Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1178
Peer-review started: May 28, 2023
First decision: June 13, 2023
Revised: June 24, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 15, 2023
Processing time: 74 Days and 15.8 Hours
During the global coronavirus disease 2019 (COVID-19) pandemic, people worldwide have experienced an unprecedented rise in psychological distress and anxiety. In addition to this challenging situation, the prevalence of diabetes mellitus (DM), a hidden epidemic, has been steadily increasing in recent years. Lower-middle-income countries have faced significant barriers in providing accessible prenatal care and promoting a healthy diet for pregnant women, and the pandemic has made these challenges even more difficult to overcome. Preg
Core Tip: The coronavirus disease 2019 pandemic has caused a rise in psychological distress on a global scale, overlapping with an increase in cases of diabetes mellitus. Women, in particular those residing in lower-middle-income countries, stumble upon difficulties for a decent prenatal care and maintaining nutritious diets. Pregnant women who have a higher susceptibility to gestational diabetes may face long-term health consequences for both them and their unborn child. Recent studies suggest a potential link between the pandemic and elevated rates of gestational diabetes. Additional research is necessary to establish a conclusive correlation between the impact of pandemic-induced stress, gestational gain weight, and the outcomes of pregnancies affected by gestational diabetes.
- Citation: Mendez Y, Alpuing Radilla LA, Delgadillo Chabolla LE, Castillo Cruz A, Luna J, Surani S. Gestational diabetes mellitus and COVID-19: The epidemic during the pandemic. World J Diabetes 2023; 14(8): 1178-1193
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1178.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1178
When the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) as a pandemic on March 11, 2020, the global count surpassed 118000 confirmed cases across 114 countries, resulting in 4291 reported deaths[1]. This rapid escalation of cases caught many by surprise, as the virus displayed high transmissibility and lacked effective control measures[2]. As a result, societal norms underwent a paradigm shift, compelling individuals to isolate themselves indoors to mitigate the viral spread and prevent transmission to their loved ones. Adapting to this new reality presented significant challenges, particularly for those balancing remote work with caregiving responsibilities for their families, including children, elderly relatives, and individuals with special needs. Unfortunately, the mounting responsibilities and pressures had a detrimental impact on mental well-being. During the initial year of the COVID-19 pandemic, there was a substantial 25% increase in global anxiety and depression rates[3].
During the pandemic, the focus on the overwhelming stress and challenges has inadvertently overshadowed the ongoing threat of diabetes mellitus (DM). In 2019, diabetes contributed to a staggering 1.5 million deaths globally, with nearly half of those occurring before the age of 70. Alarmingly, lower-middle-income countries witnessed a concerning 13% increase in mortality rates related to diabetes. This highlights the urgent need for attention and action to combat this pervasive disease, especially in the context of the current global crisis[4].
Accessing prenatal care is crucial for ensuring a healthy pregnancy, but this can pose challenges in lower-middle-income countries. Additionally, the pandemic has added stress factors for women, such as limited access to a healthy diet and opportunities for regular exercise. A review conducted by Park et al[5] highlighted the adverse effects of COVID-19 on physical activity, with varying impacts observed among different sub-populations.
Furthermore, an internet-based cross-sectional survey demonstrated that Spanish pregnant women had reduced access to exercise, negatively affecting their well-being during the pandemic[6]. Consequently, the risk of developing complications such as hypertension, preeclampsia, and gestational DM (GDM) is elevated. If a woman has pre-existing diabetes (type 1 or type 2) prior to pregnancy, it further amplifies the likelihood of complications, including premature delivery, frequently associated with polyhydramnios[7]. Pregnant women diagnosed with gestational diabetes often encounter challenges in the diagnostic process, as it typically relies on prenatal screening rather than solely relying on signs and symptoms. While gestational diabetes is a frequent pregnancy complication, it can have detrimental effects on the health of both the mother and the newborn, potentially impacting the child’s long-term health outcomes[8]. Hence, it is of utmost importance for mothers to receive ongoing postpartum follow-up, as demonstrated by a 23-year cohort study conducted by Auvinen et al[9]. The study unveiled a consistent association between gestational diabetes and the subsequent onset of type 2 DM, emphasizing the need for lifelong monitoring and care.
According to the National Vital Statistics Reports of the United States, the overall incidence rate of GDM in 2020 was 7.8 per 100 births, representing a 30% increase from 2016[10]. However, it is important to note that this report was based solely on birth certificate data, which may have resulted in underreporting, potentially leading to an even higher prevalence of GDM than reported. Considering the circumstances where women are unable to attend prenatal checkups due to various limitations such as transportation issues, isolation measures, heightened stress levels, anxiety, or simply the fear of going outdoors during the pandemic, the consequences can be significant for their overall health, particularly when they are expecting a baby and lack access to prenatal care. Although limited data is available to support this, a case-controlled study conducted by Zanardo et al[11] focused on women who gave birth in the heavily impacted Northeast region of Italy, concluding that the COVID-19 pandemic had a negative impact on the prevalence of GDM in 2020 compared to 2019. This impact was especially notable among pregnant women during the initial stage of their pregnancy. Likewise, research conducted in Quebec, which was the focal point of the pandemic in Canada, indicates that a sudden alteration in lifestyle can have a significant effect on the prevalence of gestational diabetes within a population. The study observed elevated rates of GDM during the first and second waves of the pandemic in comparison to the period before the pandemic[12].
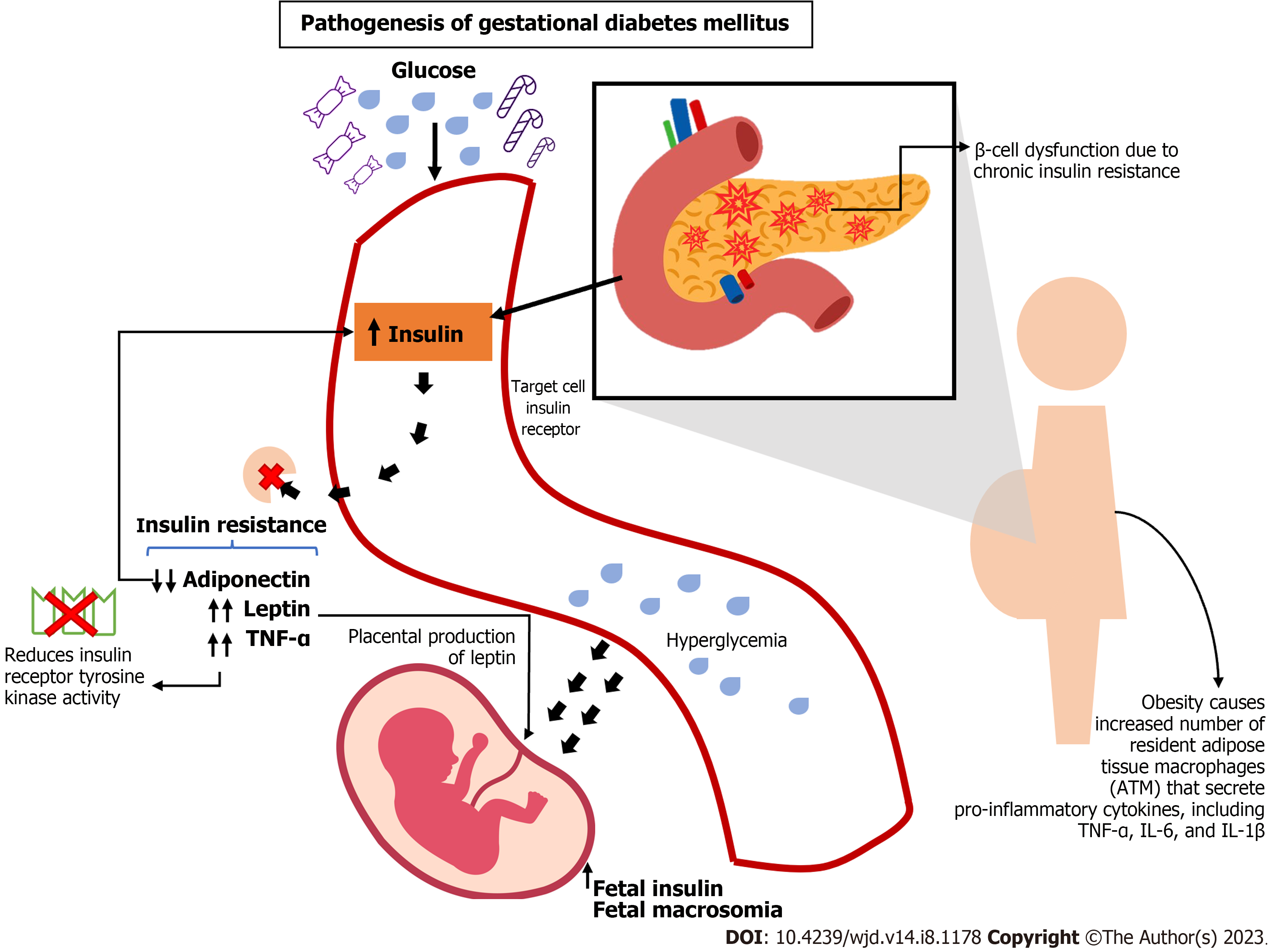
DM is a chronic metabolic disorder that affects more than 400 million people worldwide, with a rising prevalence in low- and middle-income countries[13]. During pregnancy, diabetes can lead to various complications for both the mother and the baby, including an increased risk of pre-eclampsia, preterm birth, macrosomia, and stillbirth (Figure 1).
GDM is a variant of diabetes that manifests during pregnancy, impacting approximately 10% of pregnancies globally. It is linked to a higher probability of unfavorable pregnancy outcomes and an elevated susceptibility to developing type 2 diabetes in the future[14,15] (Table 1).
| Type 2 diabetes mellitus | Gestational diabetes mellitus | |
| Occurrence | Generally, develops after age 40, but can occur at any age | Develops during pregnancy, typically after the 20th wk of gestation |
| Prevalence | Affects approximately 90% of people with diabetes | Affects approximately 2%-10% of pregnancies |
| Risk factors | Family history, obesity, physical inactivity, high blood pressure, and ethnicity | Family history, previous history of gestational diabetes, obesity, older maternal age, and certain ethnicities |
| Symptoms | Fatigue, increased thirst, frequent urination, blurred vision, slow healing wounds | Often asymptomatic, but may cause increased thirst, frequent urination, and increased hunger |
| Diagnosis | Blood tests measuring fasting blood glucose and hemoglobin A1C levels | Oral glucose tolerance test usually performed between 24-28 wk of gestation |
| Treatment | Lifestyle changes, medication, and/or insulin therapy | Lifestyle changes, close monitoring of blood glucose levels, and medication/insulin therapy if necessary |
| Potential complications | Cardiovascular disease, neuropathy, retinopathy, kidney disease, and foot ulcers | Preeclampsia, premature delivery, macrosomia, and increased risk of developing type 2 diabetes later in life |
Several risk factors have been identified that increase the likelihood of developing GDM. Nonmodifiable risk factors include age, with women over 25 years old having an increased risk, and a family history of diabetes[16]. Ethnicity is also a significant risk factor, with women of Hispanic[17], other than white European origin[18], Asian[19], and indigenous descent[20] being more likely to develop GDM. Other modifiable risk factors include being overweight or obese before pregnancy[21], excessive weight gain during pregnancy[22,23], and a sedentary lifestyle. Women with polycystic ovary syndrome[24] or a history of GDM in a previous pregnancy are also at higher risk. Early identification of these risk factors and appropriate management can help prevent or mitigate the effects of GDM (Table 2).
| Risk factors for GDM | Description |
| Increasing maternal age | Increases in gestational diabetes were seen in each maternal age group, and rates rose steadily with maternal age; in 2021, the rate for mothers aged ≥ 40 yr (15.6%) was nearly six times as high as the rate for mothers aged < 20 yr (2.7%)[16,25] |
| Past medical history of GDM in a previous pregnancy OR family history of type 2 DM | The strongest risk factor for gestational diabetes mellitus, with reported recurrence rates of up to 84%[26] |
| Race/ethnicities at increased risk for development of GDM | Women of Hispanic[17], other than white European origin[18], Asian[19], and indigenous descent[17-20] |
| Prevalence of GDM by ethnicity | The highest prevalence using the 2000 ADA diagnostic criteria among Filipinas (10.9%) and Asians (10.2%), followed by Hispanics (6.8%), non-Hispanic Whites (4.5%), and Black Americans (4.4%)[28] |
A study carried out by Teh et al[25] assessed the accuracy of various guidelines in diagnosing GDM. The study identified a history of previous GDM, maternal age of 40 years or older, and a body mass index (BMI) of 35 kg/m2 or higher as the most influential independent risk factors for GDM. The Health and Care Excellence, American Diabetes Association (ADA), and Australasian Diabetes in Pregnancy Society (ADIPS) guidelines exhibited various levels of sensitivity and specificity in diagnosing GDM, with the ADA demonstrating the highest sensitivity and the ADIPS showing the highest specificity.
Furthermore, a systematic review conducted by Kim et al[26] encompassing 13 studies examined the rates of GDM recurrence following the initial pregnancy. Recurrence rates varied from 30% to 84%, with higher rates observed among minority populations in comparison to non-Hispanic white populations. The studies did not identify consistent risk factors for GDM recurrence. Factors such as preexisting diabetes in subsequent pregnancies, socioeconomic status, rates of postpartum diabetes screening, and interpregnancy intervals were not consistently reported in the studies.
Although the GDM recurrence rate is high, with a median rate of 47.6% observed in a study conducted at the Mayo Clinic, strategies to prevent GDM recurrence are not well established. It is imperative to do further research to evaluate the effect of interventions before, during, and after pregnancy[27].
There are two common approaches for screening GDM, including universal screening and selective screening. Universal screening is applied without restriction to high-risk pregnant women to identify all potential GDM cases. On the other hand, selective screening is based on certain risk factors and might miss over 40% of GDM cases. However, selective screening may be more cost-effective by considering that screening with glucose measurements may be less beneficial to low-risk women[28].
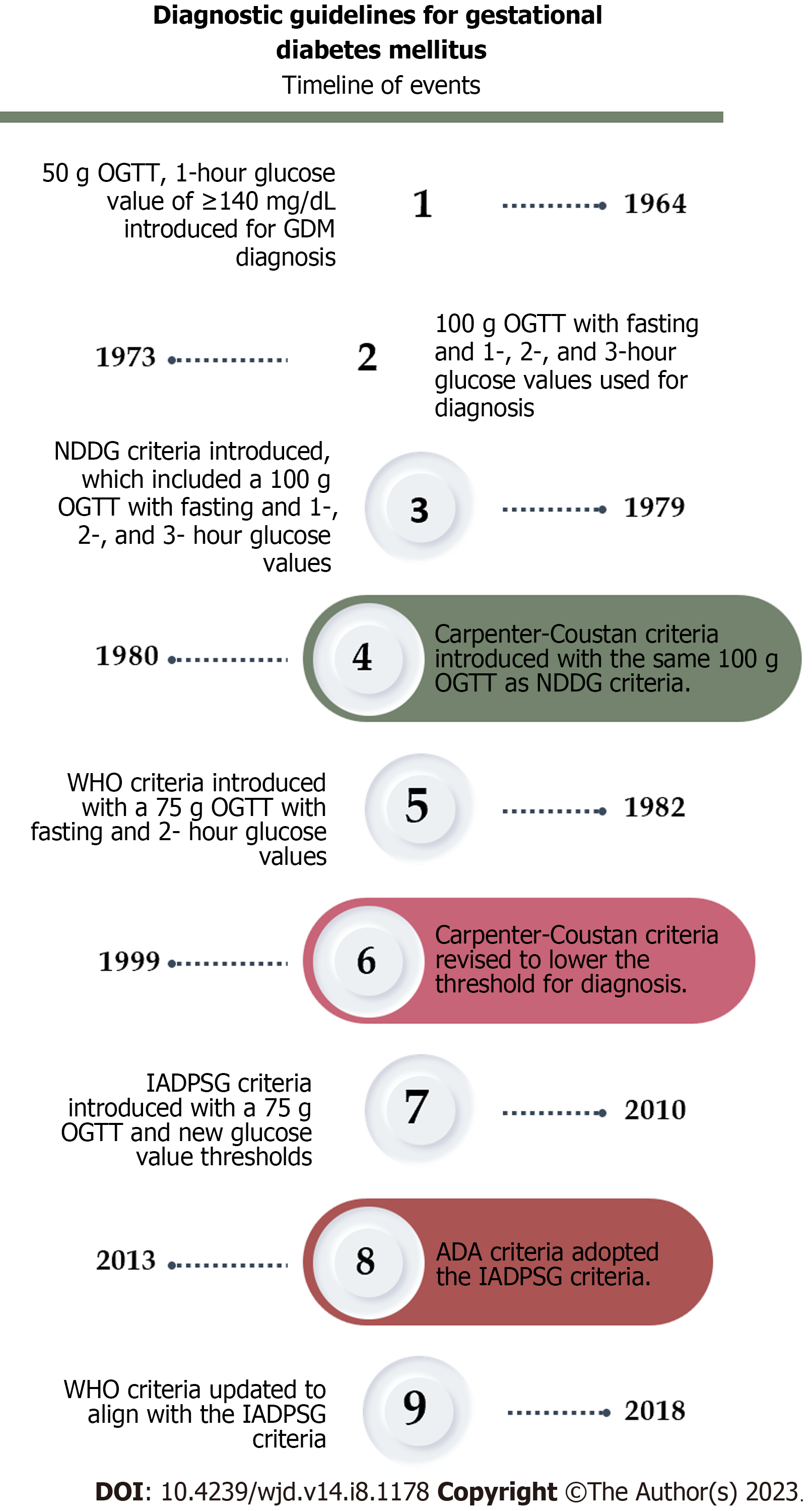
The diagnostic criteria for gestational diabetes have undergone revisions over time. The initial guideline was established in 1964 by O’Sullivan and Mahan[29], which was later modified by the National Diabetes Data Group in 1979[30] and Carpenter in 1982[31]. These frequent updates of the criteria are necessary to effectively identify women with gestational diabetes and assess their risk of perinatal complications (Figure 2).
Clinicians currently utilize the diagnostic criteria provided by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) in 2010 as the most recent standard[32]. The ADA[33] and the WHO[34] updated their guidelines in 2013 and 2018 to have the same criteria as the IADPSG. The ADA suggests using the IADPSG to improve pregnancy outcomes and offspring birth defects, rather than the prediction of subsequent maternal diabetes, based on initial data from randomized clinical trials[35].
Although they primarily support the one-step diagnosis, the American College of Obstetrics and Gynecology prefers the two-step Carpenter-Coustan screening, especially in patients with known risk factors[36]. Subsequently, a randomized trial conducted by Landon et al[37] demonstrated that even the management of mild cases of GDM [characterized by abnormal oral glucose tolerance test (OGTT) results surpassing established thresholds: 1-h, 180 mg/dL (10.0 mmol/L); 2-h, 155 mg/dL (8.6 mmol/L); and 3-h, 140 mg/dL (7.8 mmol/L), along with a fasting glucose level below 95 mg/dL (5.3 mmol/L)], has the potential to reduce the risks associated with fetal overgrowth, shoulder dystocia, the necessity for cesarean section, and hypertensive disorders[37,38].
Due to the lack of consensus in monitoring recommendations for women with gestational diabetes in different regions, and the predominant reliance on laboratory criteria rather than clinical symptoms for diagnosis, prompt and accurate identification of the condition during the pandemic became challenging. Many women received telemedicine consultations as an urgent safety measure to mitigate the spread of the virus[39]. However, some of these women did not exhibit clinical symptoms, highlighting the insufficiency of telemedicine technology in diagnosing and managing gestational diabetes compared to standard care. Further research is needed to explore this area and determine its effectiveness[40].
The COVID-19 pandemic had a substantial impact on the general population[41], but it posed additional challenges for pregnant women, exacerbating risk factors for GDM. These risk factors include heightened psychological distress, such as increased levels of depression and anxiety resulting from fear of the virus and other concerns, which can negatively impact their mental health during the perinatal period[42,43]. Additionally, the implementation of quarantine measures as a response to the pandemic led to prolonged periods of isolation at home[44,45], limiting opportunities for exercise and promoting sedentary lifestyles among pregnant women[46].
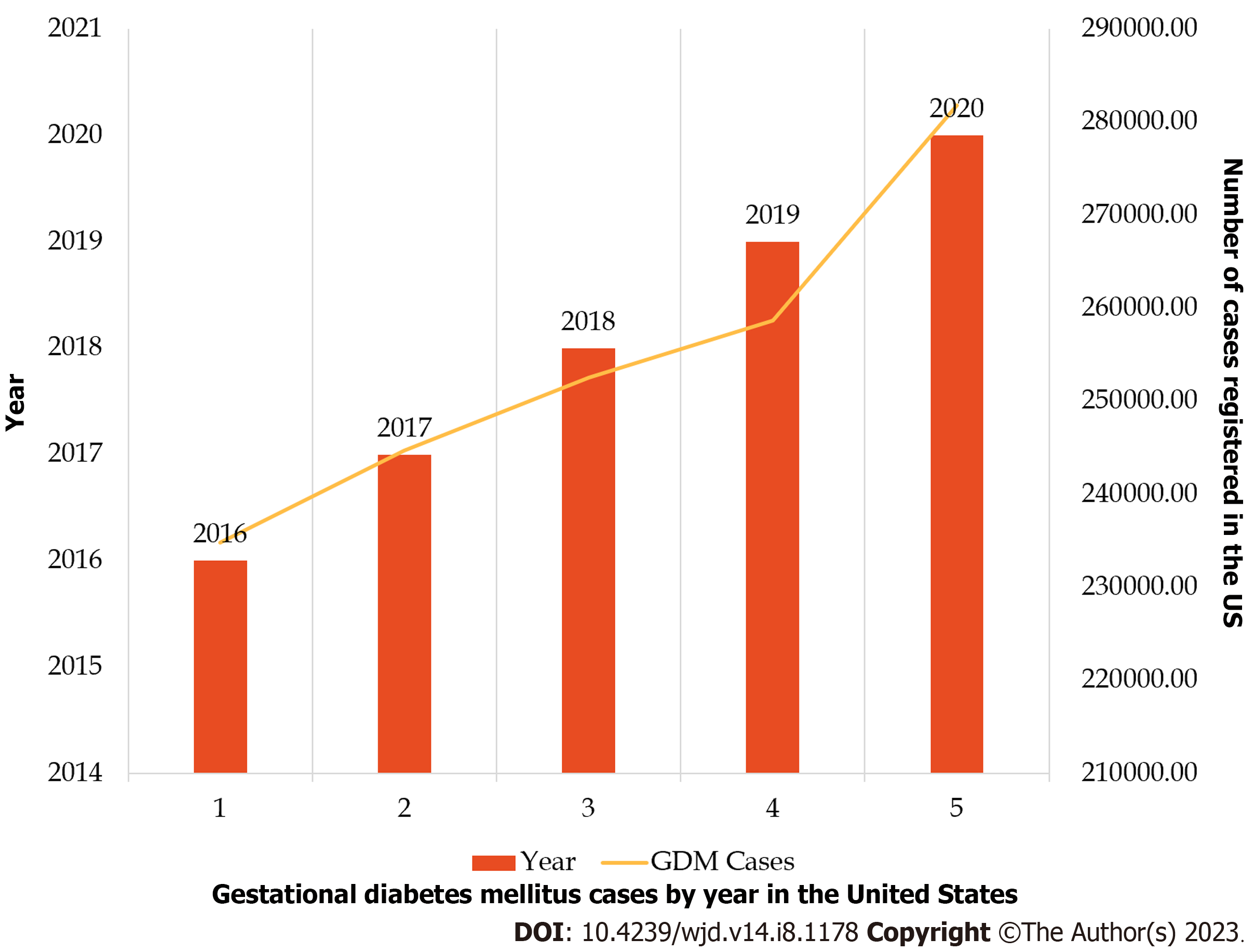
The prevalence of GDM increased at 38.9% during the COVID-19 pandemic, in comparison to pre-pandemic numbers - as demonstrated in a retrospective study by Mirsky et al[47] from data retrieved from deliveries at a single academic institution. In this study, 12.5% of patients were diagnosed with GDM during COVID-19, compared to 9.0% pre-COVID-19 (P < 0.001). But when this data was stratified by pre-pregnancy weight, no significant weight gain was shown, even among those with pre-pregnancy obesity. It was suggested then that maternal stress could have been the underlying component of gestational hyperglycemia[48]. The National Vital Statistics Reports showed data on trends for GDM from women giving birth in the United States from 2016 to 2020. Surprisingly in 2020, the rate of GDM was 7.8 per 100 births, marking a significant 30% rise compared to 2016[49].
The largest increase was seen in the annual percentage change from 2019 to 2020 (13%), surpassing the average annual percent change from 2016 to 2019 (5%). Additionally, in 2020, variations in the rate of GDM were noted based on maternal race and Hispanic origin. Non-Hispanic Asian women had the highest rate (14.9%), while non-Hispanic Black women had the lowest rate (6.5%)[49,50] (Figure 3 and Table 3). Despite a decline in the overall number of births from 2016 to 2020, the prevalence and rate of GDM indicate a resting trend.
| Year | Total births | GDM cases | GDM rate (%) | Confidence interval | Not stated cases |
| 2016 | 3945875 | 234847 | 6.0 | 5.9-6.0 | 3781 |
| 2017 | 3855500 | 244716 | 6.4 | 6.3-6.4 | 3711 |
| 2018 | 3791712 | 252522 | 6.7 | 6.6-6.7 | 2882 |
| 2019 | 3747540 | 258676 | 6.9 | 6.9-6.9 | 3284 |
| 2020 | 3613647 | 281789 | 7.8 | 7.8-7.8 | 4063 |
| Change, % | -8.6 | +19.8 | +30.0 | N/A | +7.5 |
Taking a global perspective, a comprehensive report conducted in 2021 by Wang et al[51] examined the prevalence of GDM by analyzing data from 57 studies published in PubMed. Overall, the worldwide prevalence of GDM was found to be 14.9% [95% confidence interval (CI)]. The Middle East and North Africa exhibited the highest standardized prevalence at 27.6% (95%CI: 26.9%-28.4%), followed by South-East Asia with a prevalence of 20.8% (95%CI: 20.2%-21.4%).
Due to the significant lifestyle changes during the pandemic, including reduced exercise opportunities, there is evidence to suggest that COVID-19 was linked to increased gestational weight gain (GWG) and a higher risk of excessive GWG among individuals with singleton pregnancies in the United States.
In a cross-sectional study conducted by Cao et al[52], data from United States live births between January 1, 2018 to December 31, 2020 showed an increase in GWG by 0.06 kg (after adjusting for covariates and excluding pre-pandemic trends). This increase was particularly evident among pregnant women under the age of 25, non-Hispanic Black individuals, unmarried individuals, and those with pre-pregnancy obesity.
In Italy, a study conducted during the COVID-19 lockdown revealed that pregnant women had higher BMI and experienced increased weight gain during pregnancy. The incidence of GDM also showed a significant increase, with a rate of 9.3% during the pandemic lockdown period (from March 10, 2020 to December 04, 2020), compared to 3.4% before the pandemic (June 11, 2019 to March 09, 2020) (P ≤ 0.0001)[53].
Also, a study made by Kołomańska-Bogucka et al[54], where the level of physical activity in the last trimester was evaluated, as well as the risk of postnatal depression and health habits in general, demonstrated that the COVID-19 pandemic lockdown negatively affected women even during the post-partum period, making these women be in an increased risk of developing GDM in their subsequent pregnancy. Nevertheless, some authors suggest that maternal stress is a potential factor for developing GDM[48] as some analyses of data have shown no significant weight gain in patients with gestational diabetes, even among individuals with obesity.
Conducting further investigations into the effects of lockdown measures on pregnant women, including changes in physical activity, dietary patterns, and their potential influence on maternal and neonatal outcomes, is imperative for advancing our understanding in this area. This research will contribute to a more comprehensive body of knowledge and inform evidence-based practices for optimal prenatal care.
The COVID-19 pandemic negatively impacted GDM prevalence, especially in pregnant women during their 1st trimester of gestation. An analysis made in Northeast Italy showed a 34% increase in the mean number of GDM diagnoses per month (logistic regression analysis). Hence, it was possible that exposure to stress-related factors due to the COVID-19 lockdown may have caused chronic inflammation in these pregnant women, which resulted in a higher risk of GDM[55].
It is also important to mention that maternal stress could have been exacerbated due to a lack of physical activity and sedentary behavior. Based on a survey conducted in the United Kingdom involving 553 eligible women, it was found that 79% of the participants reported an increase in sedentary behavior.
The primary reason for this decline in physical activity was a fear of leaving their homes[56]. As clinicians, it is important to encourage pregnant women to access online workout classes as an alternative to the gym or in-person classes[57]. This could potentially help them remain active and decrease their sedentary behavior. Although this could be a potential challenge, since not everyone has access to smart apps or online virtual classes, it is important to assess the patients’ availability and offer them affordable options.
GDM is a prevalent complication during pregnancy, and its misdiagnosis or inadequate control can result in substantial rates of adverse outcomes for both the neonate and the mother. The screening approaches utilized for GDM play a crucial role in its management and the prevention of future complications.
According to most health organizations, the initial prenatal visit is regarded as the optimal opportunity for screening GDM. The primary objectives of early screening are to detect patients with existing diabetes and to diagnose individuals at either low or elevated risk for GDM. The commonly employed methods for this diagnosis include measuring fasting plasma glucose (FPG), random plasma glucose (RPG), and glycosylated hemoglobin A1C (HbA1c)[58].
As per the guidelines set forth by the US Preventive Services Task Force, it is recommended to screen asymptomatic patients for GDM at 24 wk of gestational age[59]. As previously mentioned, the ADA provides two defined criteria for the diagnosis of gestational diabetes. The first approach, known as the “one-step” method, involves a 75-gram OGTT conducted between 24-28 wk of gestation, following an overnight fast of at least 8 h. The diagnosis of gestational diabetes is confirmed if any of the following values are observed: Fasting glucose: 92 mg/dL; 1-h glucose: 180 mg/dL; and 2-h glucose: 153 mg/dL.
The second approach, known as the “two-step” method[33,34], consists of a two-stage process. First, a 50-gram glucose load test (GLT) is performed (non-fasting) with plasma glucose measurement 1 h later, between 24-28 wk of gestation for women without a previous diabetes diagnosis.
If the plasma glucose level measured 1 h after the load is equal to or greater than 130, 135, or 140 mg/dL, the patient proceeds to the second stage: A 100-gram OGTT conducted after an overnight fast. In this step, the diagnosis of gestational diabetes is confirmed if at least two of the following criteria are met or exceeded among the four plasma glucose levels measured: Fasting glucose: 95 mg/dL; 1-h glucose: 180 mg/dL; 2-h glucose: 155 mg/dL; and 3-h glucose: 140 mg/dL.
The ACOG[36] proposes a two-step approach for diagnosing gestational diabetes using an universal screening with a 50-g GLT followed by a target diagnostic test with 100-g OGTT. The diagnosis criteria for GDM based on these tests are as follows: GLT criteria: (1) GLT result > 130 mg/dL; (2) GLT result > 135 mg/dL; and (3) GLT result > 140 mg/dL; OGTT criteria: (1) Fasting glucose > 95 mg/dL; (2) 1-h glucose > 180 mg/dL; (3) 2-h glucose > 155 mg/dL; and (4) 3-h glucose > 140 mg/dL.
However, the WHO supports the use of the one-step approach using a 75-gram OGTT, similar to the recommendations of the ADA and the IADPSG. Diagnosis is made if the following thresholds are met or exceeded: Fasting glucose levels of ≥ 92-125 mg/dL, 1-h glucose levels of ≥ 180 mg/dL, and 2-h glucose levels of ≥ 153-199 mg/dL[60].
Consequently, there is a lack of consensus regarding the optimal approach for diagnosing GDM, as both the “one-step” and “two-step” methods have their advantages and limitations. The diagnosis of GDM presented significant challenges worldwide during the pandemic, requiring adaptations to screening tests in order to comply with social distancing recommendations and minimize the exposure of pregnant women to COVID-19[61].
The pandemic hindered routine prenatal care by disrupting the traditional face-to-face communication and reducing the access to laboratory testing[62]. In addition, women with diabetes may also be exposed to COVID-19 more often due to intensive monitoring. This includes additional education sessions, glucose monitoring, and fetal ultrasounds, all of which take place in healthcare settings where COVID-19 is more likely to be transmitted[63].
Although pregnant women are not more likely to contract severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, in comparison to the general population, they have been observed to experience a more severe course of the disease. Additionally, adverse effects on newborns have been reported. As a result, the classification of pregnant women as a high-risk group has led to temporary modifications in screening tests to minimize their extended hospital stays[64].
The completion of routine OGTTs has become challenging for healthcare providers due to factors such as self-isolation, limited public transport, social distancing measures, and specific laboratory requirements. The ability to care for a large number of pregnant women with mild hyperglycemia has been reduced due to understaffing in healthcare facilities caused by isolation, illness, or relocation[65].
During the pandemic, certain countries chose to adapt the algorithm used for diagnosing GDM. For instance, Canada adjusted their clinical guidelines with the aim of minimizing the amount of time that pregnant women spend in clinics, thus reducing their risk of prolonged exposure. They employed a non-fasting glucose screening approach, using criteria such as HbA1c ≥ 5.7% (39 mmol/mol) and/or random venous plasma glucose levels ≥ 11.1 mmol/L. As a result, they decided to avoid conducting OGTTs, reducing the frequency of GDM diagnosis to 1.7% according to Mcintyre et al[66].
In contrast, Australia and New Zealand revised their guidelines in 2020. The updated criteria for diagnosis included an HbA1c level of ≥ 5.9% or a fasting blood glucose level of ≥ 5.1 mmol/L. Nevertheless, the findings of a retrospective study by Zhu et al[67] indicated that the screening test performance for GDM, using the mentioned criteria, was suboptimal. Specifically, the study found that 25.3% of cases either remained undiagnosed or did not receive appropriate treatment.
When evaluating the diagnosis of GDM, it is important to note that the available evidence supporting the use of RPG, HbA1c, and FPG assessments is relatively limited compared to the gold standard OGTT. However, it is worth highlighting that RPG has shown promising outcomes as a predictive tool for GDM diagnosis during the first trimester. This suggests its potential usefulness in identifying women at risk of developing GDM later in pregnancy[68].
In various study cohorts, the measurement of HbA1c during the first trimester has demonstrated its potential in identifying patients at high risk of developing GDM and other adverse pregnancy outcomes. These studies have utilized HbA1c thresholds ranging from 39-41 mmol/mol. Specifically, pregnant individuals with HbA1c levels equal to or exceeding 39 mmol/mol (5.7%) in the first trimester have been found to have a significantly higher risk, approximately five times greater, of developing GDM compared to those below this threshold. While there may be clinical value in identifying such high-risk pregnancies, it is important to acknowledge that the routine use of HbA1c as a predictor of GDM is not widely supported due to its limited specificity.
There is considerable overlap in the distribution of HbA1c levels during the first trimester between pregnancies with gestational diabetes and those without[69]. This suggests that HbA1c has limited usefulness in pregnancy, mainly due to the increased turnover of red blood cells. This increased turnover can result in an underestimation of glucose intolerance, particularly in women with anemia[70].
A comprehensive analysis of published guidelines in May 2020 highlighted consensus on three key aspects concerning the screening and management of GDM during the COVID-19 crisis. First, it was recommended to explore alternative screening methods, such as fasting blood glucose, HbA1c, or RPG, for GDM screening between 24-28 wk of gestation instead of the OGTT. Second, it was advised to delay postpartum screening tests for a period of 4-12 wk until the conclusion of the COVID-19 crisis or reschedule them for 6-12 mo after childbirth. Lastly, the use of telemedicine and telecare was encouraged wherever possible, helping remote medical consultations and patient monitoring[71].
The global challenge lies in the inconsistent and controversial approaches to screening and diagnosing GDM. The decision to avoid OGTTs in order to reduce the risk of SARS-CoV-2 transmission can have significant consequences for maternal-fetal complications related to GDM.
The COVID-19 pandemic raised substantial concerns regarding pregnant women at a considerable risk of experiencing adverse outcomes for themselves, as well as their neonates and fetuses. Measures such as social distancing, lockdowns, quarantines, and reduced in-person clinic visits were implemented to mitigate the risk of infection. However, these changes also had an impact on the management and diagnosis of GDM. As a result, further reorganization and adjustment of the healthcare system were necessary to ensure appropriate care for pregnant women during this challenging time.
The exploration of innovative digital alternatives, including telemedicine, telephone calls, internet/web-based platforms, and smartphone/mobile app-based interventions, played a crucial role in facilitating the diagnosis, management, and control of GDM. These technologies offered promising solutions to overcome the challenges derived by the pandemic and ensured effective healthcare delivery for individuals with GDM[72].
The utilization of telehealth services displayed several advantages, including mitigating the risk of COVID-19 exposure, enhancing healthcare accessibility, and reducing expenses associated with travel and parking[73]. A study conducted by Munda et al[74] further revealed that transitioning clinic visits to telehealth did not compromise glycemic control or lead to adverse neonatal outcomes. This highlights the efficacy of telehealth in providing obstetric care. Nevertheless, it is crucial to recognize that there are challenges associated with the implementation of telehealth.
Kozica-Olenski et al[75] conducted a study to examine the experiences and acceptability of utilizing telehealth for diabetes management during pregnancy. The results indicated that women encountered various challenges, including issues with internet quality, connectivity problems, and audio and video clarity, which occasionally disrupted the continuity of care.
Further challenges included the occurrence of delays in acquiring insulin prescriptions and inadequate access to nutrition and lifestyle guidance. These barriers were especially prominent among individuals who did not primarily speak English. Addressing the needs of non-English speakers or those with limited health literacy became even more complex within the realm of telehealth. These challenges were associated with an augmented administrative workload for healthcare providers[76]. Besides, more than half of the women surveyed in the study conducted by Kozica-Olenski et al[75] expressed a lack of confidence or comfort in self-monitoring their weight, blood pressure, or fundal height. Some participants reported not having the necessary equipment to perform these measurements effectively.
During the early phases of the pandemic, healthcare professionals encountered challenges due to uncertainties surrounding the virus and limited familiarity with telehealth practices. The transition to telehealth presented various obstacles, including increased work demands, difficulties in reaching and communicating with patients (who were often distracted or engaged in multitasking), and a decline in patients' perceived importance and responsibility during telephone consultations[77,78]. In general, women expressed satisfaction with telephone consultations as it provided them with access to quality care during the pandemic. However, a considerable majority of women clearly preferred in-person maternity care and felt that telehealth compromised the overall quality of their healthcare experience[75,79].
The management of GDM encompasses both non-pharmacological and pharmacological interventions. Research indicates that the majority of women (70%-90%) can effectively control GDM through lifestyle modifications, making medical and nutritional therapy the primary approach for treatment[80]. When lifestyle modifications alone do not effectively normalize blood glucose levels, pharmacological intervention becomes necessary. Insulin continues to be the primary treatment option, but oral medications have become increasingly popular. This is attributed to factors such as the cost of insulin, discomfort associated with multiple injections, and the requirement for frequent office visits for dose adjustments[81].
The primary objective in managing GDM is to ensure appropriate fetal growth while promoting steady weight gain and maintaining stable blood glucose levels. This requires maintaining euglycemia, defined as a fasting glucose level of ≤ 90-95 mg/dL and postprandial glucose levels of ≤ 140 mg/dL at 1 h or ≤ 120 mg/dL at 2 h[82].
Carbohydrates (CHO) are vital for providing energy to pregnant women and their developing fetus. The ADA recommends a minimum daily carbohydrate intake of 175 g to prevent the harmful effects of ketosis on the fetus. Ensuring an adequate carbohydrate supply is crucial for maintaining optimal health during pregnancy[83]. However, the selection of CHO with a low glycemic index (LGI) holds greater significance in the management of GDM, as emphasized in a meta-analysis conducted by Xu and Ye[84]. Their study revealed that a LGI diet, while maintaining the same level of carbohydrate restriction, resulted in significant reductions in FPG and 2-h postprandial glucose levels compared to a high glycemic index (HGI) diet. These findings underscore the importance of considering the glycemic index when designing dietary interventions for GDM.
Additionally, in a randomized controlled trial conducted by Moses et al[85], it was observed that patients following a LGI diet had a notably lower percentage of individuals requiring hypoglycemic medications compared to those on a HGI diet. Importantly, patients in the HGI group were able to avoid insulin use by transitioning them to the LGI group. These findings highlight the potential benefits of implementing a LGI diet in the management of GDM[86].
Although the primary focus of medical nutritional therapy (MNT) in GDM is to ensure adequate caloric intake for fetal development and maintain euglycemia in the mother, research from randomized controlled trials indicates that fetal growth is primarily influenced by fatty acids rather than glucose as an independent variable[87]. According to a prospective cohort study conducted by de Lima et al[88], a higher consumption of n-3 fatty acids was associated with a decreased likelihood of having a neonate with a large gestational age (LGA). Additionally, women with a higher intake of polyunsaturated fatty acids, including both n-3 and n-6, and a higher ratio of polyunsaturated fats to saturated fats (P/S), as well as a higher ratio of hypocholesterolemic to hypercholesterolemic fatty acids (h/H), had a significantly lower probability of giving birth to a neonate with macrosomia, with a potential reduction of up to 49%. These findings emphasize the importance of considering the impact of fatty acids on fetal growth and the potential influence of dietary factors on the outcomes of GDM.
During pregnancy, there is a reduction in protein breakdown, which is essential for supporting the growth of both the mother and the fetus. Interestingly, the difference in nitrogen loss between a normal pregnancy and one affected by GDM is minimal. However, this changes when advanced GDM necessitates intensive MNT and the use of hypoglycemic medications. Consequently, despite an increase in tissue synthesis, the ADA recommends a minimum daily protein requirement of only 71 g. The relationship between protein intake and LGA remains inconclusive, although one study found a correlation between leucine and birth weight in both GDM and normal pregnancies.
In the management of GDM, increasing protein intake is beneficial, regardless of the protein source, including plant-based options, lean meats, and fish. However, individuals following a vegetarian or vegan diet should be cautious and ensure adequate protein intake by supplementing with iron and cyanocobalamin and carefully planning their meals. This precaution is crucial to prevent the risk of inadequate protein intake among individuals adhering to a vegan diet[83,89].
While the majority of patients diagnosed with GDM can achieve normal blood glucose levels through MNT in the first week, there is a subset of approximately 20% of women who require treatment with hypoglycemic agents. Insulin is the preferred initial therapy due to its safety for the fetus, as it does not cross the placenta. It is important to note that the United States Food and Drug Administration has not approved oral agents for the treatment of GDM, further establishing insulin as the primary choice. The American College of Obstetricians and Gynecologists recommends considering the use of metformin only in specific scenarios, such as when the patient declines insulin therapy, faces financial constraints related to insulin costs, or expresses concerns about compliance[90].
The Society of Maternal-Fetal Medicine has raised concerns about the use of metformin in managing GDM due to its potential impact on fetal development. This is attributed to metformin’s ability to suppress mitochondrial respiration, inhibit growth, and affect gluconeogenic responses. However, there is currently no definitive evidence regarding the long-term fetal prognosis associated with metformin use. A study conducted by Landi et al[91] found that metformin treatment was associated with a reduced risk of planned cesarean section, hypoglycemia, and large-for-gestational-age infants compared to insulin treatment. In contrast, the Metformin in Gestational Diabetes Trial conducted by Rowan et al[92] involved 751 women with GDM and showed comparable glycemic control among the study groups. However, it also revealed a higher risk of preterm delivery in the metformin group. These conflicting results underscore the importance of conducting long-term studies to establish the comparative effectiveness of insulin and metformin. Despite these considerations, the ADA recommends the use of metformin in cases of GDM only when patients decline insulin treatment or face challenges with insulin compliance, such as financial constraints or language barriers[83].
The initial dosage of metformin is 500 mg taken once at night for the first week, followed by an increase to 500 mg taken twice daily. The maximum recommended dose of metformin is 2500 mg/d. For extended-release metformin, the maximum dose is 2000 mg[90].
Glyburide functions by binding to the ATP-sensitive potassium channel complex in pancreatic beta cells, leading to an increased secretion of insulin and subsequent reduction in blood glucose levels. Several studies have indicated that glyburide has similar safety profiles to insulin in terms of neonatal outcomes, as demonstrated by Langer et al[93] in their randomized clinical trial. The study found comparable glycemic control between glyburide and insulin, with no significant difference in neonatal adverse events. However, it is important to note that despite these findings, other studies have reported higher rates of neonatal intensive care unit admissions for fetal hypoglycemia and a higher incidence of failure to achieve optimal glycemic control in patients treated with glyburide[94].
Regular insulin and neutral protamine Hagedorn are commonly utilized insulin types in pregnancy, and their safety has been established through numerous human studies. Attaining optimal glycemic control is crucial to mitigate the risk of hyperglycemia during pregnancy. When choosing an insulin regimen, it is important to replicate the physiological insulin secretion pattern and customize it based on the individual patient’s condition. For example, some patients may only require a basal insulin dose if they have elevated fasting or postprandial plasma glucose levels, but not both. The selection of insulin type should be determined on a case-by-case basis to minimize the risk of hypoglycemia, particularly when using rapid-acting and long-acting insulins[95]. For further details on the distinct types of insulin and oral agents, as well as additional information regarding glyburide, please refer to Table 4[96].
| Drug class | Drug | Dosing |
| Insulin | ||
| Rapid-acting insulin | Insulin lispro | First trimester 0.7 units/kg/d. 14-18 wk 0.8 units/kg/d. 26-27 wk 0.9 units/kg/d. 36-37 wk until delivery 1 unit/kg/d[95] |
| Insulin aspart | ||
| Short-acting insulin | Regular insulin | First trimester 0.7 units/kg/d. 14-18 wk 0.8 units/kg/d. 26-27 wk 0.9 units/kg/d. 36-37 wk until delivery 1 unit/kg/d[95] |
| Intermediate-acting insulin | NPH | Two thirds can be given prebreakfast and the remaining one third can be given during the pre-evening meal[95] |
| Long-acting insulin | Detemir | 50% of total daily dose can be given in the pre-evening meal and the remaining 50% can be given as a basal insulin[95] |
| Glargine | ||
| Oral agents | ||
| Biguanide | Metformin | 500 mg once or twice daily with an increase over 1 to 2 wk to a maximum daily dose of 2500 mg. 2000 mg if using metformin of extended release[90,92] |
| Sulfonylurea | Glyburide | Starting dose of 2.5 to 5 mg once daily with an increase to a maximum dose of 20 mg/d[96] |
Nutrition and physical activity are widely recognized as crucial components in managing blood glucose levels, especially in the treatment of GDM. However, when these lifestyle approaches prove insufficient in achieving normal blood glucose levels, pharmacological therapy may be required. Interestingly, during the COVID-19 pandemic, there has been an observed increase in GDM cases, both during the first wave (March 1, 2020 to August 22, 2020) and second wave (August 23, 2020 to March 31, 2021). This increase has been particularly notable among women who were previously considered to be at minimal risk for hyperglycemia. These women were between 25 to 34 years of age, with a high socioeconomic status and no existing comorbidities, and experienced significant health impacts during the initial year of the pandemic. The reasons for this increase may be attributed to changes in screening protocols or lifestyle factors[97].
Several authors have documented the impact of the COVID-19 pandemic on physical activity levels, noting a decrease in physical activity and an increase in sedentary behavior[5,54]. This observation was corroborated by a study conducted by Hillyard et al[56], which revealed a significant 79% rise in sedentary behavior among 553 pregnant women during the pandemic. The main reported factor contributing to this pronounced shift in behavior was the fear of venturing outside the home due to the COVID-19 pandemic.
As healthcare providers, it is our duty to educate the population on the prevention of GDM. A crucial aspect of prevention involves assessing women’s lifestyles and providing comprehensive counseling to foster optimal conditions for pregnancy. According to a systematic review by Laredo-Aguilera et al[98], pregnant women diagnosed with GDM can derive advantages from participating in moderate-intensity exercise for a minimum of 20-50 min, at least twice a week. However, the review did not identify a specific exercise type due to the varied range of exercises mentioned in the studies analyzed.
In recent years, there has been extensive research exploring the effects of the COVID-19 pandemic on mental health. Numerous authors have published reviews and case reports documenting an increase in anxiety, depression, stress, and psychosis attributed to several factors. These factors include work-related stress, the implementation of lockdown measures, the closure of public facilities, adherence to social distancing requirements, quarantine measures, the fear of contracting the virus, the disruption of traditional celebrations, and the promotion of safety behaviors, among other influences[99-102].
The literature review has provided valuable insights into the relationship between mental health disorders, particularly anxiety and depression, and GDM. Multiple articles have contributed to our understanding of this link, with the majority of the reviewed literature supporting the presence of an association. It suggests that pregnant women with a history of mental health disorders have an elevated risk of developing GDM, and likewise, women diagnosed with GDM are at a higher risk of experiencing symptoms of depression and anxiety[103-106].
In 2022, Trinh et al[107] conducted a study that revealed a decline in the utilization of mental healthcare services by women during pregnancy, followed by an increase in utilization during the postpartum period. This finding emphasizes the importance of implementing consistent psychological intervention measures to identify and address mental health disorders in pregnant women, regardless of whether they have GDM or not. By implementing such measures, women can ensure a safe pregnancy and enhance their overall pregnancy outcomes.
Having open and educational conversations with patients about the potential benefits of physical activity as a treatment for GDM and its role in managing mental health conditions is crucial. Equally important is discussing the potential risks and benefits of incorporating pharmacotherapy into the treatment plan. By engaging in these discussions, patients can make informed decisions about their healthcare journey[108].
The COVID-19 pandemic has had a profound impact on pregnant women, exacerbating the risk factors for GDM and complicating its management. Psychological factors, such as increased levels of depression and anxiety, have affected the mental health of pregnant women, potentially contributing to the development or worsening of GDM. Quarantine measures and reduced physical activity have also increased the risk for this condition.
A recent retrospective study by Mirsky et al[48] showed an increase in GDM diagnoses during the pandemic, but no significant rise in GWG, even among individuals with obesity. The study suggests that maternal stress may be a contributing factor, but further research is needed. Also, recent evidence suggests that the degree of SARS-Cov-2 and placental involvement could be crucial factors for adverse outcomes in pregnancy, including placental inflammation and vascular damage caused by the virus, hence putting the patient at risk for GDM[109]. However, further histology, immunohistochemistry, and molecular genetics analyses are needed to contribute to the understanding of the epidemiological changes[110].
The COVID-19 pandemic has underscored the need for resilient healthcare systems in effectively managing and supporting pregnant women with GDM. Lessons learned from this crisis should inform future strategies for prevention, diagnosis, and management, while considering the impact of external factors on maternal and fetal health. By applying this knowledge, we can enhance the care provided to pregnant women and their infants, aiming for positive health outcomes.
We would like to thank Ilse Ruiz BS, for the valuable proofreading input and assistance in refining the grammar of our article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American College of Physician; American College of Chest Physician.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jena MK, India; Nayak S, Trinidad and Tobago; Islam MS, South Africa S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Centers for Disease Control and Prevention. CDC timeline. [cited 4 April 2023]. Available from: https://www.cdc.gov/museum/timeline/index.html. |
| 2. | Ke R, Sanche S, Romero-Severson E, Hengartner N. Fast spread of COVID-19 in Europe and the US suggests the necessity of early, strong and comprehensive interventions. medRxiv 2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 3. | World Health Organization. COVID-19 pandemic triggers 25% increase in prevalence of anxiety and depression worldwide. [cited 9 May 2023]. Available from: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide. |
| 4. | World Health Organization. Diabetes. [cited 10 May 2023]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. |
| 5. | Park AH, Zhong S, Yang H, Jeong J, Lee C. Impact of COVID-19 on physical activity: A rapid review. J Glob Health. 2022;12:05003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 6. | Biviá-Roig G, La Rosa VL, Gómez-Tébar M, Serrano-Raya L, Amer-Cuenca JJ, Caruso S, Commodari E, Barrasa-Shaw A, Lisón JF. Analysis of the Impact of the Confinement Resulting from COVID-19 on the Lifestyle and Psychological Wellbeing of Spanish Pregnant Women: An Internet-Based Cross-Sectional Survey. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 7. | Köck K, Köck F, Klein K, Bancher-Todesca D, Helmer H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J Matern Fetal Neonatal Med. 2010;23:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia. 2020;63:508-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Auvinen AM, Luiro K, Jokelainen J, Järvelä I, Knip M, Auvinen J, Tapanainen JS. Type 1 and type 2 diabetes after gestational diabetes: a 23 year cohort study. Diabetologia. 2020;63:2123-2128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Centers for Disease Control and Prevention. Gestational Diabetes. [cited 14 May 2023]. Available from: https://www.cdc.gov/diabetes/basics/gestational.html. |
| 11. | Zanardo V, Tortora D, Sandri A, Severino L, Mesirca P, Straface G. COVID-19 pandemic: Impact on gestational diabetes mellitus prevalence. Diabetes Res Clin Pract. 2022;183:109149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Auger N, Wei SQ, Dayan N, Ukah UV, Quach C, Lewin A, Healy-Profitós J, Ayoub A, Chang J, Luu TM. Impact of Covid-19 on rates of gestational diabetes in a North American pandemic epicenter. Acta Diabetol. 2023;60:257-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Pan American Health Organization. Diabetes. [cited 14 May 2023]. Available from: https://www.paho.org/en/topics/diabetes. |
| 14. | Sweeting A, Wong J, Murphy HR, Ross GP. A Clinical Update on Gestational Diabetes Mellitus. Endocr Rev. 2022;43:763-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 397] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 15. | Daly B, Toulis KA, Thomas N, Gokhale K, Martin J, Webber J, Keerthy D, Jolly K, Saravanan P, Nirantharakumar K. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med. 2018;15:e1002488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 16. | QuickStats: Percentage of Mothers with Gestational Diabetes,* by Maternal Age - National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023;72:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Buchanan TA, Xiang A, Kjos SL, Lee WP, Trigo E, Nader I, Bergner EA, Palmer JP, Peters RK. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes. 1998;47:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Girgis CM, Gunton JE, Cheung NW. The influence of ethnicity on the development of type 2 diabetes mellitus in women with gestational diabetes: a prospective study and review of the literature. ISRN Endocrinol. 2012;2012:341638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35:1492-1498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 20. | Chamberlain C, McNamara B, Williams ED, Yore D, Oldenburg B, Oats J, Eades S. Diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand and the United States. Diabetes Metab Res Rev. 2013;29:241-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Lucovnik M, Blickstein I, Verdenik I, Steblovnik L, Trojner Bregar A, Tul N. Impact of pre-gravid body mass index and body mass index change on preeclampsia and gestational diabetes in singleton and twin pregnancies. J Matern Fetal Neonatal Med. 2014;27:1901-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Rahnemaei FA, Abdi F, Kazemian E, Shaterian N, Behesht Aeen F. Association between body mass index in the first half of pregnancy and gestational diabetes: A systematic review. SAGE Open Med. 2022;10:20503121221109911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 395] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 24. | Ashrafi M, Sheikhan F, Arabipoor A, Hosseini R, Nourbakhsh F, Zolfaghari Z. Gestational diabetes mellitus risk factors in women with polycystic ovary syndrome (PCOS). Eur J Obstet Gynecol Reprod Biol. 2014;181:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol. 2011;51:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care. 2007;30:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 27. | Egan AM, Enninga EAL, Alrahmani L, Weaver AL, Sarras MP, Ruano R. Recurrent Gestational Diabetes Mellitus: A Narrative Review and Single-Center Experience. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep. 2016;16:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 870] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 29. | O'sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278-285. [PubMed] |
| 30. | Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4004] [Cited by in RCA: 3877] [Article Influence: 84.3] [Reference Citation Analysis (1)] |
| 31. | Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1342] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 32. | International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2777] [Cited by in RCA: 3176] [Article Influence: 211.7] [Reference Citation Analysis (1)] |
| 33. | American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1419] [Article Influence: 473.0] [Reference Citation Analysis (1)] |
| 34. | Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103:341-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 568] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 35. | Lowe WL Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, McCance D, Hamilton J, Nodzenski M, Talbot O, Brickman WJ, Clayton P, Ma RC, Tam WH, Dyer AR, Catalano PM, Lowe LP, Metzger BE; HAPO Follow-up Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care. 2019;42:372-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 36. | ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018;131:e49-e64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 1152] [Article Influence: 164.6] [Reference Citation Analysis (0)] |
| 37. | Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM Jr, Sciscione A, Catalano P, Harper M, Saade G, Lain KY, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GB; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1568] [Cited by in RCA: 1484] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 38. | Noctor E, Dunne FP. Type 2 diabetes after gestational diabetes: The influence of changing diagnostic criteria. World J Diabetes. 2015;6:234-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (2)] |
| 39. | Nakagawa K, Umazume T, Mayama M, Chiba K, Saito Y, Kawaguchi S, Morikawa M, Yoshino M, Watari H. Feasibility and safety of urgently initiated maternal telemedicine in response to the spread of COVID-19: A 1-month report. J Obstet Gynaecol Res. 2020;46:1967-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Ming WK, Mackillop LH, Farmer AJ, Loerup L, Bartlett K, Levy JC, Tarassenko L, Velardo C, Kenworthy Y, Hirst JE. Telemedicine Technologies for Diabetes in Pregnancy: A Systematic Review and Meta-Analysis. J Med Internet Res. 2016;18:e290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 41. | Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4389] [Article Influence: 877.8] [Reference Citation Analysis (1)] |
| 42. | Haruna M, Nishi D. Perinatal mental health and COVID-19 in Japan. Psychiatry Clin Neurosci. 2020;74:502-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Berthelot N, Lemieux R, Garon-Bissonnette J, Drouin-Maziade C, Martel É, Maziade M. Uptrend in distress and psychiatric symptomatology in pregnant women during the coronavirus disease 2019 pandemic. Acta Obstet Gynecol Scand. 2020;99:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 44. | Rodríguez-Fernández P, González-Santos J, Santamaría-Peláez M, Soto-Cámara R, Sánchez-González E, González-Bernal JJ. Psychological Effects of Home Confinement and Social Distancing Derived from COVID-19 in the General Population-A Systematic Review. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 45. | Riley V, Ellis N, Mackay L, Taylor J. The impact of COVID-19 restrictions on women's pregnancy and postpartum experience in England: A qualitative exploration. Midwifery. 2021;101:103061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Park S, Marcotte RT, Staudenmayer JW, Strath SJ, Freedson PS, Chasan-Taber L. The impact of the COVID-19 pandemic on physical activity and sedentary behavior during pregnancy: a prospective study. BMC Pregnancy Childbirth. 2022;22:899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 47. | Mirsky EL, Mastronardi A, Paudel AM, Young M, Zite N, Maples J. Comparison of the Prevalence of Gestational Diabetes Pre-COVID-19 Pandemic Versus During COVID-19 [A196]. Obstet Gynecol. 2022;. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Mirsky EL, Mastronardi AM, Paudel A, Young ML, Zite NB, Maples JM. The COVID-19 pandemic and prevalence of gestational diabetes: Does gestational weight gain matter? Am J Obstet Gynecol MFM. 2023;5:100899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 49. | Gregory EC, Ely DM. Trends and Characteristics in Gestational Diabetes: United States, 2016-2020. Natl Vital Stat Rep. 2022;71:1-15. [PubMed] [DOI] [Full Text] |
| 50. | Osterman M, Hamilton B, Martin JA, Driscoll AK, Valenzuela CP. Births: Final Data for 2020. Natl Vital Stat Rep. 2021;70:1-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 51. | Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, Hoegfeldt CA, Elise Powe C, Immanuel J, Karuranga S, Divakar H, Levitt N, Li C, Simmons D, Yang X; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract. 2022;183:109050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 546] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 52. | Cao W, Sun S, Danilack VA. Analysis of Gestational Weight Gain During the COVID-19 Pandemic in the US. JAMA Netw Open. 2022;5:e2230954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | La Verde M, Torella M, Riemma G, Narciso G, Iavarone I, Gliubizzi L, Palma M, Morlando M, Colacurci N, De Franciscis P. Incidence of gestational diabetes mellitus before and after the Covid-19 lockdown: A retrospective cohort study. J Obstet Gynaecol Res. 2022;48:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 54. | Kołomańska-Bogucka D, Pławiak N, Mazur-Bialy AI. The Impact of the COVID-19 Pandemic on the Level of Physical Activity, Emotional State, and Health Habits of Women in Late Pregnancy and Early Puerperium. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Baz B, Riveline JP, Gautier JF. Endocrinology of pregnancy: Gestational diabetes mellitus: definition, aetiological and clinical aspects. Eur J Endocrinol. 2016;174:R43-R51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 56. | Hillyard M, Sinclair M, Murphy M, Casson K, Mulligan C. The impact of COVID-19 on the physical activity and sedentary behaviour levels of pregnant women with gestational diabetes. PLoS One. 2021;16:e0254364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 57. | Liu R, Menhas R, Dai J, Saqib ZA, Peng X. Fitness Apps, Live Streaming Workout Classes, and Virtual Reality Fitness for Physical Activity During the COVID-19 Lockdown: An Empirical Study. Front Public Health. 2022;10:852311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Nouhjah S, Jahanfar S, Shahbazian H. Temporary changes in clinical guidelines of gestational diabetes screening and management during COVID-19 outbreak: A narrative review. Diabetes Metab Syndr. 2020;14:939-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Gestational Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 60. | Hassanzadeh Rad A, Fakhre Yaseri A. The association between COVID-19 and gestational diabetes mellitus: A narrative review. J Ren Endocrinol. 2022;8:e17062. [DOI] [Full Text] |
| 61. | Keating N, Carpenter K, McCarthy K, Coveney C, McAuliffe F, Mahony R, Walsh J, Hatunic M, Higgins M. Clinical Outcomes Following a Change in Gestational Diabetes Mellitus Diagnostic Criteria Due to the COVID-19 Pandemic: A Case-Control Study. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Nouhjah S, Jahanfar S. Challenges of diabetes care management in developing countries with a high incidence of COVID-19: A brief report. Diabetes Metab Syndr. 2020;14:731-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | van Gemert TE, Moses RG, Pape AV, Morris GJ. Gestational diabetes mellitus testing in the COVID-19 pandemic: The problems with simplifying the diagnostic process. Aust N Z J Obstet Gynaecol. 2020;60:671-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Molina-Vega M, Gutiérrez-Repiso C, Lima-Rubio F, Suárez-Arana M, Linares-Pineda TM, Cobos Díaz A, Tinahones FJ, Morcillo S, Picón-César MJ. Impact of the Gestational Diabetes Diagnostic Criteria during the Pandemic: An Observational Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Meek CL, Lindsay RS, Scott EM, Aiken CE, Myers J, Reynolds RM, Simmons D, Yamamoto JM, McCance DR, Murphy HR. Approaches to screening for hyperglycaemia in pregnant women during and after the COVID-19 pandemic. Diabet Med. 2021;38:e14380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | McIntyre HD, Gibbons KS, Ma RCW, Tam WH, Sacks DA, Lowe J, Madsen LR, Catalano PM. Testing for gestational diabetes during the COVID-19 pandemic. An evaluation of proposed protocols for the United Kingdom, Canada and Australia. Diabetes Res Clin Pract. 2020;167:108353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 67. | Zhu S, Meehan T, Veerasingham M, Sivanesan K. COVID-19 pandemic gestational diabetes screening guidelines: A retrospective study in Australian women. Diabetes Metab Syndr. 2021;15:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 68. | Meek CL, Murphy HR, Simmons D. Random plasma glucose in early pregnancy is a better predictor of gestational diabetes diagnosis than maternal obesity. Diabetologia. 2016;59:445-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | D'Arcy RJ, Cooke IE, McKinley M, McCance DR, Graham UM. First-trimester glycaemic markers as predictors of gestational diabetes and its associated adverse outcomes: A prospective cohort study. Diabet Med. 2023;40:e15019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 70. | Edelson PK, James KE, Leong A, Arenas J, Cayford M, Callahan MJ, Bernstein SN, Tangren JS, Hivert MF, Higgins JM, Nathan DM, Powe CE. Longitudinal Changes in the Relationship Between Hemoglobin A1c and Glucose Tolerance Across Pregnancy and Postpartum. J Clin Endocrinol Metab. 2020;105:e1999-e2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Panaitescu AM, Ciobanu AM, Popa M, Duta I, Gica N, Peltecu G, Veduta A. Screening for Gestational Diabetes during the COVID-19 Pandemic-Current Recommendations and Their Consequences. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Eberle C, Stichling S. Telemedical Approaches to Managing Gestational Diabetes Mellitus During COVID-19: Systematic Review. JMIR Pediatr Parent. 2021;4:e28630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Xie W, Dai P, Qin Y, Wu M, Yang B, Yu X. Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: an updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth. 2020;20:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 74. | Munda A, Indihar BŠ, Okanovič G, Zorko K, Steblovnik L, Barlovič DP. Maternal and Perinatal Outcomes During the COVID-19 Epidemic in Pregnancies Complicated by Gestational Diabetes. Zdr Varst. 2023;62:22-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 75. | Kozica-Olenski SL, Soldatos G, Marlow L, Cooray SD, Boyle JA. Exploring the acceptability and experience of receiving diabetes and pregnancy care via telehealth during the COVID-19 pandemic: a qualitative study. BMC Pregnancy Childbirth. 2022;22:932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 76. | Asefa A, Semaan A, Delvaux T, Huysmans E, Galle A, Sacks E, Bohren MA, Morgan A, Sadler M, Vedam S, Benova L. The impact of COVID-19 on the provision of respectful maternity care: Findings from a global survey of health workers. Women Birth. 2022;35:378-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 77. | DeNicola N, Grossman D, Marko K, Sonalkar S, Butler Tobah YS, Ganju N, Witkop CT, Henderson JT, Butler JL, Lowery C. Telehealth Interventions to Improve Obstetric and Gynecologic Health Outcomes: A Systematic Review. Obstet Gynecol. 2020;135:371-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 78. | Karavadra B, Stockl A, Prosser-Snelling E, Simpson P, Morris E. Women's perceptions of COVID-19 and their healthcare experiences: a qualitative thematic analysis of a national survey of pregnant women in the United Kingdom. BMC Pregnancy Childbirth. 2020;20:600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 79. | Pérez-Ferre N, Galindo M, Fernández MD, Velasco V, Runkle I, de la Cruz MJ, Martín Rojas-Marcos P, Del Valle L, Calle-Pascual AL. The outcomes of gestational diabetes mellitus after a telecare approach are not inferior to traditional outpatient clinic visits. Int J Endocrinol. 2010;2010:386941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Ustick J, Chakos K, Jia H, Hanneke R, DiPiazza B, Koenig MD, Ma J, Man B, Tussing-Humphreys L, Burton TCJ. Associations between plant-based diets, plant foods and botanical supplements with gestational diabetes mellitus: a systematic review protocol. BMJ Open. 2023;13:e068829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 81. | Igwesi-Chidobe CN, Okechi PC, Emmanuel GN, Ozumba BC. Community-based non-pharmacological interventions for pregnant women with gestational diabetes mellitus: a systematic review. BMC Womens Health. 2022;22:482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 82. | Hernandez TL, Mande A, Barbour LA. Nutrition therapy within and beyond gestational diabetes. Diabetes Res Clin Pract. 2018;145:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 83. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Jeffrie Seley J, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S254-S266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 184] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 84. | Xu J, Ye S. Influence of low-glycemic index diet for gestational diabetes: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2020;33:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Moses RG, Barker M, Winter M, Petocz P, Brand-Miller JC. Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care. 2009;32:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 86. | Filardi T, Panimolle F, Crescioli C, Lenzi A, Morano S. Gestational Diabetes Mellitus: The Impact of Carbohydrate Quality in Diet. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 87. | Kapur K, Kapur A, Hod M. Nutrition Management of Gestational Diabetes Mellitus. Ann Nutr Metab. 2021;1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | de Lima MC, Santos IDS, Crivellenti LC, Sartorelli DS. A better quality of maternal dietary fat reduces the chance of large-for-gestational-age infants: A prospective cohort study. Nutrition. 2021;91-92:111367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 90. | Oskovi-Kaplan ZA, Ozgu-Erdinc AS. Management of Gestational Diabetes Mellitus. Adv Exp Med Biol. 2021;1307:257-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Landi SN, Radke S, Boggess K, Engel SM, Stürmer T, Howe AS, Jonsson Funk M. Comparative effectiveness of metformin versus insulin for gestational diabetes in New Zealand. Pharmacoepidemiol Drug Saf. 2019;28:1609-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 689] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 93. | Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 469] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 94. | Mukerji G, Feig DS. Pharmacological Management of Gestational Diabetes Mellitus. Drugs. 2017;77:1723-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Mukherjee SM, Dawson A. Diabetes: how to manage gestational diabetes mellitus. Drugs Context. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 96. | Caritis SN, Hebert MF. A pharmacologic approach to the use of glyburide in pregnancy. Obstet Gynecol. 2013;121:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Mussa J, Meltzer S, Bond R, Garfield N, Dasgupta K. Trends in National Canadian Guideline Recommendations for the Screening and Diagnosis of Gestational Diabetes Mellitus over the Years: A Scoping Review. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 98. | Laredo-Aguilera JA, Gallardo-Bravo M, Rabanales-Sotos JA, Cobo-Cuenca AI, Carmona-Torres JM. Physical Activity Programs during Pregnancy Are Effective for the Control of Gestational Diabetes Mellitus. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 99. | Pandey K, Thurman M, Johnson SD, Acharya A, Johnston M, Klug EA, Olwenyi OA, Rajaiah R, Byrareddy SN. Mental Health Issues During and After COVID-19 Vaccine Era. Brain Res Bull. 2021;176:161-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 100. | Chandra PS, Shiva L, Nagendrappa S, Ganjekar S, Thippeswamy H. COVID 19 related Psychosis as an interface of fears, socio-cultural issues and vulnerability- case report of two women from India. Psychiatry Res. 2020;290:113136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | Correa-Palacio AF, Hernandez-Huerta D, Gómez-Arnau J, Loeck C, Caballero I. Affective psychosis after COVID-19 infection in a previously healthy patient: a case report. Psychiatry Res. 2020;290:113115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 102. | Moradian S, Teufel M, Jahre L, Musche V, Fink M, Dinse H, Schweda A, Weismüller B, Dörrie N, Tan S, Skoda EM, Bäuerle A. Mental health burden of patients with diabetes before and after the initial outbreak of COVID-19: predictors of mental health impairment. BMC Public Health. 2021;21:2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Fischer S, Morales-Suárez-Varela M. The Bidirectional Relationship between Gestational Diabetes and Depression in Pregnant Women: A Systematic Search and Review. Healthcare (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 104. | OuYang H, Chen B, Abdulrahman AM, Li L, Wu N. Associations between Gestational Diabetes and Anxiety or Depression: A Systematic Review. J Diabetes Res. 2021;2021:9959779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 105. | Minschart C, De Weerdt K, Elegeert A, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, Vercammen C, Maes T, Dufraimont E, De Block C, Jacquemyn Y, Mekahli F, De Clippel K, Van Den Bruel A, Loccufier A, Laenen A, Devlieger R, Mathieu C, Benhalima K. Antenatal Depression and Risk of Gestational Diabetes, Adverse Pregnancy Outcomes, and Postpartum Quality of Life. J Clin Endocrinol Metab. 2021;106:e3110-e3124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 106. | Wilson CA, Newham J, Rankin J, Ismail K, Simonoff E, Reynolds RM, Stoll N, Howard LM. Is there an increased risk of perinatal mental disorder in women with gestational diabetes? A systematic review and meta-analysis. Diabet Med. 2020;37:602-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 107. | Trinh NTH, Nordeng HME, Bandoli G, Eberhard-Gran M, Lupattelli A. Antidepressant and mental health care utilization in pregnant women with depression and/or anxiety: An interrupted time-series analysis. J Affect Disord. 2022;308:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 108. | Lin IH, Huang CY, Chou SH, Shih CL. Efficacy of Prenatal Yoga in the Treatment of Depression and Anxiety during Pregnancy: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 1020] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 110. | Tosto V, Meyyazhagan A, Alqasem M, Tsibizova V, Di Renzo GC. SARS-CoV-2 Footprints in the Placenta: What We Know after Three Years of the Pandemic. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |