Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1226
Peer-review started: May 31, 2023
First decision: June 13, 2023
Revised: June 24, 2023
Accepted: July 7, 2023
Article in press: July 7, 2023
Published online: August 15, 2023
Processing time: 72 Days and 2.5 Hours
Diabetic peripheral neuropathy (DPN) is a common chronic complication of diabetes mellitus. One of the most common types is distal symmetric poly-neuropathy, which begins as bilateral symmetry pain and hyperesthesia and gradually progresses into hypoesthesia with nerve fibre disorder and is frequently accompanied by depression and anxiety. Notably, more than half of patients with DPN can be asymptomatic, which tends to delay early detection. Furthermore, the study of adverse outcomes showed that DPN is a prominent risk factor for foot ulceration, gangrene and nontraumatic amputation, which decreases quality of life. Thus, it is essential to develop convenient diagnostic biomarkers with high sensitivity for screening and early intervention. It has been reported that there may be common pathways for microvascular and macrovascular complications of diabetes. The pathogenesis of both disorders involves vascular endothelial dys-function. Emerging evidence indicates that traditional and novel cardiovascular-related biomarkers have the potential to characterize patients by subclinical disease status and improve risk prediction. Additionally, beyond traditional cardiovascular-related biomarkers, novel cardiovascular-related biomarkers have been linked to diabetes and its complications. In this review, we evaluate the association between major traditional and nontraditional car-diovascular-related biomarkers of DPN, such as cardiac troponin T, B-type natriuretic peptide, C-reactive protein, myeloperoxidase, and homocysteine, and assess the evidence for early risk factor-based management strategies to reduce the incidence and slow the progression of DPN.
Core Tip: Emerging evidence indicates that traditional and novel cardiovascular-related biomarkers have the potential to characterize patients by subclinical disease status and improve risk prediction. Additionally, beyond traditional cardiovascular-related biomarkers, novel cardiovascular-related biomarkers have been linked to diabetes and its complications. In this paper, we review the association between major traditional and nontraditional cardiovascular-related biomarkers and diabetic peripheral neuropathy (DPN) and assess the evidence for early risk factor-based management strategies to reduce the incidence and slow the progression of DPN.
- Citation: Cheng MK, Guo YY, Kang XN, Zhang L, Wang D, Ren HH, Yuan G. Advances in cardiovascular-related biomarkers to predict diabetic peripheral neuropathy. World J Diabetes 2023; 14(8): 1226-1233
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1226.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1226
Diabetes is one of the most severe and prevalent chronic diseases worldwide. The International Diabetes Federation estimates that approximately 536.6 million adults worldwide will have diabetes in 2021, and the number is predicted to increase to 783.2 million by 2045[1]. Diabetic neuropathy is a common chronic complication of diabetes mellitus. More than 50% of patients with diabetes will develop diabetic neuropathy during their disease course, 30%-40% of whom experience neuropathic pain symptoms, and the major neuropathy is diabetic peripheral neuropathy (DPN)[2]. A survey of patients with type 2 diabetes in 14 countries showed that the overall prevalence of DPN was 26.71%, indicating that more than a quarter of patients with type 2 diabetes in these 14 countries had DPN as a complication[3]. DPN can pro-duce many symptoms, predominantly sensory impairment, which begins as bilateral symmetry pain and hyperesthesia and gradually progresses into hypoesthesia with nerve fibre disorder and is frequently accompanied by depression and anxiety. These symptoms typically start in the toes and feet and spread to the upper limbs. Studies of adverse outcomes show that DPN is the prominent risk factor for foot ulceration, gangrene and nontraumatic amputation, which diminishes quality of life[4]. Importantly, more than half of patients with DPN are asymptomatic[5]. Therefore, the early symptoms of the insidious disease are not easily detectable. Fortunately, early screening and intervention can effectively reduce the probability of amputation[6]. On the other hand, DPN imposes a physical and mental burden on patients and increases societal expenditure. According to statistics, the annual cost of treating DPN and its complications in the United States is estimated to be between 4.6 and 13.7 billion United States dollars, and DPN treatment accounts for 27% of total diabetes treatment expenditures each year[7]. DPN can suppress the immune function of the body[8], and this damage can also affect the neurological development of the next generation during pregnancy and induce congenital autism[9]. Therefore, it is essential to develop convenient diagnostic methods with high sensitivity.
Currently, the clinical diagnosis of DPN is based on the existence of signs and indications of peripheral nerve damage after excluding other causes of neuropathy[10]. Medical history, symptoms and physical examination can help doctors diagnose patients. When patients have atypical symptoms, electrophysiological examination can be used to assist in the diagnosis. Nerve conduction studies are the gold standard for the diagnosis of diabetic neuropathy. By measuring tactile and vibration sensations and proprioception, abnormally myelinated nerve fibres can be evaluated. Typically, this method is not suitable for small-fibre neuropathy[11]. A diagnosis of small-fibre neuropathy is made by measuring the intraepidermal nerve fibre density, but this test is invasive and is usually used for research purposes[12]. The other method used to diagnose small-fibre neuropathy is corneal confocal microscopy (CCM). CCM is used to diagnose DPN by observing corneal nerve fibres. Research has found that CCM data are related to a change in the severity of neuropathic pain and quality of life[13]. However, this method will also diagnose patients with other retinopathies. Moreover, this detection method requires professional personnel and equipment.
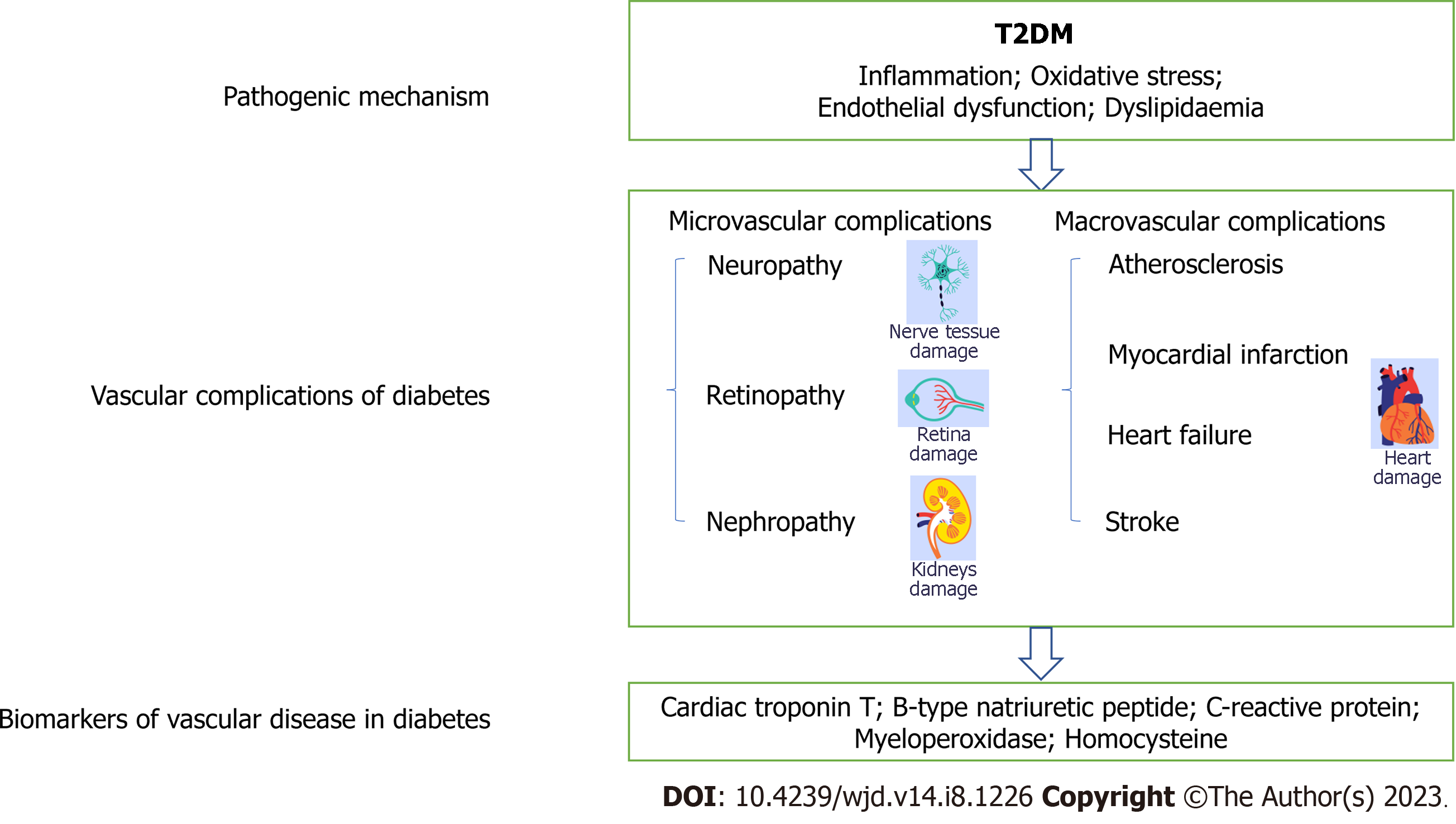
As an emerging diagnostic method, studies on biological markers of DPN have developed rapidly. Serum biomarkers can not only objectively reflect the pathological changes and pathogenesis of tissue cells but are also easy to detect, and have the capability of early prediction and high reproducibility[14]. Therefore, identifying serological markers for the early diagnosis of DPN is of great clinical significance. Although both the precise aetiology and pathogenesis of DPN are complex and not fully understood, DPN is mainly known to be associated with chronic hyperglycaemia, metabolic disorders (oxidative stress, lipid metabolism, increased end-products of advanced glycation, enhanced polyol and hexosamine pathways), the inflammatory response, and axonal degeneration[15]. The microvascular and macrovascular complications of diabetes may share common pathways. The increase in late glycosylation end products is a pathogenic mechanism of diabetes, which can damage endothelial cells and lead to endothelial dysfunction. Excessive production of reactive oxygen species (ROS) and NO can exacerbate oxidative stress and endothelial injury. Insulin resistance is a common phenomenon in diabetic patients that not only reduces the body’s sensitivity to insulin but also inhibits the anti-inflammatory and antioxidant effects of insulin. All of these factors can induce microangiopathy and cardiovascular disease[16]. Studies have demonstrated that patients with microvascular disease (MVD) have a higher risk of developing cardiovascular-related disease than patients without MVD[17], such as those with diabetic polyneuropathy[18]. This suggests that the development of macrovascular and microvascular lesions in diabetes are closely related. In recent years, domestic and foreign scholars have explored whether cardiovascular-related biomarkers can predict diabetic microangiopathy.
Finding the delicate and precise biomarkers has been a top priority in order to reduce the negative effects and financial burden of DPN. Other excellent reviews have summarized markers of DPN. There are numerous biomarkers that can be utilized to diagnose DPN, such as inflammatory markers[19], nerve tissue damage factors[20], and oxidative stress markers[21]. Some researchers have used machine learning techniques combined with novel biomarkers to diagnose DPN, which can effectively improve the efficiency of physicians[22]. However, there is still no single marker that can be widely used for clinical diagnosis. Therefore, this is the first summary of cardiovascular-related markers that can be used to diagnose DPN (Table 1), such as cardiac troponin C and B-type natriuretic peptide (BNP)[23], and the underlying pathological mechanisms are briefly described in the review (Figure 1). We hope to provide a new direction for the clinical diagnosis of DPN to protect against this common and cruel disease.
| Biomarker candidate | Sample source | Quantitative method | Role in human body | Change | Ref. |
| hs-cTnT | Human serum/plasma | ECLI | A marker of myocardial injury | Increased | [27,28] |
| BNP/NT-proBNP | Human serum/plasma | CLIA | Exclude the diagnosis of left ventricular heart failure | Increased | [29-32,35] |
| hs-CRP | Human serum | ELISA/LEITA | A nonspecific inflammatory marker that can predict the early myocardial damage | Increased | [39-42] |
| MPO | Human serum | ELISA | An inflammatory factor in coronary artery disease | Increased | [47] |
| Hcy | Human plasma | FPIA/CLIA | Induction of vascular oxidative stress and endothelial cell damage | Increased | [51-53] |
Troponin is a contractile protein present in the fine myofilaments of cardiac cells. It is composed of three subunits: cardiac troponin C, cardiac troponin I, and cardiac troponin T (cTnT). cTnT is found mainly in fine myofilaments. Troponin is a marker of myocardial injury. In general, patients with myocardial injury may have elevated troponin levels in their bodies[24]. It has been shown that increased troponin I levels can occur in obese mice with myocardial injury[25]. DPN is a microangiopathy, and a decrease in neural blood supply can result in the deterioration of axons and Schwann cells in DPN. Simultaneously, microvascular circulation disorders may partly affect the blood supply to the myocardium, leading to myocardial damage, and changes in coronary microcirculation can lead to coronary microvascular disfunction, affecting the levels of troponin in the body[26]. Jende et al[27] indirectly confirmed that microangiopathy contributes to nerve damage in type 2 diabetes and demonstrated the potential value of troponin as a marker of nerve damage in diabetic patients. A recent cross-sectional study showed that high-sensitivity cardiac troponin (hs-cTnT) is independently associated with peripheral neuropathy, regardless of diabetes mellitus diagnosis, and is a biomarker of end-organ injury, including peripheral neuropathy[28]. Therefore, when using hs-cTnT to screen DPN patients, other diseases that may cause peripheral neuropathy should be ruled out in advance. In conclusion, these studies showed that troponin could be a useful indicator for predicting the incidence of DPN, which needs to be confirmed in large-sample studies.
BNP is a natural hormone with biological activity synthesized by myocardial cells that is mainly secreted by the ventricle but also exists in brain tissue. BNP alters sodium excretion and vasodilation and inhibits sympathetic nerve activity. When left ventricular diastolic function is impaired, the myocardium rapidly synthesizes BNP and releases it into the blood to help regulate heart function. It is an essential indicator in the diagnosis of heart failure, patient management, and risk assessment of clinical events. Low BNP values can exclude the diagnosis of left ventricular heart failure. Multiple studies have shown that changes in BNP levels are associated with diabetic microvascular complications[29-31]. A cross-sectional study of patients with type 2 diabetes in China showed that circulating BNP levels were significantly increased in patients with neuropathy. Researchers found that BNP levels were positively correlated with vibration perception threshold values, suggesting that high BNP levels are a risk factor for DPN and that monitoring BNP levels can predict the risk of DPN. The best cut-off value for predicting DPN was a circulating BNP level of 15.18 pg/mL (sensitivity 78.7%, specificity 48.2%)[32].
N-terminal BNP (NT-proBNP) is the inactive N-terminal fragment of the BNP prohormone (proBNP) and is secreted mainly when the load of ventricular cells increases before and after division. BNP and NT-proBNP are essential markers in diagnosing acute and chronic heart failure. Studies have shown that the combination of NT-proBNP and its receptor can regulate blood pressure, blood volume, sodium balance, and glucose and fat metabolism. Natriuretic peptides bind to receptors located in adipose tissue to induce lipolysis in adipocytes, regulate fat distribution, and promote the absorption of more oxygen and glucose by adipose tissue[33,34]. Therefore, NT-proBNP can affect insulin and glucose metabolism in the body. A German study showed that the level of NT-proBNP is negatively correlated with the risk of type 2 diabetes. The correlation is more significant in female patients, and the higher the concentration of NT-proBNP is, the higher the risk of diabetes patients having large vessel and MVD. Therefore, NT-proBNP can be used as a biomarker to predict the risk of microvascular and macrovascular complications of diabetes[35].
C-reactive protein (CRP) is a nonspecific inflammatory marker produced by liver cells when the body is exposed to inflammatory stimulation, such as microbial infection or tissue damage. It can be used to identify bacterial and viral infections and assess the severity of infection. In addition, CRP is a marker of early myocardial injury that can be elevated within hours of the onset of myocardial damage. High-sensitivity CRP (hs-CRP) predicts the risk of cardiac events in asymptomatic populations and can assess the outcome of patients with acute coronary syndromes[36,37]. Chronic hyperglycaemia can induce vascular endothelial cell injury and an inflammatory response leading to DPN, while the inflammatory cytokines tumor necrosis factor-alpha and interleukin-6 can stimulate CRP synthesis in vivo[38]. Studies by Tang et al[39] showed that hs-CRP levels were significantly positively correlated with the occurrence of diabetic nephropathy and can be used to predict and diagnose diabetic nephropathy in the clinic. Chuengsamarn et al[40] found that hs-CRP levels are correlated with the occurrence of chronic vascular complications of diabetes and can be used to predict the occurrence of diabetic microangiopathy. Another study found a positive correlation between hs-CRP levels and urinary albumin excretion, an indicator of diabetic nephropathy[41]. A prospective study has shown that baseline hs-CRP levels can predict the occurrence and improve the predictive efficacy of microvascular complications in type 2 diabetes[42]. In summary, these studies suggest that hs-CRP levels are associated with diabetic microvascular complications. In addition, hs-CRP levels have only been shown to be related to the development of diabetic nephropathy and diabetic retinopathy. However, the mechanisms of microvascular damage in the three diseases are similar, so it is reasonable to speculate that CRP is associated with the development of DPN. In the future, it is necessary to further explore the relationship between hs-CRP and DPN and determine whether hs-CRP can be used as a biomarker for diagnosing DPN.
Myeloperoxidase (MPO) is a haem protease secreted mainly by activated neutrophils and macrophages. Changes in MPO levels in vivo can reflect the activity and functional status of neutrophils. MPO not only kills microorganisms that are phagocytosed by cells but also participates in regulating inflammatory responses. It is also an inflammatory factor in coronary artery disease, and a study showed that elevated plasma MPO levels are associated with inflammatory status in patients who suffer from acute heart attacks[43]. MPO can promote the formation of lesions in acute coronary syndrome and affect the stability of atherosclerotic plaques[44]. Additionally, it can be used to predict the risk of recent myocardial infarction in patients with coronary heart disease[45]. Moreover, MPO can catalyse hydrogen peroxide to produce ROS. When the balance of oxidants and antioxidants in the body is disrupted, oxidative stress can occur. Inflammation and oxidative stress are involved in the occurrence and development of DPN. Long-term hyperglycaemia can lead to increased nonenzymatic glycosylation end products and produce a large number of oxygen free radicals, thus aggravating oxidative stress and inflammation[46]. A prospective cohort study in Germany showed that a higher level of MPO was independently associated with DPN, suggesting that MPO may be involved in the occurrence of DPN and can be used as a biomarker[47]. However, the mechanism underlying MPO and its role in the development of DPN still needs further study. In addition, the specificity and sensitivity of MPO to diagnose DPN needs to be verified in more extensive cohort studies.
Homocysteine (Hcy) is a sulfur-containing amino acid in the human body and an essential intermediate product in methionine and cysteine metabolism. Hcy content increases when metabolic disorders occur in the body, which makes it an important indicator to measure the health status of patients. Hcy has toxic effects on neurons and vascular endothelial cells. The underlying mechanism may be that Hcy can produce a large amount of oxygen free radicals, thus causing endothelial cell damage. Studies have shown that increased Hcy can induce vascular oxidative stress and mediate arterial inflammation and atherosclerosis[48]. Hcy can also interfere with glutathione synthesis by inhibiting the activity of glutathione peroxidase, disrupting the redox balance in the body and leading to excessive proliferation of vascular endothelial cells. In recent years, people have begun to explore the relationship between increased homocysteinemia and diabetic microangiopathy. The potential mechanism involved increased Hcy-induced oxidative stress and vascular endothelial growth factor (VEGF)[49,50]. Hcy can inhibit VEGF-induced vascular endothelial cell proliferation and migration. A Canadian study showed that diabetic patients with higher Hcy levels were more likely to develop diabetic neuropathy than those with lower Hcy levels. However, the potential influence of diet and lifestyle needs to be further clarified[51]. A cross-sectional study of patients with type 2 diabetes in China showed that plasma Hcy concentration was independently associated with the development of DPN and found that the threshold for distinguishing neuropathies by Hcy was lower than average[52]. González et al[53] found that plasma Hcy content was significantly correlated with the presence and degree of DPN and that the risk of DPN increased by 23% for every 1 μmol increase in plasma Hcy. In conclusion, several studies have shown that Hcy may be a promising biomarker for DPN. However, the elevation of Hcy can also occur in older adults with poor nutrition. Therefore, future studies need to verify the predictive value of Hcy and explore the best diagnostic thresholds in different age groups.
DPN can not only reduce the quality of life of patients but also cause disability and death in severe cases. Therefore, more sensitive diagnostic methods are needed. In recent years, many studies on DPN markers have emerged, and many DPN-related molecules have been found. In this review, biomarkers of cardiovascular-related DPN have been summarized, including cTnT, BNP, CRP, MPO, and Hcy. These results encourage further studies to identify the value of these biomarkers, thus improving the diagnostic efficacy of markers, achieving the goal of early diagnosis and early treatment, and improving patient prognosis. At present, although no single marker can be used for the accurate diagnosis and disease assessment of DPN, abnormalities in known markers in combination with traditional diagnostic methods for DPN can still direct clinicians to pay attention to high-risk groups as early as possible to achieve early diagnosis and early intervention. Computer simulation technology is widely used in medical research[54], not only to improve the efficiency of researchers but also to reduce costs. In recent years, many researchers have conducted preliminary studies using computer simulation techniques before conducting in vivo investigations[55]. Future studies will use machine learning technology to screen biomarkers in combination with artificial intelligence[56]. Currently, the treatment of DPN is limited to symptomatic treatment, and there is a lack of effective prevention and treatment measures. Exploring DPN-related markers can not only be used for the accurate diagnosis of DPN but also provide new ideas for treating DPN. Studying the pathogenesis of DPN and discovering related biomarkers will provide a new direction for diagnosing and treating DPN in the future.
I would like to express my gratitude to all those who helped me during the writing of this review.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ammarullah MI, Indonesia; Gutiérrez-Cuevas J, Mexico; Cai L, United States S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4760] [Article Influence: 1586.7] [Reference Citation Analysis (36)] |
| 2. | Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O'Brien PC, Melton LJ 3rd, Service FJ. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 915] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 3. | Lu Y, Xing P, Cai X, Luo D, Li R, Lloyd C, Sartorius N, Li M. Prevalence and Risk Factors for Diabetic Peripheral Neuropathy in Type 2 Diabetic Patients From 14 Countries: Estimates of the INTERPRET-DD Study. Front Public Health. 2020;8:534372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, Tesfaye S. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 5. | Fujita Y, Murakami T, Nakamura A. Recent Advances in Biomarkers and Regenerative Medicine for Diabetic Neuropathy. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1841] [Cited by in RCA: 1784] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 7. | Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 456] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 8. | Hagen KM, Ousman SS. Aging and the immune response in diabetic peripheral neuropathy. J Neuroimmunol. 2021;355:577574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Afif IY, Manik AR, Munthe K, Maula MI, Ammarullah MI, Jamari J, Winarni TI. Physiological Effect of Deep Pressure in Reducing Anxiety of Children with ASD during Traveling: A Public Transportation Setting. Bioengineering (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | American Diabetes Association Professional Practice Committee. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S185-S194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | Yu Y. Gold Standard for Diagnosis of DPN. Front Endocrinol (Lausanne). 2021;12:719356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 859] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 13. | Kalteniece A, Ferdousi M, Azmi S, Mubita WM, Marshall A, Lauria G, Faber CG, Soran H, Malik RA. Corneal confocal microscopy detects small nerve fibre damage in patients with painful diabetic neuropathy. Sci Rep. 2020;10:3371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Benn M. Peripheral Neuropathy-Time for Better Biomarkers? Clin Chem. 2020;66:638-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17:400-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 253] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 16. | Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1600] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 17. | Kaze AD, Santhanam P, Erqou S, Bertoni AG, Ahima RS, Echouffo-Tcheugui JB. Microvascular disease and cardiovascular outcomes among individuals with type 2 diabetes. Diabetes Res Clin Pract. 2021;176:108859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Bjerg L, Nicolaisen SK, Christensen DH, Nielsen JS, Andersen ST, Jørgensen ME, Jensen TS, Sandbæk A, Andersen H, Beck-Nielsen H, Sørensen HT, Witte DR, Thomsen RW, Charles M. Diabetic Polyneuropathy Early in Type 2 Diabetes Is Associated With Higher Incidence Rate of Cardiovascular Disease: Results From Two Danish Cohort Studies. Diabetes Care. 2021;44:1714-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Zheng H, Sun W, Zhang Q, Zhang Y, Ji L, Liu X, Zhu X, Ye H, Xiong Q, Li Y, Lu B, Zhang S. Proinflammatory cytokines predict the incidence of diabetic peripheral neuropathy over 5 years in Chinese type 2 diabetes patients: A prospective cohort study. EClinicalMedicine. 2021;31:100649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Li YP, Yan ZQ, Han LP, Yin AL, Xu JY, Zhai YR, Hao S, Zhang L, Xie Y. The Association Between Phosphorylated Neurofilament Heavy Chain (pNF-H) and Small Fiber Neuropathy (SFN) in Patients with Impaired Glucose Tolerance. Diabetes Ther. 2020;11:71-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Fei Z, Gao W, Xu X, Sheng H, Qu S, Cui R. Serum superoxide dismutase activity: a sensitive, convenient, and economical indicator associated with the prevalence of chronic type 2 diabetic complications, especially in men. Free Radic Res. 2021;55:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Buccheri E, Dell'Aquila D, Russo M. Artificial intelligence in health data analysis: The Darwinian evolution theory suggests an extremely simple and zero-cost large-scale screening tool for prediabetes and type 2 diabetes. Diabetes Res Clin Pract. 2021;174:108722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Wong YK, Cheung CYY, Tang CS, Hai JSH, Lee CH, Lau KK, Au KW, Cheung BMY, Sham PC, Xu A, Lam KSL, Tse HF. High-sensitivity troponin I and B-type natriuretic peptide biomarkers for prediction of cardiovascular events in patients with coronary artery disease with and without diabetes mellitus. Cardiovasc Diabetol. 2019;18:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Tveit SH, Myhre PL, Omland T. The clinical importance of high-sensitivity cardiac troponin measurements for risk prediction in non-cardiac surgery. Expert Rev Mol Diagn. 2023;23:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Gutiérrez-Cuevas J, Sandoval-Rodríguez A, Monroy-Ramírez HC, Vazquez-Del Mercado M, Santos-García A, Armendáriz-Borunda J. Prolonged-release pirfenidone prevents obesity-induced cardiac steatosis and fibrosis in a mouse NASH model. Cardiovasc Drugs Ther. 2021;35:927-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Nelson MD, Wei J, Bairey Merz CN. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur Heart J. 2018;39:850-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Jende JME, Groener JB, Kender Z, Hahn A, Morgenstern J, Heiland S, Nawroth PP, Bendszus M, Kopf S, Kurz FT. Troponin T Parallels Structural Nerve Damage in Type 2 Diabetes: A Cross-sectional Study Using Magnetic Resonance Neurography. Diabetes. 2020;69:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Hicks CW, Wang D, Daya NR, Windham BG, Ballantyne CM, Matsushita K, Selvin E. Associations of Cardiac, Kidney, and Diabetes Biomarkers With Peripheral Neuropathy among Older Adults in the Atherosclerosis Risk in Communities (ARIC) Study. Clin Chem. 2020;66:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Beer S, Golay S, Bardy D, Feihl F, Gaillard RC, Bachmann C, Waeber B, Ruiz J. Increased plasma levels of N-terminal brain natriuretic peptide (NT-proBNP) in type 2 diabetic patients with vascular complications. Diabetes Metab. 2005;31:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Jurado J, Ybarra J, Ferrandiz M, Comerma L, Pou JM. Amino-terminal brain natriuretic peptide is related to the presence of diabetic polyneuropathy independently of cardiovascular disease. Diabetes Care. 2007;30:e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Hamano K, Nakadaira I, Suzuki J, Gonai M. N-terminal fragment of probrain natriuretic peptide is associated with diabetes microvascular complications in type 2 diabetes. Vasc Health Risk Manag. 2014;10:585-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Yan P, Wan Q, Zhang Z, Xu Y, Miao Y, Chen P, Gao C. Association between Circulating B-Type Natriuretic Peptide and Diabetic Peripheral Neuropathy: A Cross-Sectional Study of a Chinese Type 2 Diabetic Population. J Diabetes Res. 2020;2020:3436549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Gruden G, Landi A, Bruno G. Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research. Diabetes Care. 2014;37:2899-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Birukov A, Eichelmann F, Kuxhaus O, Polemiti E, Fritsche A, Wirth J, Boeing H, Weikert C, Schulze MB. Opposing Associations of NT-proBNP With Risks of Diabetes and Diabetes-Related Complications. Diabetes Care. 2020;43:2930-2937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Li G, Zhang R. Correlation Analysis of Acute Coronary Syndrome with Serum IL-18, MMP-9, hs-CRP, and Plasma FIB. Biomed Res Int. 2022;2022:5984184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Wang C, Wu Y, Su Y, Mao B, Luo Y, Yan Y, Hu K, Lu Y, Che W, Wan M. Elevated levels of sIL-2R, TNF-α and hs-CRP are independent risk factors for post percutaneous coronary intervention coronary slow flow in patients with non-ST segment elevation acute coronary syndrome. Int J Cardiovasc Imaging. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1708] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 39. | Tang M, Cao H, Wei XH, Zhen Q, Liu F, Wang YF, Fan NG, Peng YD. Association Between High-Sensitivity C-Reactive Protein and Diabetic Kidney Disease in Patients With Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 2022;13:885516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Chuengsamarn S, Rattanamongkolgul S, Sittithumcharee G, Jirawatnotai S. Association of serum high-sensitivity C-reactive protein with metabolic control and diabetic chronic vascular complications in patients with type 2 diabetes. Diabetes Metab Syndr. 2017;11:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Choudhary N, Ahlawat RS. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran J Kidney Dis. 2008;2:72-79. [PubMed] |
| 42. | Aryan Z, Ghajar A, Faghihi-Kashani S, Afarideh M, Nakhjavani M, Esteghamati A. Baseline High-Sensitivity C-Reactive Protein Predicts Macrovascular and Microvascular Complications of Type 2 Diabetes: A Population-Based Study. Ann Nutr Metab. 2018;72:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Zhang N, Wang JX, Wu XY, Cui Y, Zou ZH, Liu Y, Gao J. Correlation Analysis of Plasma Myeloperoxidase Level With Global Registry of Acute Coronary Events Score and Prognosis in Patients With Acute Non-ST-Segment Elevation Myocardial Infarction. Front Med (Lausanne). 2022;9:828174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Chen Q, Chen S, Dai Y, Wang X, Ding F, Zhang R, Shen W, Hu W, Lu L, Pan W. Serum MPO levels and activities are associated with angiographic coronary atherosclerotic plaque progression in type 2 diabetic patients. BMC Cardiovasc Disord. 2022;22:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 45. | Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Münzel T, Simoons ML, Hamm CW; CAPTURE Investigators. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 719] [Article Influence: 32.7] [Reference Citation Analysis (1)] |
| 46. | Stirban A, Gawlowski T, Roden M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol Metab. 2014;3:94-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 47. | Herder C, Kannenberg JM, Huth C, Carstensen-Kirberg M, Rathmann W, Koenig W, Strom A, Bönhof GJ, Heier M, Thorand B, Peters A, Roden M, Meisinger C, Ziegler D. Myeloperoxidase, superoxide dismutase-3, cardiometabolic risk factors, and distal sensorimotor polyneuropathy: The KORA F4/FF4 study. Diabetes Metab Res Rev. 2018;34:e3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 2007;9:1941-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Lehotský J, Tothová B, Kovalská M, Dobrota D, Beňová A, Kalenská D, Kaplán P. Role of Homocysteine in the Ischemic Stroke and Development of Ischemic Tolerance. Front Neurosci. 2016;10:538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 50. | Pan L, Yu G, Huang J, Zheng X, Xu Y. Homocysteine inhibits angiogenesis through cytoskeleton remodeling. Biosci Rep. 2017;37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Bruce SG, Young TK. Prevalence and risk factors for neuropathy in a Canadian First Nation community. Diabetes Care. 2008;31:1837-1841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Jianbo L, Yuche C, Ming S, Jingrong T, Qing D, Yu Z, Jiawei C, Hongxing W. Association of homocysteine with peripheral neuropathy in Chinese patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;93:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | González R, Pedro T, Martinez-Hervas S, Civera M, Priego MA, Catalá M, Chaves FJ, Ascaso JF, Carmena R, Real JT. Plasma homocysteine levels are independently associated with the severity of peripheral polyneuropathy in type 2 diabetic subjects. J Peripher Nerv Syst. 2012;17:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Ammarullah MI, Hartono R, Supriyono T, Santoso G, Sugiharto S, Permana MS. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 55. | Jamari J, Ammarullah MI, Santoso G, Sugiharto S, Supriyono T, Permana MS, Winarni TI, van der Heide E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: review over the past 30 years. Heliyon. 2022;8:e12050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 56. | Li W, Guo J, Chen J, Yao H, Mao R, Li C, Zhang G, Chen Z, Xu X, Wang C. Identification of Immune Infiltration and the Potential Biomarkers in Diabetic Peripheral Neuropathy through Bioinformatics and Machine Learning Methods. Biomolecules. 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |