Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.977
Peer-review started: January 9, 2023
First decision: January 17, 2023
Revised: January 31, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: July 15, 2023
Processing time: 184 Days and 15.6 Hours
Obesity and type 2 diabetes mellitus (T2DM) are chronic pathologies with a high incidence worldwide. They share some pathological mechanisms, including hyperinsulinemia, the production and release of hormones, and hyperglycemia. The above, over time, affects other systems of the human body by causing tissue hypoxia, low-grade inflammation, and oxidative stress, which lay the pathophysiological groundwork for cancer. The leading causes of death globally are T2DM and cancer. Other main alterations of this pathological triad include the accumulation of advanced glycation end products and the release of endogenous alarmins due to cell death (i.e., damage-associated molecular patterns) such as the intracellular proteins high-mobility group box protein 1 and protein S100 that bind to the receptor for advanced glycation products (RAGE) - a multiligand receptor involved in inflammatory and metabolic and neoplastic processes. This review analyzes the latest advanced reports on the role of RAGE in the development of obesity, T2DM, and cancer, with an aim to understand the intracellular signaling mechanisms linked with cancer initiation. This review also explores inflammation, oxidative stress, hypoxia, cellular senescence, RAGE ligands, tumor microenvironment changes, and the “cancer hallmarks” of the leading tumors associated with T2DM. The assimilation of this information could aid in the development of diagnostic and therapeutic approaches to lower the morbidity and mortality associated with these diseases.
Core Tip: The receptor for advanced glycation products (RAGE) is involved in every stage of the pathophysiological pathways that lead to the progression of obesity, type 2 diabetes, and cancer. This article provides a focused discussion on the stages of obesity leading to the development of metabolic diseases and provides a broad overview of the contribution of RAGE to the development of diabetes and cancer.
- Citation: Garza-Campos A, Prieto-Correa JR, Domínguez-Rosales JA, Hernández-Nazará ZH. Implications of receptor for advanced glycation end products for progression from obesity to diabetes and from diabetes to cancer. World J Diabetes 2023; 14(7): 977-994
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/977.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.977
Obesity, diabetes, and cancer are chronic diseases, the prevalences of which have all increased in parallel, and are leading causes of death worldwide[1]. However, the forecasts for these health problems are not encouraging. For example, the prevalence of diabetes is estimated to increase by 2045, specifically in middle-income countries to 21.1%, in high-income countries to 12.2%, and in low-income countries to 11.9%. Meanwhile, the incidence of malignant neoplasms in people under 50 years of age is also rising[2,3].
Although esophageal adenocarcinoma has a direct link to obesity, and pancreatic cancer can debut with type 2 diabetes mellitus (T2DM), there is an evident connection between the three disorders. Moreover, there is confusion about their shared lifestyle risk factors, including sedentariness and consumption of highly processed foods[4-6]. Regarding the common pathological mechanisms of obesity, T2DM, and cancer, expansion of adipose tissue (AT) results in the production of excess estrogen, adipokines, and inflammatory molecules that can lead to systemic or localized low-grade inflammation. In addition, omental and visceral adiposity is related to hyperinsulinemia and increased levels of insulin-like growth factor-1 (IGF-1)[7]. The metabolic abnormalities and lipo-glucotoxicity associated with insulin resistance and T2DM also cause an increase in inflammatory cytokines and oxidative stress. As a result, neoplastic processes can be triggered by T2DM and, likewise, obesity[8].
The pathogenic mechanisms that link obesity, T2DM, and cancer are complex and multifactorial. Because there is a notion of progression from obesity to T2DM towards cancer, our motivation for this review was to provide a detailed and up-to-date discussion on these mechanisms in the context of a single molecule known as the receptor for advanced glycation end products (RAGE). As such, this narrative review incorporates the conceptual framework and reports on findings extracted from two literature databases, the Reference Citation Analysis (https://www.reference
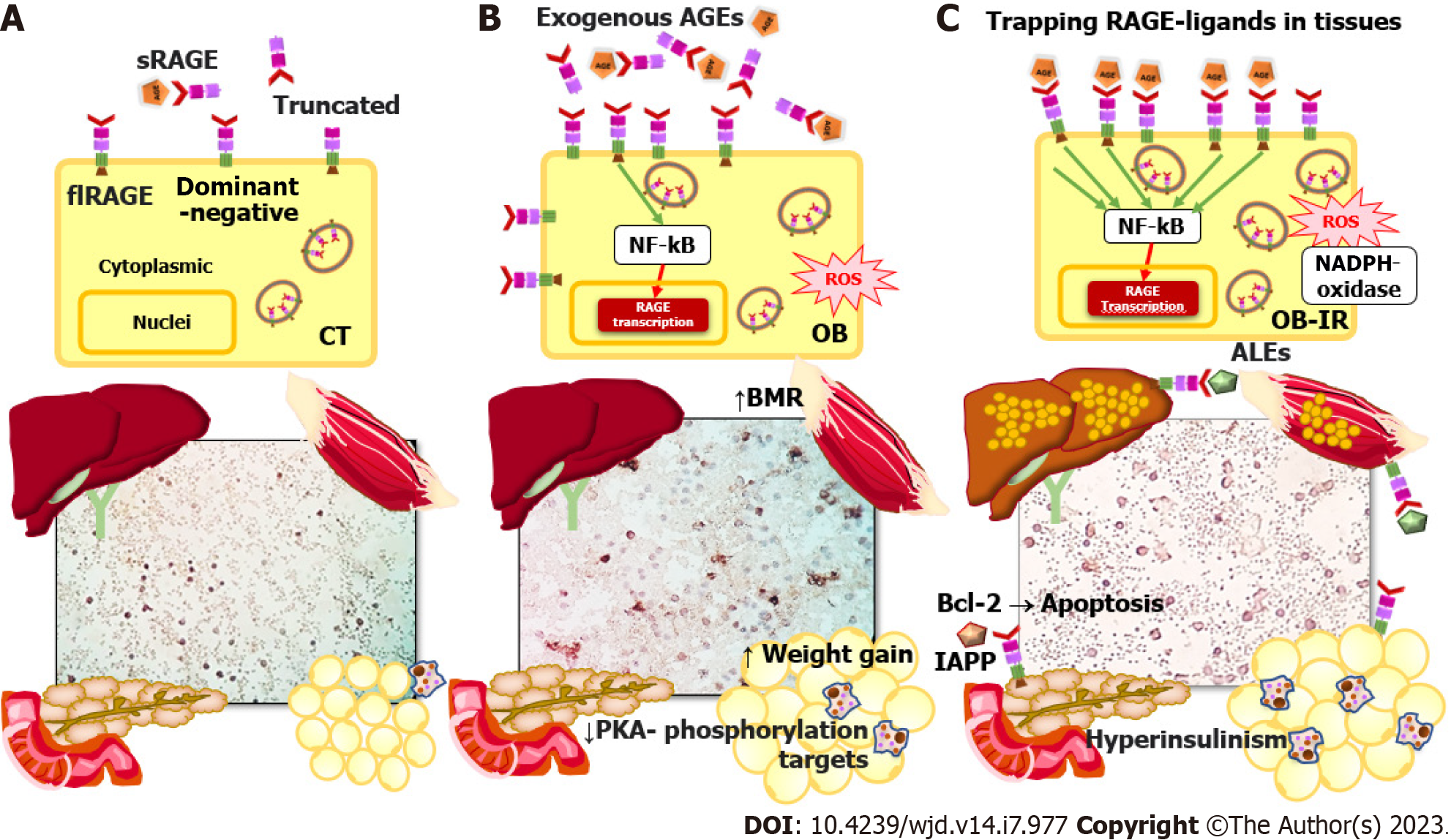
RAGE is an immunoglobulin superfamily member and a type I pattern-recognition receptor. It is also a sensitive environmental sensor with several endogenous and external ligands. Furthermore, it is a widely expressed modulator of inflammatory and oxidative stress pathways with vast metabolic implications[9]. RAGE isoforms include soluble forms (sRAGE) that act as decoy receptors, sequester circulating ligands, and attenuate membrane RAGE signaling[10]. Soluble forms derived from membrane-localized RAGE are released into the circulation by proteolytic cleavage (cRAGE), and endogenously secreted RAGE (esRAGE) is formed by alternative splicing. In addition to the sRAGE isoforms and the full-length membrane receptor (flRAGE) - the only isoform that participates in signal transduction, there are also the dominant-negative isoforms lacking the cytoplasmic tail and the truncated isoform lacking the V-type immunoglobulin domain[11] (Figure 1A).
Initially, the function of RAGE was established in the context of chronic disease, specifically T2DM and its complications, in which persistent hyperglycemia triggers inflammation, oxidative stress, and endothelial damage[12,13]. However, there is more evidence that an increase in RAGE ligands is present in the early stages of metabolic dysfunction in obesity[14,15].
The most recognized ligands of RAGE are the advanced glycosylation end products (AGEs) and lipid oxidation adducts (ALEs). These are taken in from diet or produced by endogenous metabolism through non-enzymatic and spontaneous Maillard-type reactions in which proteins and nucleic acids react with carbohydrates, lipids, or their intermediate metabolites[16,17].
Foods cooked by roasting, grilling, frying, drying, heating, or adding artificial colorants, salt, oil, or sugar are often present in ultra-processed foods to make them suitable to store[6]. In addition to those above, an increase in the diet’s caloric, fat, and glycemic indices leads to a significant rise in the levels of circulating AGEs. Some exogenous-derived food AGEs are Nδ-(5-hydro-5-methil-4-imidazolon-2-il)-ornithina (MG-H1), Nε-carboxyethyl lysine (CEL), and Nε-carboxymethyl lysine (CML), in addition to the precursor methylglyoxal[18-20].
The problem gets worse when an individual also consumes other substances like alcohol and tobacco. Cigarettes are a source of AGEs, and smoking them causes RAGE expression to rise, which is linked to airway inflammation in chronic obstructive pulmonary disease and causes sRAGE to decrease in smoke-induced cardiovascular disease[21,22]. The increase in mitochondrial-derived reactive oxygen species (ROS) caused by the RAGE pathway in smoke-exposed skeletal muscle is one of the hypothesized mechanisms in this regard[23]. In vitro, oral squamous cell carcinoma treated with cigarette smoke extract showed an increase in RAGE with a link to a rise in invasive ability[24]. Additionally, RAGE is elevated in alcoholic liver disease, affecting blood triglycerides, low-density lipoprotein cholesterol, and alanine transaminase levels. RAGE also contributes to the accumulation of lipid droplets in the liver and modifies the expression of SREBP1, a transcription factor involved in lipid homeostasis[25].
Serum AGE accumulation from the diet can lead to cross-link formation that irreversibly changes endogenous proteins independent of glycemic control. Birukov et al[26] found that in people with prediabetes and T2DM, there were significant variations in the levels of AGEs in the skin. Additionally, AGE measurements in that study were related to factors such as waist circumference, glycated hemoglobin (commonly known as hemoglobin A1c) levels, C-reactive protein levels, and vascular stiffness. Further research is required to determine the sensitivity and accuracy of testing AGE accumulation and its relationship to disease status.
In addition to the above, other natural substances such as catechols, myeloperoxidase systems, and the polyol pathway are implicated in producing endogenous AGEs in obesity and states of insulin resistance[27,28]. Likewise, the link between AGEs in obesity and T2DM is the accumulation of lipids and their oxidized products. Thus, the accumulation of free fatty acids and subsequent ALE production aids in the progression of obesity to T2DM[29,30]. Oxidative stress promotes the lipoperoxidation of membranes and the production of metabolites such as 4-hydroxyl-trans2-nonenal, acrolein, aldehydes such as malondialdehyde (MDA), and ketoaldehydes such as 4-oxo-trans-2-nonenal. These may start with obesity and insulin resistance and can result in the creation of endogenous ALEs like MDA-Lys[17,31,32]. Further studies are required on the mechanism by which the progression from obesity to T2DM is affected by the ALEs-RAGE interaction and their aldehyde precursors produced by lipid peroxidation.
In this regard, in obese subjects, RAGE induces migration of macrophages because of the rise in lipid peroxidation and the accumulation of ALEs in renal tissue that leads to kidney injury[33]. Patients with T2DM have high levels of ALE (MDA-Lys), which induces the activation and adherence of monocytes to endothelial cells by increasing the expression of monocyte chemotactic protein-1 (MCP-1) and activating the nuclear factor-kappa B (NF-kB) pathway causing inflammation[34]. Recent comprehensive reviews have addressed endogenous and exogenous AGE and ALE formation in obesity[17], T2DM, and cancer[28,35,36].
There is consistent evidence regarding how ultra-processed foods, ALEs, and AGEs disrupt the microbiota causing dysbiosis, the subsequent translocation of lipopolysaccharide (LPS), and endotoxemia[37,38]. Likewise, dysbiosis is related to obesity, low-grade inflammation, and the progression of insulin resistance and T2DM[39]. However, few publications implicate RAGE as an LPS ligand to mediate inflammatory processes in obesity[40]. This issue needs further investigation, and an exciting future research opportunity may focus on T2DM prevention with respect to the relationship between AGEs/ALEs, RAGE, and dysbiosis.
According to the most recent definitions, chronic low-grade inflammation begins when molecules and metabolites, resulting from altered cell function and structure and foods, stimulate receptors and activate their signaling cascades with dysregulated energy homeostasis. To this end, RAGE mediates danger signals to the body and metabolic stress characteristic of innate immune systems, since RAGE detects ligands from microbes via exogenous pathogen-associated molecular patterns such as LPS. Furthermore, damage-associated molecular pattern (DAMP) ligands are derived from endogenous sources such as high-mobility group box protein 1 (HMGB1), S100/calgranulins, amyloid deposits like β-amyloid peptide, and macrophage-1 antigen[41].
AGE and ALE metabolites can be considered DAMPs that are not derived from exogenous sources such as the diet, and the term “metabolism-associated molecular pattern” is proposed for these specific ligands. It is essential to differentiate between them and demonstrate that both endogenous and external components are involved in these responses[42]. An opportunity for experts in the field is to reach a consensus with respect to the classification of all exogenous and endogenous ligands for pattern-recognition receptors.
Several investigations in human subjects have found an association between obesity and low circulating AGE levels[43]. Complex detoxification and clearance kinetics of AGEs could lead to inconsistent study results. The concept of entrapment of AGE in tissues proposes that AGEs are no longer circulating because they are trapped in tissues as metabolic risks increase in individuals[44-46] (Figure 1C).
For instance, high RAGE expression in AT is implicated in its dysfunction and is evidence of a link between RAGE signaling and the progression of obesity to associated metabolic disorder. A high level of RAGE expression in human epicardial AT is related to its thickening, low glucose transporter type 4 expression, and high HMGB1 expression[47]. In this context, visceral omental AT and fetal membrane samples from women with gestational diabetes revealed higher levels of RAGE and the HMGB1 ligand, respectively[48]. RAGE signaling pathway proteins were also found to be expressed differently in omental and subcutaneous biopsies from obese people with healthy phenotypes. Subcutaneous AT showed a higher correlation between the RAGE signaling axis, inflammatory markers, and the homeostatic model assessment of insulin resistance (HOMA-IR)[49]. A study with a murine RAGE (-/-) model demonstrated protection against inflammation and oxidative stress and protection against insulin resistance. Interestingly, this model showed that the most beneficial characteristics of RAGE knockout were found in female mice[50]. Additionally, RAGE is related to the adaptive thermogenesis function of brown AT through the decline in energy expenditure caused by a high-fat diet, possibly mediated via the accumulation of AGEs[51,52] (Figure 1B).
In addition to dysregulation in AT discussed above, chronic inflammation also plays a pivotal role in obesity-related insulin resistance that leads to metabolic dysfunction in the liver and muscle. Insulin resistance is characterized by alterations in insulin signaling in sensitive tissues, hyperinsulinemia with defects in glucose uptake in muscle and AT, impaired suppression of hepatic glucose production, and ectopic accumulation of fat in the muscle and liver through re-esterification of fatty acids from AT[53,54] (Figure 1C). To this end, an increase in AGE accumulation in liver biopsies has been linked to RAGE expression, lipid accumulation, and the degree of liver damage without association with the measurements of sRAGE and circulating serum AGEs[55,56]. These studies demonstrate how RAGE affects hepatic conditions caused by the accumulation of AGEs in tissue in non-alcoholic liver disease.
RAGE expression and the accumulation of AGEs are linked to weight gain, inflammation, and oxidative stress markers in human muscle tissue[57]. For instance, one study demonstrated that RAGE expression and the accumulation of AGEs in skeletal muscle in a fructose-supplemented murine model were related to alterations in the oral glucose tolerance test curve, increased triglycerides, inflammatory response, increased basal metabolic rate, and resting metabolic rate[58]. Moreover, chronic AGE exposure is linked to sarcopenia[59]. However, the implications of obesity- and T2DM-induced RAGE expression in muscle tissue are less well explored in humans[60].
Along with the mechanism of trapping excess RAGE ligands in tissues, it is known that the sRAGE form eliminates dangerous circulating ligands and functions as a competitive inhibitor of ligands that might bind to cellular RAGE, supported by studies in which sRAGE levels were found to be low[61-64]. The role of sRAGE in metabolic diseases is debatable because it depends on the degree of disease development and the levels of cell and tissue damage[65]. The cRAGE levels are initially high in acute conditions, triggered by cleavage of flRAGE, which increases its AGE-binding activity. The main variations of sRAGE are attributed to the production of cRAGE shedding by metalloproteinases[66] to compensate for the increase in AGEs in the early stages of low-grade inflammation[67-69]. As the concentration of sRAGE decreases, sequestration and competitive inhibition of ligands decrease and as such they can reach cellular flRAGE, leading to an inflammatory response and subsequent tissue damage[68-70] (Figure 1C).
In prediabetes, plasma levels of sRAGE and esRAGE are all negatively correlated with the HOMA-IR index of insulin resistance and MDA. This correlation matches their reduction as insulin resistance develops in an oxidative environment[67]. Another study with similar results comparing healthy people to those with prediabetes and T2DM found low levels of esRAGE and an inverse linkage with S100A12[71]. Miranda et al[62] showed that all RAGE isoforms were lower when grouped by pancreatic dysfunction (i.e., healthy controls, individuals with glucose intolerance, and those with T2DM). Thus, according to the above, the negative correlation of sRAGE with RAGE ligands or increase of the AGE/esRAGE index seems to be more related to individuals with obesity-related insulin resistance and early T2DM[72], and low cRAGE concentrations are a marker of aging[72,73]. Even the elevated AGE/esRAGE index could distinguish between those with non-alcoholic fatty liver disease without T2DM and healthy individuals[74]. Further studies are needed to determine the precise interactions between sRAGE, esRAGE, cRAGE, and their ligands in these disease states.
Since sRAGE and resting energy expenditure are related, one of the most recent discoveries regarding the expression of soluble variants is sRAGE’s contribution to adaptive negative energy balance. In an investigation of the influence of sRAGE on the change in energy expenditure that occurs during weight loss, it was found that, under caloric restriction, adaptive changes arise that slow down energy expenditure. Specifically, after a 3-mo intervention for weight loss due to caloric restriction, energy expenditure increased by 52.6 kcal/d for each 100 pg/mL increase in basal sRAGE levels. Increases in esRAGE and cRAGE similarly translated to concomitant rises in energy expenditure, by 181.6 kcal/d and 56.1 kcal, respectively. This finding illustrates the potential impact of a RAGE feedback mechanism, in which a reduction in sRAGE could slow energy expenditure during weight loss[75]. Furthermore, one mechanism by which RAGE controls energy expenditure is through the suppression of adaptative thermogenesis in white and brown AT via the decline of β-adrenergic signaling in adipocytes blocking protein kinase A (PKA) phosphorylation targets[76].
Still more, the subcellular localization of RAGE can change, a process related to oligomerization in the membrane after RAGE interaction with ligands[77]. A previous study demonstrated increased localization of RAGE in the cell membrane, rather than the cytoplasm, in peripheral blood mononuclear cells of obese individuals with insulin resistance compared with healthy individuals. As such, sRAGE correlates negatively with the HOMA-IR index and tissue damage markers[78] (Figure 1A-C). Peripheral blood mononuclear cells may provide an accessible platform to study the relationship between ligands and cellular RAGE, detect systemic inflammation, and relate these to tissue damage. The preceding argument needs to be tested by additional research.
In T2DM, the pancreas loses its ability to secrete enough insulin in response to meals. One of the mechanisms of pancreas failure is low-grade systemic inflammation. The activating signaling of RAGE in response to ligand binding results in RAGE autoregulation through the increase of its synthesis, which is mediated by NF-kB[79]. In vivo and in vitro models have shown that oxidative stress and inflammation are induced by AGE stimuli through NF-kB activation and the formation of ROS, respectively[80]. These events are evidenced by the increase in the inflammatory serum marker C-reactive protein, particularly in obesity[81]. Some antioxidants and drugs can modulate the AGEs-RAGE axis and the activation of NF-kB, leading to the reduction of lipid peroxidation products in obesity models[82-84].
RAGE expression in the pancreas may be an essential mechanism for the development of T2DM in humans, based on evidence from both in vitro and in vivo glycolipotoxicity studies[85-87]. In a rodent model of diet-induced hyperglycemia, endogenous AGE products are produced, and RAGE expression is observed in pancreatic islets[87]. RAGE inhibition prevented the increase of its expression, and decreased B cell lymphoma-2 (Bcl-2) expression and apoptosis of beta cells treated with glycation serum. However, RAGE inhibition did not restore the ability of the beta cells to secrete insulin in response to glucose[85]. RAGE endocytosis regulated by Rab31 ligand can inhibit apoptosis mediated by the pAkt/Bcl-2 pathway in beta cells treated with glycation serum[88]. In another study, the pancreas of db/db transgenic mice that lack the leptin receptor but express RAGE (+/+) have less beta cell mass and less apoptosis, is glucose intolerant, and has decreased insulin secretion. Likewise, when the MIN6 pancreatic beta cell line was treated with palmitate or oleate and leptin antagonists to induce RAGE expression, pancreatic damage occurred[86]. Another mouse model of diabetes induced by streptozotocin and a high-cholesterol diet treated with the water-soluble carotenoid crocin showed attenuated atrophic effects in pancreatic tissue and decreased blood glucose levels through decreases in the expression of RAGE and LOX-1[89].
DAMP/RAGE reports such as the activation of S100b/RAGE and the subsequent loss of beta cells by apoptosis via NADPH oxidase and the protection of sRAGE against amyloid deposition, beta cell loss, and glucose intolerance demonstrate that they interact[90,91]. All of these findings suggest that RAGE can lead to pancreatic failure and the progression of T2DM.
Several studies have shown that the incidence of various malignancies increases in patients with T2DM. However, more rigorous statistical analyses of observational studies demonstrate a more significant association of T2DM with colorectal, pancreatic, hepatocellular, breast, and endometrial carcinomas. Even so, there are biases in these studies that make it challenging to study the confounding variables of T2DM leading to cancer[92]. A more recent study included statistical analysis of the “Mendelian randomization” studies to analyze genetic data from large-scale international consortia. Ultimately, it allowed to link a possible causal relationship between genetically predicted T2DM and endometrial and pancreatic cancer risks, and between the variable fasting insulin levels and breast cancer risk. In addition, numerous studies have demonstrated the impact of glycemic traits on the emergence of different malignancies, establishing a relationship between T2DM and cancer[93].
Metabolic and hormonal factors found in patients with obesity, insulin resistance, and T2DM, such as hyperinsulinism, hyperglycemia, IGF-1, adipokines, and estrogens, all of which are closely related to inflammation and oxidative stress, function in the long-term as risk factors that support transformation to neoplastic cells in diabetic patients[94].
The increase in estrogen levels in obese patients is due to the positive regulation of the aromatase enzyme, encoded by CYP19A1 and secreted by cells of the tumor stromal microenvironment. The activation mechanisms are triggered in response to hypoxia, with activation of hypoxia-inducible factor-1 alpha (HIF-1α), fat tissue hormones (e.g., adipokine leptin, which increases aromatase expression by phosphorylating serine at position 485 of AMPK and inhibiting the aromatase suppressor), and inflammation processes[95]. Estrogen receptors are transcriptional factors of DNA reprogramming that transduce extranuclear signals, resulting in the regulation of ion channels or kinase cascades such as PKC/PKA/PI3-K/MAPK. The metabolic effects of estrogen on both tumor and normal cells are survival, cell proliferation, and immunomodulation[96]. Estrogens are the most relevant risk factor for endometrial and breast cancers, especially in postmenopausal women. Recently, studies have shown that the microbiota is a source of estrogen-like compounds or estrogen mimics that could be involved in cancer progression[97].
The insulin receptor (IR) and insulin receptor substrate (IRS) are phosphorylated at Ser/Thr residues by inflammatory cytokines and oxidative stress, resulting in insulin resistance and compensatory hyperinsulinemia[98]. Insulin induces proliferation in tissues not involved in metabolism. Binding to its receptor (i.e., IR) activates the RAS/RAF/MAPK kinase-dependent/ERK signaling pathways and increases cell survival and migration[99]. Another mechanism is mediated by IGF-1, a hormone structurally and functionally similar to insulin that binds to IR and its receptor (i.e., IGFR). This receptor, like IR, activates pathways that increase cell proliferation, and insulin enhances the liver’s production of IGF-1, elevating the mitogenic activity of cancer cells expressing the IGFR[7,100]. The nuclear protein HMGA1 contributes to the potentiation of insulin action. In addition, the HMGA1 protein overexpressed in triple-negative breast cancer cells functions in chromatin remodeling and gene expression regulation, indirectly promoting enhanced IR expression through the inhibitory effect on p53 expression, which usually keeps IR expression turned off.
Although hyperglycemia is the primary cause of T2DM pathophysiological abnormalities, it also contributes to the development of cancer through several processes that either directly or indirectly harm DNA, RNA, lipids, and proteins. The production of ROS, accumulation of mutations and inhibition of their repair, alteration of the immune system, alteration of metabolism, and activation of oncogenes and inactivation tumor suppressor genes are some of the carcinogenic effects that result from the formation of AGEs through non-enzymatic reactions and the subsequent activation of RAGE[101]. Endogenous AGEs are categorized according to their precursor as follows: Glyoxal (GO)-derived compounds including glyoxal lysine dimer, N7-(carboxymethyl)arginine, and CML; methylglyoxal-derived, including MG-H1, methylglyoxal lysine, argpyrimidine, and CEL; 3-deoxyglucosone-derived, including pyrraline, pentosidine, and deoxyglucosone lysine dimer; and derivatives of glucose, fructose, and glyceraldehyde that form DNA adducts or cross-link with lysine or arginine altering protein structure and function[102]. These non-enzymatic protein modifications elevate oxidative stress and inflammation by binding with cell surface receptors such as RAGE. Exogenous AGEs play a role in the progression of cancer in addition to endogenous AGEs[29,103,104]. The metabolism and pathogenic effects of endogenous and exogenous AGEs have recently been the subject of extensive reviews[105].
Interactions between RAGE and its ligands in T2DM result in various cellular responses, including activation of signaling pathways that cause oxidative stress and inflammation, which in turn cause various pathophysiological effects such as apoptosis, autophagy[106], senescence, and osteogenic differentiation[107], remodeling processes of the extracellular matrix, and activation of fibroblasts significant in vascular, neuronal[108], and musculoskeletal processes[109]. AGEs in T2DM accumulate in the extracellular matrix, forming cross-links with type I collagen and allowing long-lasting activation of RAGE. This also initiates a complex signaling network that allows the formation of ROS, activates the signaling pathway through ERK1/2 which then phosphorylates and activates NF-kB, and directly induces inflammation. Another alternative signaling pathway to the AGE-RAGE/ERK1-2/PKC pathway involves Rap-1, which induces inflammation, remodeling of the extracellular matrix, and oxidative stress[110].
Hypoxia is frequent in solid malignant neoplasms due to the high proliferation of neoplastic cells, which does not allow rapid vascularization of neoplastic tissue so that the oxygen demand exceeds the supply. Another factor is the formation of new blood vessels that do not have the integrity of their vascular wall; a continuous outflow of blood results in tissue oxygenation deficiency[111]. Under these conditions, a series of genes regulated by HIF-1α are activated, allowing survival through the expression of genes that promote angiogenesis, metabolic reprogramming, lipid accumulation[112], inhibition of apoptosis, invasion, and metastasis. HIF-1α also promotes inflammation via NF-kB signaling in hypoxic environments. In this tumor niche with inflammation, hypoxia, and cell death, DAMPs activate the NF-kB pathway mediated by RAGE, thereby amplifying HIF-1α activity[113]. In this hypoxic setting, stromal cells are also affected by RAGE; this is the case in adipocytes, where the AGE/RAGE/NF-kB pathway is activated and prolongs the inflammatory and hypoxic processes. Other effects of hypoxia include the stimulation of cell adhesion mediated by MCP-1, chemotaxis, and the polarization of macrophages towards a proinflammatory phenotype, specifically through the RAGE/NF-kB pathway in tryptophan hydroxylase 1 monocytes[114].
Cell death is a physiological process that keeps tissues healthy by systematically removing damaged cells to prevent an immune response. Although necrosis is a kind of cell death, it is pathological and only happens when there has been a significant tissue injury coupled with an immune response. Non-pathological cell death can take many forms, including apoptosis, necroptosis, and autophagy[115]. RAGE is involved in all three death pathways and can be activated by AGEs, HMGB1, and S100. In normal tissues, both the intrinsic and extrinsic apoptosis pathways are activated[116], and ROS, NF-kB, and MAPK mediate the stimulation. High levels of ROS induce the apoptosis pathway, but if they are low, autophagy is activated. Reduced HMGB1 activates Beclin-1-mediated autophagy pathways, but if oxidized, it activates apoptosis[117].
RAGE promotes cancer cell autophagy, which eventually permits survival by utilizing nutrients through the catabolism of their cellular components in a blood-free environment with no access to external nutrients and hypoxia. RAGE-dependent signaling pathways that promote autophagy involve PI3K, NF-kB/Beclin-1, PKC, and/or RAF/p38-MAPK/ERK[118]. Likewise, in cancer cells, apoptosis is inhibited, which indirectly allows cell perpetuation and survival. The pathways that inhibit apoptosis start with the binding of HMBG1/RAGE, which induces the formation of ROS and activation of NF-kB; another pathway involves Akt and matrix metalloproteinase-9[119].
Cell senescence is present in T2DM and cancer. Frequently occurring in tissues undergoing metabolic shock, chronic inflammation, or oxidative stress, cell senescence is a physiological response that aims to prevent genomic instability and the consequent DNA damage that leads to metabolic reprogramming. In addition, senescence relates to decreasing immune surveillance, thus facilitating cancer initiation and progression[109,119-121]. The same markers found in the carcinogenesis process discussed above, such as IGF, HIF-1α, AGEs, and RAGE, were discovered in a proteomics study looking for plasma proteins that indicate a senescence-related decline in health[122]. In a model of endothelial senescence induced by protein products of advanced oxidation, the presence of modified p53 at amino acid K386 by SUMOylation was associated with evasion of apoptosis[123].
RAGE aids in the removal of endotoxins and debris from apoptotic bodies during the processes of oxidative stress, hypoxia, and inflammation. Cellular damage occurs that causes the release of intracellular molecules that, outside the cell, behave as alarmins, specifically the S100 and HMBG1 proteins, also known as DAMPs, which act as endogenous RAGE ligands[124]. These proteins are also known as “moonlighting proteins” since they have various functions depending on their location. For example, when the HMGB1 protein locates inside the nucleus, it organizes chromatin[125]. In contrast, S100 is a protein that functions as a Ca2+ sensor[126], and like HMGB1, when located extracellularly, it functions as an alarmin. Tumor initiation and progression, as well as tissue damage, are significantly influenced by endogenous DAMP/RAGE ligand signaling. Numerous malignancies, including colorectal[127], hepatocarcinoma[128], pancreatic[129], breast, and endometrial cancers, overexpress HMGB1 and S100[35].
The primary ligands that bind to RAGE in cancer cells, such as AGEs, HMGB1, and S100, activate several signaling pathways such as PI3K/Akt, ERK 1/2, JAK/STAT, Ras/MAPK, Rac/cdc42, p14/p42, p38, and SAP/JNK/MAPK, and transcription factors such as NF-kB, STAT3, HIF-1α, AP-1, and CREB[118,130], and thus activate a series of genes whose functions are essential in the initiation, promotion, and extension of various malignant neoplasms. These functions are known as “cancer hallmarks” and include cell proliferation, inhibition of apoptosis, inhibition of tumor suppressor genes, evasion of immunity, increased survival, invasion, metastasis, angiogenesis, genomic instability due to failure to repair mutations, and metabolic dysregulation[35,124] (Table 1).
| Neoplasia | Ligands and signaling pathway | Molecule expressed | Cancer hallmarks | TS/AM/CL | Ref. |
| Breast cancer | AGE/RAGE, ERK1/2; Akt, c-fos | IL-8/CXCR1/2 | Migration and invasion | CL; CAFs, TNBC (MDA-MB-231 cells) | Santolla et al[137] |
| HMGA1 | Cell proliferation, metastasis, and EMT | CL; TNBC (MDA-MB-231 and Hs578) | Shah et al[138] | ||
| HMGB1/RAGE | Motility, migration, invasion, and dysregulation of metabolism | TS; human breast cancer, AM; NOD/SCID mice, CL; human breast cancer cells (MCF-7, T-470, BT474, MDA-MB-231, ZR-75-30, BT549) and human fibroblast cells HFL1 | Chen et al[139] | ||
| HMGB1/PI3K/Akt | PD-L1 | Cell proliferation, migration, invasion, and T-cell apoptosis | CL; human breast cancer cells (MDA-MB-231 P, MDA-MB-231 BM) | Amornsupak et al[140] | |
| HMGB1, PI3K/Akt, mTOR | HIF-1α, VEGF | Migration and angiogenesis | TS; human breast cancer CL; human breast cancer cells MCF-7 | He et al[141] | |
| HMGB1/RAGE | Downregulation of miR-205 | Cell growth, invasion, and EMT | TS; human breast cancer CL; TNBC (MDA-MB-231, MDA-MB-453, MDA-MB-468) and NTNBC (MCF-7, MCF-10F) | Wang et al[142] | |
| HMGB1/RAGE, ERK 1/2, CREB | Bone metastasis and neurite outgrowth of nervous system cells | AM; 4T1 mice CL; mouse breast cancer 4T1, primary rat nervous system cells DRG, rat DRG/mouse neuroblastoma hybrid cells F11, immortalized rat DRG neuronal cells 50B11 | Okui et al[143] | ||
| S100A14/RAGE, NF-kB | CCL2/CXCL5 | Migration, invasion, and lung metastasis | TS; human breast cancer and paired adjacent breast normal, metastatic lymph node, and non-metastatic lymph node AM; BALB/c, BALB/c, SCID beige, C57BL/6J, CMV-CreC57BL/6J, S200-/- and S100A14-/- PyMT mice CL; human breast cancer cells MCF7, MCF10A, T47D, SKBR3, BT549, MDA-MB-231, MCF10AT, MCFCA1h, MCFCA1α and mouse breast cancer cells 4T1 | Li et al[144] | |
| S100A7/RAGE, PI3K/Akt, ERK1/2, STAT3 | IGF-1 | Angiogenesis | CL; human breast cancer cells MCF-7, T47D, and HUVECs cells | Muoio et al[145] | |
| S100A7/RAGE, cPLA | PGE2, CD163+ | Immunosuppression, M2-macrophages, CD4+, CD8+, and T cells | AM; NOD SCID gamma mice CL; human breast cancer cells MDA-MB-231, MDA-MB-468 and mouse mammary cancer cells MVT-1 | Mishra et al[146] | |
| S100A8/A9-RAGE, FAK, Akt, Hippo-YAK | FLNA, CTGF, Cyr61 | Cell proliferation and migration | CL; HEK293T and TNBC (MDA-MB-23 and BT-549) | Rigiracciolo et al[147] | |
| LPS/S100A7/TLR4/RAGE | Migration and invasion | AM; orthotopic breast cancer C57BL/6 mice model CL; murine mammary cancer cells EO771, MTV-1, murine metastatic mammary cells EO.2, human breast carcinoma cells SUM 159, MDA-MB-231 and MDA-MB-468 | Wilkie et al[148] | ||
| acHMGB1/RAGE, S100A4/RAGE, Gas6/AXL | CXCR4, CXCL12, CCL2, CD151 and α3β1-integrin | Cell proliferation, invasion, intravasation, and EMT | AM; murine orthotopic mammary cancer CL; human MSCs, geminin overexpressing breast tumors Gem197, Gem240, Gem256, Gem257 and Gem270 cells, CAFs, and M0- TAMs and M2-TAMs | Ryan et al[149] | |
| Colorectal cancer | S100A16 | Cancer prognostic marker | TS; human colorectal cancer | Sun et al[150] | |
| HMGB1/RAGE | PD-1 | Cancer prognostic marker | TS; human colorectal cancer CL; human colorectal cancer cells SW480, and SW620 | Huang et al[151] | |
| S100B/RAGE, NF-kB | VEGF-A | Proliferation, migration, and angiogenesis | CL; human colon cancer cells HCT116 | Zheng et al[152] | |
| IGF1R-Ras/RAGE-HMGB1, | Oncogenesis | TS; Human colorectal from diabetic patients | Niu et al[153] | ||
| AGEs/RAGE, KLF5 | MDM upregulation and RB and p53 downregulation | Cancer initiation and development | AM; diabetic mouse model and CL; human colon cancer cells HCT116 | Wang et al[154] | |
| TCTP, HMGB1/RAGE, NF-kB | Invasion and metastasis | TS; human colorectal AM; tumor xenografts BALB/c nude mice CL; human colon adenocarcinoma cells LoVo | Huang et al[155] | ||
| S100A9/RAGE/TLR4 | Arg-1, iNOS, IL-10 and ROS | Immune suppression and MDSC chemotaxis | TS; human colorectal cancer and normal colon CL; Human colorectal cells LoVo, and MDSCs | Huang et al[156] | |
| HMGB1/RAGE, Kras/Yap1 | Cell proliferation | CL; human colorectal cancer cells HCT116 and SW480 | Qian et al[157] | ||
| S100B/RAGE, p38/pAkt/mTOR | VEGF-R2, iNOS, VEGF | Cell proliferation, migration, invasion, and angiogenesis | CL; human colon adenocarcinoma cells CaCo | Seguella et al[158] | |
| HMGB1/RAGE, pERK1/2, pDRP1 | Cell viability, autophagy, and chemoresistance | TS; human colorectal AM; athymic nude BALBC/c mice CL; human colorectal cells SW480, SW620, and LoVo | Huang et al[159] | ||
| Hepatocellular carcinoma | S100A9-TLR4/RAGE-ROS, | NET | Cell proliferation, invasion, and metastasis | TS; HBV+ and HBV-hepatocellular carcinoma AM; BALB/c mice and C57BL/6 mice CL; human liver cells QSG-7701, human hepatocellular carcinoma cells HepG2.2.15, mouse hepatocellular carcinoma cells H22 and HUVEC cells | Zhan et al[160] |
| HMGB1/RAGE | Cell proliferation and tumor differentiation | TS; primary hepatocellular carcinoma | Ando et al[161] | ||
| HMGB1/RAGE, ATG7 | Cell proliferation, fibrosis, and autophagy | TS; mouse hepatocellular carcinoma AM; Atg7, RAGE, HMGB1 transgenic C57BL/6Jmouse | Khambu et al[128] | ||
| HMGB1/RAGE, JNK, OCT4/TGFb1 | miR-21, CD44 | Migration and invasion | TS; human hepatocellular carcinoma AM; BALB/c nu/nu mice CL; human hepatocellular carcinoma cells HepG2, HCCLM3, Huh7, SMMC7721 and MHCC97H | Li et al[162] | |
| S100A4/RAGE, b-catenin | OCT4, SOX2, CD44 and Nanog (stem cell-associated genes) | Fibrosis and carcinogenesis | TS; human hepatocellular carcinoma AM; S100a4-EGFP, S100A4+/+GFP, S100A4-/- transgenic mice. CL; human hepatocellular carcinoma cells Huh7 and murine liver cancer cells Hep1-6 | Li et al[163] | |
| HMGB1/RAGE, ERK1/2 | CXCL2, IL-8, TNF, IL-6, IL-10, IL-23-p19 | Macrophage activation and inflammation | AM; primary murine hepatocytes from male C57BI/6J mice, and primary murine splenocytes from male C57BI/CJ CC; murine hepatoma cells Hepa1-6 and Hep-56.1D, human hepatoma cells HepG2, RAW 264.7 macrophages and monocytic cells THP1 | Bachmann et al[164] | |
| HMGB1/RAGE, NF-kB | circRNA 101368, miR-200a | Cell migration | TS; human hepatocellular carcinoma CL; human hepatocellular carcinoma cells HCCLM3, MHCC97L, SMMC7721, Hep3B, HepG2 cells, and normal hepatocyte cells THLE-3 | Li et al[165] | |
| Pancreatic cancer | RAGE | NET | Neutrophil autophagy | TS; human pancreatic carcinoma AM; Wild type C57BL6 mice and RAGE-/- C57BL6 mice, orthotopic pancreatic cancer model, CL; murine pancreatic cancer cells Panc02, MDSCs cells | Boone et al[166] |
| RAGE, PI3K/AKT/mTOR | Cell viability | CL; human pancreatic cancer cells MIA Paca-2, BxPC-3, AsPC-1, HPAC, PANC-1, MIA PacaGEMR | Lan et al[167] | ||
| RAGE, ERK1/2/Akt | Alpha 2 and alpha 1 integrin downregulation | Cell proliferation, invasion, and migration | CL; human pancreatic cancer cells Panc-1 | Swami et al[129] | |
| AGE/RAGE, TGFb1 | a-SMA, collagen 1, IL-6 | Fibrosis and EMT | TS; human pancreatic ductal adenocarcinoma AM; WT-C57BL/6 and RG-C57BL/6 mice CL; primary PSC, human pancreatic ductal adenocarcinoma cells BxPC-3 and AsPC-1 | Uchida et al[168] | |
| HMGB1/RAGE, PI3K/Akt | Atg5, Beclin-1, LC3-II | Autophagia and apoptosis inhibition | CL; human pancreatic cancer cells MIA Paca-2 and MIA PacaGEMR | Chen et al[169] |
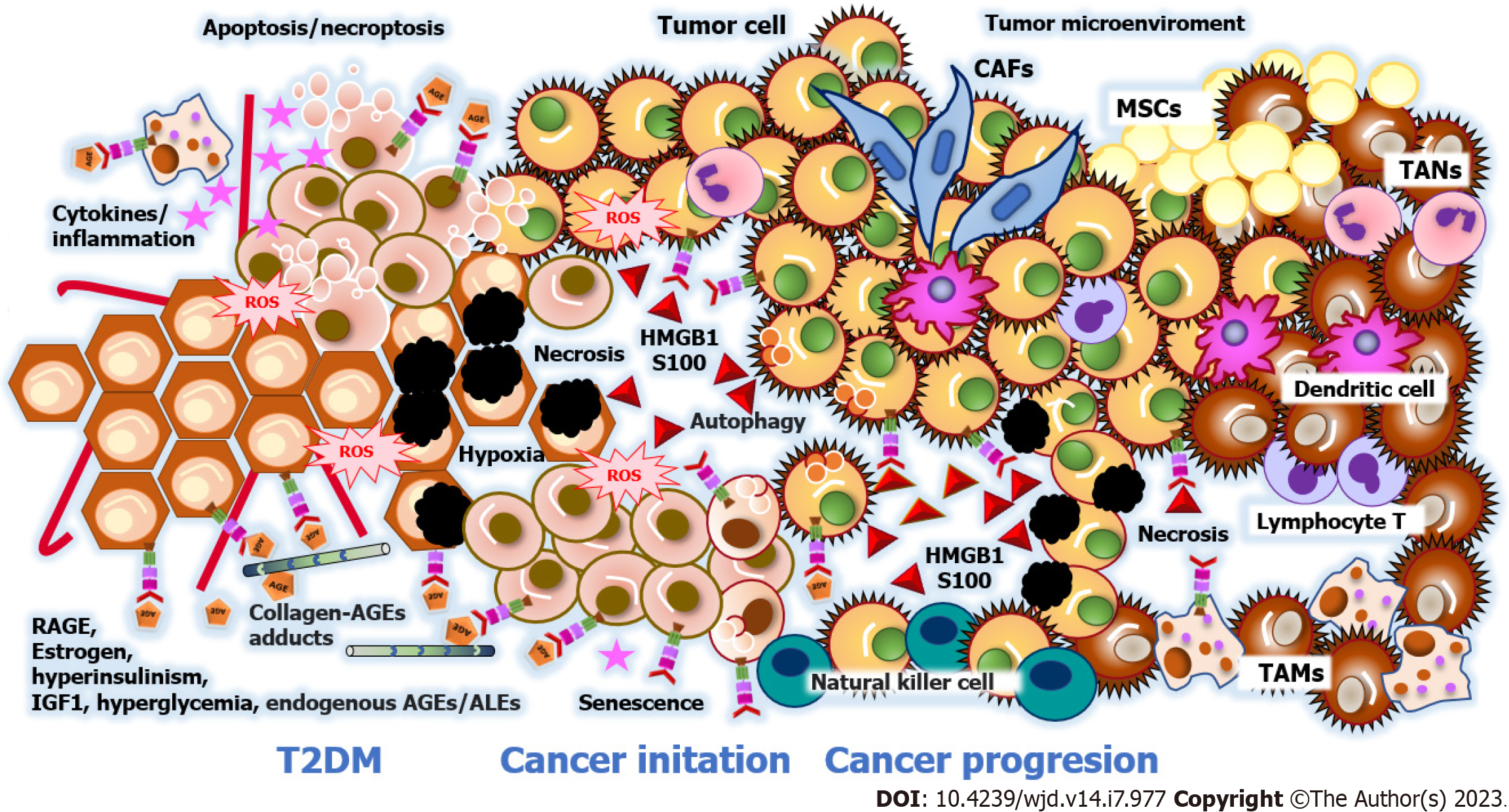
Tumorigenesis is the process by which healthy cells develop the capacity to become cancerous cells, which implies, in addition to genetic and epigenetic alterations in DNA, the formation of the tumor microenvironment. The tumor microenvironment is determined by the interaction between resident immune cells, mesenchymal stromal cells, and tumor cells, the paracrine signaling between them, and the anatomical niche built-up by the extracellular matrix and blood vessels. In addition to cancer-affected fibroblasts, the tumor microenvironment contains infiltrating tumor-associated macrophages that promote tumor survival[131,132]. The tumor microenvironment includes the extracellular matrix, blood vascular structures, and paracrine signaling between stromal cells and tumor cells (Figure 2).
Recent studies have revealed that the involved cells and specialized three-dimensional structures are unique to each tumor by tissue[133-135]. Table 1 outlines the traits of the tumor microenvironment in hepatocarcinoma, colorectal, breast, and pancreatic cancers with RAGE implications. These findings demonstrate that RAGE promotes different adaptive phenomena for the survival, initiation, and progression of malignant tumors. Nevertheless, it is necessary to mention that RAGE overexpression varies in cancer related to T2DM because of the cellular heterogeneity of the neoplastic process. The Human Protein Atlas database shows RAGE detection rates in malignant cells by immunohistochemistry as follows: Hepatocarcinoma at 50%; pancreatic cancer at 33.3%; breast cancer at 25%; endometrial cancer at 16.6%; and colorectal cancer at 8.3%[136].
RAGE is an environmental sensor with complex and multiple functions involved in every stage along the patho-physiological pathways that lead to the progression of obesity, T2DM, and cancer. Therefore, it is crucial to analyze each of the processes that RAGE is involved in, as the assimilation of this information could help in developing more accurate diagnostic and treatment approaches. For instance, this review has highlighted how RAGE acts from the earliest stages of the initiation and development of obesity, T2DM, and cancer. Recognizing all participating RAGE isoforms in their tissue and cellular locations could predict the progression points and provide diagnostic markers. In this manner, we would also be able to distinguish between a patient who is obese, has a low grade of inflammation, and is on the frontline of developing T2DM or most likely to respond to nutritional intervention.
On the other hand, RAGE participates in the initiation of neoplastic processes. Since its presence indicates cellular senescence and the presence of cancer cells with more aggressive activity, it is not surprising related to a poor prognosis and has potential as a cancer biomarker to help predict patient outcomes. Since RAGE participates even in the first stages, it has potential as a preventive and immunomodulator for therapeutic purposes to reduce morbidity and mortality associated with the development of obesity, T2DM, and cancer. Inhibitors of RAGE may be helpful in the treatment of obesity and diabetes mellitus. Studies have shown that RAGE is overexpressed in AT. Obesity is well known to contribute to inflammation and insulin resistance, which are hallmarks of obesity and diabetes. RAGE inhibitors could reduce inflammation and improve insulin sensitivity in obesity and T2DM; however, the majority of RAGE inhibitor studies have focused on cancer treatment. Some RAGE inhibitors under study are cromolyn, RAP, RAGE peptide antagonist, and gefitinib. While there are currently no RAGE-specific therapies approved for use in humans, there are pre-clinical studies investigating the potential of RAGE inhibitors as a treatment for various diseases. We review herein the topically relevant literature, delimiting by process, organ, and tissue to provide a progressive and systemic overview. It should be read and generalized with caution, as there are still many gaps in the knowledge about RAGE since most studies are experimental-based (in mice) and cross-sectional studies (in humans).
The authors acknowledge Ruelas-Cinco EC for providing some photographic images of RAGE immunocytochemistry in peripheral blood mononuclear cells from her thesis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pahlavani HA, Iran; Preziosi F, Italy; Srinivasu PN, India; Yang JS, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhao S
| 1. | World Health Organization. Noncommunicable diseases. [cited 5 December 2022]. Available from: https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1. |
| 2. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4814] [Article Influence: 1604.7] [Reference Citation Analysis (36)] |
| 3. | Ugai T, Sasamoto N, Lee HY, Ando M, Song M, Tamimi RM, Kawachi I, Campbell PT, Giovannucci EL, Weiderpass E, Rebbeck TR, Ogino S. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19:656-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 283] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 4. | Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3505] [Cited by in RCA: 3681] [Article Influence: 216.5] [Reference Citation Analysis (1)] |
| 5. | Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 780] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Lane MM, Davis JA, Beattie S, Gómez-Donoso C, Loughman A, O'Neil A, Jacka F, Berk M, Page R, Marx W, Rocks T. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes Rev. 2021;22:e13146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 332] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 7. | Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 430] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 8. | van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med. 2013;71:174-187. [PubMed] |
| 9. | Chuah YK, Basir R, Talib H, Tie TH, Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflam. 2013;2013:403460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009;52:2251-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 12. | Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 506] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Clynes R, Moser B, Yan SF, Ramasamy R, Herold K, Schmidt AM. Receptor for AGE (RAGE): weaving tangled webs within the inflammatory response. Curr Mol Med. 2007;7:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Feng Z, Zhu L, Wu J. RAGE signalling in obesity and diabetes: focus on the adipose tissue macrophage. Adipocyte. 2020;9:563-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Arivazhagan L, Popp CJ, Ruiz HH, Wilson RA, Manigrasso MB, Shekhtman A, Ramasamy R, Sevick MA, Schmidt AM. The RAGE/DIAPH1 axis: mediator of obesity and proposed biomarker of human cardiometabolic disease. Cardiovasc Res. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Bettiga A, Fiorio F, Di Marco F, Trevisani F, Romani A, Porrini E, Salonia A, Montorsi F, Vago R. The Modern Western Diet Rich in Advanced Glycation End-Products (AGEs): An Overview of Its Impact on Obesity and Early Progression of Renal Pathology. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Arivazhagan L, López-Díez R, Shekhtman A, Ramasamy R, Schmidt AM. Glycation and a Spark of ALEs (Advanced Lipoxidation End Products) - Igniting RAGE/Diaphanous-1 and Cardiometabolic Disease. Front Cardiovasc Med. 2022;9:937071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Nemet I, Varga-Defterdarović L, Turk Z. Methylglyoxal in food and living organisms. Mol Nutr Food Res. 2006;50:1105-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 551] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 20. | Scheijen JLJM, Clevers E, Engelen L, Dagnelie PC, Brouns F, Stehouwer CDA, Schalkwijk CG. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016;190:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 21. | Robinson AB, Stogsdill JA, Lewis JB, Wood TT, Reynolds PR. RAGE and tobacco smoke: insights into modeling chronic obstructive pulmonary disease. Front Physiol. 2012;3:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Prasad K, Dhar I, Caspar-Bell G. Role of Advanced Glycation End Products and Its Receptors in the Pathogenesis of Cigarette Smoke-Induced Cardiovascular Disease. Int J Angiol. 2015;24:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Kwon OS, Decker ST, Zhao J, Hoidal JR, Heuckstadt T, Sanders KA, Richardson RS, Layec G. The receptor for advanced glycation end products (RAGE) is involved in mitochondrial function and cigarette smoke-induced oxidative stress. Free Radic Biol Med. 2023;195:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Chapman S, Mick M, Hall P, Mejia C, Sue S, Abdul Wase B, Nguyen MA, Whisenant EC, Wilcox SH, Winden D, Reynolds PR, Arroyo JA. Cigarette smoke extract induces oral squamous cell carcinoma cell invasion in a receptor for advanced glycation end-products-dependent manner. Eur J Oral Sci. 2018;126:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Li Y, Qin M, Zhong W, Liu C, Deng G, Yang M, Li J, Ye H, Shi H, Wu C, Lin H, Chen Y, Huang S, Zhou C, Lv Z, Gao L. RAGE promotes dysregulation of iron and lipid metabolism in alcoholic liver disease. Redox Biol. 2023;59:102559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 26. | Birukov A, Cuadrat R, Polemiti E, Eichelmann F, Schulze MB. Advanced glycation end-products, measured as skin autofluorescence, associate with vascular stiffness in diabetic, pre-diabetic and normoglycemic individuals: a cross-sectional study. Cardiovasc Diabetol. 2021;20:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Fujiwara Y, Kiyota N, Tsurushima K, Yoshitomi M, Mera K, Sakashita N, Takeya M, Ikeda T, Araki T, Nohara T, Nagai R. Natural compounds containing a catechol group enhance the formation of Nε-(carboxymethyl)lysine of the Maillard reaction. Free Radic Biol Med. 2011;50:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 273] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 325] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 30. | Ryder E, Pedreañez A, Vargas R, Peña C, Fernandez E, Diez-Ewald M, Mosquera J. Increased proinflammatory markers and lipoperoxidation in obese individuals: Inicial inflammatory events? Diabetes Metab Syndr. 2015;9:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Mishra S, Mishra BB. Study of Lipid Peroxidation, Nitric Oxide End Product, and Trace Element Status in Type 2 Diabetes Mellitus with and without Complications. Int J Appl Basic Med Res. 2017;7:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Jaganjac M, Tirosh O, Cohen G, Sasson S, Zarkovic N. Reactive aldehydes--second messengers of free radicals in diabetes mellitus. Free Radic Res. 2013;47 Suppl 1:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 33. | Iacobini C, Menini S, Ricci C, Scipioni A, Sansoni V, Mazzitelli G, Cordone S, Pesce C, Pugliese F, Pricci F, Pugliese G. Advanced lipoxidation end-products mediate lipid-induced glomerular injury: role of receptor-mediated mechanisms. J Pathol. 2009;218:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Shanmugam N, Figarola JL, Li Y, Swiderski PM, Rahbar S, Natarajan R. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes. 2008;57:879-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 257] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 35. | Palanissami G, Paul SFD. RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer-a Review. Horm Cancer. 2018;9:295-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 36. | van Dongen KCW, Kappetein L, Miro Estruch I, Belzer C, Beekmann K, Rietjens IMCM. Differences in kinetics and dynamics of endogenous versus exogenous advanced glycation end products (AGEs) and their precursors. Food Chem Toxicol. 2022;164:112987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Lyte JM, Gabler NK, Hollis JH. Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross-over study. Lipids Health Dis. 2016;15:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Li Y, Peng Y, Shen Y, Zhang Y, Liu L, Yang X. Dietary polyphenols: regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit Rev Food Sci Nutr. 2022;1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 39. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4564] [Article Influence: 253.6] [Reference Citation Analysis (1)] |
| 40. | Wang L, Wu J, Guo X, Huang X, Huang Q. RAGE Plays a Role in LPS-Induced NF-κB Activation and Endothelial Hyperpermeability. Sensors (Basel). 2017;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 42. | Wang X, Wang Y, Antony V, Sun H, Liang G. Metabolism-Associated Molecular Patterns (MAMPs). Trends Endocrinol Metab. 2020;31:712-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 43. | Turki Jalil A, Alameri AA, Iqbal Doewes R, El-Sehrawy AA, Ahmad I, Ramaiah P, Kadhim MM, Kzar HH, Sivaraman R, Romero-Parra RM, Ansari MJ, Fakri Mustafa Y. Circulating and dietary advanced glycation end products and obesity in an adult population: A paradox of their detrimental effects in obesity. Front Endocrinol (Lausanne). 2022;13:966590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 44. | Ruiz HH, Ramasamy R, Schmidt AM. Advanced Glycation End Products: Building on the Concept of the "Common Soil" in Metabolic Disease. Endocrinology. 2020;161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 45. | Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, Rensen SS, Buurman WA, Greve JW, Blaak EE, van Zandvoort MA, Bierhaus A, Stehouwer CD, Schalkwijk CG. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 46. | Sebeková K, Krivošíková Z, Gajdoš M. Total plasma Nε-(carboxymethyl)lysine and sRAGE levels are inversely associated with a number of metabolic syndrome risk factors in non-diabetic young-to-middle-aged medication-free subjects. Clin Chem Lab Med. 2014;52:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Dozio E, Vianello E, Briganti S, Lamont J, Tacchini L, Schmitz G, Corsi Romanelli MM. Expression of the Receptor for Advanced Glycation End Products in Epicardial Fat: Link with Tissue Thickness and Local Insulin Resistance in Coronary Artery Disease. J Diabetes Res. 2016;2016:2327341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Santangelo C, Filardi T, Perrone G, Mariani M, Mari E, Scazzocchio B, Masella R, Brunelli R, Lenzi A, Zicari A, Morano S. Cross-talk between fetal membranes and visceral adipose tissue involves HMGB1-RAGE and VIP-VPAC2 pathways in human gestational diabetes mellitus. Acta Diabetol. 2019;56:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Ruiz HH, Nguyen A, Wang C, He L, Li H, Hallowell P, McNamara C, Schmidt AM. AGE/RAGE/DIAPH1 axis is associated with immunometabolic markers and risk of insulin resistance in subcutaneous but not omental adipose tissue in human obesity. Int J Obes (Lond). 2021;45:2083-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Du Z, Wu J, Feng Z, Ma X, Zhang T, Shu X, Xu J, Wang L, Luo M. RAGE displays sex-specific differences in obesity-induced adipose tissue insulin resistance. Biol Sex Differ. 2022;13:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 51. | Song F, Hurtado del Pozo C, Rosario R, Zou YS, Ananthakrishnan R, Xu X, Patel PR, Benoit VM, Yan SF, Li H, Friedman RA, Kim JK, Ramasamy R, Ferrante AW Jr, Schmidt AM. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63:1948-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 52. | Ding YS, Malik N, Mendoza S, Tuchman D, Del Pozo CH, Diez RL, Schmidt AM. PET imaging study of brown adipose tissue (BAT) activity in mice devoid of receptor for advanced glycation end products (RAGE). J Biosci. 2019;44. [PubMed] |
| 53. | da Silva Rosa SC, Nayak N, Caymo AM, Gordon JW. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol Rep. 2020;8:e14607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 54. | Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 367] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 55. | Priken K, Tapia G, Cadagan C, Quezada N, Torres J, D'Espessailles A, Pettinelli P. Higher hepatic advanced glycation end products and liver damage markers are associated with nonalcoholic steatohepatitis. Nutr Res. 2022;104:71-81. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 56. | Gaens KH, Niessen PM, Rensen SS, Buurman WA, Greve JW, Driessen A, Wolfs MG, Hofker MH, Bloemen JG, Dejong CH, Stehouwer CD, Schalkwijk CG. Endogenous formation of Nε-(carboxymethyl)lysine is increased in fatty livers and induces inflammatory markers in an in vitro model of hepatic steatosis. J Hepatol. 2012;56:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | de la Maza MP, Uribarri J, Olivares D, Hirsch S, Leiva L, Barrera G, Bunout D. Weight increase is associated with skeletal muscle immunostaining for advanced glycation end products, receptor for advanced glycation end products, and oxidation injury. Rejuvenation Res. 2008;11:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Rai AK, Jaiswal N, Maurya CK, Sharma A, Ahmad I, Ahmad S, Gupta AP, Gayen JR, Tamrakar AK. Fructose-induced AGEs-RAGE signaling in skeletal muscle contributes to impairment of glucose homeostasis. J Nutr Biochem. 2019;71:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Dozio E, Vettoretti S, Lungarella G, Messa P, Corsi Romanelli MM. Sarcopenia in Chronic Kidney Disease: Focus on Advanced Glycation End Products as Mediators and Markers of Oxidative Stress. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Riuzzi F, Sorci G, Sagheddu R, Chiappalupi S, Salvadori L, Donato R. RAGE in the pathophysiology of skeletal muscle. J Cachexia Sarcopenia Muscle. 2018;9:1213-1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 61. | Basta G, Sironi AM, Lazzerini G, Del Turco S, Buzzigoli E, Casolaro A, Natali A, Ferrannini E, Gastaldelli A. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab. 2006;91:4628-4634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 62. | Miranda ER, Somal VS, Mey JT, Blackburn BK, Wang E, Farabi S, Karstoft K, Fealy CE, Kashyap S, Kirwan JP, Quinn L, Solomon TPJ, Haus JM. Circulating soluble RAGE isoforms are attenuated in obese, impaired-glucose-tolerant individuals and are associated with the development of type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313:E631-E640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Momma H, Niu K, Kobayashi Y, Huang C, Chujo M, Otomo A, Tadaura H, Miyata T, Nagatomi R. Higher serum soluble receptor for advanced glycation end product levels and lower prevalence of metabolic syndrome among Japanese adult men: a cross-sectional study. Diabetol Metab Syndr. 2014;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Zaki M, Kamal S, Kholousi S, El-Bassyouni HT, Yousef W, Reyad H, Mohamed R, Basha WA. Serum soluble receptor of advanced glycation end products and risk of metabolic syndrome in Egyptian obese women. EXCLI J. 2017;16:973-980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 65. | Biswas SK, Mohtarin S, Mudi SR, Anwar T, Banu LA, Alam SM, Fariduddin M, Arslan MI. Relationship of Soluble RAGE with Insulin Resistance and Beta Cell Function during Development of Type 2 Diabetes Mellitus. J Diabetes Res. 2015;2015:150325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Hudson BI, Dong C, Gardener H, Elkind MS, Wright CB, Goldberg R, Sacco RL, Rundek T. Serum levels of soluble receptor for advanced glycation end-products and metabolic syndrome: the Northern Manhattan Study. Metabolism. 2014;63:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Huang M, Que Y, Shen X. Correlation of the plasma levels of soluble RAGE and endogenous secretory RAGE with oxidative stress in pre-diabetic patients. J Diabetes Complications. 2015;29:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Prasad K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases? Mol Cell Biochem. 2019;451:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 69. | Erusalimsky JD. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021;42:101958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 70. | Prasad K, Khan AS, Bhanumathy KK. Does AGE-RAGE Stress Play a Role in the Development of Coronary Artery Disease in Obesity? Int J Angiol. 2022;31:1-9. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 71. | Di Pino A, Urbano F, Zagami RM, Filippello A, Di Mauro S, Piro S, Purrello F, Rabuazzo AM. Low Endogenous Secretory Receptor for Advanced Glycation End-Products Levels Are Associated With Inflammation and Carotid Atherosclerosis in Prediabetes. J Clin Endocrinol Metab. 2016;101:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Sabbatinelli J, Castiglione S, Macrì F, Giuliani A, Ramini D, Vinci MC, Tortato E, Bonfigli AR, Olivieri F, Raucci A. Circulating levels of AGEs and soluble RAGE isoforms are associated with all-cause mortality and development of cardiovascular complications in type 2 diabetes: a retrospective cohort study. Cardiovasc Diabetol. 2022;21:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 73. | Scavello F, Tedesco CC, Castiglione S, Maciag A, Sangalli E, Veglia F, Spinetti G, Puca AA, Raucci A. Modulation of soluble receptor for advanced glycation end products isoforms and advanced glycation end products in long-living individuals. Biomark Med. 2021;15:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Palma-Duran SA, Kontogianni MD, Vlassopoulos A, Zhao S, Margariti A, Georgoulis M, Papatheodoridis G, Combet E. Serum levels of advanced glycation end-products (AGEs) and the decoy soluble receptor for AGEs (sRAGE) can identify non-alcoholic fatty liver disease in age-, sex- and BMI-matched normo-glycemic adults. Metabolism. 2018;83:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | Popp CJ, Zhou B, Manigrasso MB, Li H, Curran M, Hu L, St-Jules DE, Alemán JO, Vanegas SM, Jay M, Bergman M, Segal E, Sevick MA, Schmidt AM. Soluble Receptor for Advanced Glycation End Products (sRAGE) Isoforms Predict Changes in Resting Energy Expenditure in Adults with Obesity during Weight Loss. Curr Dev Nutr. 2022;6:nzac046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Hurtado Del Pozo C, Ruiz HH, Arivazhagan L, Aranda JF, Shim C, Daya P, Derk J, MacLean M, He M, Frye L, Friedline RH, Noh HL, Kim JK, Friedman RA, Ramasamy R, Schmidt AM. A Receptor of the Immunoglobulin Superfamily Regulates Adaptive Thermogenesis. Cell Rep. 2019;28:773-791.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 77. | Popa I, Ganea E, Petrescu SM. Expression and subcellular localization of RAGE in melanoma cells. Biochem Cell Biol. 2014;92:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Ruelas Cinco EDC, Ruíz Madrigal B, Domínguez Rosales JA, Maldonado González M, De la Cruz Color L, Ramírez Meza SM, Torres Baranda JR, Martínez López E, Hernández Nazará ZH. Expression of the receptor of advanced glycation end-products (RAGE) and membranal location in peripheral blood mononuclear cells (PBMC) in obesity and insulin resistance. Iran J Basic Med Sci. 2019;22:623-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 79. | Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;272:16498-16506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 394] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 80. | Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889-9897. [PubMed] |
| 81. | Corica D, Aversa T, Ruggeri RM, Cristani M, Alibrandi A, Pepe G, De Luca F, Wasniewska M. Could AGE/RAGE-Related Oxidative Homeostasis Dysregulation Enhance Susceptibility to Pathogenesis of Cardio-Metabolic Complications in Childhood Obesity? Front Endocrinol (Lausanne). 2019;10:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Xia B, Zhu R, Zhang H, Chen B, Liu Y, Dai X, Ye Z, Zhao D, Mo F, Gao S, Wang XD, Bromme D, Wang L, Wang X, Zhang D. Lycopene Improves Bone Quality and Regulates AGE/RAGE/NF-кB Signaling Pathway in High-Fat Diet-Induced Obese Mice. Oxid Med Cell Longev. 2022;2022:3697067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Pereira ENGDS, Araujo BP, Rodrigues KL, Silvares RR, Martins CSM, Flores EEI, Fernandes-Santos C, Daliry A. Simvastatin Improves Microcirculatory Function in Nonalcoholic Fatty Liver Disease and Downregulates Oxidative and ALE-RAGE Stress. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 84. | Ji J, Feng M, Huang Y, Niu X. Liraglutide inhibits receptor for advanced glycation end products (RAGE)/reduced form of nicotinamide-adenine dinucleotide phosphate (NAPDH) signaling to ameliorate non-alcoholic fatty liver disease (NAFLD) in vivo and vitro. Bioengineered. 2022;13:5091-5102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 85. | Zhu Y, Shu T, Lin Y, Wang H, Yang J, Shi Y, Han X. Inhibition of the receptor for advanced glycation endproducts (RAGE) protects pancreatic β-cells. Biochem Biophys Res Commun. 2011;404:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 86. | Han D, Yamamoto Y, Munesue S, Motoyoshi S, Saito H, Win MT, Watanabe T, Tsuneyama K, Yamamoto H. Induction of receptor for advanced glycation end products by insufficient leptin action triggers pancreatic β-cell failure in type 2 diabetes. Genes Cells. 2013;18:302-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Kehm R, Rückriemen J, Weber D, Deubel S, Grune T, Höhn A. Endogenous advanced glycation end products in pancreatic islets after short-term carbohydrate intervention in obese, diabetes-prone mice. Nutr Diabetes. 2019;9:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Bai R, Zhang T, Gao Y, Shu T, Zhou Y, Wang F, Chang X, Tang W, Zhu Y, Han X. Rab31, a receptor of advanced glycation end products (RAGE) interacting protein, inhibits AGE induced pancreatic β-cell apoptosis through the pAKT/BCL2 pathway. Endocr J. 2022;69:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Bayatpoor ME, Mirzaee S, Karami Abd M, Mohammadi MT, Shahyad S, Bahari Z, Raouf Sarshoori J. Crocin treatment decreased pancreatic atrophy, LOX-1 and RAGE mRNA expression of pancreas tissue in cholesterol-fed and streptozotocin-induced diabetic rats. J Complement Integr Med. 2019;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Lee BW, Chae HY, Kwon SJ, Park SY, Ihm J, Ihm SH. RAGE ligands induce apoptotic cell death of pancreatic β-cells via oxidative stress. Int J Mol Med. 2010;26:813-818. [PubMed] |
| 91. | Abedini A, Cao P, Plesner A, Zhang J, He M, Derk J, Patil SA, Rosario R, Lonier J, Song F, Koh H, Li H, Raleigh DP, Schmidt AM. RAGE binds preamyloid IAPP intermediates and mediates pancreatic β cell proteotoxicity. J Clin Invest. 2018;128:682-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 92. | Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 564] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 93. | Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, Rizos EC, Monori G, Ward HA, Kyrgiou M, Gunter MJ, Tsilidis KK. Type 2 Diabetes and Cancer: An Umbrella Review of Observational and Mendelian Randomization Studies. Cancer Epidemiol Biomarkers Prev. 2021;30:1218-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 94. | Dashti SG, Simpson JA, Viallon V, Karahalios A, Moreno-Betancur M, Brasky T, Pan K, Rohan TE, Shadyab AH, Thomson CA, Wild RA, Wassertheil-Smoller S, Ho GYF, Strickler HD, English DR, Gunter MJ. Adiposity and breast, endometrial, and colorectal cancer risk in postmenopausal women: Quantification of the mediating effects of leptin, C-reactive protein, fasting insulin, and estradiol. Cancer Med. 2022;11:1145-1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 95. | Brown KA. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol. 2021;17:350-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 96. | Mahboobifard F, Pourgholami MH, Jorjani M, Dargahi L, Amiri M, Sadeghi S, Tehrani FR. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed Pharmacother. 2022;156:113808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 97. | Parida S, Sharma D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 98. | Scully T, Ettela A, LeRoith D, Gallagher EJ. Obesity, Type 2 Diabetes, and Cancer Risk. Front Oncol. 2020;10:615375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 99. | Kang C, LeRoith D, Gallagher EJ. Diabetes, Obesity, and Breast Cancer. Endocrinology. 2018;159:3801-3812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (40)] |
| 100. | Hopkins BD, Goncalves MD, Cantley LC. Insulin-PI3K signalling: an evolutionarily insulated metabolic driver of cancer. Nat Rev Endocrinol. 2020;16:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 101. | Ramteke P, Deb A, Shepal V, Bhat MK. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 102. | Lai SWT, Lopez Gonzalez EJ, Zoukari T, Ki P, Shuck SC. Methylglyoxal and Its Adducts: Induction, Repair, and Association with Disease. Chem Res Toxicol. 2022;35:1720-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 103. | Adeshara KA, Bangar N, Diwan AG, Tupe RS. Plasma glycation adducts and various RAGE isoforms are intricately associated with oxidative stress and inflammatory markers in type 2 diabetes patients with vascular complications. Diabetes Metab Syndr. 2022;16:102441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 104. | Eva TA, Barua N, Chowdhury MM, Yeasmin S, Rakib A, Islam MR, Emran TB, Simal-Gandara J. Perspectives on signaling for biological- and processed food-related advanced glycation end-products and its role in cancer progression. Crit Rev Food Sci Nutr. 2022;62:2655-2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 105. | Rao NL, Kotian GB, Shetty JK, Shelley BP, Dmello MK, Lobo EC, Shankar SP, Almeida SD, Shah SR. Receptor for Advanced Glycation End Product, Organ Crosstalk, and Pathomechanism Targets for Comprehensive Molecular Therapeutics in Diabetic Ischemic Stroke. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 106. | Li S, Yang D, Gao X, Yao S, Wang S, Zhu J, Shu J. Argpyrimidine bonded to RAGE regulates autophagy and cell cycle to cause periodontal destruction. J Cell Physiol. 2022;237:4460-4476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 107. | Wang Y, Jiang C, Shang Z, Qiu G, Yuan G, Xu K, Hou Q, He Y, Liu Y. AGEs/RAGE Promote Osteogenic Differentiation in Rat Bone Marrow-Derived Endothelial Progenitor Cells via MAPK Signaling. J Diabetes Res. 2022;2022:4067812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 108. | Gottschalk G, Peterson D, Knox K, Maynard M, Whelan RJ, Roy A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE). Mol Cell Neurosci. 2022;120:103731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 109. | Teissier T, Temkin V, Pollak RD, Cox LS. Crosstalk Between Senescent Bone Cells and the Bone Tissue Microenvironment Influences Bone Fragility During Chronological Age and in Diabetes. Front Physiol. 2022;13:812157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Burr SD, Dorroh CC, Stewart JA Jr. Rap1a Activity Elevated the Impact of Endogenous AGEs in Diabetic Collagen to Stimulate Increased Myofibroblast Transition and Oxidative Stress. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 111. | Taneja S, Vetter SW, Leclerc E. Hypoxia and the Receptor for Advanced Glycation End Products (RAGE) Signaling in Cancer. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 112. | Seo J, Yun JE, Kim SJ, Chun YS. Lipid metabolic reprogramming by hypoxia-inducible factor-1 in the hypoxic tumour microenvironment. Pflugers Arch. 2022;474:591-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 113. | Nie Y, Yang D, Oppenheim JJ. Alarmins and Antitumor Immunity. Clin Ther. 2016;38:1042-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 114. | Zhou J, Bai W, Liu Q, Cui J, Zhang W. Intermittent Hypoxia Enhances THP-1 Monocyte Adhesion and Chemotaxis and Promotes M1 Macrophage Polarization via RAGE. Biomed Res Int. 2018;2018:1650456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 115. | D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 1488] [Article Influence: 248.0] [Reference Citation Analysis (0)] |
| 116. | Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 876] [Cited by in RCA: 887] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 117. | Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299-5310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 118. | Waghela BN, Vaidya FU, Ranjan K, Chhipa AS, Tiwari BS, Pathak C. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol Cell Biochem. 2021;476:585-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 119. | Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, Bierhaus A, Lotze MT, Zeh HJ. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 276] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 120. | Kumar V, Agrawal R, Pandey A, Kopf S, Hoeffgen M, Kaymak S, Bandapalli OR, Gorbunova V, Seluanov A, Mall MA, Herzig S, Nawroth PP. Compromised DNA repair is responsible for diabetes-associated fibrosis. EMBO J. 2020;39:e103477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 121. | Melia F, Udomjarumanee P, Zinovkin D, Arghiani N, Pranjol MZI. Pro-tumorigenic role of type 2 diabetes-induced cellular senescence in colorectal cancer. Front Oncol. 2022;12:975644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 122. | Moaddel R, Ubaida-Mohien C, Tanaka T, Lyashkov A, Basisty N, Schilling B, Semba RD, Franceschi C, Gorospe M, Ferrucci L. Proteomics in aging research: A roadmap to clinical, translational research. Aging Cell. 2021;20:e13325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 123. | Chen Y, Liu Z, Chen H, Huang X, Lei Y, Liang Q, Wei J, Zhang Q, Guo X, Huang Q. p53 SUMOylation Mediates AOPP-Induced Endothelial Senescence and Apoptosis Evasion. Front Cardiovasc Med. 2021;8:795747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 124. | Garay-Sevilla ME, Gomez-Ojeda A, González I, Luévano-Contreras C, Rojas A. Contribution of RAGE axis activation to the association between metabolic syndrome and cancer. Mol Cell Biochem. 2021;476:1555-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Pujals M, Resar L, Villanueva J. HMGA1, Moonlighting Protein Function, and Cellular Real Estate: Location, Location, Location! Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15:96-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 620] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 127. | Yu GH, Li SF, Wei R, Jiang Z. Diabetes and Colorectal Cancer Risk: Clinical and Therapeutic Implications. J Diabetes Res. 2022;2022:1747326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 128. | Khambu B, Hong H, Liu S, Liu G, Chen X, Dong Z, Wan J, Yin XM. The HMGB1-RAGE axis modulates the growth of autophagy-deficient hepatic tumors. Cell Death Dis. 2020;11:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 129. | Swami P, Thiyagarajan S, Vidger A, Indurthi VSK, Vetter SW, Leclerc E. RAGE Up-Regulation Differently Affects Cell Proliferation and Migration in Pancreatic Cancer Cells. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 130. | El-Far AH, Sroga G, Jaouni SKA, Mousa SA. Role and Mechanisms of RAGE-Ligand Complexes and RAGE-Inhibitors in Cancer Progression. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 131. | Rojas A, Schneider I, Lindner C, Gonzalez I, Morales MA. The RAGE/multiligand axis: a new actor in tumor biology. Biosci Rep. 2022;42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 132. | Muthyalaiah YS, Jonnalagadda B, John CM, Arockiasamy S. Impact of Advanced Glycation End products (AGEs) and its receptor (RAGE) on cancer metabolic signaling pathways and its progression. Glycoconj J. 2021;38:717-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 133. | Ennis CS, Llevenes P, Qiu Y, Dries R, Denis GV. The crosstalk within the breast tumor microenvironment in type II diabetes: Implications for cancer disparities. Front Endocrinol (Lausanne). 2022;13:1044670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 134. | Azizian-Farsani F, Abedpoor N, Hasan Sheikhha M, Gure AO, Nasr-Esfahani MH, Ghaedi K. Receptor for Advanced Glycation End Products Acts as a Fuel to Colorectal Cancer Development. Front Oncol. 2020;10:552283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 135. | Mollace A, Coluccio ML, Donato G, Mollace V, Malara N. Cross-talks in colon cancer between RAGE/AGEs axis and inflammation/immunotherapy. Oncotarget. 2021;12:1281-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 136. | Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, Sanli K, von Feilitzen K, Oksvold P, Lundberg E, Hober S, Nilsson P, Mattsson J, Schwenk JM, Brunnström H, Glimelius B, Sjöblom T, Edqvist PH, Djureinovic D, Micke P, Lindskog C, Mardinoglu A, Ponten F. A pathology atlas of the human cancer transcriptome. Science. 2017;357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1721] [Cited by in RCA: 2353] [Article Influence: 294.1] [Reference Citation Analysis (0)] |
| 137. | Santolla MF, Talia M, Cirillo F, Scordamaglia D, De Rosis S, Spinelli A, Miglietta AM, Nardo B, Filippelli G, De Francesco EM, Belfiore A, Lappano R, Maggiolini M. The AGEs/RAGE Transduction Signaling Prompts IL-8/CXCR1/2-Mediated Interaction between Cancer-Associated Fibroblasts (CAFs) and Breast Cancer Cells. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 138. | Shah SN, Cope L, Poh W, Belton A, Roy S, Talbot CC Jr, Sukumar S, Huso DL, Resar LM. HMGA1: a master regulator of tumor progression in triple-negative breast cancer cells. PLoS One. 2013;8:e63419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 139. | Chen Y, Cai L, Guo X, Li Z, Liao X, Zhang X, Huang L, He J. HMGB1-activated fibroblasts promote breast cancer cells metastasis via RAGE/aerobic glycolysis. Neoplasma. 2021;68:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 140. | Amornsupak K, Thongchot S, Thinyakul C, Box C, Hedayat S, Thuwajit P, Eccles SA, Thuwajit C. HMGB1 mediates invasion and PD-L1 expression through RAGE-PI3K/AKT signaling pathway in MDA-MB-231 breast cancer cells. BMC Cancer. 2022;22:578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 141. | He H, Wang X, Chen J, Sun L, Sun H, Xie K. High-Mobility Group Box 1 (HMGB1) Promotes Angiogenesis and Tumor Migration by Regulating Hypoxia-Inducible Factor 1 (HIF-1α) Expression via the Phosphatidylinositol 3-Kinase (PI3K)/AKT Signaling Pathway in Breast Cancer Cells. Med Sci Monit. 2019;25:2352-2360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 142. | Wang L, Kang FB, Wang J, Yang C, He DW. Downregulation of miR-205 contributes to epithelial-mesenchymal transition and invasion in triple-negative breast cancer by targeting HMGB1-RAGE signaling pathway. Anticancer Drugs. 2019;30:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 143. | Okui T, Hiasa M, Ryumon S, Ono K, Kunisada Y, Ibaragi S, Sasaki A, Roodman GD, White FA, Yoneda T. The HMGB1/RAGE axis induces bone pain associated with colonization of 4T1 mouse breast cancer in bone. J Bone Oncol. 2021;26:100330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 144. | Li X, Wang M, Gong T, Lei X, Hu T, Tian M, Ding F, Ma F, Chen H, Liu Z. A S100A14-CCL2/CXCL5 signaling axis drives breast cancer metastasis. Theranostics. 2020;10:5687-5703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 145. | Muoio MG, Talia M, Lappano R, Sims AH, Vella V, Cirillo F, Manzella L, Giuliano M, Maggiolini M, Belfiore A, De Francesco EM. Activation of the S100A7/RAGE Pathway by IGF-1 Contributes to Angiogenesis in Breast Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 146. | Mishra S, Charan M, Shukla RK, Agarwal P, Misri S, Verma AK, Ahirwar DK, Siddiqui J, Kaul K, Sahu N, Vyas K, Garg AA, Khan A, Miles WO, Song JW, Bhutani N, Ganju RK. cPLA2 blockade attenuates S100A7-mediated breast tumorigenicity by inhibiting the immunosuppressive tumor microenvironment. J Exp Clin Cancer Res. 2022;41:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 147. | Rigiracciolo DC, Nohata N, Lappano R, Cirillo F, Talia M, Adame-Garcia SR, Arang N, Lubrano S, De Francesco EM, Belfiore A, Gutkind JS, Maggiolini M. Focal Adhesion Kinase (FAK)-Hippo/YAP transduction signaling mediates the stimulatory effects exerted by S100A8/A9-RAGE system in triple-negative breast cancer (TNBC). J Exp Clin Cancer Res. 2022;41:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 148. | Wilkie T, Verma AK, Zhao H, Charan M, Ahirwar DK, Kant S, Pancholi V, Mishra S, Ganju RK. Lipopolysaccharide from the commensal microbiota of the breast enhances cancer growth: role of S100A7 and TLR4. Mol Oncol. 2022;16:1508-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 149. | Ryan D, Koziol J, ElShamy WM. Targeting AXL and RAGE to prevent geminin overexpression-induced triple-negative breast cancer metastasis. Sci Rep. 2019;9:19150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 150. | Sun X, Wang T, Zhang C, Ning K, Guan ZR, Chen SX, Hong TT, Hua D. S100A16 is a prognostic marker for colorectal cancer. J Surg Oncol. 2018;117:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 151. | Huang CY, Chiang SF, Ke TW, Chen TW, Lan YC, You YS, Shiau AC, Chen WT, Chao KSC. Cytosolic high-mobility group box protein 1 (HMGB1) and/or PD-1+ TILs in the tumor microenvironment may be contributing prognostic biomarkers for patients with locally advanced rectal cancer who have undergone neoadjuvant chemoradiotherapy. Cancer Immunol Immunother. 2018;67:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 152. | Zheng J, Zhu W, He F, Li Z, Cai N, Wang HH. An Aptamer-Based Antagonist against the Receptor for Advanced Glycation End-Products (RAGE) Blocks Development of Colorectal Cancer. Mediators Inflamm. 2021;2021:9958051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 153. | Niu S, Zhao ZG, Lyu XM, Zhao M, Wang XZ, Liu WN, Zhao W, Zhang XH, Wang Y. [The expression and significance of IGF1R-Ras/RAGE-HMGB1 pathway in colorectal cancer patients with type 2 diabetes mellitus]. Zhonghua Zhong Liu Za Zhi. 2020;42:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 154. | Wang P, Lu YC, Li YF, Wang L, Lee SC. Advanced Glycation End Products Increase MDM2 Expression via Transcription Factor KLF5. J Diabetes Res. 2018;2018:3274084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 155. | Huang M, Geng Y, Deng Q, Li R, Shao X, Zhang Z, Xu W, Wu Y, Ma Q. Translationally controlled tumor protein affects colorectal cancer metastasis through the high mobility group box 1-dependent pathway. Int J Oncol. 2018;53:1481-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 156. | Huang M, Wu R, Chen L, Peng Q, Li S, Zhang Y, Zhou L, Duan L. S100A9 Regulates MDSCs-Mediated Immune Suppression via the RAGE and TLR4 Signaling Pathways in Colorectal Carcinoma. Front Immunol. 2019;10:2243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 157. | Qian F, Xiao J, Gai L, Zhu J. HMGB1-RAGE signaling facilitates Ras-dependent Yap1 expression to drive colorectal cancer stemness and development. Mol Carcinog. 2019;58:500-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 158. | Seguella L, Capuano R, Pesce M, Annunziata G, de Conno B, Sarnelli G, Aurino L, Esposito G. S100B Protein Stimulates Proliferation and Angiogenic Mediators Release through RAGE/pAkt/mTOR Pathway in Human Colon Adenocarcinoma Caco-2 Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 159. | Huang CY, Chiang SF, Chen WT, Ke TW, Chen TW, You YS, Lin CY, Chao KSC, Huang CY. HMGB1 promotes ERK-mediated mitochondrial Drp1 phosphorylation for chemoresistance through RAGE in colorectal cancer. Cell Death Dis. 2018;9:1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 160. | Zhan X, Wu R, Kong XH, You Y, He K, Sun XY, Huang Y, Chen WX, Duan L. Elevated neutrophil extracellular traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the growth and metastasis of hepatocellular carcinoma. Cancer Commun (Lond). 2023;43:225-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 161. | Ando K, Sakoda M, Ueno S, Hiwatashi K, Iino S, Minami K, Kawasaki Y, Hashiguchi M, Tanoue K, Mataki Y, Kurahara H, Maemura K, Shinchi H, Natsugoe S. Clinical Implication of the Relationship Between High Mobility Group Box-1 and Tumor Differentiation in Hepatocellular Carcinoma. Anticancer Res. 2018;38:3411-3418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 162. | Li J, Ren H, Wang J, Zhang P, Shi X. Extracellular HMGB1 promotes CD44 expression in hepatocellular carcinoma via regulating miR-21. Aging (Albany NY). 2021;13:8380-8395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 163. | Li Y, Wang J, Song K, Liu S, Zhang H, Wang F, Ni C, Zhai W, Liang J, Qin Z, Zhang J. S100A4 promotes hepatocellular carcinogenesis by intensifying fibrosis-associated cancer cell stemness. Oncoimmunology. 2020;9:1725355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 164. | Bachmann M, Lamprecht L, Gonther S, Pfeilschifter J, Mühl H. A murine cellular model of necroinflammation displays RAGE-dependent cytokine induction that connects to hepatoma cell injury. J Cell Mol Med. 2020;24:10356-10366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 165. | Li S, Gu H, Huang Y, Peng Q, Zhou R, Yi P, Chen R, Huang Z, Hu X, Tang D. Circular RNA 101368/miR-200a axis modulates the migration of hepatocellular carcinoma through HMGB1/RAGE signaling. Cell Cycle. 2018;17:2349-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 166. | Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, Singhi AD, Kang R, Tang D, Lotze MT, Zeh HJ. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015;22:326-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 167. | Lan CY, Chen SY, Kuo CW, Lu CC, Yen GC. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food Drug Anal. 2019;27:887-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 168. | Uchida C, Mizukami H, Hara Y, Saito T, Umetsu S, Igawa A, Osonoi S, Kudoh K, Yamamoto Y, Yamamoto H, Yagihashi S, Hakamada K. Diabetes in Humans Activates Pancreatic Stellate Cells via RAGE in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 169. | Chen SY, Hsu YH, Wang SY, Chen YY, Hong CJ, Yen GC. Lucidone inhibits autophagy and MDR1 via HMGB1/RAGE/PI3K/Akt signaling pathway in pancreatic cancer cells. Phytother Res. 2022;36:1664-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |