Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1249
Peer-review started: May 21, 2023
First decision: June 1, 2023
Revised: June 30, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 15, 2023
Processing time: 82 Days and 8.6 Hours
Obesity usually causes diabetes mellitus (DM) and is a serious danger to human health. Type 2 DM (T2DM) mostly occurs along with obesity. Foodborne obesity-induced DM is caused by an excessive long-term diet and surplus energy. Bariatric surgery can improve the symptoms of T2DM in some obese patients. But different types of bariatric surgery may have different effects.
To investigate the effect of bariatric surgery on glucose and lipid metabolism and liver and kidney function in rats.
Male Sprague-Dawley rats aged 6-8 wk underwent Roux-en-Y gastric bypass surgery (RYGB), sleeve gastrectomy (SG), or gastric banding (GB). Glucose and insulin tolerance tests, analyses of biochemical parameters, histological examina
In comparison to the sham operation group, the RYGB, SG, and GB groups had decreased body weight and food intake, reduced glucose intolerance and insulin insensitivity, downregulated biochemical parameters, alleviated morphological changes in the liver and kidneys, and decreased levels of protein kinase C β/ P66shc. The effect in the RYGB group was better than that in the SG and GB groups.
These results suggest that RYGB, SG and GB may be helpful for the treatment of foodborne obesity-induced DM.
Core Tip: Bariatric surgery can improve the symptoms of type 2 diabetes mellitus (DM) in some obese patients. But different types of bariatric surgery may have different effects. In the current study, in comparison to the sham operation group, the Roux-en-Y gastric bypass surgery (RYGB), sleeve gastrectomy (SG), and gastric banding (GB) groups had decreased body weight and food intake, reduced glucose intolerance and insulin insensitivity, downregulated biochemical parameters, alleviated morphological changes in the liver and kidneys, and decreased levels of protein kinase C β/P66shc. The effect in the RYGB group was better than that in the SG and GB groups. Thus, bariatric surgeries are a helpful tool for the treatment of foodborn obesity-induced DM.
- Citation: Long H, Zhao L, Xiao ZS, Li SX, Huang QL, Xiao S, Wu LL. Impact of bariatric surgery on glucose and lipid metabolism and liver and kidney function in food-induced obese diabetic rats. World J Diabetes 2023; 14(8): 1249-1258
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1249.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1249
Diabetes mellitus (DM), a well-known chronic metabolic disease, is a substantial global problem characterized by a common outcome of hyperglycemia. Type 2 DM (T2DM), accounting for > 90% of all cases of DM, is considered the chief cause of diabetic complications, including diabetic nephropathy, diabetic neuropathy, cardiovascular disease, and diabetic retinopathy[1].
It is widely acknowledged that obesity, increasingly influenced by lifestyle factors, is an essential risk factor for the development of T2DM[2,3]. Obesity is involved in the upregulated circulating free fatty acids that play a distinct role in progressive dysfunction of pancreatic beta cells, damaging their ability to compensate for insulin resistance (IR) and thus contributing to T2DM pathogenesis[4]. The growing prevalence of T2DM has resulted in various approaches focusing on discovering novel therapeutic targets for treating hyperglycemia.
Higher blood glucose is an absolute risk factor for all-cause mortality, and bariatric surgery can increase survival rates of patients with obesity[5,6]. Bariatric surgery can promote sustained weight loss and is more efficacious than traditional medical strategies for long-acting control of T2DM[7]. Bariatric surgery quickly diminishes IR and blood glucose before any fathomable weight loss[8,9]. Bariatric surgery can ameliorate glycemic control and glucose homeostasis in patients with T2DM[10]. However, the underlying mechanism remains to be investigated.
Protein kinase C (PKC), a member of a family of serine/threonine protein kinases consisting of > 12 members, exerts a pivotal role in intracellular crosstalk and signal transduction[11]. It has been reported that pharmacological blockade or gene deletion of PKCβ can decrease infarct size, protect ischemic myocardium, and promote ventricular functional re
In the current study, we investigated the effects of bariatric surgery, including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and gastric banding (GB), on the foodborne obesity diabetic rats and the possible mechanisms related to the PKCβ/P66shc signaling pathway.
Male Sprague-Dawley rats aged 6-8 wk with a body weight of 200-220 g were purchased from the Guangdong Medical Laboratory Animal Center and adapted to the environment for 7 d before the experiments. All rats were housed in specific pathogen-free cages with a 12-h light/dark cycle and had free access to drinking water and food.
All the rats were randomly divided into four groups: RYGB, SG, GB, and sham operation (SO), with 10 rats in each group. The rats were anesthetized with 0.5% pentobarbital (45 mg/kg) by intraperitoneal injection before the operation.
In the RYGB group, rats were routinely fasted with water 12 h before the operation. After weighing the rats, anesthesia was induced by intraperitoneal injection of 3% pentobarbital (0.15 mL/100 g). When the limbs were soft, we used an animal shaver to prepare the operation area, dipped a sterile cotton swab in iodophor, and disinfected the skin around the incision area three times. At the sterile area about 3 cm below the sternum of the rat, a longitudinal incision was made, the abdominal cavity was opened layer by layer, and an electrocoagulator was used to stop bleeding as needed. After entering the abdominal cavity, we confirmed the position of the distal ligament of Treitz. At a distance of 10-15 cm from the distal end of the ligament, we freed a segment of the small intestine to avoid damaging the mesenteric vessels and intestinal serosa, separated and cut off the jejunum, wiped off the digestive fluid with a cotton swab, and disinfected the distal and proximal intestinal tubes with iodophor. End-to-side full-thickness anastomosis was performed at the proximal jejunum 10 cm below the distal jejunum to keep the anastomosis unobstructed. We gently exposed the stomach body, esophagus, and cardia with a sterile cotton swab, and used the electrocoagulator to dissociate the stomach towards the lower part of the cardia. The stomach was severed at 10 cm at the distal end of the cardia, and < 20% of the gastric sacs were retained. The proximal gastric sacs were anastomosed with the distal jejunum laterally. After confirming the patency of the anastomosis, the distal remnant stomach was closed by suture. Both gastrojejunostomy and jejunojejunostomy were sutured with 6-0 absorbable sutures. Before closing the abdominal cavity, we flushed the abdominal cavity with normal saline solution containing gentamicin three times. Intermittent suture was used for abdominal closure.
In the SG group, preoperative treatment was the same as that in the RYGB group. We cut the skin and subcutaneous tissue layer by layer, exposed the esophagus, stomach, duodenum, and other organs, freed the stomach from the abdominal cavity, and ligated the vessels of the greater curvature according to the scope of resection. And 75%-80% of the whole stomach volume was cut off, including the fundus and most of the stomach body tissue. After resection, we cleaned the contents of the remnant stomach with a cotton swab. The stump stomach was sutured and closed, and the abdominal cavity was washed with physiological saline. After dipping the physiological saline with a cotton ball, the stump stomach was covered with part of the omentum, and the abdominal cavity was closed layer by layer.
In the GB group, preoperative treatment was the same as that in the RYGB group. The midline incision of the upper abdomen was 3 cm long. The inner diameter of the lower part of the upper gastric cardia was bound and fixed to one third of the original position with a buckle type silicon tape at 1 cm below the cardia. The proximal end formed a 20% small gastric sac. Routine abdominal closure was performed.
In the SO group, rats were fasted 12 h before the operation, and the jejunum and gastric body were cut at the same position as that in the RYGB group, and then the broken end was cut and anastomosed. The cross section of the gastrointestinal tract was performed at the site where gastrotomy was performed in RYGB, and the anastomosis was performed at the original cutting site. The operation time should be the same as that of RYGB, and normal saline containing gentamicin was used to wash the abdomen. The physiological flow of food through the gastrointestinal tract remained intact. After surgery, the rats were placed into a single cage, and wound care was applied.
At 4 wk, the oral glucose tolerance test (OGTT) was performed as previously described[16] by glucose gavage (5 g D-glucose/kg) following an overnight fast. After the tail of rats was pierced using a needle, a drop of venous blood was collected to determine blood glucose concentration, and 300 μL of tail vein blood was gathered and centrifuged for 15 min at 2000 rpm to collect the serum for the measurement of insulin. Blood glucose was determined using a glucometer (Roche Diagnostics, Switzerland) following glucose gavage for 0 h, 1 h, and 2 h, and insulin was monitored at the same time points. The insulin tolerance test (ITT) was performed as previously described[17]. After fasting for 8 h, the rats were intraperitoneally injected with insulin (0.5 U/kg). The area under the curve (AUC) of the OGTT and ITT was also calculated.
At 4 wk, blood samples gathered from the abdominal aorta of rats under anesthesia were centrifuged for 15 min at 3000 rpm to determine the serum concentrations of triglyceride (TG), total bile acids (TBA), alanine aminotransferase (ALT), and aspartate aminotransaminase (AST) with a microplate reader (Reitman-Frankel colorimetric assay) (Nanjing Jiancheng Corp., Nanjing, China).
Histological examination was performed as previously described[17]. Liver and kidney tissues were fixed in 4% paraformaldehyde solution, following embedding in paraffin blocks. Tissue sections were obtained using a microtome, and after staining with hematoxylin and eosin (HE), they were examined under a microscope (Olympus, Japan).
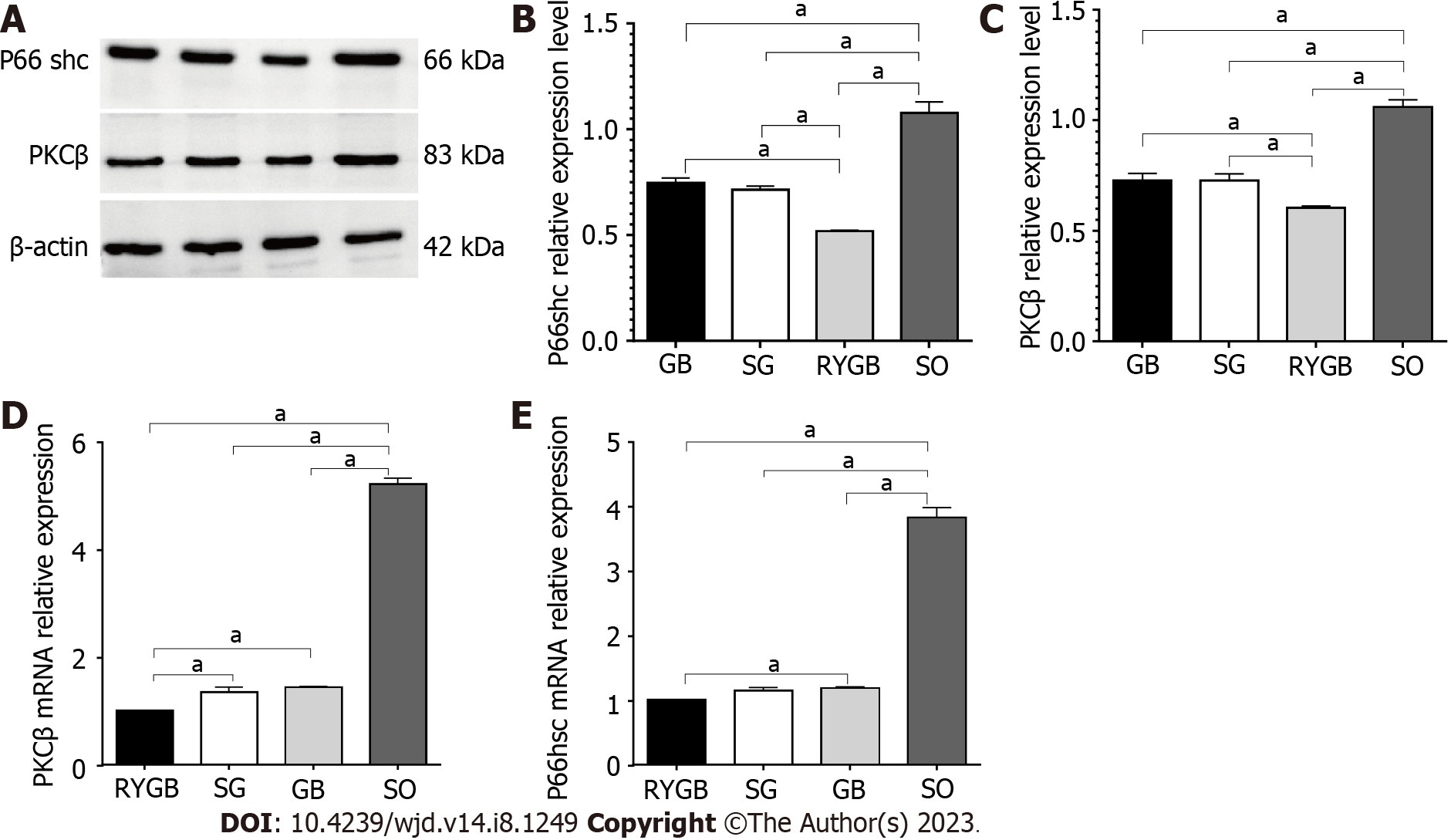
Western blot was performed as previously described[18]. Generally, protein samples were separated using 10% SDS-PAGE at 90 V for nearly 1.5 h, followed by electroblotting onto polyvinylidene difluoridemembranes for 2 h at 300 mA. The membranes were blocked with 5% bovine serum albumin (#9048-46-8, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) diluted in Tris-HCl buffer supplemented with 0.1% Tween-20 (TBST; pH = 7.4), followed by incubation with the primary antibodies rabbit anti-PKCβ (1:1000, #ab32026, Abcam Cambridge, United Kingdom) and rabbit anti-P66shc (1:1000, #ab33770, Abcam), overnight at 4 °C. The membranes were washed three times with TBST for 5 min each and then incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5000, #ab288151, Abcam) diluted in TBST for 1 h at room temperature. After the membranes were washed with TBST three times for 5 min each, an enhanced chemiluminescence solution (Bio-Rad, Hercules, CA, United States) was used to visualize the immunoreactive signals. Band intensity was quantified using ImageJ 5.0.
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed as previously described[17]. After blood collection, the rats were killed, the liver and kidney tissues were quickly removed, and 15-20 mg of pancreatic tail tissue was separated on the ice bed for fluorescent qPCR detection. Liver and kidney tissues were treated with TRIzol reagent, cut, and homogenized on ice until complete lysis. The complementary DNA (cDNA) was generated based on the RNA templates by using a Transcriptor Reverse Transcriptase kit (Roche Applied Science, IN, United States). The mRNA levels of P66shc and PKCβ were determined by qPCR using a KAPA SYBR FAST qPCR Kit (Kapa Biosystems, Inc., MA, United States) and the Miniopticon™ Real-Time PCR Detection System (Bio-Rad, CA, United States). β-actin was used as the internal control. The relative mRNA level of each gene was analyzed by the 2-ΔΔCt method. The sequences of each primer is shown in Table 1.
| Gene | 5’ to 3’ | Product length (bp) | |
| P66 shc | Forward | TTGCCCCTCCTCCAGGACAT | 196 |
| Reverse | CGCAACCCATGTACCGAACC | ||
| PKCβ | Forward | GACTTCATTTGGGGCTTCGGG | 140 |
| Reverse | TTGCTCCGTGGGTCATCAGA | ||
| β-actin | Forward | GTTGACATCCGTAAAGAC | 168 |
| Reverse | ACCAATCCACACAGAGTA |
Data are shown as the mean ± SEM and were analyzed using GraphPad Prism v7.03 by one-way analysis of variance with the Dunnett’s post-hoc test. Results were considered statistically significant at P < 0.05.
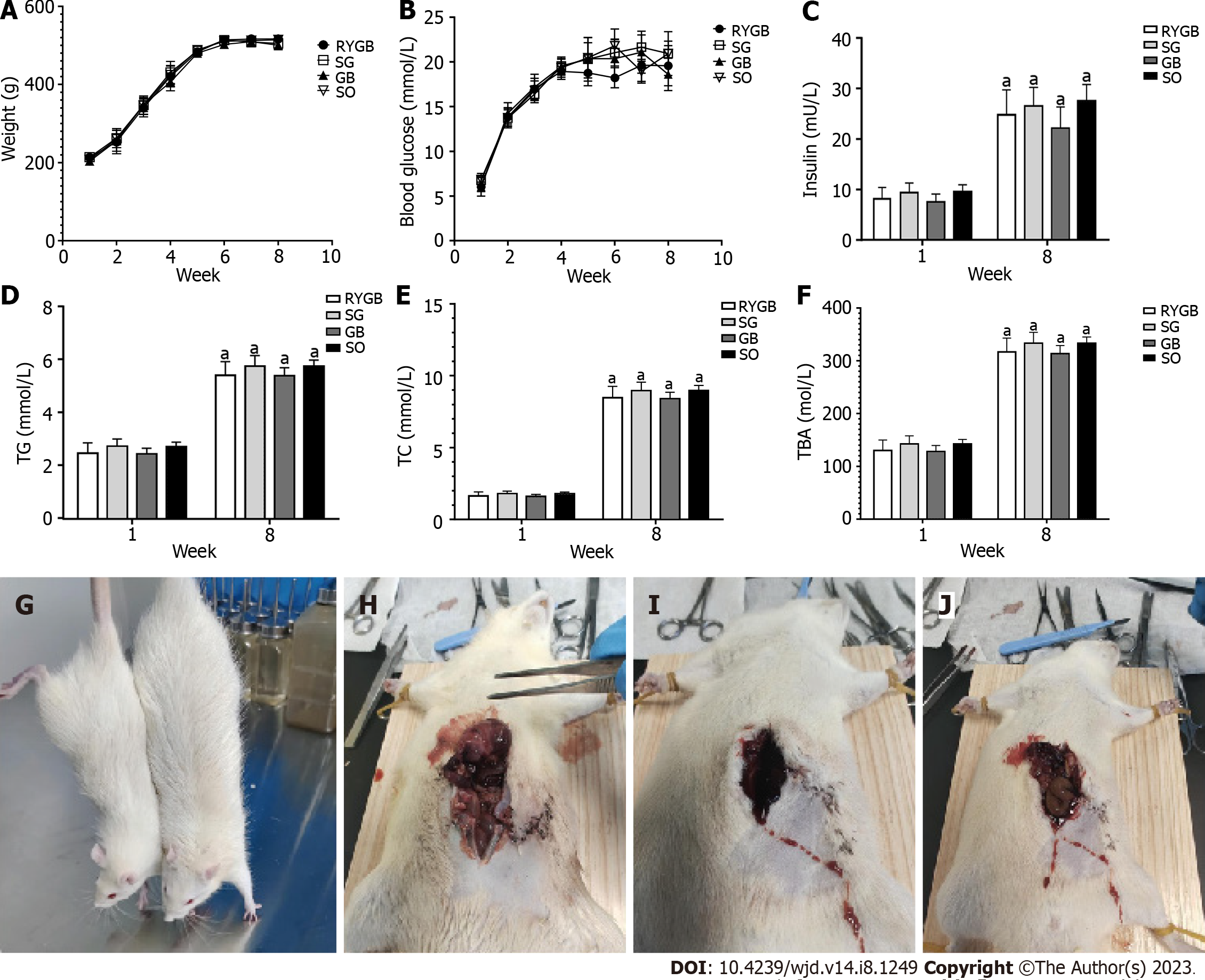
The levels of serum total cholesterol (TC), TG, IR, and TBA were significantly upregulated in all four groups after the induction of foodborne obesity-induced diabetes at week 8 compared with before (week 1).
After 8 wk, body weight, blood glucose, and serum levels of TC, TG, IR, and TBA were not significantly different among the four groups (P > 0.05) (Figures 1A-F). One rat in the RYGB group died of massive hemorrhage during surgery, and one died on postoperative day 4. Anatomical analysis showed that the cause of death might be anastomotic leakage (Figures 1G and H), and one died of wound infection (Figure 1I). Seven survived until the end of the experiment. Two rats in the SG group died of wound infection 1 wk after the operation (Figure 1J). Eight survived until the end of the experiment. All rats in the GB and SO groups survived.
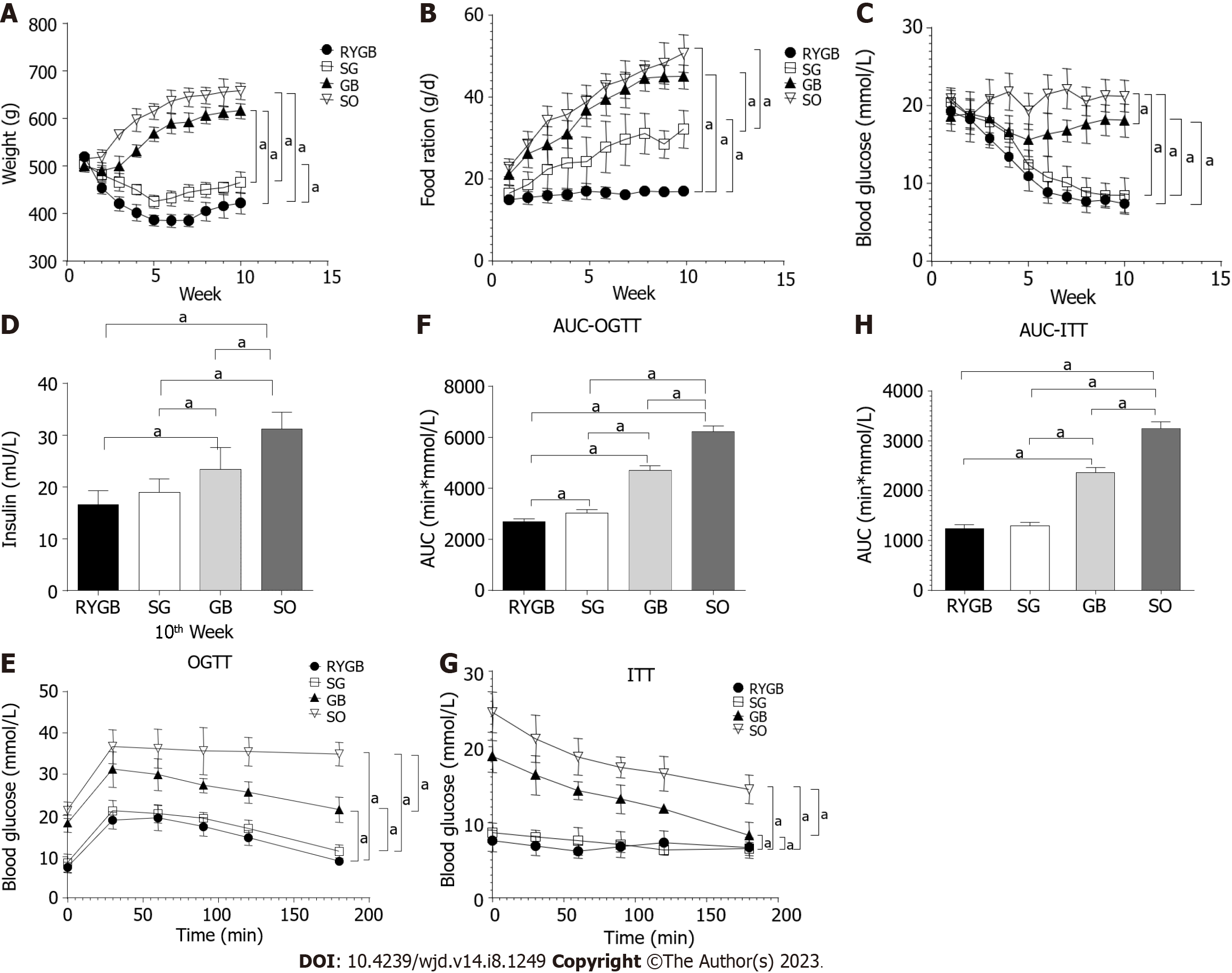
In comparison to the SO group, the body weight in the RYGB, SG, and GB groups was significantly decreased. Among the latter three groups, body weight was lowest in the RYGB group and highest in the GB group (P < 0.05) (Figure 2A). There was a significant difference in body weight between any two of the groups. In comparison to the SO group, food intake in the RYGB, SG, and GB groups was significantly decreased. Among the latter three groups, food intake was lowest in the RYGB group and highest in the GB group (P < 0.05) (Figure 2B). There was a significant difference in food intake between any two of the groups.
In comparison to the SO group, fasting blood glucose in the RYGB, SG, and GB groups was significantly decreased (P < 0.05). Among the latter three groups, fasting blood glucose was lowest in the RYGB group and highest in the GB group (Figure 2C). There was a significant difference in fasting blood glucose between any two of the groups.
In comparison to the SO group, fasting blood IR level in the RYGB, SG, and GB groups was significantly decreased (P < 0.05). Among the latter three groups, fasting blood IR level in RYGB group was significantly than those in the GB and SG groups, but with no significant difference between the RYGB and SG groups (Figure 2D). In comparison to the SO group, blood glucose level in the OGTT in the RYGB, SG, and GB groups was significantly decreased (P < 0.05). Among the latter three groups, blood glucose level in the OGTT was lowest in the RYGB group and highest in the GB group. There was a significant difference in blood glucose level in the OGTT between any two of the groups (Figure 2E). A similar pattern of AUC of the OGTT was observed (Figure 2F).
In comparison to the SO group, the blood glucose level in the ITT in the RYGB, SG, and GB groups was significantly decreased (P < 0.05). Among the latter three groups, blood glucose level in the ITT was lowest in the RYGB group and highest in the GB group (Figure 2G). There was a significant difference in blood glucose level in the ITT between any two of the groups. A similar pattern of AUC of the ITT was observed (Figure 2H).
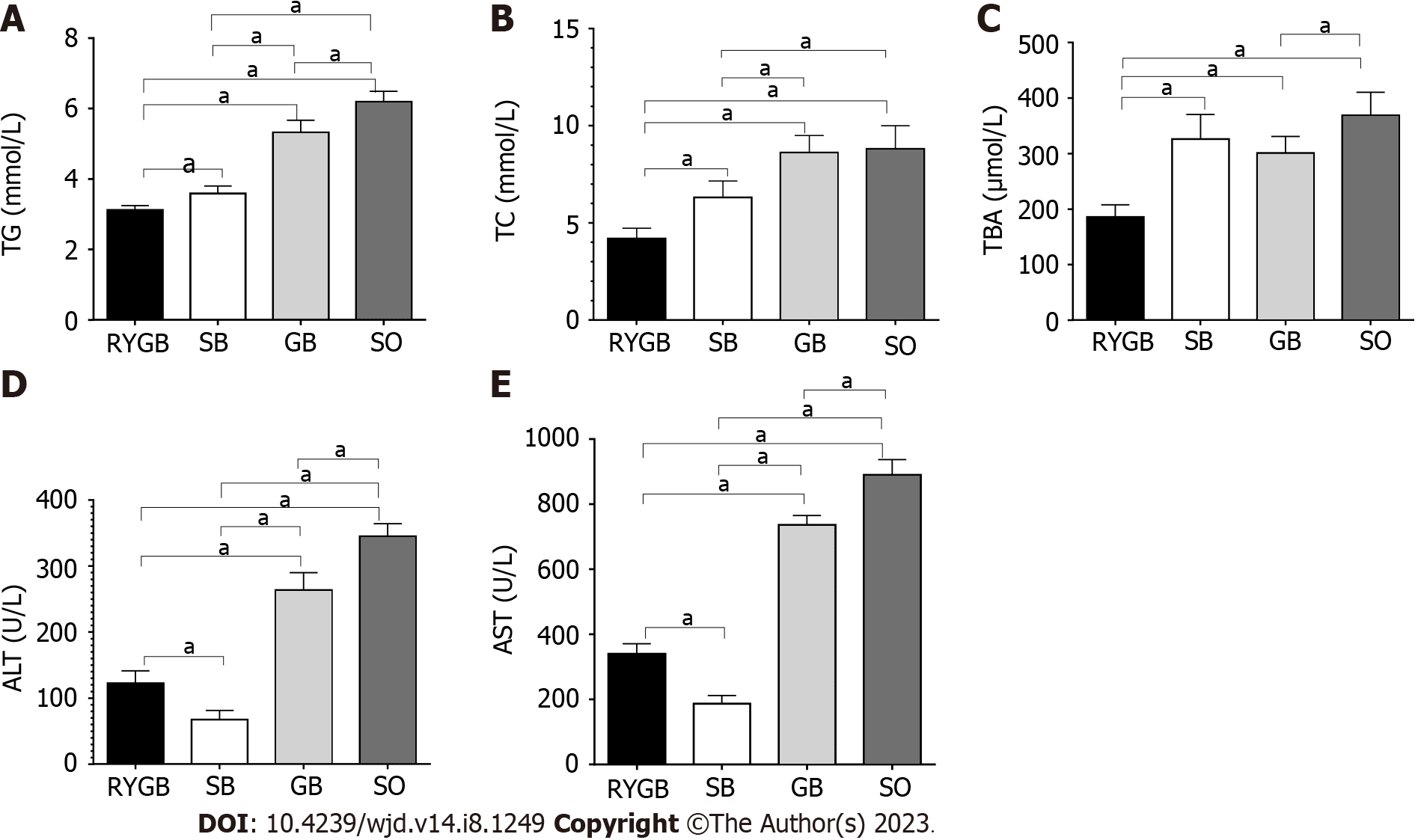
In comparison to the SO group, the serum levels of TG, TC, TBA, ALT, and AST were significantly decreased in the RYGB, SG, and GB groups (P < 0.05). Among the latter three groups, the serum levels of TG, TC, TBA, ALT, and AST were lowest in the RYGB group and highest in the GB group (Figure 3). There was a significant difference in the serum levels of TG, TC, TBA, ALT, and AST between any two of the groups.
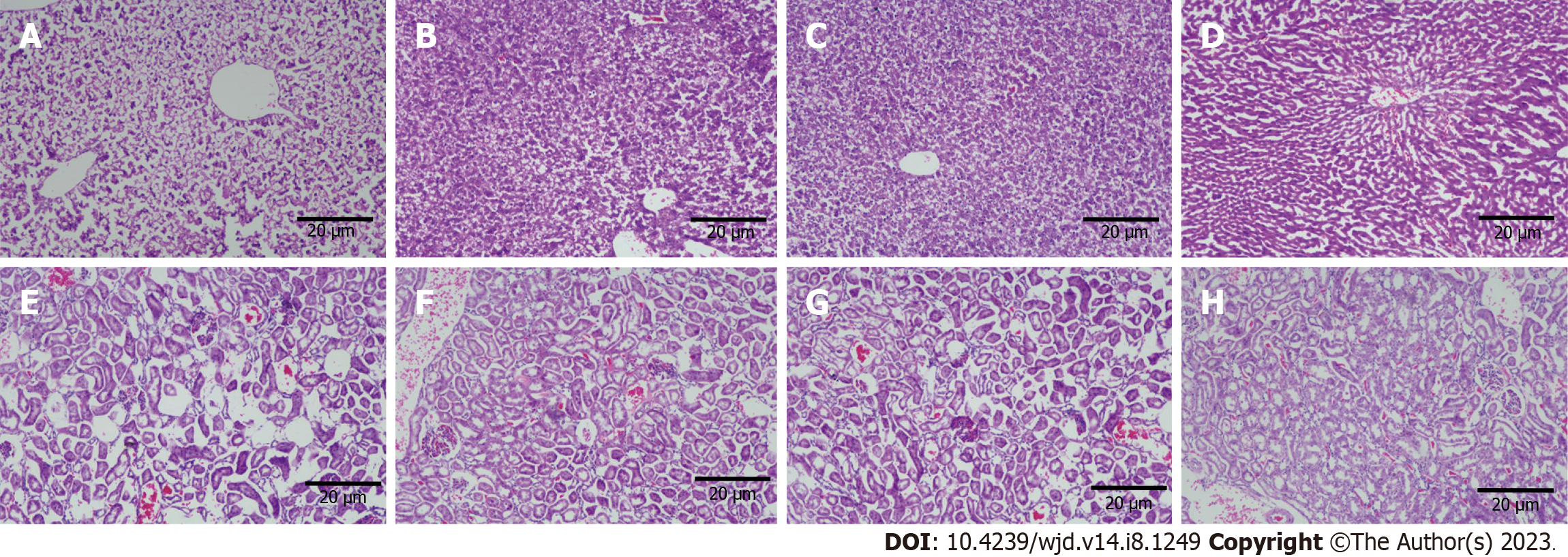
Grossly, the liver volume of rats in the SO group increased, with a milky white appearance, the edge became blunt, and the texture was soft; in the RYGB group, the liver was small and bright red, with sharp edges. HE staining showed a large number of fatty vacuoles in the liver of rats in the SO group, hepatic cord disorder, and narrowing of hepatic sinuses, indicating the presence of severe fatty liver. Compared with the SO group, the number of fatty vacuoles in the SG and GB groups was significantly reduced, the hepatic cord was unclear, and the shape was curved, indicating mild fatty liver. In the RYGB group, the liver lobules were normal, the hepatic cords were orderly arranged, the hepatic sinuses were normal, and no obvious degeneration of hepatocytes was observed (Figures 4A-D).
In the SO group, renal tubular lesions were more obvious, and epithelial cells showed vacuolar lesions, glomerular mesangial injury, and shedding. The lesions in the SG and GB groups were significantly alleviated, and there was no obvious kidney disease in the RYGB group (Figures 4E-H).
In comparison to the SO group, the protein expression levels of P66shc and PKCβ were significantly decreased in the RYGB, SG, and GB groups (P < 0.05). Among the latter three groups, the protein expression levels of P66shc and PKCβ were lowest in the RYGB group and highest in the GB group (Figures 5A-C). There was a significant difference in the protein expression levels of P66shc and PKCβ between any two of the groups.
In comparison to the SO group, the mRNA levels of P66shc and PKCβ were significantly decreased in the RYGB, SG, and GB groups (P < 0.05). Among the latter three groups, the mRNA levels of P66shc and PKCβ were lowest in the RYGB group and highest in the GB group (Figures 5D and E). There was a significant difference in the mRNA expression levels of P66shc and PKCβ between any two of the groups.
In previous studies, bariatric surgery promoted sustained weight loss, and achieved glycemic control and glucose homeostasis in patients with T2DM[7,10]. In the current study, we revealed that bariatric surgery, including RYGB, SG, and GB, can decrease body weight and food intake, reduce glucose intolerance and insulin insensitivity, downregulate biochemical parameters, alleviate morphological changes in the liver and kidneys, and diminish the expression levels of PKCβ and P66shc, suggesting that bariatric surgery may be a novel treatment for foodborne obesity-induced DM.
It is well-known that obesity predicts progression to T2DM, characterized by increased blood glucose, glucose intolerance, and IR[19,20]. Here, we observed that bariatric surgery decreased body weight and food intake, and reduced glucose intolerance and insulin insensitivity.
In complex diseases, including T2DM, there are multiple genes involved, affecting biological function in groups rather than alone. Therefore, to understand the signaling pathways involved in the pathological mechanisms and identify which of these pathways are affected in each patient may provide a better understanding of T2DM and could lead to new strategies for diagnosing, treating, and preventing this disease. It has been reported that acupuncture induced improvement of oxidative stress by regulating PKCβ/P66shc signaling in obese diabetic rats[21]. Here, we observed that bariatric surgery decreased the expression levels of PKCβ and P66shc.
Although these results look promising, each bariatric surgery, including RYGB, SG, and GB, has its own advantages and disadvantages. For RYGB, it has a small wound size, low risk, and good prognosis, and is generally less prone to recurrence. The way of food flow after surgery can also promote insulin secretion, effectively reduce the apoptosis of islet cells, restore the function of islets, and thus effectively treat diabetes. However, some rats undergoing RYGB will have abdominal discomfort, local inflammation of the anastomosis, and high blood sugar, which is easy to lead to incomplete healing of the surgical incision, infection, intestinal adhesion, and other complications. Some rats may also experience symptoms such as gastric paresis, gastrointestinal dysfunction, abdominal distension, and inability to eat, mainly related to the postoperative reduction of gastric volume.
For SG, it can effectively control T2DM and obesity related complications. By reducing the volume of the stomach, this surgery can reduce weight, improve T2DM, and reduce the risk of obesity related cardiovascular and cerebrovascular complications. However, SG completely removes the fundus of the stomach and may increase the risk of developing gastroesophageal reflux disease.
For GB, like SG, it is a surgical method of reducing weight by reducing food intake. It reduces the entry passage for food by installing binding straps. The surgical damage is minimal, and there is no need to modify the digestive tract, resulting in faster postoperative recovery. However, the restraining strap is prone to displacement and expansion, and the surgical effect is not very good, resulting in limited weight loss.
Bariatric surgery may be a novel treatment for foodborne obesity-induced diabetes.
Obesity usually causes diabetes mellitus (DM) and endangers human health seriously, and type 2 DM (T2DM) usually occurs along with obesity. Foodborne obesity-induced DM is caused by the excessive long-term diet and surplus energy.
Bariatric surgery can improve the symptoms of T2DM in some obese patients, but different types of bariatric surgery may have different effects.
To investigate the effect of different types of bariatric surgery on glucose and lipid metabolism, and liver and kidney function in rats, and to explore the underlying mechanisms.
Male Sprague-Dawley rats aged 6-8 wk underwent Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), or gastric banding (GB). Glucose and insulin tolerance tests, analysis of biochemical parameters, histological examination, western blot, and quantitative real-time polymerase chain reaction were conducted.
In comparison to the sham operation group, the RYGB, SG, and GB groups had decreased body weight and food intake, reduced glucose intolerance and insulin insensitivity, downregulated biochemical parameters, alleviated morphological changes in the liver and kidneys, and decreased levels of protein kinase C (PKC)β/P66shc. Among the three groups, the effect in the RYGB group was better than that in the SG and GB groups.
Bariatric surgeries, including RYGB, SG, and GB, can modulate the glucose and lipid metabolism, and liver and kidney function in food-derived obese diabetic rats via mediating the PKCβ/P66shc pathway.
Bariatric surgery may be helpful for the treatment of foodborne obesity-induced DM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dayan CM, United Kingdom; SantaCruz-Calvo S, United States; Horowitz M, Australia S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of Diabetes 2017. J Diabetes Res. 2018;2018:3086167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 311] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 2. | Grant B, Sandelson M, Agyemang-Prempeh B, Zalin A. Managing obesity in people with type 2 diabetes. Clin Med (Lond). 2021;21:e327-e231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Carbone S, Del Buono MG, Ozemek C, Lavie CJ. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis. 2019;62:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 4. | Johnston LW, Harris SB, Retnakaran R, Giacca A, Liu Z, Bazinet RP, Hanley AJ. Association of NEFA composition with insulin sensitivity and beta cell function in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort. Diabetologia. 2018;61:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2142] [Cited by in RCA: 2022] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 6. | Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: A systematic review and meta-analysis. PLoS Med. 2020;17:e1003206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 7. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1276] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 8. | Anhê FF, Varin TV, Schertzer JD, Marette A. The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery. Can J Diabetes. 2017;41:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Kapeluto J, Tchernof A, Biertho L. Surgery for Diabetes: Clinical and Mechanistic Aspects. Can J Diabetes. 2017;41:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Albaugh VL, Axelrod C, Belmont KP, Kirwan JP. Physiology Reconfigured: How Does Bariatric Surgery Lead to Diabetes Remission? Endocrinol Metab Clin North Am. 2023;52:49-64. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332 (Pt 2):281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1179] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 12. | Kong L, Andrassy M, Chang JS, Huang C, Asai T, Szabolcs MJ, Homma S, Liu R, Zou YS, Leitges M, Yan SD, Ramasamy R, Schmidt AM, Yan SF. PKCbeta modulates ischemia-reperfusion injury in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H1862-H1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1338] [Cited by in RCA: 1279] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 14. | Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 693] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 15. | Paneni F, Mocharla P, Akhmedov A, Costantino S, Osto E, Volpe M, Lüscher TF, Cosentino F. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res. 2012;111:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Bai XP, Du WJ, Xing HB, Yang GH, Bai R. Influence of ursodeoxycholic acid on blood glucose, insulin and GLP-1 in rats with liver fibrosis induced by bile duct ligation. Diabetol Metab Syndr. 2023;15:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Chien YH, Yu YH, Chen YW. Taiwanese green propolis ameliorates metabolic syndrome via remodeling of white adipose tissue and modulation of gut microbiota in diet-induced obese mice. Biomed Pharmacother. 2023;160:114386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Chang YQ, Zhou GJ, Wen HM, He DQ, Xu CL, Chen YR, Li YH, Chen SX, Xiao ZJ, Xie M. Treatment of radiation-induced brain injury with bisdemethoxycurcumin. Neural Regen Res. 2023;18:416-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 19. | Wilkinson L, Yi N, Mehta T, Judd S, Garvey WT. Development and validation of a model for predicting incident type 2 diabetes using quantitative clinical data and a Bayesian logistic model: A nationwide cohort and modeling study. PLoS Med. 2020;17:e1003232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, Guo Y, Cardel M, Pearson TA, DeBoer MD. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: the Atherosclerosis Risk In Communities Study and Jackson Heart Study. Diabetologia. 2017;60:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Yin G, Shen GM, Jiang AJ, Li JY. [Acupuncture intervention induced improvement of oxidative stress by regulating PKCβ/P66shc signaling in obese diabetic rats]. Zhen Ci Yan Jiu. 2021;46:642-648. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |