Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1234
Peer-review started: April 20, 2023
First decision: June 1, 2023
Revised: June 12, 2023
Accepted: July 7, 2023
Article in press: July 7, 2023
Published online: August 15, 2023
Processing time: 113 Days and 3.5 Hours
Dysregulated microRNA (miRNA) is crucial in the progression of diabetic nephropathy (DN).
To investigate the potential molecular mechanism of Icariin (ICA) in regulating endoplasmic reticulum (ER) stress-mediated apoptosis in high glucose (HG)-induced primary rat kidney cells (PRKs), with emphasis on the role of miR-503 and sirtuin 4 (SIRT4) in this process.
Single intraperitoneal injection of streptozotocin (65 mg/kg) in Sprague-Dawley rats induce DN in the in vivo hyperglycemic model. Glucose-treated PRKs were used as an in vitro HG model. An immunofluorescence assay identified isolated PRKs. Cell Counting Kit-8 and flow cytometry analyzed the effect of ICA treatment on cell viability and apoptosis, respectively. Real-time quantitative polymerase chain reaction and western blot analyzed the levels of ER stress-related proteins. Dual luciferase analysis of miR-503 binding to downstream SIRT4 was performed.
ICA treatment alleviated the upregulated miR-503 expression in vivo (DN) and in vitro (HG). Mechanistically, ICA reduced HG-induced miR-503 overexpression, thereby counteracting its function in downregulating SIRT4 levels. ICA regulated the miR-503/SIRT4 axis and subsequent ER stress to alleviate HG-induced PRKs injury.
ICA reduced HG-mediated inhibition of cell viability, promotion of apoptosis, and ER stress in PRKs. These effects involved regulation of the miR-503/SIRT4 axis. These findings indicate the potential of ICA to treat DN, and implicate miR-503 as a viable target for therapeutic interventions in DN.
Core Tip: Icariin (ICA) has shown promise as a potential therapeutic agent for diabetes mellitus (DM) by regulating the miR-503-5p/sirtuin 4 (SIRT4) axis and subsequent endoplasmic reticulum (ER) stress. This study found that ICA treatment reduced high glucose-induced inhibition of cell viability, promotion of apoptosis, and ER stress in primary rat kidney cells. Mechanistically, ICA inhibited the upregulation of miR-503-5p and subsequently restored SIRT4 levels, thereby alleviating high glucose-induced injury in cells. These findings implicate ICA as a candidate drug for the treatment of DM and miR-503-5p as a potential therapeutic target for this disease.
- Citation: Su BL, Wang LL, Zhang LY, Zhang S, Li Q, Chen GY. Potential role of microRNA-503 in Icariin-mediated prevention of high glucose-induced endoplasmic reticulum stress. World J Diabetes 2023; 14(8): 1234-1248
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1234.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1234
Elevated blood glucose levels are a defining feature of diabetes mellitus (DM), one of the most prevalent chronic illnesses[1]. Over the last few decades, the incidence of diabetic nephropathy (DN) due to DM has risen in tandem with the growing number of individuals diagnosed with diabetes[2,3]. DN has become a threat to public health security[4,5]. However, the pathogenesis of DN is still unclear. Some studies have reported that endoplasmic reticulum (ER) stress may be a major factor in the development of DN[6]. The reason is that many properties of DN, such as hyperglycemia, proteinuria, advanced glycosylation end products, and increased free fatty acids, can trigger unfolded protein responses in kidney cells[7].
Traditional Chinese medicine (TCM) and its bioactive ingredients have been used in cancer treatment, with multi-target pharmacological effects and few side effects[8]. As a traditional TCM, Icariin (ICA) has been proven by many recent studies to have a good therapeutic effect on DN. For example, Ding et al[9] showed that ICA can protect podocytes from streptozotocin (STZ)-induced damage by inhibiting the inflammasome[9]. Zang et al[10] described that ICA mitigates the adverse impact of hyperglycemia on renal tubular epithelial cells by regulating the miR-122-5p/forkhead box P2 axis[10].
As an important member of the non-coding RNA family, microRNAs (miRNAs) play important roles in various biological processes, and their multiple regulatory mechanisms in DN have attracted increasing attention[11,12]. As two examples, the enhanced expression of miR-133b can attenuate the degree of renal fibrosis, thereby inhibiting the development of DN[13], and the inhibition of miR-503 expression can improve the renal tissue injury caused by DN[14]. By binding to the 3′ untranslated region (UTR) of downstream targets and suppressing their transcriptional activity and translation, miRNAs contribute to the regulation of cellular functions and can disrupt the onset and progression of DN[15,16]. Studies have shown that miR-503 targeting E2F transcription factor 3 causes podocyte injury[17], and miR-195 targeting Toll-like receptor 4 alleviates symptoms in DN rats[18].
The current investigation established a DM cell model induced by elevated glucose [high glucose (HG)] to investigate the mechanism and impact of ICA on DM, with a focus on regulating the miR-503/sirtuin 4 (SIRT4) axis.
Twelve specific pathogen-free, 10-week-old, female Sprague-Dawley rats weighing 180-200 g were procured from the Laboratory Animal Center at Guangzhou University of Chinese Medicine (Guangzhou, China). The rats were randomly divided into two groups: Control (n = 6) and DM (n = 6). The DM group of rats were fasted for 12 h and then each received a single intraperitoneal injection of STZ (55 mg/kg). Blood samples were collected from the tail vein 72 h later, and a fasting blood glucose level of > 16.7 mmol/L for 3 consecutive days confirmed the establishment of DM. The STZ-induced hyperglycemic rats were observed for an additional 4 wk to allow the development of DN-related changes. All rats were kept in a polystyrene cage with ad libitum access to standard rat food and water, in a room with a 12-h light/dark cycle and humidity maintained at 50% ± 5%. At the end of the experiment, the rats were euthanized with an intraperitoneal injection of sodium pentobarbital (200 mg/kg body weight). The animal experiments were approved by the Animal Care and Use Committee of Guangzhou University of TCM (approval No. GZTCMF1-2022053) and were performed in accordance with the National Institutes of Health guidelines.
Rat kidney tissue was harvested under aseptic conditions, ground into powder under liquid nitrogen and dissolved in TRIZOL reagent (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, United States). The initial total RNA was quantified with Qubit RNA Assay Kit (Invitrogen). The Epicentre Ribo-Zero™ Magnetic Kit (Illumina, San Diego, CA, United States) was used to separate and remove ribosomal RNA from total RNA, to ensure extraction with an RNA integrity number ≥ 7 and a ratio of 28 s to 18 s RNA ≥ 1.5:1. First and second strand cDNAs were synthesized, and 45 μL AMPure XP Beads were used for polymerase chain reaction (PCR) amplification. The final complete transcriptome library was prepared and computer-tested by Beijing Boao JingDian Biotechnology Co., Ltd. (Beijing, China). The sequencing results were analyzed, and differentially expressed genes were identified based on a |log2(fold-change, FC) | ≥ 1.0 and a P value ≤ 0.05.
The isolation and culture of primary rat kidney cells (PRKs) were performed carried out following an established protocol[19]. Kidneys were obtained from additional 10-week-old healthy Sprague-Dawley rats (n = 3). The renal cortex of the extracted tissue was sectioned into tissue fragments of approximately 1 mm3 and digested with collagenase type I (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, United States). Cells were isolated by density gradient centrifugation using Ficoll (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and incubated in 37 °C. The collected PRKs were cultured in DMEM/F-12 supplemented with 10% fetal bovine serum, antibiotics, and growth factors in a humidified incubator at 37 °C in an atmosphere of 5% CO2. The culture medium was changed every other day, and the cells were passaged when they reached approximately 80% confluence. The PRKs used in this study were not used beyond the third passage to maintain their primary cell characteristics.
As previously described[20], PRKs were incubated with antibodies to α-smooth muscle actin (α-SMA; 1:250 dilution; ab124964, Abcam, Cambridge United Kingdom) and vimentin (1:300 dilution; ab45939, Abcam) for 12 h at 4 °C, followed by a 1 h treatment at 37 °C with antibodies labeled with Alexa Fluor® 488 (1:250 dilution; ab150077, Abcam) or 647 (1:150 dilution; 150075, Abcam). Finally, PRKs were stained for 5 min at 20 °C with 4',6-diamidino-2-phenylindole (0.5 μg/mL; Beyotime, Shanghai, China). Images were captured (magnification × 400) using a model BX53 fluorescence microscope (Olympus, Tokyo, Japan).
MiR-503 mimics/inhibitors and overexpression (ov) SIRT4 plasmids were transfected into PRKs for 4 h using Lipofectamine® 3000 (Invitrogen). GenePharma Biotechnology Co., Ltd. (Shanghai, China) synthesized the aforementioned RNAs. The sequences of miR-503 mimics/inhibitors are listed in Table 1. For HG treatment, PRKs were exposed to 5.5 mmol/L glucose (Sigma-Aldrich) as the control or 25 mmol/L glucose (HG) for 24 h to simulate cell growth in either normal or DM conditions, respectively, as previously described[21,22]. According to previous studies, ICA was used in HG treatment at doses of 10 μΜ (low, L), 25 μΜ (medium, M), and 50 μΜ (high, H)[23,24]. Treatment of PRKs with HG or ICA was performed 48 h after cell transfection.
| miRNA | Sequence (5’-3’) |
| miR-503 mimic | TAGCAGCGGGAACAGTACTGCAG |
| miR-503 mimic NC | GACAGAGACACGAGCGGCTGTAT |
| miR-503 inhibitor | CTGCAGTACTGTTCCCGCTGCTA |
| miR-503 inhibitor NC | TAGTAACCGTCTTTCCCCTGGGC |
PRKs were cultured for 24 h at a density of 5 × 103 cells/well in 96 well plates. The cell viability rate was evaluated using Cell Counting Kit-8 reagent (Beyotime, Shanghai, China) at 37 °C for 1 h. The reagent was added at 0 and 24 h as per the manufacturer's instructions. The determinations were done by measuring absorbance at 450 nm.
Total RNA was extracted from kidney tissues or PRKs using TRIzol reagent. After centrifugation at 12000 ×g at 4 °C for 10 min in a model JIDI-17RS high speed refrigerated centrifuge (Guangzhou JiDi Instrument Co., Ltd., Guangzhou, China), RNA were reverse-transcribed to cDNA and analysis using One Step SYBR Green real-time quantitative PCR (RT-qPCR) Kit (Biomarker, Beijing, China). The conditions used were 95 °C for 1 min followed by 40 cycles of 95 °C for 5 s and 62 °C for 30 s. Sequences of the primer pairs used for amplification are shown in Table 2. SIRT4 or miR-503 levels were normalized to β-actin or U6, and determined using the 2−ΔΔCt method[25].
| Primer | Sequence (5’-3’) |
| miR-151-3p-F | ACACTCCAGCTGGGCUAGACUGAGGCUCC |
| miR-151-3p-R | CTCAACTGGTGTCGTGGA |
| miR-19b-3p-F | ACACTCCAGCTGGGUGUGCAAAUCCAUGCAA |
| miR-19b-3p-R | CTCAACTGGTGTCGTGGA |
| miR-106b-5p-F | ACACTCCAGCTGGGUAAAGUGCUGACAGU |
| miR-106b-5p-R | CTCAACTGGTGTCGTGGA |
| miR-30e-5p-F | ACACTCCAGCTGGGUGUAAACAUCCUUGAC |
| miR-30e-5p-R | CTCAACTGGTGTCGTGGA |
| miR-122-5p-F | ACACTCCAGCTGGGUGGAGUGUGACAAUGG |
| miR-122-5p-R | CTCAACTGGTGTCGTGGA |
| miR-29a-3p-F | ACACTCCAGCTGGGUAGCACCAUCUGAAAU |
| miR-29a-3p-R | CTCAACTGGTGTCGTGGA |
| miR-29c-3p-F | ACACTCCAGCTGGGUAGCACCAUUUGAAAU |
| miR-29c-3p-R | CTCAACTGGTGTCGTGGA |
| miR-497-5p-F | ACACTCCAGCTGGGCAGCAGCACACUGUGG |
| miR-497-5p-R | CTCAACTGGTGTCGTGGA |
| miR-101a-3p-F | ACACTCCAGCTGGGUACAGUACUGUGAUA |
| miR-101a-3p-R | CTCAACTGGTGTCGTGGA |
| miR-741-3p-F | ACACTCCAGCTGGGAAAGAUGCCACGCUAU |
| miR-741-3p-R | CTCAACTGGTGTCGTGGA |
| miR-503-F | ACACTCCAGCTGGGTAGCAGCGGGAACAGTA |
| miR-503-R | CTCAACTGGTGTCGTGGA |
| miR-671-F | ACACTCCAGCTGGGUCCGGUUCUCAGGGC |
| miR-671-R | CTCAACTGGTGTCGTGGA |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
| SIRT4-F | TCCTGGGAGTGGACAGAATGA |
| SIRT4-R | CTGTGGATCCATGGGAACGC |
| Caspase 12-F | TGCCAATTCCGACAAACAGC |
| Caspase 12-R | CTGGATTCCCTGAGGAACTGT |
| GRP78-F | AACCCAGATGAGGCTGTAGCA |
| GRP78-R | ACATCAAGCAGAACCAGGTCAC |
| CHOP-F | CCAGCAGAGGTCACAAGCAC |
| CHOP-R | CGCACTGACCACTCTGTTTC |
| β-actin-F | CACCCGCGAGTACAACCTTC |
| β-actin-R | CCCATACCCACCATCACACC |
Annexin V-fluorescein isothiocyanate (BD Biosciences, Santa Clara, CA, United States) and propidium iodide (BD) were incubated with PRKs (5 × 104 cells/mL) in the dark for 12 min at 25 °C, then analyzed with FACSAria™ Fusion using FACSDiva software (BD).
Denatured proteins extracted from PRKs were resolved by 10% SDS-PAGE (Beyotime). The resolved protein bands were subsequently transferred onto PVDF membranes. The membranes were incubated for 12 h at 4 °C with primary antibodies to SIRT4 (1:800 dilution; ab231137, Abcam), caspase 12 (1:800 dilution; ab62484, Abcam), C/EBP-homologous protein (CHOP; 1:1200 dilution, #5554; Cell Signaling Technology, Beverly, MA, United States), glucose regulated protein 78 (GRP78; 1:1000 dilution; ab108615, Abcam), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2000 dilution; ab8245, Abcam). The membranes were then incubated with goat anti-rabbit antibody (1:12000 dilution; ab205718, Abcam) for 2 h at 25 °C. Immunoreactive bands were detected using an ECL system (Millipore, Temecula, CA, United States).
The 3’-UTR fragments of SIRT4 were amplified and cloned into the psi-CHECK-2 Luciferase reporter vector (Promega Corporation, Madison, WI, United States). PRKs were then transfected with miR-503 mimic/mimic NC and the psi-CHECK-2 containing either the wild type or mutant (Mut) of SIRT4 using Lipofectamine® 2000 (Invitrogen) as per the manufacturer’s instructions. After 48 h, luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega Corporation).
The data are expressed as mean ± SD of triplicate measurements. Statistical analysis was conducted by one-way analysis of variance followed by Bonferroni post hoc tests. Independent two-group comparisons were analyzed using the Student's t-test. The significance level was set at P < 0.05.
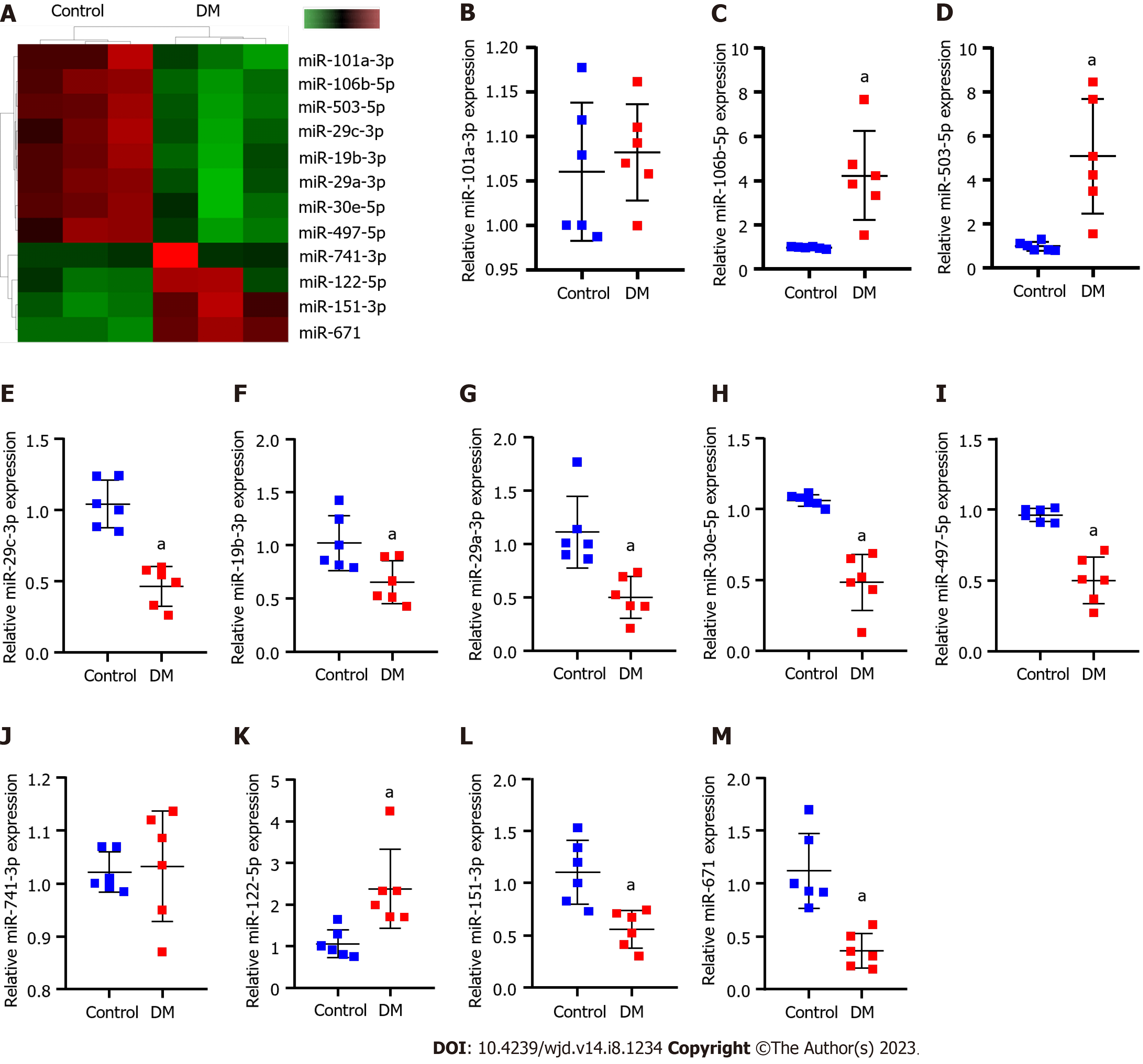
Sequencing of kidney tissues from normal and DM model rats revealed that 754 miRNAs were expressed. Among them, 12 miRNAs were differentially expressed (Figure 1A and Table 3). RT-qPCR results revealed that compared with normal rats, miR-106b-5p, miR-122-5p and miR-503 were significantly upregulated, miR-151-3p, miR-19b-3p, miR-30e-5p, miR-29a/c-3p, miR-497-5p and miR-671 were significantly downregulated, and miR-101a-3p and miR-741-3p was not significantly different in DM rats (Figure 1B-M).
| miRNA_id | Log2 FC | P value |
| rno-miR-151-3p | > 1.0 | < 0.001 |
| rno-miR-19b-3p | < -1.0 | < 0.001 |
| rno-miR-106b-5p | < -1.0 | < 0.001 |
| rno-miR-30e-5p | < -1.0 | < 0.001 |
| rno-miR-122-5p | > 1.0 | < 0.001 |
| rno-miR-29a-3p | < -1.0 | < 0.001 |
| rno-miR-29c-3p | < -1.0 | < 0.001 |
| rno-miR-497-5p | < -1.0 | < 0.001 |
| rno-miR-101a-3p | < -1.0 | < 0.001 |
| rno-miR-741-3p | > 1.0 | < 0.001 |
| rno-miR-503 | < -1.0 | < 0.001 |
| rno-miR-671 | > 1.0 | < 0.001 |
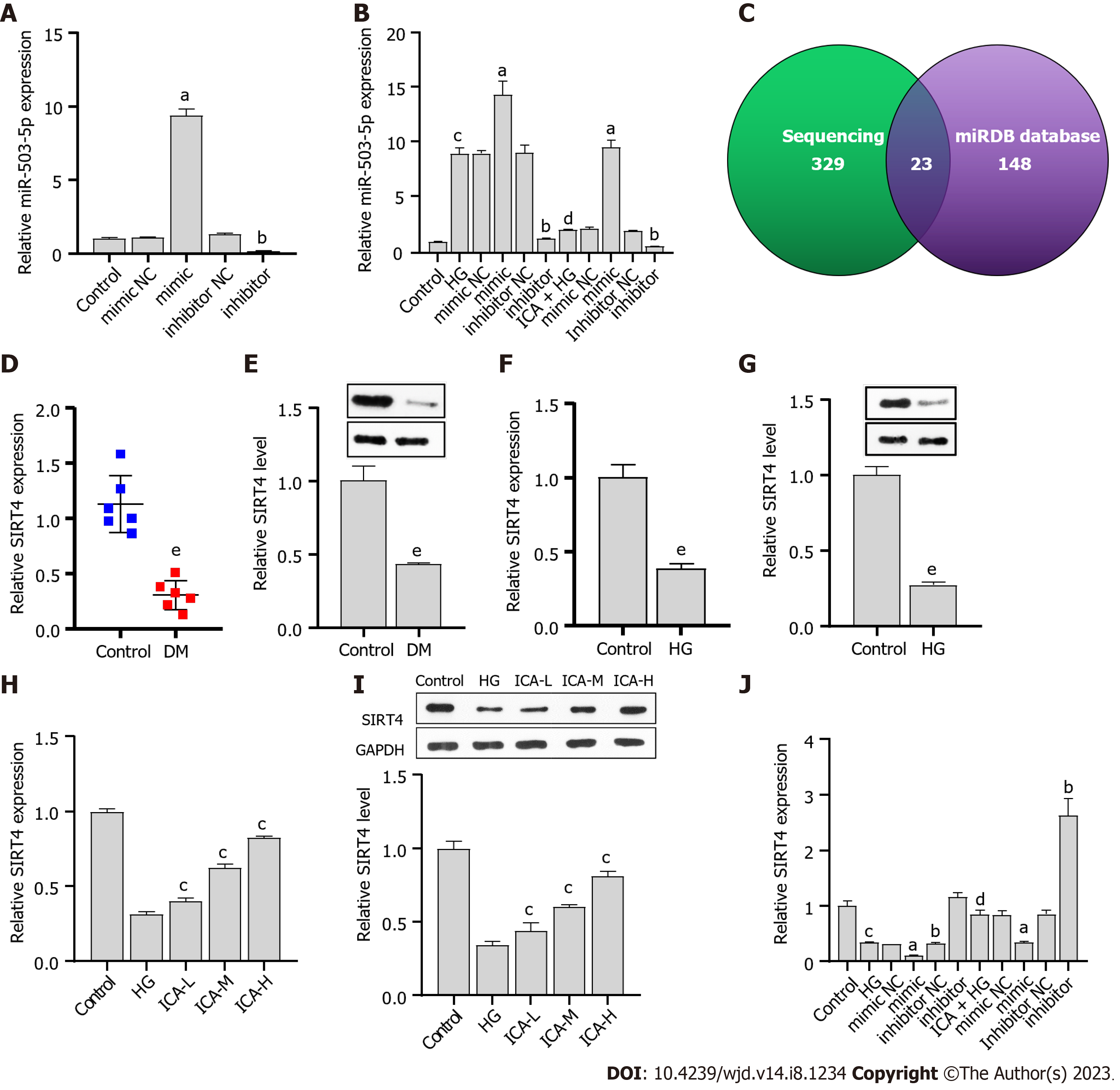
Isolated PRKs were subjected to immunofluorescence (IF) staining for vimentin and α-SMA. Fluorescence microscopy of these samples revealed the high expression of both vimentin and α-SMA in the PRKs (Figure 2A), indicating that the PRKs used in this study were effective. In vitro, the expression of miRNAs in the HG-induced cell model was consistent with the in vivo results (Figure 2B). Among the miRNAs, miR-503 had the highest expression in vivo and in vitro, and was explored as a potential therapeutic target for DM. Furthermore, the expression (Figure 2C) and protein levels (Figure 2D) of ER stress associated factors caspase 12, CHOP, and GRP78 were significantly upregulated in HG-induced PRKs. The expression of miR-503 upregulated by HG was decreased in PRKs after the addition of ICA; the effect of high doses of ICA was most obvious (Figure 2E). In addition, the high expression and protein levels of HG-induced caspase 12, CHOP, and GRP78 progressively decreased in the low, medium, and high doses of ICA (Figure 2F and G). Thus, miR-503 may be a therapeutic target for DM. The effects of HG-induced reduction in PRKs cell viability (Figure 2H) and increased apoptosis (Figure 2I) were reversed by ICA. Notably, low, medium, and high doses of ICA did not significantly change the activity of normal PRKs (Figure 2J). Therefore, ICA at a dose of 50 μM was used as the therapeutic dose in subsequent molecular mechanism experiments.
The synthesized miR-503 mimic/inhibitor was transfected into PRKs. The results showed that miR-503 was upregulated in the miR-503 mimic transfection group and downregulated in the miR-503 inhibitor transfection group (Figure 3A). These results confirmed the efficacy of the synthesized miR-503 mimic/inhibitor. The effect of HG-induced promotion of miR-503 expression was enhanced by mimic and reversed by inhibitor. However, ICA treatment inhibited the effect of HG-mediated promotion of miR-503 expression, which was reversed by mimic and enhanced by inhibitor (Figure 3B). These results confirmed that miR-503 may be a key pathway for ICA to improve HG-induced cell injury. The mechanism of miR-503 is still unclear.
The sequencing results showed that 329 mRNAs could potentially bind to miR-503. Joint analysis of the sequencing results with the miRDB database (Figure 3C) revealed the intersection of 23 mRNAs (Table 4). Among them, prior results have suggested that SIRT4 can prevent glucose-induced apoptosis and cell damage[26,27]. We observed that SIRT4 expression and protein levels were downregulated in DM renal tissue or HG-induced PRKs (Figure 3D-G). The HG-induced downregulations were gradually recovered after low, medium, and high doses of ICA treatment (Figure 3H and I). The inhibitory effect of HG treatment on SIRT4 expression was enhanced by mimic and reversed by inhibitor. The inhibitory effect of ICA treatment on HG-induced SIRT4 expression was reversed by mimic and enhanced by inhibitor (Figure 3J). Therefore, the expression of miR-503 is negatively correlated with SIRT4.
| No. | Gene_id |
| 1 | SLC12A1 |
| 2 | SIRT4 |
| 3 | MLYCD |
| 4 | FBXL22 |
| 5 | CCND1 |
| 6 | MAP2K1 |
| 7 | PCP4 |
| 8 | RPS6KA3 |
| 9 | EMC6 |
| 10 | PPFIA3 |
| 11 | TTC17 |
| 12 | RAB11FIP1 |
| 13 | GALNT2 |
| 14 | NCS1 |
| 15 | APLN |
| 16 | CEP85L |
| 17 | CLSTN1 |
| 18 | KIF1C |
| 19 | PIGQ |
| 20 | BTRC |
| 21 | LOC691995 |
| 22 | EGLN3 |
| 23 | NABP1 |
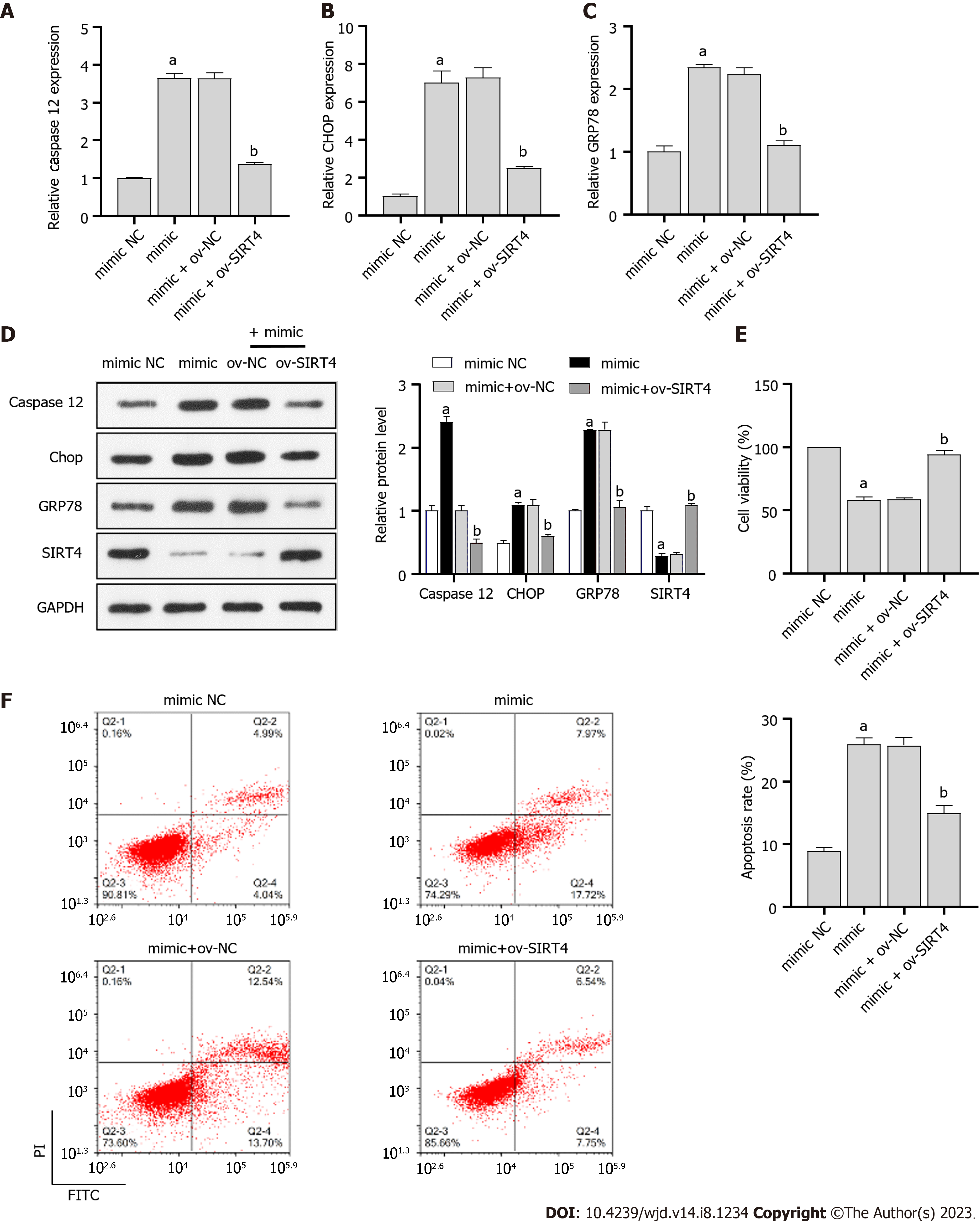
Typically, miRNAs bind to the 3’-UTR of downstream targets and inhibit transcriptional activity and translational levels, thereby affecting DN progression[28,29]. We constructed a SIRT4 overexpressing plasmid and transfected it into PRKs cells. The resulting expression and protein level of SIRT4 were upregulated in the ov-SIRT4 group (Figure 4A and B), confirming the successful ov-SIRT4. Sequencing results showed that miR-503 had a potential binding site with SIRT4 (Figure 4C), Dual luciferase results revealed that SIRT4 is a direct target of miR-503 (Figure 4D). The inhibition of SIRT4 expression by miR-503 mimic was reversed after transfection with ov-SIRT4 (Figure 4E and F). The findings indicate that miR-503 directly interacts with the 3'-UTR of SIRT4, inhibiting its transcriptional activity. Furthermore, our results suggest that the mechanism by which ICA restores viability and inhibits apoptosis of HG-induced PRKs cells is related to the miR-503/SIRT4 axis.
To investigate the role of miR-503/SIRT4 axis in ICA treatment of HG-induced injury in PRKs, miR-503 mimic and ov-SIRT4 were co-transfected into PRKs. In PRKs co-treated with HG and ICA, miR-503 mimic promoted the expression/protein levels of ER stress associated factors caspase 12, CHOP, and GRP78 (Figure 5A-C) and inhibited the expression/protein levels of SIRT4 (Figure 5D). However, after co-transfection with ov-SIRT4, the effect of miR-503 mimic was reversed. In addition, in PRKs co-treated with HG and ICA, the inhibition of cell viability (Figure 5E) and promotion of apoptosis (Figure 5F) by miR-503 mimic were reversed after co-transfection with ov-SIRT4.
Recent research has suggested that improving protein folding may attenuate renal injury and that pharmacological treatment of ER stress is a promising therapeutic approach to prevent or halt the progression of kidney disease[6,30]. Studies have suggested that TCM is a safer treatment[31,32]. The use of TCM as combination therapy has been increasingly reported[33,34]. ICA is a flavonol glycoside isolated from Epimedium[35]. Long-term administration of ICA significantly improves behavioral performance[36], reduces neuronal apoptosis, and inhibits ER stress signaling[37]. Protein misfolding and ER stress are evident in various kidney diseases, including DN and chronic kidney disease[38,39]. Therefore, studying the effects of ICA on HG-treated PRKs may help uncover its potential cellular mechanisms, which could further the understanding of the potential effectiveness and reliability of ICA. The findings could suggest new avenues for potential therapeutic interventions relevant to DN.
We demonstrated HG-induced inhibition of proliferation, promotion of apoptosis, and ER stress in PRKs, suggesting that HG can induce renal cell damage, which may contribute to mechanisms relevant to DN[40]. These results are similar to previous results of ICA for ER stress in renal cells[41]. Since the overexpression of miR-503 may be the cause of the pathogenesis of DN[14], miR-503 was selected as a potential therapeutic target for DN from miRNA sequencing results. The expression of miR-503 increased after HG treatment, but was reversed under the action of ICA. Therefore, miR-503 may play an essential role in processes relevant to DN. To investigate the role of miR-503 in the pathology of HG-induced PRKs injury, we overexpressed and inhibited miR-503, and measured the levels of ER stress markers. The results demonstrated that the miR-503 mimic can accelerate HG-induced ER stress, while miR-503 inhibitor can reduce HG-induced ER stress.
It is well known that miRNAs function via targeting to the 3’-UTR of downstream targets[42]. Here, we report the first evidence of miR-503 directly targeting SIRT4. Through this mechanism, miR-503 regulates SIRT4-mediated growth of PRKs and ER stress. SIRT4 downregulation blocks the improvement and protection of renal function by forkhead box M1 in mice[26]. SIRT4 overexpression increases podocyte proliferation and inhibits apoptosis[27]. This could be attributed to the fact that SIRT4 induced by stresses and contributes to cell survival after stress[43]. In the present study, we observed HG-induced downregulation of SIRT4 expression and protein level in PRKs, which was increased after ICA treatment. Moreover, overexpression of SIRT4 reversed ER stress induced by miR-503 mimic. The dual luciferase assay revealed that SIRT4 is a direct target of miR-503. Therefore, HG-mediated upregulation of miR-503 inhibits the transcriptional activity and translation level of downstream target SIRT4, which damages PRKs and produces ER stress. However, ICA treatment blocked HG treatment, promoted the change of miR-503/SIRT4 axis in cells, and protected PRKs from HG-induced cell damage and ER stress.
We present novel evidence supporting the role of the ICA/miR-503/SIRT4 axis in the pathogenesis of HG-induced PRKs. Our findings expand the current understanding of HG-induced pathogenesis and suggest new potential molecular targets that could be explored in future therapeutic interventions related to conditions such as DN.
The study has several limitations. Firstly, while our results show a correlation between ICA, miR-503, and mechanisms related to DN, the causative role of miR-503 in the pathology of DN remains to be established. Secondly, although ICA treatment has shown promising effects in our in vitro study, the lack of clinical data supporting the therapeutic efficacy of ICA or miR-503 in conditions like DN is a clear limitation. Finally, while we have demonstrated an interplay between ICA and the miR-503-SIRT4 axis, validation in PRKs only does not provide broader insights. Subsequent studies are needed to expand the experimental scope in different cell types associated with diabetes (e.g., podocytes or β-cells).
The in vivo and in vitro data demonstrate that ICA has the ability to safeguard PRKs against damage caused by HG-induced injury and ER stress. This protective effect is attributed to the ability of ICA to increase the proliferation of PRKs, while simultaneously inhibiting apoptosis. This is achieved by regulating the miR-503-SIRT4 axis. Moreover, the study findings provide an understanding of the molecular mechanisms potentially underlying the development of DN, specifically the role of miRNAs and their downstream targets. Based on these findings, ICA should be further explored in animal models to test whether it can still protect the kidney from diabetes as a reliable and efficient candidate for the treatment of DN.
The study focuses on the effects of high glucose (HG) levels in diabetes and its implications on kidney function. It builds upon existing literature indicating that protein misfolding and endoplasmic reticulum (ER) stress are prevalent in kidney diseases, including diabetic nephropathy (DN) and chronic kidney disease. In addition, the flavonol glycoside Icariin (ICA), a traditional Chinese medicine (TCM), has shown potential in attenuating ER stress, which may potentially prevent or halt the progression of kidney diseases.
The research aimed to explore the potential of ICA as a treatment for HG-induced cellular damage, focusing on its interaction with specific microRNAs and proteins that contribute to renal cell damage. Given the increasing interest in the use of TCM as a safer and efficient alternative therapy, understanding the cellular mechanisms of ICA could provide valuable insights for potential therapeutic interventions in DN.
The primary goal was to investigate the effects of ICA on HG-treated kidney cells, with a particular emphasis on the role of miR-503 and sirtuin 4 (SIRT4) in the pathogenesis of HG-induced kidney injury. The study's findings could further the understanding of the mechanisms of ICA, paving the way for future research into its effectiveness and reliability as a treatment for DN.
The research used in vitro experiments to study the effects of ICA on HG-induced cell injury and ER stress in renal cells. The methods adopted included overexpression and inhibition of miR-503 and measuring levels of ER stress markers. The study also conducted a dual luciferase assay to determine whether SIRT4 is a direct target of miR-503.
The study identified that ICA ameliorates HG-induced cell injury by reducing the expression of miR-503. This research also discovered that miR-503 directly targets SIRT4, a novel finding in the field. However, some problems remain, such as establishing the causative role of miR-503 in DN and validating the results in other cell types associated with diabetes.
This study proposes a new theory suggesting the role of the ICA/miR-503/SIRT4 axis in the pathogenesis of HG-induced renal cell injury. Additionally, it presents a new method of using ICA treatment to regulate the miR-503/SIRT4 axis, thereby protecting cells from HG-induced cell damage and ER stress.
Future research should focus on validating these findings in animal models and different diabetic-associated cell types like podocytes or β-cells. Further studies should also explore the therapeutic efficacy of Icariin or miR-503 in clinical settings for conditions like DN, ultimately advancing our understanding of the ICA/miR-503/SIRT4 axis's role in managing diabetes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aslam M, India; Cai L, United States; Liu YQ, United States; Horowitz M, Australia S-Editor: Fan JR L-Editor: A P-Editor: Chen YX
| 1. | Defeudis G, Mazzilli R, Tenuta M, Rossini G, Zamponi V, Olana S, Faggiano A, Pozzilli P, Isidori AM, Gianfrilli D. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab Res Rev. 2022;38:e3494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 2. | Giralt-López A, Molina-Van den Bosch M, Vergara A, García-Carro C, Seron D, Jacobs-Cachá C, Soler MJ. Revisiting Experimental Models of Diabetic Nephropathy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep. 2019;21:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 402] [Article Influence: 67.0] [Reference Citation Analysis (1)] |
| 4. | Wada T, Mori-Anai K, Kawaguchi Y, Katsumata H, Tsuda H, Iida M, Arakawa K, Jardine MJ. Renal, cardiovascular and safety outcomes of canagliflozin in patients with type 2 diabetes and nephropathy in East and South-East Asian countries: Results from the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation Trial. J Diabetes Investig. 2022;13:54-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Jacob SR, Raveendran R, Kannan S. Causes, comorbidities and current status of chronic kidney disease: A community perspective from North Kerala. J Family Med Prim Care. 2019;8:2859-2863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Victor P, Umapathy D, George L, Juttada U, Ganesh GV, Amin KN, Viswanathan V, Ramkumar KM. Crosstalk between endoplasmic reticulum stress and oxidative stress in the progression of diabetic nephropathy. Cell Stress Chaperones. 2021;26:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Fan Y, Lee K, Wang N, He JC. The Role of Endoplasmic Reticulum Stress in Diabetic Nephropathy. Curr Diab Rep. 2017;17:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Wang S, Fu JL, Hao HF, Jiao YN, Li PP, Han SY. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res. 2021;170:105728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 9. | Ding X, Zhao H, Qiao C. Icariin protects podocytes from NLRP3 activation by Sesn2-induced mitophagy through the Keap1-Nrf2/HO-1 axis in diabetic nephropathy. Phytomedicine. 2022;99:154005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Zang L, Gao F, Huang A, Zhang Y, Luo Y, Chen L, Mao N. Icariin inhibits epithelial mesenchymal transition of renal tubular epithelial cells via regulating the miR-122-5p/FOXP2 axis in diabetic nephropathy rats. J Pharmacol Sci. 2022;148:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Kaur P, Kotru S, Singh S, Munshi A. miRNA signatures in diabetic retinopathy and nephropathy: delineating underlying mechanisms. J Physiol Biochem. 2022;78:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Colhoun HM, Marcovecchio ML. Biomarkers of diabetic kidney disease. Diabetologia. 2018;61:996-1011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 13. | Sun Z, Ma Y, Chen F, Wang S, Chen B, Shi J. miR-133b and miR-199b knockdown attenuate TGF-β1-induced epithelial to mesenchymal transition and renal fibrosis by targeting SIRT1 in diabetic nephropathy. Eur J Pharmacol. 2018;837:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Zhu X, Zhang C, Fan Q, Liu X, Yang G, Jiang Y, Wang L. Inhibiting MicroRNA-503 and MicroRNA-181d with Losartan Ameliorates Diabetic Nephropathy in KKAy Mice. Med Sci Monit. 2016;22:3902-3909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Li Y, Xu Y, Hou Y, Li R. Construction and Bioinformatics Analysis of the miRNA-mRNA Regulatory Network in Diabetic Nephropathy. J Healthc Eng. 2021;2021:8161701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Yarahmadi A, Shahrokhi SZ, Mostafavi-Pour Z, Azarpira N. MicroRNAs in diabetic nephropathy: From molecular mechanisms to new therapeutic targets of treatment. Biochem Pharmacol. 2021;189:114301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Zha F, Bai L, Tang B, Li J, Wang Y, Zheng P, Ji T, Bai S. MicroRNA-503 contributes to podocyte injury via targeting E2F3 in diabetic nephropathy. J Cell Biochem. 2019;120:12574-12581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Zhu LL, Wang HY, Tang T. Effects of miR-195 on diabetic nephropathy rats through targeting TLR4 and blocking NF-κB pathway. Eur Rev Med Pharmacol Sci. 2021;25:1522-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Song Y, Bai Z, Zhang Y, Chen J, Chen M, Zhang X, Mai H, Wang B, Lin Y, Gu S. Protective effects of endothelial progenitor cell microvesicles on Ang IIinduced rat kidney cell injury. Mol Med Rep. 2022;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Mai H, Huang Z, Zhang X, Zhang Y, Chen J, Chen M, Song Y, Wang B, Lin Y, Gu S. Protective effects of endothelial progenitor cell microvesicles carrying miR985p on angiotensin IIinduced rat kidney cell injury. Exp Ther Med. 2022;24:702. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Wang L, Li H. MiR-770-5p facilitates podocyte apoptosis and inflammation in diabetic nephropathy by targeting TIMP3. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Li A, Peng R, Sun Y, Liu H, Peng H, Zhang Z. LincRNA 1700020I14Rik alleviates cell proliferation and fibrosis in diabetic nephropathy via miR-34a-5p/Sirt1/HIF-1α signaling. Cell Death Dis. 2018;9:461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 23. | Jia Z, Wang K, Zhang Y, Duan Y, Xiao K, Liu S, Ding X. Icariin Ameliorates Diabetic Renal Tubulointerstitial Fibrosis by Restoring Autophagy via Regulation of the miR-192-5p/GLP-1R Pathway. Front Pharmacol. 2021;12:720387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Zhou YD, Hou JG, Yang G, Jiang S, Chen C, Wang Z, Liu YY, Ren S, Li W. Icariin ameliorates cisplatin-induced cytotoxicity in human embryonic kidney 293 cells by suppressing ROS-mediated PI3K/Akt pathway. Biomed Pharmacother. 2019;109:2309-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133995] [Article Influence: 5583.1] [Reference Citation Analysis (1)] |
| 26. | Xu X, Zhang L, Hua F, Zhang C, Mi X, Qin N, Wang J, Zhu A, Qin Z, Zhou F. FOXM1-activated SIRT4 inhibits NF-κB signaling and NLRP3 inflammasome to alleviate kidney injury and podocyte pyroptosis in diabetic nephropathy. Exp Cell Res. 2021;408:112863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 27. | Shi JX, Wang QJ, Li H, Huang Q. SIRT4 overexpression protects against diabetic nephropathy by inhibiting podocyte apoptosis. Exp Ther Med. 2017;13:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Cao H, Rao X, Jia J, Yan T, Li D. Identification of tubulointerstitial genes and ceRNA networks involved in diabetic nephropathy via integrated bioinformatics approaches. Hereditas. 2022;159:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Guo M, Dai Y, Jiang L, Gao J. Bioinformatics Analysis of the Mechanisms of Diabetic Nephropathy via Novel Biomarkers and Competing Endogenous RNA Network. Front Endocrinol (Lausanne). 2022;13:934022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 30. | Ricciardi CA, Gnudi L. The endoplasmic reticulum stress and the unfolded protein response in kidney disease: Implications for vascular growth factors. J Cell Mol Med. 2020;24:12910-12919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Wang WY, Zhou H, Wang YF, Sang BS, Liu L. Current Policies and Measures on the Development of Traditional Chinese Medicine in China. Pharmacol Res. 2021;163:105187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 32. | Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, Cai H. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 328] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 33. | Ren YY, Zhang XR, Li TN, Zeng YJ, Wang J, Huang QW. Galla Chinensis, a Traditional Chinese Medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J Ethnopharmacol. 2021;278:114247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Zhang Y, Lu P, Qin H, Zhang Y, Sun X, Song X, Liu J, Peng H, Liu Y, Nwafor EO, Li J, Liu Z. Traditional Chinese medicine combined with pulmonary drug delivery system and idiopathic pulmonary fibrosis: Rationale and therapeutic potential. Biomed Pharmacother. 2021;133:111072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 35. | Mo ZT, Liao YL, Zheng J, Li WN. Icariin protects neurons from endoplasmic reticulum stress-induced apoptosis after OGD/R injury via suppressing IRE1α-XBP1 signaling pathway. Life Sci. 2020;255:117847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Li F, Zhang Y, Lu X, Shi J, Gong Q. Icariin improves the cognitive function of APP/PS1 mice via suppressing endoplasmic reticulum stress. Life Sci. 2019;234:116739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Su B, Cheng D, Chen G, Zhang S, Wang L, Wu X, Tang S. Icariin Attenuation of Diabetic Kidney Disease Through Inhibition of Endoplasmic Reticulum Stress via G Protein-Coupled Estrogen Receptors. J Biomed Nanotechnol. 2022;18:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Ni L, Yuan C. The Mitochondrial-Associated Endoplasmic Reticulum Membrane and Its Role in Diabetic Nephropathy. Oxid Med Cell Longev. 2021;2021:8054817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Cybulsky AV. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol. 2017;13:681-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 404] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 40. | Wu C, Yang G, Pan Y, Wang L, Tu P, Zheng S, Guo Y, Ma Y. Icariin promotes the repair of PC12 cells by inhibiting endoplasmic reticulum stress. BMC Complement Med Ther. 2021;21:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Gupta V, Agarwal VK, Mathur S, Tripathi VN, Agarwal A, Bhalla M, Khadwal A. Modified Levinson's test in rapid diagnosis of tuberculous meningitis. Indian Pediatr. 1994;31:301-304. [PubMed] |
| 42. | Chen S, Zhang Y, Ding X, Li W. Identification of lncRNA/circRNA-miRNA-mRNA ceRNA Network as Biomarkers for Hepatocellular Carcinoma. Front Genet. 2022;13:838869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 43. | Jeong SM, Hwang S, Seong RH. SIRT4 regulates cancer cell survival and growth after stress. Biochem Biophys Res Commun. 2016;470:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |