, Morrison D, Brody R, Urbina E, Attina T, Trasande L, Trachtman H. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr Res. 2017;81:857-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 33. | Li AJ, Xue J, Lin S, Al-Malki AL, Al-Ghamdi MA, Kumosani TA, Kannan K. Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia. Environ Res. 2018;166:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Duan Y, Yao Y, Wang B, Han L, Wang L, Sun H, Chen L. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: A case-control study. Environ Pollut. 2018;243:1719-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 35. | Zhang W, Xia W, Liu W, Li X, Hu J, Zhang B, Xu S, Zhou Y, Li J, Cai Z, Li Y. Exposure to Bisphenol a Substitutes and Gestational Diabetes Mellitus: A Prospective Cohort Study in China. Front Endocrinol (Lausanne). 2019;10:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Lee I, Kim S, Park S, Mok S, Jeong Y, Moon HB, Lee J, Kim HJ, Choi G, Choi S, Kim SY, Lee A, Park J, Choi K. Association of urinary phthalate metabolites and phenolics with adipokines and insulin resistance related markers among women of reproductive age. Sci Total Environ. 2019;688:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Rancière F, Botton J, Slama R, Lacroix MZ, Debrauwer L, Charles MA, Roussel R, Balkau B, Magliano DJ; D. E.S.I.R. Study Group. Exposure to Bisphenol A and Bisphenol S and Incident Type 2 Diabetes: A Case-Cohort Study in the French Cohort D.E.S.I.R. Environ Health Perspect. 2019;127:107013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 38. | van der Meer TP, Chung MK, van Faassen M, Makris KC, van Beek AP, Kema IP, Wolffenbuttel BHR, van Vliet-Ostaptchouk JV, Patel CJ. Temporal exposure and consistency of endocrine disrupting chemicals in a longitudinal study of individuals with impaired fasting glucose. Environ Res. 2021;197:110901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Tang P, Liang J, Liao Q, Huang H, Guo X, Lin M, Liu B, Wei B, Zeng X, Liu S, Huang D, Qiu X. Associations of bisphenol exposure with the risk of gestational diabetes mellitus: a nested case-control study in Guangxi, China. Environ Sci Pollut Res Int. 2023;30:25170-25180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Lee S, Karvonen-Gutierrez C, Mukherjee B, Herman WH, Harlow SD, Park SK. Urinary concentrations of phenols and parabens and incident diabetes in midlife women: The Study of Women's Health Across the Nation. Environ Epidemiol. 2021;5:e171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Duan Y, Sun H, Yao Y, Han L, Chen L. Perturbation of serum metabolome in relation to type 2 diabetes mellitus and urinary levels of phthalate metabolites and bisphenols. Environ Int. 2021;155:106609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | An H, Yu H, Wei Y, Liu F, Ye J. Disrupted metabolic pathways and potential human diseases induced by bisphenol S. Environ Toxicol Pharmacol. 2021;88:103751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Moreno-Gómez-Toledano R, Vélez-Vélez E, Arenas MI, Saura M, Bosch RJ. Association between urinary concentrations of bisphenol A substitutes and diabetes in adults. World J Diabetes. 2022;13:521-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (1)] |
| 44. | Zhu Y, Hedderson MM, Calafat AM, Alexeeff SE, Feng J, Quesenberry CP, Ferrara A. Urinary Phenols in Early to Midpregnancy and Risk of Gestational Diabetes Mellitus: A Longitudinal Study in a Multiracial Cohort. Diabetes. 2022;71:2539-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 45. | Conroy-Ben O, Garcia I, Teske SS. In silico binding of 4,4'-bisphenols predicts in vitro estrogenic and antiandrogenic activity. Environ Toxicol. 2018;33:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Marroqui L, Martinez-Pinna J, Castellano-Muñoz M, Dos Santos RS, Medina-Gali RM, Soriano S, Quesada I, Gustafsson JA, Encinar JA, Nadal A. Bisphenol-S and Bisphenol-F alter mouse pancreatic β-cell ion channel expression and activity and insulin release through an estrogen receptor ERβ mediated pathway. Chemosphere. 2021;265:129051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Wei P, Jiang G, Wang H, Ru S, Zhao F. Bisphenol AF exposure causes fasting hyperglycemia in zebrafish (Danio rerio) by interfering with glycometabolic networks. Aquat Toxicol. 2021;241:106000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Gayrard V, Lacroix MZ, Grandin FC, Collet SH, Mila H, Viguié C, Gély CA, Rabozzi B, Bouchard M, Léandri R, Toutain PL, Picard-Hagen N. Oral Systemic Bioavailability of Bisphenol A and Bisphenol S in Pigs. Environ Health Perspect. 2019;127:77005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 49. | Danzl E, Sei K, Soda S, Ike M, Fujita M. Biodegradation of bisphenol A, bisphenol F and bisphenol S in seawater. Int J Environ Res Public Health. 2009;6:1472-1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 50. | Holeček M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 51. | Tuck ML. Nitric oxide in diabetes mellitus. J Hypertens. 2003;21:1081-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric Oxide and Endothelial Dysfunction. Crit Care Clin. 2020;36:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |