Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1163
Peer-review started: February 28, 2023
First decision: May 8, 2023
Revised: May 19, 2023
Accepted: July 5, 2023
Article in press: July 5, 2023
Published online: August 15, 2023
Processing time: 164 Days and 1.6 Hours
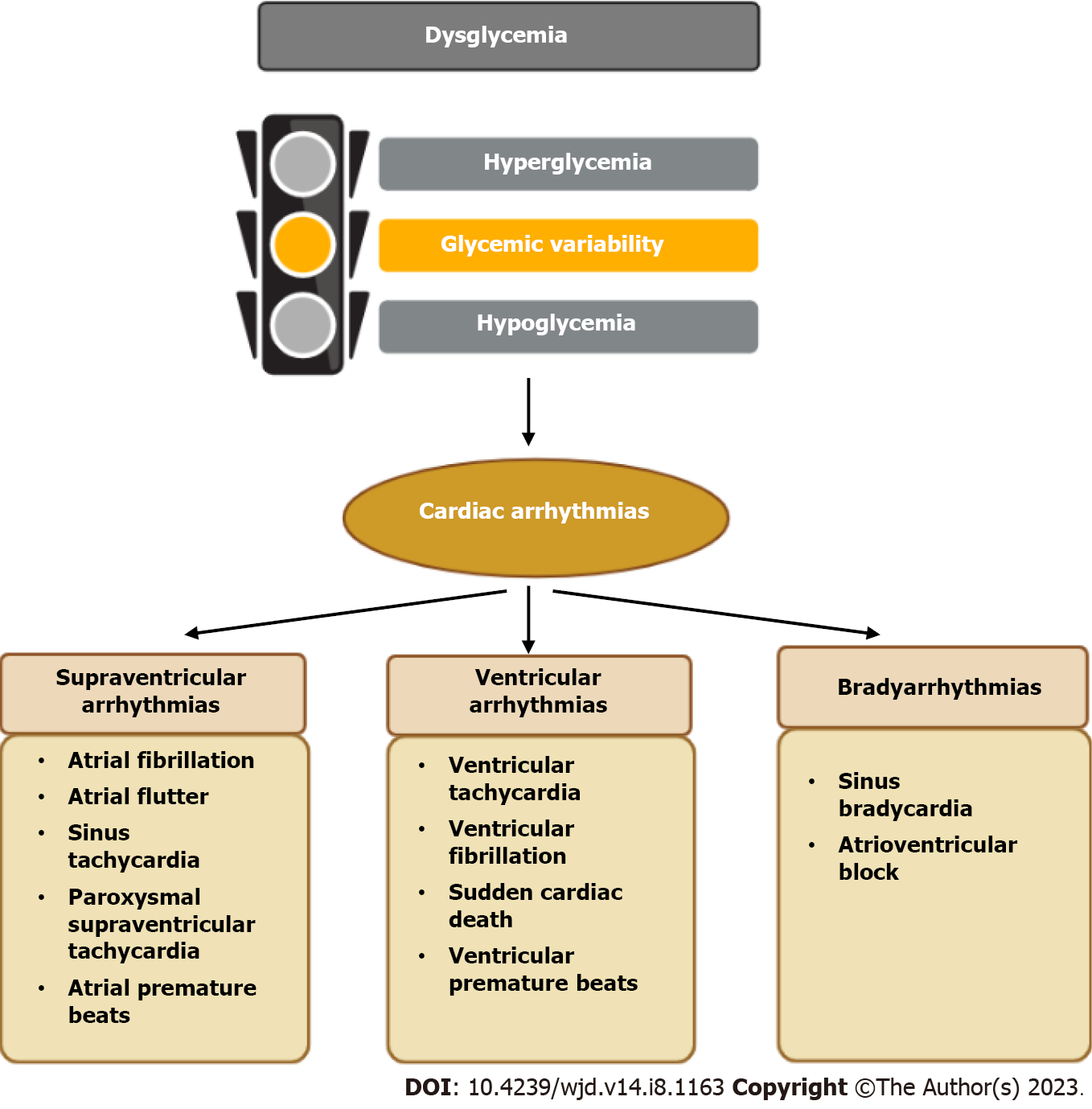
Disorders in glucose metabolism can be divided into three separate but interrelated domains, namely hyperglycemia, hypoglycemia, and glycemic variability. Intensive glycemic control in patients with diabetes might increase the risk of hypoglycemic incidents and glucose fluctuations. These three dysglycemic states occur not only amongst patients with diabetes, but are frequently present in other clinical settings, such as during critically ill. A growing body of evidence has focused on the relationships between these dysglycemic domains with cardiac arrhythmias, including supraventricular arrhythmias (primarily atrial fibrillation), ventricular arrhythmias (malignant ventricular arrhythmias and QT interval prolongation), and bradyarrhythmias (bradycardia and heart block). Different mechanisms by which these dysglycemic states might provoke cardiac arr-hythmias have been identified in experimental studies. A customized glycemic control strategy to minimize the risk of hyperglycemia, hypoglycemia and glucose variability is of the utmost importance in order to mitigate the risk of cardiac arrhythmias.
Core Tip: Different mechanisms by which these dysglycemic states might provoke cardiac arrhythmias have been identified in experimental studies. A customized glycemic control strategy to minimize the risk of hyperglycemia, hypoglycemia and glucose variability is of the utmost importance in order to mitigate the risk of cardiac arrhythmias.
- Citation: Sun DK, Zhang N, Liu Y, Qiu JC, Tse G, Li GP, Roever L, Liu T. Dysglycemia and arrhythmias. World J Diabetes 2023; 14(8): 1163-1177
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1163.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1163
Hyperglycemia, hypoglycemia, and glycemic variability (GV) represent three important domains of dysglycemia. Type 2 diabetes mellitus (T2DM) is a metabolic disease that is diagnosed mainly on the basis of sustained hyperglycemia, which has been associated with a number of adverse health outcomes, such as coronary artery disease, stroke and cardiac arrhythmias[1-3]. Epidemiologic studies have demonstrated that the incidence of many diabetic complications is directly associated with the degree of hyperglycemia[1]. However, overcorrection of hyperglycemia may lead to episodes of hypoglycemia, then increasing the risk of fatal adverse events, and that has been observed in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. The ACCORD study was terminated early due to the observation of significant increased mortality in the intensive glycemic control group, and the majority of excess deaths in this group has been attributed to either unexpected deaths or witnessed deaths due to arrhythmias[1]. Glycemic variability is an integral component of glucose homoeostasis, which can represent the presence of excess glycemic excursions, namely the hyperglycemic spikes and hypoglycemic incidents[4]. Increased GV has been linked to the development of cardiac arrhythmias, including atrial fibrillation (AF) and ventricular tachy-arrhythmias[5,6]. High GV appears to exert more detrimental effects than persistent hyperglycemia on the pathogenesis of diabetic complications[7,8], and has also been associated with an increased risk of cardiac arrhythmias compared to those with good glycemic control[9]. Overall, each of these three dysglycemic states has been associated with an increased risk of certain types of cardiac arrhythmias. Therefore, in this review, we explore the epidemiology of three dysglycemic states and arrhythmias, identify potential mechanisms, consider what additional research is required, and suggest how the issue might be approached in clinical practice (Figure 1).
This review focused on the associations between hyperglycemia, hypoglycemia, and GV with cardiac arrhythmias, which was implemented within databases of PubMed and EMBASE, from April 1975 to November 2022. The literature search was conducted using the following keywords: “hyperglycemia”, “hypoglycemia”, “glycemic variability”, “glucose variability”, “glucose fluctuation”, “dysglycemia”, “diabetes”, “type 2 diabetes”, “type 1 diabetes”, “arrhythmia”, “tachycardia”, “bradycardia”, “premature beat”, “ectopic”, “flutter”, “fibrillation”, “atrioventricular block”, “sudden cardiac death”, and “QT prolongation”. Studies that focused on the associations between three dysglycemia domains with cardiac arrhythmias were included in this review, without study design restriction. Duplicate records and studies without full-text access were excluded. Two reviewers (D.S. and N.Z.) independently conducted the literature search and study selection, and discrepancies were resolved by a third author (T.L.).
Hyperglycemia mainly includes impaired fasting blood glucose (IFG), impaired glucose tolerance (IGT), and overt diabetes[10]. IFG was defined as a fasting plasma glucose (FPG) level between 110 mg/dL and 125 mg/dL, according to the 2006 World Health Organization guidelines. IGT was defined as FPG < 126 mg/dL with 2-h plasma glucose after a 75-g oral glucose challenge of 140-199 mg/dL. Patients with DM were defined as those with a history of physician-confirmed diabetes or history of oral hypoglycemic agents or insulin use[10]. Hypoglycemia is defined as blood glucose concentration less than 70 mg/dL[11,12]. Glycemic variability refers to intraday or daily blood glucose fluctuation, and months or years of blood glucose fluctuation. At present, the definition of GV is very vague, and it is mainly measured by indicators such as mean blood glucose and standard deviation, J index and coefficient of variation, postprandial hyperglycemia and mean amplitude of glucose excursion[7].
A total of 1929 records were identified, after excluding duplicates (n = 509) and those irrelevant (n = 1296), 124 studies were included in this review. An overview of studies included in this review is showed in Supplementary Tables 1-3.
Chronic hyperglycemia is a hallmark and remains one of the most important pathophysiologic features of DM. Previous studies have observed multiple types of cardiac arrhythmias in patients with diabetes, including supraventricular and ventricular arrhythmias[2]. An overview of studies evaluating the association between hyperglycemia and cardiac arrhythmias is provided in Supplementary Table 1.
Studies have found that patients with diabetes are more likely to develop AF, atrial flutter, and paroxysmal supraventricular tachycardia than those without diabetes[13,14]. Current researches on hyperglycemia and sup-raventricular arrhythmias have mainly focused on the association between abnormal state of glucose metabolism, including IFG, IGT, and DM, with the risk of AF[13,15,16].
Hyperglycemia and AF: Epidemiology of hyperglycemia-related AF: A cross-sectional study found a two-fold increased risk of incident non-valvular AF amongst individuals with an elevated blood glucose levels compared to those with normal glucose levels[17]. IFG has also been identified as a risk factor for AF in healthy Asian populations[15]. In patients with IGT, FPG but not progression to diabetes is one of the predictors for AF, and a one mmol/L increase in baseline FPG has been associated with a 33% increased risk of AF[16]. As for the overt DM, numerous studies have shown that diabetes is associated with an increased risk of AF and has been considered a risk factor for AF in healthy individuals and hospitalized patients[13,18,19]. Diabetes is included in the CHA2DS2-VASc, CHARGE-AF, and MR DASH scores which have been established to predict the chances of developing AF[20,21]. A relevant meta-analysis showed that the risk of AF was 34% higher in patients with DM than non-DM patients[22]. In addition, hyperglycemia is also associated with the development of AF in some certain clinical settings. A retrospective cohort study with an average 11-year follow-up has identified that fasting hyperglycemia was independently correlated with new-onset AF in patients with acute myocardial infarction (AMI)[23]. Moreover, another retrospective analysis suggested that stress hyperglycemia is associated with an increased prevalence of AF in AMI[24]. A prospective study has demonstrated that tighter glycemic control in diabetic coronary artery bypass grafting (CABG) patients is associated with a lower incidence of perioperative AF[25].
Hyperglycemia or DM could not only increase the incidence of AF but also affect the prognosis of patients with AF. Studies have shown a significant correlation between admitted blood glucose levels and increased mortality of hospitalized patients with AF[26]. Moreover, diabetes has been considered a significant predictor of ischemic stroke, major bleeding, and heart failure in patients with non-valvular AF[27]. In AF patients with DM, a positive linear correlation has been identified between glycated hemoglobin (HbA1c) levels with all-cause and cardiovascular mortality[28]. However, a previous study has reported that there was no significant association between diabetes and future hospitalization for AF[29]. The different results may be related to population differences and different study designs. Overall, hyperglycemia has a significant impact on the prognosis of AF, therefore, glycemic control should be paid more attention among patients with AF.
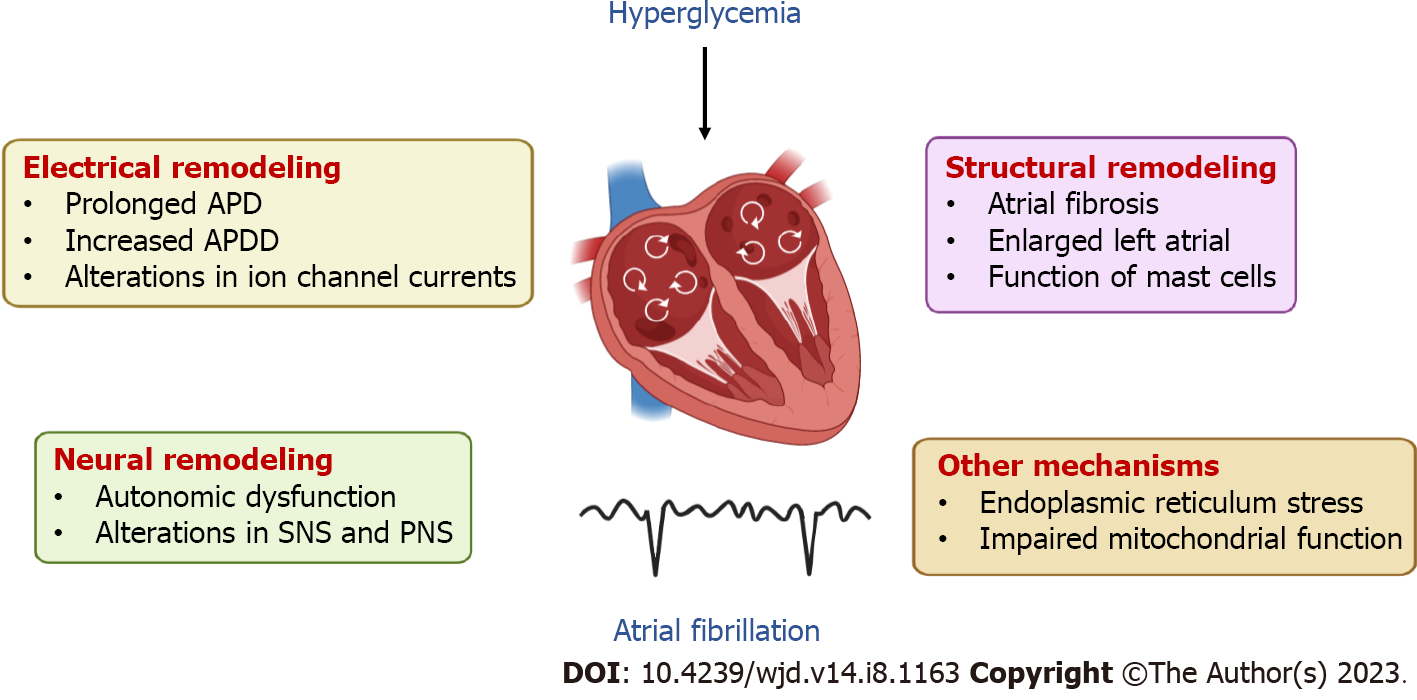
Mechanisms of hyperglycemia-related AF: Electropathology is identified as the underlying cause of AF. Abnormal electrical activity of pulmonary veins and non-pulmonary veins trigger foci is a significant mechanism of AF, which causes electrical, structural and neural remodeling. Patients with AF and abnormal glucose metabolism have significantly longer total activation times and lower atrial voltages in the left and right atrial than those without. Moreover, patients with abnormal glucose metabolism are more likely to have greater chances of AF relapse after catheter ablation[30]. Therefore, as the main character of abnormal glucose metabolism, hyperglycemia may change the atrial substrate to some extent, which increases the susceptibility to the occurrence and maintenance of AF[30].
Electrical remodeling: Accumulating evidence from basic studies has suggested that the effect of hyperglycemia on the atrium was related to electrical remodeling[31]. Prolonged action potential duration (APD) and increased APD dispersion (APDD) were observed in animal models of type 1 diabetes (T1DM), indicating increased susceptibility to AF and difference of refractoriness in atrium[32,33]. Furthermore, the densities of Na+ current (INa) were reduced and the densities of L-type Ca2+ channel (ICa-L) were increased in the atrial of alloxan-induced diabetic rabbits[32]. In rat models of T2DM, similar prolongation of APD and increased susceptibility to AF were observed[34,35]. However, atrial myocytes isolated from Zucker diabetic fatty rats had decreased current densities of transient outward K+ current (Ito), ultrafast delayed rectifier K+ current (IKur) and ICa-L[34]. The different changes in densities of ICa-L in these studies may be related to the type of model[32,34]. Additionally, the ion channel protein (Kv4.3, Kv1.5 and Cav1.2) expression in HL-1 cells treated with advanced glycation end products was significantly down-regulated, indicating that hyperglycemia can directly affect the electrical remodeling of cardiomyocytes through alterations in ion channels[34].
Structural remodeling: Fibrosis is a major feature of the atrial structural remodeling[31]. A large body of studies have demonstrated that hyperglycemia contributes to atrial interstitial fibrosis in animal models[32,34-36]. Transforming growth factor beta 1, the main profibrotic cytokine, presented with an increased expression in the atrium of diabetic rabbits[32]. It also has been shown that diabetic rats have significantly enlarged left atria[34,37]. Furthermore, Studies have shown that mast cells could contribute to the development of AF by enhancing inflammation and fibrosis in diabetic mice, and AF can be attenuated by the deletion of mast cells[38].
Neural remodeling: A prospective study has shown that there was a significant association between reduced heart rate recovery and new-onset AF in patients with T2DM, suggesting that autonomic dysfunction may play an important role in DM-related AF[39]. In a previous animal study, sympathetic nerve stimulation significantly increased the incidence of AF in streptozotocin (STZ)-induced DM rats, while parasympathetic nerve stimulation increased the risk of AF both in the DM rats and control rats[40]. Besides, both the stimulation of sympathetic nerve and parasympathetic nerve decreased the atrial effective refractory period, and the former also increased atrial effective refractory period heterogeneity in DM rats[40]. These alterations in the autonomic nervous system are likely to be associated with the increased incidence of AF in diabetes.
Other mechanisms: Cellular researches have showed that hyperglycemia could induce endoplasmic reticulum stress in atrial cardiomyocytes, in which mitofusin-2 (Mfn-2) plays a major role[41]. Mfn-2 downregulation was found to prevent mitochondrial Ca2+ overload-mediated mitochondrial dysfunction and subsequent cardiomyocyte death[41]. Furthermore, clinical studies have found that the maximum ability to oxidize fatty acids and glutamate of myocardial mitochondria is impaired, and mitochondrial H2O2 release is increased in patients with T2DM. Disruption of myocardial oxidative balance and mitochondrial metabolism may affect the normal function of the atrium[42], which may also contribute to the development of AF (Figure 2).
Based on these mechanisms, some upstream therapies have been proposed to prevent the development of AF in patients with diabetes. For example, the sodium glucose co-transporter-2 inhibitor (SGLT2i), empaglifozin, can ameliorate atrial structural and electrical remodeling as well as improve mitochondrial function in T2DM animal models[43]. In addition, Lee et al[44] have confirmed the benefit of SGLT2i in prevention of incident AF among T2DM patients, compared with dipeptidyl peptidase-4 inhibitor (DDP-4i). These findings consistently suggested that SGLT2i may play an important role in the prevention of T2DM-related AF. Apart from SGLT2i, thiazolidinedione[37,45] and allopurinol[36] have also been demonstrated to have protective effects on prevention of incident AF in patients with diabetes. However, a prior populationbased study has observed that sulphonylurea use was associated with a higher risk of incident AF compared to metformin in T2DM patients, especially in males and those older than 65 years[46]. Future large-scale studies are needed to optimize the prevention strategy of DM-related AF, especially in higher risk population.
Hyperglycemia and other supraventricular arrhythmias: Unlike AF, evidences regarding the association between hyperglycemia and other supraventricular arrhythmias are less frequently reported. Agarwal et al[2] have conducted a cross-sectional study including 100 patients of T2DM, and found that sinus tachycardia is one of the common arrhythmias in T2DM patients, with a prevalence of 32%. In addition, previous studies also suggested that the risk of symptomatic paroxysmal supraventricular tachycardia[14] and atrial flutter[13] was higher among DM patients, in relation to the non-DM population.
Hyperglycemia and ventricular arrhythmias: Hyperglycemia or diabetes has been associated with a higher risk of ventricular arrhythmias and sudden cardiac death (SCD) in some studies. Besides, as a well-established marker for ventricular arrhythmia, QT prolongation has also been observed in hyperglycemic settings[47-49].
Ventricular tachycardia, ventricular fibrillation, and SCD: A retrospective study has shown that HbA1c levels are associated with spontaneous ventricular tachycardia (VT) in high-risk male patients, independently of QT interval duration[50]. Moreover, another study also suggested that hyperglycemia on admission was significantly associated with early VT after myocardial infarction[51]. As for ventricular fibrillation (VF), Movahed et al. have observed that the prevalence of VF in patients with T2DM was significantly increased after adjusting for potential confounders[52]. Additionally, in a prior prospective study conducted among women in the United States, diabetes significantly increased the risk of SCD[53]. Another study also showed that the risk of sudden cardiac arrest was significantly reduced in DM patients taking antidiabetic medications than those without, indicating that hyperglycemia may play an important role in sudden cardiac arrest[54,55].
QT prolongation: Using The Third National Health and Nutrition Examination Surveys (NHANES III), Brown and colleagues have found that there was a 1.2-fold increased risk of developing QTc prolongation in patients with IFG than those with normal glucose tolerance (NGT)[56]. Meanwhile, patients with established diabetes experienced a 1.6-fold increased risk of QTc prolongation than those with NGT[56]. Another cross-sectional study also suggested that mean blood glucose level is an independent risk factor for QTc prolongation[57]. In addition to the observational studies mentioned above, there are many experimental studies investigating the relationship between hyperglycemia and QT interval. Through hyperglycemic clamp tests, studies have found that acute hyperglycemia increased the QTc interval in healthy individuals and patients with T1DM[58,59]. Besides, hyperglycemia has also been associated with increased QTc dispersion (QTd) among healthy individuals, which reflects the inhomogeneity of myocardial repolarization[58]. However, a prospective study (n = 26) failed to observe a significant change in QTc or QTd among patients with newly diagnosed T2DM[60], which may be due to the small sample size, short follow-up duration and unknown confounding factors.
Mechanisms of hyperglycemia-related ventricular arrhythmias: Alterations of ion channels: Studies have found that diabetes prolongs cardiomyocyte APD, and changes in APD are mainly due to alterations in ion channels[61,62]. Meo et al[61] have observed that hyperglycemia in STZ-treated mice was associated with prolongation of the QT interval, enhanced temporal dispersion of electrical recovery, and susceptibility to ventricular arrhythmias, compared with controls. According to Meo et al[61], the density of voltage-gated K+ current (Kv) currents were decreased in STZ myocytes, in comparison to cells from normoglycemic mice. In another study using diabetic rabbits model, the inhibition of rapid delayed rectifier K+ current (IKr) was found to be a major ionic factor in APD prolongation[62]. However, in diabetic rats model, researchers have suggested that the DM-related APD prolongation was mainly due to the reduction in Ito[63,64].
Compared with the control group, the density of Na+ current in ventricular myocytes of diabetic rabbits and HEK-293 T cells exposed to high glucose was reduced[65,66]. Reduced INa amplitude also has been shown to be a key determinant of diabetic ventricular conduction impairment[65]. Furthermore, studies on posttranslational modification have found that hyperglycemia increases the O-GlcNAcylation of cardiac Nav1.5 expression, resulting in abnormal Nav1.5 expression and distribution, as well as alteration of QT interval[66]. Studies have shown conflicting results on the alterations of ICa-L in DM animal models[61,62,67]. Further research is needed to investigate the association between hyperglycemia and alterations of cardiac calcium channels.
Connexins: As a component of gap junctions, play crucial roles in electrical coupling between cardiomyocytes and affect myocardial electrical propagation velocity and coordinated contraction[68]. The connexin 43 (Cx43) isoform is predomainantly expressed in ventricles. Researches in diabetic animal models have observed an association between enhanced Cx43 phosphorylation mediated by ε-PKC with a decrease in myocardial conduction velocity[69,70]. However, other studies also suggested that hyperglycemia promotes the nitrification of Cx43 tyrosine rather than phosphorylation[71]. Moreover, a study has shown that the content of total Cx43 increases in short-term diabetic rat hearts and does not significantly change in long-term diabetic rat hearts, while others showed the opposite result[69,70,72]. This may be related to the different modeling time and measurement methods.
Oxidative stress: Oxidative stress is involved in hyperglycemia-related ventricular arrhythmias through multiple pathways. The excess reactive oxygen species (ROS) induced by hyperglycemia in cardiomyocytes impaired the function of the ion channel[73,74]. Previous studies have shown that hyperglycemia induces sarcoplasmic reticulum Ca2+ release events and downregulates the functional expression of most K+ channels through Calcium/calmodulin-dependent protein kinase II (CaMKII) activation[75,76]. The properties of INa are also found to be altered by oxidative stress[77]. Oxidative modification of tyrosine-mediated signaling may play an important role in the mechanism of Cx43 alteration in diabetes[71]. Additionally, glutathione oxidation enhances APD heterogeneity and increases the arrhythmia score index in diabetic animal models[78].
Hormones: Hyperglycemia causes alterations in the levels of hormones in vivo, which are involved in the pathogenesis of arrhythmias. A significant negative correlation was observed between serum-free thyroxine and glucose concentration[79]. Thyroid hormone reduced the expression of ε-PKC in non-diabetic rat hearts, resulting in the downregulation of Cx43, which is related to increased myocardial conduction and arrhythmia susceptibility[69]. Moreover, it has been found that reduction of the ventricular fibrillation threshold in diabetes is associated with altered sympathetic activity in myocardium, and there was a significantly greater decline of ventricular fibrillation threshold in response to epinephrine infusion in diabetic dogs[80].
Studies have shown an increased incidence of cardiac conduction abnormalities in patients with diabetes[81]. Right bundle branch block, bifascicular block and high degree atrioventricular block (AVB) are more likely to occur in diabetic patients[81]. It has been found that T2DM is independently associated with third-degree AVB in multivariable model[82,83]. In addition, the prevalence of diabetes in patients with pacemakers is six to ten times higher than the general population due to severe bradycardiac arrhythmias[84].
Hypoglycemia and cardiac arrhythmias: Hypoglycemia is a frequent and feared adverse effect among individuals treated with insulin and insulin secretagogue drugs. The average incidence of mild hypoglycemia is 1-2 episodes per patient per week in T1DM and 0.3-0.7 episodes per patient per week in insulin-treated T2DM[85]. It has been reported that the insulin-mediated hypoglycemic events account for approximately 100000 emergency department visits per year in the United States[86]. A pivotal report by Tattersall et al[87] first presented evidence that hypoglycemia was implicated in the sudden overnight death of young individuals with T1DM. This mode of death has been described as the dead-in-bed syndrome since then, and thought to be caused by fatal hypoglycemia-induced arrhythmias during sleep. Generally, hypoglycemia can affect cardiac repolarization and cardiac electrophysiology[88], inducing various types of arrhythmias, which has been considered as a proarrhythmic event. Information about the studies regarding the association between hypoglycemia and cardiac arrhythmias is presented in Supplementary Table 2.
Hypoglycemia and supraventricular arrhythmias: Several types of supraventricular arrhythmias have been observed during hypoglycemia, including sinus tachycardia[89], atrial premature beats[90,91], AF[92-94], and non-paroxysmal atrioventricular junctional tachycardia[95].
Hypoglycemia and AF: Hypoglycemia-induced AF was first reported in nondiabetic patients undergoing insulin shock therapy for some psychiatric illnesses[96]. Several other case reports have also observed the epidodes of AF during hypoglycemia in both diabetics and nondiabetics, which reverted to sinus rhythm after intravenous dextrose[93,97]. In a recent prospective study, 21 insulin-treated T2DM patients were monitored with continuous glucose monitoring (mean ± SD, 118 d ± 6 d) and an implantable cardiac monitor for a one-year follow-up. The researchers have found that the time spent in hypoglycemia was higher during nighttime than daytime, and the AF accounted for 22% of episodes of potentially clinically significant arrhythmias[92]. In another nationwide population-based Korean study, 1509280 participants with T2DM and free of baseline AF were included. After a mean follow-up of 8.5 years, the incidence of AF was significantly higher in patients with severe hypoglycemia than those without, where the severe hypoglycemia was defined as any hypoglycemic events requiring the assistance of another person to actively administer carbohydrates, other corrective actions, hospitalization, or medical care. This study also observed that previous severe hypoglycemia was a significant risk factor for the development of AF after adjusting for potential confounders[94]. The association between hypoglycemia and AF is not limited to DM patients. Humos et al[98] have assessed the impact of hypoglycemia in patients with ST-elevation myocardial infarction (STEMI) using the National Inpatient Sample database, and found that the risk of AF was significantly higher in STEMI patients with in-hospital hypoglycemia, compared to those without hypoglycemia.
These case reports and clinical studies suggest an association between hypoglycemia and AF. However, the animal study exploring the underlying mechanism of hypoglycemia-induced AF is still scarce. Vardas et al[99] observed that the incidence of induced AF, sustained AF (> 3 min), and the susceptibility to AF was significantly higher in the hypo-glycemic group than the normoglycemic and hyperglycemic groups in ex vivo dog models. According to the Vardas et al[99], the refractory period of the atrium was significantly shorter under hypoglycemia, which might be due to the hypercatecholaminemia in hypoglycemic settings and then contribute to the profibrillatory electrophysiologic changes. Future basic studies are needed to elucidate the mechanisms of hypoglycemia-induced AF.
Hypoglycemia and other supraventricular arrhythmias: Increased heart rate is usually an initial electrocardiographic manifestation caused by hypoglycemia. In an interventional study where 119 individuals underwent experimentally-induced hypoglycemia, the mean heart rate increased from 62.2 bpm ± 9.6 bpm at baseline to 70.6 bpm ± 11.7 bpm during hypoglycemia, and recovered to baseline after oral ingestion of glucose[100]. Individuals may develop sinus tachycardia if the hypoglycemia is not corrected promptly, as seen in an animal study[89]. Atrial premature beats is also one of the common arrhythmias among patients with hypoglycemia, and has been reported to have a nearly fourfold higher risk during nocturnal hypoglycemia, compared with euglycemia[90]. There have been sporadic case reports of other supraventricular arrhythmias. For example, Pezzarossa and colleagues have reported a case of a non-diabetic patient presented with non-paroxysmal atrioventricular junctional tachycardia during postprandial hypoglycemia, and the sinus rhythm was promptly restored with the correction of hypoglycemia[95].
Hypoglycemia and ventricular arrhythmias: Compared with supraventricular arrhythmias, much interest has been focused on the potential for hypoglycemia to cause dangerous and life-threatening ventricular arrhythmias. The hypoglycemia-induced ventricular arrhythmias and related electrophysiological events include ventricular premature beats (VPBs)[90,91,101], QT prolongation[89,100-102], VT[103,104], VF[98], and SCD[1].
Epidemiology of ventricular arrhythmias during hypoglycemia: Hypoglycemia-induced VPBs have been observed both in the spontaneous hypoglycemia[90] and experimentally-induced hypoglycemia settings[101], and among individuals with T1DM[91], T2DM and non-diabetes[101]. Although being a less dangerous arrhythmia, more attention should be paid to the occurrence of VBPs, especially in DM patients, which could facilitate the early identification of the onset of hypoglycemia. Moreover, the presence of VPBs has been shown to be associated with a significantly increased risk of SCD in middle-aged men[105].
QT prolongation: Consistent evidence from different types of studies have established that hypoglycemia could induce QT prolongation. Profound hypoglycemia has been observed to cause significant QT prolongation in animal models[89,102]. Corresponding to these basic experiments, hypoglycemia causes longer QT intervals in humans, irrespective of the diabetes status. In an experimentally-induced hypoglycemic study, the insulin-treated T2DM patients and matched controls underwent a sequential hyperglycemic and hypoglycemic clamp, both groups experienced progressively increased QTc interval (corrected by Fridericia’s formula) prolongations during hypoglycemia[101]. In another interventional study enrolling 119 individuals underwent routine insulin-induced hypoglycemia testing for clinically suspected pituitary dysfunction, Kacheva et al[100]. observed that the QTc (Bazett’s formula) increased from 415.1 ms ± 21.9 ms at baseline to 444.9 ms ± 26.5 ms during hypoglycemia, accompanied by a significant increase of QT dispersion (QTd). In addition, Fitzpatrick et al[106] also provided pooled evidence in their meta-analysis which supported the association between hypoglycemia and QTc prolongation among patients with T1DM and T2DM. Besides the severity of hypoglycemia, the time of onset also plays an important role in the development of QTc prolongation. Tsujimoto et al[107] retrospectively assessed the ECG characteristics among patients presenting to the emergency department with hypoglycemia, and found that the incidence of abnormal QT prolongation during severe hypoglycemia was significantly higher in the night-time, particularly in the early morning. In addition, both the depth and duration of hypoglycemia have been associated with the severity of QT prolongation[90,108].
It is well recognized that prolongation of the QT interval can lead to life-threatening arrhythmias such as torsades de pointes (TdP). Thus, monitoring of the QT interval is critical in patients with hypoglycemia. Duration of the QT interval naturally varies inversely with heart rate; hence, a corrected QT (QTc) that is adjusted for heart rate is most predictive of proarrhythmic potential and has been widely used in clinical practice. However, key aspects of QTc monitoring lack standardization in some clinical settings, including hypoglycemia[109]. In a previous study where 21 T1DM patients underwent continuous glucose and ECG monitoring for 72 h, the researchers have confirmed previous findings of prolonged QTc corrected by Bazett’s formula during spontaneous hypoglycemia, but not by Fridericia’s formula and the nomogram method, which suggested that Bazett’s formula might result in overcorrection of QTc while both Fridericia’s formula and the nomogram method might undercorrect the QTc during hypoglycemia[110]. Therefore, future studies are needed to standardize QTc monitoring in hypoglecemia settings.
VT, VF, and SCD: A causal relationship between hypoglycemia and fatal arrhythmias is usually difficult to demonstrate in clinical practice, since simultaneous monitoring of cardiac rhythm and blood glucose levels is seldom undertaken, even in intensive-care settings[85]. Chelliah et al[103] have reported a case of a frail old man who developed VT intraoperatively with a random blood glucose level of 2.9 mmol/L, which was aborted immediately on correction of hypoglycemia. In another cohort study, 94 patients with T2DM and established cardiovascular disease underwent concomitant continuous glucose monitoring and Holter monitoring for five days. It was found that patients experiencing episodes of hypoglycemia had a significantly higher number of VT than those without[104]. Using the National Inpatient Sample database, Humos et al[98] have observed a significantly higher risk of ventricular fibrillation (OR, 1.80; 95%CI, 1.41-2.30) and cardiogenic shock (OR, 1.72; 95%CI, 1.39-2.13) among patients hospitalized with STEMI and complicated with hypoglycemia, in relation to those without in-hospital hypoglycemia. In the ACCORD trial, the incidence of hypoglycemia requiring assistance was over three times higher in the intensive control group. Furthermore, the intensive glycemic control resulted in a significant increase in all-cause mortality, mainly driven by a 35% increase in cardiovascular mortality compared with the standard therapy, which led to an early termination of the trial. The majority of excess deaths in the intensive treatment group of ACCORD were caused by cardiac events, the most common of which was unexpected, that is, sudden death[1].
Mechanisms of hypoglycemia-related ventricular arrhythmias: The mechanism of hypoglycemia-induced ventricular arrhythmias is multifactorial, involving the inhibition of cardiac ion channel, altered levels of electrolytes (potassium) and hormones (epinephrine and norepinephrine), and also related to the underlying diseases of the patient. First, hypoglycemia itself inhibits the rapid component of the cardiac delayed rectifier K+ current (IKr), which is one of the main repolarizing K+ channels in human myocytes and encoded by human ether-a-go-go-related gene (HERG)[74]. Blockade of the IKr leads to a longer action potential, then causes QT interval prolongation, which is recognized as a marker of the propensity to develop early afterdepolarizations and VT[111]. Second, the development of hypokalemia during hypoglycemia has been observed both in the basic study and clinical study[89,100]. Hypokalemia could prolong the QT interval, and promote the development of calcium overload and associated electrical instability (early and delayed afterdepolarizations). Third, the catecholamine surge induced by hypoglycemia plays an important role in the development of ventricular arrhythmias. Plasma epinephrine and norepinephrine significantly increased during severe hypoglycemia[89,100], which may lower serum potassium[112], cause QT prolongation, calcium overload, early and delayed afterdepolarizations[113]. Fourth, the underlying diseases, such as left ventricular hypertrophy, myocardial infarction, autonomic neuropathy and diabetes, may reduce the tolerance of myocardial tissue for the further proarrhythmic action of hypoglycemia, as described in a review by Nordin[113]. Fifth, both the clinical and experimental evidence suggests that hypoglycemia can cause ischemia of myocardial tissue, which is known to be highly proarrhythmic. Libby et al[114] have observed that the size of myocardial infarctions in dogs with hypoglycemia was larger than those with normoglycemia. Consistently, Desouza et al[115] also confirmed that hypoglycemia is more likely to be associated with cardiac ischemia and symptoms than normoglycemia and hyperglycemia in humans.
Hypoglycemia and other arrhythmias: Apart from tachyarrhythmias, bradyarrhythmias induced by hypoglycemia, such as bradycardia and AVB, also have been observed in preclinical and clinical studies. In a previous study where 25 insulin-treated T2DM patients underwent 5 d of simultaneous Holter and continuous interstitial glucose monitoring, the researchers found that bradycardia was eightfold higher during nocturnal hypoglycemia compared with euglycemia. However, during the daytime, no bradycardia was observed in this study[90]. Other case reports have also noted bradycardia during hypoglycemic episodes[116,117]. Bradycardia then could cause action potential and QT interval prolongation, and increase the risk of early afterdepolarizations. According to the Reno et al[89], different types of AVB, from first- to third- degree, have been observed during hypoglycemia in rat models.
Bradycardia is more likely to be induced by nocturnal hypoglycemia, which may be explained by sympathetic withdrawal followed by vagal overcompensation. Chow et al[90] have observed an initial vagal withdrawal during hypoglycemia resulting in an increase in heart rate, but with more prolonged hypoglycemia, vagal reactivation then resulted in relative bradycardia.
GV includes short-term variability and long-term variability in blood glucose[118]. Short-term GV refers to intraday or daily blood glucose fluctuation, and long-term GV refers to months or years of blood glucose fluctuation, which is supposed to predict complications of diabetes[119,120]. Currently, only a few studies have investigated the relationship between GV and arrhythmias, which mainly focused on AF and QT prolongation. Information about the studies regarding the association between the GV and cardiac arrhythmias is presented in Supplementary Table 3.
Glycemic variability and AF: Epidemiology of GV-related AF: A retrospective cohort study with a median follow-up of 6.9 years has found that higher HbA1c variability was associated with an increased risk of new-onset AF in patients with T2DM, which indicated that long-term GV has the potential to be one of the early predictors of new-onset AF[9]. By measuring the HbA1c variability score, another retrospective cohort study including 27246 subjects in Taiwan also showed that high GV was independently associated with the occurrence of new-onset AF in patients with T2DM[121]. Both the HbA1c variability score and HbA1c variability are measures of long-term GV, and these two studies have suggested the predictive value of long-term blood glucose fluctuation in the development of incident AF[9,121]. However, the effect of short-term fluctuation in blood glucose on AF has rarely been studied. More research is needed to investigate the relationship between short-term GV and AF.
The relationship between GV and AF has also been observed in patients with underlying cardiovascular diseases and in those undergoing cardiac surgery. Xia et al[122] have conducted an observational study enrolling 864 patients with acute coronary syndrome, and found that higher GV during hospitalization was associated with a higher incidence of AF, compared to the lower GV group. Furthermore, a retrospective study on patients undergoing CABG showed that every 10% increased in GV in the first 24 h after surgery, the risk of postoperative AF (POAF) increased by 16%[123]. Another Singapore study also showed that wider perioperative glycemic fluctuations in patients undergoing CABG represented an independent risk factor of POAF[124].
In general, studies on the association between GV and AF, especially in healthy people and patients with T1DM, are relatively deficient. Different measures of blood glucose fluctuation have their own advantages. More research is needed to explore the relationship between GV and AF in different populations and evaluate the effect of different measures of GV on this relationship.
Mechanisms of GV-related AF: Cardiac fibrosis: In a prior study evaluating the association between glucose fluctuations and AF in diabetic rats, Saito et al[125] have induced the glucose fluctuations by fasting for 24 h and additional regular insulin injections, and observed that the degree of myocardial fibrosis in DM rats with glucose fluctuations was significantly increased compared with the uncontrolled or controlled DM group. Moreover, the interatrial conduction time in glucose fluctuation group was significantly longer than the other two groups, and the rate of AF induction was also the highest, which suggested that higher GV increased the incidence of AF by promoting cardiac fibrosis. In another similar study, where the glycemic fluctuations was induced by subcutaneous injection of insulin and intraperitoneal injection of glucose, the researchers found that glycemic fluctuations worsened myocardial fibrosis in diabetic rats[126].
Oxidative stress: A prior basic study has found that the level of ROS significantly increased in the myocardium of DM rats with glucose fluctuations, compared to the controls. Meanwhile, this study also suggested that elevated ROS levels caused by upregulation of thioredoxin-interacting protein (Txnip) may be a mechanism of glucose fluctuations-induced fibrosis[125]. In another study, Ying et al[126] have demonstrated that blood glucose variability can aggravate heart tissue fibrosis, possibly involving oxidative stress by inhibiting protein kinase B (AKT) signaling path.
Consistent with these basic experiments, clinical studies also confirmed the role of oxidative stress in the development of GV-related AF. Chang and colleagues evaluated the relationship between GV and oxidative stress markers among 34 T2DM patients, and found that both the short-term GV measure (mean amplitude of glycemic excursions, MAGE) and long-term GV measure (standard deviation of HbA1c) were positively associated with the level of plasma 8-iso-prostaglandin F2α (8-iso-PGF2α), a marker of oxidative stress[127]. In another case-control study, a more accurate measurement of urinary 8-iso-PGF2α excretion was used to represent the oxidative stress level in vivo, which also confirmed the previous findings[128]. Similarly, another cross-sectional study has included 68 patients with T2DM, where the plasma oxidant capacity was measured with the diacron-reactive oxygen metabolites test, the researchers found that higher levels of daily and day-to-day GV was associated with an increased level of oxidative stress[129]. Oxidative stress plays a substantial role in the cardiac electrical and structural remodeling, increasing the susceptibility to AF, which might represent an important mechanism of GV-related AF.
Autonomic neuropathy: Abnormal autonomic activity is generally recognized to play an important role in the development and maintenance of AF[130]. A study has showed a significant correlation between MAGE and cardiac autonomic neuropathy in patients with newly diagnosed T2DM[131]. Other cross-sectional studies have also shown that GV in patients with T2DM is negatively correlated with baroreflex sensitivity and heart rate variability parameters[132,133]. These studies suggested that GV-mediated abnormal autonomic activity might contribute to the development of AF.
Glycemic variability and other supraventricular arrhythmias: Zhang et al[134] evaluated the association between GV and arrhythmia in middle-aged and elderly T2DM patients (n = 107), and found that compared with the middle-aged group, elderly patients have greater GV and are more likely to develop atrial premature beat, couplets of atrial premature beat and atrial tachycardia.
Epidemiology of GV-related ventricular arrhythmias: Studies reporting the asso
Mechanisms of GV-related ventricular arrhythmias: Overall, the effects of GV on ventricular arrhythmias are similar to those of AF, to some extent. Firstly, higher GV exacerbates left ventricular fibrosis in diabetic rats, which might increase the predisposition to ventricular arrhythmias[125]. Secondly, oxidative stress plays an important role in the GV-related ventricular fibrosis[126]. Oxidative stress associated with GV observed in clinical studies may also potentially cause ventricular remodeling[127-129]. Thirdly, cardiac autonomic neuropathy induced by higher GV may also contribute to the development of ventricular arrhythmias[136].
In this study, although we have applied a comprehensive search strategy using relevant keywords regarding the associations between three dysglycemic states with cardiac arrhythmias, it is possible that we missed some data, because we did not search the grey literature or the google scholar, and the search was limited to papers published in English. Besides, some of the included studies were conducted with specific target populations that may not be generalizable to all populations and, thus, our results should be extrapolated with caution. In addition, the definitions of hyperglycemia, hypoglycemia, and GV are consistent in most of the included studies, whereas some minor difference existed in a few studies.
Chronic hyperglycemia and diabetes have been associated with a higher risk of AF and considered as important risk factors of AF. Therefore, study exploring novel predictors for new-onset AF among patients with diabetes is of the utmost importance. Besides, evidence regarding the associations between hyperglycemia and supraventricular arrhythmias are mainly focused on AF. Only a few studies have reported the association between hyperglycemia and other sup-raventricular arrhythmias, such as sinus tachycardia and atrial flutter[2,13]. More studies are needed to further evaluate the associations between hyperglycemia and other arrhythmias.
Although both case reports and clinical studies have suggested an association between hypoglycemia and increased risk of AF, little is known about the underlying mechanisms, which highlights the need for future basic studies to elucidate the mechanisms of hypoglycemia-induced AF. In addition, a causal relationship between hypoglycemia and fatal arrhythmias is usually difficult to demonstrate in clinical practice, since long-term simultaneous monitoring of cardiac rhythm and blood glucose levels is seldom undertaken[85], which might result in underestimation of the incidence of hypoglycemia-induced fatal arrhythmias. Therefore, long-term, large-scale, prospective studies are needed to elucidate the real incidence of various types of arrhythmias during the episodes of hypoglycemia. Besides, susceptibility to cardiac arrhythmias during hypoglycemia seems to be confined to a few individuals[137], thus identifying individuals who are at increased risk of arrhythmias during hypoglycemia is important and possesses great clinical significance.
Hyperglycemia, hypoglycemia, and GV has been associated with various types of arrhythmias, through different mechanisms. It is important to establish a customized glycemic control strategy that takes individual characteristics into account, such as age and underlying diseases, in order to maintain a healthy glycemic homoeostasis and minimize the risk of cardiac arrhythmias.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carlier S, Belgium; Mishra AK, United States; Horowitz M, Australia S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6292] [Cited by in RCA: 5614] [Article Influence: 330.2] [Reference Citation Analysis (0)] |
| 2. | Agarwal G, Singh SK. Arrhythmias in Type 2 Diabetes Mellitus. Indian J Endocrinol Metab. 2017;21:715-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Lee TTL, Hui JMH, Lee YHA, Satti DI, Shum YKL, Kiu PTH, Wai AKC, Liu T, Wong WT, Chan JSK, Cheung BMY, Wong ICK, Cheng SH, Tse G. Sulfonylurea Is Associated With Higher Risks of Ventricular Arrhythmia or Sudden Cardiac Death Compared With Metformin: A Population-Based Cohort Study. J Am Heart Assoc. 2022;11:e026289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 5. | Lee S, Jeevaratnam K, Liu T, Chang D, Chang C, Wong WT, Wong ICK, Lip GYH, Tse G. Risk stratification of cardiac arrhythmias and sudden cardiac death in type 2 diabetes mellitus patients receiving insulin therapy: A population-based cohort study. Clin Cardiol. 2021;44:1602-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Lee S, Zhou J, Wong WT, Liu T, Wu WKK, Wong ICK, Zhang Q, Tse G. Glycemic and lipid variability for predicting complications and mortality in diabetes mellitus using machine learning. BMC Endocr Disord. 2021;21:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Frontoni S, Di Bartolo P, Avogaro A, Bosi E, Paolisso G, Ceriello A. Glucose variability: An emerging target for the treatment of diabetes mellitus. Diabetes Res Clin Pract. 2013;102:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Zhang N, Wang Y, Tse G, Li G, Wu S, Liu T. Association of Visit-to-Visit Variability in Fasting Plasma Glucose with Digestive Cancer Risk. Oxid Med Cell Longev. 2022;2022:4530894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 9. | Gu J, Fan YQ, Zhang JF, Wang CQ. Impact of long-term glycemic variability on development of atrial fibrillation in type 2 diabetic patients. Anatol J Cardiol. 2017;18:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2379] [Cited by in RCA: 2592] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 11. | Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 746] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 12. | McCall AL, Lieb DC, Gianchandani R, MacMaster H, Maynard GA, Murad MH, Seaquist E, Wolfsdorf JI, Wright RF, Wiercioch W. Management of Individuals With Diabetes at High Risk for Hypoglycemia: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2023;108:529-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 13. | Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 14. | Go AS, Hlatky MA, Liu TI, Fan D, Garcia EA, Sung SH, Solomon MD. Contemporary Burden and Correlates of Symptomatic Paroxysmal Supraventricular Tachycardia. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Lee SS, Ae Kong K, Kim D, Lim YM, Yang PS, Yi JE, Kim M, Kwon K, Bum Pyun W, Joung B, Park J. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J. 2017;38:2599-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Latini R, Staszewsky L, Sun JL, Bethel MA, Disertori M, Haffner SM, Holman RR, Chang F, Giles TD, Maggioni AP, Rutten GE, Standl E, Thomas L, Tognoni G, Califf RM, McMurray JJ. Incidence of atrial fibrillation in a population with impaired glucose tolerance: the contribution of glucose metabolism and other risk factors. A post hoc analysis of the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research trial. Am Heart J. 2013;166:935-40.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Fu L, Deng H, Lin WD, He SF, Liu FZ, Liu Y, Zhan XZ, Fang XH, Liao HT, Wei W, Liao ZL, Tang LH, Fu ZY, Zheng MR, Wu SL, Xue YM. Association between elevated blood glucose level and non-valvular atrial fibrillation: a report from the Guangzhou heart study. BMC Cardiovasc Disord. 2019;19:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Kaneko H, Itoh H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Takeda N, Morita H, Yasunaga H, Komuro I. Fasting plasma glucose and subsequent cardiovascular disease among young adults: Analysis of a nationwide epidemiological database. Atherosclerosis. 2021;319:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840-844. [PubMed] |
| 20. | Christophersen IE, Yin X, Larson MG, Lubitz SA, Magnani JW, McManus DD, Ellinor PT, Benjamin EJ. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation in the Framingham Heart Study. Am Heart J. 2016;178:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Mitrega K, Lip GYH, Sredniawa B, Sokal A, Streb W, Przyludzki K, Zdrojewski T, Wierucki L, Rutkowski M, Bandosz P, Kazmierczak J, Grodzicki T, Opolski G, Kalarus Z. Predicting Silent Atrial Fibrillation in the Elderly: A Report from the NOMED-AF Cross-Sectional Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 393] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 23. | Li M, Gao Y, Guo K, Wu Z, Lao Y, Li J, Huang X, Feng L, Dong J, Yuan Y. Association Between Fasting Hyperglycemia and New-Onset Atrial Fibrillation in Patients With Acute Myocardial Infarction and the Impact on Short- and Long-Term Prognosis. Front Cardiovasc Med. 2021;8:667527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Koracevic GP, Petrovic S, Damjanovic M, Stanojlovic T. Association of stress hyperglycemia and atrial fibrillation in myocardial infarction. Wien Klin Wochenschr. 2008;120:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 507] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 26. | Akirov A, Grossman A, Shochat T, Shimon I. Hyperglycemia on admission and hospitalization outcomes in patients with atrial fibrillation. Clin Cardiol. 2017;40:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Krittayaphong R, Aroonsiriwattana S, Ngamjanyaporn P, Patmuk T, Kaewkumdee P; COOL-AF Investigators. Outcomes of patients with atrial fibrillation with and without diabetes: A propensity score matching of the COOL-AF registry. Int J Clin Pract. 2021;75:e14671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Papazoglou AS, Kartas A, Samaras A, Vouloagkas I, Vrana E, Moysidis DV, Akrivos E, Kotzampasis G, Baroutidou A, Papanastasiou A, Liampas E, Botis M, Karagiannidis E, Stalikas N, Karvounis H, Tzikas A, Giannakoulas G. Prognostic significance of diabetes mellitus in patients with atrial fibrillation. Cardiovasc Diabetol. 2021;20:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Chao TF, Suenari K, Chang SL, Lin YJ, Lo LW, Hu YF, Tuan TC, Tai CT, Tsao HM, Li CH, Ueng KC, Wu TJ, Chen SA. Atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation associated with diabetes mellitus or impaired fasting glucose. Am J Cardiol. 2010;106:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Zhang Q, Liu T, Ng CY, Li G. Diabetes mellitus and atrial remodeling: mechanisms and potential upstream therapies. Cardiovasc Ther. 2014;32:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Liu C, Fu H, Li J, Yang W, Cheng L, Liu T, Li G. Hyperglycemia aggravates atrial interstitial fibrosis, ionic remodeling and vulnerability to atrial fibrillation in diabetic rabbits. Anadolu Kardiyol Derg. 2012;12:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Watanabe M, Yokoshiki H, Mitsuyama H, Mizukami K, Ono T, Tsutsui H. Conduction and refractory disorders in the diabetic atrium. Am J Physiol Heart Circ Physiol. 2012;303:H86-H95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Fu L, Rao F, Lian F, Yang H, Kuang S, Wu S, Deng C, Xue Y. Mechanism of electrical remodeling of atrial myocytes and its influence on susceptibility to atrial fibrillation in diabetic rats. Life Sci. 2019;239:116903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Kato T, Yamashita T, Sekiguchi A, Sagara K, Takamura M, Takata S, Kaneko S, Aizawa T, Fu LT. What are arrhythmogenic substrates in diabetic rat atria? J Cardiovasc Electrophysiol. 2006;17:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Zhao J, Qiu J, Li J, Liang X, Zhang Z, Zhang X, Fu H, Korantzopoulos P, Letsas KP, Tse G, Li G, Liu T. Xanthine Oxidase Inhibitor Allopurinol Prevents Oxidative Stress-Mediated Atrial Remodeling in Alloxan-Induced Diabetes Mellitus Rabbits. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Zhang Z, Zhang X, Meng L, Gong M, Li J, Shi W, Qiu J, Yang Y, Zhao J, Suo Y, Liang X, Wang X, Tse G, Jiang N, Li G, Zhao Y, Liu T. Pioglitazone Inhibits Diabetes-Induced Atrial Mitochondrial Oxidative Stress and Improves Mitochondrial Biogenesis, Dynamics, and Function Through the PPAR-γ/PGC-1α Signaling Pathway. Front Pharmacol. 2021;12:658362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Uemura K, Kondo H, Ishii Y, Kobukata M, Haraguchi M, Imamura T, Otsubo T, Ikebe-Ebata Y, Abe I, Ayabe R, Saito S, Aoki K, Nagano-Torigoe Y, Akioka H, Shinohara T, Teshima Y, Masaki T, Yufu K, Nakagawa M, Takahashi N. Mast Cells Play an Important Role in the Pathogenesis of Hyperglycemia-Induced Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016;27:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Negishi K, Seicean S, Negishi T, Yingchoncharoen T, Aljaroudi W, Marwick TH. Relation of heart-rate recovery to new onset heart failure and atrial fibrillation in patients with diabetes mellitus and preserved ejection fraction. Am J Cardiol. 2013;111:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Otake H, Suzuki H, Honda T, Maruyama Y. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int Heart J. 2009;50:627-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Yuan M, Gong M, Zhang Z, Meng L, Tse G, Zhao Y, Bao Q, Zhang Y, Yuan M, Liu X, Li G, Liu T. Hyperglycemia Induces Endoplasmic Reticulum Stress in Atrial Cardiomyocytes, and Mitofusin-2 Downregulation Prevents Mitochondrial Dysfunction and Subsequent Cell Death. Oxid Med Cell Longev. 2020;2020:6569728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 42. | Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54:1891-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 43. | Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 44. | Lee S, Zhou J, Leung KSK, Wai AKC, Jeevaratnam K, King E, Liu T, Wong WT, Chang C, Wong ICK, Cheung BMY, Tse G, Zhang Q. Comparison of Sodium-Glucose Cotransporter-2 Inhibitor and Dipeptidyl Peptidase-4 Inhibitor on the Risks of New-Onset Atrial Fibrillation, Stroke and Mortality in Diabetic Patients: A Propensity Score-Matched Study in Hong Kong. Cardiovasc Drugs Ther. 2023;37:561-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 45. | Zhang Z, Zhang X, Korantzopoulos P, Letsas KP, Tse G, Gong M, Meng L, Li G, Liu T. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord. 2017;17:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 46. | Zhou J, Zhang G, Chang C, Chou OHI, Lee S, Leung KSK, Wong WT, Liu T, Wai AKC, Cheng SH, Zhang Q, Tse G. Metformin versus sulphonylureas for new onset atrial fibrillation and stroke in type 2 diabetes mellitus: a population-based study. Acta Diabetol. 2022;59:697-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 47. | Niemeijer MN, van den Berg ME, Eijgelsheim M, van Herpen G, Stricker BH, Kors JA, Rijnbeek PR. Short-term QT variability markers for the prediction of ventricular arrhythmias and sudden cardiac death: a systematic review. Heart. 2014;100:1831-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Warnier MJ, Holtkamp FA, Rutten FH, Hoes AW, de Boer A, Mol PG, De Bruin ML. Safety information on QT-interval prolongation: comparison of European Union and United States drug labeling. Drug Discov Today. 2014;19:1294-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Moskovitz JB, Hayes BD, Martinez JP, Mattu A, Brady WJ. Electrocardiographic implications of the prolonged QT interval. Am J Emerg Med. 2013;31:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Chen-Scarabelli C, Scarabelli TM. Suboptimal glycemic control, independently of QT interval duration, is associated with increased risk of ventricular arrhythmias in a high-risk population. Pacing Clin Electrophysiol. 2006;29:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Tran HV, Gore JM, Darling CE, Ash AS, Kiefe CI, Goldberg RJ. Hyperglycemia and risk of ventricular tachycardia among patients hospitalized with acute myocardial infarction. Cardiovasc Diabetol. 2018;17:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Movahed MR, Hashemzadeh M, Jamal M. Increased prevalence of ventricular fibrillation in patients with type 2 diabetes mellitus. Heart Vessels. 2007;22:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 54. | Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, Lemaitre RN. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord. 2010;11:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Kim YG, Roh SY, Han KD, Jeong JH, Choi YY, Min K, Shim J, Choi JI, Kim YH. Hypertension and diabetes including their earlier stage are associated with increased risk of sudden cardiac arrest. Sci Rep. 2022;12:12307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 56. | Brown DW, Giles WH, Greenlund KJ, Valdez R, Croft JB. Impaired fasting glucose, diabetes mellitus, and cardiovascular disease risk factors are associated with prolonged QTc duration. Results from the Third National Health and Nutrition Examination Survey. J Cardiovasc Risk. 2001;8:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Ninkovic VM, Ninkovic SM, Miloradovic V, Stanojevic D, Babic M, Giga V, Dobric M, Trenell MI, Lalic N, Seferovic PM, Jakovljevic DG. Prevalence and risk factors for prolonged QT interval and QT dispersion in patients with type 2 diabetes. Acta Diabetol. 2016;53:737-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Marfella R, Nappo F, De Angelis L, Siniscalchi M, Rossi F, Giugliano D. The effect of acute hyperglycaemia on QTc duration in healthy man. Diabetologia. 2000;43:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Gordin D, Forsblom C, Rönnback M, Groop PH. Acute hyperglycaemia disturbs cardiac repolarization in Type 1 diabetes. Diabet Med. 2008;25:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Kumar R, Fisher M, Whitaker R, Macfarlane PW. Effect of controlling hyperglycemia with diet on QT abnormalities in newly diagnosed patients with type 2 diabetes. Diabetes Care. 2004;27:2767-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Meo M, Meste O, Signore S, Sorrentino A, Cannata A, Zhou Y, Matsuda A, Luciani M, Kannappan R, Goichberg P, Leri A, Anversa P, Rota M. Reduction in Kv Current Enhances the Temporal Dispersion of the Action Potential in Diabetic Myocytes: Insights From a Novel Repolarization Algorithm. J Am Heart Assoc. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Zhang Y, Xiao J, Lin H, Luo X, Wang H, Bai Y, Wang J, Zhang H, Yang B, Wang Z. Ionic mechanisms underlying abnormal QT prolongation and the associated arrhythmias in diabetic rabbits: a role of rapid delayed rectifier K+ current. Cell Physiol Biochem. 2007;19:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Tsuchida K, Watajima H. Potassium currents in ventricular myocytes from genetically diabetic rats. Am J Physiol. 1997;273:E695-E700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Sato T, Kobayashi T, Kuno A, Miki T, Tanno M, Kouzu H, Itoh T, Ishikawa S, Kojima T, Miura T, Tohse N. Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am J Physiol Heart Circ Physiol. 2014;306:H1054-H1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Stables CL, Musa H, Mitra A, Bhushal S, Deo M, Guerrero-Serna G, Mironov S, Zarzoso M, Vikstrom KL, Cawthorn W, Pandit SV. Reduced Na⁺ current density underlies impaired propagation in the diabetic rabbit ventricle. J Mol Cell Cardiol. 2014;69:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Yu P, Hu L, Xie J, Chen S, Huang L, Xu Z, Liu X, Zhou Q, Yuan P, Yan X, Jin J, Shen Y, Zhu W, Fu L, Chen Q, Yu J, Hu J, Cao Q, Wan R, Hong K. O-GlcNAcylation of cardiac Nav1.5 contributes to the development of arrhythmias in diabetic hearts. Int J Cardiol. 2018;260:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Jourdon P, Feuvray D. Calcium and potassium currents in ventricular myocytes isolated from diabetic rats. J Physiol. 1993;470:411-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Inoguchi T, Yu HY, Imamura M, Kakimoto M, Kuroki T, Maruyama T, Nawata H. Altered gap junction activity in cardiovascular tissues of diabetes. Med Electron Microsc. 2001;34:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Lin H, Mitasikova M, Dlugosova K, Okruhlicova L, Imanaga I, Ogawa K, Weismann P, Tribulova N. Thyroid hormones suppress epsilon-PKC signalling, down-regulate connexin-43 and increase lethal arrhythmia susceptibility in non-diabetic and diabetic rat hearts. J Physiol Pharmacol. 2008;59:271-285. [PubMed] |
| 70. | Lin H, Ogawa K, Imanaga I, Tribulova N. Remodeling of connexin 43 in the diabetic rat heart. Mol Cell Biochem. 2006;290:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Joshi MS, Mihm MJ, Cook AC, Schanbacher BL, Bauer JA. Alterations in connexin 43 during diabetic cardiomyopathy: competition of tyrosine nitration versus phosphorylation. J Diabetes. 2015;7:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Howarth FC, Chandler NJ, Kharche S, Tellez JO, Greener ID, Yamanushi TT, Billeter R, Boyett MR, Zhang H, Dobrzynski H. Effects of streptozotocin-induced diabetes on connexin43 mRNA and protein expression in ventricular muscle. Mol Cell Biochem. 2008;319:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Zhang Y, Xiao J, Wang H, Luo X, Wang J, Villeneuve LR, Zhang H, Bai Y, Yang B, Wang Z. Restoring depressed HERG K+ channel function as a mechanism for insulin treatment of abnormal QT prolongation and associated arrhythmias in diabetic rabbits. Am J Physiol Heart Circ Physiol. 2006;291:H1446-H1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Han H, Wang J, Wang H, Yang B, Wang Z. Impairment of human ether-à-go-go-related gene (HERG) K+ channel function by hypoglycemia and hyperglycemia. Similar phenotypes but different mechanisms. J Biol Chem. 2003;278:10417-10426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Lu S, Liao Z, Lu X, Katschinski DM, Mercola M, Chen J, Heller Brown J, Molkentin JD, Bossuyt J, Bers DM. Hyperglycemia Acutely Increases Cytosolic Reactive Oxygen Species via O-linked GlcNAcylation and CaMKII Activation in Mouse Ventricular Myocytes. Circ Res. 2020;126:e80-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 76. | Hegyi B, Borst JM, Bailey LRJ, Shen EY, Lucena AJ, Navedo MF, Bossuyt J, Bers DM. Hyperglycemia regulates cardiac K(+) channels via O-GlcNAc-CaMKII and NOX2-ROS-PKC pathways. Basic Res Cardiol. 2020;115:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 77. | Sovari AA. Cellular and Molecular Mechanisms of Arrhythmia by Oxidative Stress. Cardiol Res Pract. 2016;2016:9656078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 78. | Xie C, Biary N, Tocchetti CG, Aon MA, Paolocci N, Kauffman J, Akar FG. Glutathione oxidation unmasks proarrhythmic vulnerability of chronically hyperglycemic guinea pigs. Am J Physiol Heart Circ Physiol. 2013;304:H916-H926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Zhang L, Parratt JR, Beastall GH, Pyne NJ, Furman BL. Streptozotocin diabetes protects against arrhythmias in rat isolated hearts: role of hypothyroidism. Eur J Pharmacol. 2002;435:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Fusilli L, Lyons M, Patel B, Torres R, Hernandez F, Regan T. Ventricular vulnerability in diabetes and myocardial norepinephrine release. Am J Med Sci. 1989;298:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 81. | Movahed MR. Diabetes as a risk factor for cardiac conduction defects: a review. Diabetes Obes Metab. 2007;9:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Movahed MR, Hashemzadeh M, Jamal MM. Increased prevalence of third-degree atrioventricular block in patients with type II diabetes mellitus. Chest. 2005;128:2611-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Haxha S, Halili A, Malmborg M, Pedersen-Bjergaard U, Philbert BT, Lindhardt TB, Hoejberg S, Schjerning AM, Ruwald MH, Gislason GH, Torp-Pedersen C, Bang CN. Type 2 diabetes mellitus and higher rate of complete atrioventricular block: a Danish Nationwide Registry. Eur Heart J. 2023;44:752-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 84. | Hasslacher C, Wahl P. Diabetes prevalence in patients with bradycardiac arrhythmias. Acta Diabetol Lat. 1977;14:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 86. | Geller AI, Shehab N, Lovegrove MC, Kegler SR, Weidenbach KN, Ryan GJ, Budnitz DS. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. 2014;174:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 87. | Tattersall RB, Gill GV. Unexplained deaths of type 1 diabetic patients. Diabet Med. 1991;8:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 198] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 88. | Landstedt-Hallin L, Englund A, Adamson U, Lins PE. Increased QT dispersion during hypoglycaemia in patients with type 2 diabetes mellitus. J Intern Med. 1999;246:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Reno CM, Daphna-Iken D, Chen YS, VanderWeele J, Jethi K, Fisher SJ. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes. 2013;62:3570-3581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 90. | Chow E, Bernjak A, Williams S, Fawdry RA, Hibbert S, Freeman J, Sheridan PJ, Heller SR. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63:1738-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 91. | Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the 'dead in bed' syndrome revisited. Diabetologia. 2009;52:42-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 92. | Andersen A, Bagger JI, Sørensen SK, Baldassarre MPA, Pedersen-Bjergaard U, Forman JL, Gislason G, Lindhardt TB, Knop FK, Vilsbøll T. Associations of hypoglycemia, glycemic variability and risk of cardiac arrhythmias in insulin-treated patients with type 2 diabetes: a prospective, observational study. Cardiovasc Diabetol. 2021;20:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 93. | Celebi S, Celebi OO, Aydogdu S, Diker E. A peculiar medical cardioversion of atrial fibrillation with glucose infusion--a rare cause of atrial fibrillation: hypoglycemia. Am J Emerg Med. 2011;29:134.e1-134.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 94. | Ko SH, Park YM, Yun JS, Cha SA, Choi EK, Han K, Han E, Lee YH, Ahn YB. Severe hypoglycemia is a risk factor for atrial fibrillation in type 2 diabetes mellitus: Nationwide population-based cohort study. J Diabetes Complications. 2018;32:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 95. | Pezzarossa A, Baggi V, Manca C, Bolognesi R. Non-paroxysmal arterioventricular junctional tachycardia during postprandial hypoglycaemia in an obese non-diabetic patient. J Intern Med. 1993;234:325-327. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 96. | Odeh M, Oliven A, Bassan H. Transient atrial fibrillation precipitated by hypoglycemia. Ann Emerg Med. 1990;19:565-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Collier A, Matthews DM, Young RJ, Clarke BF. Transient atrial fibrillation precipitated by hypoglycaemia: two case reports. Postgrad Med J. 1987;63:895-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Humos B, Mahfoud Z, Dargham S, Al Suwaidi J, Jneid H, Abi Khalil C. Hypoglycemia is associated with a higher risk of mortality and arrhythmias in ST-elevation myocardial infarction, irrespective of diabetes. Front Cardiovasc Med. 2022;9:940035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 99. | Vardas PE, Vemmos K, Sideris DA, Moulopoulos SD. Susceptibility of the right and left canine atria to fibrillation in hyperglycemia and hypoglycemia. J Electrocardiol. 1993;26:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Kacheva S, Karges B, Göller K, Marx N, Mischke K, Karges W. QT prolongation caused by insulin-induced hypoglycaemia - An interventional study in 119 individuals. Diabetes Res Clin Pract. 2017;123:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Andersen A, Bagger JI, Baldassarre MPA, Christensen MB, Abelin KU, Faber J, Pedersen-Bjergaard U, Holst JJ, Lindhardt TB, Gislason G, Knop FK, Vilsbøll T. Acute hypoglycemia and risk of cardiac arrhythmias in insulin-treated type 2 diabetes and controls. Eur J Endocrinol. 2021;185:343-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Reno CM, Bayles J, Huang Y, Oxspring M, Hirahara AM, Dosdall DJ, Fisher SJ. Severe Hypoglycemia-Induced Fatal Cardiac Arrhythmias Are Mediated by the Parasympathetic Nervous System in Rats. Diabetes. 2019;68:2107-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 103. | Chelliah YR. Ventricular arrhythmias associated with hypoglycaemia. Anaesth Intensive Care. 2000;28:698-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Pistrosch F, Ganz X, Bornstein SR, Birkenfeld AL, Henkel E, Hanefeld M. Risk of and risk factors for hypoglycemia and associated arrhythmias in patients with type 2 diabetes and cardiovascular disease: a cohort study under real-world conditions. Acta Diabetol. 2015;52:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 105. | Svendsen JH, Goette A, Dobreanu D, Marinskis G, Mabo P, Blomström-Lundqvist C; Scientific Initiative Committee, European Heart Rhythm Association. Outpatient evaluation and management of patients with ventricular premature beats or non-sustained ventricular tachycardia. Europace. 2012;14:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 106. | Fitzpatrick C, Chatterjee S, Seidu S, Bodicoat DH, Ng GA, Davies MJ, Khunti K. Association of hypoglycaemia and risk of cardiac arrhythmia in patients with diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:2169-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 107. | Tsujimoto T, Yamamoto-Honda R, Kajio H, Kishimoto M, Noto H, Hachiya R, Kimura A, Kakei M, Noda M. High risk of abnormal QT prolongation in the early morning in diabetic and non-diabetic patients with severe hypoglycemia. Ann Med. 2015;47:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 108. | Lipponen JA, Kemppainen J, Karjalainen PA, Laitinen T, Mikola H, Kärki T, Tarvainen MP. Dynamic estimation of cardiac repolarization characteristics during hypoglycemia in healthy and diabetic subjects. Physiol Meas. 2011;32:649-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Richardson DR, Parish PC, Tan X, Fabricio J, Andreini CL, Hicks CH, Jensen BC, Muluneh B, Zeidner JF. Association of QTc Formula With the Clinical Management of Patients With Cancer. JAMA Oncol. 2022;8:1616-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 110. | Christensen TF, Tarnow L, Randløv J, Kristensen LE, Struijk JJ, Eldrup E, Hejlesen OK. QT interval prolongation during spontaneous episodes of hypoglycaemia in type 1 diabetes: the impact of heart rate correction. Diabetologia. 2010;53:2036-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 111. | Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1357] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 112. | Lee SP, Yeoh L, Harris ND, Davies CM, Robinson RT, Leathard A, Newman C, Macdonald IA, Heller SR. Influence of autonomic neuropathy on QTc interval lengthening during hypoglycemia in type 1 diabetes. Diabetes. 2004;53:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 113. | Nordin C. The case for hypoglycaemia as a proarrhythmic event: basic and clinical evidence. Diabetologia. 2010;53:1552-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 114. | Libby P, Maroko PR, Braunwald E. The effect of hypoglycemia on myocardial ischemic injury during acute experimental coronary artery occlusion. Circulation. 1975;51:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 283] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 116. | Bolognesi R, Tsialtas D, Bolognesi MG, Giumelli C. Marked sinus bradycardia and QT prolongation in a diabetic patient with severe hypoglycemia. J Diabetes Complications. 2011;25:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Navarro-Gutiérrez S, González-Martínez F, Fernández-Pérez MT, García-Moreno MT, Ballester-Vidal MR, Pulido-Morillo FJ. Bradycardia related to hypoglycaemia. Eur J Emerg Med. 2003;10:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 118. | Ravi R, Balasubramaniam V, Kuppusamy G, Ponnusankar S. Current concepts and clinical importance of glycemic variability. Diabetes Metab Syndr. 2021;15:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 119. | Wadén J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH; Finnish Diabetic Nephropathy Study Group. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58:2649-2655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 120. | Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, Heatlie G, Loke Y, Rutter MK, Mamas MA. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care. 2015;38:2354-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 121. | Hsu JC, Yang YY, Chuang SL, Yu CC, Lin LY. Higher long-term visit-to-visit glycemic variability predicts new-onset atrial fibrillation in patients with diabetes mellitus. Cardiovasc Diabetol. 2021;20:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 122. | Xia J, Xu J, Li B, Liu Z, Hao H, Yin C, Xu D. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin Chim Acta. 2017;466:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 123. | Clement KC, Alejo D, DiNatale J, Whitman GJR, Matthew TL, Clement SC, Lawton JS. Increased glucose variability is associated with atrial fibrillation after coronary artery bypass. J Card Surg. 2019;34:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 124. | Sim MA, Liu W, Chew STH, Ti LK. Wider perioperative glycemic fluctuations increase risk of postoperative atrial fibrillation and ICU length of stay. PLoS One. 2018;13:e0198533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 125. | Saito S, Teshima Y, Fukui A, Kondo H, Nishio S, Nakagawa M, Saikawa T, Takahashi N. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc Res. 2014;104:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 126. | Ying C, Liu T, Ling H, Cheng M, Zhou X, Wang S, Mao Y, Chen L, Zhang R, Li W. Glucose variability aggravates cardiac fibrosis by altering AKT signalling path. Diab Vasc Dis Res. 2017;14:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 127. | Chang CM, Hsieh CJ, Huang JC, Huang IC. Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus. Acta Diabetol. 2012;49 Suppl 1:S171-S177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 128. | Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1796] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 129. | Ohara M, Fukui T, Ouchi M, Watanabe K, Suzuki T, Yamamoto S, Yamamoto T, Hayashi T, Oba K, Hirano T. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res Clin Pract. 2016;122:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 130. | Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 583] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 131. | Xu W, Zhu Y, Yang X, Deng H, Yan J, Lin S, Yang H, Chen H, Weng J. Glycemic variability is an important risk factor for cardiovascular autonomic neuropathy in newly diagnosed type 2 diabetic patients. Int J Cardiol. 2016;215:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 132. | Matsutani D, Sakamoto M, Iuchi H, Minato S, Suzuki H, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Glycemic variability in continuous glucose monitoring is inversely associated with baroreflex sensitivity in type 2 diabetes: a preliminary report. Cardiovasc Diabetol. 2018;17:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 133. | Kalopita S, Liatis S, Thomakos P, Vlahodimitris I, Stathi C, Katsilambros N, Tentolouris N, Makrilakis K. Relationship between autonomic nervous system function and continuous interstitial glucose measurement in patients with type 2 diabetes. J Diabetes Res. 2014;2014:835392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 134. | Zhang J, Yang J, Liu L, Li L, Cui J, Wu S, Tang K. Significant abnormal glycemic variability increased the risk for arrhythmias in elderly type 2 diabetic patients. BMC Endocr Disord. 2021;21:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Su JB, Yang XH, Zhang XL, Cai HL, Huang HY, Zhao LH, Xu F, Chen T, Cheng XB, Wang XQ, Lu Y. The association of long-term glycaemic variability versus sustained chronic hyperglycaemia with heart rate-corrected QT interval in patients with type 2 diabetes. PLoS One. 2017;12:e0183055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 136. | Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 816] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 137. | Campbell M, Heller SR, Jacques RM. Response to Comment on Novodvorsky et al Diurnal Differences in Risk of Cardiac Arrhythmias During Spontaneous Hypoglycemia in Young People With Type 1 Diabetes. Diabetes Care. 2018;41:e65-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |