Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1289
Peer-review started: February 27, 2023
First decision: April 11, 2023
Revised: April 24, 2023
Accepted: May 16, 2023
Article in press: May 16, 2023
Published online: August 15, 2023
Processing time: 164 Days and 15.3 Hours
Hepatocellular carcinoma (HCC) is among the commonest malignancies associated with significant cancer-related death. The identification of chemo-preventive agents following HCC treatments with the potential to lower the risk of HCC adverse course is intriguing. Metformin, a first-line agent used in the treatment of type 2 diabetes mellitus (T2DM), has been associated with inhibition of HCC growth.
To determine whether metformin can prevent adverse events (i.e., death, tumor progression, and recurrence) after any HCC treatment in T2DM patients.
A systematic review of the published literature was undertaken focused on the role of metformin on outcomes in patients with T2DM and HCC receiving any tumor therapy. A search of the PubMed and Cochrane Central Register of Con-trolled Trials Databases was conducted.
A total of 13 studies (n = 14886 patients) were included in this review. With regard to the risk of death, a decreased risk was reported in cases receiving metformin, although this decrease was not statistically significant [odds ratio (OR) = 0.89, P = 0.42]. When only patients treated with curative strategies were considered, a more marked correlation between metformin and favorable cases was reported (OR = 0.70, P = 0.068). When analyzing palliative treatment, there was no statistical significance in terms of the correlation between metformin and favorable cases (OR = 0.74, P = 0.66). As for the risks of progressive disease and recurrence, no obvious correlation between metformin use and reduced risk was reported. When sub-analyses were performed for patients from different regions, the results for patients from Eastern countries showed a tendency for decreased risk of death in T2DM cases receiving metformin (OR = 0.69, P = 0.17), but the same was not seen in patients from Western countries (OR = 1.19, P = 0.31).
Metformin failed to show a marked impact in preventing adverse effects after HCC treatment. A trend was reported in T2DM cases receiving curative therapies in relation to the risk of death, especially in patients from Eastern regions. Great heterogeneity was reported among the different studies. Further large studies are required to definitively clarify the real impact of metformin as a chemopreventive agent for HCC.
Core Tip: The identification of chemopreventive agents following hepatocellular carcinoma (HCC) treatments with the potential to lower the risk of its adverse course is of paramount relevance. Among them, metformin has been recently examined in this setting. The present systematic review and meta-analysis aim to determine the role of metformin in preventing HCC adverse events (i.e., death, tumor progression, and recurrence). Metformin only showed statistical significance as a protective factor for the risk of death in patients receiving curative therapies for HCC, but failed as a protective agent for progressive disease and recurrence. Further large studies are required to definitively clarify the real impact of metformin as a chemopreventive agent for HCC.
- Citation: Cigrovski Berkovic M, Giovanardi F, Mrzljak A, Lai Q. Prognostic role of metformin in diabetes mellitus type 2 patients with hepatocellular carcinoma: A systematic review and meta-analysis. World J Diabetes 2023; 14(8): 1289-1300
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1289.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1289
Hepatocellular carcinoma (HCC), with an estimated incidence of > 1 million cases, is among the commonest malignancies worldwide and is still associated with significant cancer-related death[1,2]. The major risk factor for developing HCC is advanced liver disease due to various etiologies such as alcohol or viral disease (hepatitis C and B). In addition, non-alcoholic steatohepatitis (NASH), associated with metabolic syndrome, obesity, and diabetes mellitus, is becoming increasingly important for HCC development. In addition, it represents the fastest-growing cause of HCC in westernized and sedentary-lifestyle regions of the world. Moreover, the comorbidities associated with nonalcoholic fatty liver disease (NAFLD) and cardiovascular diseases promote HCC development and negatively influence patients’ outcomes[3-6]. Indeed, while HCC predominantly occurs in the setting of chronic liver disease and cirrhosis (80%), in up to 20% of cases, especially those with metabolic syndrome [obesity, type 2 diabetes mellitus (T2DM), and NAFLD] it emerges much earlier, during non-cirrhotic liver disease stages[7].
Several proposed mechanisms explain the relationship between NAFLD-associated comorbidities and HCC deve-lopment. Among them, insulin resistance, insulin-like growth factor (IGF) related factors, chronic inflammation and proinflammatory cytokines, oxidative stress, dysbiosis of gut microbiota, intrahepatic fat accumulation, inhibition of cell apoptosis and autophagy together with enhanced angiogenesis might play a key link[8-10]. A large retrospective cohort study following over 85000 patients with NAFLD and concomitant diabetes for an average of 10 years showed that good glycemic control (hemoglobin A1c < 7%) not only results in a reduction of micro- and macrovascular diabetes-related complications but can also lower the risk of HCC by 31% [hazard ratio = 0.69; 95% confidence interval (CI): 0.62-0.78][11].
In addition to being a significant risk factor for HCC development, diabetes mellitus has been linked to unfavorable prognosis in HCC patients, including recurrence of HCC after curative approaches and mortality[12]. When possible, surgery, including hepatic resection or liver transplantation, is the first line of treatment, but unfortunately, the incidence of tumor recurrence is still high. Therefore, the role of adjuvant therapy to preclude relapse is a medical need, and different chemopreventive agents following hepatic resection with the potential to lower the risk of HCC recurrence are being investigated[13].
Metformin, a first-line agent used in the treatment of T2DM, has been associated with inhibition of the growth of different cancer types[14,15]. A cohort study by Libby et al[16] analyzing patients with T2DM showed that new users of metformin might have a lower risk of overall incident cancer by 30% to 50% while on standard clinical doses of metformin (1500-2250 mg/d in adults).
Similarly, data from epidemiological studies suggest metformin might also lower the risk of HCC in diabetic patients[17-21]. In addition, as an adjuvant treatment for different cancers, metformin might also improve patients’ survival by acting synergistically with chemo- and radiotherapy. On the other hand, data on HCC patients treated with sorafenib suggest tumor aggressiveness and therapy resistance in the case of chronic metformin use[22-25], while a recent study, using propensity score matching, suggested improved survival and reduced HCC recurrence in hepatitis B virus-induced HCC patients with T2DM receiving metformin[26]. Overall, whether metformin improves long-term outcomes in the HCC setting is still unclear. Therefore, we performed a systematic review and meta-analysis to further examine its chemopreventive role in HCC patients.
A systematic review of the published literature was undertaken focused on the role of metformin in patients with T2DM and HCC receiving any tumor therapy. The search strategy was performed following the PRISMA guidelines[27]. The study has been registered on the International Prospective Register of Systematic Reviews (code CRD42023416686).
The specific research questions formulated in the present study included the following Patients, Intervention, Comparator, Outcome components: Patient: Patient with HCC and T2DM receiving metformin. Intervention: Any HCC therapy. Comparison: Patient with HCC with T2DM not receiving metformin. Outcome: Death, or progressive disease, or recurrence.
A search of the PubMed and Cochrane Central Register of Controlled Trials Databases was conducted using the following terms: (Recurrence or death or surv*) and (diabetes or DM2 or T2DM) and (metformin) and (HCC or hepatocellular cancer or hepatocellular carcinoma or hepatoma). The search period was from “2000/01/01” to “2022/08/11”.
The systematic qualitative review included only English studies involving human patients. Published reports were excluded based on several criteria: (1) Data on animal models; (2) Lacked enough clinical details; and (3) Had non-primary source data (e.g., review articles, non-clinical studies, letters to the editor, expert opinions, and conference summaries). In the case of studies originating from the same center, the possible overlap of clinical cases was examined, and the most informative study was considered eligible.
Following a full-text review of the eligible studies, two independent authors (Giovanardi F and Lai Q) performed the data extraction and crosschecked all outcomes. During the selection of articles and extraction of data, potential discrepancies were resolved following a consensus with a third reviewer (Mrzljak A). Collected data included the first author of the publication, year of publication, country, and the number of treated patients and those with recurrence according to the different therapies adopted.
Selected studies were systematically reviewed with the intent to identify potential sources of bias. The quality of each study was assessed using the Risk of Bias In Non-randomized Studies of Interventions (Robins-I) tool[28].
Study results were expressed as odds ratio (OR) with 95%CIs. The statistical heterogeneity was evaluated with the Higgins statistic squared (I2). I2 values of 0%-25% were considered an index of low heterogeneity between studies, 26%-50%: Moderate heterogeneity, and ≥ 51%: High heterogeneity. The fixed-effects model was used when low or moderate (0%-50%) heterogeneity was detected between studies, while the random effects model was preferred when high heterogeneity was present. Subgroup analyses (for different types of HCC treatment and different ethnicities) were used to investigate the source of the heterogeneity.
Sensitivity analysis was used to assess the stability of the study. The funnel plot was used to evaluate publication bias. The rank correlation test and the regression test, using the standard error of the observed outcomes as predictor, were used to check for funnel plot asymmetry. A value P < 0.05 was considered indicative of statistical significance. The meta-analysis was performed using OpenMetaAnalyst (http://www.cebm.brown.edu/openmeta/index.html).
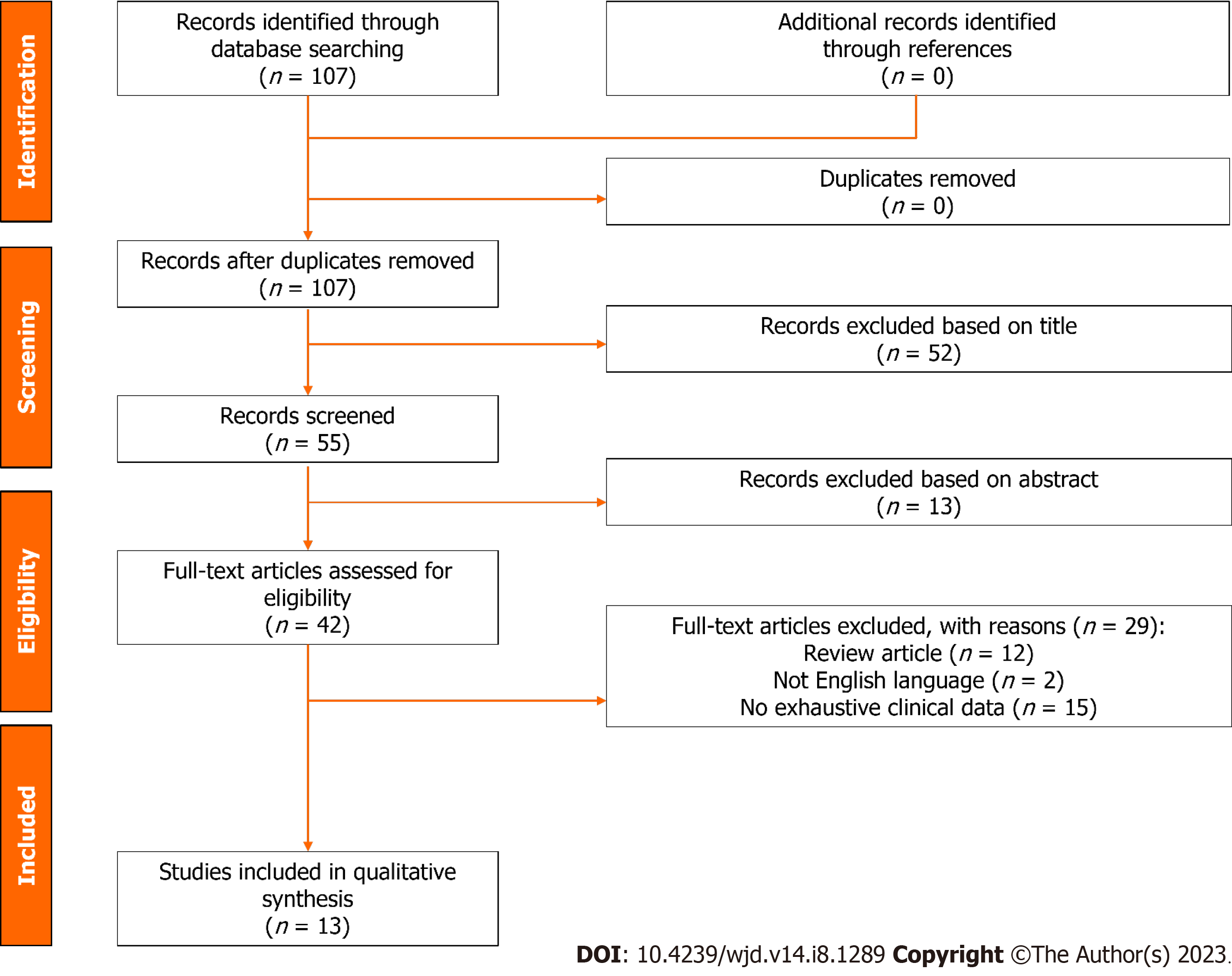
The PRISMA flow diagram schematically depicts the article selection process (Figure 1). Among the 107 articles screened, a total of 13 studies were finally included in this review[24-26,29-38]. All the studies included in the analytic cohort were published during the last decade. Eight articles (61.5%) were from Asia, of which three (23.1%) were from Taiwan or Korea, respectively. The remaining studies were from Europe (n = 3, 23.1%) and North America (n = 2, 15.4%) (Table 1).
| Ref. | City | Country | Study period | Design | N | Therapy | DM (no metformin) | Number of events | DM (metformin) | Number of events |
| Chen et al[29], 2011 | Taichung | Taiwan | June 2003 to July 2009 | Retro | 53 | RFA | 32 | 18 | 21 | 6 |
| Bhat et al[25], 2014 | Rochester | United States | January 2005 to June 2011 | Retro | 263 | No therapy | 207 | 133 | 56 | 37 |
| Jang et al[30], 2015 | Seoul | Korea | March 2003 to December 2012 | Retro | 48 | RT | 29 | 13 | 19 | 5 |
| Casadei Gardini et al[31], 2015 | Meldola | Italy | March 2008 to August 2014 | Retro | 42 | Sorafenib | 11 | 5 | 31 | 22 |
| Seo et al[32], 2016 | Seoul | Korea | January 2005 to December 2011 | Retro | 751 | HR | 218 | 111 | 533 | 169 |
| Chan et al[33], 2017 | Taipei | Taiwan | January 1995 to December 2011 | Retro | 4610 | HR | 2978 | 1335 | 1632 | 612 |
| 7813 | No surgery | 5884 | 3858 | 1929 | 1437 | |||||
| Casadei Gardini et al[24], 2017 | Meldola | Italy | May 2007 to September 2015 | Retro | 86 | Sorafenib | 34 | 26 | 52 | 45 |
| Chung et al[34], 2018 | Seoul | Korea | January 2009 to December 2016 | Retro | 63 | Sorafenib + HR | 23 | 23 | 40 | 30 |
| 31 | Sorafenib + LT | 17 | 14 | 14 | 13 | |||||
| Luo et al[26], 2020 | Nanchang | China | January 2000 to December 2013 | Retro | 250 | HR | 184 | 129 | 66 | 49 |
| Cho et al[35], 2021 | Kaohsiung | Taiwan | April 2001 to June 2016 | Retro | 222 | HR | 86 | 15 | 136 | 26 |
| Tangjarusritaratorn et al[36], 2021 | Bangkok | Thailand | January 2006 to June 2014 | Prosp | 327 | Multiple therapies | 165 | 84 | 162 | 60 |
| Elsayed et al[37], 2021 | Atlanta | United States | 2014-2018 | Retro | 40 | TARE | 21 | 8 | 19 | 8 |
| Hydes et al[38], 2022 | Birmingham | United Kingdom | January 2007 to March 2012 | Prosp | 287 | Multiple therapies | 139 | 58 | 148 | 63 |
| Total | 14886 | 10028 | 5830 | 4858 | 2582 |
Eleven (84.6%) studies were retrospective analyses, while two (15.4%) were prospective studies. The ROBINS-I tool quality assessment showed that all the studies had a low risk of bias (Figure 2).
Data extracted from the selected articles are reported in detail in Tables 1-3. All the studies investigated the risk of death for any reason observed after any HCC treatment (Table 1)[24-26,29-38]. In seven (53.8%) studies, a curative therapy (i.e., thermoablation, resection or transplantation) was performed[26,29,32,34,37]. The risk of progressive tumor disease was reported in four (30.8%) studies (Table 2)[24,30,31,34]. Tumor recurrence was investigated in three (23.1%) studies (Table 3)[29,33,35]. Overall, only one (7.7%) study was based on a population of patients including more than 1000 cases[33].
| Ref. | City | Country | Study period | Design | N | Therapy | DM (no metformin) | Number of events | DM (metformin) | Number of events |
| Jang et al[30], 2015 | Seoul | Korea | March 2003 to December 2012 | Retro | 48 | RT | 29 | 24 | 19 | 9 |
| Casadei Gardini et al[31], 2015 | Meldola | Italy | March 2008 to August 2014 | Retro | 42 | Sorafenib | 11 | 9 | 31 | 28 |
| Casadei Gardini et al[24], 2017 | Meldola | Italy | May 2007 to September 2015 | Retro | 86 | Sorafenib | 34 | 33 | 52 | 47 |
| Chung et al[34], 2018 | Seoul | Korea | January 2009 to December 2016 | Retro | 63 | Sorafenib + HR | 23 | 23 | 40 | 40 |
| 31 | Sorafenib + LT | 17 | 17 | 14 | 14 | |||||
| Total | 270 | 114 | 106 | 156 | 138 |
| Ref. | City | Country | Study period | Design | N | Therapy | DM (no metformin) | Number of events | DM (metformin) | Number of events |
| Chen et al[29], 2011 | Taichung | Taiwan | June 2003 to July 2009 | Retro | 53 | RFA | 32 | 18 | 21 | 12 |
| Chan et al[33], 2017 | Taipei | Taiwan | January 1995 to December 2011 | Retro | 4610 | HR | 2978 | 1615 | 1632 | 698 |
| 7813 | No surgery | 5884 | 2668 | 1929 | 982 | |||||
| Cho et al[35], 2021 | Kaohsiung | Taiwan | April 2001 to June 2016 | Retro | 222 | HR | 86 | 52 | 136 | 85 |
| Total | 12698 | 8980 | 4353 | 3718 | 1777 |
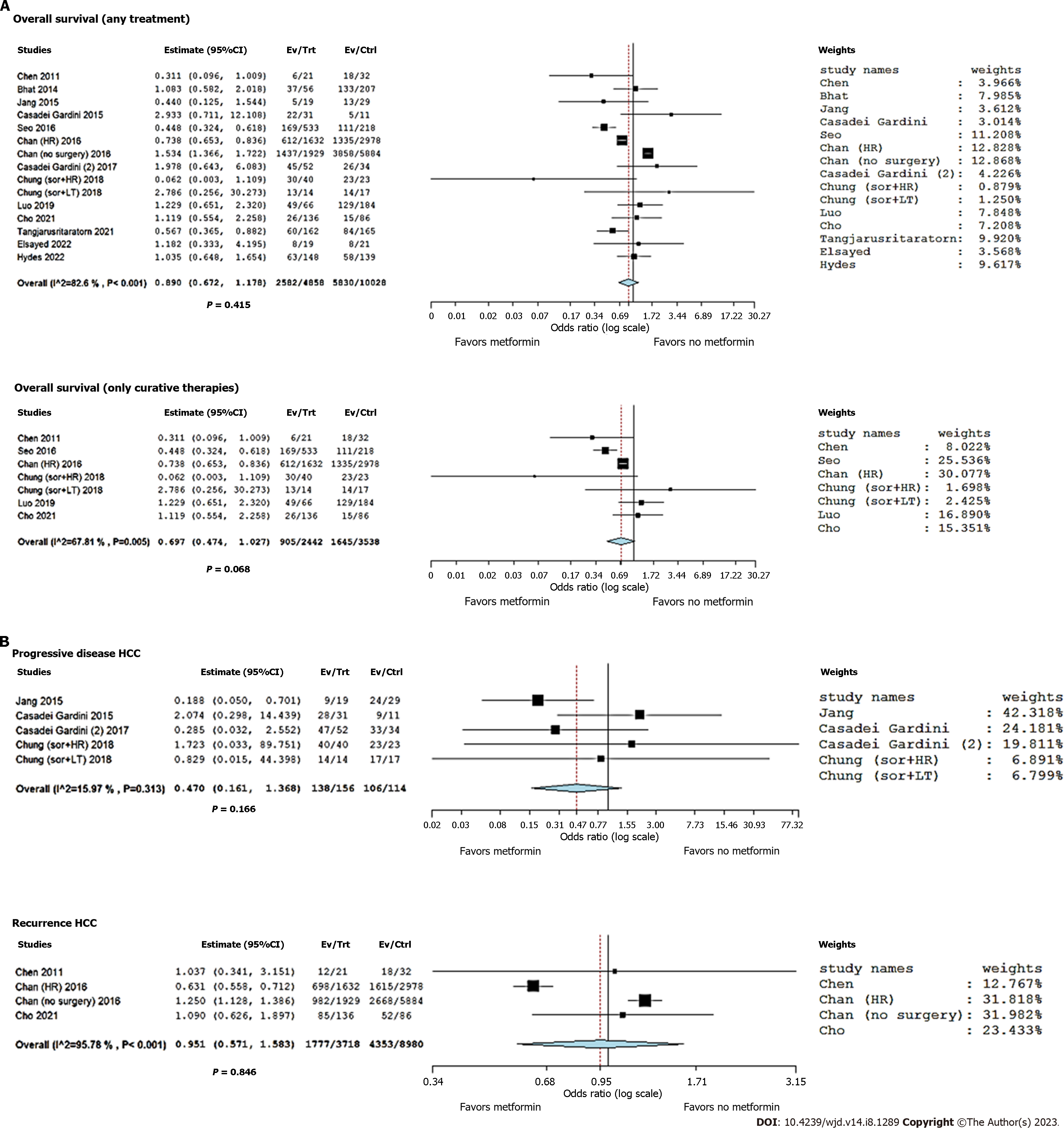
According to the data shown in Table 1, 13 studies reported post-treatment death rates in HCC patients with T2DM treated or not treated with metformin. A total of 14886 patients were considered, with 8412 (56.5%) deaths. In detail, 2582/4858 (53.1%) and 5830/10028 (58.1%) deaths were observed in the metformin group and no metformin group, respectively.
Great heterogeneity was observed among the selected studies, with an I2 = 82.6% (P < 0.001). The summary OR (95%CI) showed a decreased risk of death in T2DM cases receiving metformin, although this value did not reach statistical significance (OR = 0.89, 95%CI: 0.67-1.18; P = 0.42) (Figure 3A). Sensitivity analysis indicated no change in the direction of effect when any one study was excluded from the meta-analysis. Funnel plot did not indicate a significant risk of publication bias (Figure 4). Neither the rank correlation nor the regression test indicated any funnel plot asymmetry (P = 0.20 and P = 0.86, respectively).
When only patients treated with curative strategies were considered, the heterogeneity reduced; however, it remained relevant (I2 = 67.8%, P = 0.005). The summary OR (95%CI) showed a more marked correlation between metformin and favorable cases, with an OR = 0.70 (95%CI: 0.47-1.03; P = 0.068) (Figure 3A). Despite the correlation between metformin and positive clinical course being more evident, statistical significance was not reached in this sub-analysis.
When only patients treated with palliative strategies were considered, the heterogeneity was high (I2 = 90.8%, P < 0.001). The summary OR (95%CI) did not show statistical significance in terms of the correlation between metformin and favorable cases, with an OR = 0.74 (95%CI: 0.19-2.85; P = 0.66). A sub-analysis focused on the region in which the studies were published was also performed. In patients from Eastern countries, great heterogeneity was observed among the studies, with an I2 = 67.9% (P < 0.001). The summary OR (95%CI) showed a decreased risk of death in T2DM cases receiving metformin, although this value did not reach statistical significance (OR = 0.69, 95%CI: 0.40-1.17; P = 0.17). In Western patients, heterogeneity was not present (I2 = 0; P = 0.60). Also in this case, the summary OR (95%CI) did not show any correlation between metformin and favorable cases (OR = 1.19, 95%CI: 0.85-1.65; P = 0.31).
According to the data shown in Table 2, four studies reported post-treatment progressive disease rates in HCC patients with T2DM treated or not treated with metformin[24,30,31,34]. A total of 270 patients were considered, with 244 (90.4%) patients having progressive disease. In detail, 138/156 (88.5%) and 106/114 (93.0%) patients with progressive disease were observed in the metformin and no metformin group, respectively.
Low heterogeneity was observed among the selected studies, with an I2 = 16.0% (P = 0.31). The summary OR (95%CI) showed a decreased risk of progressive disease in T2DM cases receiving metformin, although this correlation did not reach statistical significance (OR = 0.47, 95%CI: 0.16-1.37; P = 0.17) (Figure 3B).
According to the data shown in Table 3, three studies reported post-treatment recurrence rates in HCC patients with T2DM receiving or not receiving metformin[29,33,35]. A total of 12698 patients were considered, with 6130 (48.3%) recurrences. In detail, 1777/3718 (47.8%) and 4353/8980 (48.5%) recurrences were observed in the metformin and no metformin group, respectively. Great heterogeneity was observed among the selected studies, with an I2 = 95.8% (P < 0.001). The summary OR (95%CI) showed no evident correlation between metformin use and reduced risk of recurrence (OR = 0.95, 95%CI: 0.57-1.58; P = 0.85) (Figure 3B).
HCC, even after potentially curative treatment, is still associated with significant mortality. Therefore, identifying adjuvant agents that might decrease this risk is important. As T2DM imposes a significant risk for HCC, the role of metformin in this setting is of relevance. Data including information on 14886 patients with T2DM and HCC included in 13 studies, regardless of the treatment option used, showed a numerical decrease in the death rate in those on metformin, although no statistical significance was reported. When the survival results were analyzed for T2DM patients treated with curative strategies, similar results were reported, although the suggested correlation between the use of metformin and favorable prognosis was close to statistical significance (P = 0.068).
The results mentioned were promising but never reached statistical significance, showing that the real protective effect of metformin is questionable. Unfortunately, the present study could not definitively clarify this potential protective effect due to several confounding factors related to tumor burden and the severity of cirrhosis, which are not well described in the studied population. Therefore, it was impossible to perform a meta-regression focused on these aspects.
Moreover, there was no specific investigation on the HCC-related death concerning the timing of curative treatment
Due to these promising results, metformin has also been used in non-diabetic cancer patients, and available data suggest its benefits in terms of Ki-67 reduction (positive effects on tumor cell proliferation and apoptosis) and insulin level reduction when given to non-diabetic breast cancer patients in standard doses[40,41]. Whether the suppressive effect of metformin on cancer is caused by a direct preventive effect or is due to the cancer-diabetes association, relying on lowering hyperglycemia and insulin levels, remains unclear[42]. An interesting clinical study suggests metformin has a preventive role in colorectal precancerous lesions in non-diabetic patients even when used in very low doses (250 mg/d)[43]. The actual mechanism behind the metformin anti-tumor effect is still intriguing. In the case of HCC, activating adenosine monophosphate-activated protein kinase and increasing p53 gene expression, which then induces the senescence of cancer cells, might be the key player in the anti-tumor role[44]. Besides inhibition of the mechanistic target of rapamycin signaling, effects on insulin and IGF-1 are also interesting potential pathways[45,46]. Data regarding metformin use in non-diabetic HCC patients is lacking. Also, in the present meta-analysis, all the enrolled cases had a diagnosis of T2DM. The correlation between metformin and HCC appears intriguing for numerous reasons related to the connection of T2DM and metabolic associated fatty liver disease/NASH and obesity, together with the potential lowering of hyperglycemia and hyperinsulinemia known factors in HCC development.
The effects of metformin on tumor progression and tumor recurrence are best studied in pancreatic cancer. A recently published meta-analysis including 38772 patients with T2DM and pancreatic adenocarcinoma showed a significant survival benefit of those taking metformin during early and mixed stages of the disease, for patients receiving surgical treatment but not for those at an advanced stage or those receiving chemotherapy[47]. Similar improvements in survival were described earlier by Li et al[48] and related only to patients with locally advanced pancreatic cancer and coexisting T2DM when taking metformin.
In the present study, patients with T2DM and HCC receiving metformin had a decreased risk of progressive disease, although this correlation did not reach statistical significance (OR = 0.47, 95%CI: 0.16-1.37; P = 0.17). In addition, the role of metformin on tumor recurrence showed no evident correlation between its use and reduced risk of recurrence (OR = 0.95, 95%CI: 0.57-1.58; P = 0.85). The negative results might relate to the previously reported biases deriving from the high rate of heterogeneity observed among the studies. Moreover, only a few studies explored the role of metformin on tumor progression and recurrence, with a consequently limited number of cases investigated.
As previously reported, the present study has some limitations. First, studies examining the effect of metformin in HCC patients have been carried out exclusively in T2DM patients. No specific studies investigating non-diabetic cases have been published, therefore identifying a potential new area of interest to be explored with specific prospective research. Second, the heterogeneity of the studies limits our ability to clarify the real effect of metformin, mainly on outcomes (i.e., recurrence and progressive disease) with a limited number of enrolled cases. Therefore, more studies are needed specifically focused on these aspects, to better clarify whether the role of metformin is marginal for the risk of post-curative recurrence. Third, despite the potential effect on death prevention after curative therapies, many confounders were reported, requiring a meta-regression to clarify the real positive effect of metformin. Unfortunately, in many cases, the data needed to perform such an analysis is insufficient or lacking. New studies to clarify these aspects or an individual participant data meta-analysis are required. Other relevant aspects not explorable in the extracted studies are the metformin dose, HCC-related death, and the time-to-event data to perform inferential analyses. Also, in this case, the publication of new studies on these aspects or an individual participant data meta-analysis are needed. Lastly, HCC patients often present an underlying liver disease, with different degrees of severity. Metformin is contraindicated in patients which severe liver injury, therefore adding a potential bias in the results reported.
In conclusion, no definitive answer can be given on the real protective effect of metformin in diabetic patients receiving therapies for HCC. A trend for a protective effect regarding death after curative treatments has been reported. However, many confounders exist, reducing the relevance of the reported results. More studies are needed to resolve these relevant confounders.
Hepatocellular carcinoma (HCC) is a common malignancy associated with significant cancer-related death. Therefore, it is important to identify chemopreventive potential to lower the risk of an HCC adverse course. Metformin has been associated with a lower risk of HCC development, but its role in prevention of death, tumor progression, and recurrence after any HCC treatment in type 2 diabetes mellitus (T2DM) patients is still inconclusive.
Metformin is a first-line therapeutic option for T2DM, with expanded re-purposing in the treatment of different cancer types. Whether it can improve long-term outcomes in the HCC setting is still unclear. Therefore, we performed a systematic review and meta-analysis to further explore its chemopreventive role in HCC patients.
We focused on the role of metformin in patients with T2DM and HCC in terms of outcomes (death, or progressive disease, or recurrence) receiving any tumor therapy. Moreover, we performed subgroup analyses (including different types of HCC treatment and different ethnicities).
We performed a systematic review via a search of PubMed and Cochrane Central Register of Controlled Trials Databases of the published literature focused on the role of metformin in patients with T2DM and HCC receiving any tumor therapy.
We included 13 studies (n = 14886 patients) in this review. A decreased risk was reported in cases receiving metformin, although this value did not reach statistical significance [odds ratio (OR) = 0.89, P = 0.42]. When only patients treated with curative strategies were considered, a more marked correlation between metformin and favorable cases was reported (OR = 0.70, P = 0.068). In the case of a palliative treatment, there was no correlation between metformin and favorable cases (OR = 0.74, P = 0.66). With regard to the risk of progressive disease and recurrence, no obvious correlation between metformin use and reduced risk was reported. Moreover, there was a tendency for a decreased risk of death with metformin use in patients from Eastern countries (OR = 0.69, P = 0.17), but the same was not seen in patients from Western countries (OR = 1.19; P = 0.31).
Metformin failed to have a relevant impact on preventing adverse effects after HCC treatment. A trend was reported in T2DM cases receiving curative therapies in relation to the risk of death.
Further large studies are required to definitively clarify the real impact of metformin as a chemopreventive agent for HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fang ZH, China; Liao J, China; Mostafavinia A, Iran S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Chen YX
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3176] [Article Influence: 529.3] [Reference Citation Analysis (37)] |
| 2. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 3. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1500] [Article Influence: 187.5] [Reference Citation Analysis (0)] |
| 4. | Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996-1005.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 685] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 5. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1713] [Article Influence: 244.7] [Reference Citation Analysis (0)] |
| 6. | Zhang C, Liu S, Yang M. Hepatocellular Carcinoma and Obesity, Type 2 Diabetes Mellitus, Cardiovascular Disease: Causing Factors, Molecular Links, and Treatment Options. Front Endocrinol (Lausanne). 2021;12:808526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Lee DH, Lee JM. Primary malignant tumours in the non-cirrhotic liver. Eur J Radiol. 2017;95:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Sakurai Y, Kubota N, Takamoto I, Obata A, Iwamoto M, Hayashi T, Aihara M, Kubota T, Nishihara H, Kadowaki T. Role of insulin receptor substrates in the progression of hepatocellular carcinoma. Sci Rep. 2017;7:5387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Hamouda HA, Mansour SM, Elyamany MF. Vitamin D Combined with Pioglitazone Mitigates Type-2 Diabetes-induced Hepatic Injury Through Targeting Inflammation, Apoptosis, and Oxidative Stress. Inflammation. 2022;45:156-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Suresh D, Srinivas AN, Kumar DP. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front Oncol. 2020;10:601710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 11. | Kramer JR, Natarajan Y, Dai J, Yu X, Li L, El-Serag HB, Kanwal F. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2022;75:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 12. | Fujita K, Iwama H, Miyoshi H, Tani J, Oura K, Tadokoro T, Sakamoto T, Nomura T, Morishita A, Yoneyama H, Masaki T. Diabetes mellitus and metformin in hepatocellular carcinoma. World J Gastroenterol. 2016;22:6100-6113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 595] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 14. | Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 16. | Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 809] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 17. | Cunha V, Cotrim HP, Rocha R, Carvalho K, Lins-Kusterer L. Metformin in the prevention of hepatocellular carcinoma in diabetic patients: A systematic review. Ann Hepatol. 2020;19:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 19. | Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 20. | Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 22. | Siddharth S, Kuppusamy P, Wu Q, Nagalingam A, Saxena NK, Sharma D. Metformin Enhances the Anti-Cancer Efficacy of Sorafenib via Suppressing MAPK/ERK/Stat3 Axis in Hepatocellular Carcinoma. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Kim EH, Kim MS, Cho CK, Jung WG, Jeong YK, Jeong JH. Low and high linear energy transfer radiation sensitization of HCC cells by metformin. J Radiat Res. 2014;55:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Casadei Gardini A, Faloppi L, De Matteis S, Foschi FG, Silvestris N, Tovoli F, Palmieri V, Marisi G, Brunetti O, Vespasiani-Gentilucci U, Perrone G, Valgiusti M, Granato AM, Ercolani G, Negrini G, Tamburini E, Aprile G, Passardi A, Santini D, Cascinu S, Frassineti GL, Scartozzi M. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur J Cancer. 2017;86:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Bhat M, Chaiteerakij R, Harmsen WS, Schleck CD, Yang JD, Giama NH, Therneau TM, Gores GJ, Roberts LR. Metformin does not improve survival in patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20:15750-15755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Luo CS, Lin Y, Zhou WP, Shi J. Survival advantage associated with metformin usage in hepatocellular carcinoma patients with diabetes mellitus receiving radical resection: a propensity score matching analysis. Eur J Gastroenterol Hepatol. 2020;32:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5298] [Article Influence: 529.8] [Reference Citation Analysis (1)] |
| 28. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10881] [Article Influence: 1209.0] [Reference Citation Analysis (2)] |
| 29. | Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Jang WI, Kim MS, Lim JS, Yoo HJ, Seo YS, Han CJ, Park SC, Kay CS, Kim M, Jang HS, Lee DS, Chang AR, Park HJ. Survival Advantage Associated with Metformin Usage in Hepatocellular Carcinoma Patients Receiving Radiotherapy: A Propensity Score Matching Analysis. Anticancer Res. 2015;35:5047-5054. [PubMed] |
| 31. | Casadei Gardini A, Marisi G, Scarpi E, Scartozzi M, Faloppi L, Silvestris N, Masi G, Vivaldi C, Brunetti O, Tamberi S, Foschi FG, Tamburini E, Tenti E, Ricca Rosellini S, Ulivi P, Cascinu S, Nanni O, Frassineti GL. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin Pharmacother. 2015;16:2719-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Seo YS, Kim YJ, Kim MS, Suh KS, Kim SB, Han CJ, Jang WI, Kang SH, Tchoe HJ, Park CM, Jo AJ, Kim HJ, Choi JA, Choi HJ, Polak MN, Ko MJ. Association of Metformin Use With Cancer-Specific Mortality in Hepatocellular Carcinoma After Curative Resection: A Nationwide Population-Based Study. Medicine (Baltimore). 2016;95:e3527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Chan KM, Kuo CF, Hsu JT, Chiou MJ, Wang YC, Wu TH, Lee CF, Wu TJ, Chou HS, Lee WC. Metformin confers risk reduction for developing hepatocellular carcinoma recurrence after liver resection. Liver Int. 2017;37:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Chung YK, Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Ryoo BY, Lee SG. Absence of antitumor effects of metformin in sorafenib-treated patients with hepatocellular carcinoma recurrence after hepatic resection and liver transplantation. Ann Hepatobiliary Pancreat Surg. 2018;22:297-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Cho WR, Wang CC, Tsai MY, Chou CK, Liu YW, Wu YJ, Lin MT, Chen KD, Chuang CH, Huang PY, Hu TH, Tsai MC. Impact of metformin use on the recurrence of hepatocellular carcinoma after initial liver resection in diabetic patients. PLoS One. 2021;16:e0247231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Tangjarusritaratorn T, Tangjittipokin W, Kunavisarut T. Incidence and Survival of Hepatocellular Carcinoma in Type 2 Diabetes Patients with Cirrhosis Who Were Treated with and without Metformin. Diabetes Metab Syndr Obes. 2021;14:1563-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Elsayed M, Wagstaff W, Behbahani K, Villalobos A, Bercu Z, Majdalany BS, Akce M, Schuster DM, Mao H, Kokabi N. Improved Tumor Response in Patients on Metformin Undergoing Yttrium-90 Radioembolization Segmentectomy for Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2021;44:1937-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Hydes TJ, Cuthbertson DJ, Graef S, Berhane S, Teng M, Skowronska A, Singh P, Dhanaraj S, Tahrani A, Johnson PJ. The Impact of Diabetes and Glucose-Lowering Therapies on Hepatocellular Carcinoma Incidence and Overall Survival. Clin Ther. 2022;44:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 39. | Spillane S, Bennett K, Sharp L, Barron TI. A cohort study of metformin exposure and survival in patients with stage I-III colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1364-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Goodwin PJ, Pritchard KI, Ennis M, Clemons M, Graham M, Fantus IG. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 41. | Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L, Jellema G, Deharo S, Hardie DG, Pusztai L, Moulder-Thompson S, Dewar JA, Thompson AM. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011;128:783-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 42. | Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48:R31-R43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 43. | Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, Koide T, Tokoro C, Abe Y, Inamori M, Nakagama H, Nakajima A. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila). 2010;3:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 44. | Yi G, He Z, Zhou X, Xian L, Yuan T, Jia X, Hong J, He L, Liu J. Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int J Oncol. 2013;43:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804-10812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 46. | Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 47. | Shi YQ, Zhou XC, Du P, Yin MY, Xu L, Chen WJ, Xu CF. Relationships are between metformin use and survival in pancreatic cancer patients concurrent with diabetes: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e21687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Li X, Li T, Liu Z, Gou S, Wang C. The effect of metformin on survival of patients with pancreatic cancer: a meta-analysis. Sci Rep. 2017;7:5825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |