Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1212
Peer-review started: March 28, 2023
First decision: April 26, 2023
Revised: May 19, 2023
Accepted: July 6, 2023
Article in press: July 6, 2023
Published online: August 15, 2023
Processing time: 135 Days and 16.5 Hours
The occurrence of diabetes mellitus (DM) in pancreatitis is being increasingly recognized lately. Diabetes can develop not only with chronic pancreatitis but even after the first episode of acute pancreatitis (AP). The incidence of diabetes after AP varies from 18% to 23% in 3 years and reaches up to 40% over 5 years. The exact pathogenesis of diabetes after AP is poorly understood and various mechanisms proposed include loss of islet cell mass, AP-induced autoimmunity, and alterations in the insulin incretin axis. Risk factors associated with increased risk of diabetes includes male sex, recurrent attacks of pancreatitis, presence of pancreatic exocrine insufficiency and level of pancreatitic necrosis. Diagnosis of post-pancreatitis DM (PPDM) is often excluded. Treatment includes a trial of oral antidiabetic drugs in mild diabetes. Often, insulin is required in uncontrolled diabetes. Given the lack of awareness of this metabolic disorder after AP, this review will evaluate current information on epidemiology, risk factors, diagnosis and management of PPDM and identify the knowledge gaps.
Core Tip: Diabetes mellitus (DM) due to diseases of the exocrine pancreas, diabetes of exocrine pancreas (DEP), is a common but underrecognized clinical entity. Post-pancreatitis DM (PPDM), which develop after pancreatitis, is classified as a subtype of DEP. The PPDM can develop even after acute pancreatitis; it is termed as post-acute pancreatitis DM. It differs in pathogenesis and natural history from type 1 and type 2 DM. There is a loss of pancreatic endocrine tissue, fibrosis and a component of autoimmunity. Both insulin deficiency and resistance play a role in this process. There are a number of knowledge gaps in diagnostic criteria, natural history and treatment options of PPDM.
- Citation: Manrai M, Singh AK, Birda CL, Shah J, Dutta A, Bhadada SK, Kochhar R. Diabetes mellitus as a consequence of acute severe pancreatitis: Unraveling the mystery. World J Diabetes 2023; 14(8): 1212-1225
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1212.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1212
Metabolic abnormalities in, during and after an episode of acute pancreatitis (AP) are frequent. Diabetes mellitus (DM) due to pancreatic diseases is a commonly seen disorder. Earlier, terminologies like ‘pancreatic diabetes’ or ‘pan-creatogenic diabetes’ were used to describe diabetes after pancreatic diseases[1,2]. Subsequently, American Diabetes Association (ADA) gave the term ‘type 3c diabetes’ in 2002 which later was abandoned[3,4]. Recently, ADA come up with a unified nomenclature of diabetes of exocrine pancreas which include 3 subtypes: (1) Post-pancreatitis DM (PPDM); (2) Pancreatic cancer-related diabetes; and (3) Cystic fibrosis-related diabetes[5].
Until recently, it was considered that PPDM is associated with chronic pancreatitis only. In 2014, Das et al[6] reported in a systematic review that PPDM can develop in pancreatitis patients even after a single episode of AP, identified as post-AP DM (PPDM-A). Subsequently a number of high-quality population-based studies confirmed these findings[7,8]. Despite a number of available studies, PPDM-A remains an under-recognized entity for most physicians, gastroenterologists, surgeons, and endocrinologists. This review consists of diagnostic criteria, epidemiology, pathophysiology, natural course of DM related to AP. We also discuss the predictors, screening recommendations and management of the same.
Often, an episode of AP is not limited to a single episode in a number of patients. A systematic review in 2015 looked at this aspect and found that recurrent AP developed in 21% of the patients within 1 year of the initial episode of AP[9]. Also, chronic pancreatitis developed in 36% of patients after recurrent AP. Dysglycemia can develop in any of these subtypes of pancreatitis. It has been suggested that pathophysiological mechanisms of diabetes are different in these extreme forms of pancreatitis. Since there are two main types of pancreatitis i.e., AP and chronic pancreatitis, PPDM is also subdivided into two types: (1) PPDM-A; and (2) post-chronic pancreatitis DM[10].
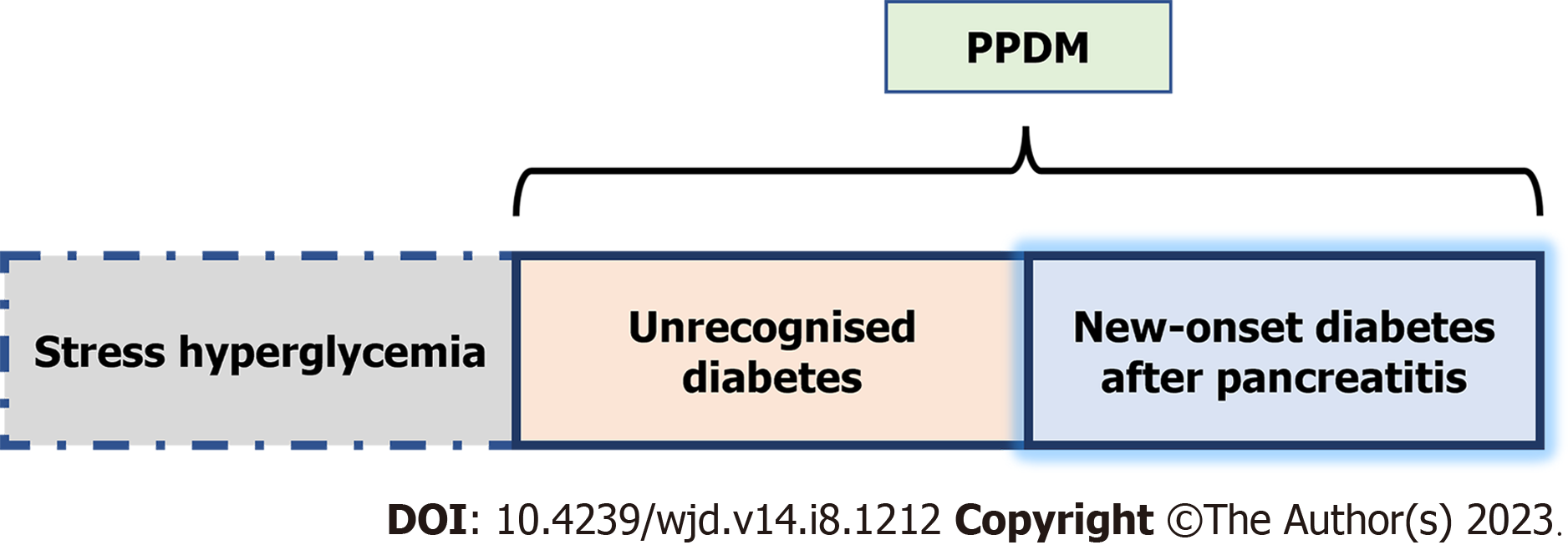
Irrespective of the type of pancreatitis, dysglycemia could be a manifestation of stress hyperglycemia, unrecognized diabetes or new-onset diabetes after pancreatitis[5] (Figure 1). Stress hyperglycemia is defined as an elevated level of blood glucose, without elevated glycated hemoglobin A1c (HbA1c ≥ 6.5%), during the course of pancreatitis and/or within 3 mo after hospital admission in patients without a previous diagnosis of diabetes. This stress hyperglycemia is usually transient, and the elevated levels of blood glucose normalize during the follow up period.
Unrecognized diabetes can be unveiled during an episode of pancreatitis and is defined as elevated glycated HbA1c ≥ 6.5% above the diabetes diagnostic threshold, first detected during the course of pancreatitis and/or within 3 mo after hospital admission[5]. New onset diabetes after pancreatitis (NODAP) acknowledges the metabolic effect of acute or chronic pancreatitis on previously normal glucose homeostasis[5]. The NODAP excludes the diabetic patients diagnosed during the episodes of pancreatitis or up to 3 mo after hospital discharge. PPDM includes patients with diabetes in the setting of pancreatitis irrespective of the timing of diabetes onset (Figure 1). In this review, the DM developing after AP i.e., PPDM-A was focused as a point of discussion.
The diagnosis of PPDM should be suspected in all patients with a history of pancreatitis and fulfilling the diagnostic criteria for diabetes by the ADA. The diagnosis of PPDM is more of a diagnosis of exclusion, after excluding the more common stress hyperglycemia, type 1 and type 2 diabetes. The diagnosis of PPDM-A is made in patients with first or recurrent episodes of pancreatitis without clinical or imaging features of chronic pancreatitis. Petrov and Yadav[10] proposed a stepwise and practical algorithm for diagnosing PPDM[10] (Figure 2).
The global incidence of AP 34 cases/100000 of population based on pooled incidence[10,11]. Of these 6 cases/100000 will develop PPDM-A[10]. The course in AP can be mild, moderately severe, or severe which dictates duration of hospitalization and long-term sequelae. More than 80% of patients have a mild course with a hospitalization required for less than a week, while those with moderate to severe AP experience pancreatic necrosis and a protracted hospital course[12]. Long-term sequelae of AP include exocrine pancreatic insufficiency, complications related to pancreatic necrotic collections, and recurrent attacks of AP in up to 21% patients[13,14].
During hospitalization, hyperglycemia could be seen in up to 51% of patients during AP. Hyperglycemia in the early phase may arise from multiple mechanisms such as uncontrolled pre-existing DM, damage to endocrine pancreas or metabolic stress of critical illness[15]. Hyperglycemia is usually considered as a transient complication of AP. However, two recent meta-analyses have revealed the high incidence of AP related to DM observing that approximately 18%-23% patients of AP will develop DM within three years of discharge[6,16]. However, in longitudinal studies with more than five years of follow-up, the cumulative incidence rate of PPDM-A is up to 40%[16]. Moreover, the precise time of onset of pancreatic endocrine dysfunction cannot be determined. Some studies have demonstrated resolution while others have revealed persistence of endocrine dysfunction[7,17]. To summarize, post pancreatitis endocrine dysfunction is common, however it may be reversible in some cases.
The exact mechanism of PPDM-A is poorly understood. However various mechanisms have been proposed which may lead to diabetes due to loss of islet cell mass, AP induced autoimmunity, alterations in insulin incretin axis and common risk factors. In patients with acute necrotizing pancreatitis, the loss of beta cell of islets of Langerhans leads to insulin deficiency resulting in DM. As a result of pancreatic necrosis, reduction or loss in the production and secretion of insulin as well as other islet hormones is expected. Since, a subset of patients with non-necrotizing AP also develop DM during short-term follow up, the pathophysiology of PPDM seems to be dependent on multiple factors other than immediate loss of islets secondary to necrosis of pancreatitis tissue[16]. It has been observed that the insulin requirements in PPDM-A are akin to type 1 diabetes. Hence, the role of autoimmunity is considered as an important factor in this process. In patients with type 1 diabetes, autoantibodies like glutamic acid decarboxylase (70%-80%), insulin associated antibodies, insulinoma associated autoantigen 2, zinc transporter, and/or tetraspanin 7 cause immune mediated beta cell destruction. There is a possibility that post-pancreatitis there could be immune activation, which though is less clearly defined, which destroys β-cells in pancreas. Some reports have also demonstrated generation of β-cell autoantibody in patients with PPDM-A[18]. However, no available study has evaluated the frequency of autoimmunity following an episode of AP.
The AP and type 2 DM share common risk factors like obesity and hypertriglyceridemia. These factors are independently associated with an increased risk for severe pancreatitis which may partly explain PPDM-A[19,20]. The prevalence of obesity is seen in 42% cases of PPDM-A while in type 2 diabetes obesity is seen in 48% cases supporting the evidence of obesity as a high risk for DM after an episode of AP[21]. Although similar data are not available for hypertriglyceridemia, these factors are likely to contribute towards the development of PPDM-A[22].
Pancreatic exocrine insufficiency may occur in up to 30% of the patients within three years of an episode of AP[23]. In such patients, the incretin-insulin axis is disrupted leading to insufficient incretin hormone production. This leads to reduce secretion of incretin hormones, glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1, and subsequently leads to PPDM-A[24].
In the past, several studies ranging from single center studies to systematic reviews and meta-analyses, had looked at the development of PPDM-A. A recent systematic review by Zhi et al[16] revealed that the incidence of new-onset DM post-AP on follow up was 23% overall. The authors also found that patients with severe pancreatitis had higher incidence of DM as compared to mild AP (39% vs 14%). In addition, they found that alcohol related pancreatitis had higher incidence of PPDM-A as compared to biliary pancreatitis (28% vs 12%). In a previous systematic review by Das et al[6] a similar incidence of PPDM-A was noted. It was also seen that the risk of diabetes increased two-fold at 5 years as compared to 12 mo, on follow up. A few studies have shown higher incidence ranging from 37% to 60.2%[7-10] while others have shown lower incidence, from 10.9% to 22.5%[7,17].
The etiology, severity and degree of pancreatic necrosis are considered important risk factors for development of PPDM-A[16]. In theory, it is believed that the extent of pancreatic necrosis determines the development of PPDM-A, even though some studies have found no association between post-pancreatitis diabetes and severity of pancreatitis[12,13,25-27]. These studies conjecture other mechanisms like autoimmunity and development of insulin resistance leading to PPDM-A. However, most studies have shown a strong association between severity of pancreatitis, extent of pancreatic necrosis and development of diabetes[16,25,27]. Vipperla et al[27] considered the risk of new-onset diabetes as determined by severity of AP. They derived the results from the general population and calculated the risk of new-onset diabetes after mild AP as 7%-15% and 30%-50% after severe or necrotizing pancreatitis over a period of 3 to 5 years[27]. The extent of necrosis and number of functionally active beta cells were considered major determinants for development of diabetes. Table 1 summarizes all the studies examined the endocrine insufficiency after AP episodes[8,17,25-60].
| Ref. | Study design | Number of patients evaluated for endocrine function | Severity of AP | Etiology of pancreatitis | Follow up period | Test to diagnose endocrine function | Pre-diabetic (%) | Diabetes (%) | Insulin treatment (%) | Comment |
| Ohlsén et al[28], 1968, Sweden | Prospective case control | 23 | - | - | Not stated | IV GTT, glucose infusion test | 4 (17) | 0 (0) | 0 (0) | - |
| Johansen and Ornshlot[29], 1972, Denmark | Prospective cohort | 22 | - | Alcohol 4 (18%). Biliary 11 (50%). Others 3 (14%). Idiopathic 4 (18%) | 24 | OGTT | 0 (0) | 4 (18) | - | - |
| Olszewski et al[30], 1978, Poland | Prospective case control | 25 | - | - | 12 | OGTT, BI | - | 7 (28) | - | - |
| Seligson et al[31], 1982, Sweden | Prospective cohort | 9 | All severe | - | 63 | OGTT | 3 (33) | 2 (22) | - | - |
| Angelini et al[32], 1984, Italy | Prospective cohort | 19 | All severe | - | 25 | OGTT | 7 (36) | 2 (10.5) | - | - |
| Eriksson et al[33], 1992, Finland | Prospective cohort | 36 | Mild: 16 (44%). Severe: 20 (56%) | Alcohol: 28 (78%). Biliary: 2 (6%). Post-ERCP: 2 (6%). Idiopathic: 4 (10%) | 74 | OGTT | 4 (11) | 19 (53) | 9 (25) | Diabetes was more in surgical necrosectomy compared to conservative approach (100% vs 26%, P = 0.0004) |
| Doepel et al[34], 1993, Finland | Prospective cohort | 37 | All severe | Alcohol: 28 (76%). Biliary: 3 (8%). Post-ERCP: 2 (5%). Idiopathic: 4 (11%) | 74 | BG, C-peptide, HbA1, OGGT | - | 20 (54) | 9 (45) | Diabetes was more in surgical necrosectomy compared to no necrosectomy (100% vs 29.1%, P < 0.005). Diabetes was more common with alcohol related pancreatitis compared to other etiologies (64% vs 22%, P < 0.05) |
| Angelini et al[35], 1993, Italy | Prospective cohort | 118 | Mild: 35 (30%). Severe: 83 (70%) | 53 | OGTT | - | 9 (8) | - | - | |
| Appelros et al[36], 2001, Sweden | Prospective cohort | 35 | All severe | - | 83 | Questionnaire, BG, HbA1c | 4 (11) | 15 (43) | 9 (26) | No difference in incidence of diabetes based on etiologies |
| Malecka-Panas et al[37], 2002, Poland | Prospective cohort | 82 | Mild: 54 (66%). Severe: 28 (34%) | - | 56 | OGTT, RIA insulin measurements | 2 (2) | 15 (16) | 6 (7) | - |
| Ibars et al[17], 2002, Spain | Prospective cohort | 55 | Mild: 45 (71%). Severe AP 18 (24%) | - | 1, 6 and 12 | OGTT, arginine test | 7 (13) | 6 (11) | - | - |
| Halonen et al[38], 2003, Finland | Prospective cohort | 145 | All severe | Alcohol: 113 (78%). Others: 32 (22%) | 66 | Questionnaire | - | 68 (47) | - | - |
| Boreham and Ammori[39], 2003, United Kingdom | Prospective cohort | 23 | Mild: 16 (70%). Severe: 7 (30%) | - | 3 | FBG | - | 4 (17) | 1 (4) | - |
| Szentkereszty et al[40], 2004, Hungary | Prospective cohort | 22 | All severe | - | 38 | Questionnaire | - | 3 (14) | - | - |
| Sabater et al[41], 2004, Spain | Prospective cohort | 27 | All severe | - | 12 | Cardinal symptoms identification, 2 basal BG, OGTT in patients with lower BG | - | - | 4 (15) | - |
| Hochman et al[42], 2006, Canada | Prospective cohort | 25 | All severe | Alcohol: 4 (16%). Biliary: 12 (48%). HTG: 2 (8%). Idiopathic: 7 (28%) | 24 and 36 | Questionnaire | - | 8 (19) | 5 (20) | - |
| Symersky et al[43], 2006, Netherland | Prospective cohort | 34 | Mild: 22 (65%). Severe: 12 (35%) | - | 55 | OGTT | - | - | 3 (9) | Endocrine insufficiency develops independent of severity of AP |
| Kaya et al[44], 2007, Turkey | Prospective cohort | 112 | Mild: 136 (68%). Severe: 63 (32%) | - | 12 | OGTT | 27 (24) | 13 (21) | - | No association between endocrine insufficiency and necrosis or disease severity |
| Yasuda et al[45], 2008, Japan | Prospective cohort | 41 | All severe | - | 56 | FBG | - | 16 (39) | 4 (9) | No difference in etiology, presence of necrosis or alcohol intake among development of diabetes vs no diabetes |
| Pelli et al[46], 2009, Finland | Prospective cohort | 46 | Mild: 41 (76%). Severe: 13 (24%) | - | 23 (median) | FBG, plasma HbA, OGTT | 12 (20.1) | 5 (10.8) | - | - |
| Gupta et al[47], 2009, India | Prospective cohort | 30 | All severe | Alcohol: 10 (33.3%). Biliary: 12 (40%). Alcohol + biliary 3 (10%). Idiopathic 5 (16.6%) | 31 | FBG, postprandial sugar level, OGTT, fasting serum C-peptide | 6 (20) | 6 (20) | 6 (100) | No effect of etiology of pancreatitis on the incidence of endocrine insufficiency |
| Andersson et al[48], 2010, Sweden | Prospective cohort | 39 | Mild: 26 (65%). Severe: 14 (35%) | - | 45 | FBG, C-peptide, insulin, OGTT | 13 (33) | 9 (23) | - | - |
| Uomo et al[49], 2010, Italy | Prospective cohort | 38 | All severe | Alcohol 0 (0%). Others: 38 (100%) | 179 | FBG, OGTT | - | 6 (16) | - | No relationship between extent of pancreatic necrosis and endocrine insufficiency |
| Wu et al[50], 2011 China | Prospective case control | 59 | Mild: 24 (41%). Severe: 35 (59%) | Gallstone 42 (71%). Hyperlipemia 7 (12%). Alcoholic 7 (12%). Idiopathic 3 (5%) | 42 | FBG, HbA1c, FBI, C-peptide. HOMA | 14 (23.7) | 5 (8) | - | Possible risk factors for endocrine dysfunction were pancreatic surgery, pancreatic necrosis, family history of diabetes, obesity, alcohol abuse, smoking and hyperlipidemia |
| Garip et al[51], 2013, Turkey | Retrospective cohort | 96 | - | - | 32 | OGTT | 5 (5.2) | 33 (43) | - | Severe disease and necrosis was associated with development of new onset diabetes |
| Vujasinovic et al[52], 2014, Slovenia | Retrospective cohort | 100 | Mild: 67 (67%). Moderate: 15 (15%). Severe: 18 (18%) | Alcohol: 42 (42%). Biliary: 36 (36%). Idiopathic: 12 (12%). Others: 10 (10%) | 32 | OGTT, HbA1c | - | 14 (14) | - | Severe disease was associated with development of diabetes |
| Ho et al[53], 2015, Taiwan | Retrospective population-based database study | 12284 | Mild: 11519 (93.8%). Severe: 665 (6.2%) | Biliary: 6556 (53.3%). Alcohol 5728 (46.7%) | - | ICD-9-CM code for diabetes | - | 618 (5) | - | Alcohol associated AP and ≥ 2 admissions for AP were predictors of new onset diabetes mellitus |
| Chandrasekaran et al[54], 2015, India | Prospective cohort study | 35 | All severe | Alcohol: 19. Gallstone: 11. Idiopathic: 5 | 26.6 | OGTT | - | 17 (48.5) | 12 (34.3) | - |
| Winter Gasparoto et al[55], 2015 | Retrospective cohort study | 16 | - | Biliary: 10 (62.5%). Alcohol: 4 (25.0%). HTG: 2 (12.5%) | 34.8 | OGTT, C-peptide, HOMA (homeostasis model assessment) | 7 (43.7) | 5 (31) | - | |
| Yuan et al[56], 2017, China | Retrospective cohort study | 310 | Mild: 261 (84.19). Moderate: 39 (12.58). Severe: 10 (3.23) | Biliary: 153 (49.35). Hyperlipidemia: 32 (10.32). Alcohol: 15 (4.84). Others: 110 (35.48) | - | FBG | 34 (11) | 35 (11.3) | - | Hyperlipidemia and fatty liver were predictors of abnormal FBG. Abnormal FBG was not different between alcohol and biliary pancreatitis |
| Lee et al[8], 2016, Taiwan | Retrospective population-based database study | 3187 | Mild: 2932 (92%). Severe: 255 (8%) | - | - | ICD-9-CM code for diabetes | - | 324 | - | - |
| Umapathy et al[57], 2016, United States | Retrospective cohort study | 73 | - | - | 3 yr (median) | 33 (45) | Risk of endocrine insufficiency was associated with extent of necrosis 2/3rd develop diabetes during index admission | |||
| Vipperla et al[27], 2016, United States | Retrospective cohort study | 101 | - | - | 34.5 | WHO criteria of OGTT | - | 28 (28) | - | Risk of diabetes increased with severity of disease |
| Nikkola et al[58], 2017, Finland | Prospective cohort study | 47 | - | - | 126 | FBG, OGTT | 13 (27.6) | 7 (15) | 7 (15) | Pancreatogenic diabetes develops in recurrent AP only |
| Tu et al[26], 2017, China | Prospective cohort study | 113 | Mild: 10 (8.8%). Moderate: 12 (10.6%). Severe: 91 (80.6%) | Alcohol: 3 (2.7%). Biliary: 65 (57.5%). Hyper TG: 39 (34.5%). Others 6 (5.3%) | 42.9 | FBG, OGTT | 33 (29.2) | 34 (30.1) | - | Extent of pancreatic necrosis > 50%, walled-off necrosis and insulin resistance were independent risk factors for new onset diabetes after AP |
| Tu et al[25], 2018, China | Prospective cohort study | 256 | Mild: 54 (21.1%). Moderate: 42 (16.4%). Severe: 160 (62.5%) | Alcohol: 7 (2.7%). Gallstone: 147 (57.5%). Hyperlipemia: 88 (34.5%). Others: 14 (5.3%) | 42.9 | FBG, OGTT | - | 154 (60.2) | - | Incidence of pancreatic necrosis was higher in diabetics (64.7% and 53.0%, P = 0.06). Necrotic debridement (PCD or surgical necrosectomy) were higher in diabetes (66.3% vs 33.7%, P = 0.02) |
| Phillips et al[59], 2020, United States | Prospective cohort study | 186 | Mild: 120 (64.5%). Moderate: 40 (21.5%). Severe: 26 (14.0%) | Alcohol: 17 (9.1%). Biliary: 84 (45.2). Idiopathic 26 (16.1%). Post-ERCP 23 (12.4%). Other 17 (9.1%). Hyper TG 15 (8.1%) | 12 | Questionnaire | - | 9 (4.8) | - | |
| Man et al[60], 2022, Romania | Prospective cohort study | 329 | Mild: 117 (35.6%). Moderate: 167 (50.8%). Severe: 45 (13.7%) | Alcohol: 87 (26.4%). Biliary: 217 (66.7%) | 1, 3 and 12 mo | FBG, OGTT, HbA1c | - | 29 (8.8) | - | Obesity and pancreatic necrosis > 50% were risk factors for new onset diabetes |
A population-based study from Taiwan confirmed higher risk of PPDM-A in both men and women. The risk was significantly more for males [adjusted hazard ratio (aHR) = 3.21; 95% confidence interval (CI): 2.59-3.98] compared to females (aHR = 1.58, 95%CI: 1.14-2.20)[8]. The COSMOS study also identified higher prevalence of PPDM-A in men (1.32 per 1000 general population) compared to women (0.93 per 1000 general population)[61]. Other population-based studies also demonstrated similar results with higher incidence of PPDM-A among men[62,63].
The risk of PPDM-A is also age dependent, with increased risk of PPDM-A among younger population. A United Kingdom population-based study showed that individuals aged 30-39 [odds ratio (OR): 1.68; 95%CI: 1.20-2.35)] and 20-29 (OR = 4.25, 95%CI: 2.58-7.01) and a history of disease of exocrine pancreas had a higher risk of newly diagnosed diabetes compared with general population[62]. Individual with age group between 40-59 years has similar risk of developing PPDM and type 2 DM while those with age 60-79 years had increased risk of type 2 DM than PPDM. Bendor et al[61] in a population-based study also noted that individuals with age < 40 years and a history of AP had higher risk of developing DM [adjusted OR (aOR) = 4.65, 95%CI: 2.48-8.72) compared to the general population.
Evidence suggests that the probability of diabetes increases with the number of AP episodes. Lee et al[8] in a population-based study analyzed 12284 individuals with initial attack of AP. They found that two or more recurrent attacks of pancreatitis significantly increased the risk of PPDM-A (OR = 1.94, 95%CI: 1.48-2.40). Similarly, COSMOS study found that one recurrence of pancreatitis was not associated with the increased risk of PPDM-A (aHR = 0.93, 95%CI: 0.56-1.52)[61]. However, two (aHR = 1.9, 95%CI: 1.04-3.76) and more recurrences (aHR = 2.77, 95%CI: 1.34-5.72) were associated with significantly increased risk of PPDM-A.
A number of studies suggest that patients with DM have increased prevalence of exocrine pancreatic insufficiency. However, the reverse was not discovered until recently. Cho et al[64] in a national population-based study investigated patients of pancreatitis, both acute and chronic, without a history of both DM and exocrine pancreatic insufficiency. Study revealed that exocrine pancreatic dysfunction was associated with higher risk of PPDM-A (aHR = 2.51, 95%CI: 1.38-4.58). This association was independent of severity and etiology of AP.
A number of other factors are shown to be associated with increased risk of PPDM-A. Deposition of intra-pancreatic fat after an episode of pancreatitis is a risk factor for PPDM, independent of obesity or visceral fat[65]. Yuan et al[56] identified the presence of hyperlipidemia and fatty liver as predictors of follow-up abnormal fasting blood sugar on Kaplan-Meier analysis with 2.52- and 1.87-fold increased risk, respectively, compared to absence of these conditions.
Etiology of pancreatitis as a risk factor for development of diabetes has been evaluated by a number of studies. Doepel et al[34] in a small study identified alcohol as the risk factor for the development of diabetes after severe pancreatitis. Subsequent population-based study by Ho et al[53] also found a similar association with alcohol oriented pancreatitis and risk of PPDM-A. Though a number of studies have not found such association with etiology of pancreatitis and risk of PPDM-A[56], a meta-analysis with meta-regression found no evidence to suggest a differential effect of alcohol or gallstone etiology on the risk of PPDM-A[6].
Severity of AP has long been considered as a risk factor of development of PPDM-A. Das et al[6] in a meta-analysis identified minimal effect of severity of pancreatitis on the development of PPDM-A. Subsequently, large population-based study by Lee et al[8] showed that risk of PPDM-A did not change significantly for mild (aHR = 2.10, 95%CI: 1.92-2.41) and severe disease (aHR = 2.22; 95%CI: 1.50-3.29). These findings were further confirmed in a large population-based study by Shen et al[7]. Though, severity of AP has no effect of PPDM-A, the amount of necrosis and requirement of surgical necrosectomy have been identified as predictors of PPDM-A in a number of studies[25-27].
Incidence of endocrine insufficiency varies from 4.8% to 60.2% after the initial episode of AP. Similarly, exocrine insufficiency develops in 0% to 35%[26,49]. Literature on the development of both exocrine and endocrine insufficiency after episodes of AP is limited. Ho et al[53] in a nationwide cohort study identified that 3% patients develop both exocrine and endocrine insufficiency after an initial episode of AP. The incidence of both exocrine and endocrine insufficiency was less than the individual endocrine (5%) or exocrine insufficiency (45.7%) after the first episode of AP. The study identified that alcohol-associated AP (OR = 1.804; 95%CI: 1.345-2.263; P < 0.001), and ≥ 2 readmissions for AP (OR = 3.190; 95%CI: 2.317-4.063; P < 0.001) were independent predictors for development of both exocrine and endocrine insufficiencies after AP. These risks were similar to the risk factors for development of individual endocrine or exocrine insufficiency[53].
Uomo et al[49] during long term follow up of AP patients managed non-surgically, found that exocrine insufficiency was temporary in patients with endocrine insufficiency. Huang et al[66] also found in the meta-analysis that exocrine insufficiency decreases from 62% to 35% during follow up after AP. These differences in incidences of exocrine insufficiency could be multifactorial and driven by the symptomatic nature, test used for screening, level of pancreatic necrosis during the episode of AP, pancreatic resection during necrosectomy etc.
Das et al[6] in a meta-analysis evaluated the concomitant development of exocrine and endocrine insufficiency after AP. The pooled prevalence of exocrine and endocrine insufficiency after AP was 29% and 43%, respectively. The prevalence of concomitant pancreatic exocrine insufficiency in newly developed prediabetes/DM was 40%. To summarize, the initial exocrine insufficiency after AP being transient recovers in a majority of patients and concomitant endocrine and exocrine insufficiency develops in 3%-17%.
It is well established that AP can lead to DM, however the reverse is less well studied. Epidemiological studies have reported increased incidence of AP in patients with DM. A United States insurance claims database reported 2.83-fold increased risk of AP in diabetic cohort compared to non-diabetic counterpart[67]. In Taiwan, Lee et al[8] reported 1.95-fold higher incidence of AP in diabetics compared to non-diabetics. Same study also reported even higher HR in those who had a history of hyperglycemic episodes so there might be a severity-response relationship[8]. Another study from the United Kingdom reported 1.49-fold higher incidence of AP in patients with type 2 DM[68]. Proposed patho-physiology of increased incidence of AP in DM includes: (1) Chronic hyperglycemia leads to formation of reactive oxygen species, lipid peroxidation and may result in episodes of pancreatitis; (2) Association of comorbid risk factors like obesity, hypertriglyceridemia and gall stone disease which may independently precipitate pancreatitis; (3) Enhanced ryanodine receptor function leading to alteration in calcium metabolism; (4) Certain medications [dipeptidyl peptidase-4 (DPP-4) inhibitors] may enhance AP risk when used for the treatment[8].
Another study has reported structural changes in pancreas in patients with diabetes. Authors found reduced weight and volume of pancreas in patients with type 1 DM, when fibrosis without significant inflammation and ductal changes were observed in autopsy reports. Fecal elastase levels were low in these patients but there were no symptoms of pancreatitis. There were no significant changes in patients with type 2 DM. This study highlighted the complex interplay between exocrine and endocrine pancreas[69]. These studies suggest a bidirectional relationship between DM and AP. More studies are needed for a better understanding of this complex interplay between exocrine and endocrine pancreas.
Currently there are no evidence-based guidelines for screening of diabetes after AP. Though diabetes can develop more frequently after necrotizing pancreatitis requiring necrosectomy, it can also develop even after an episode of mild pancreatitis. So, screening for diabetes should be done in every patient of AP even in the absence of robust risk factors for the development of the same.
One more dilemma is the timing and frequency of screening tests. A proposed approach is of frequent screening for the first year (HbA1c in 6 mo intervals) after hospital discharge. Subsequent, screening should be done on annual basis[70]. The rationale of frequent screening for the first year is based on the observation of new-onset of pre-diabetes or diabetes in 20% of the patient within 6 mo of an episode of AP[70]. However, further population-based studies regarding actual prevalence, time course of NODAP, cost and effectiveness of screening tests are needed for definite recommendation of screening in these patients.
There are no evidence-based guidelines available regarding the treatment of PPDM-A in the absence of clinical trials. Current treatment is typically adapted using a similar paradigm as used in type 2 DM, however, PPDM-A is more difficult to control than type 2 DM. A large United Kingdom based study showed that mean HbA1c level was significantly higher at the time of diagnosis in patients with PPDM-A compared to type 2 DM (8.3% ± 2.4 % vs 7.9% ± 2%; P = 0.002)[62]. The difference of mean HbA1c level remained statistically significant at 1 year (7.1% ± 1.5 % vs 6.8% ± 1.2%, P < 0.001) and at 5 years (7.6 ± 1.7 vs 7.2 ± 1.4, P < 0.001) of follow-up. The proportion of patients with poor glycemic control (defined with HbA1c ≥ 7%) was higher at 1 year (aOR = 1.3) and at 5 years (aOR = 1.7) compared to type 2 DM. Same study also reported that a higher number of patients were on insulin therapy for glycemic control after 5 years of diagnosis in the PPDM-A group (20.1%) compared to type 2 DM (4.1%)[62]. In the absence of defined guidelines and prospective studies, metformin is most commonly used as a first line therapy in PPDM-A as in type 2 DM. Metformin is associated with reduced risk of hypoglycemia which is one of the main concerns in patients with PPDM-A[71].
Metformin is also associated with reduced risk of pancreatic neoplasia with anti-neoplastic properties. Even in patients with established pancreatic carcinoma, metformin is associated with better surgical and overall clinical outcomes[72-75]. As pancreatic carcinoma is also one of the dreaded long-term complications of chronic pancreatitis, these added benefits of metformin make its usually first line therapy in the management of PPDM. However, some gastro-intestinal side effects like nausea, diarrhea and weight loss might become more prominent in some patients with pancreatitis resulting in poor tolerability[71]. Incretins based therapy (glucagon like peptide 1 receptor agonists and DPP-4 inhibitors) have also been tried in PPDM-A, however, incretins can precipitate AP and they are more likely to be associated with gastro-intestinal side effects so preferably avoided in the treatment of PPDM-A[76,77]. Additional post-marketing surveillance studies are needed to confirm the safety of these medications in this setting.
Sulphonylureas are again not good choice because of poor beta cell reserve in PPDM-A. Thiazolidinediones are generally avoided given the risk of fluid overload, and risk of fracture, however this is an insulin sensitizing drug and can actually improve glycemic variability in patients on insulin injection[78]. Sodium-glucose cotransporter-2 inhibitors use in PPDM-A is limited due to the associated muscle/weight loss, which may be undesirable in already malnourished patients. In a nutshell, most oral antidiabetic drugs are recommended in mild PPDM-A with HbA1c < 8%. Despite the use of oral anti-diabetic agents, most patients require insulin at the onset or later, given the progressive nature of the disease. Insulin being an anabolic hormone, is associated with weight gain, a beneficial effect to combat malnutrition. Basal bolus regime, similar to being used in type 1 DM, are frequently used in PPDM-A according to pre-meal glucose levels and carbohydrate intake. Some patients require basal only, basal plus oral antidiabetic drugs or basal plus regimes. In the presence of associated alpha cell injury and blunted glucagon response to hypoglycemia, careful titration of insulin dose is required to prevent hypoglycemic episodes. Due to the complex issues in the management of hyper- and hypoglycemia in patients with PPDM-A, it is also called ‘brittle diabetes’. Frequent blood glucose monitoring is the cornerstone of management.
Additionally, treatment of concurrent pancreatic exocrine dysfunction which can occur in up-to one third of patients as a sequel of AP, might also be associated with better glycemic control as shown in patients with chronic pancreatitis, by stimulating the incretin hormone response[79].
The pathophysiology of PPDM-A is incompletely understood. Currently the diagnosis of PPDM-A is mainly based on the chronological sequence of pancreatitis diagnosed before the onset of diabetes. Comparative studies of more common subtypes of diabetes like type 1 and type 2 diabetes are lacking. Large prospective epidemiological studies focusing on incidence, risk factors and natural history as well as studies focusing on tailored approaches for diagnosing, screening, preventive and treatment strategies are lacking. Studies with continuous glucose monitoring while using oral anti diabetic drugs and/or insulin can give insights into glycemic management in such patients. Randomized trials comparing insulin vs oral antidiabetic drugs in AP are warranted but may not be ethically viable.
To address these knowledge gaps, the National Institute of Diabetes and Digestive and Kidney Diseases of the United States recently formed a collaborative network referred as Type 1 Diabetes after Acute Pancreatitis Consortium. The objective of this consortium is to conduct large prospective observational studies to focus on pathophysiology, incidence, natural history and identification of risk factors. One such trial is Diabetes RElated to Acute Pancreatitis and its Mechanisms, NCT05197920. Animal models should also be developed to accurately replicate AP related diabetes, to better characterize the pathophysiology of the disease and provide a platform to investigate potential therapeutic interventions. Also, interdisciplinary and collaborative work is needed to address the screening, preventive and treatment approaches.
PPDM-A is increasingly being recognized as a long-term sequel of diseases of exocrine pancreas after the episodes of AP. This is a distinct clinical entity as mechanisms and natural history are different from type 1 and type 2 DM. As there is necrosis and fibrosis involving both the exocrine and endocrine pancreas; concomitant exocrine dysfunction is common. There is involvement of all the subtypes of islet cells of Langerhans which explains the brittle nature of diabetes. The pathophysiology is poorly understood, large prospective cohort and animal studies are needed for better understanding. Also, interdisciplinary and collaborative work is needed to address screening, preventive and treatment approaches.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aslam M, India; Gornik I, Croatia; Xiao B, China; Islam MS, South Africa S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Harley V. Experimental Pathological Evidence proving the Existence of Pancreatic Diabetes. J Anat Physiol. 1892;26:204-219. [PubMed] |
| 2. | Deckert T. Late diabetic manifestations in "pancreatogenic" diabetes mellitus. Acta Med Scand. 1960;168:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S33-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 399] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3444] [Article Influence: 313.1] [Reference Citation Analysis (16)] |
| 5. | Petrov MS. Diabetes of the exocrine pancreas: American Diabetes Association-compliant lexicon. Pancreatology. 2017;17:523-526. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of Diabetes Mellitus after First-Attack Acute Pancreatitis: A National Population-Based Study. Am J Gastroenterol. 2015;110:1698-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Lee YK, Huang MY, Hsu CY, Su YC. Bidirectional Relationship Between Diabetes and Acute Pancreatitis: A Population-Based Cohort Study in Taiwan. Medicine (Baltimore). 2016;95:e2448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. 2015;149:1490-1500.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 525] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 11. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 474] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 12. | Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 515] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 13. | Yadav D, O'Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (2)] |
| 14. | Ahmed Ali U, Issa Y, Hagenaars JC, Bakker OJ, van Goor H, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Brink MA, Schaapherder AF, Dejong CH, Spanier BW, Heisterkamp J, van der Harst E, van Eijck CH, Besselink MG, Gooszen HG, van Santvoort HC, Boermeester MA; Dutch Pancreatitis Study Group. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Xiu F, Stanojcic M, Diao L, Jeschke MG. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol. 2014;2014:486403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of New Onset Diabetes Mellitus Secondary to Acute Pancreatitis: A Systematic Review and Meta-Analysis. Front Physiol. 2019;10:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Ibars EP, Sánchez de Rojas EA, Quereda LA, Ramis RF, Sanjuan VM, Peris RT. Pancreatic function after acute biliary pancreatitis: does it change? World J Surg. 2002;26:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kahara T, Takamura T, Otoda T, Ishikura K, Matsushita E. Transient anti-GAD antibody positivity and acute pancreatitis with pancreas tail swelling in a patient with susceptible haplotype for type 1 diabetes mellitus. Intern Med. 2009;48:1897-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology. 2020;20:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 20. | Smeets XJNM, Knoester I, Grooteman KV, Singh VK, Banks PA, Papachristou GI, Duarte-Rojo A, Robles-Diaz G, Kievit W, Besselink MGH, Verdonk RC, Van Santvoort HC, Drenth JPH, Belias M, Van Geenen EJM; Dutch Pancreatitis Study Group. The association between obesity and outcomes in acute pancreatitis: an individual patient data meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, Pandol SJ, Yadav D, Chari ST; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer(CPDPC). Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 22. | Szentesi A, Párniczky A, Vincze Á, Bajor J, Gódi S, Sarlós P, Gede N, Izbéki F, Halász A, Márta K, Dobszai D, Török I, Farkas H, Papp M, Varga M, Hamvas J, Novák J, Mickevicius A, Maldonado ER, Sallinen V, Illés D, Kui B, Erőss B, Czakó L, Takács T, Hegyi P. Multiple Hits in Acute Pancreatitis: Components of Metabolic Syndrome Synergize Each Other's Deteriorating Effects. Front Physiol. 2019;10:1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Hollemans RA, Hallensleben NDL, Mager DJ, Kelder JC, Besselink MG, Bruno MJ, Verdonk RC, van Santvoort HC; Dutch Pancreatitis Study Group. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology. 2018;18:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010;1:8-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 493] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 25. | Tu J, Yang Y, Zhang J, Yang Q, Lu G, Li B, Tong Z, Ke L, Li W, Li J. Effect of the disease severity on the risk of developing new-onset diabetes after acute pancreatitis. Medicine (Baltimore). 2018;97:e10713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Tu J, Zhang J, Ke L, Yang Y, Yang Q, Lu G, Li B, Tong Z, Li W, Li J. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol. 2017;17:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Vipperla K, Papachristou GI, Slivka A, Whitcomb DC, Yadav D. Risk of New-Onset Diabetes Is Determined by Severity of Acute Pancreatitis. Pancreas. 2016;45:e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Ohlsén P. Endocrine and exocrine pancreatic function in pancreatitis. Acta Med Scand Suppl. 1968;484:1-99. [PubMed] |
| 29. | Johansen K, Ornsholt J. Frequency of diabetes after acute pancreatitis. Metabolism. 1972;21:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Olszewski S, Kinalska I, Długosz J, Stasiewicz J, Gabryelewicz A. The glucose tolerance, insulin response and pancreatic exocrine function in patients after acute pancreatitis. Endokrinologie. 1978;71:183-191. [PubMed] |
| 31. | Seligson U, Ihre T, Lundh G. Prognosis in acute haemorrhagic, necrotizing pancreatitis. Acta Chir Scand. 1982;148:423-429. [PubMed] |
| 32. | Angelini G, Pederzoli P, Caliari S, Fratton S, Brocco G, Marzoli G, Bovo P, Cavallini G, Scuro LA. Long-term outcome of acute necrohemorrhagic pancreatitis. A 4-year follow-up. Digestion. 1984;30:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Eriksson J, Doepel M, Widén E, Halme L, Ekstrand A, Groop L, Höckerstedt K. Pancreatic surgery, not pancreatitis, is the primary cause of diabetes after acute fulminant pancreatitis. Gut. 1992;33:843-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Doepel M, Eriksson J, Halme L, Kumpulainen T, Höckerstedt K. Good long-term results in patients surviving severe acute pancreatitis. Br J Surg. 1993;80:1583-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Angelini G, Cavallini G, Pederzoli P, Bovo P, Bassi C, Di Francesco V, Frulloni L, Sgarbi D, Talamini G, Castagnini A. Long-term outcome of acute pancreatitis: a prospective study with 118 patients. Digestion. 1993;54:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Appelros S, Lindgren S, Borgström A. Short and long term outcome of severe acute pancreatitis. Eur J Surg. 2001;167:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Malecka-Panas E, Gasiorowska A, Kropiwnicka A, Zlobinska A, Drzewoski J. Endocrine pancreatic function in patients after acute pancreatitis. Hepatogastroenterology. 2002;49:1707-1712. [PubMed] |
| 38. | Halonen KI, Pettilä V, Leppäniemi AK, Kemppainen EA, Puolakkainen PA, Haapiainen RK. Long-term health-related quality of life in survivors of severe acute pancreatitis. Intensive Care Med. 2003;29:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Szentkereszty Z, Agnes C, Kotán R, Gulácsi S, Kerekes L, Nagy Z, Czako D, Sápy P. Quality of life following acute necrotizing pancreatitis. Hepatogastroenterology. 2004;51:1172-1174. [PubMed] |
| 41. | Sabater L, Pareja E, Aparisi L, Calvete J, Camps B, Sastre J, Artigues E, Oviedo M, Trullenque R, Lledó S. Pancreatic function after severe acute biliary pancreatitis: the role of necrosectomy. Pancreas. 2004;28:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Hochman D, Louie B, Bailey R. Determination of patient quality of life following severe acute pancreatitis. Can J Surg. 2006;49:101-106. [PubMed] |
| 43. | Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP. 2006;7:447-453. [PubMed] |
| 44. | Kaya E, Dervisoglu A, Polat C. Evaluation of diagnostic findings and scoring systems in outcome prediction in acute pancreatitis. World J Gastroenterol. 2007;13:3090-3094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Kuroda Y. Long-term outcome of severe acute pancreatitis. J Hepatobiliary Pancreat Surg. 2008;15:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Pelli H, Lappalainen-Lehto R, Piironen A, Järvinen S, Sand J, Nordback I. Pancreatic damage after the first episode of acute alcoholic pancreatitis and its association with the later recurrence rate. Pancreatology. 2009;9:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Gupta R, Wig JD, Bhasin DK, Singh P, Suri S, Kang M, Rana SS, Rana S. Severe acute pancreatitis: the life after. J Gastrointest Surg. 2009;13:1328-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Andersson B, Pendse ML, Andersson R. Pancreatic function, quality of life and costs at long-term follow-up after acute pancreatitis. World J Gastroenterol. 2010;16:4944-4951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Uomo G, Gallucci F, Madrid E, Miraglia S, Manes G, Rabitti PG. Pancreatic functional impairment following acute necrotizing pancreatitis: long-term outcome of a non-surgically treated series. Dig Liver Dis. 2010;42:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Wu D, Xu Y, Zeng Y, Wang X. Endocrine pancreatic function changes after acute pancreatitis. Pancreas. 2011;40:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Garip G, Sarandöl E, Kaya E. Effects of disease severity and necrosis on pancreatic dysfunction after acute pancreatitis. World J Gastroenterol. 2013;19:8065-8070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Vujasinovic M, Tepes B, Makuc J, Rudolf S, Zaletel J, Vidmar T, Seruga M, Birsa B. Pancreatic exocrine insufficiency, diabetes mellitus and serum nutritional markers after acute pancreatitis. World J Gastroenterol. 2014;20:18432-18438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 53. | Ho TW, Wu JM, Kuo TC, Yang CY, Lai HS, Hsieh SH, Lai F, Tien YW. Change of Both Endocrine and Exocrine Insufficiencies After Acute Pancreatitis in Non-Diabetic Patients: A Nationwide Population-Based Study. Medicine (Baltimore). 2015;94:e1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Chandrasekaran P, Gupta R, Shenvi S, Kang M, Rana SS, Singh R, Bhasin DK. Prospective comparison of long term outcomes in patients with severe acute pancreatitis managed by operative and non operative measures. Pancreatology. 2015;15:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Winter Gasparoto RC, Racy Mde C, De Campos T. Long-term outcomes after acute necrotizing pancreatitis: what happens to the pancreas and to the patient? JOP. 2015;16:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 56. | Yuan L, Tang M, Huang L, Gao Y, Li X. Risk Factors of Hyperglycemia in Patients After a First Episode of Acute Pancreatitis: A Retrospective Cohort. Pancreas. 2017;46:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Umapathy C, Raina A, Saligram S, Tang G, Papachristou GI, Rabinovitz M, Chennat J, Zeh H, Zureikat AH, Hogg ME, Lee KK, Saul MI, Whitcomb DC, Slivka A, Yadav D. Natural History After Acute Necrotizing Pancreatitis: a Large US Tertiary Care Experience. J Gastrointest Surg. 2016;20:1844-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Nikkola J, Laukkarinen J, Lahtela J, Seppänen H, Järvinen S, Nordback I, Sand J. The Long-term Prospective Follow-up of Pancreatic Function After the First Episode of Acute Alcoholic Pancreatitis: Recurrence Predisposes One to Pancreatic Dysfunction and Pancreatogenic Diabetes. J Clin Gastroenterol. 2017;51:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Phillips AE, Ooka K, Pothoulakis I, Paragomi P, Komara N, Lahooti A, Harb D, Mays M, Koutroumpakis F, Stello K, Greer PJ, Whitcomb DC, Papachristou GI. Assessment of Weight Loss and Gastrointestinal Symptoms Suggestive of Exocrine Pancreatic Dysfunction After Acute Pancreatitis. Clin Transl Gastroenterol. 2020;11:e00283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Man T, Seicean R, Lucaciu L, Istrate A, Seicean A. Risk factors for new-onset diabetes mellitus following acute pancreatitis: a prospective study. Eur Rev Med Pharmacol Sci. 2022;26:5745-5754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 61. | Bendor CD, Bardugo A, Zucker I, Cukierman-Yaffe T, Lutski M, Derazne E, Shohat T, Mosenzon O, Tzur D, Sapir A, Pinhas-Hamiel O, Kibbey RG, Raz I, Afek A, Gerstein HC, Tirosh A, Twig G. Childhood Pancreatitis and Risk for Incident Diabetes in Adulthood. Diabetes Care. 2020;43:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Woodmansey C, McGovern AP, McCullough KA, Whyte MB, Munro NM, Correa AC, Gatenby PAC, Jones SA, de Lusignan S. Incidence, Demographics, and Clinical Characteristics of Diabetes of the Exocrine Pancreas (Type 3c): A Retrospective Cohort Study. Diabetes Care. 2017;40:1486-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 63. | Cho J, Scragg R, Petrov MS. The influence of cholecystectomy and recurrent biliary events on the risk of post-pancreatitis diabetes mellitus: a nationwide cohort study in patients with first attack of acute pancreatitis. HPB (Oxford). 2021;23:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Cho J, Scragg R, Pandol SJ, Petrov MS. Exocrine Pancreatic Dysfunction Increases the Risk of New-Onset Diabetes Mellitus: Results of a Nationwide Cohort Study. Clin Transl Sci. 2021;14:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Singh RG, Cervantes A, Kim JU, Nguyen NN, DeSouza SV, Dokpuang D, Lu J, Petrov MS. Intrapancreatic fat deposition and visceral fat volume are associated with the presence of diabetes after acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2019;316:G806-G815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 66. | Huang W, de la Iglesia-García D, Baston-Rey I, Calviño-Suarez C, Lariño-Noia J, Iglesias-Garcia J, Shi N, Zhang X, Cai W, Deng L, Moore D, Singh VK, Xia Q, Windsor JA, Domínguez-Muñoz JE, Sutton R. Exocrine Pancreatic Insufficiency Following Acute Pancreatitis: Systematic Review and Meta-Analysis. Dig Dis Sci. 2019;64:1985-2005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 67. | Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 329] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 68. | Girman CJ, Kou TD, Cai B, Alexander CM, O'Neill EA, Williams-Herman DE, Katz L. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab. 2010;12:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 69. | Mohapatra S, Majumder S, Smyrk TC, Zhang L, Matveyenko A, Kudva YC, Chari ST. Diabetes Mellitus Is Associated With an Exocrine Pancreatopathy: Conclusions From a Review of Literature. Pancreas. 2016;45:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 70. | Bharmal SH, Cho J, Alarcon Ramos GC, Ko J, Stuart CE, Modesto AE, Singh RG, Petrov MS. Trajectories of glycaemia following acute pancreatitis: a prospective longitudinal cohort study with 24 months follow-up. J Gastroenterol. 2020;55:775-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 71. | Nasri H, Rafieian-Kopaei M. Metformin: Current knowledge. J Res Med Sci. 2014;19:658-664. [PubMed] |
| 72. | Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 73. | Lee SH, Yoon SH, Lee HS, Chung MJ, Park JY, Park SW, Song SY, Chung JB, Bang S. Can metformin change the prognosis of pancreatic cancer? Retrospective study for pancreatic cancer patients with pre-existing diabetes mellitus type 2. Dig Liver Dis. 2016;48:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Cerullo M, Gani F, Chen SY, Canner J, Pawlik TM. Metformin Use Is Associated with Improved Survival in Patients Undergoing Resection for Pancreatic Cancer. J Gastrointest Surg. 2016;20:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Yang J, Yang H, Cao L, Yin Y, Shen Y, Zhu W. Prognostic value of metformin in cancers: An updated meta-analysis based on 80 cohort studies. Medicine (Baltimore). 2022;101:e31799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 76. | Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98:271-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 77. | Soranna D, Bosetti C, Casula M, Tragni E, Catapano AL, Vecchia CL, Merlino L, Corrao G. Incretin-based drugs and risk of acute pancreatitis: A nested-case control study within a healthcare database. Diabetes Res Clin Pract. 2015;108:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Kung J, Henry RR. Thiazolidinedione safety. Expert Opin Drug Saf. 2012;11:565-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 79. | Beglinger S, Drewe J, Schirra J, Göke B, D'Amato M, Beglinger C. Role of fat hydrolysis in regulating glucagon-like Peptide-1 secretion. J Clin Endocrinol Metab. 2010;95:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |